Abstract
A 14-year-old female domestic shorthair cat was presented to Tehran University Veterinary Teaching Hospital for a persistent fever, anorexia, intermittent vomiting, weight loss and weakness. The main clinical signs were pale mucous membranes, dehydration and splenomegaly. The complete blood count and serum biochemistry tests revealed non-regenerative anaemia, thrombocytopenia and increased alkaline phosphatase (ALP) activity. An enzyme-linked immunosorbent assay (ELISA) test for feline leukaemia virus was negative. Blood film and bone marrow examination revealed a large number of immature eosinophils with variable sizes and numbers of faintly azurophilic granules. Cytochemical staining of blood film demonstrated 70% positive cells for ALP activity. Four percent CD34 positive cells were detected by flow cytometry. As eosinophilic leukaemia is difficult to identify by light microscopy, well-defined diagnostic criteria and the use of flow cytometry and cytochemical staining can improve the ability to correctly diagnose this type of leukaemia in cats.
Eosinophilic leukaemia is extremely rare in cats and is characterised by a rapid progression of disease and death (Finlay 1985). Blood eosinophil counts are markedly increased with many immature cells present. Splenomegaly and immature eosinophilic infiltration in multiple organs have also been described (Holzworth 1960, Finlay 1985). Chronic eosinophilic leukaemia (CEL) is particularly difficult to differentiate from the hypereosinophilic leukaemia syndrome (HES). Indeed, the two conditions may represent different patterns of a similar neoplastic process (Thrall et al 2004, McManus 2005).
A 14-year-old female domestic shorthair (DSH) cat was presented to Tehran University Veterinary Teaching Hospital with a 1-week history of persistent fever, anorexia, intermittent vomiting, weight loss (not emaciated) and weakness. Physical examination revealed pale mucous membranes, dehydration and splenomegaly, which were confirmed by abdominal radiography.
Laboratory evaluation included a complete blood count (Vet Hema-Screen 18; Hospitex Diagnostics, Italy), serum biochemical profile (Eppendorf EPOS Analyzer 5060, Germany), bone marrow aspiration, faecal flotation test for the detection of parasitic infections (direct flotation method with saturated sugar solution) and an antigen-capture enzyme-linked immunosorbent assay (ELISA) test for FeLV P27 protein (Pet Check FeLV ELISA; Synbiotics, San Diego, California). The following cytochemical stains on blood smears were performed using a kit (Merck, Germany): leukocyte alkaline phosphatase (LAP), Sudan black B (SBB), chloroacetate esterase (CAE) and α-naphthyl butyrate esterase (α-NBE).
Flow cytometric analyses using antibodies against human CD34, CD11b and CD33 (Partec PAS III, Germany) were conducted with EDTA-anticoagulated blood samples from control animals as well as the patient in duplicate to evaluate if there would be any cross reactions with feline antigens.
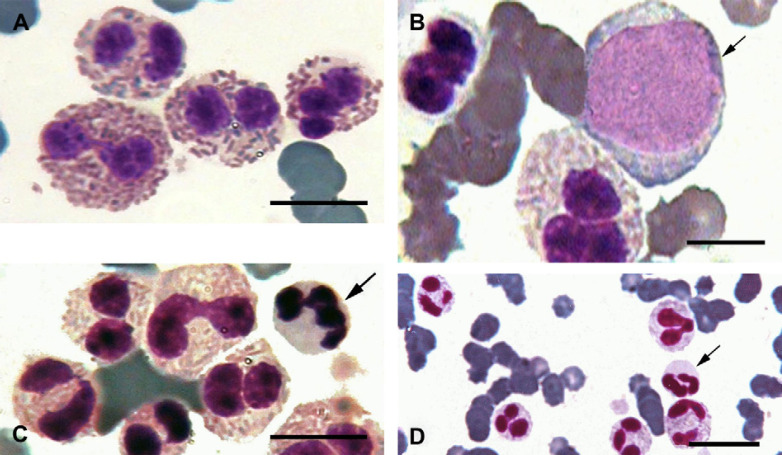
Abnormalities noted in the complete blood count included macrocytosis and non-regenerative anaemia characterised by a packed cell volume of 20% and lack of reticulocytosis. In addition, leukopenia, neutrophilia and marked eosinophilia (180,818 cells/μl) were found (Table 1). The peripheral blood demonstrated a large number of immature eosinophils with variable numbers of faintly azurophilic granules (Fig 1).
Table 1.
