Abstract
Introduction:
Change in the balance of Bcl-2 family proteins is one of the main reasons for resistance of tumor cells to ABT-199. In this study, the effect of dihydroartemisinin on cell growth, apoptosis and sensitivity of the AML cells to ABT-199 was investigated.
Methods:
Cell proliferation and survival were assessed by trypan blue staining and MTT assay, respectively. Cell apoptosis was measured by Hoechst 33342 staining and caspase-3 activity assay. The expression levels of Bcl-2, Mcl-1 and Bax mRNA were tested by qRT-PCR.
Results:
Our data showed that combination therapy significantly reduced the IC50 value and synergistically decreased the AML cell survival and growth compared with dihydroartemisinin or ABT-199 alone. Treatment with each of ABT-199 or dihydroartemisinin alone clearly enhanced the Bax mRNA expression and inhibited the expression of Mcl-1 and Bcl-2 mRNA. Inhibition of Mcl-1 mRNA by dihydroartemisinin was associated with enhancement of apoptosis induced by ABT-199 in AML cells.
Conclusion:
In conclusion, dihydroartemisinin not only triggers the intrinsic pathway of apoptosis, but also can increase the sensitivity of the AML cells to ABT-199 via suppression of Mcl-1 expression.
Key Words: ABT-199, AML, Apoptosis- Bcl-2, Dihydroartemisinin, Mcl-1
Introduction
Acute myeloid leukemia (AML) is malignant clonal diseases characterized by the uncontrolled proliferation of immature myeloid cells with a reduced capacity to differentiate into mature cells [1-3]. Despite initial sensitivity to standard chemotherapy, long-term survival rate is only 30–40%, and the majority of patients eventually relapse. The main limitation in treatment of AML patients is the development of primary and secondary drug resistance. Therefore, novel therapeutic strategies are required for complete cure of AML [1-3].
The intrinsic pathway of apoptosis (mitochondrial) is regulated by Bcl-2 family proteins, which include anti-apoptotic and pro-apoptotic proteins [4-6]. Increased expression of Bcl-2 family anti-apoptotic proteins such as Bcl-2, Bcl-xL and Mcl-1 has been observed in many hematological malignancies such as AML, which is associated with increased survival and resistance to cell apoptosis [4-6]. Therefore, targeting these proteins is considered as a therapeutic strategy in cancer.
ABT-199 is a synthetic BH3 domain mimic of BH3-only pro-apoptotic proteins, which induces the mitochondrial pathway of apoptosis by binding to Bcl-2 and not Bcl-xL and Mcl-1 proteins [7, 5]. ABT-199 shows promising anticancer activity in AML patients, but resistance occurs rapidly [8]. Reports show that ABT-199 reduces the association of the proapoptotic protein Bim with Bcl-2 without affecting Bcl-xL. Also, in AML cells resistant to ABT-199, it has been observed that the relationship between Bim and Mcl-1 is strengthened and the intrinsic pathway of apoptosis is not induced [9]. These evidences show that the change in the balance of Bcl-2 family proteins is one of the main reasons for resistance to ABT-199. Therefore, simultaneous suppression of Bcl-2 and Mcl-1 is an attractive strategy for sensitizing cells resistant to ABT-199 [9].
Artemisinin is the active ingredient of Artemisia annua plant, which is used as an antimalarial drug. Artemisinin and its strongest derivative, dihydroartemisinin, in addition to antimalarial properties, also show anticancer properties [10]. Numerous laboratory and clinical evidences have shown that artemisinin and its derivatives play a role in controlling the growth and migration of cancer cells by mediating the systems of oxidative stress, DNA damage and repair, apoptosis and angiogenesis [10]. In addition, it has been found that these compounds change the expression of different genes involved in apoptosis, such as Bcl-2, Mcl-1 and Bax, by changing different cell signaling pathways [10]. However, the combined effect of these compounds with ABT-199 on AML cells has not been investigated so far. In this study, the effect of dihydroartemisinin on the sensitivity of AML cells to ABT-199 has been investigated.
