Abstract
Feline mammary fibroepithelial hyperplasia (FMFH) following a single injection of depot medroxyprogesterone acetate (MPA) was observed in eight intact young queens. The repository compound is marketed as a veterinary product by a local pharmaceutical company with an indication for contraception in cats. The drug was administered according to the recommended doses and injection frequencies. Serum hormone assays performed immediately before neutering and 3 weeks after neutering detected persistently high levels of progesterone suggesting that depot MPA was still exerting its influence. No corpora lutea were found in those cases ruling out ovaries as the main site of progesterone. Immunohistochemistry performed on the hyperplastic mammary glands detected progesterone receptors in the nuclei of ductal cells, and growth hormone (GH) and insulin-like growth factor-I (IGF-I) in the cytoplasm of ductal epithelium. Overdosing should be considered here as the animals received at least 10 mg/kg of depot MPA in a single injection. Progestin-induced local synthesis of GH and IGF-I in mammary epithelial cells is suggested as one of the pathogenic mechanisms involved in the development of FMFH.
Feline mammary fibroepithelial hyperplasia (FMFH) is a growth disturbance of cats characterised by rapid, non-neoplastic proliferation of ductal epithelium and stroma of the mammary gland resulting in enlargement of one, several or all the mammary glands (Allen 1973). It is seen mainly in young, sexually intact queens at the time of puberty, during the first oestral cycle, pregnancy or pseudopregnancy. Current evidence indicates that FMFH represents a hormone-dependent lesion as high levels of endogenous progesterone induce an exaggerated proliferative response of the mammary glandular tissue (Hayden and Johnson 1986). A number of hormones have been implicated in the pathogenesis of this condition (Hayden and Johnson 1986, Mol et al 1996, Martin de las Mulas et al 2000, Ordás et al 2004) including synthetic progestins such as megestrol acetate and acetate medroxyprogesterone (MPA) (Hayden et al 1989).
MPA is commercially available as a repositol injectable product used as a contraceptive drug for dogs and cats. Currently, there are no products licensed to be used in the prevention or suppression of oestrus in queens in the USA and UK (Noakes et al 2001). Nevertheless, in other countries, parenterally administered progesterone-containing compounds are still available in the market for this purpose (Romagnoli and Concannon 2003). In Brazil, contraceptive therapy with depot injectable MPA has been used over the years in feline veterinary clinical practice. Recently, clusters of cases of FMFH have been described in this country. There is an increasing number of anecdotal reports from many local university veterinary hospitals and private practitioners of young cats treated with a single dose of MPA that developed mammary fibroepithelial hyperplasia.
The purpose of the present study is to report the signalment, history, clinical, pathological and immunohistochemical findings, management and outcome of eight cats that developed mammary fibroepithelial hyperplasia after the improper use of a single injection of MPA.
Materials and methods
Animals
From 1999 to 2003, eight cats with mammary fibroepithelial hyperplasia were admitted in three Brazilian university veterinary hospitals. Each of these animals received a single subcutaneous injection of depot MPA. One of these establishments has an annual caseload of approximately 900 cats. Similar statistical data were not available for the other two institutions.
Vaginal exfoliative cytology
Vaginal exfoliative cytology samples were obtained with a cotton swab from two animals (cases 7 and 8) when presented for the first time to the referring veterinarian. Smears were stained with Wright's Giemsa stain. The phase of the ovarian cycle for these animals was determined according to the microscopic findings in those smears.
Serum hormone assays
In two animals (cases 7 and 8), blood samples were taken for serum steroid hormone assays (progesterone and oestrogen) on two different occasions. The first set of samples for those assays was collected 19 days (case 8) and 30 days (case 7) after mammary gland enlargement was first noticed by the owners. Only progesterone levels were measured in the second set of hormonal analyses, ie, 19 days after neutering (cases 7 and 8), with an interval of 26 days (case 8) and 28 days (case 7) between the first and the second set of assays. Measurements of hormonal concentrations were undertaken through an immunoassay immunofluorimetric method for progesterone and radioimmunoassay for oestrogen. Each of those samples was analysed in the automated machine AutoDelfia™ (Perkin Elmer Brazil, Wallac, Turku, Finland).
Pathology
Surgical biopsy, necropsy, light microscopy
Samples of the affected mammary glands were obtained through surgical biopsy (cases 7 and 8), mastectomy (cases 2 and 5) or necropsy (cases 3, 4 and 8) and submitted for histopathology. One of the necropsied animals died spontaneously (case 4) and the other two (cases 3 and 8) were humanely euthanased at the owners' request. In two animals (cases 1 and 6), the condition was diagnosed during the first evaluation of the patients at the hospitals based only on the history and clinical picture. Mammary tissues of six animals (cases 2, 3, 4, 5, 7 and 8) and fragments of different organs including the ovaries and uteri from two queens (cases 7 and 8) were fixed in 10% buffered formalin and routinely processed for light microscopy.
