Abstract
Fifteen cats were evaluated with urethral obstruction. Penile trauma by catheterization was the major indication for perineal urethrostomy. Ten cats had developed a urethral stricture and five had rupture of the urethra following medical management. All cats had abnormalities in penis and/or prepuce and/or scrotal sacs including hyperemia or swelling. Perineal urethrostomy was performed in all cases and they were evaluated for 6 months after surgery. Few complications were noted. Urinary tract infection was the most frequent complication observed. The clients considered their cats to have a good quality of life following surgery.
Perineal urethrostomy is a surgical procedure performed in male cats to create a permanent opening between the pelvic urethra and the skin in the perineal region. Indications include recurrent urethral obstruction non-responsive to nutritional or medical therapy and urethral obstruction that cannot be relieved by catheterization and reverse flushing, including urolithiasis, urethral strictures, urethral trauma or neoplasia. Urethral strictures may occur with chronic urethritis or repeated trauma from catheterization. Likewise, perineal urethrostomy is indicated if the stricture is within the penile urethra. If the location of the obstruction is unknown, contrast radiography may be necessary (Osborne et al 1991, Smith et al 1991, Barsanti et al 1994).
Several techniques and modifications for perineal urethrostomy have been described since the early 1960s. The goal of these techniques is to create a permanent opening three to four times larger than the penile urethra (Carbone 1963, 1967, Christensen 1964, Manziano and Manziano 1966, Blake 1968, Wilson and Harrison 1971, Caywood and Raffe 1984). The Wilson and Harrison technique has been reported to have a lower rate of complications (Smith and Schiller 1978).
Perineal urethrostomy in the cat, when indicated and when properly performed, is beneficial to the cat, rewarding to the surgeon, and plagued with few complications. Complications reported after perineal urethrostomy include hemorrhage from erectile tissue, wound dehiscence, urine leakage into the perineal tissue, recurrent cystitis, ascending urinary tract infection (UTI), urethral stricture, urinary and fecal incontinence, perineal hernia, and rectourethral fistula. Although many of these complications are uncommon or rare, recurring bacterial UTI is frequently a long-term postoperative problem (Smith et al 1991, Smith 2002, Bass et al 2005).
This study aimed to determine the indications for, and the complications in, 15 cats where perineal urethrostomy was performed until 6 months after the surgical procedure.
Material and methods
Fifteen feline perineal urethrostomies were performed at the University Rural of Rio de Janeiro and Gatos and Gatos Veterinary Clinic. Follow-up evaluation at least 6 months postoperatively was available from the medical records and verbal contact. All cats had urethral obstruction and these cats had been catheterized at least once before being evaluated.
Data collected included signalment, clinical signs and preoperative diagnosis based on the history and physical examination findings, as well as further investigative tests including urinalysis, bacteriological urine culture, complete blood count, biochemical profile (serum urea nitrogen, creatinine and potassium) and survey and contrast abdominal radiography. Urinalysis and bacteriological urine culture was performed in first day and in six month.
Cats with fluid, electrolyte and acid–base imbalances caused by urethral obstruction were preoperatively stabilized by intravenous fluid therapy and cystocentesis. Perineal urethrostomies were performed on the cats using the Wilson and Harrison technique (Wilson and Harrison 1971).
Postoperative treatment consisted of the use of analgesics (0.1 mg/kg meloxicam) for 5 days, an Elizabethan collar for 15 days and antibiotics for 3 weeks. Sutures were removed 15 days following surgery. Elizabethan collars were used postoperatively to prevent licking and self-mutilation of the surgical site.
Results
Fifteen cats with urethral obstruction were evaluated. Their age ranged from 8 to 120 months and body weight from 3.22 to 6.51 kg. All cats had urethral obstruction that could not be relieved by catheterization and reverse flushing. When referred, these cats had penile urethral trauma caused by inadequate or recurrent catheterization. All cats had abnormalities in the penis and/or prepuce (Table 1). Catheterization of the urethra proved impossible in five of 15 cats.
Table 1.
