Abstract
Background:
Acute Myeloid Leukaemia (AML) is considered to be an extremely heterogeneous malignancy of bone marrow and blood. The first line of therapy for AML is prolonged chemotherapy. Due to the presence of molecular heterogeneity in AML as confirmed by next-generation sequencing, researchers are planning to develop newer strategies of therapy.
Objective:
In the present study we have explored the anti-cancer potentiality of the hydro-ethanolic extract (50% and 70%) of the whole flower of Nymphaea caerulea against the Acute Myeloid Leukaemia cell line, THP-1 with control of normal human kidney epithelial cell line (HEK 293). The present study is a novel contribution to the existing scientific knowledge as at present no study as an anti-leukaemic agent is available on N. caerulea (blue lotus) extract and exploring its action mechanism on in-vitro cell line model.
Methods:
Some targeted cytokine and apoptotic genes genes to deduce the anti-cancer mechanism of action of the crude extract (hydro-ethanolic extract (50% and 70%) of the whole flower) were selected as Interferon (IFN) γ, Interleukins – IL-6, IL-8, IL- 10, IL-1β, Transforming Growth Factor (TGF β1), Tumor Necrosis Factor (TNF α), Caspase 3(CAS 3), Caspase 9 (CAS 9), CD95 (Fas), Tumor Necrosis Factor Receptor 1 (TNFRSF1A) to observe relative fold changes of the expression using Real-Time PCR with housekeeping gene β-actin. Cellular cytopathic effect (CPE), cell viability assay by methylene blue assay, and cell cytotoxicity of the crude extract against the THP-1 cell line were also studied along with it’s bio-active compositional analysis of the extract was explored using ultra-performance liquid chromatography followed by mass spectra.
Results:
The N. caerulea flower extract is capable of inducing apoptosis in AML and it can balance cytokine alterations in such diseases.
Conclusions:
Nymphaea caerulea flower extract appears to be a good anti-leukemia agent.
Key Words: Nymphaea caerulea, Acute Myeloid Leukaemia, THP-1 cell line, LC-MS analysis, anti-cancer activity
Introduction
The latest data sheet from WHO, 2022 [1] indicated 10 million cancer deaths in 2020, affecting one out of six people. WHO observes that the primary reasons for the rising cases of death due to cancer are worse prognosis and limited access to diagnosis and therapeutic measures at the proper time [1]. The most common cases of cancer are of breast, colon and rectum, and lung, whereas breast cancer and leukemia are considered to be the most neglected forms of cancer [1].
As of now, there are no curative measures to treat leukemia and therefore, researchers all over the world are trying to develop innovative therapeutic measures and technologies for the targeted or precision therapy of leukemia [2, 3]. Many studies are being done on immunotherapies to curb the progression of these deadly diseases from the initial stages in some sub-types of leukemia [4].
Among the varied treatment procedures, alternative or complementary medicines are also considered to be effective measures where conventional therapy fails [4]. Past literature survey has shown that hypertension is a potent risk factor for the disease of erectile dysfunction. The study demonstrated that Nymphaea lotus Linn. can alleviate the sexual dysfunction in the hypertensive male rat [5]. Nymphaea caerulea is a perennial aquatic plant that grows on the shore of lakes and is commonly named as blue lotus, Egyptian lotus and blue water lily. According to traditional medicine literatures, the blue lotus have several healing or medicinal properties such as a tranquilizer, a significant detoxicant, aphrodisiac, act as traditional or complementary medicine for dyspepsia, diarrhea, urinary passage associated problems, palpitations of heart, enteritis, and fever [6]. According to the past historic literatures, when the Napolean expedited Nile River, they found three types of water lilies namely Nymphaea caerulea, Nymphaea lotos (white in colour), and Nelumbo nucifera (pink in colour). In the early literatures, Nymphaea caerulea was named in varied names such as N. stellata, N. scutifolia [7].
It is evident that the geographical difference leads to the development of heterogeneous cytogenetic nature among the different hematological neoplasms. A study which explored about the cytogenetic profile of the adult patients suffering from Acute Myeloid Leukaemia (AML) in Egypt reported that the median age of the patients was 36.5 years, and among them 53.3% were males whereas 46.7% were females and the median WBC count was 42.3 × 109/L [8]. Leukaemia is the most common hematological neoplasm and nearly 50% of them are AML cases in Egypt. The major risk factors identified by scientists in a study are agricultural chemicals and electromagnetic fields and therefore in the past 10 years the cases of hematological neoplasms are constantly rising [9]. Findings on Leukaemia reported by National Institute of Health of the Republic of Armenia within the period of 2012 - 2018 indicated that there were 259 new cases of ALL and 478 AML [10]. However, the morbidity indicators obtained from the registered incidence of AML was found to be few in numbers in population based study in Kazakhstan [11].
As mentioned earlier, that the sacred blue lotus N. caerulea is found in several numbers along the banks of River Nile. The flower comprises several important phytochemicals namely Liensinine, Iso- Liensinine, Neferine, Lotusine, Pronuciferine, Rutin, Hyperin etc [12]. Many medicinal properties of blue lotus which are reported are antioxidant activity, antisteroids, anti-pyretic, anti-viral, anti-inflammatory, for treatment against erectile dysfunctions, against diabetic complications, as an anti-ageing, showing hepato-protective activity, etc [12]. Blue lotus exhibited cytotoxic and apoptotic activities on triple-negative breast cancer cells via ROS generation and p38 MAPK/JNK activation pathway [13]. N. nucifera also showed anti-cancer activity against lung cancer cell line (NSCLC) and also lowered the ratio of B-cell lymphoma 2/Bcl-2-associated X protein (Bcl-2/Bax) pointing towards the pro-apoptosis nature of nuciferin [14].
In the present study, we have explored the anti-cancer activity for the first time of the ethanolic extracts of Nymphaea caerulea flower against the cancer cell line THP-1 monocytes and normal HEK-293 cell line. The bioactive composition analysis was also carried out with the aid of ultra-performance liquid chromatography followed by mass spectrometry.
Materials and Methods
Plant Sample collection
50 g of dried petals of the blue lotus was purchased from authenticated herbal product production company (approved by ISO 9000-2015; 100% organic herbal; GMP certified and HACCP certified), Green Earth Products Pvt. Ltd. India (approved by fssai 23318008001334), collected and packed in August 2022 (Figure 1).
Figure 1.

Dry Flower of Blue lotus (N. caerulea) Procured from Green Earth Private Ltd
Cell lines
The cell line, THP -1 was procured from National Centre for Cell Science (NCCS), Pune, India. The cells were transported within sterile RPMI 1640 media with 10% fetal bovine serum concentration. The human embryonic kidney cell line (HEK 293) was also procured from NCCS, Pune, and it was transported in sterile DMEM media with 10% fetal bovine serum concentration. The cell lines were authenticated using sixteen short tandem repeat (STR) loci which was amplified using commercially available AmpFISTR Identifier Plus PCR Amplification kit from Applied Bio-systems. The cell line sample was processed using the Applied Bio-system® 3500 genetic analyser. The data obtained was analysed using Gene Mapper® ID –X v1.5 software (Applied Bio-systems). For confirmation, appropriate positive and negative controls were used. Mycoplasma testing was done using Mycoplasma PCR method/ Hoechst staining and it was confirmed not detected.
Chemicals
Rose well Park Memorial Institute 1640 (RPMI) 1X (Ref: 11875 -093; Lot: 2436312) and Dulbecco’s Modified Eagle Medium (DMEM) (1X) along with Glutamax were purchased from Gibco, UK (Ref: 10567-014; Lot No: 2522563) for cell culture purposes of THP-1 and HEK 293 respectively. F12 (1X) nutrient mixture Ham + L-glutamine supplement (Product code: AL025A; Lot No. 0000512083) for the growth of the cell line, was also purchased from Himedia Pvt. Ltd., India. It was required for the culture and maintenance of the HEK 293 cell line. Fetal Bovine Serum (FBS) (Product code: RM1112-500ML, Himedia Pvt. Ltd., India), antibiotic–antimycotic solution (Penicillin/Streptomycin/ Amphotericin B) (A002A – 100ML, Lot No. 0000506599, Himedia Pvt. Ltd., India) and Phosphate Buffer saline (PBS, 1X) (Ref: 10010-031; Lot no. 2276680; Gibco, UK) were also procured. The MTT assay EZ count kit was bought from Hi Media, India (Cat No: CCK003). Molecular biology reagents and RNA iso plus were purchased from Takara (TAKARA, USA; Cat. No. 9108; Lot No. ALZ1011N), and the reverse transcriptase cDNA synthesis kit (Bio-Rad, USA, Cat. No. 1708841; Batch No. 64449565) and the iTaq SYBR green supermix RT-PCR reagents (Cat. No. 1725121; Batch No. 64464415) were purchased from Bio-Rad, USA.