Immunohistochemistry
Formalin-fixed, paraffin embedded tissue sections of the mammary glands of five animals (cases 2, 4, 5, 7 and 8) were analysed for the presence of oestrogen receptors, progesterone receptors, growth hormone (GH) and insulin-like growth factor-I (IGF-I) using an avidin–biotin-peroxidase complex (ABC) technique for monoclonal antibodies as previously described (Martin de las Mulas et al 2000, Ordás et al 2004).
Surgical procedures for the treatment of FMFH included neutering (ovariectomy as in case 3 or ovariohysterectomy as in cases 1, 2, 4, 6, 7 and 8), mastectomy (cases 2, 3 and 5) or both (cases 2 and 3). Ultrasonographic examination of the affected mammary glands was undertaken in one of the affected queens (case 7). Quantification of the levels of MPA in the pharmaceutical product used in those cats was not undertaken as a standard sample was not available and also because multiple lots of that medication were used on different occasions.
Results
A summary of the clinical and pathological findings, therapy and clinical outcome in eight cats with feline mammary fibroadenomatous change is given in Table 1.
Table 1.
Clinical and pathological findings, therapy and outcome for eight cats with feline mammary fibroepithelial hyperplasia
| Case | Age at presentation | History and clinical signs | Therapy | Follow-up | |||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 10 months | Symmetrical, bilateral enlargement of both mammary chains; 1 SC injection of MPA (10.8 mg/kg) at 9 months of age | OVH | Continuous expansive growth and marked enlargement of all mammary glands after OVH; died spontaneously 3 days after neutering | |||||
| 2 | 9 months | Asymmetrical enlargement of the axillary mammary glands during 2–3 days; 1 SC injection MPA (20.8 mg/kg) at 6 months of age | OVH MT RM1 | Continuous expansive growth and marked enlargement of LM1 after OVH/MTR1; complete regression 2 months after OVH/MTR1; no recurrence 24 months neutering | |||||
| 3 | ±1 year | 1 SC injection MPA (13.2 mg/kg) during the first oestrus; symmetrical and bilateral enlargement of both mammary chains 15 days after dosing | OV MT RM1 | Partial regression 1 month after OV/MT; recurrence 2 months after OV/MT; euthanasia was carried out | |||||
| 4 | 1 year | Asymmetrical, bilateral enlargement of both mammary chains; 1 contraceptive SC injection (18.5 mg/kg); pregnancy (2 foetuses); no consistent information about the time of dosing | OVH | Died spontaneously 3 days after OVH | |||||
| 5 | 6 months | 1 SC injection of MPA (12.5 mg/kg); asymmetrical, bilateral enlargement of both mammary chains 2–3 days after dosing; expansive growth continued during the following 30 days | MT RM1–RM4 | Complete regression and no recurrence 24 months after MT | |||||
| 6 | 8 months | Asymmetrical, bilateral enlargement of both mammary chains 15 days after contraceptive SC injection (12.8 mg/kg); pregnancy (28–35 days) (2 foetuses) | OVH | Continuous expansive growth and marked enlargement of mammary glands, ulceration of the overlying skin during the following month after OVH; complete regression 6 months after OVH | |||||
| 7 | 16 months | Asymmetrical, bilateral enlargement of abdominal and inguinal mammary glands 1 month after contraceptive SC injection (17.8 mg/kg) | OVH | Continuous regression up to 2 months after OVH | |||||
| 8 | 7 months | Bilateral enlargement of both mammary chains 1.5 months after contraceptive SC injection (25 mg/kg) | OVH | Partial regression of RLM3, RLM4 and marked ulceration of axillary glands and progressive weight loss during 2 months after OVH; euthanasia was carried out |
OVH=ovariohysterectomy, OV=ovariectomy, SC=subcutaneous, MPA=medroxyprogesterone acetate, MT=mastectomy, RM1=first right mammary gland, LM1=first left mammary gland, RLM3=third right and left mammary glands, RLM4=fourth right and left mammary glands, RM1–RM4=entire right mammary chain.
Case histories and clinical findings
Eight sexually intact cross-bred queens aged between 6 and 16 months (cases 1 to 8) developed mammary fibroepithelial hyperplasia after a single subcutaneous injection of depot MPA for prevention of oestrus. Doses and injection frequencies used in those cases were in accordance with those recommended by a local pharmaceutical company. Doses varied from 10.8 mg/kg to 25 mg/kg (Table 1). Two of those patients were primiparous queens (cases 4 and 6) while one of them (case 7) had recently undergone parturition. In two queens (cases 3 and 5), an injection of MPA was given when the animals were showing behavioural signs typical of oestrus–pro-oestrus. Two animals (cases 4 and 6) were pregnant when MPA was given. In those cases, pregnancy was diagnosed only during neutering. The other queens (cases 1, 2, 7 and 8) were in anoestrous when MPA was administered. Two animals (cases 7 and 8) belonged to the same client and were kept together with a group of 32 cats. Of those, six out of 26 female cats were dosed with MPA. Two out of those six queens that received a single injection of this synthetic progestin in late March 2003 (early fall, southern hemisphere) developed massive enlargement of mammary glands 30 days (April 2003) (case 7) and 45 days (May 2003) (case 8) after the dosage. Both queens were presented to the referring veterinarian at the same time with the same clinical complaint, ie, FMFH (Fig 1). In one of those animals (case 7), MPA was dosed during late lactation. This owner reported that signs of oestrus had never been observed in one of the queens (case 8).