Materials and Methods
Cell culture
The human AML cell lines U937 and KG-1 were purchased from the Iranian Pasteur Institute (Tehran, Iran). The cells were maintained in RPMI-1640 medium (Sigma-Aldrich, St. Louis, MO, USA) supplemented with 1% penicillin- Streptomycin (Sigma-Aldrich), 2 mM glutamine, 20% fetal bovine serum (Invitrogen, Carlsbad, CA, USA). The cells were cultured in suspension at a concentration of 105-106 cells/ml and incubated at 37oC containing 5% CO2
MTT assay
Cell toxicity was assessed by 3-(4, 5-dimethylthiazol-2-yl) 2, 5-diphenyl tetrazolium bromide (MTT) assay. The Experiment was divided into 5 groups: blank control, solvent control, ABT-199, dihydroartemisinin, ABT-199+ dihydroartemisinin. The AML cell lines were grown in 96-well plates at a density of 2×104 cells per well. After 24 h of adhesion, the cells were treated with different concentrations of ABT-199, dihydroartemisinin for 24 h. Next, 10 µl of MTT solution (5 mg/ml) (Sigma-Aldrich) was added into each well and then incubated for another 4 h. The medium was removed, and 150 μl of DMSO was added to each well to dissolve the sediment. All mixtures were gentle shaken for 15 minutes. The optical density (OD) for each well plate was calculated by a micro-plate reader spectrophotometer (Awareness Technology, Palm City, FL, USA) at a reference wavelength of 490 nm. In the next experiments the IC50 doses of drugs were used.
Combination index study
The combination index (CI) analysis based upon the Chou-Talalay method was used to calculate the interaction between ABT-199 and dihydroartemisinin [11, 12]. The all data obtained with the MTT assay was converted to Fraction affected (Fa; where Fa = 1 is 0% cell survival and Fa = 0 is 100% cell survival) and analyzed with the CompuSyn 1.0 software (ComboSyn Inc., Paramus, NJ, USA). Additive, synergistic and antagonistic effects are indicated by CI=1, CI<1 and CI>1, respectively.
Cell proliferation assay
The antiproliferative effects of ABT-199 and dihydroartemisinin were measured by trypan blue staining. In Brief, each cancer cell lines were seeded in 6-well culture plates at a density of 4×104 cells per well and treated with ABT-199 and dihydroartemisinin, alone and in combination for five days. At various time points, all cells were harvested and next cell suspensions were stained with equal volumes of 0.4% trypan blue (Merck KGaA, Darmstadt, Germany) for 3 min. Next, the number of unstained cells (viable cells) was counted using an inverted microscope (Nikon Instrument Inc., Melville, NY, USA) and a hematocytometer. Cell viability was considered as a percent of the blank control group.
RT-qPCR
Following treatments, total cellular RNA was extracted from the leukemic cells by using RNA extraction reagent (Parstous, Tehran, Iran) as described by the manufacturer’s protocol. Then, 1 μg of total RNA was reverse transcribed into complementary DNA (cDNA) by using of MMLV reverse transcriptase and oligo-dT primer following the manufacturer’s instructions (Parstous). Relative gene mRNA expression was quantified by RT-qPCR using SYBR green PCR Master Mix (Parstous) and the LightCycler 96 system (Roche Diagnostic GmbH, Mannhein, Germany). The RT-qPCR was performed in a 20 μl reaction system containing 1 μl of cDNA template, 12 μl of SYBR green reagent, 0.2 μM of each of the primers and 6 μl of nuclease-free distilled water. The sequences of primers used for PCR are listed in Table 1. The RT-qPCR condition was 95oC for 10 min followed by 35 cycles at 95oC for 20 sec and 60 oC for 1 min. Relative gene expression was determined with the 2-(ΔΔCt) method [13, 14], using β-actin as an internal control.
Table 1.
Sequence of Primers Used in Real-Time PCR
| Genes | Sequence (5'→3') |
|---|---|
| β-actin FW | GACATCCGCAAAGACCTGTA |
| β-actin RV | GGAGCAATGATCTTGATCTTCA |
| Bcl-2 FW | GGATGCCTTTGTGGAACTG |
| Bcl-2 RV | CAGCCAGGAGAAATCAAACAG |
| Bax FW | GCTTCAGGGTTTCATCCAG |
| Bax RV | TTACTGTCCAGTTCGTCCC |
| Mcl-1 FW | TAGTTAAACAAAGAGGCTGGGA |
| Mcl-1 RV | CCTTCTAGGTCCTCTACATGG |
Apoptotic morphologic changes
IC50 concentration of ABT-199 and dihydroartemisinin were used to treat the U937 and KG-1 cells for 24 h. After treatment, the cells were washed and fixed in 4% formaldehyde for 15 minute at room temperature. The fixed cells were washed with PBS and then stained with 5 μg⁄mL Hoechst 33342 (Sigma-Aldrich) for 10 minute according to the manufacturer’s protocol. Finally, by using a fluorescence microscope (Olympus, Tokyo, Japan), the morphological changes in the Hoechst 33342-stained nuclei of cells were observed. U937 and KG-1cells with fragmented or condensed nuclei were considered as apoptotic cells.
