Abstract
Xanthine urolithiasis was found in a 4-year-old spayed female Himalayan cat with a 10-month history of intermittent haematuria and dysuria. Ultrasonographs indicated the existence of several calculi in the bladder that were undetectable by survey radiographic examination. Four bladder stones were removed by cystotomy. The stones were spherical brownish-yellow and their surface was smooth and glossy. Quantitative mineral analysis showed a representative urolith to be composed of more than 95% xanthine. Ultrasonographic examination of the bladder 4.5 months postoperatively indicated the recurrence of urolithiasis. Analysis of purine concentration in urine and blood showed that the cat excreted excessive amounts of xanthine. In order to test the hypothesis that xanthinuria was caused by a homozygote of the inherited mutant allele of a gene responsible for deficiency of enzyme activity in purine degradation pathway, the allele composition of xanthine dehydrogenase (XDH) gene (one of the candidate genes for hereditary xanthinuria) was evaluated. The cat with xanthinuria was a heterozygote of the polymorphism. A single nucleotide polymorphism analysis of the cat XDH gene strongly indicated that the XDH gene of the patient cat was composed of two kinds of alleles and ruled out the hypothesis that the cat inherited the same recessive XDH allele suggesting no activity from a single ancestor.
Xanthine urolithiasis is uncommon in small companion animals (Thumchai et al 1996, Houston et al 2003) and is usually associated with allopurinol administration (Osborne et al 1996, 1999). Therapy with allopurinol causes xanthinuria in animals eating a diet containing purine precursors. However, in a few dogs including Cavalier King Charles spaniels (van Zuilen et al 1997) and Dachshunds (Kucera et al 1997, Flegel et al 1998) that had not been treated with allopurinol, xanthinuria and xanthine urolithiasis have been reported. A family analysis of Cavalier King Charles spaniels with xanthinuria indicated that the disorder was inherited in an autosomal recessive mode (van Zuilen et al 1997). Although several cases of feline xanthine urolithiasis have been documented in cats untreated with allopurinol (Osborne et al 1996, 1999), naturally occurring xanthine urolithiasis has been reported in only one cat (White et al 1997). In the present study, we report a case of xanthine urolithiasis in a cat untreated with allopurinol. In order to scan the gene for causative mutation of a candidate gene, we also evaluate the allele composition of a gene coding xanthine dehydrogenase (XDH) for hereditary xanthinuria.
Case report
A 4.2 kg (9.2 lb) 4-year-old spayed female Himalayan cat was referred to the Veterinary Teaching Hospital of Nippon Veterinary and Life Science University with a 10-month history of intermittent haematuria and dysuria. The cat was housed indoors, and was fed a commercially available canned food. The cat was vaccinated periodically. The results of a complete blood count and serum biochemical analysis were within established reference ranges for the laboratory used. Urinalysis using the urine collected by manual expression after abdominal palpation demonstrated haematuria, a pH of 7.0, and a specific gravity of 1.024. No crystals or bacteria were found on sediment examination. Survey of abdominal radiographs showed no apparent reason for haematuria. However, ultrasound examination showed several calculi in the bladder and a thickened bladder wall. A cystotomy was performed because of suspected urolithiasis. Four bladder stones were removed during surgery. The stones were spherical brownish-yellow and their surface was smooth and glossy (Fig 1). Quantitative mineral analysis by infrared spectrophotometry showed that the urolith composition was greater than 95% xanthine (Marpy-lifetech, Osaka, Japan).
Fig 1.

Picture of a urolith containing 95% xanthine.
The cat recovered well from the surgical procedure, and was discharged from hospital 3 days after the operation. The cat was fed a canned food with high moisture content in an attempt to reduce urinary xanthine concentration. The cat remained free of clinical signs and 4.5 months later, a follow-up ultrasonographic examination was performed. Surprisingly, several calculi were present in the bladder.
