Abstract
Background.
The authors assessed the clinical effectiveness of analgesics to manage acute pain after dental extractions and pain associated with irreversible pulpitis in children.
Types of Studies Reviewed.
The authors searched MEDLINE, Embase, Cochrane Central Register of Controlled Trials, and US Clinical Trials registry from inception through November 2020. They included randomized controlled trials comparing any pharmacologic interventions with each other and a placebo in pediatric participants undergoing dental extractions or experiencing irreversible pulpitis. After duplicate screening and data abstraction, the authors conducted random-effects meta-analyses. They assessed risk of bias using the Cochrane Risk of Bias 2.0 tool and certainty of the evidence using the Grading of Recommendations Assessment, Development and Evaluation approach.
Results.
The authors included 6 randomized controlled trials reporting 8 comparisons. Ibuprofen may reduce pain intensity compared with acetaminophen (mean difference [MD], 0.27 points; 95% CI, −0.13 to 0.68; low certainty) and a placebo (MD, −0.19 points; 95% CI, −0.58 to 0.21; low certainty). Acetaminophen may reduce pain intensity compared with a placebo (MD, −0.13 points; 95% CI, −0.52 to 0.26; low certainty). Acetaminophen and ibuprofen combined probably reduce pain intensity compared with acetaminophen alone (MD, −0.75 points; 95% CI, −1.22 to −0.27; moderate certainty) and ibuprofen alone (MD, −0.01 points; 95% CI, −0.53 to 0.51; moderate certainty). There was very low certainty evidence regarding adverse effects.
Practical Implications.
Several pharmacologic interventions alone or in combination may provide a beneficial effect when managing acute dental pain in children. There is a paucity of evidence regarding the use of analgesics to manage irreversible pulpitis.
Keywords: Acute dental pain, dental extraction, toothache, irreversible pulpitis, systematic review, nonsteroidal antiinflammatory, acetaminophen
Management of acute pain in children is changing with emergence of novel pharmacologic evidence. In the United States, to gain approval to administer a drug in a pediatric population, the US Food and Drug Administration (FDA) requires provision of evidence of the drug’s effectiveness in this population. Randomized controlled trials (RCTs) examining the effectiveness of pharmacologic interventions, specifically analgesics, in children, however, remain scarce.
Nonsteroidal anti-inflammatory drugs (NSAIDs), acetaminophen, and opioids have been common medication choices for the treatment of acute dental pain in children.1,2 NSAIDs are frequently used drugs owing to their anti-inflammatory, analgesic, antipyretic, and antiplatelet properties.3 Examples of the major categories of NSAIDs are salicylic acids (aspirin), propionic acids (ibuprofen, naproxen), and selective cyclooxygenase-2 inhibitors (celecoxib).3 The adverse events associated with the uptake of NSAIDs include increased incidence of cardiovascular events, inhibition of platelet function, decreased kidney blood flow, and gastritis with pain and bleeding.4
Acetaminophen is an analgesic often prescribed for mild to moderate pain and is an antipyretic.4 This analgesic can be administered orally in tablet, capsule, or liquid form, as well as intravenously and via the rectum.5 Optimization of pain management has been shown when acetaminophen and NSAIDs are either combined or staggered, which is a therapeutic strategy termed multimodal therapy.1,6–8
Opioid analgesics usually are considered for acute pain of either moderate or severe intensity. Oral opioid agents include codeine, morphine, oxycodone, hydrocodone, and tramadol. Since 2016, there has been a marked decrease in prescribing of oral opioids for the management of acute pain, including acute dental pain.9–11 As of April 2017, the use of codeine in the United States in children 12 years or younger was prohibited by the FDA.12 Evidence suggests that in dentistry, oral opioids may offer no advantage over appropriately dosed NSAIDs.13
Although oral health care providers have been decreasing the prescription of opioid-containing medications to children owing to concerns related to the risk of harm, they continue to prescribe them. To date, to our knowledge, there has been no systematic review (SR) and meta-analysis that describes and compares the effect of analgesics, including opioid-containing medications, being prescribed for the management of acute dental pain in children. Furthermore, there is no clinical practice guideline that provides recommendations to assist patients and clinicians in determining the most appropriate use of pharmacologic strategies for the management of acute dental pain. Therefore, we conducted this SR and meta-analysis to describe the evidence that was used to inform the development of a clinical practice guideline for the management of acute dental pain in the pediatric population produced by the American Dental Association (ADA) Council on Scientific Affairs, the ADA Science and Research Institute, and the University of Pittsburgh’s and the University of Pennsylvania’s schools of dental medicine in partnership with the FDA.
The objective of this SR and meta-analysis was to assess the comparative effectiveness of analgesic treatments for the management of pain after dental extraction (simple and surgical tooth extraction) and irreversible pulpitis in the pediatric population (≤ 12 years). We included both patient populations because tooth extraction and irreversible pulpitis are known causes of acute dental pain.
METHODS
For this SR, we followed the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses checklist (eTable 1, available online at the end of the article).14 We did not register this SR and meta-analysis; however, we followed preestablished methodology outlined in the plan for clinical practice guideline development and defined eligibility criteria determined by the recommendation questions posed by the guideline panel. The recommendation questions were informed using the report from the National Academies of Sciences, Engineering, and Medicine titled Framing Opioid Prescribing Guidelines for Acute Pain: Developing the Evidence.15
Eligibility criteria
We included RCTs that compared the effectiveness of any analgesic treatment against another analgesic or a placebo, except for treatments administered intravenously, in patients 12 years or younger undergoing simple or surgical tooth extraction or experiencing irreversible pulpitis. We included any outcome of effectiveness and safety related to acute pain reported in these RCTs. We limited the inclusion criteria to peer-reviewed articles published in English. All decisions regarding the eligibility criteria were made by the panel of experts including dentists and dental surgeons with professional experience in the management of acute dental pain to create an updated clinical practice guideline for the management of acute dental pain in children.
Information sources
We performed searches in MEDLINE, Embase, Cochrane Central Register of Controlled Trials, and the US Clinical Trials registry from inception through November 2020. We conducted a search strategy that included a combination of key and free terms reflecting concepts related to acute pain (for example, tooth extraction, pulpitis, toothache) and analgesic therapy (for example, analgesics, analgesia) and a filter for RCTs (eTable 2, available online at the end of this article).
Study selection
Using SR software (Covidence; Vertias Health Innovation) and after undergoing training and calibration exercises, unfixed pairs of reviewers (M.A., S.I., D.T., L.H.) independently screened all titles and abstracts, followed by full texts of trials that were identified as potentially eligible. A third reviewer (A.M.) resolved conflicts.
Data collection
For each eligible trial, pairs of reviewers (M.A., S.I., D.T., L.H.) extracted data independently using a standardized, pilot-tested data extraction form after undergoing training and calibration exercises. Reviewers collected information on trial characteristics (for example, design), patient characteristics (for example, age, sex, and country), and outcomes (that is, all outcomes of effectiveness and safety related to acute dental pain). Pairs of reviewers resolved discrepancies in data collection forms via discussion and, when necessary, with adjudication by a third reviewer (A.M.).
Risk of bias of individual studies
After undergoing training and calibration exercises, for each eligible trial and outcome, pairs of reviewers (M.A., S.I.) used a modified version of RoB 2.0, the Cochrane tool for assessing risk of bias in randomized trials, to rate trials as at low risk of bias, probably at low risk of bias, probably at high risk of bias, or at high risk of bias across the following domains: bias arising from the randomization process, bias due to deviations from the intended intervention, bias due to missing data, bias due to measurement of the outcome, and bias in selection of the reported results.16 This modified version of RoB 2.0 has been used and reported in other peer-reviewed articles.17–19 Reviewers resolved discrepancies via discussion and, when necessary, with adjudication by a third reviewer (A.M.).
