History and physical findings
An 11-year-old neutered male domestic crossbred cat was referred with a 2-month history of progressive stertor. Although upper respiratory tracts signs and ‘snuffling’ had been present for approximately 6 months, they had become much more evident over the preceding 8-weeks. Recently, the owners had reported that the cat apparently had not been able to smell its food. A course of clindamycin had no beneficial effect. The cat also had a history of variable polyuria and polydipsia. Haematology and a panel of serum biochemistries had been undertaken by the referring veterinarian and were unremarkable.
On general physical examination, the cat was in good body condition (4.8 kg) and was bright and alert. An upper respiratory tract stertor was audible, the inspiratory effort was exaggerated and the cat occasionally breathed through its mouth. During examination of the oral cavity, the soft palate was observed to deviate ventrally. Furthermore, a mass could be palpated through the soft palate immediately caudal to the hard palate. The rest of the physical examination was unremarkable, including the peripheral lymph nodes and kidneys.
Our assessment was that the cat had a disease process involving the caudal portion of the nasal cavity and/or adjacent nasopharynx. The differential diagnosis included tumours (eg, lymphoma, adenocarcinoma, etc), fungal lesions (cryptococcosis, aspergillosis, etc), polyp(s), or foreign body granuloma (eg, surrounding a grass awn or fish bone) (Malik et al 1997b, Hunt et al 2002, White et al 2005). Although it is possible to obtain needle aspirates or core biopsies from a lesion such as this via the soft palate, or to actually excise the lesion through an incision in the soft palate, our preferred initial approach is retroflexed nasopharyngoscopy (to visualise the lesion) combined with digital manipulation of the mass and a vigorous nasal flush (Hunt et al 2002). In the absence of a suitable endoscope, it is sometimes possible to retract the soft palate forward using a spay hook or Babcock's tissue forceps to a caudal nasopharyngeal mass (eg, a polyp) and thereby access the lesion (Hunt et al 2002).
Investigation
The cat was anaesthetised to facilitate endoscopic examination of the nasopharynx and obtain representative material for laboratory testing. Following induction of general anaesthesia with propofol, intubation with a cuffed Portex-type endotracheal tube (internal diameter 4.5 mm), maintenance of anaesthesia with isoflurane and desensitisation of the pharyngeal region with topical lignocaine, the cat was positioned in dorsal recumbency and prepared for retroflexed endoscopy. Swabs were packed in the laryngopharynx.
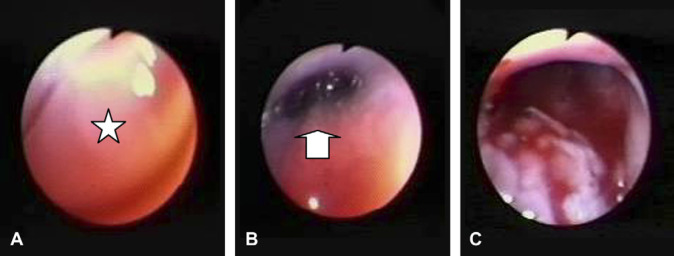
Nasopharyngoscopy was performed with a retroflexed paediatric gastroscope. The choanae could not be visualised due to the presence of a large white mass in the nasopharynx (Fig 1A). A vigorous flush using sequential 5–10 ml aliquots of saline via the left nostril dislodged mucoid material, which was collected for laboratory investigations. It was not possible initially to flush any saline via the right nostril, however, vigorous digital palpation of the lesion through the soft palate permitted flushing via the right naris to dislodge several plaques of white tissue. These were subsequently retrieved and submitted for cytological and histological assessment. It was then possible to visualise the choanae (Fig 1B), and to appreciate the haemorrhage from where the tissue specimens had been detached from the pharyngeal wall (Fig 1C).
Fig 1.
Posterior rhinoscopy of the cat of the case report. (A) Shows a fleshy white rounded lesion (star) occupying the entire nasopharyngeal region. (B) Illustrates a slightly oblique view of the choane (arrow) that was possible after the mass was dislodged by digital manipulation and vigorous flushing. Haemorrhage from the site of attachment of the mass to the pharyngeal wall can be appreciated in (C).
