Abstract
This is the first report of feline solitary plasmacytoma of bone. We describe the clinical, clinico-pathological, radiographic and pathological findings of two successfully treated cats with long-term follow-up. The first case presented with spinal pain and neurological deficits. Radiographs demonstrated sclerosis of lumbar vertebra L6 and a myelogram confirmed interference to flow of contrast in the L4–7 region. A biopsy of L6 revealed neoplastic plasma cell infiltration. There was no evidence of paraproteinaemia on serum protein electrophoresis. The cat underwent hypofractionated megavoltage radiotherapy. Clinical signs resolved completely and 4 years after diagnosis the cat remains well and has no electrophoretically detectable paraproteinaemia. The second case presented with neurological deficits of the tail and spinal radiographs revealed extensive osteolysis of the sacrum. A biopsy of sacral bone demonstrated neoplastic plasma cell infiltration. The animal was normoglobulinaemic. The cat improved clinically with induction chemotherapy (melphalan and methylprednisolone). The same chemotherapeutics were continued at maintenance doses for 4.3 years, at which time there was recurrence of neurological deficits and a palpable sacral mass. Cytological examination of a fine needle aspirate confirmed recurrence of plasma cell neoplasia. A low concentration monoclonal paraproteinaemia was detected. Vincristine was administered resulting in resolution of neurological deficits and a palpably smaller sacral mass. Eighteen months into vincristine therapy, there was recurrence of clinical signs and the cat was euthanased, more than 6 years after the initial diagnosis.
Case 1
A 6-year-old female-neutered domestic shorthair cat was presented to the referring veterinarian with a 1-week history of hindlimb incoordination. The previous medical history was otherwise unremarkable. Clinical examination revealed bilateral hindlimb proprioceptive deficits and pain was elicited on palpation of the caudal lumbar spine. Investigations revealed adequate renal concentrating ability (urine specific gravity 1.050), a granulocytic leukocytosis (white blood cell count 23.6×109/l, reference range 5–18.9×109/l), a marginally raised total protein (92 g/l, reference range 57–89 g/l) and raised globulins (63 g/l, reference range 28–51 g/l). Serum protein electrophoresis (SPE) did not reveal a paraproteinaemia. Serological assays for feline immunodeficiency virus and Toxoplasma species were negative, as was an antigen capture ELISA for feline leukaemia virus (Witness; Rhone-Merieux). Initial treatments included 0.15 mg/kg betamethasone (Betsolan; Schering-Plough) subcutaneously and 4 days later ketoprofen (Ketofen; Merial) 1.25 mg/kg daily per os for 3 days.
Fifteen days after the onset of clinical signs, the patient was referred to Davies Veterinary Specialists. The owner reported little or no observed benefit to the preceding medication. Full clinical and neurological examinations revealed bilateral hindlimb ataxia and proprioceptive deficits with pain elicited on palpation of the caudal lumbar spine. Haematological and serum biochemical examinations were unremarkable. Radiographs revealed extensive sclerosis and areas of lysis within L6, although the vertebral end plates appeared unremarkable (Fig. 1). A lumbar myelogram revealed thinning of the dorsal and ventral contrast columns over the region of L4–7, but in particular at L6. Survey radiographs of the chest and abdomen were unremarkable and no other skeletal lesions were observed. A dorsal laminectomy from L5–7 revealed dorsal displacement of the spinal cord over an abnormal L6 ventral spinal canal floor. The L6 vertebral body was biopsied. The original histopathological report was of a plasmacytoid neoplasm and this was subsequently confirmed when sections were reviewed by a joint-panel of histopathologists (SH, KCS, TJS, PEM) who were involved in diagnosing feline myeloma-related disorders in a large case series (Mellor et al 2006; Fig. 2).