Plant extraction of bioactive constituents
10 g of dried blue lotus petals was weighed and cut down into small pieces and extracted using 100 mL of 50% and 70% ethanol (molecular biology grade purity) for 72 hours in dark conditions. Following extraction, the crude extract was filtered through Whatman filter paper no. 1 and then sterilized using a 0.22 micron PES membrane filter (Merck, Millipore). The extract was then lyophilized and the crude powder was stored at 4ºC in a refrigerator for further biological assays [15].
To calculate the yield analysis of the extract, the dried content of the extract was weighed and then calculated using the formula:
W2 – W1 / 100mL where, W2 is the weight of the extract along with the container, W1 is the weight of the container alone and W0 is the weight of the initial dried plant sample.
Culturing of Cell Lines
THP-1 cell line, peripheral blood monocyte isolated from acute monocytic leukaemia patient, a non-adherent cell line with passage number 28 was seeded in a T25cm2 cell culture flask with filtered sterile RPMI 1640 media supplemented with 10% FBS and antibiotic–antimycotic solution. The cells were allowed to become confluent at 37ºC, 5% carbon dioxide, and humidified atmosphere for the next 48 hours. When the cells reached a confluence of more than 80%, the cells were re-suspended with fresh media in a 12-well culture plate for experimental purposes. The plate was incubated at 37ºC, with 5% carbon dioxide in the humidified atmosphere for the next 24 hours [16].
The human embryonic kidney cell line (HEK 293) is an adherent cell line of passage number 27 that was seeded in a T25cm2 cell culture flask with filtered sterile DMEM + F12 along with 10% FBS and antibiotic–antimycotic solution and incubated at 37ºC, 5% carbon-dioxide in the humidified atmosphere for the next 48 – 72 hours. HEK 293 cells take time to adhere to the base but grow rapidly once it gets adhered to the base. When the cells reached 80% confluence, it was trypsinized and re-suspended in a 12-well culture plate and allowed to grow to maintain the above-mentioned culture conditions. The cells were allowed to grow until it reaches a confluence of 106 to 108 [17].
Inoculation of Extracts and Vehicle Control
Following incubation, the experimental sets were prepared for both the THP-1 and HEK 293 cell lines to study the anti-cancer activities as well as to observe any deleterious effect in normal cells (control) - cells were kept intact and allowed to grow freely for the next 24 hours. There were also vehicle control sets in which cells were inoculated with 50µL of 50% and 70% ethanol for the next 24 hours; following that cells were inoculated with 50% and 70% ethanolic extract of blue lotus. Then the plates were rotated clockwise and anti-clockwise for proper mixing, and then kept in a carbon-dioxide incubator at 5% CO2 level, 37ºC temperature in a humidified environment [18].
Cytopathic effect Study
After 24 hours of incubation, the cell morphology was observed under an inverted microscope under 40 X magnification and the photographs were recorded for the cell size and shape in all the experimental sets with both cancer and normal cell line [18].
Cell viability Assay
A viable Cell counting assay was done following the modified methylene blue assay. The modified stain comprises 1X PBS solution, glutaraldehyde, and methylene blue stain. After discarding the media, 300 µL of stain was added to each well and incubated for 1 hour at 37ºC. Following incubation, the stain was discarded and the cells were washed with PBS (1X) and observed under the inverted microscope in 40 X magnification and the photographs were recorded of the viable and non–viable cells. The viable cells appeared blue while the non-viable cells appeared white under the inverted microscope [18].
Gene Expression Assay
The cells after staining of different experimental sets of both the cell lines were washed with PBS (1X) and harvested with RNA iso plus following the protocol of the manufacturer. The RNA was dissolved in 60µL of nuclease-free water and the yield was quantified using A260/280 ratio of UV-Vis spectrophotometer. cDNA was synthesized using the reverse transcriptase kit following the instructions with thermal cycler T100 (BIO-RAD, USA). The cytokines expression was analyzed Interferon (IFN) γ, Interleukins – IL-6, IL-8, IL- 10, IL-1β, Transforming Growth Factor (TGF β1), Tumor Necrosis Factor (TNF α), Caspase 3(CAS 3), Caspase 9 (CAS 9), CD95 (Fas), Tumor Necrosis Factor Receptor 1 (TNFRSF1A) gene as a relative fold change of the expression of housekeeping gene β-actin using Real-Time PCR (BIO-RAD, USA) [18].
Cytotoxicity Assay
The cytotoxicity of the extracts was assayed using an MTT assay kit in a 96-well microtiter plate. The assay consisted of the following controls namely medium control (without THP-1 cell line), cell control (THP-1 cell line + media), and the vehicle control containing the 50% and 70% ethanol-based extracts. The inoculum volumes of the extracts were 2, 4, 6, 8, 10, 12, and 15 µL of the extracts in triplicates keeping the final volume of the reaction per well to be 100µL. After completion of dilution, the plate was kept in the carbon- dioxide incubator (5%) at 37ºC for the next 24 hrs. 10µL of MTT solution was added to each well, mixed thoroughly, and kept at incubation for the next 4 hours. After completion of incubation, the plate was observed under the inverted microscope to observe formazan crystal formation. Thereafter, 100 µL of solubilization solution was added to each of the wells and kept at overnight incubation to maintain the above-mentioned condition. The absorbance of the 96 well plates was recorded using an ELISA plate reader (Roboniks, India) and thereafter the data was graphically plotted [18].
Characterization of bioactive compounds by LCESI-QTOF-MS/MS
The bioactive constituents of the extract were analyzed by reverse-phase ultra-performance liquid chromatography and electrospray ionization mass spectrometry. The instrument that was used for this analysis was the Waters Acquity UPLC system and Waters Acquity SQD mass spectrometer (Single quadrupole mass spectrometer) system (Waters TM, USA). The electrospray ionization source of the MS system operated in the positive ion mode. Based on the experimental m/z value of the analyte obtained, it was identified with the m/z value of the standard compounds mentioned in the previous literature [19].
Statistical Analysis
The T-test and P-value with 95% of Confidence Interval of each parameter of cytokines were studied and given in Table 1 for the experimental sets, both row-wise and column wise interaction were analyzed. The software used for the analysis was MedCalc, easy to use statistical software, version 22.
Table 1.