Cytological examination of mucus and particulate material initially retrieved from the nasopharyngeal region revealed numerous inflammatory cells, numerous bacterial rods and scattered spherical, capsulate, narrow-necked, budding yeasts. The inflammatory cells comprised 49% neutrophils, 49% lymphoid cells (the majority of which were medium-large, nucleolated, basophilic lymphoid cells) and the occasional macrophage. ‘Crush’ preparations from the white fleshy material flushed subsequent to disruption of the nasopharyngeal mass revealed sheets of easily damaged, basophilic, nucleolated, large lymphoid cells, scattered small lymphocytes, macrophages and numerous lymphoglandular bodies. The preliminary cytological diagnosis was concurrent cryptococcosis and lymphoma. A moderately heavy growth of a Cryptococcus species was observed after 24 h on birdseed agar incubated at room temperature. Testing on Canavanine glycine bromothymol blue (CGB) agar established that the isolate was C neoformans. Histopathology results from portions of the nasopharyngeal mass were pending.
Questions
What are the “pros” and “cons” of performing a vigorous nasal flush?
What is your assessment of the cytological and microbiological findings from material harvested from the nasopharyngeal region? Does the cat have nasopharyngeal cryptococcosis or lymphosarcoma, or both?
How would you manage the patient?
Answers on page 448.
Answers to What is Your Diagnosis? on page 447 Chronic progressive stertor in an 11-year-old cat
1. What are the “pros” and “cons” of performing a vigorous nasal flush?
Recently, it has become popular to obtain biopsies from nasopharyngeal lesions using biopsy forceps pre-placed in the endoscope prior to retroflexion. This technique is elegant, and allows multiple small tissue specimens to be obtained at little risk to the patient. It requires considerably more skill than endoscopy alone, as the biopsy instrument cannot be advanced through the endoscope after it has been retroflexed 180°, so the forceps must be pre-placed, extending a finite distance ahead of the scope, which is then retracted rostrally (after insertion) to engage the mass.
In contrast, we favour a vigorous nasal flush technique, sometimes combined with digital massage through the soft palate. This is our preferred method for a number of reasons, despite the availability of a suitable endoscope and biopsy forceps:
we are accustomed to this approach, and can do it quickly and efficiently
it can be combined with the use of a Tom-cat catheter, infant feeding tube or an embolectomy catheter, to dislodge material located in the choanae
there is no danger of damaging the scope by moving the biopsy forceps while the tip is fully retroflexed
the size of biopsies obtained with cup-shaped forceps is limited in relation to the ‘chunks’ of material typically obtained with a traumatic flush; furthermore, material obtained by grasping forceps may be superficial and not representative of the entire lesion
by debulking the lesion, immediate relief of the upper airway obstruction occurs, so that the patient can recover more safely from anaesthesia
the technique requires no special equipment and can therefore be utilised in practices without an endoscope and in situations when the animal cannot be sent to a referral centre
Some cats with caudal nasal and nasopharyngeal lymphoma/granuloma have destruction of the cribriform plate while others have extensive forebrain involvement despite no overt signs. Some clinicians consider that as a result of this, forced flushing has the potential of causing iatrogenic damage to the central nervous system. However, the authors and many colleagues in Australia have used this technique on a very large number of cats without significant adverse sequellae being observed.
2. What is your assessment of the cytological and microbiological findings from material harvested from the nasopharyngeal region?
The physical findings were indicative of a disease process centred in the nasopharyngeal region. The presence of stertor is strongly suggestive of a lesion here, and in this instance, the mass was actually palpable through the soft palate and apparent on visual inspection of the oral cavity. These findings provided a clear direction for the diagnostic investigation, as the way forward was obviously to obtain a representative tissue specimen(s) from this lesion for laboratory studies. Although a wide variety of disease processes can produce a space occupying lesion in this location, in Australia the three most common aetiologies are nasal lymphoma (the most common nasal malignancy in the cat), nasopharyngeal mycotic disease (most commonly cryptococcosis or aspergillosis) and a nasopharyngeal polyp (Hunt et al 2002). However, polyps arising from the opening of the auditory tube are usually situated more caudally, arise from a stalk and are amenable to removal via traction. Less common disease conditions affecting this anatomical region include other tumours (eg, adenocarcinoma), foreign bodies and foreign body granulomas.