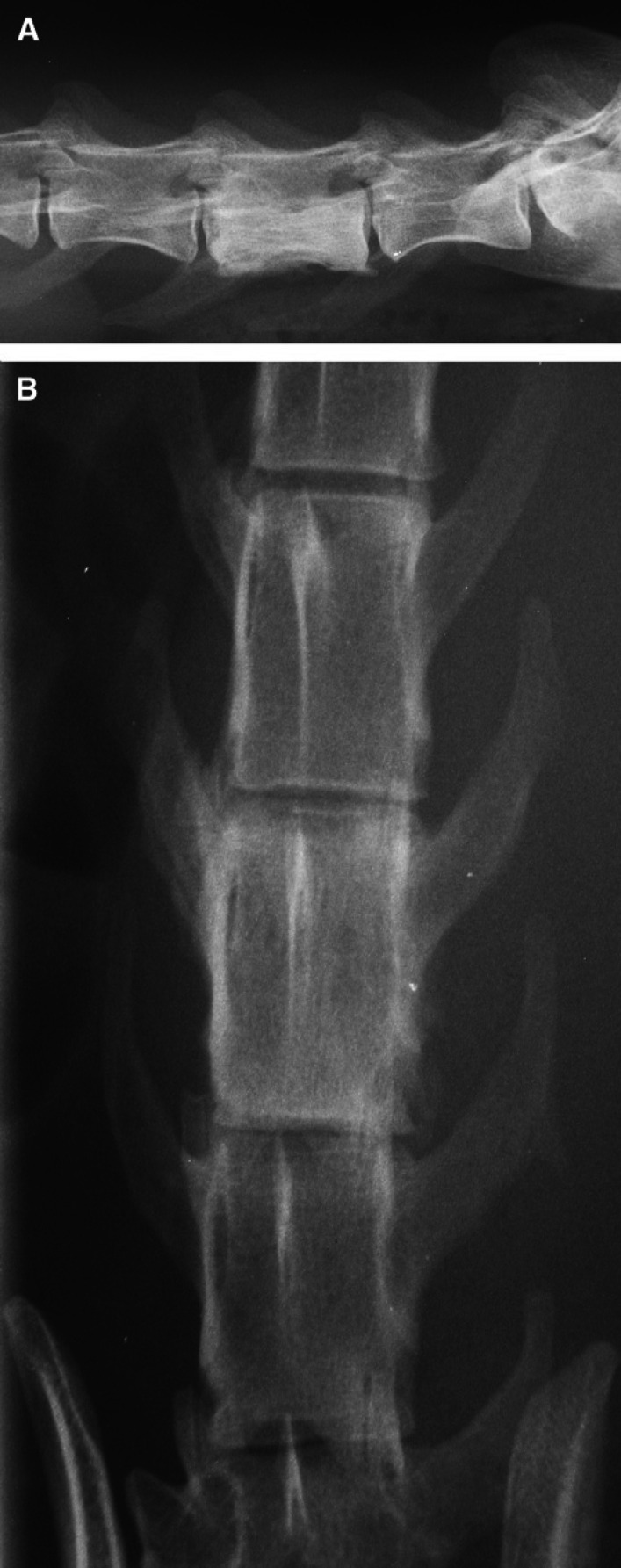
Fig 1.
Case 1 – survey lateral and ventro-dorsal spinal radiographs. On these views taken by the referring veterinarian 8 days after the onset of clinical signs, there is marked sclerosis of the lumbar vertebra L6 body. This is attributable to palisading new bone formation, most notable at the ventral and lateral margins of the L6 body. Mild to moderate osteolysis is also evident in these locations. The ventral margin of the vertebral canal appears indented due to new bone formation. The vertebral end plates appear unremarkable.
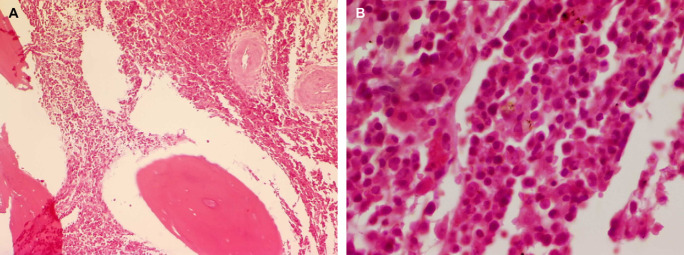
Fig 2.