Data synthesis
We summarized the effect of interventions on dichotomous outcomes using the risk ratio. When the incidence of the outcome was low across studies (for example, no events in several study groups), we used the risk difference. For continuous outcomes, we used the mean difference. When studies reported the same outcome using scales with different ranges, we converted all data for that outcome to the scale most frequently reported among the included studies before conducting analyses. We built 95% CIs around all reported estimates.
We performed random-effect meta-analyses weighting studies according to the inverse of their variance, using Review Manager Version 5.4 (Cocharane Collaboration). If a study did not provide an effect estimate for an outcome, we used Review Manager to calculate this effect estimate from the data reported in the study. We also generated forest plots to illustrate all effect estimates, even if only 1 study was informing the outcome of interest. We considered the random-effects model because we could not assume that all studies included in the meta-analysis were estimating a single true underlying effect and because we intended to generalize the results beyond the included studies.20
Certainty of the evidence
We assessed the certainty of the evidence using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach. Two methodologists (M.A., S.I.) with experience using GRADE rated each domain for each comparison and outcome independently, resolving discrepancies via discussion and, when necessary, with adjudication by a third methodologist (R.B.-P.). We rated the certainty as high, moderate, low, or very low, while taking into consideration risk of bias, inconsistency (heterogeneity), indirectness, publication bias, and imprecision. We used a minimally contextualized approach with a null effect threshold to rate the certainty that there is a benefit or a harm.21 When the point estimate was close to the null effect, we rated our certainty that there was a trivial effect (that is, no important difference) using a threshold of 10% of the baseline risk for dichotomous outcomes and 10% of the scale range for continuous outcomes.22 Although the effect estimates we present may be statistically significant, these may not be clinically important on the basis of the established thresholds. For dichotomous outcomes pooled using the risk ratio, we calculated absolute estimates of effect using the mean baseline risk across trials. To facilitate interpretation of results for dichotomous outcomes, we calculated absolute effects and presented them in natural frequencies (that is, per 100 patients). We created GRADE Summary of Findings tables using GRADEpro (McMaster University and Evidence Prime).
RESULTS
Our search across all databases yielded 4,716 records to screen, of which we assessed 858 at full-text level. Six RCTs proved to be eligible (Figure).23–28
Figure.
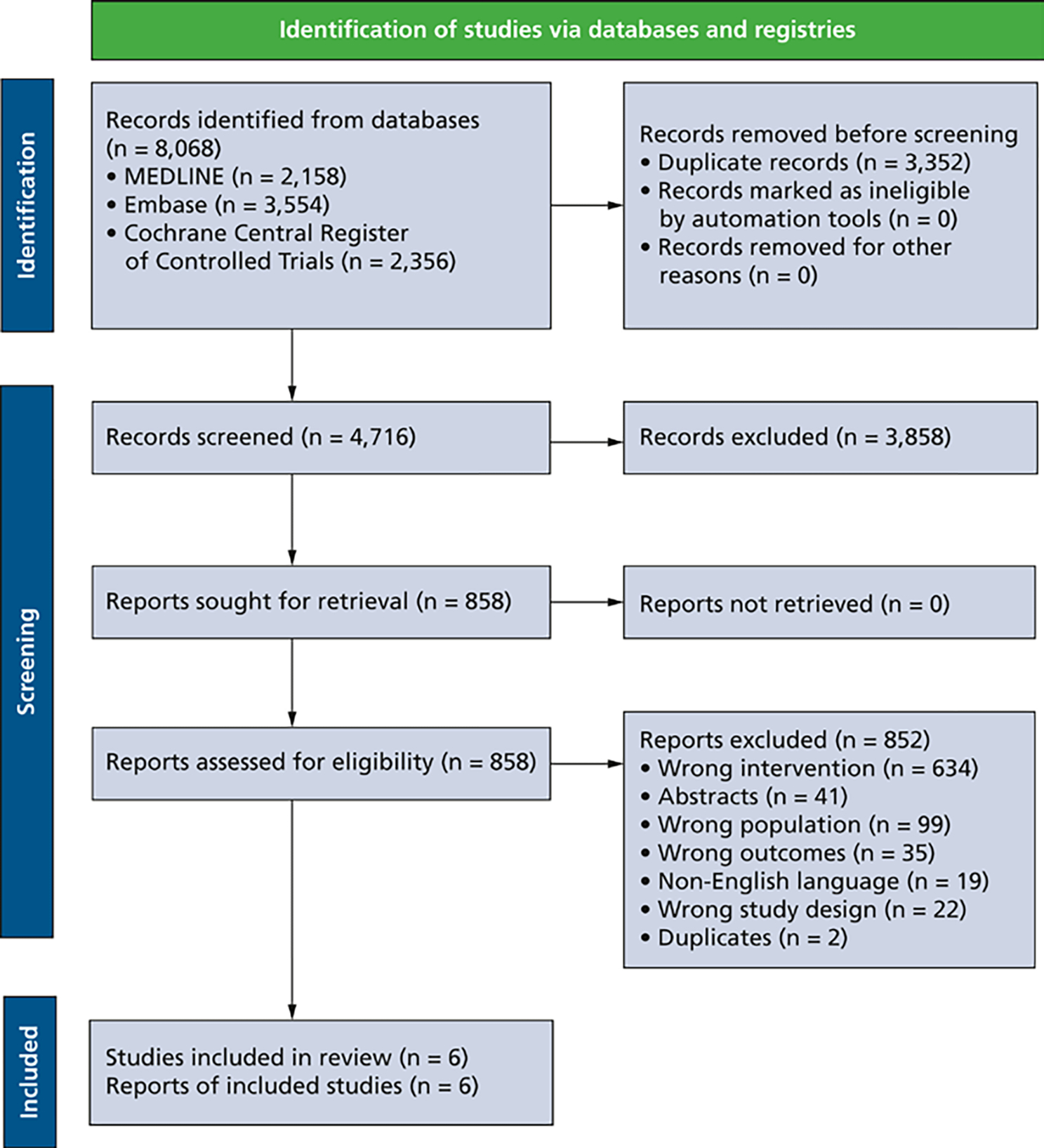
Preferred Reporting Items for Systematic Reviews and Meta-Analyses14 flowchart of study identification and selection.
Characteristics of included studies
The number of participants randomized ranged from 45 through 201. Mean (SD) age across studies ranged from 5.5 (1.9) years through 9.3 (2.3) years. In all trials, participants underwent simple tooth extractions, and in one-half of the trials, participants also underwent surgical tooth extractions (Table 1). No studies reported on the effect of analgesics for the management of irreversible pulpitis.
Table 1.