Clinical evaluation of lower urinary tract before perineal urethrostomy in 15 cats
| Animals | Urethral obstruction | Penis | Urethral catheterization | Scrotal sacs | Prepuce |
|---|---|---|---|---|---|
| Cat 1 | Partial | Warped | Impossible | Normal | Normal |
| Cat 2 | Partial | Hyperemia/swollen | Difficult | Normal | Normal |
| Cat 3 | Partial | Hyperemia | Difficult | Normal | Normal |
| Cat 4 | Partial | Hyperemia/swollen | Difficult | Normal | Normal |
| Cat 5 | Partial | Hyperemia/swollen | Difficult | Swollen | Swollen |
| Cat 6 | Total | Hyperemia/swollen | Difficult | Swollen | Swollen |
| Cat 7 | Partial | Hyperemia | Difficult | Normal | Normal |
| Cat 8 | Total | Swollen | Impossible | Normal | Normal |
| Cat 9 | Total | Swollen/hyperemia | Impossible | Swollen/hyperemia/hematoma | Swollen/hyperemia/hematoma |
| Cat 10 | Total | Swollen/hyperemia | Difficult | Swollen | Swollen |
| Cat 11 | Total | Normal | Impossible | dermatitis | dermatitis |
| Cat 12 | Total | Swollen/warped | Impossible | Swollen | Swollen |
| Cat 13 | Partial | Swollen/warped | Difficult | Normal | Normal |
| Cat 14 | Partial | Hyperemia | Difficult | Normal | Normal |
| Cat 15 | Total | Hyperemia | Impossible | Normal | Normal |
Preoperative clinical signs included dysuria in 15 cats, hematuria in two cats, pollakiuria in 12 cats and urinary incontinence in one cat. The bacterial UTI was present in nine cats. Seven cats had uremia, none had hyperkalemia.
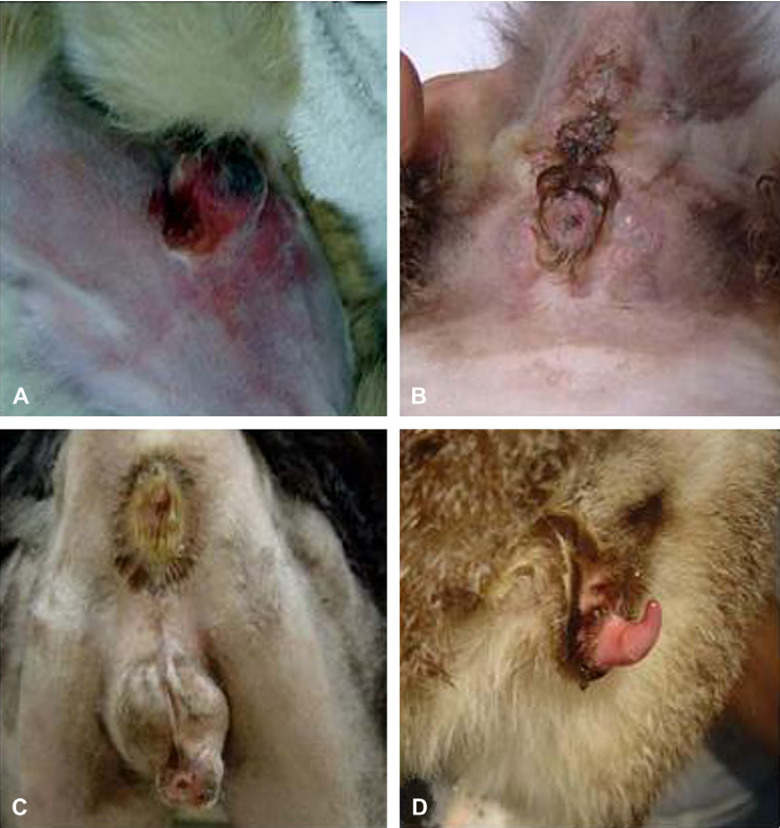
All cats had abnormalities in the penis and/or prepuce and/or scrotal sacs including hyperemia or swelling. One cat had a hematoma of the perineal region after medical management in another veterinary clinic (Fig 1A). One cat had signs of irritation of the skin due to urine scalding resulting from urinary incontinence due to a partial urethral stricture (Fig 1B). One castrated cat had swollen scrotal sacs (Fig 1C). Three cats had penile deviation; the penis was deviated dorsally in one cat (Fig 1D).
Fig 1.