Case 1 – histopathological section of a surgical biopsy of the lumbar vertebral body L6. There is extensive infiltration of inter-trabecular spaces by a neoplastic round cell population. The infiltrating cells have abundant eosinophilic cytoplasm with eccentrically located hyperchromatic round-to-oval nuclei consistent with plasmacytoid cells (haematoxylin and eosin stain, original magnification ×200 (A) and ×1000 (B)).
The patient's post-surgical recovery was unremarkable, with progressive improvement in hindlimb ataxia. After 2 weeks, manually planned, hypofractionated radiotherapy was instituted using a 4 MeV linear accelerator. The field size was determined from spinal radiographs. Radiation was delivered over a 6×4 cm area centred on the L6 vertebra with 1 cm margins via parallel opposed beams. The treated tissue received three doses of 4 Gy once weekly to a total of 12 Gy. This dose schedule was empirically derived by one of the co-authors (MJB) based upon observations made using hypofractionated radiotherapy in the management of tumours affecting this site (Laing et al 1989, Rusbridge et al 1999). No side-effects were noted during radiotherapy and the cat's hindlimb locomotor ability continued to improve. At follow-up 2 months post-radiotherapy, the cat appeared to be normal with respect to gait, general clinical and neurological examinations. No medications were prescribed. At 4.1 years after initial diagnosis, clinical and neurological examinations were unremarkable. Haematological and serum biochemical examinations showed no significant abnormalities except marginal increases in total protein (84.5 g/l, reference range 60–80 g/l) and globulin (49.4 g/l, reference range 25–45 g/l). SPE did not reveal a monoclonal paraprotein.
Case 2
A 7-year-old male-neutered domestic longhair cat was presented to the referring veterinarian with a 3-day history of a hunched posture and decreased tail movement. Previous medical history was unremarkable. Clinical examination revealed decreased tail sensation, movement and muscle tone and a palpable mass in the sacral region. Haematological and serum biochemical examinations (including total protein and globulin) were unremarkable. Radiographs of the thorax were unremarkable. A survey of the lateral lumbosacral spinal radiograph revealed extensive destruction of the sacrum (Fig. 3). An incisional biopsy of the sacral bone revealed sheets of proliferating plasma cells demonstrating pleomorphism and high mitotic activity (IDEXX Laboratories, Wetherby, UK). The original biopsy specimen was not available for review.
Fig 3.
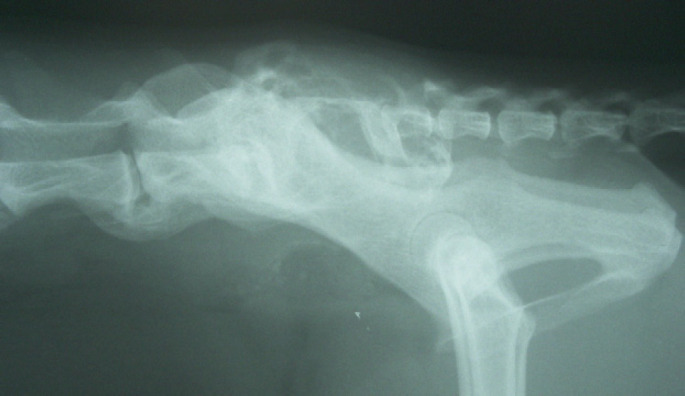
Case 2 – survey lateral spinal radiograph. On this view taken by the referring veterinarian at first presentation, the sacral silhouette appears poorly defined, with the cranial sacral end plate most clearly evident. The sacrum is virtually ablated by osteolysis, and surrounded by extensive ‘wispy’ new bone formation that is seen superimposed over the sacroiliac joint and extending into the soft tissue dorsal to the wings of the ilia and caudally into the pelvis to the level of the acetabulae. There is dorsal displacement of the tail. The first coccygeal vertebra appears unaffected. Smooth bridging spondylosis is observed from L6 to S1. The contours of the ilia appear unremarkable.