Fig 1.

Feline mammary fibroepithelial hyperplasia. There is enlargement of the mammary glands of variable severity. The abdominal and inguinal mammary glands (case 7, right) or all the mammary glands (case 8, left) are affected. Those queens belonged to the same client and were presented to the referring veterinarian on the same day. Both animals were dosed with a single dose of acetate medroxyprogesterone.
According to the owners' information, the initial onset of mammary hypertrophy after MPA administration varied from 2 days to 3 months after dosing. The clinical picture consisted of enlargement of the mammary glands of variable severity in which only the axillary mammary glands (case 2), the abdominal and inguinal mammary glands (case 7) or all the mammary glands were affected at the same time (cases 1, 3, 4, 5, 6 and 8) (Figs 1 and 2). Growth rate of the mammary lesions ranged from 2 days to 1 month. Some cats developed sudden and rapid expansive growth of the mammary glands. In other animals, mammary enlargement was slow and progressive. The mammary masses were sharply circumscribed and had clearly delineated borders. When multiple mammary glands were involved in the same animal, those lesions varied in size (cases 2, 4, 5, 6 and 7). In three cases (cases 1, 3 and 8), similar degrees of mammary enlargement were noticed symmetrically and bilaterally. Hypertrophic mammary glands ranged from 5 cm×5 cm to 8 cm×8 cm. They were soft and fluctuant or firm and resilient. In four cases (cases 1, 4, 6 and 8), the overlying skin was tense and erythematous or dark and necrotic. In some cases, the nipples were difficult to locate since the marked enlargement of the mammary glands stretched and flattened those adnexal structures. Only one, several or all enlarged mammary glands had an alopecic, moist, ulcerated, bleeding or exudative surface covered with pus. Those inflammatory and necrotic lesions were more severe in the axillary and inguinal mammary glands (Fig 2). Ultrasonography of the affected mammary glands showed a homogenous tissue that had the typical imaging appearance of mammary tissue without any evidence of malignancy. No other clinical signs were observed except for cases 2, 4 and 8. Those animals showed signs of systemic illness when presented to the referring veterinarian including depression (cases 2 and 4), fever (case 2) and pallor of the mucous membranes (case 2). One cat (case 8) showed progressive weight loss even after neutering.
Fig 2.
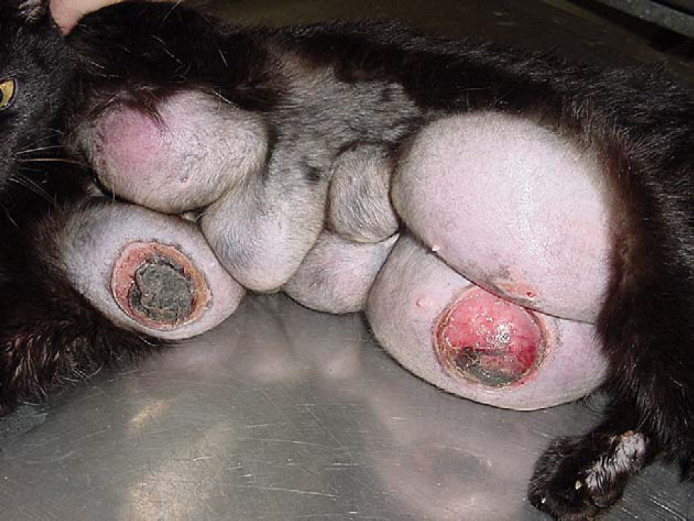
Feline mammary fibroepithelial hyperplasia. Case 6. This queen was presented for consultation with the clinical complaint of marked enlargement of all the mammary glands after receiving a single dose of acetate medroxyprogesterone. Continuous, expansive growth of the affected mammary glands with ulceration of the tense skin covering the axillary and inguinal mammary glands was observed even after neutering. The affected area was shaved.
Vaginal exfoliative cytology
In two animals (cases 7 and 8), vaginal cytology was performed 19 days (case 8) and 30 days (case 7) and after mammary gland enlargement was noticed. In those cases, numerous parabasal and intermediate cells were observed in the smears corresponding to approximately 70% of the total number of cells recovered from the vaginal mucosa. In one animal (case 7), some anucleated superficial cells and bacteria were also observed. In one patient (case 8), parabasal cells corresponded to more than 60% of the total population of cells obtained from the vagina. The cytological findings in those smears were consistent with those described in the anoestrus (Henson 2001).
Laboratory findings
Serum hormonal assays
Results of serum analyses for progesterone and oestrogen in two cats with feline mammary fibroadenomatous change are given in Table 2.
Table 2.