Characteristics of the included studies.
| STUDY | STUDY DESIGN | COUNTRY | PARTICIPANTS RANDOMIZED, NO. | AGE, Y | SEX, FEMALE, % | TYPE OF EXTRACTION | INTERVENTIONS | TIME OF ADMINISTRATION | COINTERVENTIONS | OUTCOMES |
|---|---|---|---|---|---|---|---|---|---|---|
| Moore and Colleagues,27 1985 | Parallel group | United States | 45 | Range, 5–12 | 45.45 | Simple tooth extraction | Acetaminophen (240 mg or 360 mg) Acetaminophen (240 mg) with codeine (24 mg) Ibuprofen (200 mg) Placebo |
After extraction | Not administered | Pain intensity (4 h), pain relief (4 h), global efficacy rating (4 h), adverse effects (4 h) |
| McGaw and Colleagues,26 1987 | Parallel group | Canada | 123 | Range, 8–16* | 58.14 | Simple tooth extraction, surgical tooth extraction | Ibuprofen (200 mg in suspension) Acetaminophen (240 mg or 360 mg) Placebo |
After extraction | Not reported | Pain intensity (4 h), pain relief (4 h), global efficacy rating (4 h), adverse effects (4 h) |
| Elhakim,23 1993 | Parallel group | Egypt | 60 | Mean (SD), 5.47 (1.94) | 40.00 | Simple tooth extraction, surgical tooth extraction | Acetaminophen (10 mg/kg) Placebo |
After extraction | General anesthesia | Presence of pain (15 min and 30 min), adverse effects (1 h) |
| Primosch and Colleagues,28 1995 | Parallel group | United States | 60 | Mean (SD), 6.64 (1.76) | 36.67 | Simple tooth extraction | Ibuprofen (10–15 mg/kg in suspension) Acetaminophen (15–20 mg/kg) Placebo |
Before extraction | 2% lidocaine with 1:100,000 epinephrine | Presence of pain (within 7 h postoperatively), rescue analgesia (within 7 h postoperatively) |
| Gazal and Mackie,24 2007 | Parallel group | United Kingdom | 201 | Mean (SD), 6.64 (2.44) | Not reported | Simple tooth extraction, surgical tooth extraction | Acetaminophen (15 mg/kg) Acetaminophen (20 mg/kg) Ibuprofen (5 mg/kg) Acetaminophen (15 mg/kg) and ibuprofen (5 mg/kg) |
Before extraction | General anesthesia | Pain intensity (15 min after recovery from anaesthesia) |
| Kharouba and Colleagues,25 2019 | Parallel group | Israel | 105 | Mean (SD), 9.25 (2.25) | 54.55 | Simple tooth extraction | Acetaminophen (15 mg/kg) Ibuprofen (15 mg/kg) Placebo |
Before extraction | 2% lidocaine with 1:100,000 epinephrine | Pain intensity (4 h and 24 h), rescue analgesia (4 h) |
The range of 8–16 years (outside of age range of ≤ 12 years) was accounted for in the certainty of the evidence assessments.
Risk of bias in included studies
The risk of bias domains most often judged at high or probably high risk of bias across studies were missing outcome data and selective reporting of the results (eTable 3, available online at the end of this article).
Effects of interventions
Pain Intensity (Scale From 1 [None]-4 [Severe])
Ibuprofen, acetaminophen, and combination
Ibuprofen may reduce pain intensity at 4 hours by a trivial amount compared with acetaminophen (mean difference [MD], 0.27 points; 95% CI, −0.13 to 0.68; low certainty) (Table 2; eFigure 1, available online at the end of this article) and a placebo (MD, −0.19 points; 95% CI, −0.58 to 0.21; low certainty) (Table 3; eFigure 2, available online at the end of this article). Acetaminophen may reduce pain intensity at 4 hours by a trivial amount compared with a placebo (MD, −0.13 points; 95% CI, −0.52 to 0.26; low certainty) (Table 4; eFigure 3, available online at the end of this article). Acetaminophen (15 mg/kg) and ibuprofen (5 mg/kg) probably reduces pain intensity 15 minutes after recovery from general anesthesia compared with acetaminophen (15 mg/kg or 20 mg/kg) by an important amount (MD, −0.75 points; 95% CI, −1.22 to 0.27; moderate certainty) (eTable 4, eFigure 4, available online at the end of this article) and ibuprofen (5 mg/kg) by a trivial amount (MD, −0.01 points; 95% CI, −0.53 to 0.51; moderate certainty) (eTable 5, eFigure 5, available online at the end of this article).
Table 2.
Ibuprofen vs acetaminophen for acute dental pain in children.
| OUTCOME | PARTICIPANTS, NO. (STUDIES) | RELATIVE EFFECT (95% CI)* | ANTICIPATED ABSOLUTE EFFECTS (95% CI) |
CERTAINTY (GRADE†) | WHAT HAPPENS | ||
|---|---|---|---|---|---|---|---|
| With Ibuprofen | With Acetaminophen | Difference | |||||
| Any Adverse Effect Assessed With Proportion of Participants Experiencing Dizziness and Mild Stomach Upset; Follow-up, 4 H | 109 (2 RCTs‡)§ | Not estimable | 1.8% | 1.9% | 0% more (6% fewer to 6% more) | Very low¶,# | There is very low certainty evidence regarding the difference between acetaminophen and ibuprofen for the incidents of adverse effects at 4 h. |
| Rescue Analgesia Assessed With Proportion of Patients Requiring Rescue Analgesia; Follow-up, 4 H | 76 (1 RCT) | RR**, 3.45 (0.80 to 14.92) | 6.1% | 20.9% (4.8% to 90.4%) | 14.8% more (1.2% fewer to 84.4% more) | Low†† | Acetaminophen may increase the need for rescue analgesia at 4 h by an important amount compared with ibuprofen. |
| Rescue Analgesia Assessed With Proportion of Patients Requiring Rescue Analgesia; Follow-up, 7 H | 40 (1 RCT) | RR, 0.75 (0.19 to 2.93) | 20.0% | 15.0% (3.8% to 58.6%) | 5.0% fewer (16.2% fewer to 38.6% more) | Low‡‡ | Acetaminophen may decrease the need for rescue analgesia at 7 h by an important amount compared with ibuprofen. |
| Pain Intensity Assessed With Scale From 1 (None) Through 4 (Severe); Follow-up, 4 H | 335 (4 RCTs) | –**** | Median pain intensity, 1.77 points | – | MD,§§ 0.27 points higher (0.13 lower to 0.68 higher) | Low¶¶,##,*** | Ibuprofen may reduce pain intensity at 4 h by a negligible amount compared with acetaminophen. |
| Pain Relief Assessed With Scale From 1 (Complete) Through 5 (None); Follow-up, 4 H | 109 (2 RCTs) | Acetaminophen provided less pain relief at 4 h than ibuprofen (weighted MD, 0.20 points). By comparison, the mean pain relief in the ibuprofen group was 1.76 points. SDs and exact P values were not provided; 1 of the included studies stated that there was no significant difference between interventions. | Moderate‡‡‡ | There is probably a negligible difference between the pain relief provided by acetaminophen and ibuprofen at 4 h. | |||
| Global Subjective Efficacy Rating Assessed With Scale From 1 (Excellent) Through 5 (Discontinued); Follow-up, 4 H | 109 (2 RCTs) | Acetaminophen was rated as less efficacious than ibuprofen at 4 h (weighted MD, 0.53 points). By comparison, the mean global subjective efficacy rating in the ibuprofen group was 2.11 points. SDs and exact P values were not provided; 1 of the included studies stated that there was a statistically significant difference between interventions favoring ibuprofen (P < .05). | Moderate†††,‡‡‡ | Acetaminophen is probably importantly less efficacious than ibuprofen at 4 h. | |||
| Total Pain Relief Assessed With Time-Weighted Scale From 4 (Best) Through 20 (Worst); Follow-up, 4 H | 84 (1 RCT) | Acetaminophen provided decreased total pain relief compared with ibuprofen (MD, 1.01 points). By comparison, the mean total pain relief score in the ibuprofen group was 9.93 points. SDs were not provided; authors reported that this was a statistically significant difference (P < .05). | Low§§§,¶¶¶ | Ibuprofen may have a negligible benefit compared with acetaminophen on total pain relief at 4 h. | |||
| Summed Pain Intensity Difference Assessed With 12-Point Time-Weighted Scale; Follow-up, 4 H | 84 (1 RCT) | Acetaminophen decreased the summed pain intensity difference from baseline compared with ibuprofen (MD, −0.94 points). By comparison, the mean summed pain intensity difference score in the ibuprofen group was 4.66 points. SDs were not provided; authors stated that although there was a trend in favor of ibuprofen, it was not statistically significant (P = .058). | Low§§§,¶¶¶ | Ibuprofen may have a negligible benefit compared with acetaminophen on summed pain intensity difference scores at 4 h. | |||
| Pain Intensity Assessed With Scale From 1 (None) Through 4 (Severe); Follow-up, 24 H | 76 (1 RCT) | Acetaminophen increased pain intensity compared with ibuprofen (MD, 0.22 points). By comparison, the mean pain intensity in the ibuprofen group was 1.18 points. SDs and exact P values were not provided; authors stated that there were no significant differences between groups. | Low§§§,¶¶¶ | Ibuprofen may have a negligible benefit compared with acetaminophen on pain intensity measured at 24 h. | |||
| Presence of Pain Assessed With Proportion of Patients Exhibiting Pain-Related Behaviors; Follow-up, 7 H | 40 (1 RCT) | RR, 1.17 (0.48 to 2.86) | 30.0% | 35.1% (14.4% to 85.8%) | 5.1% more (15.6% fewer to 55.8% more) | Low### | Acetaminophen may be associated with an importantly higher incidence of pain at 7 hours compared to ibuprofen. |
The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
GRADE: Grading of Recommendations Assessment, Development and Evaluation. The GRADE Working Group grades of evidence are as follows. High certainty: Very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: Moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: Very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect.21,22
RCT: Randomized controlled trial.