To evaluate abnormalities in purine metabolism, analysis of purine concentration in urine and blood was performed by high performance liquid chromatography (SRL, Tokyo, Japan) (Table 1). When xanthine concentration in urine collected by manual expression was measured in comparison with that of creatinine, the cat was found to excrete an excessive amount of xanthine in urine, and this condition was diagnosed as xanthinuria. The xanthine level was also elevated in blood, and was 18 times higher than the level from healthy control cats. However, the hypoxanthine concentration in the cat's blood sample was significantly lower than that from the control cats. On the other hand, the concentrations of uric acid in both blood and urine were similar between the patient and healthy control cats.
Table 1.
Concentration of purine metabolites in blood and urine from a feline xanthine urolithiasis patient and normal controls
| Patient | Control | |
|---|---|---|
| Serum | ||
| Hypoxanthine (μM) | 4.63 | 19.02 |
| Xanthine (μM) | 12.82 | 0.70 |
| Uric acid (mg/dl) | 0.2 | 0.17 |
| Urine | ||
| Xanthine/creatinine (μmol/mmol) | 63.3 | 0.072 |
| Uric acid/creatinine (mg/mg) | 0.035 | 0.034 |
Materials and methods for gene coding
Preparation of genomic DNA from blood samples
Blood samples were collected via venepuncture from the patient with xanthine urolithiasis and 50 control cats. After erythrocytes were lysed in lysis buffer (10 mM Tris (pH 7.5), 0.32 M sucrose, 5 mM MgCl2, 1% TritonX-100), cell pellets were resuspended and incubated overnight at 37°C in an extraction buffer (100 mM Tris (pH 8.0), 150 mM NaCl, 10 mM EDTA, 0.5% SDS, 100 μg/ml proteinase K), and were subjected to extraction with phenol and chloroform. The DNA was subsequently precipitated in ethanol. DNA samples were dissolved in TE buffer (10 mM Tris (pH 8.0), 1 mM EDTA) and stored at −30°C until use.
Primers for PCR analysis
Primers for PCR amplification of feline XDH gene were designed on the sequence of feline XDH cDNA. After determination of the sequences for counterpart of the human XDH gene in the feline XDH gene including a DNA fragment from exon 15 to exon 17, feline-specific primers were constructed for amplifying the polymorphic region in feline XDH gene. The sequences of the primer pairs were as follows: 5′-AGCTTCTTCTTCAAGGTCGACCTGACAGT-3′ with 5′-GGAATGTCGACACAGTACACGGCCTC-3′ for feline XDH gene from exon 15 to exon 17 and 5′-CAGATTCTAGAGGAAACCACTG-3′ with 5′-CCCGAATTCGCAACAGTAACAACTGTTC-3′ for the polymorphic region in intron 15 of feline XDH gene.
Polymerase chain reaction technique
Polymerase chain reaction (PCR) amplification was performed using 100 ng of genomic DNA samples for the template as described in the manufacturer's protocol. For amplifying the feline XDH gene from exon 15 to exon 17 including two introns, PCR was carried out in 20 μl of a reaction mixture consisting of 1× LA PCR buffer (Takara Bio Inc, Ohtsu, Japan), 2.5 mM MgCl2, 0.25 mM each of deoxyribonucleoside triphosphate (dNTPs), 0.2 μM each of primer and 1 unit of TaqDNA polymerase (TaKaRa LA Taq™, Takara Bio Inc). The PCR programme involved an initial denaturation step of 2 min at 94°C followed by 30 cycles of two-step reaction of 20 s at 94°C for denaturation and 15 min at 68°C for annealing and extension. For amplification of genomic DNA fragment including polymorphic region in the intron 15 of feline XDH gene, PCR was performed in 20 μl of a reaction mixture consisting of 20 mM Tris–HCl (pH 8.4), 1.5 mM MgCl2, 50 mM KCl, 0.2 mM each of dNTPs, 0.5 μM each of primer and 0.5 units of TaqDNA polymerase (Invitrogen Corporation, CA, USA). After an initial denaturation step of 2 min at 94°C, three-step PCR programme was carried out by 35 cycles of 30 s at 94°C for denaturation, 30 s at 58°C for annealing and 40 s at 72°C for extension. The PCR programme was followed by a final extension of 7 min at 72°C and a cooling phase at 4°C. Amplified PCR products were separated by gel electrophoresis in a 2% agarose gel, and were evaluated by ethidium bromide staining and ultraviolet transillumination.