Concentrations of progesterone and oestrogen in two cats with feline mammary fibroepithelial hyperplasia
| Case | Progesterone a (ng/ml) | |||
|---|---|---|---|---|
| Oestrogen b , c (pg/ml) | First hormonal analysis c | Second hormonal analysis d | ||
| 7 | 0.706 | 0.826 | 17 | |
| 8 | 1.300 | 1.180 | 20 | |
Reference values—queens in anoestrus and neutered queens: below 1.0 ng/ml; queens with follicular-phase ovaries: less than 0.15 ng/ml; queens with luteal-phase ovaries: greater than 1.87 ng/ml (Edqvist and Forsberg 1997)
2Reference values—queens in the interfollicular phase: 10 pg/ml; queens during folliculogenesis: 60 pg/ml (Colorado State University Reproductive Endocrinology Laboratory, Colorado State University, Fort Collins, CO, USA, web site, http://www.cvmbs.colostate.edu/physio/arbl_endolab/reference_values.html, 2001)
cSamples collected 19 days (case 8) and 30 days (case 7) after mammary gland enlargement was noticed by the owners.
dSamples collected 26 days (case 8) and 28 days (case 7) after the first hormonal analysis and 19 days after neutering.
Pathology
Necropsy findings
Grossly, the external surface of the affected mammary glands was homogeneous or multilobulated and the lesions were encapsulated. The enlarged glands adhered tightly to the overlying skin. The cut surface bulged and was diffusely white, white-yellow or pink, shiny and homogeneous. In one animal (case 8), multiple small, well-circumscribed, finely lobulated, contiguous, yellowish areas were observed on a whitish, homogeneous, glistening background (Fig 3). In one case (case 4), there were extensive areas of red or dark-red discoloration on cut section corresponding to foci of necrosis in the hyperplastic mammary glands. Those necrotic areas were well demarcated and could be seen through the darkened, devitalised covering skin. In one case (case 8), there was an abscess (2 cm in diameter) in the inguinal mammary gland. No corpora lutea were found in those cases in which the ovaries were available for gross examination (cases 7 and 8). The walls of the uterine horns from one animal (case 8) were mildly thickened and the endometrial surface was diffusely rough and wrinkled. The body condition of this queen was thin (2.0 kg). The mammary glands of this cat weighed 460 g, approximately 25% of the total body weight. Each of the pregnant queens (cases 4 and 6) had two foetuses in the gravid uterus. In case 6, gestation was estimated at between 28 and 35 days based on the size of the foetuses.
Fig 3.

Feline mammary fibroepithelial hyperplasia. Case 8. Mammary gland, cut surface. Multiple small, well-circumscribed, finely lobulated, contiguous, yellowish areas (corresponding to hyperplastic mammary ductal epithelium on histology) on a whitish, homogeneous, glistening background (that corresponds microscopically to proliferated interlobular stroma).
Histology
Microscopic findings in the affected mammary tissues consisted of diffuse fibroepithelial hyperplasia characterised by varying degrees of proliferation of both intralobular mammary duct epithelium and interlobular stroma composed of fibroblasts and collagen fibres. Myoepithelial cells were concentrically disposed around proliferating ducts. Extensive areas of coagulative necrosis and haemorrhage occurred contiguous to the ulcerated and acutely inflamed overlying skin. In the biopsy of the mammary gland from one of the cats (case 7), there were also focal zones of squamous metaplasia of the hyperplastic epithelium of the ducts near an ulcerated and acutely inflamed area of mammary tissue (Fig 4). No corpora lutea were observed in those cases in which samples of ovarian tissue were collected for histology (cases 7 and 8). Those two animals were neutered 65 days after the injection of depot MPA. In one patient (case 8), increased secretory activity of the endometrium was observed.
Fig 4.
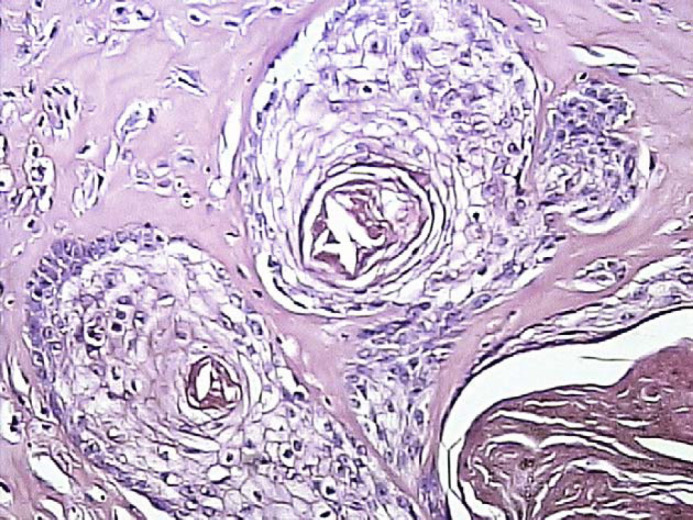
Feline mammary fibroepithelial hyperplasia. Case 7. Squamous metaplasia of the hyperplastic epithelium of the ducts (haematoxylin and eosin, magnification ×400).