Caspases-3 activity assay
Induction of caspase-3 activity was determined using a colorimetric caspase activity Kit according to the manufacturer’s instruction (Abnova, Taipei, Taiwan). Briefly, the cells were resuspended in 50 µl of cooled lysis buffer and incubate on ice for 15 min. The cell suspension was centrifuged at 9,000 g for 2 min. Subsequently, 50 µl reaction buffer and 5 µl of caspase-3 (DEVD-pNA) substrate was added to each well and incubated at 37°C for 24 h. The absorbance was determined using a microplate ELISA reader (Awareness Technology, Palm City, FL, USA) at 405 nm.
Statistical Analysis
Data were presented as mean ± standard deviation (SD). Analysis of variance (ANOVA) followed by t-test was used to explored the significant differences between groups. A p-value less than 0.05 were considered significant. All statistical analyses were performed using GraphPad Prism software.
Results
Dihydroartemisinin increased the sensitivity of AML cells to ABT-199
To explore the effect of dihydroartemisinin on the sensitivity of the AML cells to ABT-199, a combination treatment of dihydroartemisinin and ABT-199 on U937 and KG-1 cells was studied. The results of MTT assay (Figure 1) demonstrated that treatment whit either dihydroartemisinin or ABT-199 alone, markedly decreased the cell survival in a dose-dependent way (p<0.05, relative to the blank control). As shown in Table 2, the IC50 values for dihydroartemisinin and ABT-199 for 24 h of treatment were 14.95 and 0.12 µM in U937 cells, and 11.26 and 0.18 µM in KG-1 cells, respectively. Moreover, the dihydroartemisinin in combination with ABT-199 further reduced the cell survival rate and lowered the IC50 value (p< 0.05, relative to the single therapy). These results suggest that dihydroartemisinin can increase the sensitivity of the AML cells to ABT-199.
Figure 1.
Effect of Dihydroartemisinin and ABT-199 Combination on Cell Survival. The U937 and KG-1 cells were exposed to dihydroartemisinin (DHA) (A) and ABT-199 (B) at indicated concentrations. After 24 h, the cell survival rate was measured using MTT assay. The cell survival curves were plotted using GraphPad 6.1 software (C, D, F and G). Data are showed as mean±SD of three experiments. The combination index (CI) values were determined using the fractional affected (Fa) values of MTT assay and CalcuSyn software (E and H).
Table 2.
IC50 Values of the ABT-199 Alone and in Combination with Dihydroartemisinin in AML Cells
| IC50 (24 h) | ||
|---|---|---|
| U937 | KG-1 | |
| ABT-199 | 0.12 | 0.18 |
| Dihydroartemisinin | 14.95 | 11.26 |
| Combination | 0.09* | 0.11* |
The combination effect of dihydroartemisinin and ABT-199 on AML cells was synergistic
CI analysis based on the non-constant method of Chou-Talalay was performed to assess the drugs interaction. The CI–Fa curves indicated that combination treatment was synergistic (CI<1) when dihydroartemisinin (0–0.32 µM) combined with ABT-199 (0–0.32 µM) (Figure 1). Our results showed that the best mean CI value of 24 h was observed at 0.32 µM ABT-199 in combination with 0.32 µM dihydroartemisinin (CI=0.70) with Fa level of 0.96 in U937 cells (Figure 1). Moreover, in KG-1 cells the best mean CI value was observed at 0.16 µM ABT-199 in combination with 0.16 µM dihydroartemisinin (CI=0.71) with Fa level of 0.58 (Figure 1).
Dihydroartemisinin augmented the growth inhibitory effect of ABT-199
We next studied whether dihydroartemisinin and ABT-199 could restrain the proliferation of AML cells. The cells were exposed to dihydroartemisinin and ABT-199, alone and in combination and cell viability was then determined using trypan blue assay over a period of 5 days. The results are presented as the percentage of viable cells relative to total number of cells. The cell growth curve revealed that compared with the control groups, the viability of the cells in dihydroartemisinin, ABT-199 and combinatorial group significantly reduced in a time-dependent manner. In U937 cells, the cell growth in dihydroartemisinin, ABT-199 and combinatorial group reduced to 62 %, 59 % and 54 %, respectively, and then to a further 26 %, 21 % and 19 % at the end of the experiment (p<0.05; Figure 2). Similar results were observed in the KG-1 cell line.
Figure 2.