Perineal region abnormalities in obstructed cats. (A) Hematoma in a perineal region in a cat. (B) Signs of irritation of the skin from urine. This cat had urinary incontinence because it had a partial urethral stricture. (C) Castrated male cat with the scrotal sacs swollen. (D) Penis warped with the tip to dorsal side.
Survey radiographic abnormalities of the lower urinary tract were detected in three of 15 cats. The abnormalities were urethral dilation in one cat and uroabdomen in two cats. None had radiolucent urocystoliths.
Contrast radiography was performed in 10 cats. Contrast radiographic abnormalities of the lower urinary tract were detected in six cats. Of these six cats, narrowing of the penile urethra was observed in one cat (Fig 2A) and urethral rupture was observed in five cats (Fig 2B). Inability to insert a catheter into the urethral opening occurred in five cats because the urethral opening had severe stricture (Fig 3). Four cats did not show abnormalities on contrast studies due to the urethral stricture being in the penile tip. Although it was possible to pass a catheter into the tip, it was not possible to detect any urethral abnormalities proximal to this.
Fig 2.
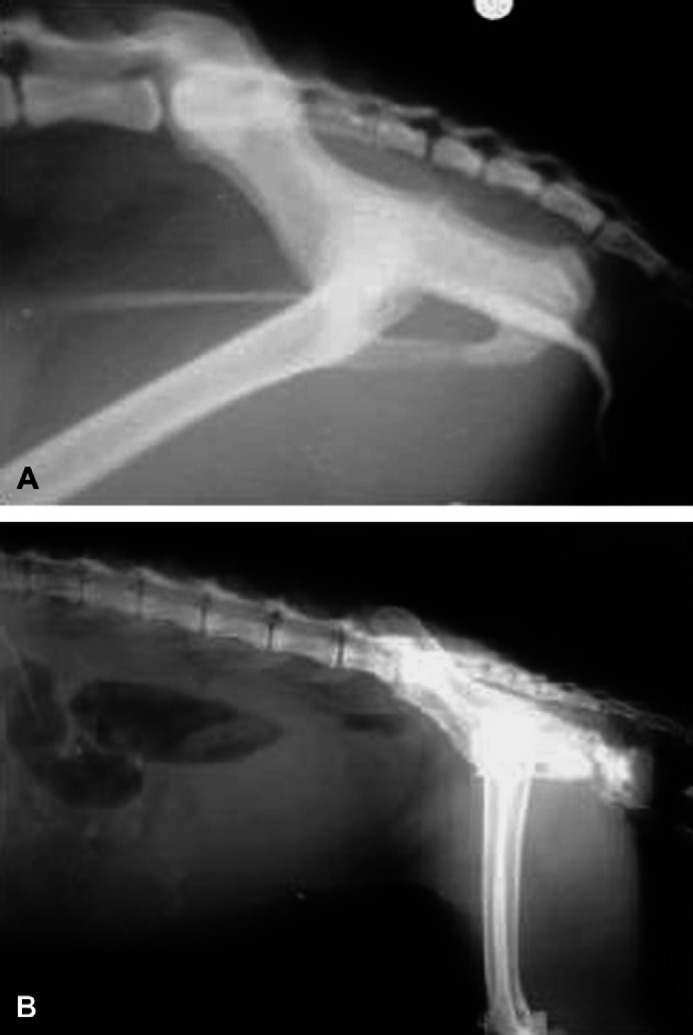
Positive contrast urethrogram abnormalities of the lower urinary tract. (A) Urethral stricture at the ischial arch in one cat. (B) Urethral rupture in a cat. Positive contrast urethrogram shows free contrast agent in the subcutaneous region.
Fig 3.