Immunohistochemistry
Immunohistochemistry from five cases (cases 2, 4, 5, 7 and 8) revealed the presence of progesterone receptors in the nuclei of ductal epithelium (Fig 5). Epithelial cells had large, round or oval deeply stained nuclei. The amount of immunoreactivity products to progesterone receptors was variable among those cases. The number of positively stained nuclei ranged from 5–20% (cases 2, 4 and 7) to more than 60% (cases 5 and 8). Growth hormone (GH) and insulin-like growth factor-I (IGF-I) were found in the cytoplasm of ductal epithelium. Brownish, homogeneous cytoplasmic immunostaining indicated the presence of GH (Fig 6). Immunostained GH and IGF-I-producing epithelial cells varied from 5–20% (cases 2, 5 and 7) to 20–60% (cases 4 and 8). No oestrogen receptors were detected. Fibroblasts and myoepithelial cells were consistently unreactive for the immunohistochemical methods used in all cases.
Fig 5.
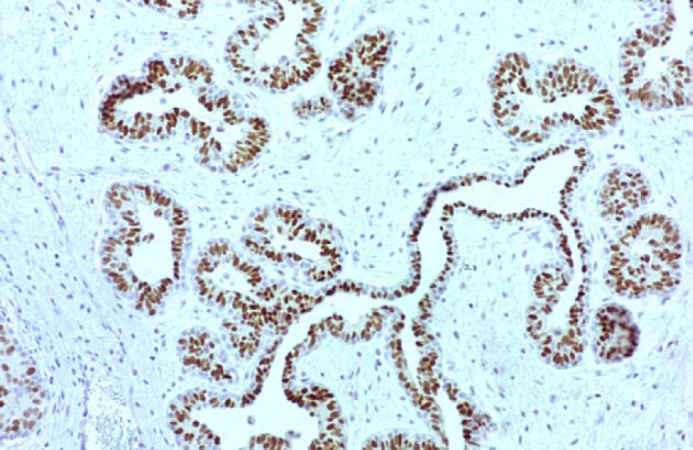
Feline mammary fibroepithelial hyperplasia. Case 5. Progesterone receptors are present in the nuclei of ductal epithelial cells. Spindle cells (myoepithelial cells and fibroblasts) in the surrounding stroma are unreactive (ABC immunohistochemical method, magnification ×100).
Fig 6.
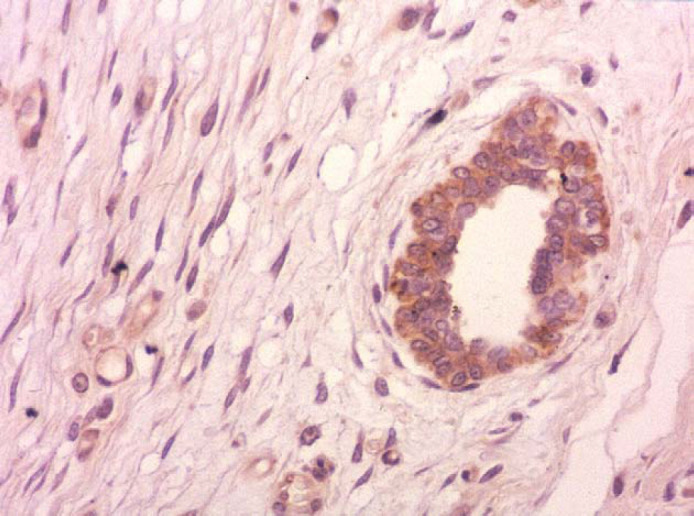
Feline mammary fibroepithelial hyperplasia. Case 5. Growth hormone (GH) is present in the cytoplasm of ductal epithelium. Spindle cells (myoepithelial cells and fibroblasts) in the surrounding stroma are unreactive (ABC immunohistochemical method, magnification ×400).
Therapy, follow-up and outcome
Treatment consisted of neutering or mastectomy (or both). Broad spectrum antibiotics and topical anti-inflammatory and analgesic gels were prescribed to those animals with extensive ulcerative lesions on the skin overlying the enlarged mammary glands. One anaemic animal (case 2) required a blood transfusion. The clinical outcome and the effectiveness of the therapy for feline mammary hyperplasia in the affected queens were variable. In two cases (cases 3 and 8), there was only partial regression of the hyperplastic lesions on the mammary glands 1–2 months after surgery. These animals were euthanased because of guarded prognosis and unresponsiveness to treatment. Two animals (cases 1 and 4) died spontaneously a few days after the surgery. Four animals (cases 2, 5, 6 and 8) fully recovered from this disease approximately 2 months after surgery.