The reported adverse effects included dizziness and mild stomach upset.
One study was at a high risk of bias owing to missing outcome data and selective outcome reporting. Another study was probably at a high risk of bias owing to selective outcome reporting because the outcome of adverse effects was not prespecified in the Methods section and the number of participants analyzed was unclear. Therefore, we rated down 1 level owing to risk of bias.
Using a threshold of 0.18% (based on 10% of the baseline risk, that is, the risk with ibuprofen), the lower bound of the 95% CI suggests an important benefit of acetaminophen, whereas the upper bound of the 95% CI suggests an important benefit of ibuprofen. Therefore, we rated down 2 levels owing to imprecision.
RR: Risk ratio.
Using a threshold of 0.61% (based on 10% of the baseline risk, that is, the risk with ibuprofen), the lower bound of the 95% CI suggests an important difference favoring acetaminophen, whereas the upper bound of the 95% CI suggests an important difference favoring ibuprofen. Therefore, we rated down 2 levels owing to imprecision.
Using a threshold of 2% (based on 10% of the baseline risk, that is, the risk with ibuprofen), the lower bound of the 95% CI suggests an important difference favoring acetaminophen whereas the upper bound of the 95% CI suggests an important difference favoring ibuprofen. Therefore, we rated down 2 levels owing to imprecision.
MD: Mean difference.
Three included studies were at a high risk of bias owing to selection of the reported results because they did not report measures of variability. One study was also at a high risk of bias owing to missing outcome data. Therefore, we rated down 1 level owing to risk of bias.
There is moderate statistical heterogeneity (I2 = 46%; P = .14). However, the 95% CIs of the effect estimates overlap, so we did not rate down for inconsistency.
Using a threshold of 0.3 points (based on 10% of the range of the scale), the lower bound of the 95% CI suggests a negligible difference favoring acetaminophen, whereas the upper bound of the 95% CI suggests an important difference favoring ibuprofen. Therefore, we rated down 1 level owing to imprecision.
All included studies were at a high risk of bias owing to selection of the reported results because they did not report measures of variability. One study was also at a high risk of bias owing to missing outcome data. Therefore, we rated down 1 level owing to risk of bias.
The effect estimates were different in magnitude, but they both showed an important effect favoring ibuprofen. Therefore, we did not rate down for inconsistency.
There was a high risk of selection bias owing to the lack of reported measures of variability. Therefore, we rated down 1 level owing to risk of bias.
The optimal information size of 100 participants was not met. Therefore, we rated down 1 level owing to imprecision.
Using a threshold of 3% (based on 10% of the baseline risk, that is, the risk with ibuprofen), the lower bound of the 95% CI suggests an important difference favoring acetaminophen, whereas the upper bound of the 95% CI suggests an important difference favoring ibuprofen. Therefore, we rated down 2 levels owing to imprecision.
–: Data not generated.
Table 3.
Ibuprofen vs a placebo for acute dental pain in children.
| OUTCOME | PARTICIPANTS, NO. (STUDIES) | RELATIVE EFFECT (95% CI)* | ANTICIPATED ABSOLUTE EFFECTS (95% CI) |
CERTAINTY (GRADE†) | WHAT HAPPENS | ||
|---|---|---|---|---|---|---|---|
| With a Placebo | With Ibuprofen | Difference | |||||
| Any Adverse Effects Assessed With Proportion of Participants Experiencing Any Adverse Event Including Dizziness, Drowsiness, and Mild Stomach Upset; Follow-up, 4 H | 106 (2 RCTs‡) | Not estimable | 3.9% | 1.8% | 2% fewer (9% fewer to 5% more) | Very low§,¶ | There is very low certainty evidence regarding the incidence of adverse effects when comparing ibuprofen with a placebo. |
| Rescue Analgesia Assessed With Proportion of Patients Requiring Rescue Analgesia; Longest Follow-up, Range 4–7 H | 102 (2 RCTs) | RR,# 0.39 (0.06 to 2.71) | 32.7% | 12.7% (2% to 88.5%) | 19.9% fewer (30.7% fewer to 55.8% more) | Low**,†† | Ibuprofen may decrease the need for rescue analgesia by an important amount from 4 through 7 h compared with a placebo. |
| Pain Intensity Assessed With Scale From 1 (None) Through 4 (Severe); Follow-up, 4 H | 168 (3 RCTs) | –§§§ | Median pain intensity, 2.02 points | – | MD,‡‡ 0.19 points lower (0.58 lower to 0.21 higher) | Low§§,¶¶ | Ibuprofen may reduce pain intensity at 4 h by a negligible amount compared with a placebo. |
| Pain Relief Assessed With Scale From 1 (Complete) Through 5 (None); Follow-up, 4 H | 106 (2 RCTs) | Ibuprofen provided increased pain relief at 4 h compared with a placebo (weighted MD, −0.49 points). By comparison, the mean pain relief in the placebo group was 2.28 points. SDs and exact P values were not provided; authors stated that there was no significant difference between interventions. | Low§§,## | Ibuprofen may increase pain relief by an important amount at 4 h compared with a placebo. | |||
| Global Subjective Efficacy Rating Assessed With Scale From 1 (Excellent) Through 5 (Discontinued); Follow-up, 4 H | 106 (2 RCTs) | Ibuprofen was rated as more efficacious than a placebo at 4 h (weighted MD, −0.97 points). By comparison, the mean global subjective efficacy in the placebo group was 3.09 points. The authors stated that there was a statistically significant difference between interventions favoring ibuprofen (P < .05). | Moderate§§ | Ibuprofen is probably more efficacious than a placebo at 4 h by an important amount. | |||
| Total Pain Relief Assessed With Time-Weighted Scale From 4 (Best) Through 20 (Worst); Follow-up, 4 H | 80 (1 RCT) | Ibuprofen provided increased total pain relief compared with a placebo (MD, −2.55 points). By comparison, the mean total pain relief score in the placebo group was 12.48 points. SDs were not provided; authors reported that this was a statistically significant difference (P < .05). | Low***,††† | Ibuprofen may provide importantly more total pain relief than a placebo at 4 h. | |||
| Summed Pain Intensity Difference Assessed With 12-Point Time-Weighted Scale; Follow-up, 4 H | 80 (1 RCT) | Ibuprofen increased the summed pain intensity difference from baseline compared with a placebo (MD, 1.28 points). By comparison, the mean summed pain intensity score in the placebo group was 3.39 points. SDs were not provided; authors stated that although there was a trend in favor of ibuprofen, it was not statistically significant (P = .058). | Low***,††† | Ibuprofen may increase the summed pain intensity difference from baseline scores at 4 h by an important amountcompared with a placebo. | |||
| Pain Intensity Assessed With Scale From 1 (None) Through 4 (Severe); Follow-up, 24 H | 62 (1 RCT) | Ibuprofen decreased pain intensity compared with a placebo (MD, −0.12 points). By comparison, the mean pain intensity in the placebo group was 1.3 points. SDs and exact P values were not provided; authors stated that there were no significant differences between groups. | Low***,††† | Ibuprofen may have a negligible benefit compared a placebo on pain intensity at 24 h. | |||
| Presence of Pain; Follow-up, 7 H | 40 (1 RCT) | RR, 0.75 (0.32 to 1.77) | 40.0% | 30.0% (12.8% to 70.8%) | 10.0% fewer (27.2% fewer to 30.8% more) | Low‡‡‡ | Ibuprofen may be associated with an importantly lower incidence of pain at 7 h compared a placebo. |
The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
GRADE: Grading of Recommendations Assessment, Development and Evaluation. The GRADE Working Group grades of evidence are as follows. High certainty: Very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: Moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: Very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect.21,22
RCT: Randomized controlled trial.