Complete blood count data
| Parameter | Patient | Reference range * |
|---|---|---|
| Haematocrit (%) | 20 | 25.0–45.0 |
| Haemoglobin (g/dl) | 6.9 | 8.5–16.5 |
| Total red cell count (×106/μl) | 3.1 | 5.5–9.5 |
| Mean corpuscule volume (fl) | 64.5 | 41.0–55.0 |
| Mean corpuscular haemoglobin concentration (%) | 34.5 | 31.0–35.0 |
| Reticulocytes (×103/μl) | 5 | <15 |
| Aggregate | 40 | <200 |
| Punctate | 100 | 250–700 |
| Platelets (×103/μl) | ||
| White blood cells (/μl) | 203,625 | 6000–19,000 |
| Neutrophils (/μl) | 18,326 | 3000–12,500 |
| Lymphocytes (/μl) | 2443 | 1300–8000 |
| Eosinophils (/μl) | 180,818 | 0–1500 |
| Segmented (/μl) | 169,008 | 0–1500 |
| Bands (/μl) | 6720 | 0 |
| Metamyelocysts (/μl) | 4072 | 0 |
| Myeloblast (/μl) | 1018 | 0 |
| Monocyte (/μl) | 0 | 0–650 |
All reference ranges (95% quantile) were calculated based on data obtained from pet cats (>10 years of age; Tehran, Iran).
Fig 1.
Wright–Giemsa stained blood film. (A) Feline eosinophils with characteristic rod-shaped granules. (B) Immature eosinophils and eosinophilic precursors in the peripheral blood. (C) and (D) Arrows indicate neutrophils among eosinophils in the peripheral blood. Scale bars, 5 μm in panels (A)–(C) and 10 μm in panel (D).
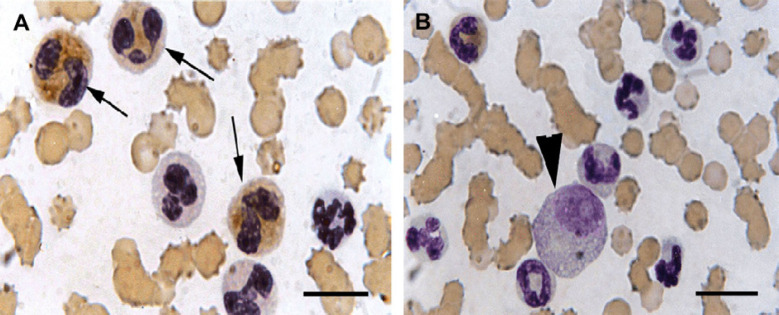
The cells had abundant cytoplasm with numerous azurophilic granules, variable numbers of poorly staining eosinophilic granules and round-oval nuclei with finely stippled chromatin and prominent nucleoli (Figs 1 and 2). These cells were difficult to identify as to their origin on the routine stained blood smear and cytochemical staining of these cells indicated positive LAP activity (Fig 2), but were negative for SBB, CAE and α-NBE (data not shown).
Fig 2.
LAP staining of the peripheral blood. (A) Blood film demonstrated 70% positive cells for ALP activity (arrows). (B) “Immature eosinophils' and eosinophilic precursors' round-oval nuclei” with finely stippled chromatin and prominent nucleoli (arrowhead). Scale bars, 10 μm.
For flow cytometric analysis, blood samples from three age-matched clinically healthy cats were used as a control. The patient had 4% CD34 positive cells detected by flow cytometry whereas peripheral blood cells were negative for CD34 in the control cats. The patient and control cats were negative for both CD11b and CD33. The bone marrow aspirate was highly cellular with an increased myeloid-to-erythroid (M:E) ratio (15.1:1) because of an increase in granulopoiesis, especially of eosinophils. The eosinophilic cell type was dominant and the pertinent precursors were increased but the differential estimation of marrow myeloid lineage was impossible because of the existing myelodysplasia. The percentages of proliferating and maturing pool of myeloid lineage were 74 and 26, respectively. The number of marrow megakaryocytes was three per low-power field (10×), suggesting megakaryocytic hypoplasia (Thrall et al 2004).
Serum biochemical abnormalities included marked increase in alkaline phosphatase (ALP) (1627 U/l; range, 10–84 U/l) and mild increase in glucose (134 mg/dl; range, 62–105 mg/dl). Results for serum alanine aminotransferase (ALT), blood urea nitrogen (BUN), cholesterol and total protein were within the reference ranges. The faecal flotation test and the serum FeLV ELISA test were negative. Treatment consisted of intravenous Ringer's solution, intramuscular cefazolin (Keflin) (100 mg every 12 h for 3 days) and a single dose (1 mg/kg) of intramuscular nandrolone decanoate (Deca-Durabol).