Discussion
In the present report, the diagnosis of FMFH was based on the history, clinical signs, histological lesions, hormonal assays and response to therapy. The clinical and pathological features of cases of FMFH described in this study are similar to those previously reported (Hayden and Johnson 1986). Subcutaneous and intramuscular repositol forms of synthetic progestins are potent anti-ovulatory compounds with a progestational activity 25 times greater than that of endogenous progesterone (Hayden et al 1989). A single intramuscular injection of the depot form of MPA can maintain effective circulating levels of this compound for 6 months. Overdosage of MPA or administration of this drug during stages of the oestral cycle other than anoestrus (or both) can cause detrimental effects on cats (Concannon and Meyers-Wallen 1991). In the present report, all the queens developed mammary gland enlargement when given a single injection of depot MPA. In those cases, it is suggested that one of the main contributory factors to the development of FMFH is the potent and long-term effect of the repositol injectable forms of synthetic progestins. Variable temporal relationships between drug dosage and full development of FMFH could not be explained.
In this study, it was noticed that doses and injection frequencies of MPA for contraception in cats reported on drug leaflets from a local manufacturer did not meet ideally with those recommended in scientific manuscripts published elsewhere (Romagnoli and Concannon 2003). In some countries, dosages of this synthetic progestin used for contraception in dogs and cats are calculated based on weight classes rather than on the weight of each individual. In the cases of FMFH described here, injectable depot MPA was used in accordance with the doses and injection frequencies recommended by the pharmaceutical companies. Despite the fact that the contraceptive doses recommended by those companies were not exceeded, overdosing should be considered here as all the queens from this study received an inappropriate, excessive dose (ie, higher than 10 mg/kg and up to 25 mg/kg) in a single injection. Depot injectable MPA-based drugs currently available in the market in Brazil recommend a total dose of 50 mg for one queen every 6 months regardless of body weight. According to a recent review on the clinical use of progestins in bitches and queens, the minimal effective dose and the more appropriate dose of depot MPA to be used initially in queens is 2.0 mg/kg every 5 months (Romagnoli and Concannon 2003). Cases of FMFH associated with a single injection of a synthetic progestin have been reported only on rare occasions (Meisl et al 2003). Months to years of therapy based on synthetic progestin and repeated doses of this repository drug are required to produce FMFH and, when it happens, only one or two glands rather than the entire mammary system are affected (Hayden and Johnson 1986). Although in such a treatment for prolonged postponement of oestrus the contraceptive drug is administered once every 5–6 months, a high dose of this synthetic progestin might predispose a healthy female to develop one or more of the side effects associated with the use of this depot compound or might exacerbate a pre-existing, subclinical condition (ie, diabetes mellitus, cystic endometrial hyperplasia) into a clinically evident condition.
In this report, the adverse effects of single depot MPA injections could also be related to dosing this drug during pregnancy when progesterone levels are high. Both exogenous and endogenous progesterone would accumulate in this situation. An exacerbated response of progestin-sensitive tissues, ie, mammary gland and uterus would be expected to occur. Two animals (cases 4 and 6) were pregnant at the time of presentation and neutering. The phenomenon of spontaneous ovulation could also have contributed to the development of this disorder. The incidence of spontaneous ovulation in queens ranges from 30 to 55% (Gudermuth et al 1997). If an MPA injection is administered to a queen soon after a spontaneous ovulation, mammary hypertrophy would be expected to occur as the queen experiences serum progesterone concentrations much higher than normal.
Administration of recommended doses of MPA during the follicular phase of the oestrous cycle of cats may not necessarily produce side-effects, eg, cystic endometrial hyperplasia and FMFH. In this situation, normal amounts of oestrogen tissue receptors binding to supposedly safe concentrations of MPA-derived progesterone are unlikely to cause excessive stimulation of target organs that would lead to hyperplastic disorders. However, in two queens (cases 3 and 5), a high, single dose of MPA was given when the animals were showing behavioural signs typical of oestrus. During this stage, plasma concentration of oestrogen rapidly increases and remains elevated for 3–4 days (Shille et al 1979). This situation would contribute to an exaggerated response of the mammary gland to high levels of MPA since those tissues would be already primed by oestrogen and therefore hyperresponsive to the synthetic progestin. Higher sensitivity of the mammary tissues of some individuals to those hormones, associated with genetic or age-related factors, could also have contributed to some extent to the occurrence of FMFH. Puberty might also have played its role in the development of FMFH. In this report, young queens that experienced massive mammary enlargement were dosed with a single injection of MPA at an early age, ie, 6–16 months when puberty usually begins and sexual hormones are on the rise. Interestingly enough, one case of FMFH (case 8) described here occurred in a prepuberal queen which strengthens the hypothesis that exogenous progesterone was one of the main contributory factors for the development of FMFH.
In two animals (cases 7 and 8), the cytological findings in vaginal smears are consistent with anoestrus which corroborate the theory that the synthetic progestin is one of the most significant stimuli for the occurrence of FMFH (Mowers et al 1975). Both animals received an injection of this depot drug at the beginning of the Fall. The owner did not notice any signs of oestrus in those two cats. Those cats were kept outdoors under the effect of the solar light. It is widely known that cats are seasonally polyoestrous breeders with their sexual cycles occurring especially on late winter and spring (Löfstedt 1982). Hence, those young queens were not in the period of the year when they were more prone to be cycling and therefore high levels of endogenous progesterone were not to be expected.