Proliferation Inhibition of AML Cells. The U937 and KG-1 cells were treated with ABT-199 and dihydroartemisinin (DHA) for 1-5 day. Next, the cell viability was determined using trypan blue exclusion assay. The data are showed as mean±SD of three experiments. *p<0.05 versus blank control or solvent control
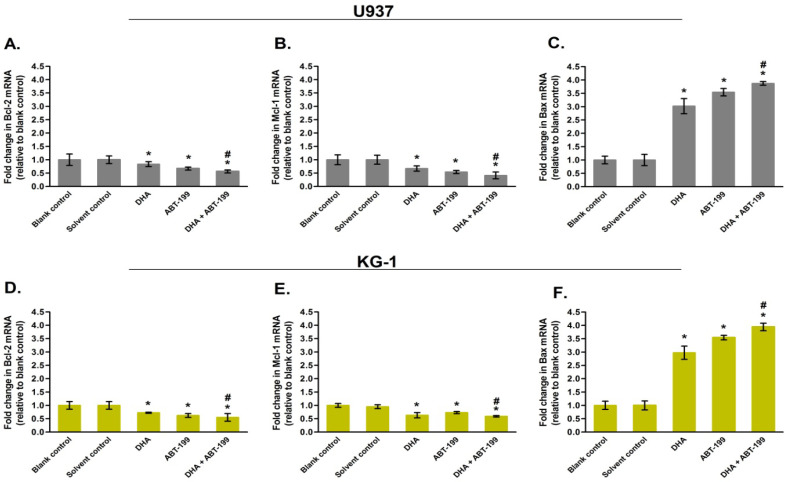
Dihydroartemisinin changed the expression of apoptotic genes
The effect of dihydroartemisinin and ABT-199 on the Mcl-1, Bcl-2 and Bax mRNA expression was determined by qRT-PCR method. The results showed that 24 h treatment of AML cells with each of dihydroartemisinin or ABT-199 decreased Bcl-2 and Mcl-1 mRNA expression, while increased the Bax mRNA expression (compared to the blank control group, p<5%). Combination therapy led to a stronger effect in gene expression levels relative to the single therapy (Figure 3, p<5%). In addition, no significant difference in mRNA expression levels was observed between the solvent control and blank control groups (p>5%). The findings were consistent across both U937 and KG1 cell lines, indicating that the treatment had comparable effects on both cell lines. (Figure 3).
Figure 3.
RT-qPCR Analysis of AML Cells. The U937 and KG-1 cells were treated with dihydroartemisinin (DHA) and ABT-199 (IC50 doses) for 24 h. Next, relative mRNA expression levels of Bcl-2 (A and D), Mcl-1 (B and E) and Bax (C and F) were determined by RT-qPCR using 2-(∆∆Ct) method. Results are presented as mean±SD (n=3). #p< 0.05 relative to single treatment; *p<0.05 relative to blank control
Dihydroartemisinin enhanced the apoptotic effect of ABT-199 in AML cells
To determine whether the inhibitory effect of treatments on cell survival was related to apoptosis, the U937 and KG-1 cells were exposed to the IC50 doses of dihydroartemisinin, ABT-199 and their combination for 24 h. Then, we performed Hoechst 33342 nuclear staining and caspase-3 activity assay. As shown in Figure 4, apoptotic cells containing nuclear morphologic changes were observed in dihydroartemisinin and ABT-199 treated cells but not in the control cells. Moreover, the number of apoptotic cells in the combination treated cells was not significantly differing from the mono-treated cells.
Figure 4.
The Effect of Dihydroartemisinin and ABT-199 on Apoptosis of AML Cells. U937 and KG-1 cells were exposed to the IC50 doses of dihydroartemisinin (DHA) and ABT-199 for 24 h. Next, the apoptosis was measured using Hoechst 33342 staining (A and B) and caspase-3 activity assay (C and D). The data are expressed as mean±SD (n=3) of three experiments. *p<0.05 compared with blank control. Arrows show the apoptotic cells
To explore the molecular mechanism of apoptosis induced by dihydroartemisinin and ABT-199, caspase-3 activity assay was conducted. The results revealed that there was a marked increase in caspase-3 activity in the cells treated with dihydroartemisinin and ABT-199 (p<0.05). The extent of caspase-3 activity in the AML cells treated with the combination of dihydroartemisinin and ABT-199 was not different relative to caspase-3 activity in the cells treated with either dihydroartemisinin or ABT-199 alone (p>0.05, Figure 4).
Because the IC50 dose of the combination group is lower than the IC50 dose of either agent alone, thus, our result show that the combination of the two agents has a stronger effect on triggering apoptosis compared to treatment with each single agent. The enhanced apoptotic effect is in consistent with the results from the MTT assay.
Discussion
Although AML patients show an initial response to chemotherapy, the long-term survival rate is only 30-40%, and the majority of patients eventually relapse. The main limitation in the treatment of AML patients is the development of primary and secondary drug resistance. Therefore, novel therapeutic strategies are required for complete cure of AML [1-3]. Increased expression of Bcl-2 family anti-apoptotic proteins such as Bcl-2, Bcl-xL and Mcl-1 has been observed in many hematological malignancies such as AML, which is associated with increased survival and resistance to cell apoptosis [15, 4-6]. Moreover, change in the balance of Bcl-2 family proteins is one of the main reasons for resistance to ABT-199 [9]. In this study, the effect of dihydroartemisinin on the sensitivity of AML cells to ABT-199 has been investigated.