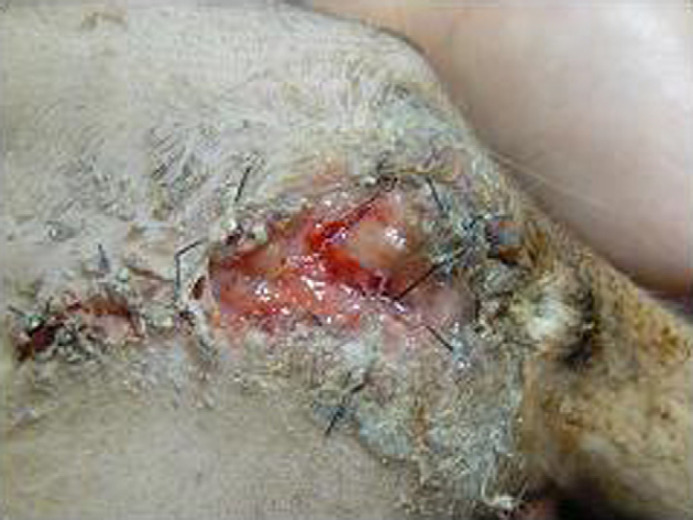
Penile opening closed. It is impossible to introduce the urinary catheter. The urethral stricture is at the penile tip.
Postoperative complications included vesical bladder atony, hemorrhage from surgical site, wound dehiscence, bacterial UTI and recurrent cystitis (Table 2). Bladder atony developed in two cats after surgery because of a chronic bladder overdistension and were treated by manual expression of the bladder and after 3 days required no assistance in voiding. Hemorrhage from the surgical site occurred in three cats and was associated with incising the ischiocavernosus and ischiourethralis muscles. Incisional dehiscence occurred in two cats on the seventh postoperative day (Fig 4). Resuturing was not performed; therefore, there were no further healing problems. Bacterial UTI developed in two cats in the fourth postoperative month and in six cats in the sixth postoperative month (Table 3). In four of eight cats, the bacteriuria did not produce clinical signs of infection. Recurrent cystitis occurred in three cats in the fourth postoperative month and in another three cats in the sixth postoperative month; all responded to symptomatic treatment. With the exception of signs associated with lower urinary tract disease, all cats remained clinically normal throughout the study. Signs of urethral obstruction were not observed in cats up to 6 months following perineal urethrostomy. Urethral stricture formation did not occur.
Table 2.
Postoperative complications in 15 cats after perineal urethrostomy
| Postoperative complications | 24 hours | Seventh day | Fifteenth day | Fourth month | Sixth month |
|---|---|---|---|---|---|
| Animals | |||||
| Hemorrhage from surgical site | 3/15 (20.0%) | 0 | 0 | 0 | 0 |
| Wound dehiscence | 0 | 2/15 (13.3%) | 0 | 0 | 0 |
| Vesical bladder atony | 2/15 (13.3%) | 0 | 0 | 0 | 0 |
| Signs of feline urinary tract disease | 6/15 (40.0%) | 8/15 (53.3%) | 0 | 3/15 (20.0%) | 3/15 (20.0%) |
| Bacterial UTI | 0 | 0 | 0 | 2/15 (13.3%) | 6/15 (40.0%) |
Fig 4.
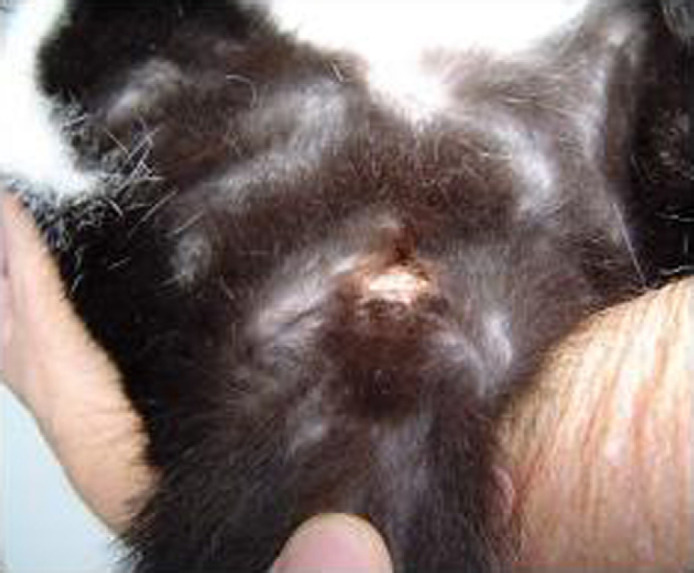
Wound dehiscence in a cat on the seventh day after the surgical procedure.
Table 3.