Previous studies have revealed the presence of both progesterone and oestrogen receptors in hyperplastic mammary glands of cats suggesting that both hormones are involved in the pathogenesis of this condition (Hayden et al 1981, Martin de las Mulas et al 2000). It has been hypothesised that fibroepithelial proliferation could be under the influence of progesterone in oestrogen-primed mammary tissues (Martin de las Mulas et al 2000). The ability of progesterone in inducing hyperplastic changes in the mammary gland of cats may depend on this oestrogen preparation suggesting that a hormone-dependent pathway is responsible for the occurrence of FMFH. It is assumed that oestrogen, occurring in high levels during oestrus and pro-oestrus, primes mammary tissue for the action of progesterone. Oestrogens may act by binding to oestrogen receptors present in ductal epithelial cells and stromal cells of the mammary gland with synthesis of intracellular receptors for progesterone. The increased expression of progesterone receptors in the mammary tissues may intensify the physiological response of the mammary gland to this hormone leading to hyperplastic changes. Similarly, oestrogens stimulate synthesis of progesterone receptors within the tissues of the reproductive tract, eg, uterus. This pathogenesis has been suggested for cystic endometrial hyperplasia and pyometra in the bitch (De Bosschere et al 2002). Progestin-induced local synthesis of GH and insulin-like growth factors (IGFs) in mammary epithelial cells has been proposed as additional pathogenic mechanisms involved in the development of FMFH (Mol et al 1996). Cumulatively, these observations provide circumstantial evidence that both endogenous and exogenous progesterone in conjunction with other hormones are involved in the development of this growth disturbance in cats. In the present report, progesterone receptors were detected immunohistochemically in the nuclei of ductal epithelium. Immunohistochemistry for oestrogen receptors was consistently negative in our cases. In a previous study, only 33% of the cases of FMFH stained positively for oestrogen receptors (Martin de las Mulas et al 2000). In our cases, the lack of reactivity of the hyperplastic mammary gland to oestrogen receptors could be explained by the absence of oestrogen receptors in those tissues when the animals were first examined and the mammary tissues were sampled for immunohistochemistry.
In this study, GH and IGF-I were detected immunohistochemically in the cytoplasm of mammary ductal cells representing local synthesis of both hormones. These findings are consistent with those of previous studies which suggest that local production of GH and IGF-I enhances the proliferation of the mammary tissue in an autocrine and/or paracrine manner, and that mammary epithelial cells are capable of producing GH locally under adequate progesterone stimulus (Van Garderen et al 1997). Synthetic progestins have the ability to induce local expression of the GH-encoding gene (GH mRNA) in the mammary gland resulting in enhanced biosynthesis of GH in the ductular epithelium. Local expression of GH mRNA, together with the expression of genes encoding for insulin-like growth factors such as IGF-I and IGF-II and their binding proteins (IGFBPs), create an environment for proliferation and differentiation of the mammary epithelium primed by progesterone (Mol et al 1996). GH is the major regulator of circulating concentrations of IGF-I that has a potential mitogenic effect on some tissues, eg, the uterus, and is capable of regulating proliferation and differentiation events, eg, in the endometrium. IGF-I might play an important role in the genesis of cystic endometrial hyperplasia in dogs (De Cock et al 2002). We suggest that GH and IGF-I could also participate in the development of FMFH.
In the present report, spontaneous resolution of FMFH without recurrence of the condition occurred in one queen. Similar cases have been described only occasionally (Hayden and Johnson 1986). The pathogenic mechanisms involved in those atypical situations remain to be determined. Another unusual clinical outcome observed in this study was one animal in which there was partial reduction of the mammary hyperplastic changes after neutering followed by recrudescence of those mammary lesions. The atypical presentation of this disorder in this particular case could be attributed to the long-lasting effect of depot synthetic progestins.
In the present study, progesterone concentrations were high both before and after neutering in two cases. No corpora lutea were found in those cases in which the ovaries were available for examination which rules out long-standing, endogenous production of progesterone by these sex organs. It is suggested that persistently elevated serum progesterone levels detected in both cases corresponded to circulating exogenous, long-term depot MPA. Additionally, smaller amounts of endogenous progesterone and 17a-hydroxyprogesterone secreted by zona reticularis of the adrenal cortex and by unknown sources of progesterone could also had played a role in the pathogenesis of this atypical case. No ectopic tissue-related sources of progesterone were detected in our cases.
Additional studies are warranted to investigate the potential adverse effects of synthetic progestins in cats must be carried out. An understanding of the mechanisms involved in the genesis of FMFH is pivotal for developing a sensible basis for its prevention and therapy.