In this study we showed that treatment with either dihydroartemisinin or ABT-199 alone, caused a significant decrease in the cell survival and proliferation, and triggered apoptosis in AML cell lines. Combination therapy significantly reduced the IC50 value and synergistically decreased the cell survival compared with dihydroartemisinin or ABT-199 alone. The IC50 in combination treatment was lower than the IC50 dose of either compound alone; therefore our data suggest that the combination therapy has a greater inhibitory effect on cell growth and apoptosis relative to the monotherapy. As yet, different studies have investigated the relationship between the expression levels Mcl-1 and Bcl-2 with ABT-199 sensitivity in tumor cells. Peirs et al. [16] found that Bcl-2 shows high expression in early T-cell precursors and gradually decreases during normal T-cell differentiation, and differences in ABT-199 sensitivity could partially be mediated by distinct stages of differentiation arrest between different molecular genetics subtypes of human T-ALL. Pan et al. [5] in a study found that HL-60 cells with high levels of Bcl-2 protein and relatively low Bcl-xL and Mcl-1 expression are very sensitive to ABT-199. The study demonstrated that ABT-199 alone decreases the association of proapoptotic protein Bim with Bcl-2, but this is compensated by increased Mcl-1 protein levels, which contribute to ABT-199 resistance. The study also showed that AML cell lines, primary patient samples, and murine primary xenografts are very sensitive to treatment with the selective Bcl-2 antagonist ABT-199. Wang et al. [17] in another study found that B-cell lymphoid cell lines with low Bcl-xL and Mcl-1 expression develop resistance to ABT-199 following chronic exposure. The study also showed that preventing PI3K/AKT/mTOR activation can overcome Mcl-1 and Bcl-xL-dependent resistance to the ABT-199 in lymphoid malignancies. Another study demonstrated that combination of ABT-199 and the proteasome inhibitor bortezomib results in strongly synergistic apoptosis induction. Mechanistically, ABT-199 mainly affected the multidomain effector Bax by liberating it from Bcl-2 inhibition. The combination with bortezomib additionally resulted in the accumulation of Bok, a Bax/Bak homologue, and of the BH3-only protein Noxa, which inhibits the Mcl-1 protein [18].
The effect of ABT-199 on Bcl-2 mRNA expression is not clear, but ABT-199 treatment results in decreased Bcl-2 protein levels, leading to on-target cell death in some cell types. In line with the above studies, other studies have investigated the role of Mcl-1 protein in resistance to ABT-199. For example, Luedtke et al. [19] investigated the anti-leukemic activity of the Mcl-1-selective inhibitor A-1210477 in combination with ABT-199 in AML cells. They found that A-1210477 synergistically induces apoptosis with ABT-199 in AML cell lines and primary patient samples. The synergistic induction of apoptosis was decreased upon Bak, Bax and Bim knockdown. While A-1210477 treatment alone also increased Mcl-1 protein levels, combination with ABT-199 reduced binding of Bim to Mcl-1. These results demonstrate that sequestration of Bim by Mcl-1, a mechanism of ABT-199 resistance, can be abrogated by combined treatment with the Mcl-1 inhibitor A-1201477. Another study showed that sustained B-cell receptor stimulation in CLL cells results in significant ABT-199 resistance, which correlates with induction of the anti-apoptotic protein Mcl-1. A major role for Mcl-1 in conferring ABT-199 resistance supported by knockdown and enforced expression experiments with primary CLL cells [20]. In this study, we showed that inhibition of Mcl-1 mRNA expression by dihydroartemisinin enhances the apoptotic effect of ABT-199 in U937 and KG-1 cells. Our results are in agreement with the above reports and demonstrate that dihydroartemisinin can increase the sensitivity of the AML cells to ABT-199 by suppressing the expression of Mcl-1 mRNA.