After an initial improvement, the condition of the cat deteriorated within a week and the owners elected euthanasia. A request for necropsy was declined.
Eosinophilic leukaemia has been reported sporadically because of both the rarity of the disease and the lack of well-established diagnostic criteria. In this report, morphology, cytochemistry and flow cytometry were used together to identify and characterise eosinophilic leukaemic cells in a cat suspected with eosinophilic leukaemia. Poorly differentiated eosinophils may resemble neutrophils, basophils or monocytes, therefore, differentiation from neutrophilic, basophilic and monocytic leukaemia based on cytochemical staining of the cells is necessary. The results of cytochemical reactions, positive LAP staining and negative SBB, CAE and α-NBE stainings, were characteristic of feline eosinophils and excluded the possibilities of neutrophils, monocytes and basophils (Facklam and Kociba 1986, Jain et al 1989, Raskin and Valencialo 2000). As peroxidase staining was not undertaken, there is still the possibility that this condition represents a mixed chronic myelogenous leukaemia with more than one cell line involved (ie, neutrophils as well as eosinophils).
Some authors have tried to differentiate particularly CEL from HES. The latter is characterised by a chronic course with mature eosinophilia, organ system dysfunction with frequent eosinophilic infiltration of the intestine, and lack of an identifiable cause of eosinophilia (Hendrick 1981, Borgfeldt et al 1988, Swenson et al 1993, Huibregtse and Turner 1994). CEL is characterised by the presence of blasts in the peripheral blood, morphological abnormalities in eosinophils, immaturity of eosinophils with abnormal granulation, high M:E ratio and significant anaemia (Hendrick 1981, Perkins 2000). In humans, CEL is a clonal proliferation of eosinophils characterised by persistent unexplained eosinophilia for more than 6 months at over 1500 cells/μl (Brito-Babapulle 1997). However, HES is an unexplained polyclonal increase in eosinophils in which, as in CEL, increases are found in both the peripheral blood and the tissues. However, this is not always apparent (McManus 2005). Morphologically, the only clue useful in distinguishing the two conditions is an increase in blast cells in eosinophilic leukaemia (McManus 2005). Recent reports suggest that the separation between the two disorders may be artificial, and that they both may represent a spectrum of the same disease (Thrall et al 2004, McManus 2005).
A marked splenomegaly was also observed in this patient, which might occur as a result of the infiltration of eosinophils along splenic sinuses (Hendrick 1981, Borgfeldt et al 1988).
Our flow cytometric results showed a cross reaction between feline and human CD34. However, the patient cells as well as those from the control animals did not react with antibodies against human CD33 and CD11b. The 4% CD34 positive cells in the patient's blood might represent blasts. The Animal Leukemia Study Group of the American Society for Veterinary Clinical Pathology proposed a blast percentage of over 30% threshold for the diagnosis of acute myeloid leukaemia (Jain et al 1991, Ledieu et al 2005). This case describes an extremely high eosinophil peripheral blood count with immature forms and a blast cell count of less than 30%, which would be consistent with a chronic myelogenous leukaemia of eosinophilic origin.
Serum ALP activity was markedly increased in this cat. The concurrent presence of normal ALT is strongly suggestive of a cholestatic cause for the increased ALP. In addition, the elevated ALP may have been due to increased production of glucocorticoids associated with neoplasms (Lassen 2004) and/or enzyme release from circulating eosinophils (Swenson et al 1993), nonetheless leukocyte-derived isoenzyme have not been described in cat.
Although, the serum ELISA test for FeLV was negative, this does not eliminate the presence of virus incorporated into bone marrow progenitor cells, resulting in a latent disease. Indeed, macrocytosis in the absence of reticulocytosis is similar to that observed in the FeLV-infected cats, which is likely related to defective erythrocytic maturation (Weiser and Kociba 1983). Molecular methods such as polymerase chain reaction would be helpful for definitive diagnosis. The majority of the references in the literature indicates most cats with eosinophilic leukaemia are FeLV negative. Alternatively, feline leukaemia virus may play a part in the pathogenesis of eosinophilic leukaemia by inducing genetic alteration in haematopoietic stem cells, but at the time when overt leukaemia develops no evidence of virus infection can be determined (Toth et al 1985, Lewis et al 1985).