DNA sequencing
After the confirmation by agarose gel electrophoresis, PCR products were purified by a PCR purification kit (QIAquick PCR Purification Kit, Qiagen, Hilden, Germany), and sequenced by dideoxy-mediated chain-termination method with a BigDye terminator kit (BigDye Terminator v1.1 Cycle Sequencing Kit, Applied Biosystems, CA, USA). Sequences were analysed on an ABI Prism 310 apparatus (Applied Biosystems, CA, USA).
Restriction fragment length polymorphism with PstI
After PCR amplifying the DNA fragments of feline XDH gene including a polymorphic site in the intron 15, PCR products were treated with restriction enzyme, PstI. Digested fragments were evaluated by agarose gel electrophoresis and ethidium bromide staining.
Results
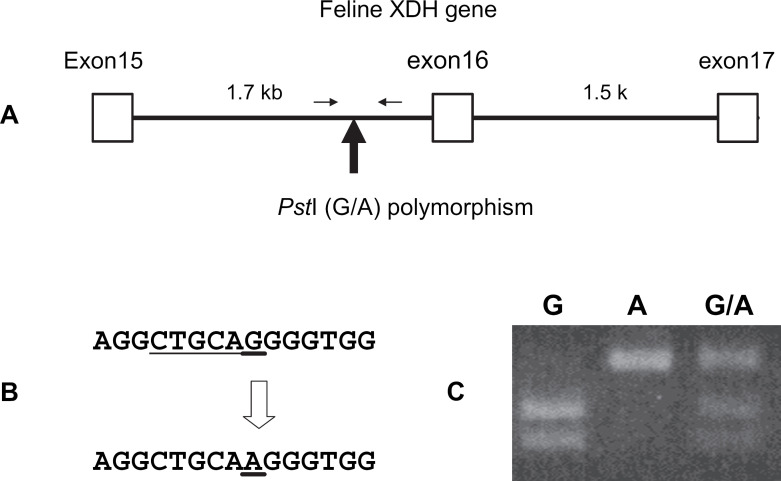
To evaluate the composition of the XDH gene in the cat patient, DNA polymorphisms were investigated in the feline XDH gene, and conditions of alleles were analysed. By the PCR method using primers constructed from a feline XDH cDNA sequence, a DNA fragment containing a part of feline XDH gene was amplified. Sequence analysis of the cloned PCR product showed that the PCR product included a DNA fragment of the counterpart of human XDH gene from exon 15 to exon 17 with two introns in feline XDH gene (GenBank accession number: DQ097516). Comparison of the DNA sequences of the cloned PCR products from several cats indicated that there was an A–G single nucleotide polymorphism (SNP) in the intron 15 region of feline XDH gene (Fig 2A). Three genotypes including homozygous A-type, homozygous G-type and heterozygous A–G-type were observed. The A–G mutation changed the digestion site of the restriction enzyme PstI. The SNP of an A–G mutation was detected by PCR–RFLP using PstI (Fig 2B and C). When the distribution of the A–G polymorphism in the cat XDH gene was analysed using 50 unrelated cats, gene frequencies of A-type and G-type alleles were 0.54 and 0.46, respectively (Table 2). This polymorphism was in Hardy–Weinberg equilibrium stating that allele frequencies in a single gene locus remain unchanged after one generation of random mating. The SNP has high variability, which makes it ideal for resolution of allele composition. The cat with xanthinuria was a heterozygote of the A-type and G-type alleles of the polymorphism.
Fig 2.
RFLP after PstI digestion in feline XDH gene. A: Map of RFLP polymorphic site in feline XDH gene. The exons encoding proteins are indicated by open boxes. The number of each exon corresponds to exon in the human XDH gene. The lines between open boxes indicate the introns. The number above each line shows the length of the intron. A solid arrow indicates the polymorphic site in the intron of feline XDH gene. Two arrows show the primer set used for amplification for PCR-RFLP analysis. B: Localisation of the A–G mutation site. The polymorphic sites are indicated by underlines and an open arrow. A digestion site with PstI is also shown below the G-type sequence in feline XDH gene. C: Gel electrophoretic patterns of RFLP after PstI digestion in feline XDH gene. Three genotypes of G homozygote, A homozygote and A–G heterozygote were distinguished by digested fragment lengths.