Changes in Cytokine Gene Expressions Alongwith Statistical Analysis
| Cytokine gene expressions (fold changes, Mean ± Standard Deviation) in different experimental sets |
P value (between different sets) with 95% Confidence Interval (THP-1) |
P value (between different rows) with 95% Confidence Interval (THP-1) |
P value (between different columns) with 95% Confidence Interval (THP-1) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sets | HEK 293 (IFN γ) | THP -1 (IFN γ) | HEK 293 (IFN γ) | THP -1 (IFN γ) | IFN γ (HEK 293 vs THP-1) | |||||
| Control | 1.00 ± 0.05 | 1.00 ± 0.05 | 1 | 1 | 1 | |||||
| N. caerulea 50 % | 0.44 ± 0.02 | 0.87 ± 0.04 | 0.0001 | 0.0245 | 0.0001 | |||||
| N. caerulea 70 % | 0.02 ± 0.00 | 0.62 ± 0.03 | < 0.0001 | 0.0004 | < 0.0001 | |||||
| ALC 70 % | 0.01 ± 0.00 | 0.27 ± 0.01 | < 0.0001 | < 0.0001 | < 0.0001 | |||||
| ALC 50 % | 0.02 ± 0.00 | 2.40 ± 0.12 | < 0.0001 | < 0.0001 | < 0.0001 | |||||
| Sets | HEK 293 (IL 6) | THP -1 (IL 6) | HEK 293 (IL 6) | THP -1 (IL 6) | IL-6 (HEK 293 vs THP-1) | |||||
| Control | 1.00 ± 0.05 | 1.00 ± 0.05 | 1 | 1 | 1 | |||||
| N. caerulea 50 % | 0.69 ± 0.03 | 1.38 ± 0.07 | 0.0008 | 0.0016 | 0.0001 | |||||
| N. caerulea 70 % | 0.15 ± 0.01 | 120.28 ± 6.01 | < 0.0001 | < 0.0001 | < 0.0001 | |||||
| ALC 70 % | 0.06 ± 0.00 | 76.02 ± 3.80 | < 0.0001 | < 0.0001 | < 0.0001 | |||||
| ALC 50 % | 0.17 ± 0.01 | 63.70 ± 3.19 | < 0.0001 | < 0.0001 | < 0.0001 | |||||
| Sets | HEK 293 (IL 8) | THP -1 (IL 8) | HEK 293 (IL 8) | THP -1 (IL 8) | IL-8 (HEK 293 vs THP-1) | |||||
| Control | 1.00 ± 0.05 | 1.00 ± 0.05 | 1 | 1 | 1 | |||||
| N. caerulea 50 % | 4.66 ± 0.23 | 0.30 ± 0.01 | < 0.0001 | < 0.0001 | < 0.0001 | |||||
| N. caerulea 70 % | 6.07 ± 0.30 | 18.54 ± 0.93 | < 0.0001 | < 0.0001 | < 0.0001 | |||||
| ALC 70 % | 2.57 ± 0.13 | 0.92 ± 0.05 | < 0.0001 | 0.1216 | < 0.0001 | |||||
| ALC 50 % | 3.03 ± 0.15 | 0.26 ± 0.01 | < 0.0001 | < 0.0001 | < 0.0001 | |||||
| HEK 293 Sets | HEK 293 (IL 10) | THP -1 (IL 10) | HEK 293 (IL 10) | THP -1 (IL 10) | IL-10 (HEK 293 vs THP-1) | |||||
| Control | 1.00 ± 0.05 | 1.00 ± 0.05 | 1 | 1 | 1 | |||||
| N. caerulea 50 % | 0.18 ± 0.01 | 3.60 ± 0.18 | < 0.0001 | < 0.0001 | < 0.0001 | |||||
| N. caerulea 70 % | 1.52 ± 0.08 | 0.52 ± 0.03 | 0.0007 | 0.0001 | < 0.0001 | |||||
| ALC 70 % | 1.03 ± 0.05 | 2.63 ± 0.13 | < 0.0001 | < 0.0001 | < 0.0001 | |||||
| ALC 50 % | 1.87 ± 0.09 | 2.91 ± 0.15 | < 0.0001 | < 0.0001 | 0.0005 | |||||
| Sets | HEK 293 (IL 1B) | THP -1 (IL 1B) | HEK 293 (IL 1B) | THP -1 (IL 1B) | IL-1β (HEK 293 vs THP-1) | |||||
| Control | 1.00 ± 0.05 | 1.00 ± 0.05 | 1 | 1 | 1 | |||||
| N. caerulea 50 % | 0.06 ± 0.00 | 0.43 ± 0.02 | < 0.0001 | 0.0001 | < 0.0001 | |||||
| N. caerulea 70 % | 0.78 ± 0.04 | 17.03 ± 0.85 | 0.004 | 0.0001 | < 0.0001 | |||||
| ALC 70 % | 0.30 ± 0.01 | 7.60 ± 0.38 | < 0.0001 | < 0.0001 | < 0.0001 | |||||
| ALC 50 % | 0.63 ± 0.03 | 0.20 ± 0.01 | 0.0004 | < 0.0001 | < 0.0001 | |||||
| Sets | HEK 293 (TGF B1) | THP -1 (TGF B1) | HEK 293 (TGF B1) | THP -1 (TGF B1) | TGF B1 (HEK 293 vs THP-1) | |||||
| Control | 1.00 ± 0.05 | 1.00 ± 0.05 | 1 | 1 | 1 | |||||
| N. caerulea 50 % | 9.54 ± 0.48 | 13.45 ± 0.67 | < 0.0001 | < 0.0001 | 0.0012 | |||||
| N. caerulea 70 % | 2.22 ± 0.11 | 0.30 ± 0.02 | 0.0001 | < 0.0001 | < 0.0001 | |||||
| ALC 70 % | 0.21 ± 0.01 | 7.24 ± 0.36 | < 0.0001 | < 0.0001 | < 0.0001 | |||||
| ALC 50 % | 0.68 ± 0.03 | 2.62 ± 0.13 | 0.0007 | < 0.0001 | < 0.0001 | |||||
| Sets | HEK 293 (TNF-α) | THP -1 (TNF-α) | HEK 293 (TNF-α) | THP -1 (TNF-α) | TNF-α (HEK 293 vs THP-1) | |||||
| Control | 1.00 ± 0.05 | 1.00 ± 0.05 | 1 | 1 | 1 | |||||
| N. caerulea 50 % | 0.01 ± 0.00 | 0.30 ± 0.01 | < 0.0001 | < 0.0001 | < 0.0001 | |||||
| N. caerulea 70 % | 0.02 ± 0.00 | 0.81 ± 0.04 | < 0.0001 | 0.0068 | < 0.0001 | |||||
| ALC 70 % | 2.17 ± 0.11 | 4.20 ± 0.21 | 0.0001 | < 0.0001 | 0.0001 | |||||
| ALC 50 % | 0.01 ± 0.00 | 3.48 ± 0.17 | < 0.0001 | < 0.0001 | < 0.0001 | |||||
| SETS | HEK 293 (CAS 3) | THP 1 (CAS 3) | HEK 293 (CAS 3) | THP 1 (CAS 3) | CAS 3 (HEK 293 vs THP-1) | |||||
| Control | 1.00 ± 0.05 | 1.00 ± 0.05 | 1 | 1 | 1 | |||||
| N. caerulea 50 % | 2.53 ± 0.13 | 173.59 ± 8.68 | < 0.0001 | < 0.0001 | < 0.0001 | |||||
| N. caerulea 70 % | 0.47 ± 0.02 | 5.65 ± 0.28 | 0.0001 | < 0.0001 | < 0.0001 | |||||
| ALC 70 % | 0.85 ± 0.04 | 1.33 ± 0.07 | 0.0154 | 0.0027 | < 0.0001 | |||||
| ALC 50 % | 0.82 ± 0.04 | 0.42 ± 0.02 | 0.0082 | < 0.0001 | < 0.0001 | |||||
| Sets | HEK 293 (CAS 9) | THP 1 (CAS 9) | HEK 293 (CAS 9) | THP 1 (CAS 9) | CAS 9 (HEK 293 vs THP-1) | |||||
| Control | 1.01 ± 0.05 | 1.00 ± 0.05 | 1 | 1 | 1 | |||||
| N. caerulea 50 % | 1.41 ± 0.07 | 7.67 ± 0.38 | 0.0012 | < 0.0001 | < 0.0001 | |||||
| N. caerulea 70 % | 0.83 ± 0.04 | 2.85 ± 0.14 | 0.01 | < 0.0001 | < 0.0001 | |||||
| ALC 70 % | 0.46 ± 0.02 | 5.44 ± 0.27 | 0.0001 | < 0.0001 | < 0.0001 | |||||
| ALC 50 % | 0.97 ± 0.05 | 8.85 ± 0.44 | 0.5032 | < 0.0001 | < 0.0001 | |||||
| Sets | HEK 293 - CD 95 (FAS) | THP 1 - CD 95 (FAS) | HEK 293 - CD 95 (FAS) | THP 1 - CD 95 (FAS) | CD 95 (HEK 293 vs THP-1) | |||||
| Control | 1.00 ± 0.05 | 1.00 ± 0.05 | 1 | 1 | 1 | |||||
| N. caerulea 50 % | 0.31 ± 0.02 | 83.20 ± 4.16 | < 0.0001 | < 0.0001 | < 0.0001 | |||||
| N. caerulea 70 % | 0.10 ± 0.01 | 0.08 ± 0.00 | < 0.0001 | < 0.0001 | < 0.0001 | |||||
| ALC 70 % | 0.10 ± 0.01 | 0.10 ± 0.00 | < 0.0001 | < 0.0001 | < 0.0001 | |||||
| ALC 50 % | 0.14 ± 0.01 | 0.03 ± 0.00 | < 0.0001 | < 0.0001 | < 0.0001 | |||||
| Sets | HEK 293 (TNFRA1) | THP 1 (TNFRA1) | HEK 293 (TNFRA1) | THP 1 (TNFRA1) | TNFRA1 (HEK 293 vs THP-1) | |||||
| Control | 1.00 ± 0.05 | 1.00 ± 0.05 | 1 | 1 | 1 | |||||
| N. caerulea 50 % | 0.94 ± 0.05 | 21.91 ± 1.10 | 0.2156 | < 0.0001 | < 0.0001 | |||||
| N. caerulea 70 % | 0.18 ± 0.01 | 0.59 ± 0.03 | 0.0003 | < 0.0001 | < 0.0001 | |||||
| ALC 70 % | 0.55 ± 0.03 | 6.17 ± 0.31 | 0.0002 | < 0.0001 | < 0.0001 | |||||
| ALC 50 % | 0.32 ± 0.02 | 1.57 ± 0.08 | < 0.0001 | < 0.0001 | < 0.0001 | |||||
Results
Yield Content analysis
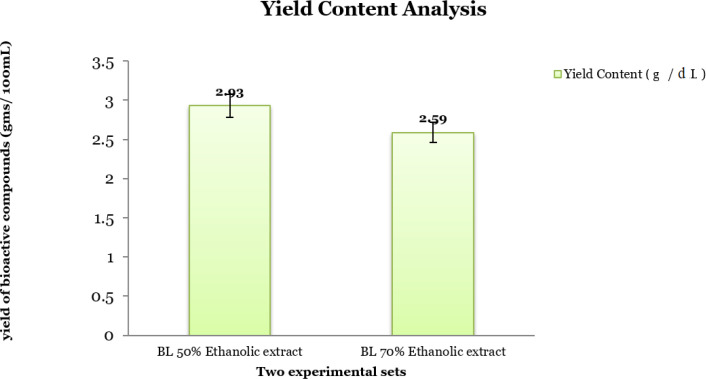
The crude ethanolic extract of different percentages, i.e., 50% and 70% depicted marginal variation in their yield content of the bioactive compounds (g/dL). It is found to be high with the 50% ethanolic solvent (2.93 g/dL) in comparison to 70% ethanolic solvent (2.59 g/dL) (Figure 2).