Post-surgical recovery was unremarkable and 3 weeks later the cat commenced chemotherapy comprising melphalan (Alkeran; GlaxoSmithKline) 0.1 mg/kg daily for 14 days, followed by alternate day use and methylprednisolone (Medrone V; Pharmacia & Upjohn) 2 mg/kg daily for 14 days, followed by 1 mg/kg daily. One month into chemotherapy, clinical examination revealed normal tail and hindlimb neurological function and haematological analysis was normal. However, 11 weeks after commencing chemotherapy, the cat appeared mildly depressed and haematological analysis revealed neutropenia (1.37×109/l, reference range 2.5–12.5×109/l). Chemotherapy was withheld for 1 week and following clinical and haematological recovery, therapy was recommenced with melphalan 0.2 mg/kg every 3 days and methylprednisolone 1 mg/kg every other day. A gradual lengthening in the chemotherapeutic dose interval followed, and 1.7 years after commencing the treatment the cat continued to receive melphalan 0.2 mg/kg every 8 days and methylprednisolone 1 mg/kg every 4 days with good owner compliance. Over this period, regular clinical and haematological examinations were unremarkable. The cat continued to receive the same doses of medication but at 4.3 years into treatment it re-presented with apparent hindlimb weakness manifested by a decreased ability to jump, as well as a palpable mass in the left dorsal sacral region. Fine needle aspirates of the mass revealed plasmacytoid neoplastic cells and this was confirmed when slides were subsequently reviewed by a joint-panel of cytologists (JA, EJV, RMP, PJM) who were involved in diagnosing feline myeloma-related disorders in a large case series (Mellor et al 2006; Fig. 4).
Fig 4.
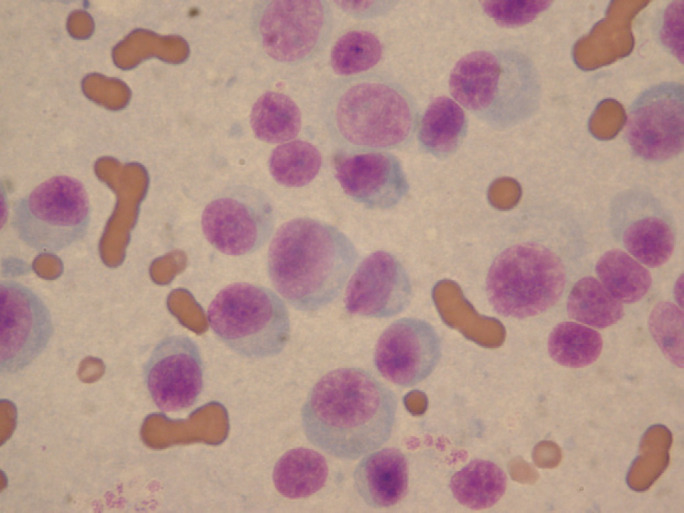
Case 2 – cytological preparation from a fine needle aspirate of the sacral mass. The aspirate was taken 4.4 years after the histopathological diagnosis of a myeloma-related disorder from a sacral biopsy. There are large numbers of round cells showing moderate anisocytosis with scant to moderate amounts of basophilic cytoplasm. A perinuclear Golgi-region clear halo is observed in many cells. Round-to-ovoid nuclei are eccentrically located. There is moderate anisokaryosis and a variable nuclear:cytoplasmic ratio. Nuclear chromatin appears coarse and small; indistinct nucleoli are present. The cells are consistent with plasmacytoid cells (May–Grünwald–Giemsa stain, original magnification ×1000).