One study was at a high risk of bias owing to missing outcome data and selective outcome reporting. Another study was probably at a high risk of bias owing to selective outcome reporting because the outcome of adverse effects was not prespecified in the Methods section and the number of participants analyzed was unclear. Therefore, we rated down 1 level owing to risk of bias.
Using a threshold of 0.39% (based on 10% of the baseline risk, that is, the risk with a placebo), the lower bound of the 95% CI suggests an important benefit of ibuprofen, whereas the upper bound of the 95% CI suggests an important benefit of a placebo. Therefore, we rated down 2 levels owing to imprecision.
RR: Risk ratio.
There is moderate statistical heterogeneity (I2 = 76%; P = .04). However, the CIs of the effect estimates overlap, so we did not rate down for inconsistency.
Using a threshold of 3.27% (based on 10% of the baseline risk, that is, the risk with a placebo), the lower bound of the 95% CI suggests an important difference favoring ibuprofen, whereas the upper bound of the 95% CI suggests an important difference favoring a placebo. Therefore, we rated down 2 levels owing to imprecision.
MD: Mean difference.
All included studies were at a high risk of bias owing to selection of the reported results because they did not report measures of variability. One study was also at a high risk of bias owing to missing outcome data. Therefore, we rated down 1 level owing to risk of bias.
Using a threshold of 0.3 points (based on 10% of the range of the scale), the lower bound of the 95% CI suggests an important difference favoring ibuprofen, whereas the upper bound of the 95% CI indicates a negligible benefit of a placebo. Therefore, we rated down 1 level owing to imprecision.
One effect estimate (SD) indicates a small but important effect (0.40) favoring ibuprofen, whereas the other effect estimate indicates a negligible benefit of ibuprofen. Therefore, we rated down 1 level owing to inconsistency.
There was a high risk of selection bias owing to the lack of reported measures of variability. Therefore, we rated down 1 level owing to risk of bias.
The optimal information size of 100 participants was not met. Therefore, we rated down 1 level owing to imprecision.
Using a threshold of 4% (based on 10% of the baseline risk, that is, the risk with a placebo), the lower bound of the 95% CI suggests an important benefit of ibuprofen, whereas the upper bound of the 95% CI suggests an important benefit of a placebo. Therefore, we rated down 2 levels owing to imprecision.
–: Data not generated.
Table 4.
Acetaminophen vs a placebo for acute dental pain in children.
| OUTCOME | PARTICIPANTS, NO. (STUDIES) | RELATIVE EFFECT (95% CI)* | ANTICIPATED ABSOLUTE EFFECTS (95% CI) |
CERTAINTY (GRADE†) | WHAT HAPPENS | ||
|---|---|---|---|---|---|---|---|
| With Placebo | With Acetaminophen | Difference | |||||
| Any Adverse Effects Assessed With Proportion of Participants Experiencing Adverse Effects; Follow-up, Range, 1–4 H | 145 (3 RCTs‡)§ | Not estimable | 4.2% | 2.7% | 2% fewer (8% fewer to 5% more) | Very low¶,# | There is very low certainty evidence regarding the incidence of adverse effects at 1–4 h when comparing acetaminophen with a placebo. |
| Rescue Analgesia Assessed With Proportion of Patients Requiring Rescue Analgesia; Longest Follow-up, Range 4–7 H | 112 (2 RCTs) | RR**, 0.55 (0.29 to 1.05) | 32.7% | 18.0% (9.5% to 34.3%) | 14.7% fewer (23.2% fewer to 1.6% more) | Very low††,‡‡ | There is very low certainty evidence on the need for rescue analgesia at 4–7 h with acetaminophen compared with a placebo. |
| Pain Intensity Assessed With Scale From 1 (None) Through 4 (Severe); Follow-up 4 H | 177 (3 RCTs) | –**** | Median pain intensity, 2.02 points | – | MD,§§ 0.13 points lower (0.52% lower to 0.26% higher) | Low¶¶,## | Acetaminophen may reduce pain intensity at 4 h by a negligible amount compared with a placebo. |
| Pain Relief Assessed With Scale From 1 (Complete) Through 5 (None); Follow-up, 4 H | 105 (2 RCTs) | Acetaminophen provided increased pain relief at 4 h compared with a placebo (weighted MD, −0.30 points). By comparison, the mean pain relief in the placebo group was 2.28 points. SDs and exact P values were not provided; authors stated that there was no significant difference between these interventions. | Moderate¶¶ | Acetaminophen probably provides negligibly more pain relief than a placebo at 4 h. | |||
| Global Subjective Efficacy Rating Assessed With Scale From 1 (Excellent) Through 5 (Discontinued); Follow-up, 4 H | 105 (2 RCTs) | Acetaminophen was rated as more efficacious than a placebo at 4 h (weighted MD, −0.44 points). By comparison, the mean global subjective efficacy rating in the placebo group was 3.09 points. SDs and exact P values were not provided; authors stated that there was no significant difference between these interventions. | Low ¶¶,*** | Acetaminophen may be importantly more efficacious than a placebo at 4 h. | |||
| Total Pain Relief Assessed With Time-Weighted Scale From 4 (Best) Through 20 (Worst); Follow-up, 4 H | 82 (1 RCT) | Acetaminophen provided increased total pain relief compared with a placebo (MD, −1.54 points). By comparison, the mean total pain relief in the placebo group was 12.48 points. SDs and exact P values were not provided; authors stated there was no significant difference between these interventions. | Low†††,‡‡‡ | There may be a negligible benefit of acetaminophen compared with a placebo with regard to total pain relief at 4 h. | |||
| Summed Pain Intensity Difference Assessed With 12-Point Time-Weighted Scale; Follow-up, 4 H | 82 (1 RCT) | Acetaminophen increased the summed pain intensity difference from baseline compared with a placebo (MD, 0.34 points). By comparison, the mean summed pain intensity difference score in the placebo group was 3.39 points. SDs and exact P values were not provided; authors stated that there was no significant difference between these interventions. | Low†††,‡‡‡ | There may be a negligible benefit of acetaminophen compared with a placebo with regard to summed pain intensity difference scores at 4 h. | |||
| Pain Intensity Assessed With Scale From 1 (None) Through 4 (Severe); Follow-up, 24 H | 72 (1 RCT) | Acetaminophen increased pain intensity compared with a placebo (MD, 0.10 points). By comparison, the mean pain intensity in the placebo group was 1.3 points. SDs and exact P values were not provided; authors stated that there were no significant differences between these interventions. | Low†††,‡‡‡ | There may be a negligible benefit of a placebo compared with acetaminophen with regard to pain intensity measured at 24 h. | |||
| Presence of Pain Assessed With Proportion of Participants Experiencing Pain; Longest Follow-up, Range 1–7 H | 80 (2 RCTs) | RR, 0.67 (0.35 to 1.28) | 42.5% | 28.5% (14.9% to 54.4%) | 14.0% fewer (27.6% fewer to 11.9% more) | Very low§§§,¶¶¶,### | There is very low certainty evidence on the presence of pain at 1–7 h for acetaminophen compared with a placebo. |
The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
GRADE: Grading of Recommendations Assessment, Development and Evaluation. The GRADE Working Group grades of evidence are as follows. High certainty: Very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: Moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: Very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect.21,22
RCT: Randomized controlled trial.