Table 2.
Distribution of RFLP with PstI in feline XDH gene
| Types | No. |
| G | 11 |
| A–G | 24 |
| A | 15 |
| Total | 50 |
Gene frequencies G: 0.46, A: 0.54 (χ2 =0.057, d.f.=1, 0.80< P<0.90).
Discussion
There had been no history of allopurinol administration to the cat described in this report. Allopurinol is a competitive inhibitor of XDH, the enzyme catalysing purine metabolism. In this case, four bladder stones estimated by ultrasonographic examination before surgery were removed. It was possible that some calculi were left in bladder or urethra after surgery in spite of repeated flushing and aspiration to remove discrete calculi and small particles of sand for lack of ultrasonographic examination immediately after the surgery. However, recurrence of bladder urolithiasis after surgery and high xanthine concentration in the patient's blood and urine suggested that congenital metabolic abnormalities caused the disease in the cat.
Xanthinuria is a rarely recognised disorder characterised by a deficiency in XDH enzyme activity observed in humans and other manmalians. XDH is an enzyme which belongs to the family of molibdo-flavo enzymes that require a molybdenum cofactor and flavin adenine dinucleotide for their catalytic activity along with aldehyde oxidase (AO) and sulphite oxidase (SO) (Simmonds et al 1995, Kisker et al 1997). XDH catalyses two steps of the purine degradation pathway, converting hypoxanthine to xanthine and xanthine to uric acid. XDH deficiency leads to excess urinary excretion of xanthine and hypoxanthine. Hypoxanthine is very soluble, but xanthine is extremely insoluble in urine at any pH (Simmonds et al 1995). Therefore, xanthinuria derived from the deficiency of XDH activity results in the formation of xanthine calculi.
Hereditary xanthinuria in humans is classified into three subtypes according to deficiencies in activities of three enzymes including XDH, AO, and SO (Simmonds et al 1995, Johnson and Wadman 1995). The categories are xanthinuria type I and II, and molybdenum cofactor deficiency. Xanthinuria type I lacks only XDH activity caused by a loss-of-function mutation in the XDH gene (Ichida et al 1997). In xanthinuria type II, both XDH and AO activities are deficient. A mutation in the gene encoding molybdenum cofactor sulphurase (MCSU/HMCS) is responsible for xanthinuria type II (Watanabe et al 2000, Ichida et al 2001). The third type involving molybdenum cofactor deficiency is caused by a loss-of-function mutation of molybdenum cofactor synthetase catalysing the first steps in molybdenum cofactor synthesis (Reiss et al 1998). This type is lacking all three enzyme activities, and is associated with severe neurological disorders in the neonatal period caused by SO deficiency. In the contrary, about 60% of human patients with xanthinuria type I and II (classical xanthinuria) usually have no symptoms and have been discovered incidentally during investigation for another disorder, on routine examination or during investigation in family studies for xanthinuria. Approximately 40% of human patients may develop urinary calculi, acute renal failure or myositis (Simmonds et al 1995). However, in bovine xanthinuria type II especially in Japanese Black cattle, the patient shows lethal growth retardation at approximately 6 months of age (Watanabe et al 2000).
In our study, although there was no information of enzyme activities related to molybdenum cofactor, the cat was diagnosed as classical xanthinuria (xanthinuria I or II), not molybdenum cofactor deficiency, as the cat grew normally and did not have neurological abnormalities. Classical xanthinuria is an autosomal recessive disorder in humans (Simmonds et al 1995). The cat with xanthinuria was thought to have acquired the disorder with xanthinuria because of a homologous mutation in the allele suggesting no XDH activity. In theory, the mutated allele in the XDH gene could have derived from one ancestor. However, contrary to our expectations, SNP analysis of the cat XDH gene strongly indicated that the XDH gene of the patient cat was composed of two kinds of alleles and ruled out the hypothesis that the cat inherited the same recessive XDH allele suggesting no activity from a single ancestor. Although it is possible for two kinds of alleles to have no enzyme activity, it is reasonable to think that XDH gene mutation is unlikely to be the cause of xanthinuria in this cat. The XDH gene is responsible for xanthinuria type I. On the other hand, MCSU gene is associated with xanthinuria type II. Xanthinuria type I and II aren't distinguishable clinically without data from an allopurinol loading test or activities of XDH, AO and SO enzymes. If the cat with xanthinuria shows xanthinuria type II, analysis of MCSU gene will be useful for determining the cause of the disease.
