Abstract
Pregnancy places a unique stress upon choline metabolism, requiring adaptations to support both maternal and fetal requirements. The impact of pregnancy and prenatal choline supplementation on choline and its metabolome in free-living, healthy adults is relatively uncharacterized. This study investigated the effect of prenatal choline supplementation on maternal and fetal biomarkers of choline metabolism among free-living pregnant persons consuming self-selected diets. Participants were randomized to supplemental choline (as choline chloride) intakes of 550 mg/d (500 mg/d d0-choline + 50 mg/d methyl-d9-choline; intervention) or 25 mg/d d9-choline (control) from gestational week (GW) 12–16 until Delivery. Fasting blood and 24-h urine samples were obtained at study Visit 1 (GW 12–16), Visit 2 (GW 20–24), and Visit 3 (GW 28–32). At Delivery, maternal and cord blood and placental tissue samples were collected. Participants randomized to 550 (vs. 25) mg supplemental choline/d achieved higher (p < .05) plasma concentrations of free choline, betaine, dimethylglycine, phosphatidylcholine (PC), and sphingomyelin at one or more study timepoint. Betaine was most responsive to prenatal choline supplementation with increases (p ≤ .001) in maternal plasma observed at Visit 2-Delivery (relative to Visit 1 and control), as well as in the placenta and cord plasma. Notably, greater plasma enrichments of d3-PC and LDL-C were observed in the intervention (vs. control) group, indicating enhanced PC synthesis through the de novo phosphatidylethanolamine N-methyltransferase pathway and lipid export. Overall, these data show that prenatal choline supplementation profoundly alters the choline metabolome, supporting pregnancy-related metabolic adaptations and revealing biomarkers for use in nutritional assessment and monitoring during pregnancy.
Keywords: choline metabolome, PEMT pathway, phosphatidylcholine, pregnancy, prenatal choline supplementation
1 |. INTRODUCTION
Pregnancy places a unique stress upon choline metabolism, requiring adaptations to support both maternal and fetal requirements.1–3 Derivatives of choline metabolism (i.e., phospholipids, betaine and acetylcholine) play a critical role in fetal development, including membrane biosynthesis, tissue expansion, genomic and cellular methylation, and neurotransmission.4,5 The liver is capable of synthesizing a choline moiety de novo via the activity of the phosphatidylethanolamine N-methyltransferase (PEMT) enzyme, catalyzing the triple methylation of phosphatidylethanolamine to phosphatidylcholine (PC). The promoter region of the PEMT gene contains an estrogen response element, and thus, the rising estrogen concentration across pregnancy results in increased PEMT activity and de novo choline production. However, a growing body of data support the notion that such maternal adaptations are inadequate to meet the high choline demands of pregnancy,2,3 and that choline intakes exceeding current dietary recommendations are required to support both maternal metabolic needs and fetal neurodevelopment.2,3,6
In rodent models, consumption of choline at intake levels required to prevent deficiency in non-pregnant dams results in a pronounced depletion of maternal hepatic choline levels, similar to those observed in non-pregnant dams consuming choline deficient diets.7,8 Such depletion during rodent pregnancy facilitates maintenance of the plasma choline supply to support biomagnification in the fetal compartment, a process actively facilitated by the placenta to meet the high demands of the developing fetus.9–12 This decline in maternal hepatic choline stores likely represents a functional inadequacy requiring increased dietary intakes; indeed, a large body of literature demonstrates that supplementing the diet of dams with choline results in increased hepatic choline metabolites,13 as well as plasma choline, phosphatidylcholine and betaine,14 and significantly enhances fetal neurodevelopment, resulting in improved offspring cognition.15
Less is known about choline metabolism and requirements during human pregnancy. A limited body of data from prospective studies indicate that plasma choline typically rises across pregnancy16–18 with biomagnification in the fetal compartment.9,16,19 In the only controlled feeding choline intervention study,2 third trimester pregnant participants randomized to consume either 480 or 930 mg choline/day exhibited a significant rise in choline over time at both intake levels, with plasma choline levels being greater relative to non-pregnant participants consuming equivalent choline intakes. Choline supplementation at the 930 versus 480 mg/d level yielded higher plasma choline and betaine during the third trimester of pregnancy2 and restored the partitioning of choline between betaine synthesis and PC synthesis via the CDP-choline pathway to that observed in non-pregnant state.3 Moreover, similar to rodent models, infants born to participants randomized to the 930 mg/d arm of this trial demonstrated improvements in cognitive function across the first year of life, relative to the 480 mg/d arm.6 These data suggest that, in humans as in rodents, prenatal choline supplementation of a nutritionally “adequate” diet with choline intakes >100 mg/d above habitual intakes in North America20–22 enhances choline supply to the fetus and improves offspring cognition. However, the impact of pregnancy and prenatal choline supplementation on longitudinal measures of the choline metabolome prior to the third trimester, and at choline intake levels more closely aligned with habitual intakes, remains relatively uncharacterized.
To address this gap, we undertook an analysis of a randomized controlled trial of choline supplementation throughout the second and third trimesters of pregnancy to longitudinally assess choline and its related metabolome in maternal and newborn biological samples. The design of this study informs upon the plasma choline metabolome and its response to prenatal supplementation in participants consuming background diets that align more closely with typical intakes of pregnant persons in North America. In addition to providing mechanistic insights, the results of this study may be useful in determining appropriate pregnancy-specific indicators of requirement for establishing Dietary Reference Intakes in pregnant persons.