References
- Allen H.L. Feline mammary hypertrophy, Veterinary Pathology 10, 1973, 501–508. [DOI] [PubMed] [Google Scholar]
- Concannon P.W., Meyers-Wallen V.N. Current and proposed methods for contraception and termination of pregnancy in dogs and cats, Journal of the American Veterinary Medical Association 198, 1991, 1214–1225. [PubMed] [Google Scholar]
- De Bosschere H., Ducatelle R., Vermeirsch H., et al. Estrogen-α and progesterone receptor expression in cystic endometrial hyperplasia and pyometra in the bitch, Animal Reproduction Science 70, 2002, 251–299. [DOI] [PubMed] [Google Scholar]
- De Cock H., Ducatelle R., Tilmant K., De Schepper J. Possible role for insulin-like growth factor-I in the pathogenesis of cystic endometrial hyperplasia pyometra complex in the bitch, Theriogenology 57, 2002, 2271–2287. [DOI] [PubMed] [Google Scholar]
- Edqvist L.-E., Forsberg M. Clinical reproductive endocrinology. Kaneko J.J., Harvey J.W., Bruss M.L. Clinical Biochemistry of Domestic Animals, 1997, Academic Press: San Diego, 589–617. [Google Scholar]
- Gudermuth D.F., Newton L., Daels P., Concannon P.W. Incidence of spontaneous ovulation in young, group-housed cats based on serum and faecal concentrations of progesterone, Journal of Reproduction and Fertility Supplement 51, 1997, 177–184. [PubMed]
- Hayden D.W., Barnes D.M., Johnson K.H. Morphologic changes in the mammary gland of megestrol acetate-treated and untreated cats: a retrospective study, Veterinary Pathology 26, 1989, 104–113. [DOI] [PubMed] [Google Scholar]
- Hayden D.W., Johnston S.J., Kiang D.T., Johnson K.H., Barnes D.M. Feline mammary hypertrophy/fibroadenoma complex: clinical and hormonal aspects, American Journal of Veterinary Research 42, 1981, 1699–1703. [PubMed] [Google Scholar]
- Hayden D.W., Johnson K.H. Feline mammary hypertrophy-fibroadenoma complex. Kirk R.W. Current Veterinary Therapy IX, 9th edn, 1986, WB Saunders: Philadelphia, 477–480. [Google Scholar]
- Henson K. Reproductive system. Raskin R.E., Meyer D.J. Atlas of Canine and Feline Cytology, 2001, WB Saunders: Philadelphia, 277–312. [Google Scholar]
- Löfstedt R.M. The estrous cycle of the domestic cat, Compendium Continuing Education 4, 1982, 52–58. [Google Scholar]
- de las Mulas J.M. Martin, Millan Y., Bautista M.J., et al. Oestrogen and progesterone receptors in feline fibroadenomatous change: an immunohistochemical study, Research in Veterinary Science 68, 2000, 15–21. [DOI] [PubMed] [Google Scholar]
- Meisl D., Hubler M., Arnold S. Treatment of fibroepithelial hyperplasia (FEH) of the mammary gland in the cat with progesterone antagonist Aglepristone (Alizine), Schweiz Arch Tierheikd 145, 2003, 130–136. [DOI] [PubMed] [Google Scholar]
- Mol J.A., Van Garderen E., Rutteman G.R., Rijnberk A. New insights in the molecular mechanism of progestin-induced proliferation of mammary epithelium: induction of the local synthesis of growth hormone (GH) in the mammary glands of dogs, cats and humans, Journal of Steroid Biochemistry and Molecular Biology 57, 1996, 67–71. [DOI] [PubMed] [Google Scholar]
- Mowers B.S., Conti P.A., Rossow C.F. Vaginal cytology, an approach to improvement of cat breeding, Veterinary Medicine/Small Animal Clinician 70, 1975, 691–696. [PubMed] [Google Scholar]
- Noakes D.E., Parkinson T.J., England G.C.W. Arthur's Veterinary Reproduction and Obstetrics, 8th edn, 2001, WB Saunders: London, pp. 839–848 [Google Scholar]
- Ordás J., Millan Y., de los Monteros A. Espinosa, Reymundo C., de las Mulas J. Martin. Immunohistochemical expression of progesterone receptors, growth hormone and insulin growth factor-I in feline fibroadenomatous change, Research in Veterinary Science 76, 2004, 227–233. [DOI] [PubMed] [Google Scholar]
- Romagnoli S., Concannon P.W. Clinical use of progestins in bitches and queens: a review. Concannon P.W., England G., Verstegen J., Linde-Forsberg C. Recent Advances in Small Animal Reproduction, 2003, International Veterinary Information Service: Ithaca NY, http://www.ivis.org, A1206.0903 (last updated: 9 Sep 2003) [Google Scholar]
- Shille V.M., Lundstrom K.E., Stabenfeldt G.H. Follicular function in the domestic cat as determined by estradiol-17 β concentrations in plasma: relation to estrus behaviour and cornification of exfoliated vaginal epithelium, Biology of Reproduction 21, 1979, 953–963. [DOI] [PubMed] [Google Scholar]
- Van Garderen E., de Wit M., Voorhout W.F., et al. Expression of growth hormone in canine mammary tissue and mammary tumors. Evidence for a potential autocrine/paracrine stimulatory loop, American Journal of Pathology 150, 1997, 1037–1047. [PMC free article] [PubMed] [Google Scholar]