In the present case, the cat was found to excrete excessive amounts of xanthine in urine, and the blood xanthine concentration was also elevated. According to the purine metabolic pathway in humans, deficient XDH activity not only induces an increase in xanthine levels, but also increases hypoxanthine, and decreases uric acid in both blood and urine (Simmonds et al 1995). The serum hypoxanthine concentration in this cat was significantly lower than that in healthy control cats. Although the hypoxanthine salvage pathway is thought to be enhanced in hereditary xanthinuria patients (Mateos et al 1987), the blood level of hypoxanthine is not below the control level. On the other hand, XDH converts xanthine to uric acid. As a consequence of deficiency of XDH activity, uric acid levels in blood and urine are expected to decrease dramatically. Human patients with classical xanthinuria are usually asymptomatic, and are diagnosed during routine medical examinations with hypouricaemia. However, hypouricaemia was not diagnosed in this cat with xanthinuria. In a previous report in a cat with xanthine urolithiasis (White et al 1997), there was no information on blood and urine examinations for purine metabolism because of accidental death prior to consultation for blood and urine sampling. In a family of Cavalier King Charles spaniels with xanthine calculi, urinary concentrations of xanthine and hypoxanthine were elevated (van Zuilen et al 1997). However, in a Dachshund with naturally occurring xanthine urolithiasis, although xanthine calculi were located in the kidney and bladder, the concentrations of both xanthine and hypoxanthine seemed to be lower than the values in healthy control dogs (Flegel et al 1998). To interpret the oxypurine profile in the present cat, understanding of exact changes in the metabolic pathway involving XDH enzyme deficiency in cats is required.
There is no specific or effective prevention therapy for xanthine urolithiasis. A high fluid intake coupled with a diet low in purine is the only and the most effective prevention treatment for classical xanthinuria in humans (Simmonds et al 1995). In this cat case, only feeding a high moisture food could manage to restrain the growth of bladder stones and the occurrence of clinical signs. To evaluate the effectiveness of a high fluid intake in the prevention therapy in cat xanthinuria, detailed monitoring of xanthine concentration in urine is required in relation to the change of fluid intake.
Feline xanthinuria is recognised to be an extremely uncommon metabolic disorder (Thumchai et al 1996, Houston et al 2003). It is possible that it is not being detected as inherited xanthinuria because classical xanthinuria usually shows no clinical signs. According to the analysis of mineral composition of feline urolith specimens, 28 of 17,383 were xanthine uroliths (Osborne et al 1999). If all xanthine urolithiases in cats are derived from hereditary XDH deficiency as an autosomal recessive inheritance, although gene frequency of the recessive mutated allele responsible for xanthine urolithiasis is appeared to be exceedingly low in feline population, the distribution of the mutated gene might increase in some inbred cats. Congenital xanthinuria might be one of the inheritary diseases to watch out for in cats.
Acknowledgements
This study was supported in part by a grant from Japan Forum on Small Animal Clinical Nutrition (JFSACN) and by ‘Academic Frontier’ Project for Private Universities: matching fund subsidy from the Ministry of Education, Culture, Sports, Science and Technology, 2005–2009.