At this time point, the cat was referred to Davies Veterinary Specialists. Urination and defecation were normal. Clinical examination revealed a palpable non-painful mass in the left dorsal sacral region, moderate hindlimb ataxia and decreased tail movement and muscle tone. Melphalan and methylprednisolone were discontinued. Treatment options considered included radiotherapy and chemotherapy. The owner elected for chemotherapy and vincristine (vincristine sulphate, Faulding DBL) 0.5 mg/m2 was given intravenously once weekly. By the third treatment, the owner reported increased hindlimb strength (manifested by an increased ability to jump). At examination, hindlimb gait had normalised, increased hindlimb strength was evident, but the tail remained paretic. The sacral mass was palpably smaller. Chemotherapy with vincristine was maintained weekly for 8 weeks then the interval was lengthened to monthly injections. Eight months into vincristine therapy, full haematological and urine examinations including urine protein electrophoresis were unremarkable. Serum biochemical analysis revealed a marginally increased globulin of 48.5 g/l (reference range 25–45 g/l). SPE revealed a small but narrow spike in the γ-globulin region (Fig. 5) suggestive of a low concentration monoclonal paraprotein, although the absolute concentration of the γ-globulin fraction was within normal limits (25.63 g/l, reference range 17–27 g/l) (Central Diagnostic Services, Dept. of Veterinary Medicine, University of Cambridge). Monthly vincristine injections continued for 18 months, at which time the cat re-presented with increasing sacral mass size and recurrence of hindlimb and tail weakness. The owners requested elective euthanasia of the cat, 6 years after initial histopathological diagnosis. Post-mortem examination was not allowed.
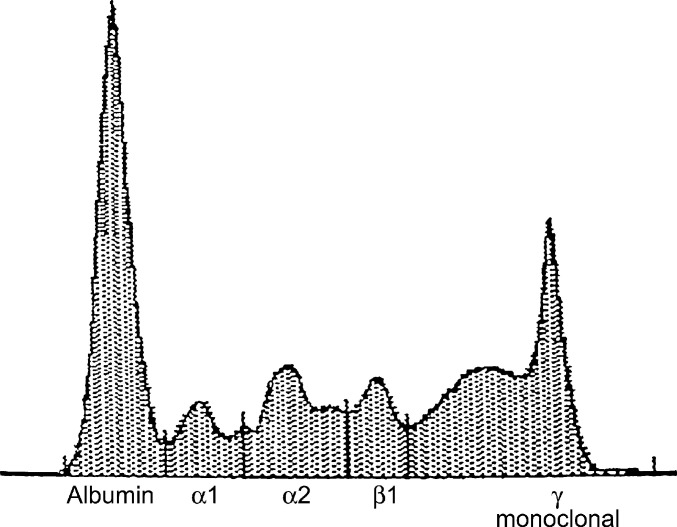
Fig 5.
Case 2 – serum protein electrophoresis. Five years after the initial histopathological diagnosis of a sacral myeloma-related disorder in this cat, SPE revealed a small but narrow spike in the γ-globulin region. Although the absolute concentration of the γ-globulin fraction was within normal limits (25.63 g/l, reference range 17–27 g/l), the spike is consistent with a low concentration paraproteinaemia.
The myeloma-related disorders (MRD) derive from the neoplastic transformation of a plasma cell or its B-lymphocyte precursors and their classification is outlined in Fig. 6 (International-Myeloma-Working-Group 2003). Solitary plasmacytoma of bone (SPB) is uncommon in humans accounting for only 3–5% of all MRD patients (International-Myeloma-Working-Group 2003). The median age of diagnosis of SPB is lower than that observed in multiple myeloma patients (55 vs. 65 years) (International-Myeloma-Working-Group 2003, Soutar et al 2004). The spine is most commonly affected (Chang et al 1994). Hyperglobulinaemia is uncommon, but serum or urine paraprotein is demonstrable in a small proportion of cases by routine electrophoresis (Soutar et al 2004) and in up to 72% of cases by the more sensitive technique of immunofixation (a form of immunoelectrophoresis) (Dimopoulos et al 2000). In human patients, a complete radiographic skeletal survey is recommended (Soutar et al 2004). Ideally, additional bone marrow aspiration and core biopsy at a separate site from the putative SPB should be carried out to demonstrate cytologically and histopathologically normal marrow elsewhere (<5% plasma cells) (Mackey 1977, Soutar et al 2004).
Fig 6.

Classification of feline myeloma-related disorders. The myeloma-related disorders derive from the neoplastic transformation of plasma cells or immunoglobulin-secreting B-lymphocyte precursors.