Figure 2.
Yield Content Analysis of the Crude Ethanolic (50 and 70 percent) Extracts of N. caerulea
Cytopathic Study
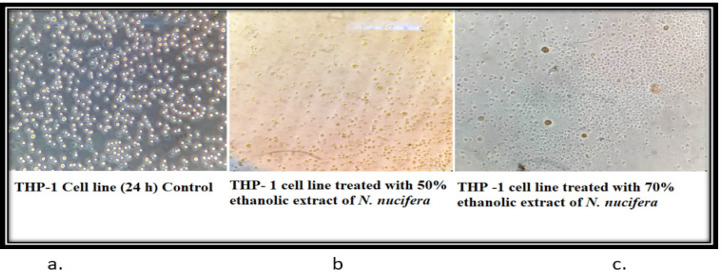
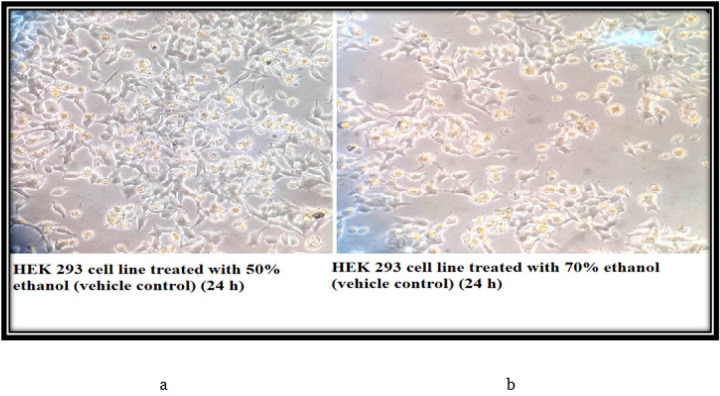
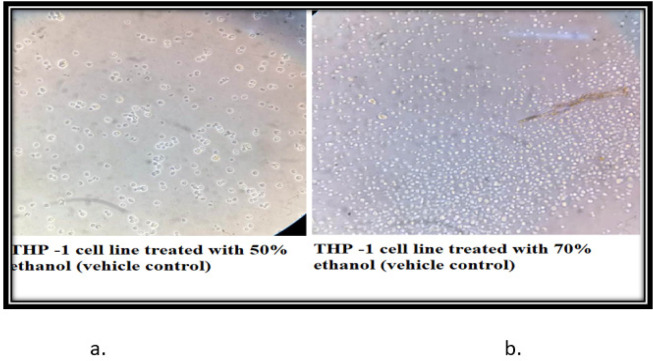
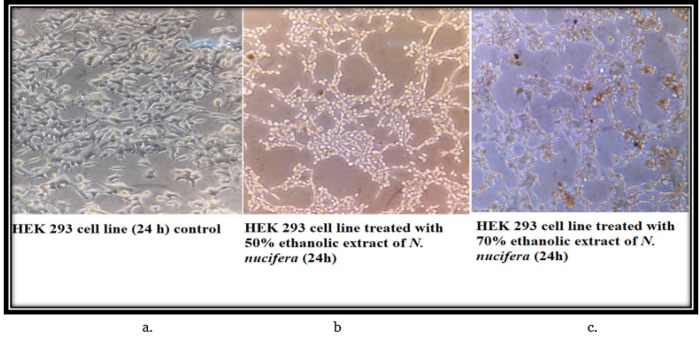
The control cells of THP-1 (24 h) showed that the morphology of the cells is round, large, and single-cell. Cells are in the active proliferation stage with increased numbers. In the 50% and 70% N. caerulea extract treated sets cells are mostly dead with altered shape, size, and numbers along with extensive cell debris. In the vehicle control sets, the cells treated with 50% alcohol are comparatively large and round in size and shape with lesser cellular debris when compared with the 70% ethanol-treated cells. The HEK 293 cell line control (24 h) set depicted proper epithelial cell-like morphology with good margins and a negligible amount of dead cells and debris. With the treatment of 50% N. caerulea extract, the cells are alive with little round-shaped epithelial morphology but with very few dead cells or cellular debris. Whereas in the 70% N. caerulea extract treated sets cells the cells are mostly dead with membrane blebs, and no proper marginations, cells have become rounded, clustered together, and have left the base of the flask, floating in the culture medium. However, in the vehicle control sets, cells treated with 50% ethanol are mostly showing proper morphology with a few numbers round dead cells in between, and in the 70% ethanol control set the number of dead cells is comparatively higher (Figures 3 – 6).
Figure 3.
a, control cells of THP-1 (24 h) showed that the morphology of the cells are round, large and single cell. Cells are in the active proliferation stage with increased numbers; b & c, In the 50% and 70% N. caerulea extract treated sets cells are mostly dead with reduced shape, size and numbers along with extensive cell debris
Figure 6.
a & b, In the vehicle control sets, cells treated with 50% ethanol are mostly showing proper morphology with few numbers of round dead cells in between and in the 70% ethanol control set the number of dead cells are comparatively higher
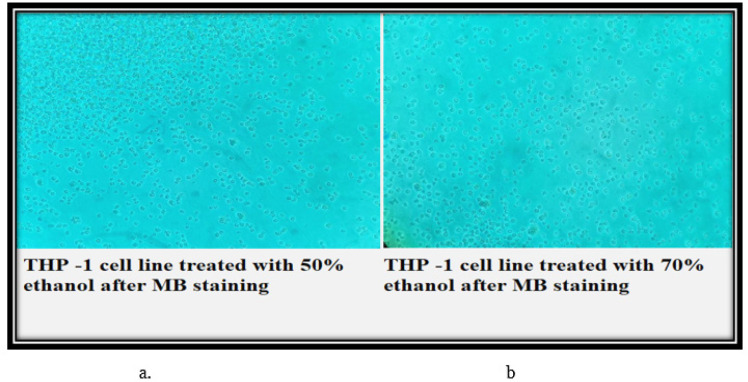
Cell viability Study
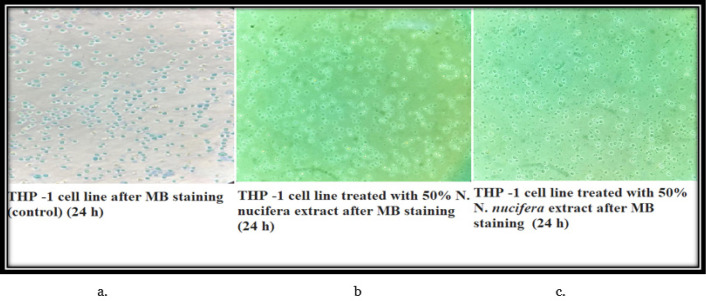
The control set THP-1 cells have all taken up the modified methylene blue stain and are showing round large single cellular morphology. Therefore, all the cells visible in the field are viable. However, in the 50% and 70% N. caerulea extract treated sets, all the cells are de-shaped with no up taking of stain indicating that all cells are dead. In the vehicle control sets (both 50% and 70% ethanol) the cells are mostly dead with no stain. In the HEK 293 cell line, the control set cells are all stained blue (live) with accurate morphology. Whereas in the 50% N. caerulea extract-treated set, the cellular morphology has little changes and is all stained but in the 70% extract-treated set the morphology of cells has become more rounded and clustered though they are alive. In the vehicle control sets (50% and 70% ethanol), cells are marginally round in shape with few clustered together but are mostly live (stained blue) (Figures 7 – 10).