We also investigated the effect of dihydroartemisinin and ABT-199 on mRNA expression by qPCR assay. The results showed that treatment with each of ABT-199 or dihydroartemisinin alone clearly enhances the Bax mRNA expression and inhibits the expression of Mcl-1 and Bcl-2 mRNA. The effect of combination treatment on gene expression was greater than single treatment. In this regard, several studies have been conducted regarding the effect of dihydroartemisinin on gene expression and drug-resistance of cancer cells. Qin et al. [21] demonstrated that dihydroartemisinin enhances Bim and Bak expressions and reduces Mcl-1 expression in hepatocellular carcinoma cells thereby triggers the intrinsic pathway of apoptosis. Hou et al. [22] showed that dihydroartemisinin significantly exerted cytotoxic effect in hematoma cells but had a low cytotoxicity in normal liver cells. This compound triggered cell cycle arrest, suppressed cell proliferation, lowered the levels of cyclin E, cyclin D1, and enhanced the levels of Kip1/p27 and Cip1/p21. Dihydroartemisinin also enhanced the Bax/Bcl-2 ratio and triggered apoptosis. Moreover, it augmented the therapeutic efficacy of the gemcitabine in hematoma cells. Results of another study showed that treatment with dihydroartemisinin lead to activation of caspases, DNA fragmentation and induction of apoptosis in T-lymphoma cells. Moreover, dihydroartemisinin enhanced the Noxa expression and activated the Bak protein. This compound also enhanced the cytotoxic effect of ionizing radiation in lymphoma cells [23]. Other study revealed that dihydroartemisinin exhibits an anti-leukemic activity and induces apoptosis in human leukemia cells. This process involves inactivation MEK/ERK signaling pathway, Mcl-1 down-regulation, release of cytochrome c and caspase activation [24]. In another research, the combination effect of artemisinin and resveratrol on HeLa and HepG2 cells was explored. Data revealed that both artemisinin and resveratrol inhibits the proliferation and invasion of HepG2 and HeLa cells. Furthermore, the combination therapy with two agents exhibited the synergistic anti-cancer activity [25]. The results of above studies are in agreement with our results and show that dihydroartemisinin can sensitize the cancer cells to anti-cancer agents, such as ABT-199, through induction of apoptosis.
The mitochondrial or intrinsic pathway of cell death is initiated by intracellular cues, such as chemotherapy, free radicals, toxins, radiation and hypoxia. Intrinsic apoptosis pathway is regulated by the pro- or anti-apoptotic members of the Bcl-2 family proteins which bound to the mitochondrial membrane [1, 26, 27]. In apoptotic conditions, the activated pro-apoptotic members such as Bax and Bak cause the mitochondrial outer membrane permeability (MOMP), release of proapoptotic proteins into the cytoplasm and activation of caspases. Bcl-2 and Mcl-1 are anti-apoptotic proteins that prevent apoptosis by inhibiting the activation of Bax and Bak on the mitochondrial outer membrane [1, 26, 27]. ABT-199 is a synthetic BH3 domain mimic, which induces the mitochondrial pathway of apoptosis by binding to Bcl-2 protein [5, 7]. ABT-199 shows promising anticancer activity in AML patients, however, cancer cells can develop resistance to ABT-199, which can limit its efficacy as an anti-cancer agent [8]. ABT-199 resistance can arise through multiple mechanisms, including up-regulation of Mcl-1, activation of the AKT pathway, and dependence on other anti-apoptotic proteins [18, 20, 28]. Also, in AML cells resistant to ABT-199, it has been observed that the relationship between Bim and Mcl-1 is strengthened and the intrinsic pathway of apoptosis is not induced. These evidences show that the change in the balance of Bcl-2 family proteins is one of the main reasons for resistance to ABT-199 [9]. Numerous evidences have shown that artemisinin and its derivatives such as dihydroartemisinin play a pivotal role in controlling the cancer cell growth and apoptosis. Moreover, these compounds change the expression of different genes involved in apoptosis, such as Bcl-2, Mcl-1 and Bax, through ERK, AKT and NF-kB cell signaling pathways [10]. In this study, we showed that dihydroartemisinin reduced the expression of Bcl-2 and Mcl-1, and enhanced the expression of Bax in AML cells. These changes were associated with inhibition of cell growth and increased apoptosis induced by ABT-199. These data suggest that dihydroartemisinin not only triggers the intrinsic pathway of apoptosis, but also can increase the sensitivity of the AML cells to ABT-199 through suppression of Mcl-1 expression.
In conclusion, our study revealed that both dihydroartemisinin and ABT-199 significantly decreases the expression levels of Mcl-1 and Bcl-2, while they enhance the expression level of Bax mRNA in AML cells. These changes in gene expression are associated with the inhibition of cell proliferation and enhancement of intrinsic pathway of apoptosis. In combination therapy, the IC50 value was significantly decreased and a greater anti-leukemic effect was observed. Since resistance to ABT-199 is associated with increased expression of Mcl-1 and Bcl-2, therefore dihydroartemisinin can increase ABT-199 sensitivity of the AML cells by inhibiting the expression levels of these anti-apoptotic proteins.
Author Contribution Statement
Study concept and design: HK and YA; Acquisition of data: RN, MP, JA and MD; Analysis and interpretation of data: HK, YA and MD; Drafting of the manuscript: RN, MP, JA and MD; Critical revision of the manuscript for important intellectual content: HK, RN and YA; Funding recipients: HK and YA.