2 |. MATERIALS AND METHODS
2.1 |. Participants
Healthy pregnant persons in their second trimester (gestational week [GW] 12–16) were recruited from Ithaca, NY and surrounding area between October 2017 and April 2019. During the screening phase, interested individuals completed a demographic questionnaire, a health history questionnaire, and a short version of the NIH Diet History Questionnaire (DHQ-III; https://www.nal.usda.gov/fnic/dietary-assessment-instruments-research) to estimate usual choline intakes. Because docosahexaenoic acid (DHA) status response to prenatal choline was also a pre-specified study outcome (to be published separately), a validated DHA/EPA food frequency screening questionnaire (DSM Nutritionals Inc) was used to assess omega-3 fatty acid intake. Interested individuals were instructed to complete these based on habitual pre-pregnancy use (excluding recent efforts to increase fish intake and/or utilize omega-3 fatty acid supplements).
Inclusion criteria included age 21 to 40 years, 12- to 16-week singleton pregnancy, pre-pregnancy BMI <32 kg/m2, intention to delivery at Cayuga Medical Center, and willingness to comply with the study protocol. Pregnant persons were excluded if they reported: current alcohol, recreational drug or tobacco usage; total (dietary + supplemental) estimated pre-pregnancy intakes exceeding 400 mg choline/d or 400 mg DHA/d; use of medications known to affect liver function; or medical conditions (e.g., diabetes, hypertension, cancer, anemia and gastrointestinal disorders) or pregnancy complications (e.g., gestational diabetes, preeclampsia). Additionally, when these pregnancy complications arose during the study period, the participants were withdrawn.
Participants gave written informed consent prior to participating in the study. The study protocol was approved by the Institutional Review Board for Human Study Participants at Cornell University (Protocol ID: 1702006936) and Cayuga Medical Center at Ithaca Institutional Review Board (Protocol number: 0320MC). This trial was registered at clinicaltrials.gov as NCT03194659. The results shown in this manuscript correspond to pre-registered secondary outcomes; the primary outcomes will be reported in a separate manuscript.
2.2 |. Study design and supplements
The study was a single-center, randomized, double-blind, parallel-group choline intervention study. Eligible pregnant persons between 12- to 16-week gestation (n = 33) were randomized to the choline intervention group (550 mg choline/d; n = 17) or control group (25 mg choline/d; n = 16) from enrollment (~beginning of second trimester) to Delivery (a duration of ~24 weeks). Three participants were withdrawn due to development of gestational diabetes (n = 2 in the intervention group; n = 1 in the control group), yielding a final sample size of 30 participants with n = 15 in each group.
Participants in the choline intervention group consumed a grape juice cocktail containing 500 mg of unlabeled d0-choline plus 50 mg of deuterium-labeled methyl-d9-choline (d9-choline) as choline chloride daily. The control group consumed a grape juice cocktail containing 25 mg of d9-choline only. The amounts of the d9-labeled choline (50 and 25 mg/d for the intervention and control groups, respectively) were chosen to account for label dilution, providing approximately the same ratio of orally consumed labeled to unlabeled choline based on the assumption that the intervention (vs. control) group would be consuming about two times more unlabeled total choline throughout the study duration. Both groups also consumed a daily 200-mg DHA supplement (DSM Nutritional Products) and a daily, choline-free prenatal vitamin and mineral supplement (Nature Made).
Choline supplements were prepared in the Human Metabolic Research Unit at Cornell University. First, d0-choline and d9-choline stock solutions were prepared separately by dissolving choline chloride (Balchem Inc.) or d9-choline chloride (Cambridge Isotope Laboratories Inc.) in NanoPure water to produce a 250 mg/ml d0-choline solution and 50 mg/ml d9-choline solution. The stock solutions were filter sterilized using 0.22 μm cellulose acetate filters (Corning Inc.), and kept at 4°C for up to 12 months; tests conducted during this time revealed negligible loss of choline (<1%). Next, choline supplements were assembled by aliquoting 2 ml of the choline stock solution and 1 ml of the d9-choline stock solution (for the choline intervention group) or 0.5 ml of the d9-choline stock solution (for the control group) into 15-ml sterilized polystyrene tubes, and filling with grape juice (Welch Foods Inc. Concord, Massachusetts). The supplement tubes were labeled such that they were indistinguishable by the personnel who interacted with the participants. Finally, all supplement tubes were packaged in Ziplock bags labeled with the participant ID numbers, kept frozen in a food-only freezer and thawed in a food-only refrigerator prior to pick-ups. Testing of the supplements that were kept for two months at room temperatures or inside a home refrigerator showed negligible loss of choline over time.
Participants were scheduled to visit the Human Metabolic Research Unit three times during the trial: GW 12–16 (Visit 1), GW 20–24 (Visit 2) and GW 28–32 (Visit 3). Prior to each visit, participants fasted 8–10 h overnight and collected 24-h urine samples using the materials provided by research personnel. At each visit, follow-up questionnaires were administered to assess changes in health status and medication/supplement use. Weight and height were measured and fasting venous blood samples were collected. At each visit, participants also received their choline supplement tubes as well as returned both empty and unconsumed tubes (Visits 2 and 3) for assessment of compliance to the study protocol, which was close to 100%. Additionally, participants completed at least two self-administered 24-hr dietary recalls (Automated Self-Administered Recall System, ASA24): a single 24-hr dietary recall completed at Visit 1 and 2–4 recalls throughout the course of the 2nd and 3rd trimester, prior to Delivery.
At Delivery, maternal (non-fasting) and cord blood samples as well as placental samples were collected at Cayuga Medical Center, Ithaca, NY. With permission from the participants, information on gestational age, delivery mode, pregnancy and delivery complications, as well as infant birth date, ethnicity, weight, length, head circumference and Apgar scores were obtained from medical charts.
2.3 |. Sample collection and processing
Fasting maternal blood samples were collected at Visits 1–3 into EDTA tubes (Becton, Dickinson and Company), kept on ice and processed within 90 min of collection. Whole blood was aliquoted from the EDTA tubes, and the rest was centrifuged at 1055× g for 15 min at 4°C to obtain plasma, which was distributed into 1.8-ml cryostat vials (CryoTube; NUNC) for analysis of choline metabolites. All samples were stored at −80°C.