The reported adverse events include nausea, vomiting, dizziness, and drowsiness.
One study was at a high risk of bias because it did not mention allocation concealment and the health care providers did not seem blinded. Another study was at a high risk of bias owing to missing outcome data and selective outcome reporting. A third study was probably at a high risk of bias owing to selective outcome reporting because the outcome of adverse effects was not prespecified in the Methods section and the number of participants analyzed was unclear. Therefore, we rated down 1 level owing to risk of bias.
Using a threshold of 0.42% (based on 10% of the baseline risk; that is, the risk with a placebo), the lower bound of the 95% CI suggests an important benefit of acetaminophen, whereas the upper bound suggests an important benefit of a placebo. Therefore, we rated down 2 levels owing to imprecision.
RR: Risk ratio.
Using a threshold of 3.27% (based on 10% of the baseline risk, that is, the risk with a placebo), the lower bound of the 95% CI suggests an important difference favoring acetaminophen, whereas the upper bound of the 95% CI suggests a negligible difference favoring a placebo. Therefore, we rated down 2 levels owing to imprecision.
Another study measured rescue analgesia but did not report results for the acetaminophen group. Therefore, we rated down 1 level owing to publication bias.
MD: Mean difference.
All included studies were at a high risk of bias owing to selection of the reported results because they did not report measures of variability. One study was also at a high risk of bias owing to missing outcome data. Therefore, we rated down 1 level owing to risk of bias.
Using a threshold of 0.3 points (based on 10% of the range of the scale), the lower bound of the 95% CI suggests an important benefit of acetaminophen, whereas the upper bound of the 95% CI suggests a negligible difference favoring a placebo. Therefore, we rated down 1 level owing to imprecision.
One of the effect estimates indicates an important difference favoring acetaminophen, whereas the other effect estimate indicates a negligible benefit of acetaminophen. Therefore, we rated down 1 level owing to inconsistency.
There was a high risk of selection bias owing to a lack of reported measures of variability. Therefore, we rated down 1 level owing to risk of bias.
The optimal information size of 100 participants was not met. Therefore, we rated down 1 level owing to imprecision.
One of the studies was at a high risk of bias because it did not mention allocation concealment and the health care providers did not seem blinded. Therefore, we rated down 1 level owing to risk of bias.
In 1 of the studies, the presence of pain was measured by a blinded observer. Therefore, we rated down 1 level owing to indirectness.
Using a threshold of 4.25% (based on 10% of the baseline risk, that is, the risk with a placebo), the lower bound of the 95% CI suggests an important difference favoring acetaminophen, whereas the upper bound of the 95% CI suggests an important difference favoring a placebo. Therefore, we rated down 1 level owing to imprecision.
–: Data not generated.
Acetaminophen with codeine
Acetaminophen (240 mg) with codeine (24 mg) may reduce pain intensity at 4 hours by a trivial amount compared with ibuprofen (200 mg) alone (MD, −0.29 points; low certainty) (eTable 6, available online at the end of this article), acetaminophen (240 mg or 360 mg) alone (MD, −0.09 points; low certainty) (eTable 7, available online at the end of this article), and a placebo (MD, −0.16 points; low certainty) (eTable 8, available online at the end of this article).
Adverse Effects
In terms of adverse effects, the certainty of the evidence was very low for the comparisons of acetaminophen vs ibuprofen, acetaminophen vs a placebo, ibuprofen vs a placebo, acetaminophen (240 mg) with codeine (24 mg) vs ibuprofen (200 mg), acetaminophen (240 mg) with codeine (24 mg) vs acetaminophen (240 mg or 360 mg), and acetaminophen (240 mg) with codeine (24 mg) vs a placebo (Tables 2–4; eTables 6–8 and eFigures 6–11, available online at the end of this article).
Pain Relief (Scale From 1 [Complete]-5 [None])
Ibuprofen and acetaminophen
Acetaminophen probably provides less pain relief at 4 hours than ibuprofen (MD, 0.2 points; moderate certainty) (Table 2) and more pain relief than a placebo (MD, −0.30 points; moderate certainty) (Table 4) by a trivial amount. Ibuprofen may increase pain relief by an important amount at 4 hours compared with a placebo (MD, −0.49 points; low certainty) (Table 3).
Acetaminophen with codeine
Acetaminophen (240 mg) with codeine (24 mg) may increase pain relief at 4 hours by a trivial amount compared with ibuprofen (200 mg) alone (MD, −0.29 points; low certainty) (eTable 6, available online at the end of this article), acetaminophen (240 mg or 360 mg) alone (MD, −0.36 points; low certainty) (eTable 7, available online at the end of this article), and a placebo (MD, −0.17 points; low certainty) (eTable 8, available online at the end of this article).
Global Efficacy Rating (Scale From 1 [Excellent]-5 [Discontinued])
Ibuprofen and acetaminophen
Acetaminophen may be less efficacious than ibuprofen at 4 hours (MD, 0.53 points; moderate certainty) (Table 2) and more efficacious than a placebo (MD, −0.44 points; low certainty) by an important amount (Table 4). Ibuprofen may be more efficacious than a placebo at 4 hours by an important amount (MD, −0.97 points; moderate certainty) (Table 3).
Acetaminophen with codeine
Acetaminophen (240 mg) with codeine (24 mg) may be less efficacious at 4 hours by a trivial amount than ibuprofen (200 mg) (MD, 0.24 points; low certainty) (eTable 6, available online at the end of this article) and more efficacious by an important amount than acetaminophen (240 mg or 360 mg) alone (MD, −0.57 points; low certainty) (eTable 7, available online at the end of this article) and a placebo (MD, −0.87 points; low certainty) (eTable 8, available online at the end of this article).
Rescue Analgesia
Ibuprofen and acetaminophen
Acetaminophen may increase the need for rescue analgesia at 4 hours (risk ratio [RR], 3.45; 95% CI, 0.80 to 14.92; low certainty) (Table 2; eFigure 12, available online at the end of this article) and decrease it at 7 hours by an important amount (RR, 0.75; 95% CI, 0.19 to 2.93; low certainty) compared with ibuprofen (Table 2; eFigure 13, available online at the end of this article). There was very low certainty evidence regarding the need for rescue analgesia at 4 through 7 hours of follow-up when comparing acetaminophen with a placebo (Table 4; eFigure 14, available online at the end of this article). Ibuprofen may decrease the need for rescue analgesia by an important amount at 4 through 7 hours of follow-up (RR, 0.39; 95% CI, 0.06 to 2.71; low certainty) compared with a placebo (Table 3; eFigure 15). Other outcomes are displayed in eFigures 16–21.