Figure 7.
a, control set THP-1 cells have all taken up the modified methylene blue stain and are showing round large single cellular morphology. Therefore, all the cells visible in the field are viable; b & c, In the 50% and 70% N. caerulea extract treated sets, all the cells are de-shaped with no up taking of stain indicating that all cells are dead
Figure 10.
a & b, In the vehicle control sets (50% and 70% ethanol), cells are marginally round in shape with few clustered together but are mostly live (stained blue)
Moreover before initiating the experiment the total yield of bioactive compositions was measured and it is found to be high with the 50% ethanolic solvent (2.93 g/dL) in comparison to 70% ethanolic solvent (2.59 g/dL) (Figure 2).
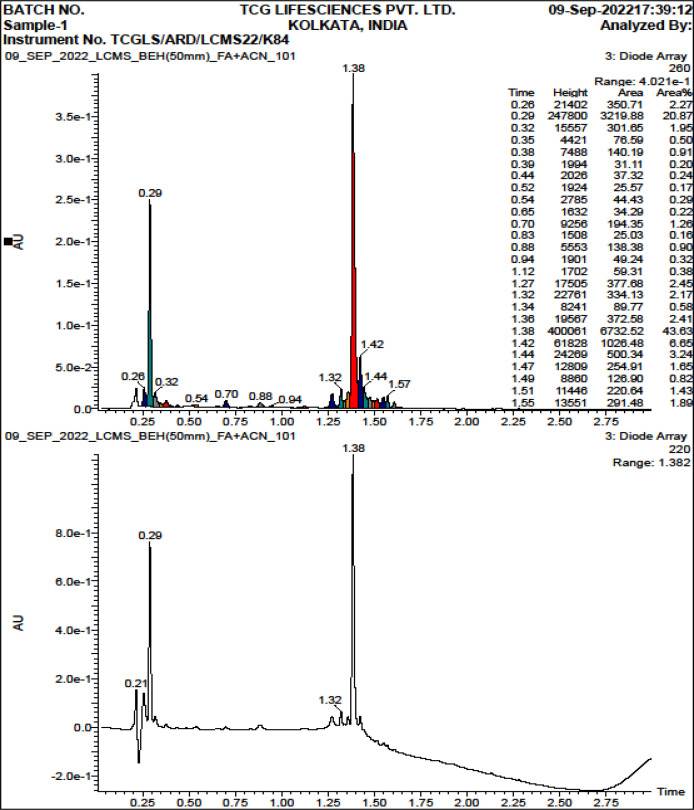
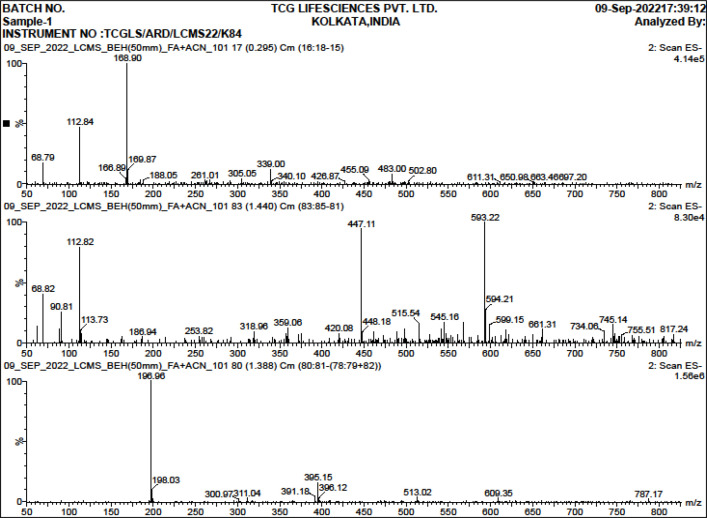
The ultra-performance liquid chromatography followed by mass spectra analysis of the crude extract revealed the presence of several bioactive constituents like Paeoniflorin, Hesperidin, Homogentisic acid, 3 –p coumaroyl – quinic acid, Syringic acid, etc (Table 2) [19, 20].
Table 2.
Phytochemical Profiling of the Crude 50% Ethanolic Extract
| SL. NO. | Experimental m/z value | Probable Compounds Identified |
|---|---|---|
| 1 | 169.87 | Gallic acid |
| 2 | 168.9 | Homogentisic acid |
| 3 | 166.89 | Vanillic acid |
| 4 | 186.94 | Brevifolin |
| 5 | 198.1 | Syringic acid |
| 6 | 305.05 | Gallocatechin |
| 7 | 339 | 3 –p coumaroyl – quinic acid |
| 8 | 340.1 | Caffeoyl glucose/ Esculin |
| 9 | 359.06 | Ellagic acid rhamnosyl |
| 10 | 447.11 | Quercetin 3–O-acetyl hexoside |
| 11 | 448.18 | delphinidin 3-O-rhamnosyl-5-Ogalactoside |
| 12 | 455.09 | delphinidin 39-O-(20-O-galloyl-60-Oacetyl-b-galactopyranoside) |
| 13 | 480.16 | Paeoniflorin |
| 14 | 483 | HHDPe-hexoside |
| 15 | 610.18 | Hesperidin |
Cytokine expression analysis
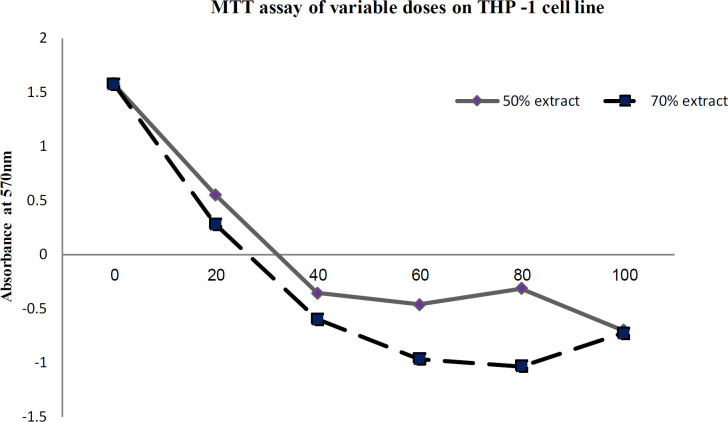
The cytokine gene expression of the targeted genes namely Interferon (IFN) γ, Interleukins – IL-6, IL-8, IL- 10, IL-1β, Transforming Growth Factor (TGF β1), Tumor Necrosis Factor (TNF α), Caspase 3(CAS 3), Caspase 9 (CAS 9), CD95 (Fas), Tumor Necrosis Factor Receptor 1 (TNFRSF1A) gene was expressed as bar diagrams as a relative fold change of the expression of housekeeping gene β-actin of the five different experimental sets (Figure 11 – 12). The MTT cytotoxicity assay showed that the effective dosage (volume) of the crude extract upon the THP-1 cell line is approximately 40µL for both extracts (50% and 70%) (Table 1).
Figure 11.
MTT Cyto-Toxicity assay of the 50% and 70% Extract of N. caerulea with Different Doses on THP-1 Cell Line
Figure 12.
UPLC Chromatogram Profile of Several Elutes Purified along with Their Retention Time (RT) of the 50% Ethanolic Extract of N. caerulea
Thus in brief, after yield content analysis of the extract we observed 2.93 g/dl and 2.59 g/dl yield of bioactive chemicals in 50% and 70% ethanol extracts of the flower respectively. When applied to the THP-1 leukemia cell line there were significant dead, particularly with 50% extract. Although some changes were present with 50% and 70% alcohol (vehicle), the changes were not so pronounced and the cells were viable (Figure 3). However, when 50% and 70% extracts were separately applied on HEK 293 normal cell line there were insignificant changes with 50% and 70% extract. IFN γ gene expression changes were not significant with the extracts as well as with the vehicle alcohols. However, IL-6, IL-8, IL-1β, TGF-β1, and TNF-α gene expressions were much less with 50% extract although they were markedly increased with 70% extract. On the contrary, IL-10 gene expression was significantly increased with 50% extract. Thus 50% extract appears to be capable of controlling an imbalance between pro-inflammatory and anti-inflammatory cytokines which is commonly found in most infectious diseases and many non-infectious diseases also.
When we studied some markers of the apoptotic pathway, we found the Cas3, Cas9, CD95, and TNFRSF1A gene expressions were more with 50% extract, which also indicates that significant apoptotic action on the leukemia cells is possible with this extract (Table 1).
Statistical Analysis
All the data of gene expression was found to be statistically significant with P- value <0.005 (Given in Table 1).