Participants collected 24-h urine samples at home for all three visits in wide-mouth polyethylene bottles (1 L; Nalgene). They were instructed to keep the urine cold and to deliver the bottles in the provided insulated thermal bag with ice packs. Upon receipt, study personnel recorded the total volume, and urine was distributed and stored at −80°C.
At the hospital, non-fasting maternal blood samples and cord blood samples were collected into EDTA tubes at the time of delivery. All blood samples were placed on ice immediately after collection, and processed for plasma within 90 min. Placenta was also obtained and processed at the hospital within 90 min of delivery. Briefly, after the amniotic sac was removed, the placenta was laid flat on a tray and visually divided into four quadrants. A full-thickness placental biopsy without maternal and fetal membranes was taken from the center of each quadrant. The biopsies were further divided into 1 cm × 1 cm × placental depth slices, snap-frozen in liquid nitrogen and stored at −80°C.
2.4 |. Analytical measurements
2.4.1 |. Water-soluble choline metabolome
Choline, betaine, dimethylglycine, acetylcholine, methionine and trimethylamine N-oxide (TMAO)
Unlabeled and deuterium-labeled forms of free choline, betaine, dimethylglycine, acetylcholine, methionine and TMAO in maternal and cord plasma, urine, placenta and choline supplements (only choline) were analyzed using stable-isotope dilution liquid chromatography tandem mass spectrometry (LC-MS/MS) as previously described23 with modifications to include measurements of acetylcholine (in placenta), methionine and TMAO (in plasma, urine, and placenta). Briefly, 100 μl of 0.1% formic acid in acetonitrile and 5 μl of an internal standard mix [d13-choline (CDN Isotopes), d3-betaine (CDN Isotopes), d3-dimethylglycine (CDN Isotopes), d13-acetylcholine (CDN Isotopes), d3-methionine (Santa Cruz Biotechnology; d3-methionine was utilized following confirmation that no endogenous label was detectable at study visits following intake of d9-choline), and 13C3-TMAO (Toronto Research Chemicals)] were added to 50 μl of plasma and urine or to 20–30 mg of placenta powderized in liquid nitrogen. Samples were homogenized (placenta), vortexed and centrifuged, and clear supernatant was removed and transferred into glass vials for subsequent LC-MS/MS analysis.
LC-MS/MS was carried out using a TSQ Quantum Ultra (ThermoElectron Corp) equipped with a Accela autosampler and pump (ThermoElectron Corp). Samples (5 μl) were injected onto a Syncronis Silica column (150 × 2.1 mm, 5 μm particle size) with matching guard column (ThermoFisher Scientific). Metabolites were separated under isocratic conditions using 19% 15 mM ammonium formate with 0.1% formic acid in water and 81% acetonitrile and a flow rate of 0.4 ml/min. Column and autosampler temperatures were at 25 and 10°C, respectively. Calibration curves were generated by serial dilutions of unlabeled metabolites in water mixed with internal standards. Positive ion SRM transitions were: m/z 104 to 60 for choline; m/z 117 to 69 for d13-choline; m/z 107 to 63 for d3-choline; m/z 110 to 66 for d6-choline; m/z 113 to 69 for d9-choline; m/z 118 to 58 for betaine; m/z 121 to 61 for d3-betaine; m/z 124 to 64 for d6-betaine; m/z 127 to 67 for d9-betaine; m/z 104 to 58 for dimethylglycine; m/z 107 to 61 for d3-dimethylglycine; m/z 110 to 64 for d6-dimethylglycine; m/z 146 to 87 for acetylcholine; m/z 159 to 91 for d13-acetylcholine; m/z 149 to 87 for d3-acetylcholine; m/z 152 to 87 for d6-acetylcholine; m/z 155 to 87 for d9-acetylcholine; m/z 153 to 133 for methionine; m/z 150 to 136 for d3-methionine; m/z 76 to 58 for TMAO; m/z 79 to 61 for 13C3-TMAO; m/z 79 to 61 for d3-TMAO; m/z 82 to 64 for d6-TMAO; and m/z 85 to 67 for d9-TMAO.
The data were acquired using the XCalibur (Thermo) software. All area ratios of the choline metabolites (Peak area of analyte/Peak area of IS) fell within the linear dynamic range of calibration standards. The samples were run with and without internal standards for determination of the enrichments of deuterium-labeled compounds. Total concentrations of each metabolite were back calculated using the enrichment percent. Intra- and inter-coefficients of variation of the controls ranged from 1.0% to 8.0% for plasma, urine and the choline supplement, and from 2.0% to 14.0% for placenta (intra-CV % only).
2.4.2 |. Lipid-soluble choline metabolome
PC, lysoPC, sphingomyelin
Unlabeled and deuterium-labeled forms of PC, lysoPC, and sphingomyelin in maternal and cord plasma and placenta were analyzed using stable-isotope dilution LC-MS/MS as previously described24 with modifications based on our equipment. Briefly, 400 μl of a methanol:chloroform mix (2:1; v/v) and d4-PC 16:0 (Avanti Polar Lipids; internal standard for PC, lysoPC and sphingomeylin) were added to 100 μl of plasma or 20–30 mg of placenta powderized in liquid nitrogen. After homogenization (for tissue), mixing, and incubation overnight at −20°C, samples were centrifuged, and the supernatant was collected and transferred into a new Eppendorf tube without disturbing the solid phase. To the pellet, 0.25 ml of methanol:chloroform:water (2:1:0.8; v/v) was added, followed by vortexing and centrifugation. The resulting supernatant was collected and combined with the first supernatant. To the pooled supernatant, chloroform (100 μl) and nanopure water (100 μl) were added; samples were mixed and then centrifuged to separate the extract into two layers. The lower chloroform layer containing the lipid-soluble choline metabolites was dried in a DNA 110 Speed Vac centrifuge (Thermo Savant).