DISCUSSION
The aim of this SR and meta-analysis was to examine the effects of analgesics on the management of acute dental pain in the pediatric population. The evidence synthesis from this SR and meta-analysis was used to inform the 2022 clinical practice guideline for the management of acute dental pain in children produced by the ADA Council on Scientific Affairs, ADA Science and Research Institute, and the University of Pittsburgh’s and the University of Pennsylvania’s schools of dental medicine in partnership with the FDA. We included 6 RCTs reporting 8 distinct comparisons for 8 different outcomes. Evidence about the comparative effects of the interventions on pain intensity suggests that ibuprofen may reduce pain intensity compared with acetaminophen and a placebo, and acetaminophen may reduce pain intensity compared with a placebo. The combination of acetaminophen and ibuprofen reduces pain intensity compared with acetaminophen and ibuprofen alone. With respect to pain relief, acetaminophen may provide less pain relief at 4 hours than ibuprofen and more pain relief than a placebo. Ibuprofen may provide more pain relief than a placebo. In terms of rescue analgesia, acetaminophen may increase the need for rescue analgesia at 4 hours of follow-up and then decrease the need for rescue analgesia at 7 hours of follow-up compared with ibuprofen. Ibuprofen may require less rescue analgesia than a placebo. The results were similar with respect to global efficacy ratings. There was very low certainty evidence regarding adverse effects.
Although the combination of acetaminophen with codeine was slightly superior to acetaminophen alone and ibuprofen alone with respect to pain intensity and pain relief at 4 hours of follow-up, the difference was trivial. As of April 2017, the FDA restricted the use of codeine and tramadol in children, as these opioids showed serious risks, including slowed or difficult breathing and death, especially in children 12 years or younger.29
We did not find studies that reported on the effectiveness of analgesics for the temporary management of irreversible pulpitis, and, therefore, we cannot make conclusions about this patient population. As the evidence addressing the management of acute pain after dental extraction in the pediatric population is limited, we were unable to construct pooled estimates across studies for several collected outcomes. The risk of bias assessment showed that the best available evidence (that is, RCTs included in this SR) has serious limitations regarding missing outcome data and selective reporting of results. With respect to the assessment of the certainty of the evidence, the risk of bias and imprecision were the domains most frequently rated down across comparisons and outcomes. Imprecision was rated down when the 95% CI was too wide (that is, the 95% CI crossed both thresholds of benefit and harm).
Our review found very low certainty evidence regarding the incidence of adverse effects when comparing ibuprofen with a placebo. A previously published SR examined adverse events associated with provision of pharmacologic agents for the management of acute, nonsurgical pain in children.1 The review suggested that single medication and medication combinations that include opioids are associated with the largest proportion of acute adverse events, ranging from 132% (> 1 adverse event per patient) for 2 mg/kg of codeine through 95% for 0.2 mg/kg of oxycodone and 60% for 0.5 mg/kg of morphine compared with 15% or fewer for 10 mg/kg of ibuprofen, 20 mg/kg of naproxen, 40 mg/kg of ketoprofen, 2 mg/kg of tramadol, or 15 mg/kg of acetaminophen. The authors of this SR did not assess the certainty of the evidence.
According to the best available evidence, which is limited in quality, reported in our SR and meta-analysis, the combination of acetaminophen and ibuprofen appears to be the most effective option for the management of acute dental pain after dental extractions in children 12 years or younger. We cannot make conclusions about adverse effects of included analgesics because the certainty of the evidence informing this outcome is very low. Therefore, high-quality evidence examining the effects of analgesics, especially adverse effects, for the management of acute dental pain commonly caused by tooth extractions and irreversible pulpitis in children is urgently needed. This evidence is necessary to better inform clinical practice and consequently improve patient outcomes.
Strengths and limitations
One strength of our SR is rigorous identification and selection of studies. We included experienced researchers in the field of health research methodology and dental pain management who informed critical aspects of the eligibility criteria, data extraction, analysis, and interpretation of results. To our knowledge, this review is the first SR and meta-analysis addressing the pharmacologic management of acute dental pain in children that assessed the certainty of the evidence using the GRADE approach. A limitation of this review is that we only included peer-reviewed studies published in English. Furthermore, by limiting our inclusion criteria to peer-reviewed studies, we may have missed unpublished theses and other research that was not submitted or accepted. However, we believe it is unlikely that our conclusions would have been different if we had included studies in a language other than English and unpublished literature.
We chose an approach to pool studies widely, in which we made an a priori decision to combine studies across characteristics of the questions, and only if heterogeneity was observed, we explored whether the differences could be explained by these characteristics. For example, although the type of cointervention and time of administration (preoperatively, postoperatively) of analgesics varied among the included studies, we did not find important heterogeneity in any of the performed meta-analyses and, therefore, decided to pool widely (and combine studies with differences in these characteristics) as originally planned. We also did not detect important heterogeneity in effect estimates due to rectal administration of acetaminophen23 compared with oral administration.26,27 This approach is consistent with high methodological standards and increases both the applicability and precision of the estimates of effect when heterogeneity is not detected.
CONCLUSIONS
To our knowledge, there are no studies addressing the effects of analgesics for the management of pain due to irreversible pulpitis in children. With regard to the effects of analgesics for the treatment of pain after dental extractions, ibuprofen may reduce pain intensity compared with a placebo and acetaminophen, whereas acetaminophen may reduce pain intensity compared with a placebo. The combination of acetaminophen and ibuprofen reduces pain intensity compared with acetaminophen and ibuprofen alone. There was very low certainty evidence with respect to adverse effects. Although several analgesics alone or in combination may provide a beneficial effect when managing acute dental pain in children, methodologically rigorous RCTs examining the effects (that is, adverse effects) of analgesics on acute dental pain commonly caused by tooth extractions and irreversible pulpitis are needed.
Supplementary Material
Disclosures.
Dr. Hersh and the Trustees of the University of Pennsylvania have received grant funding from Pfizer Consumer Healthcare and Bayer Healthcare. In addition, within the last 5 years, Dr. Hersh received consulting funds for reviewing data and sharing his expertise with the National Advertising Division of the Better Business Bureau concerning pain studies that compared Aleve and Extra Strength Tylenol. None of the other authors reported any disclosures.
This project was financially supported by the US Food and Drug Administration, US Department of Health and Human Services.
The contents of the article are those of the authors and do not necessarily represent the official views of, nor an endorsement by, the Food and Drug Administration, Health and Human Services, the US government, and the American Dental Association.
ABBREVIATION KEY
- FDA
US Food and Drug Administration
- GRADE
Grading of Recommendations Assessment, Development and Evaluation
- NSAID
Nonsteroidal anti-inflammatory drug
- RCT
Randomized controlled trial
- SR
Systematic review
Footnotes
SUPPLEMENTAL DATA
Supplemental data related to this article can be found at: https://doi.org/10.1016/j.adaj.2023.02.013.
Contributor Information
Anna Miroshnychenko, Department of Health Research Methods, Evidence and Impact, McMaster University, Hamilton, Ontario, Canada..
Maria Azab, Faculty of Health Sciences, McMaster University, Hamilton, Ontario, Canada, when the work described in this article was conducted. She now is a medical student, The Temerty Faculty of Medicine, University of Toronto, Toronto, Ontario, Canada..
Sara Ibrahim, Department of Health Research Methods, Evidence and Impact, McMaster University, Hamilton, Ontario, Canada..
Yetiani Roldan, Department of Health Research Methods, Evidence and Impact, McMaster University, Hamilton, Ontario, Canada..
Juan Pablo Diaz Martinez, Department of Health Research Methods, Evidence and Impact, McMaster University, Hamilton, Ontario, Canada..
Divyalakshmi Tamilselvan, Faculty of Health Sciences, McMaster University, Hamilton, Ontario, Canada..