Figure 4.
a & b, the cells treated with 50% alcohol are comparatively large and round in size and shape with lesser cellular debris when compared with the 70% ethanol treated cells
Figure 5.
a, The HEK 293 cell line control (24 h) set depicted proper epithelial cell like morphology with good margins and negligible amount of dead cells and debris; b, With the treatment of 50% N. caerulea extract, the cells are alive with little round shaped epithelial morphology but with very few number of dead cells or cellular debris; c, In the 70% N. caerulea extract treated sets cells the cells are mostly dead with membrane blebs, no proper marginations, cells have become rounded, clustered together and have left the base of the flask, floating in the culture medium
Figure 8.
a & b, The vehicle control sets (both 50% and 70% ethanol) the cells are mostly viable
Figure 9.
a, The control set cells are all stained blue (live) with accurate morphology; b, In the 70% extract treated set the morphology of cells have become more rounded and clustered though they are alive; c, In the 50% N. caerulea extract treated set, the cellular morphology have little changes and are all stained
Discussion
Acute Myeloid Leukaemia (AML) is considered to be an extremely aggressive hematological malignancy that is associated with the gathering of cytogenetic and molecular mutations within progenitor and or hematopoietic stem cells (HSPCs) [21]. This condition leads to the development of leukemic stem cells (LSCs) [21, 22]. It is reported that most AML patients go through a hard-line clinical course and 28% of them reported having an overall survival rate of 5 years. There are varied factors that influence the survival rate of individual patients such as associated genetic mutations, factors of individuals that comprises gender, age, exposure to radiation, and initial stage of chemotherapy [23-25]. It is also reported previously by researchers that signaling pathways of cytokines lead to tumor development of AML and its progression, and incongruence, resulting in chemo-resistance, and escaping the immune system. Therefore, we in this study tried to develop some targeted therapy towards cytokine signaling that might eradicate the LSC populace along with prevention of relapse of chemotherapy [23-25].
It has been observed the cytokine IFN γ (a pro-inflammatory cytokine) causes a reduction in AML cell production and survival and also increases unplanned clonogenicity of AML cells [26-28] Binder et al. 2018;. In the case of AML patients, the gene expression of the cytokine remains unchanged in peripheral blood (PB) levels and also reduces levels in the bone marrow (BM) [29, 27, 28]. In our study, we observed that there is a mild decrease in IFN γ, both in the N. caerulea extracts (50% and 70%) treated cells concerning control, however, the level got mildly enhanced after challenging the THP-1 cell line with 50% ethanol. When compared with the normal epithelial HEK 293 cell line, there was a reduction of the gene expression level of IFN γ among all five sets.
According to the reports of existing literature, another pro-inflammatory cytokine, IL-6 partially promotes AML cell proliferation and the level is also found to be elevated within the plasma of patients [30, 31]. Our study data revealed that there are hardly any elevations of the level of IL-6 in the 50% extract treated THP-1 cells whereas to the contrary among all the other sets the gene expression level of IL-6 is found to be profoundly high when compared with controls, especially in the 70% extract treated THP-1 cell line (120 fold). In the case of the HEK 293 cell line, there is an overall decrease of IL-6 expression in all the sets for control.
Chemo-attractant cytokine, IL-8 plays a significant role in the activation of chemoattracting neutrophils resulting in acute inflammation, therefore AML patients with a lower expression level of IL-8 were reported to have good survival outcomes [31, 27, 30]. Thus, this factor is also reported to be a prognostic biomarker for the progress of acute promyelocytic leukemia differentiation syndrome [27, 30, 32];Çelik et al. 2020. Within our study, there is a decrease in the gene expression level of IL-8 in the 50% treated THP-1 cell line, whereas the level got enhanced in the 70% extract-treated cell line (19 fold approx) concerning the control set. In all the HEK 293 cell sets, the gene expression level of IL-8 was enhanced for the control set. Therefore, 50% extract of N. caerulea is considered to have cancer-regulating properties.
Anti-inflammatory cytokine, IL-10 is reported to inhibit the enhancement of AML cells with the reduction of associated factors expressions such as Interleukins - IL-1α, IL-1β, IL-6, TNF-α and Granulocyte-macrophage colony-stimulating factor (GM-CSF) [33-35]. Moreover, it is also reported that the level of IL-10 is enhanced in the PB of AML patients when compared to normal healthy individuals [36]. Here also in our experimental data, it can be noticed that the gene expression level in the 50% extract treated cells is more (3.60 fold) when compared to the other sets concerning the control set. When the cell line, HEK 293 is considered, there is a very mild increase in the gene expression level of IL-10 compared to the control set.
Another pro-inflammatory cytokine IL-1β is reported to support the growth survival, and proliferation of AML cells in vitro and turn also enhances the expression level of IL-6, GM-CSF, and TNF factors [34, 36]. However, with AML patients the level is either unchanged or elevated in PB and remains unchanged in BM [34, 36]. The expression level of IL-1β is lower in 50% extract treated THP-1 cells (0.43 fold) and higher in 70% extract-treated cell line (17.03) when compared to normal cell control. In the HEK 293 cell line, there are no such marked changes concerning control.
TGF β, an anti-inflammatory cytokine inhibits the proliferation and survival of AML cells in vitro and the level is also reported to be lower in the BM and PB among AML patients [33, 34]. The expression level of TGF β1 is also lower in 50% extract treated THP-1 cells when compared to the other sets. There is also a slight increase in the gene expression in the 70% ethanol (vehicle control) set. In the HEK 293 cell line also there is an enhancement of gene expression of TGF β1 among the 50% extract-treated cells. However, these changes are very mild.
As previously reported TNF α, pro-inflammatory cytokine favors the development of chemoresistance of AML cells and proliferation of LSCs in vitro. The level is also found to be elevated in PB among AML patients [33, 34, 32]. In our data, there is a reduction of the gene expression in all the THP -1 cell treated sets except the one challenged with 70% alcohol. The same observations have been noticed among the HEK 293 cell line.
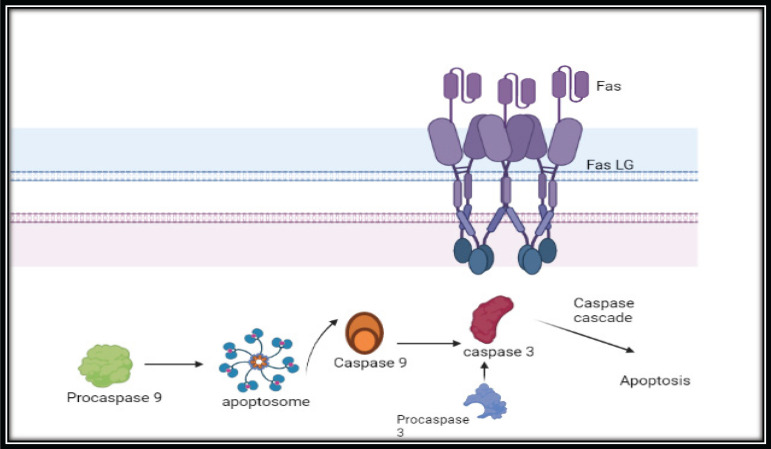
The process of apoptosis involves two core pathways namely extrinsic and intrinsic pathways [37, 38]. The extrinsic pathway involves the death receptors (DR) pathway and the intrinsic pathway is via the mitochondrial mediated pathway [38] (Figure 13). The extrinsic pathway involves the type 1 TNF receptor (TNFR1), and Fas (also called CD95/Apo-1) receptors [39, 40]. Moreover, experimental evidence has also confirmed the extensive significant role of caspases in apoptosis. Caspases are considered to be the initiator and the executioners in the pathway of apoptosis. Death effector domain (DED) comprises caspase-8 and 10 whereas caspase recruitment domains (CARD) comprise caspase-1, 2, 4, 5, 9, 11, and 12 [41, 42]. The triggering of caspase 9 leads to the activation of the caspase 3 signaling network which in turn causes the destruction of cells and concludes in apoptosis [41, 42]. In our experimental data there is an elevated gene expression level of caspase 9 (7.67 fold) in 50% extract treated THP-1 cells and followed by extensive expression of caspase 3 (173.59 fold) that may lead to apoptosis of THP-1 cells. There is also an elevation of the gene expression level of CD95 (Fas L) (83.20 fold) and TNFR1 (21.91 fold) in 50% extract treated THP-1 cells indicating the execution of extrinsic pathway.
Figure 13.