The samples were reconstituted with 500 μl hexane:chloroform:methanol (95:3:2 v/v), vortexed, sonicated and placed on Bond Elut-NH2 columns (Agilent), preconditioned with hexane. Columns were washed with chloroform, then with diethyl ether with 2% of acetic acid, and eluted with 2 ml 25 mM sodium acetate in methanol:chloroform (6:1 v/v). The eluent was transferred into glass vials and 5 μl was injected into the TSQ Quantum Ultra equipped with an adsorbosphere silica column (150 × 2.1 mm, 5 μm particle size) with matching guard (Grace Vydac, Columbia MD). Phospholipids were separated under gradient conditions with a flow rate of 400 μl/min and a mobile phase consisting of buffer A (800 ml acetonitrile: 127 ml water: 68 ml ethanol: 3 ml 1M ammonium acetate in water: 2 ml concentrated glacial acetic acid) and buffer B (500 ml acetonitrile: 500 ml water: 85 ml ethanol: 27 ml 1M ammonium acetate in water: 18 ml concentrated glacial acetic acid). The following gradient was employed: 5% buffer B from 0 to 3 min; 30% buffer B from 3 to 10 min; 60% buffer B from 10 to 14 min; 100% buffer B from 16 to 17 min; 5% buffer B from 17 to 19 min. Calibration curves were prepared by serial dilutions of the standards, mixed with the internal standard. SIM positive ions were used for detection of phospholipids: m/z 184 for PC, sphingomyelin, and lysoPC; m/z 188 for d4-PC; m/z 187 for d3-PC, d3-sphingomyelin and d3-lysoPC, m/z 190 for d6-PC, d6-sphingomyelin, and d6-lysoPC; m/z 193 for d9-PC, d9-sphingomyelin, and d9-lysoPC.
The data were acquired using XCalibur (Thermo) software. All area ratios of the choline metabolites (Peak area of analyte/Peak area of IS) in the samples fell within the linear dynamic range of calibration standards. The samples were run with and without internal standards for determination of the enrichments of deuterium-labeled compounds. Total concentrations of each metabolite were back calculated using the enrichment percent. The coefficient of variation of the controls ranged from 4.0% to 9.0% for PC, lysoPC, and sphingomyelin in placenta (intra-CV% only); from 3.0% to 9.0% for PC and sphingomyelin in plasma (intra and inter-CV%) and ~12% for lysoPC in plasma (intra- and inter-CV%).
2.4.3 |. Isotopic enrichment calculations
Administration of d9-choline allows tracing of its methyl groups (generated d3- and d6-deuterium labeled choline metabolites) and the intact choline molecule (generated d9-deuterium labeled choline metabolites) as diagramed previously.3,25,26 The enrichment percentage of isotopically labeled choline metabolites was calculated as follows:
The enrichment percentages and enrichment ratios can provide important insights into the overall fate of orally consumed d9-choline including its flow from a precursor to product pool (e.g., d6-DMG:d9-betaine) and its partitioning between the PEMT pathway and CDP-choline pathway (d3-PC:d9-PC). Importantly enrichments of d6-PC produced by the PEMT pathway were not detected, indicating that all (or nearly all) of the d9-PC was generated by the CDP-choline pathway.
2.4.4 |. Serum lipids and urinary creatinine
Because choline (as PC) plays a critical role in the export of lipids from liver into circulation, serum total cholesterol, LDL-C, HDL-C, and triglycerides were measured at Visit 1, Visit 3 and Delivery by using the Dimension Xpand chemistry analyzer (Siemens Healthcare Diagnostic). This instrument was also used to measure urinary creatinine at Visits 1–3 so that urinary metabolites could be expressed per gram creatinine.
2.5 |. Statistical analysis
Data was inspected using histograms, Q-Q plots and descriptive statistics to examine distribution, normality and outliers. Extreme values were rechecked for errors. Baseline characteristics and biochemical profiles were compared between groups using unequal variance T-test for continuous variables and Fisher’s exact test for categorical variables. Repeated measures mixed models with random intercepts and slopes and heterogenous autoregressive covariance structures were constructed to test the effect of choline supplementation on each metabolite concentration, enrichment, and enrichment ratio. Participant ID was entered as a random effect, while the intervention and study visit (Visit 1, Visit 2, Visit 3 and Delivery, modeled as a categorical variable) were entered as fixed effects. A time × intervention interaction term was included to compare the effect of the intervention at each study visit. The Tukey-Kramer post-hoc test was used to correct for multiple-hypothesis testing within each regression model. Further adjustments across separate models were not implemented because the metabolites of interest lie within the same pathway. Model residuals were inspected for evidence of heteroscedasticity and deviation from normality, and the variables were log-transformed if needed. To aid in result interpretation, model estimates obtained using the transformed data were back transformed into their original scales using the modified Cox’s method.27 Additionally, the regression models for plasma variables were further adjusted for maternal age (y) and pre-pregnancy body mass index (BMI) as covariates in a sensitivity analysis.
Total amount of excreted creatinine and choline metabolites in 24-h urine samples was calculated by multiplying urine concentrations of the metabolites by 24-h urine volume. Then, the values were divided by excreted creatinine (g) to adjust for filtration rate. Creatinine-adjusted values were used in all analysis. The effect of choline supplementation on urinary metabolites were analyzed using repeated measures mixed models in the same manner as that of plasma. The effect of the intervention on placental and cord plasma metabolites were analyzed using unequal variance T-test. All analysis was conducted using SAS software (ver 9.4). Data are presented as either mean ± SD or mean [95% CI]. p < .05 was considered statistically significant.