Preoperative and 6 months postoperative urine cultures
| Animals | Preoperative | Six months postoperative | ||
|---|---|---|---|---|
| Qualitative urine culture | Quantitative urine culture | Qualitative urine culture | Quantitative urine culture | |
| Cat 1 | Escherichia coli | <1000 | S epidermidis | 70,000 |
| E coli | ||||
| Cat 2 | E coli | >100,000 | E coli | >100,000 |
| Staphylococcus epidermidis | ||||
| Cat 3 | Sem crescimento | – | E coli | <1000 |
| Cat 4 | Enterobacter aerogenes | <1000 | E coli | >100,000 |
| S epidermidis | ||||
| Cat 5 | E coli | >100,000 | S epidermidis | >100,000 |
| E aerogenes | ||||
| Cat 6 | S epidermidis | >100,000 | E aerogenes | <1000 |
| E coli | ||||
| Cat 7 | E coli | >100,000 | E coli | <1000 |
| Cat 8 | E coli | <1000 | E coli | >100,000 |
| Cat 9 | Sem crescimento | – | E coli | <1000 |
| Cat 10 | E coli | <1000 | E aerogenes | >100,000 |
| Cat 11 | E coli | <1000 | E coli | >100,000 |
| Cat 12 | E aerogenes | >100,000 | S epidermidis | 8000 |
| S epidermidis | E coli | |||
| Cat 13 | E coli | 55,000 | Staphylococcus saprophyticus | <1000 |
| Cat 14 | E aerogenes | >100,000 | E coli | >100,000 |
| Cat 15 | Sem crescimento | – | E coli | <1000 |
Discussion
Urethral strictures may occur with chronic urethritis or repeated trauma from catheterization. Urethral rupture may occur due to trauma from catheterization and by use of inappropriate equipment for catheterization, eg, a dog urinary catheter (Barsanti et al 1994, Corgozinho and Souza 2003). In this report, most cats with urethral obstruction had urethral trauma (penile urethral rupture or stricture) because of the use of an inappropriate procedure or equipment to restore urethral patency. Penile trauma due to catheterization was the major indication for urethrostomy.
Most of the cats had been catheterized more than once before our evaluation. The repeated catheterization had predisposed the cats to urethral inflammation, trauma or stricture.
Signalment and preoperative clinical signs were similar to those previously described in cats deemed to be candidates for perineal urethrostomy (Bass et al 2005).
In this study, the preoperative incidence of bacterial UTI was high because most of the cats had been catheterized in the preoperative period; it is possible that many of these infections were catheter-related. This supports findings in other reports (Lees et al 1980, Bass et al 2005).
Survey radiography did not identify a cystic or urethral calculi in this study. The decline in the frequency of urethroliths coincides with widespread availability and use of specially formulated diets designed to minimize struvite crystalluria in cats (Lekcharoensuk et al 2002).
Contrast studies identified a urethral stricture at the ischial arch in one cat and urethral rupture in five cats. Inability to insert a catheter into the distal urethra occurred in five cats with penile trauma so that performing contrast radiography was not possible. Four cats did not show abnormalities on contrast study because the abnormality was in the urethral opening. The catheter was in the penile stricture when the radiographic studies were performed. Perineal urethrostomy was indicated for penile urethral trauma in these cats.
Bacterial UTI was the most frequent postoperative complication observed. Previous studies found that between 17 and 57% of cats that underwent perineal urethrostomy developed UTI (Smith and Schiller 1978, Gregory and Vasseur 1983, Griffin and Gregory 1992, Bass et al 2005). During the long-term follow-up period in the present study, 53% of cats developed UTI. It is possible that the increased prevalence of bacterial UTI in cats after perineal urethrostomy is attributable in part to the underlying disease process producing obstructive uropathy, because there is greater tendency towards bacterial UTI in cats with obstructive uropathy, compared with healthy cats (Griffin and Gregory 1992).
Urethral stricture, a serious complication of perineal urethrostomy, did not occur in this study in contrast with the results of another report (Bass et al 2005). Increased experience with the technique, uniform use of Elizabethan collars postoperatively, and avoidance of postoperative indwelling urethral catheters may have contributed to the lower incidence of stricture formation (Smith et al 1991).