Mass Spectra of the 50% Ethanolic Extract of N. caerulea where X Axis Denotes mass/charge (m/z) and Y axis Denotes Relative Abundance (%).The figure stands for the MSn fragmentation of bioactive compositions of the 50% ethanolic extract of N. caerulea
Other researchers have reported about the presence of several phytochemicals namely alkaloids (+++), anthraquinones (+++), cardiac glycosides (+++), flavonoids (++), phenolics (++), saponins (+) and tannins (+) [5]. The extract of blue lotus depicted antibacterial activity against E .coli, P. aeruginosa, K. pneumonia, S. aureus and S. pyogenes (Akinjogunla, et al. 2009). Very few research article are available on the bioactive composition of N. caerulea and among them one of the researcher have reported about three novel macrocyclic flavonoids namely myricetin-3’ -O- (6” - p-coumaroyl) glucoside, pentagalloyl glucose and myricetin-3-O-rhamnoside via NMR study [43]. Similarly in the year, 1999 few authors reported about the confirmation of two important compounds namely delphinidin 3’- O- (2”-O- galloyl β galactopyranoside (1) and delphinidin 3’- O- (2”-O- galloyl – 6”- O acetyl β galactopyranoside (2) [44]. According to the West African cultural beliefs, the rhizome of the plant N. caerulea was consumed during the time of famine. Moreover, the seeds and rhizome was used for the treatment of diabetes. Infusions prepared from the root and stem of the plant fights with the disease like gonorrhea and urinary passage diseases [45]. The tea prepared with rhizome was used to treat kidney and bladder disorders, also treats insomnia, problems of the intestine and this tea infusion causes drowsiness. Moreover, the decoction prepared from the flower was used to treat painful urination, reduces libido and cough problems [45]. Previously some authors have reported about the phytochemical characterization of the flower and the reported compounds are - kaempferol aglycones, quercetin, and myricetin. It also comprises of compounds like helichrysin B, naringenin, gallic acid, isosalipurposide, p-coumaric acid, and methoxybenzoic acid [45, 46]. In our study, we have also reported the presence of similar bioactive constituents of the whole flower (petals, thalamus, reproductive parts of Nelumbo nucifera) by ultra-performance liquid chromatography followed by mass spectrometry such as homogentisic acid, gallocatechin, caffeoyl glucose/ esculin, paeoniflorin, hesperidin, and several others. Thus, the reported anti-cancer activities of the N. caerulea flower might be due to the synergistic activities of all such bioactive compounds (Figures 12 and 14).
Figure 14.
Extrinsic Apoptotic Pathway
In conclusion, N. caerulea flower extract appears to be a good anti-leukemia drug against AML, and the extract is capable of inducing apoptosis of these cells as well as the extract can also control the cytokine imbalance in leukemia by balancing pro-inflammatory and anti-inflammatory cytokine gene expressions.
Limitations of the study
The study was done with crude 50% ethanolic extract of flower N. caerulea. Though phytochemical profiling has been done of the crude extract, however further study is needed on animal model with pure marker compounds reported from this study to see the difference in the anti-cancer activity of the phyto-chemicals against the crude extract.
List of abbreviations
THP-1: human monocytic cell line; HEK 293: Human Embryonic Kidney Cell line; AML: Acute myeloid leukemia; HSPCs: Hematopoietic Stem Cells; LSCs: Leukemic Stem Cells; PB: Peripheral Blood; IFN γ: Interferon Gamma; IL-6: Interleukin – 6; IL-8: Interleukin – 8; IL-10: Interleukin – 10; IL-1β: Interleukin – 1β; TGF-β1: Transforming Growth Factor- β1; TGF-β3: Transforming Growth Factor- β3; TNF-α: Tumor Necrosis Factor Alpha; GM-CSF: Granulocyte-macrophage colony-stimulating factor ; DR: Death Receptors ; TNFR1: Type 1 Tumor Necrosis Factor receptor ; DED: Death Effector Domain ; CARD: Caspase Recruitment Domains ; RT-PCR: Real Time Polymerase Chain Reaction; DNA: Deoxy Ribonucleic Acid; MTT: 3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide; HPLC: high-performance liquid chromatography; LC-MS: Liquid Chromatography and Mass Spectrometry; Earle’s BSS: Earle’s Balanced Salt Solution; DMEM: Dulbecco’s Modified Eagle Medium; RPMI: Rosewell Park Memorial Institute; RNA: Ribonucleic Acid; cDNA: complementary DNA; FBS: Fetal Bovine Serum; PBS: Phosphate Buffer Saline.
Author Contribution Statement
Author DC performed the experimental study, initially analyzed the findings and written the draft manuscript; author BS assisted her during the entire experiment and data analysis; author KP provided all the necessary administrative support for the research investigation and author SD conceptualized the experimental study, finalized the data interpretation and checked the final version of the manuscript. All authors have gone through and accepted the final version of the manuscript.
Acknowledgements
The authors would like to acknowledge the Heritage Institute of Technology for providing the laboratory setup for conducting the experimental study. We would also like to acknowledge TCG Life Sciences, Pvt. Ltd. for carrying out the LC-MS analysis of the research samples. No funding is available for this study. The study was carried out on in-vitro cell line model, therefore no scientific body or Institutional Ethical Committee permission was needed.
Ethics Approval
However, the study was ratified by Institutional Ethical Committee.
Availability of Data
All the data files produced during the study are with corresponding author and can be produced if required in future.
Conflict of Interest
The authors declare none.
References
- 1.World health organization. Key facts on cancer, 3rd february. 2020. [Accessed on 12.12.2022]. https://www.Who.Int/news-room/fact-sheets/detail/cancer .
- 2.Marçais A, Suarez F, Sibon D, Frenzel L, Hermine O, Bazarbachi A. Therapeutic options for adult t-cell leukemia/lymphoma. Curr Oncol Rep. 2013;15(5):457–64. doi: 10.1007/s11912-013-0332-6. [DOI] [PubMed] [Google Scholar]
- 3.Fiore D, Cappelli LV, Broccoli A, Zinzani PL, Chan WC, Inghirami G. Peripheral t cell lymphomas: From the bench to the clinic. Nat Rev Cancer. 2020;20(6):323–42. doi: 10.1038/s41568-020-0247-0. [DOI] [PubMed] [Google Scholar]
- 4.N’Guessan BB, Asiamah AD, Arthur NK, Frimpong-Manso S, Amoateng P, Amponsah SK, et al. Ethanolic extract of nymphaea lotus l (nymphaeaceae) leaves exhibits in vitro antioxidant, in vivo anti-inflammatory and cytotoxic activities on jurkat and mcf-7 cancer cell lines. BMC Complement Med Ther. 2021;21(1):22 . doi: 10.1186/s12906-020-03195-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kameni PM, Dzeufiet DPD, Bilanda DC, Mballa MF, Mengue NYS, Tchoupou TH, et al. Nymphaea lotus linn (nymphaeaceae) alleviates sexual disability in l-name hypertensive male rats. Evid Based Complement Alternat Med. 2019;2019:8619283. doi: 10.1155/2019/8619283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agnihotri VK, Elsohly HN, Khan SI, Smillie TJ, Khan IA, Walker LA. Antioxidant constituents of nymphaea caerulea flowers. Phytochemistry. 2008;69(10):2061–6. doi: 10.1016/j.phytochem.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 7.Harer WB. Pharmacological and biological properties of the egyptian lotus. JARCE. 1985;22:49–54. [Google Scholar]
- 8.Elnaggar MG, Mosad E, Makboul A, Shafik EA. Cytogenetic profile of adult acute myeloid leukemia in egypt: A single-center experience. Mol Cytogenet. 2022;15(1):43 . doi: 10.1186/s13039-022-00621-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hussein S, Mohamed D, Hafez R. Risk factors of hematological malignancies in upper egypt: A case–control study. Egypt J Intern Med. 2019;31:171–7. [Google Scholar]