In this case, cytological evaluation of nasal inflammatory exudates and ‘crush preparations’ of the mass were strongly suggestive of lymphoblastic lymphoma. However, the presence of budding capsulated yeast cells in the mucus complicated the picture and were compatible with concurrent nasal cryptococcosis. There is precedence in the literature for concurrent lymphoma and cryptococcosis in cats, with the suggestion of some immunodeficiency state predisposing to the development of either one or both conditions (Madewell et al 1979, Peaston and Maddison 1999, Zaisser et al 2001). On the other hand, the patient may have actually had posterior nasal lymphoma, with cryptococcal cells merely colonising the mucus trapped in this anatomically disrupted site. Asymptomatic colonisation of the nasal cavity of normal cats with C neoformans var grubii has been reported previously (Malik et al 1997a). Furthermore, cryptococcal organisms can be present in the lower airways in human patients secondary to a variety of structural lower respiratory disorders.
In order to decide between these two different possibilities, we need more information. Firstly, we need the full histological assessment of representative material flushed from the nasopharyngeal mass, to determine if the tissues are consistent with lymphoma, cryptococcal granuloma, colonisation of nasal mucus, or a combination of these alternatives. Secondly, we need to determine whether cryptococcal antigen is present in the cat's serum, as this is a very sensitive sign of cryptococcal invasion of mammalian tissues (Malik et al 1996).
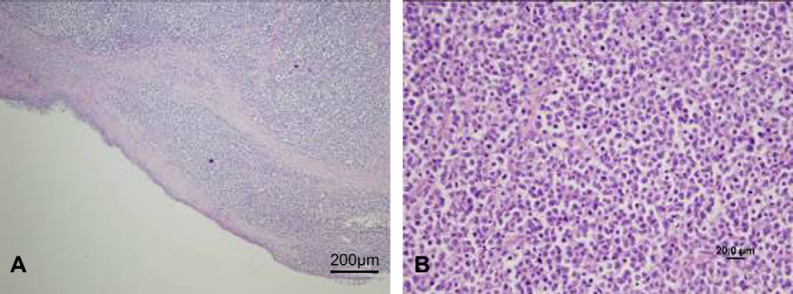
The specimens provided for histological examination comprised sheets of large round cells with large round to irregular cleaved nuclei with clumped chromatin and prominent central nucleoli, and small to moderate amounts of basophilic cytoplasm. Mitotic figures were frequent (3–5 per high powered field) and individual cell necrosis with karyorhexis as well as areas of avascular tumour necrosis were present. Interspersed histiocytic cells containing tingible bodies were prominent in some areas of the tumour. There was occasional ulceration and necrosis of the overlying flattened cuboidal to pseudostratified epithelium. Histologically, all tissues retrieved were consistent microscopically with lymphoma (Fig 2A and B).
Fig 2.
Low power photomicrograph of the nasopharyngeal lesion (A). A high power view is provided in (B).
Immunohistochemistry demonstrated the majority of cells present to be CD79a positive, with scattered CD3 positive cells throughout the lymphoid proliferation (Fig 3); this is consistent with a diagnosis of B cell lymphoma (Gabor et al 1999). Anatomically, we suspect the lymphoma arose in the lymphoid tissue of the pharyngeal tonsil (Chang et al 2006). No portion of the tissues showed evidence for cryptococcal invasion or inflammation of nasal tissue. Yeast cells could not be seen in the small amount of mucus adherent to the surface of the tumour fragments. The serum latex cryptococcal antigen agglutination test (after pronase pretreatment and heating) was negative, confirming that there had been no penetration of the upper respiratory tract epithelium by cryptococcal cells.
Fig 3.

Immunohistology of the neoplastic lymphoid tissue stained with CD79a; note that the majority of cells show staining with this B cell marker.
The final diagnosis was, therefore, lymphoblastic B cell lymphoma of the posterior nasal cavity with colonisation of nasal mucus with C neoformans. The cat had recently been tested for FIV antibodies and FeLV antigen by the referring veterinary clinic and was negative for both. It is interesting to speculate retrospectively as to whether the nasal lymphoma had been developing slowly over 6 months, or only 2 months, with the eventual development of upper respiratory obstruction precipitating detailed diagnostic investigations.
3. How would you manage this patient?
The most significant immediate problem for the patient was its nasopharyngeal lymphoma. However, the diagnostic investigation had been in part therapeutic, because ‘debulking’ the nasopharyngeal mass largely alleviated the upper respiratory obstruction.
Thus, upon recovery from anaesthesia, the cat's breathing pattern was less exaggerated and stertor was no longer apparent. This ‘bought us some time’, as it was possible to clear the nasal colonisation with cryptococci prior to starting multi-agent chemotherapy (incorporating prednisolone) with the attendant immunosuppression. In this way, we were able to guard against the possibility of invasive cryptococcal rhinitis developing.