Acknowledgements
The authors thank the Molecular and Medicine Research Center Arak University of Medical Sciences (AUMS), for providing the necessary equipment for this work.
Funding Statement
This work was supported by a grant from the Faculty of Medicine from AUMS of Iran [Grant number 4173].
Ethical approval
This research was ethically wise approved from Deputy of research and technology, Arak University of Medical Sciences, Arak, Iran [Number 4173].
Conflict of interest
The authors have no conflict of interest to declare
References
- 1.Baradaran B, Nazmabadi R, Ariyan Z, Sakhinia E, Karami H. Dual targeting of anti-apoptotic proteins enhances chemosensitivity of the acute myeloid leukemia cells. Asian Pac J Cancer Prev. 2022;23(7):2523–30. doi: 10.31557/APJCP.2022.23.7.2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maffeo B, Panuzzo C, Moraca A, Cilloni D. A leukemic target with a thousand faces: The mitochondria. Int J Mol Sci. 2023;24:17. doi: 10.3390/ijms241713069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turkalj S, Radtke FA, Vyas P. An overview of targeted therapies in acute myeloid leukemia. HemaSphere. 2023;7(6):e914. doi: 10.1097/HS9.0000000000000914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lauria F, Raspadori D, Rondelli D, Ventura MA, Fiacchini M, Visani G, et al. High bcl-2 expression in acute myeloid leukemia cells correlates with cd34 positivity and complete remission rate. Leukemia. 1997;11(12):2075–8. doi: 10.1038/sj.leu.2400854. [DOI] [PubMed] [Google Scholar]
- 5.Pan R, Hogdal LJ, Benito JM, Bucci D, Han L, Borthakur G, et al. Selective bcl-2 inhibition by abt-199 causes on-target cell death in acute myeloid leukemia. Cancer Discov. 2014;4(3):362–75. doi: 10.1158/2159-8290.CD-13-0609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davids MS, Letai A. Targeting the b-cell lymphoma/leukemia 2 family in cancer. J Clin Oncol. 2012;30(25):3127–35. doi: 10.1200/JCO.2011.37.0981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Souers AJ, Leverson JD, Boghaert ER, Ackler SL, Catron ND, Chen J, et al. Abt-199, a potent and selective bcl-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med. 2013;19(2):202–8. doi: 10.1038/nm.3048. [DOI] [PubMed] [Google Scholar]
- 8.Konopleva M, Contractor R, Tsao T, Samudio I, Ruvolo PP, Kitada S, et al. Mechanisms of apoptosis sensitivity and resistance to the bh3 mimetic abt-737 in acute myeloid leukemia. Cancer cell. 2006;10(5):375–88. doi: 10.1016/j.ccr.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 9.Niu X, Zhao J, Ma J, Xie C, Edwards H, Wang G, et al. Binding of released bim to mcl-1 is a mechanism of intrinsic resistance to abt-199 which can be overcome by combination with daunorubicin or cytarabine in aml cells. Clin Cancer Res. 2016;22(17):4440–51. doi: 10.1158/1078-0432.CCR-15-3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Efferth T. From ancient herb to modern drug: Artemisia annua and artemisinin for cancer therapy. Seminars in cancer biology. 2017;46:65–83. doi: 10.1016/j.semcancer.2017.02.009. [DOI] [PubMed] [Google Scholar]
- 11.Amri J, Molaee N, Karami H, Baazm M. Combination of two mirnas has a stronger effect on stimulating apoptosis, inhibiting cell growth, and increasing erlotinib sensitivity relative to single mirna in a549 lung cancer cells. Biotechnol Appl Biochem. 2022;69(4):1383–94. doi: 10.1002/bab.2211. [DOI] [PubMed] [Google Scholar]
- 12.Alamdari-Palangi V, Karami Z, Karami H, Baazm M. Mirna-7 replacement effect on proliferation and tarceva-sensitivity in u373-mg cell line. Asian Pac J Cancer Prev. 2020;21(6):1747–53. doi: 10.31557/APJCP.2020.21.6.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ashofteh N, Mahbod ASA, Bayat M, Karami H. Mirna-16-1 suppresses mcl-1 and bcl-2 and sensitizes chronic lymphocytic leukemia cells to bh3 mimetic abt-199. Cell J. 2022;24(8):473–80. doi: 10.22074/cellj.2022.8101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ashofteh N, Amini R, Molaee N, Karami H, Baazm M. Mirna-mediated knock-down of bcl-2 and mcl-1 increases fludarabine-sensitivity in cll-cii cells. Asian Pac J Cancer Prev. 2021;22(7):2191–8. doi: 10.31557/APJCP.2021.22.7.