Urethral obstruction must be managed with adequate equipment (eg, cat urinary catheter) and the procedure should be carried out gently to avoid urethral trauma. It should be remembered that hydropropulsion is used to remove obstructive material and not the urinary catheter. The cause of the obstruction should be identified and corrected to prevent future obstruction. When the urethral obstruction does not respond to medical management, perineal urethrostomy can be recommended as it has few complications in the hands of experienced surgeons. Bacterial UTI was the most frequent complication observed. Many cats enjoy a long-term disease-free outcome and the clients considered their cats to have a good quality of life following surgery.
References
- Barsanti J.A., Finco D.R., Scott A.B. Diseases of the lower urinary tract. Sherding R.G. The Cat: Diseases and Clinical Management, 2nd edn, 1994, Churchill Livingstone: New York, 1769–1823. [Google Scholar]
- Bass M., Howard J., Gerber B., et al. Retrospective study of indications for and outcome of perineal urethrostomy in cats, Journal of Small Animal Practice 46, 2005, 227–231. [DOI] [PubMed] [Google Scholar]
- Blake J.A. Perineal urethrostomy in cats, Journal of the American Veterinary Medical Association 152, 1968, 1499–1506. [PubMed] [Google Scholar]
- Carbone M.G. Perineal urethrostomy to relieve urethral obstruction in the male cat, Journal of the American Veterinary Medical Association 143, 1963, 34–39. [PubMed] [Google Scholar]
- Carbone M.G. A modified technique for perineal urethrostomy in the male cat, Journal of the American Veterinary Medical Association 151, 1967, 301–305. [PubMed] [Google Scholar]
- Caywood D.D., Raffe M.R. Perspectives on surgical management of feline urethral obstruction, Veterinary Clinics of North America: Small Animal Practice 14, 1984, 677–690. [DOI] [PubMed] [Google Scholar]
- Christensen N.R. Preputial urethrostomy in the male cat, Journal of the American Veterinary Medical Association 145, 1964, 903–908. [PubMed] [Google Scholar]
- Corgozinho K.B., Souza H.J.M. Condutas na desobstrução uretral. Souza H.J.M. Coletâneas em Medicina e Cirurgia Felina, 2003, L.F. Livros de Veterinária: Rio de Janeiro, 67–88. [Google Scholar]
- Gregory C.R., Vasseur P.B. Long-term examination of cats with perineal urethrostomy, Veterinary Surgery 12, 1983, 210–212. [Google Scholar]
- Griffin D.W., Gregory C.R. Prevalence of bacterial urinary tract infection after perineal urethrostomy in cats, Journal of the American Veterinary Medical Association 200, 1992, 681–684. [PubMed] [Google Scholar]
- Lees G.E., Osborne C.A., Stevens J.B., Ward G.E. Adverse effects caused by polypropylene and polyvinyl feline urinary catheters, American Journal of Veterinary Research 41, 1980, 1836–1840. [PubMed] [Google Scholar]
- Lekcharoensuk C., Osborne C.A., Lulich J.P. Evaluation of trends in frequency of urethrostomy for treatment of urethral obstruction in cats, Journal of the American Veterinary Medical Association 221, 2002, 502–505. [DOI] [PubMed] [Google Scholar]
- Manziano C.F., Manziano J.R. Perineal urethrostomy for relief of urethral blockage in the male cat, Journal of the American Veterinary Medical Association 149, 1966, 1312–1316. [PubMed] [Google Scholar]
- Osborne C.A., Caywood D.D., Johnston G.R., Polzin D.J., Lulich J.P., Kruger J.M. Perineal urethrostomy versus dietary management in prevention of recurrent lower urinary tract disease, Journal of Small Animal Practice 32, 1991, 296–305. [Google Scholar]
- Smith C.W. Perineal urethrostomy, Veterinary Clinics of North America: Small Animal Practice 32, 2002, 917–925. [DOI] [PubMed] [Google Scholar]
- Smith C.W., Schiller A.G. Perineal urethrostomy in the cats: a retrospective study of complications, Journal of the American Animal Hospital Association 14, 1978, 225–228. [Google Scholar]
- Smith C.W., Weigel R.M., Smith A.R. Perineal urethrostomy in the cat, Feline Practice 19, 1991, 20–23. [Google Scholar]
- Wilson G.P., Harrison J.W. Perineal urethrostomy in cats, Journal of the American Veterinary Medical Association 159, 1971, 1789–1793. [PubMed] [Google Scholar]