- 10.Khondkaryan L, Andreasyan D, Hakobyan Y, Bankoglu EE, Aroutiounian R, Stopper H, et al. Incidence and risk factors of acute leukemias in armenia: A population-based study. Asian Pac J Cancer Prev. 2022;23(11):3869–75. doi: 10.31557/APJCP.2022.23.11.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aitbekov R, Murzakhmetova M, Zhamanbayeva G, Zhaparkulova N, Seel H. Epidemiological features of acute myeloid leukemia in five regions of the republic of kazakhstan: Population study. Asian Pac J Cancer Prev. 2022;23(12):4163–7. doi: 10.31557/APJCP.2022.23.12.4163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paudel KR, Panth N. Phytochemical profile and biological activity of nelumbo nucifera. Evid Based Complement Alternat Med. 2015;2015:789124. doi: 10.1155/2015/789124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang X, Wang X, Wu T, Li B, Liu T, Wang R, et al. Isoliensinine induces apoptosis in triple-negative human breast cancer cells through ros generation and p38 mapk/jnk activation. Sci Rep. 2015;5:12579. doi: 10.1038/srep12579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu W, Yi DD, Guo JL, Xiang ZX, Deng LF, He L. Nuciferine, extracted from nelumbo nucifera gaertn, inhibits tumor-promoting effect of nicotine involving wnt/β-catenin signaling in non-small cell lung cancer. J Ethnopharmacol. 2015;165:83–93. doi: 10.1016/j.jep.2015.02.015. [DOI] [PubMed] [Google Scholar]
- 15.Shukla K, chaturvedi N. In vitro antioxidant properties of different parts of nelumbo nucifera gaertn. Int J Adv Pharm Biol Chem. 2016;5(2):196–201. [Google Scholar]
- 16.Nascimento CR, Rodrigues Fernandes NA, Gonzalez Maldonado LA, Rossa Junior C. Comparison of monocytic cell lines u937 and thp-1 as macrophage models for in vitro studies. Biochem Biophys Rep. 2022;32:101383. doi: 10.1016/j.bbrep.2022.101383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kannan N, Shanmuga Sundar S, Balaji S, Amuthan A, Anil Kumar NV, Balasubramanian N. Physiochemical characterization and cytotoxicity evaluation of mercury-based formulation for the development of anticancer therapeuticals. PLoS One. 2018;13(4):e0195800. doi: 10.1371/journal.pone.0195800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chatterjee D, Singh B, Pradhan AK, Paira K, Das S. Diluted Lycopodium Induced Cell Death and Clinical Improvement in Hepatocellular Carcinoma. Science Repository. 2022:5(3):2–8. [Google Scholar]
- 19.Zhu Z, Zhong B, Yang Z, Zhao W, Shi L, Aziz A, et al. Lc-esi-qtof-ms/ms characterization and estimation of the antioxidant potential of phenolic compounds from different parts of the lotus (nelumbo nucifera) seed and rhizome. ACS Omega. 2022;7(17):14630–42. doi: 10.1021/acsomega.1c07018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rajauria G, Foley B, Abu-Ghannam N. Identification and characterization of phenolic antioxidant compounds from brown irish seaweed himanthalia elongata using lc-dad–esi-ms/ms. Innov Food Sci Emerg Technol. 2016;37:261–8. [Google Scholar]
- 21.Döhner H, Weisdorf DJ, Bloomfield CD. Acute myeloid leukemia. N Engl J Med. 2015;373(12):1136–52. doi: 10.1056/NEJMra1406184. [DOI] [PubMed] [Google Scholar]
- 22.Papayannidis C, Sartor C, Marconi G, Fontana MC, Nanni J, Cristiano G, et al. Acute myeloid leukemia mutations: Therapeutic implications. Int J Mol Sci. 2019;20:11. doi: 10.3390/ijms20112721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krause DS, Scadden DT. A hostel for the hostile: The bone marrow niche in hematologic neoplasms. Haematologica. 2015;100(11):1376–87. doi: 10.3324/haematol.2014.113852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ganesan S, Mathews V, Vyas N. Microenvironment and drug resistance in acute myeloid leukemia: Do we know enough? Int J Cancer. 2022;150(9):1401–11. doi: 10.1002/ijc.33908. [DOI] [PubMed] [Google Scholar]
- 25.Zeisig BB, Fung TK, Zarowiecki M, Tsai CT, Luo H, Stanojevic B, et al. Functional reconstruction of human aml reveals stem cell origin and vulnerability of treatment-resistant mll-rearranged leukemia. Sci Transl Med. 2021;13:582. doi: 10.1126/scitranslmed.abc4822. [DOI] [PubMed] [Google Scholar]
- 26.Gu J, Huang X, Zhang Y, Bao C, Zhou Z, Jin J. Cytokine profiles in patients with newly diagnosed multiple myeloma: Survival is associated with il-6 and il-17a levels. Cytokine. 2021;138:155358. doi: 10.1016/j.cyto.2020.155358. [DOI] [PubMed] [Google Scholar]
- 27.Corradi G, Bassani B, Simonetti G, Sangaletti S, Vadakekolathu J, Fontana MC, et al. Release of ifnγ by acute myeloid leukemia cells remodels bone marrow immune microenvironment by inducing regulatory t cells. Clin Cancer Res. 2022;28(14):3141–55. doi: 10.1158/1078-0432.CCR-21-3594. [DOI] [PubMed] [Google Scholar]
- 28.Ding H, Wang G, Yu Z, Sun H, Wang L. Role of interferon-gamma (ifn-γ) and ifn-γ receptor 1/2 (ifnγr1/2) in regulation of immunity, infection, and cancer development: Ifn-γ-dependent or independent pathway. Biomed Pharmacother. 2022;155:113683. doi: 10.1016/j.biopha.2022.113683. [DOI] [PubMed] [Google Scholar]
- 29.Konopleva M, Cardama AQ, Kantarjian H, Cortes J. Molecular biology and cytogenetics of chronic myeloid leukemia. Neoplastic Diseases of the Blood. 2018:29–47. [Google Scholar]
- 30.Hassanshahi M, Hassanshahi A, Khabbazi S, Su YW, Xian CJ. Bone marrow sinusoidal endothelium: Damage and potential regeneration following cancer radiotherapy or chemotherapy. Angiogenesis. 2017;20(4):427–42. doi: 10.1007/s10456-017-9577-2. [DOI] [PubMed] [Google Scholar]
- 31.Konopleva M, Cardama A, Kantarjian H, Cortes J. Molecular biology and cytogenetics of chronic myeloid leukemia. Neoplastic Diseases of the Blood. 2018:29–47. [Google Scholar]
- 32.Islam M, Mohamed EH, Esa E, Kamaluddin NR, Zain SM, Yusoff YM, et al. Circulating cytokines and small molecules follow distinct expression patterns in acute myeloid leukaemia. Br J Cancer. 2017;117(10):1551–6. doi: 10.1038/bjc.2017.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Savage SA, Walsh MF. Myelodysplastic syndrome, acute myeloid leukemia, and cancer surveillance in fanconi anemia. Hematol Oncol Clin North Am. 2018;32(4):657–68. doi: 10.1016/j.hoc.2018.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karimdadi Sariani O, Eghbalpour S, Kazemi E, Rafiei Buzhani K, Zaker F. Pathogenic and therapeutic roles of cytokines in acute myeloid leukemia. Cytokine. 2021;142:155508. doi: 10.1016/j.cyto.2021.155508. [DOI] [PubMed] [Google Scholar]
- 35.Wu H, Li P, Shao N, Ma J, Ji M, Sun X, et al. Aberrant expression of treg-associated cytokine il-35 along with il-10 and tgf-β in acute myeloid leukemia. Oncol Lett. 2012;3(5):1119–23. doi: 10.3892/ol.2012.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grauers Wiktorin H, Aydin E, Christenson K, Issdisai N, Thorén FB, Hellstrand K, et al. Impact of il-1β and the il-1r antagonist on relapse risk and survival in aml patients undergoing immunotherapy for remission maintenance. Oncoimmunology. 2021;10(1):1944538. doi: 10.1080/2162402X.2021.1944538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kessler CS, Eisenmann C, Oberzaucher F, Forster M, Steckhan N, Meier L, et al. Ayurvedic versus conventional dietary and lifestyle counseling for mothers with burnout-syndrome: A randomized controlled pilot study including a qualitative evaluation. Complement Ther Med. 2017;34:57–65. doi: 10.1016/j.ctim.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 38.Elmore S. Apoptosis: A review of programmed cell death. Toxicol Pathol. 2007;35(4):495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jan R, Chaudhry GE. Understanding apoptosis and apoptotic pathways targeted cancer therapeutics. Adv Pharm Bull. 2019;9(2):205–18. doi: 10.15171/apb.2019.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jin Z, El-Deiry WS. Overview of cell death signaling pathways. Cancer Biol Ther. 2005;4(2):139–63. doi: 10.4161/cbt.4.2.1508. [DOI] [PubMed] [Google Scholar]
- 41.Guicciardi ME, Gores GJ. Life and death by death receptors. Faseb J. 2009;23(6):1625–37. doi: 10.1096/fj.08-111005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407(6805):770–6. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- 43.Elegami AA, Bates C, Gray AI, Mackay SP, Skellern GG, Waigh RD. Two very unusual macrocyclic flavonoids from the water lily nymphaea lotus. Phytochemistry. 2003;63(6):727–31. doi: 10.1016/s0031-9422(03)00238-3. [DOI] [PubMed] [Google Scholar]
- 44.Fossen T, Andersen ØM. Delphinidin 3′-galloylgalactosides from blue flowers of nymphaéa caerulea. Phytochemistry. 1999;50(7):1185–8. [Google Scholar]
- 45.Oosthuizen CB, Fisher M, Lall N. Underexplored Medicinal Plants from Sub-Saharan Africa: Academic Press; 2020. Nymphaea caerulea; pp. 205–10. [Google Scholar]
- 46.Zhu M, Zheng X, Shu Q, Li H, Zhong P, Zhang H, et al. Relationship between the composition of flavonoids and flower colors variation in tropical water lily (nymphaea) cultivars. PLoS One. 2012;7(4):e34335. doi: 10.1371/journal.pone.0034335. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data files produced during the study are with corresponding author and can be produced if required in future.