Accordingly, the cat was discharged the day following endoscopy and administered itraconazole prophylactically (100 mg orally once daily for 2 days; then 100 mg every other day). The cat was then started on a multi-agent chemotherapy protocol (modified Madison-Wisconsin-type protocol; Malik et al 2001), starting with l-asparaginase (450 U/kg), vincristine (0.025 mg/kg) and prednisolone (2 mg/kg orally once daily), while itraconazole was continued for a further week. The cat responded completely to chemotherapy, with resolution of all respiratory signs and weight gain (to 5.3 kg). The cat received the chemotherapy protocol for approximately 12 months, at which time its chronic renal insufficiency progressed to renal failure, eventually necessitating euthanasia. The lymphoma was in remission at the time of euthanasia, consistent with the good prognosis for many of these cases with appropriate treatment that has been reported previously (Malik et al 2001, Teske et al 2002, Malik et al 2003).
Acknowledgements
The authors wish to thank Drs Robert Boyd, David Barton and Lucinda Booth for referring this case and subsequently managing most of its lymphoma chemotherapy protocol.
References
- Chang Y., Thompson H., Reed N., Penderis J. Clinical and magnetic resonance imaging features of nasopharyngeal lymphoma in two cats with concurrent intracranial mass, Journal of Small Animal Practice 47, 2006, 678–681. [DOI] [PubMed] [Google Scholar]
- Gabor L.J., Canfield P.J., Malik R. Immunophenotypic and histological characterisation of 109 cases of feline lymphosarcoma, Australian Veterinary Journal 77, 1999, 436–441. [DOI] [PubMed] [Google Scholar]
- Hunt G.B., Perkins M., Foster S.F., Malik R. Nasopharyngeal disease: a review of 60 cases, Compendium of Continuing Education for the Practicing Veterinarian 24, 2002, 184–200. [Google Scholar]
- Malik R., Gabor L.J., Foster S.F., McCorkell B.E., Canfield P.J. Therapy for Australian cats with lymphosarcoma, Australian Veterinary Journal 79, 2001, 808–817. [DOI] [PubMed] [Google Scholar]
- Malik R., McPetrie R., Wigney D.I., Love D.N. Use of the cryptococcal latex agglutination antigen test for diagnosis and monitoring of therapy in veterinary patients with cryptococcosis, Australian Veterinary Journal 74, 1996, 358–364. [DOI] [PubMed] [Google Scholar]
- Malik R., Wigney D.I., Muir D., Love D.N. Asymptomatic carriage of Cryptococcus neoformans in the nasal cavity of dogs and cats, Journal of Medical and Veterinary Mycology 35, 1997a, 27–31. [PubMed] [Google Scholar]
- Malik R., Martin P., Wigney D.I., Church D.B., Bradley W., Bellenger C.R., Lamb W.A., Barrs V.R., Foster S., Hemsley S., Canfield P.J., Love D.N. Nasopharyngeal cryptococcosis, Australian Veterinary Journal 75, 1997b, 483–488. [DOI] [PubMed] [Google Scholar]
- Malik R., Gabor L.J., Canfield P.J. Lymphoma in Australian cats - lessons for Europe?, Journal of Feline Medicine and Surgery 5 (2), 2003, 147–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madewell B.R., Holmberg C.A., Ackerman N. Lymphosarcoma and cryptococcosis in a cat, Journal of the American Veterinary Medical Association 175, 1979, 65–68. [PubMed] [Google Scholar]
- Peaston A.E., Maddison J.E. Efficacy of doxorubicin as an induction agent for cats with lymphosarcoma, Australian Veterinary Journal 77, 1999, 442–444. [DOI] [PubMed] [Google Scholar]
- Teske E., Stratenvan G., Noortvan R., Rutteman G.R. Chemotherapy with cyclophosphamide, vincristine, and prednisolone (COP) in cats with malignant lymphoma: new results with an old protocol, Journal of Veterinary Internal Medicine 16, 2002, 179–186. [DOI] [PubMed] [Google Scholar]
- White J., Bosward K., Malik R. Episodic dyspnoea, gagging, coughing and persistent nasal discharge in an elderly cat, Australian Veterinary Practioner 35 (4), 2005, 124. [Google Scholar]
- Zaisser A., Kresken J.G., Weber A., et al. A case report of cryptococcosis in cat, Kleintierpraxis 46, 2001, 581. [Google Scholar]