3 |. RESULTS
3.1 |. Demographics and self-reported nutrient intakes
Thirty participants (N = 15, control; N = 15, intervention) completed this randomized controlled trial. Demographic and self-reported nutrient intakes are reported in Table 1. Participants in the choline intervention and control groups were similar amongst most reported variables, including self-reported race (predominantly Caucasian) and education level, and self-reported baseline intakes of dietary choline and related one-carbon nutrients. Participants in the intervention group tended to be older (p = .09) and exhibited a lower (p = .03) pre-pregnancy BMI. Participants completed additional dietary recalls throughout the study; median dietary choline and related nutrient intakes from follow-up dietary recalls (N = 102) were similar (p > .29) between the choline intervention and control groups. No significant differences were observed in the baseline concentration of blood lipids (carriers for the lipid soluble forms of choline); however, the choline intervention resulted in a higher LDL-C at Visit 3 (p = .15, unadjusted; p = .036, adjusted for Visit 1), a product of hepatic VLDL export that is dependent upon an adequate supply of PC (Table S1).
TABLE 1.
Participant demographics and self-reported nutrient intakes1
| Variables | Control (n = 15) | Intervention (n = 15) | p-Value | |||
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Age (y) | 30.5 ± 4.1 | 32.9 ± 3.4 | .09 | |||
| Race (n Caucasian) | 15 | 13 | .48 | |||
| Education level (n) | .71 | |||||
| High school | 7 | 5 | ||||
| Bachelor’s degree or higher | 8 | 10 | ||||
| Pre-pregnancy weight (lb) | 150 ± 27 | 136 ± 17 | .11 | |||
| Pre-pregnancy BMI (kg/m2) | 25 ± 4 | 22 ± 2 | .03 | |||
| Self-reported dietary intakes 2 | ||||||
| Visit 1 | Follow-up | Visit 1 | Follow-up | Visit 1 | Follow-up | |
| Choline (mg/d) | 364 ± 117 | 377 ± 112 | 320 ± 185 | 353 ± 66 | .54 | .08 |
| Folate (DFE; μg/d) | 577 ± 329 | 686 ± 223 | 626 ± 229 | 580 ± 190 | .29 | .20 |
| Vitamin B12 (μg/d) | 4.4 ± 3.0 | 4.5 ± 2.4 | 4.1 ± 2.1 | 4.7 ± 2.3 | .30 | .86 |
Values are mean ± SD.
Values are median ± SD. Between-group difference was determined by using unequal variance T-test for continuous variables and Fisher’s exact test for categorical variables. p < .05 is considered significant.
3.2 |. Choline metabolome
We assessed the impact of choline supplementation on the choline metabolome broadly in maternal plasma and urine, the placenta, and newborn cord plasma. Comprehensive assessment across maternal, placental and fetal compartments facilitates inferences about the availability of individual choline metabolites within each compartment and their responsiveness to choline supplementation. These measures inform upon potential biomarkers that may be utilized to assess choline status and adherence to supplementation, as well as potentially relevant metabolites facilitating choline’s known impact on maternal and fetal health outcomes, such as improved fetal neurodevelopment and infant cognition. Such findings may also serve as rationale to drive future research investigating the acute and long-term postnatal health impacts of the choline supplemented metabolic milieu.
3.3 |. Plasma choline metabolome response to the choline intervention across pregnancy