Leon He, Faculty of Health Sciences, McMaster University, Hamilton, Ontario, Canada, when the work described in this article was conducted. He now is a medical student, The Faculty of Medicine, University of Ottawa, Ottawa, Ontario, Canada..
Olivia Urquhart, Department of Preventative and Restorative Sciences and Center for Integrative Global Oral Health, School of Dental Medicine, University of Pennsylvania, Philadelphia, PA..
Malavika Tampi, Department of Cariology, University of Michigan School of Dentistry, Ann Arbor, MI..
Deborah E. Polk, Department of Dental Public Health, University of Pittsburgh, Pittsburgh, PA..
Paul A. Moore, Department of Dental Public Health, University of Pittsburgh, Pittsburgh, PA..
Elliot V. Hersh, Department of Oral Surgery and Pharmacology, University of Pennsylvania, Philadelphia, PA..
Alonso Carrasco-Labra, Department of Preventative and Restorative Sciences and Center for Integrative Global Oral Health, School of Dental Medicine, University of Pennsylvania, Philadelphia, PA..
Romina Brignardello-Petersen, Department of Health Research Methods, Evidence and Impact, McMaster University, Hamilton, Ontario, Canada..
References
- 1.Hartling L, Ali S, Dryden DM, et al. How safe are common analgesics for the treatment of acute pain for children? A systematic review. Pain Res Manag. 2016;2016:5346819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pergolizzi JV, Magnusson P, LeQuang JA, Gharibo C, Varrassi G. The pharmacological management of dental pain. Expert Opin Pharmacother. 2020;21(5):591–601. [DOI] [PubMed] [Google Scholar]
- 3.Ghlichloo I, Gerriets V. Nonsteroidal anti-inflammatory drugs (NSAIDs). In: StatPearls. StatPearls Publishing; 2022. [PubMed] [Google Scholar]
- 4.Abou-Atme YS, Melis M, Zawawi KH. Efficacy and safety of acetaminophen and caffeine for the management of acute dental pain: a systematic review. Saudi Dent J. 2019;31(4):417–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laskarides C Update on analgesic medication for adult and pediatric dental patients. Dent Clin North Am. 2016;60(2):347–366. [DOI] [PubMed] [Google Scholar]
- 6.Becker DE. Pain management, part 1: managing acute and postoperative dental pain. Anesth Prog. 2010;57(2):67–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moore PA, Hersh EV. Combining ibuprofen and acetaminophen for acute pain management after thirdmolar extractions: translating clinical research to dental practice. JADA. 2013;144(8):898–908. [DOI] [PubMed] [Google Scholar]
- 8.Ong CK, Seymour RA, Lirk P, Merry AF. Combining paracetamol (acetaminophen) with nonsteroidal antiinflammatory drugs: a qualitative systematic review of analgesic efficacy for acute postoperative pain. Anesth Analg. 2010;110(4):1170–1179. [DOI] [PubMed] [Google Scholar]
- 9.Okunev I, Frantsve-Hawley J, Tranby E. Trends in national opioid prescribing for dental procedures among patients enrolled in Medicaid. JADA. 2021;152(8):622–630.e3. [DOI] [PubMed] [Google Scholar]
- 10.Stein BD, Taylor EA, Sheng F, Dick AW, Vaiana M, Sorbero M. Change in per capita opioid prescriptions filled at retail pharmacies: 2008–2009 to 2017–2018. Ann Intern Med. 2022;175(2):299–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang TT, Tong J, Hersh EV, Chuang SK, Panchal N. Does prescription drug monitoring program usage affect opioid analgesic prescriptions by oral and maxillofacial surgeons after third molar surgery? Oral Surg Oral Med Oral Pathol Oral Radiol. 2021;132(1):26–31. [DOI] [PubMed] [Google Scholar]
- 12.Navin L FDA Drug Safety Podcast: FDA restricts use of prescription codeine pain and cough medicines and tramadol pain medicines in children; recommends against use in breastfeeding women. April 20, 2017. Accessed July 20, 2022. https://www.fda.gov/media/104742/download
- 13.Moore PA, Ziegler KM, Lipman RD, Aminoshariae A, Carrasco-Labra A, Mariotti A. Benefits and harms associated with analgesic medications used in the management of acute dental pain: an overview of systematic reviews. JADA. 2018;149(4):256–265.e3. [DOI] [PubMed] [Google Scholar]
- 14.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Academies of Sciences Engineering Medicine. Framing Opioid Prescribing Guidelines for Acute Pain: Developing the Evidence. National Academies Press, 2020. [PubMed] [Google Scholar]
- 16.Higgins JPT, Altman DG, Gøtzsche PC, et al. ; Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Siemieniuk RA, Bartoszko JJ, Zeraatkar D, et al. Drug treatments for covid-19: living systematic review and network meta-analysis. BMJ. 2020;370:m2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miroshnychenko A, Ibrahim S, Azab M, et al. Injectable and topical local anesthetics for acute dental pain: 2 systematic reviews. JADA. 2023;154(1):53–64.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miroshnychenko A, Ibrahim S, Azab M, et al. Acute postoperative pain due to dental extraction in the adult population: a systematic review and network meta-analysis. J Dent Res. 2023:220345221139230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tufanaru C, Munn Z, Stephenson M, Aromataris E. Fixed or random effects meta-analysis? Common methodological issues in systematic reviews of effectiveness. Int J Evid Based Healthc. 2015;13(3):196–207. [DOI] [PubMed] [Google Scholar]
- 21.Hultcrantz M, Rind D, Akl EA, et al. The GRADE Working Group clarifies the construct of certainty of evidence. J Clin Epidemiol. 2017;87:4–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zeng L, Brignardello-Petersen R, Hultcrantz M, et al. GRADE guidelines 32: GRADE offers guidance on choosing targets of GRADE certainty of evidence ratings. J Clin Epidemiol. 2021;137:163–175. [DOI] [PubMed] [Google Scholar]
- 23.Elhakim M Painless dental extraction in children. Anaesthesiol Reanim. 1993;18(3):80–82. [PubMed] [Google Scholar]
- 24.Gazal G, Mackie IC. A comparison of paracetamol, ibuprofen or their combination for pain relief following extractions in children under general anaesthesia: a randomized controlled trial. Int J Paediatr Dent. 2007;17(3):169–177. [DOI] [PubMed] [Google Scholar]
- 25.Kharouba J, Ratson T, Somri M, Blumer S. Preemptive analgesia by paracetamol, ibuprofen or placebo in pediatric dental care: a randomized controlled study. J Clin Pediatr Dent. 2019;43(1):51–55. [DOI] [PubMed] [Google Scholar]
- 26.McGaw T, Raborn W, Grace M. Analgesics in pediatric dental surgery: relative efficacy of aluminum ibuprofen suspension and acetaminophen elixir. ASDC J Dent Child. 1987;54(2):106–109. [PubMed] [Google Scholar]
- 27.Moore PA, Acs G, Hargreaves JA. Postextraction pain relief in children: a clinical trial of liquid analgesics. Int J Clin Pharmacol Ther Toxicol. 1985;23(11):573–577. [PubMed] [Google Scholar]
- 28.Primosch RE, Nichols DL, Courts FJ. Comparison of preoperative ibuprofen, acetaminophen, and placebo administration on the parental report of postextraction pain in children. Pediatr Dent. 1995;17(3):187–191. [PubMed] [Google Scholar]
- 29.FDA drug safety communication: FDA restricts use of prescription codeine pain and cough medicines and tramadol pain medicines in children; recommends against use in breastfeeding women. US Food and Drug Administration. Accessed July 20, 2022. https://www.fda.gov/Drugs/DrugSafety/ucm549679.htm [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