References
- Borgfeldt C.B., Hansen B., Manthrope R. The hypereosinophilic syndrome. Report of a case with successful medical treatment following cardiac biopsy, Scandinavian Journal of Rheumatology 17, 1988, 51–54. [DOI] [PubMed] [Google Scholar]
- Brito-Babapulle F. Clonal eosinophilic disorders and the hypereosinophilic syndrome, Blood Review 11, 1997, 129–145. [DOI] [PubMed] [Google Scholar]
- Facklam N.R., Kociba G.J. Cytochemical characterization of feline leukemic cells, Veterinary Pathology 23, 1986, 155–161. [DOI] [PubMed] [Google Scholar]
- Finlay D. Eosinophilic leukaemia in the cat: a case report, Veterinary Record 116, 1985, 567. [DOI] [PubMed] [Google Scholar]
- Hendrick M. A spectrum of hypereosinophilic syndrome examplified by six cats with eosinophilic enteritis, Veterinary Pathology 18, 1981, 188–200. [DOI] [PubMed] [Google Scholar]
- Holzworth J. Leukaemia and related neoplasm in the cat: two malignancies other than lymphoid, Journal of the American Veterinary Medical Association 136, 1960, 107–121. [PubMed] [Google Scholar]
- Huibregtse B.A., Turner J.L. Hypereosinophilic syndrome and eosinophilic leukaemia, Journal of the American Animal Hospital Association 30, 1994, 591–599. [Google Scholar]
- Jain N.C., Blue J.T., Grindem C.B., Harvey J.W., Kociba G.J., Krehbiel J.D., Latimer K.S., Raskin R.E., Thrall M.A., Zinkl J.G. Proposed criteria for classification of acute myeloid leukaemia in dogs and cats, Veterinary Clinical Pathology 20, 1991, 63–82. [DOI] [PubMed] [Google Scholar]
- Jain N.C., Kono C.S., Madewell B.R. Cytochemical studies of normal feline blood and bone marrow cells, Annals of Hematology 58, 1989, 195–199. [DOI] [PubMed] [Google Scholar]
- Lassen E.D. Laboratory evaluation of the liver. Thrall M.A. Veterinary Hematology and Clinical Chemistry, 1st edn, 2004, Lippincott, Williams and Wilkins: Philadelphia, 355–375. [Google Scholar]
- Ledieu D., Palazzi X., Marchal T., Fournel-Fleury C. Acute megakaryoblastic leukaemia with erythrophagocytosis and thrombosis in a dog, Veterinary Clinical Pathology 34, 2005, 52–56. [DOI] [PubMed] [Google Scholar]
- Lewis M.G., Kociba G.J., Rojko J.L., Stiff M.I., Haberman A.B., Velicer L.F., Olsen R.G. Retroviral-associated eosinophilic leukaemia in the cat, American Journal of Veterinary Research 46, 1985, 1066–1070. [PubMed] [Google Scholar]
- McManus P.M. Classification of myeloid neoplasms: a comparative review, Veterinary Clinical Pathology 34, 2005, 189–212. [DOI] [PubMed] [Google Scholar]
- Perkins P. Hematologic abnormalities accompanying leukaemia. Feldman B.F., Zinkl J.G., Jain N.C. Schalm's Veterinary Hematology, 5th edn, 2000, Lippincott, Williams and Wilkins: Philadelphia, 740–746. [Google Scholar]
- Raskin R.E., Valencialo A. Cytochemical test for diagnosis of leukaemia. Feldman B.F., Zinkl J.G., Jain N.C. Schalm's Veterinary Hematology, 5th edn, 2000, Lippincott, Williams and Wilkins: Philadelphia, 755–763. [Google Scholar]
- Swenson C.L., Carothers M.A., Wellman M.L., Kociba G.J. Eosinophilic leukaemia in a cat with naturally acquired feline leukaemia virus infection, Journal of the American Animal Hospital Association 29, 1993, 497–501. [Google Scholar]
- Thrall M.A., Weiser G., Jain N. Laboratory evaluation of bone marrow. Thrall M.A. Veterinary Hematology and Clinical Chemistry, 1st edn, 2004, Lippincott, Williams and Wilkins: Philadelphia, 147–178. [Google Scholar]
- Toth S.R., Nash A.S., McEwan A.M., Jarrett O. Chronic eosinophilic leukaemia in blast crisis in a cat negative for feline leukaemia virus, Veterinary Record 117, 1985, 417–420. [DOI] [PubMed] [Google Scholar]
- Weiser M.G., Kociba G.J. Erythrocyte macrocytosis in feline leukaemia virus associated anemia, Veterinary Pathology 20, 1983, 687–697. [DOI] [PubMed] [Google Scholar]