Randomization to the choline intervention group, relative to the control group, (Figure 1) as well as progression through pregnancy (Table 2) resulted in significant alterations in the choline metabolome. Results adjusted for additional covariates (maternal age, pre-pregnancy BMI) are shown in Table S2.
FIGURE 1.
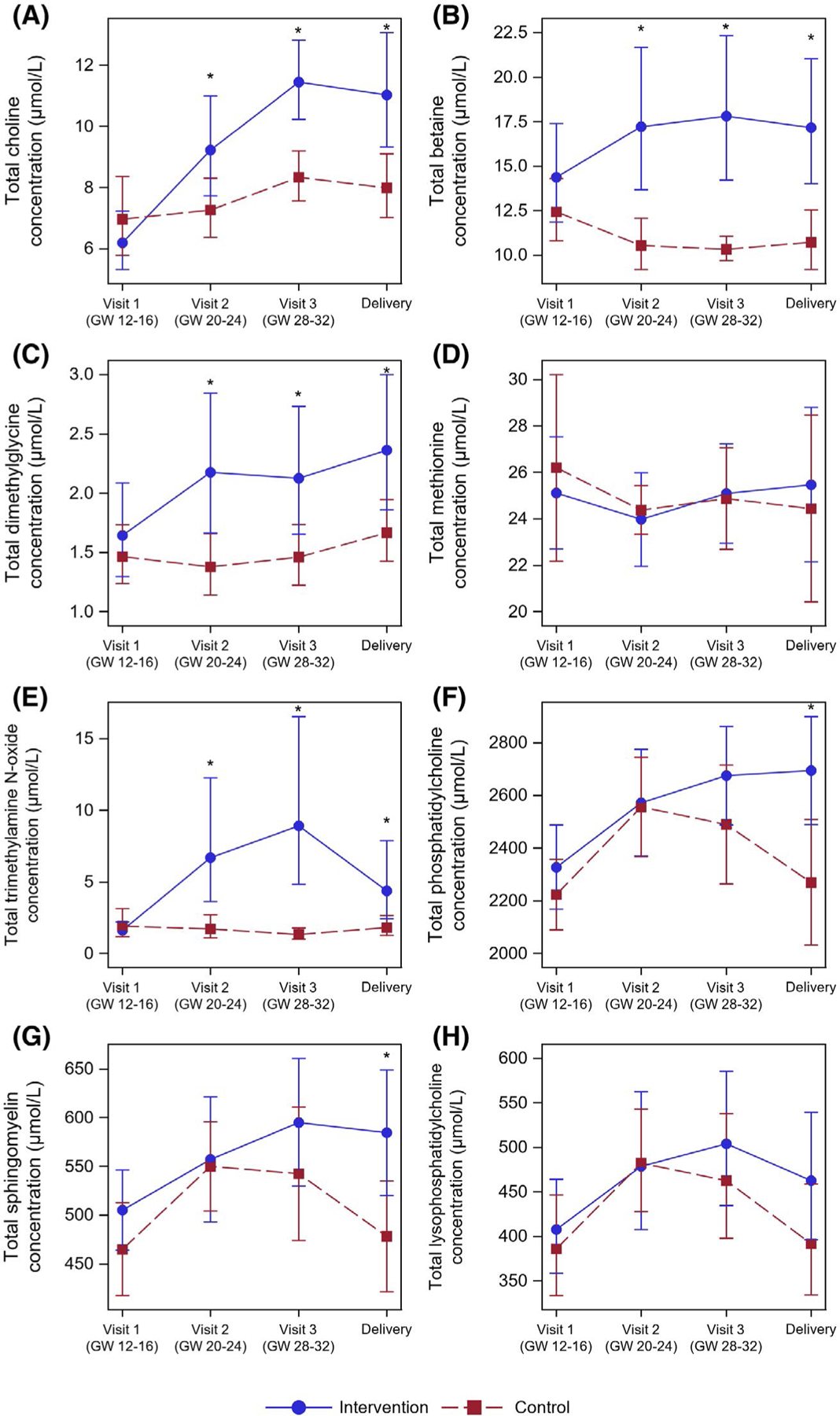
The Impact of Intervention on the Water- and Lipid-Soluble Choline Metabolome Across Pregnancy. Pregnant persons were randomized to 550 versus 25 mg of choline supplementation throughout the 2nd and 3rd trimester of pregnancy. Choline, betaine, dimethylglycine, methionine, TMAO, phosphatidylcholine, sphingomyelin and lysophosphatidylcholine were measured via liquid chromatography tandem mass spectrometry in fasting plasma samples taken at Visit 1 (GW 12–16), Visit 2 (GW 20–24), and Visit 3 (GW 28–32), and in Delivery blood samples obtained at parturition. Metabolites were analyzed using mixed linear models to assess the impact of intervention; statistically significant differences between intervention groups at each time point are indicated with a *(p < .05; following Tukey’s Post-hoc Adjustment)
TABLE 2.
Plasma total choline metabolite concentrations for the control and intervention groups at Visit 1 through delivery1
| Total2 concentrations (μmol/L) | Visit 1 (gestation week 12–16) |
Visit 2 (gestation week 20–24) |
Visit 3 (gestation week 28–32) |
Delivery |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control group | Intervention group | p-Value | Control group | Intervention group | p-Value | Control group | Intervention group | p-Value | Control group | Intervention group | p-Value | |
| Choline | 7.0a [5.80, 8.36] | 6.2a [5.32, 7.23] | .31 | 7.3a,b [6.37, 8.30] | 9.2b [7.73, 11.0] | .02 | 8.3b [7.56, 9.19] | 11.4c [10.2, 12.8] | .0002 | 8.0a,b [7.02, 9.10] | 11.0b,c [9.32, 13.1] | .02 |
| Betaine | 12.4a [10.8, 14.3] | 14.4a [11.9, 17.4] | .26 | 10.5b [9.20, 12.1] | 17.2b [13.7, 21.7] | .0007 | 10.3a,b [9.68, 11.1] | 17.8b [14.2, 22.3] | .0001 | 10.7a,b [9.19, 12.5] | 17.2a,b [14.0, 21.1] | .001 |
| Dimethylglycine | 1.46a,b [1.24, 1.73] | 1.64a [1.30, 2.09] | .57 | 1.38a [1.14, 1.66] | 2.18b [1.66, 2.85] | .006 | 1.46a,b [1.22, 1.74] | 2.13b [1.66, 2.73] | .02 | 1.67b [1.43, 1.95] | 2.36b [1.86, 3.00] | .03 |
| Methionine | 26.2a [22.2, 30.2] | 25.1a [22.7, 27.5] | .63 | 24.4a [23.3, 25.4] | 24.0a [22.0, 26.0] | .71 | 24.9a [22.7, 27.0] | 25.1a [22.9, 27.2] | .88 | 24.4a [20.4, 28.5] | 25.5a [22.1, 28.8] | .67 |
| TMAO | 1.91a [1.17, 3.12] | 1.61a [1.18, 2.19] | .98 | 1.72a [1.09, 2.70] | 6.70b,c [3.66, 12.3] | .0002 | 1.33a [1.00, 1.77] | 8.93c [4.82, 16.5] | <.0001 | 1.81a [1.25, 2.63] | 4.39b [2.43, 7.90] | .02 |
| PC | 2223a [2090, 2357] | 2328a [2169, 2488] | .29 | 2556b [2368, 2744] | 2573b [2371, 2775] | .90 | 2490b [2265, 2714] | 2675b [2487, 2863] | .16 | 2270a,b [2031, 2509] | 2695b [2490, 2899] | .01 |
| Sphingomyelin | 465a [418, 513] | 505a [464, 546] | .20 | 550b [504, 596] | 557a,b [493, 621] | .86 | 542b [474, 611] | 595b [530, 661] | .19 | 478a,b [422, 535] | 585b [520, 649] | .01 |
| LysoPC | 386a [333, 446] | 408a [358, 464] | .55 | 482b [428, 543] | 479a,b [408, 563] | .80 | 463b [398, 538] | 504b [435, 585] | .34 | 392a [334, 459] | 462a,b [396, 540] | .13 |
Abbreviations: PC, phosphatidylcholine; TMAO, trimethylamine N-oxide.