MRD are rare in cats, accounting for 0.003–0.1% of all feline malignancies reported in two large case series (Engle and Brody 1969, Carpenter et al 1987) and to the authors' knowledge, feline SPB has not been described previously. The median age of feline MRD patients was 12 years in one study (Mellor et al 2006). Feline myeloma displays a different phenotype from that classically reported in canine and human multiple myeloma, with many cats displaying marked extramedullary infiltration (eg, hepatomegaly, splenomegaly and/or other abdominal masses) (Mellor et al 2006). Although radiographic bony lesions have been described in feline MRD (Hawkins et al 1986, Sheafor et al 1996, Bienzle et al 2000, Hanna 2005), the prevalence appears to be significantly lower in comparison with human MRD (8% vs. 80%) (Mellor et al 2006). There is a shared pathophysiology between myeloma and non-cutaneous extramedullary plasmacytoma in cats – typically, production of serum paraprotein leading to hyperviscosity syndrome (Mellor et al 2006). They are usually aggressive neoplastic disorders and the overall median survival time for cats responding to combination chemotherapy is 12.3–16 months (Hanna 2005, Mellor et al 2006).
By comparison, the two relatively young patients reported herein had neither systemic clinical signs nor hyperglobulinaemia. Clinical examination and clinico-pathological findings did not reveal any of the presenting abnormalities typically observed in feline myeloma and non-cutaneous extramedullary plasmacytoma (Mellor et al 2006). Both cats had dramatic radiographic vertebral bony lesions with no radiographic evidence of other thoracic/abdominal pathology. In both cases, biopsy of the radiographic bony lesions revealed pathological evidence of neoplastic plasma cell infiltration. SPE did not demonstrate a paraproteinaemia in case 1 at follow-up (4 years after histopathological diagnosis) but did reveal a low concentration paraproteinaemia in case 2 at follow-up (5 years after histopathological diagnosis). Limitations in the investigation of these cases included partial (rather than complete) skeletal survey radiography, a lack of abdominal ultrasonography and a lack of bone marrow sampling outside of the lesional site. Immunofixation is reported to be a more sensitive technique than SPE for the detection of paraproteinaemia (Dimopoulos et al 2000), but is not a clinically available test in veterinary laboratories at the current time and was not carried out in our patients. The clinical presentation, clinico-pathologic data, radiographic and pathological findings as well as the slow disease progression over years in these two cats were consistent with a diagnosis of solitary plasmacytoma of bone.
In case 2, melphalan tablets (administered prior to referral) were divided to achieve the desired dose, although it must be noted that cytotoxic drugs in tablet form should not be split for health and safety reasons. Radiotherapy is the treatment of choice in human solitary plasmacytoma of bone (Soutar et al 2004) with 100% local control for tumours under 5 cm, and 38% control if over 5 cm (Tsang et al 2001). Large tumours may best be managed by combined chemotherapy and higher dose radiotherapy based on limited published evidence in man (Soutar et al 2004). Response may be assessed clinically, by imaging and by monitoring paraprotein levels in affected patients (Wilder et al 2002).
The majority of human solitary plasmacytoma of bone patients develop myeloma, with a median time to progression of 2–4 years (Soutar et al 2004). To the authors' knowledge, this is the first report of feline solitary plasmacytoma of bone. The prolonged survival times following radiotherapy in one cat (>4 years) and chemotherapy in the other (6 years) are encouraging.
Acknowledgements
The authors would like to thank the following people: the staff at Davies Veterinary Specialists; the referring veterinarians (Neil Palmer MRCVS, Shearbridge Veterinary Hospital, Bradford; Chess Veterinary Clinic, Rickmansworth; Joel Veterinary Clinic, Eastcote); the owners and their cats; the staff of LVL Ltd., Leeds and IDEXX Laboratories, Wetherby, D.I. Andrew Sillwood (investigations in Case 1) and to Camvet for allowing access to the linear accelerator at the Queen's Veterinary School Hospital, Cambridge, UK. This work was financially supported by the University of Cambridge Vet School Trust and Pet Plan Charitable Trust. Paul Mellor's clinical scholarship at the University of Cambridge was kindly sponsored by Hill's Pet Nutrition Ltd.