References
- Flegel T., Freistadt R., Haider W. Xanthine urolithiasis in a dachshund, Veterinary Record 143, 1998, 420–423. [DOI] [PubMed] [Google Scholar]
- Houston D.M., Moore A.E., Favrin M.G., Hoff B. Feline urethral plugs and bladder uroliths: a review of 5484 submissions 1998–2003, Canadian Veterinary Journal 44, 2003, 974–977. [PMC free article] [PubMed] [Google Scholar]
- Ichida K., Amaya Y., Kamatani N., Nishino T., Hosoya T., Sakai O. Identification of two mutations in human xanthine dehydrogenase gene responsible for classical type I xanthinuria, Journal of Clinical Investigation 99, 1997, 2391–2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichida K., Matsumura T., Sakuma R., Hosoya T., Nishino T. Mutation of human molybdenum cofactor sulfurase gene is responsible for classical xanthinuria type II, Biochemical and Biophysical Research Communications 282, 2001, 1194–1200. [DOI] [PubMed] [Google Scholar]
- Johnson J.L., Wadman S.K. Molybdenum cofactor deficiency and isolated sulfite oxidase deficiency. Scriver C.R., Beaudet A.L., Sly W.S., Valle D. 7th edn, The Metabolic and Molecular Bases of Inherited Disease Vol. 2, 1995, McGraw-Hill: New York, 2271–2283. [Google Scholar]
- Kisker C., Schindelin H., Rees D.C. Molybdenum-cofactor-containing enzymes: structure and mechanism, Annual Review of Biochemistry 66, 1997, 233–267. [DOI] [PubMed] [Google Scholar]
- Kucera J., Bulkova T., Rychla R., Jahn P. Bilateral xanthine nephrolithiasis in a dog, Journal of Small Animal Practice 38, 1997, 302–305. [DOI] [PubMed] [Google Scholar]
- Mateos F.A., Puig J.G., Jimenez M.L., Fox I.H. Hereditary xanthinuria. Evidence for enhanced hypoxanthine salvage, Journal of Clinical Investigation 79, 1987, 847–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne C.A., Lulich J.P., Ulrich L.K., Bird K.A. Feline crystalluria. Detection and interpretation, Veterinary Clinics of North America Small Animal Practice 26, 1996, 369–391. [DOI] [PubMed] [Google Scholar]
- Osborne C.A., Lulich J.P., Bartges J.W., Ulrich L.K., Koehler L.A., Bird K.A., Swanson L.L., Austin G.W., Prien E.L., Jr., Steinam K.U. Drug-induced urolithiasis, Veterinary Clinics of North America Small Animal Practice 29, 1999, 251–266. [DOI] [PubMed] [Google Scholar]
- Reiss J., Cohen N., Dorche C., Mandel H., Mendel R.R., Stallmeyer B., Zabot M.T., Dierks T. Mutations in a polycistronic nuclear gene associated with molybdenum cofactor deficiency, Nature Genetics 20, 1998, 51–53. [DOI] [PubMed] [Google Scholar]
- Simmonds H.A., Reiter S., Nishino T. Hereditary xanthinuria. Scriver C.R., Beaudet A.L., Sly W.S., Valle D. 7th edn, The Metabolic and Molecular Bases of Inherited Disease Vol. 2, 1995, McGraw-Hill: New York, 1781–1797. [Google Scholar]
- Thumchai R., Lulich J., Osborne C.A., King V.L., Lund E.M., Marsh W.E., Ulrich L.K., Koehler L.A., Bird K.A. Epizootiologic evaluation of urolithiasis in cats: 3,498 cases (1982–1992), Journal of the American Veterinary Medical Association 208, 1996, 547–551. [PubMed] [Google Scholar]
- Watanabe T., Ihara N., Itoh T., Fujita T., Sugimoto Y. Deletion mutation in Drosophila ma-l homologous, putative molybdopterin cofactor sulfurase gene is associated with bovine xanthinuria type II, Journal of Biological Chemistry 275, 2000, 21789–21792. [DOI] [PubMed] [Google Scholar]
- White R.N., Tick N.T., White H.L. Naturally occurring xanthine urolithiasis in a domestic shorthair cat, Journal of Small Animal Practice 38, 1997, 299–301. [DOI] [PubMed] [Google Scholar]
- van Zuilen C.D., Nickel R.F., van Dijk T.H., Reijngoud D.J. Xanthinuria in a family of Cavalier King Charles spaniels, Veterinary Quarterly 19, 1997, 172–174. [DOI] [PubMed] [Google Scholar]