Values are mean [95% CI]. The intervention group (n = 15) received 550 mg of choline from choline chloride (500 mg unlabeled choline + 50 mg deuterium-labeled d9-choline), while the control group (n = 15) received 25 mg of d9-choline only. Repeated measures mixed models with random intercepts and slopes and heterogenous autoregressive covariance structures were employed followed by the Tukey-Kramer post-hoc test. A time × intervention interaction term was used to obtain p-value for the effect of intervention at each time point. Superscript letters within a row indicate significant difference (p < .05) among visits within each group.
Total refers to the sum of the labeled and unlabeled metabolites.
3.3.1 |. Water-soluble choline metabolome
The choline intervention (vs. control) resulted in significantly higher plasma choline concentrations at Visit 2 (p = .02), Visit 3 (p < .005) and Delivery (p = .02) (Figure 1A). This impact of the choline intervention occurred in addition to the expected increase in plasma choline across pregnancy, as seen via the increase in plasma choline concentrations at Visit 3, relative to Visit 1 and Visit 2 (p < .05), in the control group. The choline intervention resulted in a sustained increase in plasma choline concentrations by Visit 2 through Delivery (p < .0005), relative to Visit 1, achieving peak choline concentrations at Visit 3.
The choline intervention (vs. control) resulted in significantly higher plasma concentrations of the methyl metabolites, betaine (p < .005) and dimethylglycine (p < .05) at Visit 2, Visit 3 and Delivery (Figure 1B,C). Whereas betaine concentrations in the control group exhibited a decrease from Visit 1 concentrations at Visit 2 (p < .05), concentrations at Visit 3 and Delivery were not significantly different from Visit 1 or Visit 2 (p > .20). The choline intervention group exhibited an increase in betaine concentrations at Visit 2 (p = .01), Visit 3 (p = .02) and Delivery (p = .16), relative to Visit 1. Dimethylglycine concentrations in the control group were similar across study visits, only achieving a statistically significant increase at Delivery relative to Visit 2 (p = .04). In the choline intervention group, however, dimethylglycine concentrations exhibited a sustained increase at Visit 2 through Delivery (p < .005), relative to Visit 1, consistent with increased use of betaine as a methyl donor. Plasma methionine levels were not significantly different (p > .60) between the choline intervention and control groups, and no significant changes (p > .55) were observed between study time points in either study arm (Figure 1D). Similar to betaine, the choline intervention (vs. control) yielded higher plasma TMAO at Visit 2 (p < .005), Visit 3 (p < .005) and Delivery (p < .05) (Figure 1E). No changes were observed at Visit 2 through Delivery relative to Visit 1 in the control group (p > .60), whereas the choline intervention group resulted in a significant increase in TMAO at Visit 2 through Delivery (p < .05) relative to Visit 1, though a decline at Delivery relative to Visit 3 was observed (p < .05). The within-individual changes in TMAO over time were notably variable in the intervention and suggest significant individual variation in the response to supplementation worthy of future investigation (Figure S1).
3.3.2 |. Lipid-soluble choline metabolome
The choline intervention (vs. control) yielded higher plasma PC concentrations at Visit 3 (p = .16) and Delivery (p = .01), relative to the control group (Figure 1F). Whereas the plasma PC concentrations reached their peak at Visit 2 (p < .005) and Visit 3 (p < .05), before declining to Visit 1 concentrations at Delivery in the control group (p = .97), plasma PC concentrations in the choline intervention group were increased by Visit 2 and remained elevated through Delivery (p < .05), relative to Visit 1. Products of PC metabolism, sphingomyelin and lysoPC, exhibited similar patterns to PC, with higher concentrations observed in the intervention group (vs. control) at Delivery (p = .01 and p = .13, respectively) (Figure 1G,H). In both the choline intervention and the control groups, plasma lysoPC concentrations rose by Visit 2 (p = .06 and p = .001, respectively) and Visit 3 (p = .004 and p = .004, respectively), relative to Visit 1, and returned to Visit 1 concentrations by Delivery (p = .30 and p = .99, respectively). In the control group, plasma sphingomyelin concentrations increased by Visit 2 (p = .005) and Visit 3 (p = .04), relative to Visit 1, and returned to Visit 1 concentrations by Delivery (p = .97). In the choline intervention group, plasma sphingomyelin concentrations exhibited significant increases at Visit 3 (p = .01) and Delivery (p = .04), relative to Visit 1.
3.4 |. Urinary choline metabolome response to intervention across pregnancy
Supplementation with water soluble micronutrients, such as choline, can result in increased urinary excretion of the micronutrient and related excretion products, particularly when consumed in excess. To that end, we investigated the impact of the choline intervention on the water-soluble urinary metabolome across pregnancy in 24-h urine samples, normalized to total creatinine concentrations (Table 3).
TABLE 3.
24-h urinary choline metabolite concentrations for the control and intervention groups at each visit1
| Variables | Visit 1 (gestation week 12–16) |
Visit 2 (gestation week 20–24) |
Visit 3 (gestation week 28–32) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | Intervention | p-Value | Control | Intervention | p-Value | Control | Intervention | p-Value | |
| Total2 concentrations (mg/g creatinine) | |||||||||
| Choline | 9.2a [7.36, 11.5] | 8.6a [6.69, 11.1] | .61 | 15.3b [11.9, 19.8] | 20.5b [15.7, 26.8] | .11 | 24.5c [18.3, 32.9] | 35.0c [24.8, 49.4] | .09 |
| Betaine | 22.9 [16.7, 31.5] | 15.7 [13.2, 18.7] | .08 | 22.4 [16.6, 30.4] | 17.1 [13.9, 20.9] | .21 | 28.4 [20.2, 40.0] | 18.6 [15.8, 21.9] | .10 |
| Dimethylglycine | 5.35 [3.61, 7.94] | 6.03a [4.23, 8.58] | .49 | 5.58 [3.96, 7.87] | 8.74b [6.92, 11.0] | .007 | 6.78 [4.76, 9.66] | 9.61b [7.31, 12.6] | .04 |
| Methionine | 4.99 [4.36, 5.71] | 4.90 [4.32, 5.55] | .87 | 5.18 [4.43, 6.07] | 5.27 [4.40, 6.31] | .96 | 5.88 [4.88, 7.08] | 4.94 [4.38, 5.56] | .15 |
| Trimethylamine N-oxide | 33.7 [25.0, 45.5] | 30.2a [24.9, 36.7] | .82 | 36.8 [25.9, 52.4] | 57.6b [43.3, 76.6] | .02 | 28.9 [22.7, 37.0] | 81.0b [58.5, 112] | <.0001 |
Values are Mean [95% CI]. The intervention group (n = 15) received 550 mg of choline from choline chloride (500 mg unlabeled choline + 50 mg deuterium-labeled d9-choline), while the control group (n = 15) received 25 mg of d9-choline only. Repeated measures mixed models with random intercepts and slopes and heterogenous autoregressive covariance structures were employed followed by the Tukey-Kramer post-hoc test. A time × intervention interaction term was used to obtain p-value for the intervention effect at each time point. Superscript letters within a row indicate significant difference (p < .05) among visits within each group.