References
- Bienzle D., Silverstein D.C., Chaffin K. Multiple myeloma in cats: variable presentation with different immunoglobulin isotypes in two cats, Veterinary Pathology 37, 2000, 364–369. [DOI] [PubMed] [Google Scholar]
- Carpenter J.L., Andrews L.K., Holzworth J. Tumours and tumour like lesions. Holzworth J. Diseases of the Cat: Medicine and Surgery, 1987, W.B. Saunders: Philadelphia, 406–596. [Google Scholar]
- Chang M.Y., Shih L.Y., Dunn P., Leung W.M., Chen W.J. Solitary plasmacytoma of bone, Journal of the Formosan Medical Association 93, 1994, 397–402. [PubMed] [Google Scholar]
- Dimopoulos M.A., Moulopoulos L.A., Maniatis A., Alexanian R. Solitary plasmacytoma of bone and asymptomatic multiple myeloma, Blood 96, 2000, 2037–2044. [PubMed] [Google Scholar]
- Engle G.C., Brody R.S. A retrospective study of 395 feline neoplasms, Journal of the American Animal Hospital Association 5, 1969, 21–31. [Google Scholar]
- Hanna F. Multiple myelomas in cats, Journal of Feline Medicine & Surgery 7, 2005, 275–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins E.C., Feldman B.F., Blanchard P.C. Immunoglobulin A myeloma in a cat with pleural effusion and serum hyperviscosity, Journal of the American Veterinary Medical Association 188, 1986, 876–878. [PubMed] [Google Scholar]
- International-Myeloma-Working-Group Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working Group, British Journal of Haematology 121, 2003, 749–757. [PubMed] [Google Scholar]
- Laing E.J., Fitzpatrick P.J., Norris A.M., Mosseri A., Rider W.D., Binnington A.G., Baur A., Valli V.E. Half-body radiotherapy. Evaluation of the technique in normal dogs, Journal of Veterinary Internal Medicine 3, 1989, 96–101. [DOI] [PubMed] [Google Scholar]
- Mackey L. Haematology of the cat. Archer R.K., Jeffcott L.B. Comparative Clinical Haematology, 1977, Blackwell Scientific Publications: Oxford, 441–482. [Google Scholar]
- Mellor P.J., Haugland S., Murphy S., Smith K.C., Holloway A., Archer J., Powell R.M., Polton G., Tasker S., McCormick D., Tempest M.E., McNeil P.E., Scase T.J., Knott C.D., Bonfanti U., Villiers E.J., Argyle D.J., Herrtage M.E., Day M.J. Feline myeloma-related disorders commonly present as extramedullary neoplasms in contrast to human myeloma: 24 cases with clinical follow up, Journal of Veterinary Internal Medicine 20, 2006, 1376–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusbridge C., Wheeler S.J., Lamb C.R., Page R.L., Carmichael S., Brearley M.J., Bjornson A.P. Vertebral plasma cell tumors in 8 dogs, Journal of Veterinary Internal Medicine 13, 1999, 126–133. [DOI] [PubMed] [Google Scholar]
- Sheafor S.E., Gamblin R.M., Couto C.G. Hypercalcaemia in two cats with multiple myeloma, Journal of the American Animal Hospital Association 32, 1996, 503–508. [DOI] [PubMed] [Google Scholar]
- Soutar R., Lucraft H., Jackson G., Reece A., Bird J., Low E., Samson D. Guidelines on the diagnosis and management of solitary plasmacytoma of bone and solitary extramedullary plasmacytoma, British Journal of Haematology 124, 2004, 717–726. [DOI] [PubMed] [Google Scholar]
- Tsang R.W., Gospodarowicz M.K., Pintilie M., Bezjak A., Wells W., Hodgson D.C., Stewart A.K. Solitary plasmacytoma treated with radiotherapy: impact of tumor size on outcome, International Journal of Radiation Oncology, Biology, Physics 50, 2001, 113–120. [DOI] [PubMed] [Google Scholar]
- Wilder R.B., Ha C.S., Cox J.D., Weber D., Delasalle K., Alexanian R. Persistence of myeloma protein for more than one year after radiotherapy is an adverse prognostic factor in solitary plasmacytoma of bone, Cancer 94, 2002, 1532–1537. [DOI] [PubMed] [Google Scholar]