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Efferth T, Benakis A, Romero MR, Tomicic M, Rauh R, Steinbach D, et al. Enhancement of cytotoxicity of artemisinins toward cancer cells by ferrous iron. Free Radic Biol Med. 2004;37(7):998–1009. doi: 10.1016/j.freeradbiomed.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 16.Peirs S, Matthijssens F, Goossens S, Van de Walle I, Ruggero K, de Bock CE, et al. Abt-199 mediated inhibition of bcl-2 as a novel therapeutic strategy in t-cell acute lymphoblastic leukemia. Blood. 2014;124(25):3738–47. doi: 10.1182/blood-2014-05-574566. [DOI] [PubMed] [Google Scholar]
- 17.Wang Q, Wan J, Zhang W, Hao S. Mcl-1 or bcl-xl-dependent resistance to the bcl-2 antagonist (abt-199) can be overcome by specific inhibitor as single agents and in combination with abt-199 in acute myeloid leukemia cells. Leuk Lymphoma. 2019;60(9):2170–80. doi: 10.1080/10428194.2018.1563694. [DOI] [PubMed] [Google Scholar]
- 18.Muenchow A, Weller S, Hinterleitner C, Malenke E, Bugl S, Wirths S, et al. The bcl-2 selective inhibitor abt-199 sensitizes soft tissue sarcomas to proteasome inhibition by a concerted mechanism requiring bax and noxa. Cell Death Dis. 2020;11(8):701. doi: 10.1038/s41419-020-02910-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luedtke DA, Niu X, Pan Y, Zhao J, Liu S, Edwards H, et al. Inhibition of mcl-1 enhances cell death induced by the bcl-2-selective inhibitor abt-199 in acute myeloid leukemia cells. Signal Transduct Target Ther. 2017;2:17012. doi: 10.1038/sigtrans.2017.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bojarczuk K, Sasi BK, Gobessi S, Innocenti I, Pozzato G, Laurenti L, et al. Bcr signaling inhibitors differ in their ability to overcome mcl-1-mediated resistance of cll b cells to abt-199. Blood. 2016;127(25):3192–201. doi: 10.1182/blood-2015-10-675009. [DOI] [PubMed] [Google Scholar]
- 21.Qin G, Zhao C, Zhang L, Liu H, Quan Y, Chai L, et al. Dihydroartemisinin induces apoptosis preferentially via a bim-mediated intrinsic pathway in hepatocarcinoma cells. Apoptosis. 2015;20(8):1072–86. doi: 10.1007/s10495-015-1132-2. [DOI] [PubMed] [Google Scholar]
- 22.Hou J, Wang D, Zhang R, Wang H. Experimental therapy of hepatoma with artemisinin and its derivatives: In vitro and in vivo activity, chemosensitization, and mechanisms of action. Clin Cancer Res. 2008;14(17):5519–30. doi: 10.1158/1078-0432.CCR-08-0197. [DOI] [PubMed] [Google Scholar]
- 23.Handrick R, Ontikatze T, Bauer KD, Freier F, Rubel A, Durig J, et al. Dihydroartemisinin induces apoptosis by a bak-dependent intrinsic pathway. Mol Cancer Ther. 2010;9(9):2497–510. doi: 10.1158/1535-7163.MCT-10-0051. [DOI] [PubMed] [Google Scholar]
- 24.Gao N, Budhraja A, Cheng S, Liu E-H, Huang C, Chen J, et al. Interruption of the mek/erk signaling cascade promotes dihydroartemisinin-induced apoptosis in vitro and in vivo. Apoptosis. 2011;16(5):511–23. doi: 10.1007/s10495-011-0580-6. [DOI] [PubMed] [Google Scholar]
- 25.Li P, Yang S, Dou M, Chen Y, Zhang J, Zhao X. Synergic effects of artemisinin and resveratrol in cancer cells. J Cancer Res Clin Oncol. 2014;140(12):2065–75. doi: 10.1007/s00432-014-1771-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shahverdi M, Amri J, Karami H, Baazm M. Knockdown of myeloid cell leukemia-1 by microrna-101 increases sensitivity of a549 lung cancer cells to etoposide. Iran J Med Sci. 2021;46(4):298–307. doi: 10.30476/ijms.2020.83173.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karami H, Baradaran B, Esfahani A, Sakhinia M, Sakhinia E. Therapeutic effects of myeloid cell leukemia-1 sirna on human acute myeloid leukemia cells. Adv Pharm Bull. 2014;4(3):243–8. doi: 10.5681/apb.2014.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bodo J, Zhao X, Durkin L, Souers AJ, Phillips DC, Smith MR, et al. Acquired resistance to venetoclax (abt-199) in t(14;18) positive lymphoma cells. Oncotarget. 2016;7(43):70000–10. doi: 10.18632/oncotarget.12132. [DOI] [PMC free article] [PubMed] [Google Scholar]