Total refers to the sum of the labeled and unlabeled metabolites.
Progression through pregnancy, regardless of choline intervention arm, resulted in a progressive increase in total urinary choline concentrations (p < .005, for all within-study arm pairwise comparisons); the choline intervention group tended to have higher urinary choline excretion at Visit 2 (p = .11) and Visit 3 (p = .09), relative to the control group, though the magnitude of the effect of supplementation was less pronounced than the effect of pregnancy progression. Urinary betaine concentrations exhibited no significant differences between study arms or study visits (p > .05). The choline intervention yielded significantly higher urinary dimethylglycine concentrations at Visit 2 (p = .007) and Visit 3 (p = .04), relative to the control group. While the control group exhibited no significant changes in urinary dimethylglycine concentrations across study visits, the choline intervention group resulted in significant increases in Visit 2 (p = .005) and Visit 3 (p = .004) relative to Visit 1. Urinary methionine excretion was not significantly different between study arms or visits (p > .15). As expected, the choline intervention arm yielded significantly higher urinary TMAO concentrations at Visit 2 (p = .02) and Visit 3 (p < .001) relative to the control group. No differences between study visits were observed in the control group (p > .50), whereas increases in TMAO were observed as Visit 2 (p < .005) and Visit 3 (p < .000005) in the choline intervention group, relative to Visit 1.
3.5 |. Placental and newborn cord plasma choline metabolome response to intervention
Similar to its effect on the plasma choline metabolome in the maternal compartment, choline intervention resulted in significant changes in the choline metabolome in the placenta (per unit weight) and newborn cord plasma, relative to the control group (Table 4).
TABLE 4.
Placental and newborn cord plasma choline metabolite concentrations for the control and intervention groups1
| Variables | Placenta |
Newborn cord plasma |
||||
|---|---|---|---|---|---|---|
| Control, n = 15 | Intervention, n = 13 | p-Value | Control, n = 15 | Intervention, n = 12 | p-Value | |
| Total2 concentrations | (nmol/g) | (μmol/L) | ||||
| Choline | 1888 [606, 2170] | 1796 [1593, 1999] | .57 | 48.4 [35.6, 61.3] | 67.5 [25.3, 110] | .36 |
| Betaine | 35.8 [30.3, 41.4] | 48.8 [42.6, 55.0] | .002 | 24.4 [22.4, 26.5] | 31.4 [28.4, 34.5] | .0005 |
| Dimethylglycine | 2.20 [2.03, 2.37] | 2.85 [2.17, 3.54] | .06 | 2.09 [1.75, 2.42] | 2.82 [1.97, 3.67] | .10 |
| Methionine | – | – | – | 41.2 [37.4, 45.0] | 40.5 [36.1, 44.9] | .80 |
| Trimethylamine N-oxide | 1.41 [0.97, 1.86] | 5.09 [2.62, 7.57] | .01 | 1.70 [1.15, 2.26] | 4.83 [1.99, 7.67] | .04 |
| Phosphatidylcholine | 15315 [14261, 16370] | 15014 [14094, 15935] | .65 | 989 [875, 1103] | 1045 [779, 1311] | .68 |
| Sphingomyelin | 2776 [2616, 2936] | 2851 [2671, 3030] | .51 | 266 [234, 298] | 261 [212, 311] | .87 |
| Acetylcholine | 90.1 [72.6, 108] | 105 [86.4, 123] | .23 | – | – | – |
| LysoPC | 760 [733, 788] | 766 [720, 812] | .83 | 175 [148, 202] | 188 [123, 253] | .68 |
Values are Mean [95% CI]. The intervention group received 550 mg of choline from choline chloride (500 mg unlabeled choline + 50 mg deuterium-labeled d9-choline), while the control group received 25 mg of d9-choline only. For each compartment, unequal variance T-test was used to compare between groups with cut-off p values of <.05.
Total refers to the sum of the labeled and unlabeled metabolites.
3.5.1 |. Placenta
The choline intervention yielded higher concentrations of the choline-derived metabolites, betaine (p = .002), dimethylglycine (p = .06), and TMAO (p = .01). The choline intervention did not result in significantly higher concentrations of placental choline, PC, sphingomyelin or acetylcholine (p > .16) or any of the lipid-soluble choline metabolites (p > .25).
3.5.2 |. Newborn cord plasma
While the choline intervention yielded higher newborn cord plasma choline concentrations in magnitude, concentrations were highly variable in the intervention group, and these did not achieve statistical significance (p = .36). The choline intervention yielded higher plasma betaine concentrations (p = .0005) and borderline higher dimethylglycine concentrations (p = .10). Similar to the maternal plasma, there was no effect of the choline intervention on newborn cord plasma methionine concentrations (p = .80). Fetal cord plasma TMAO concentrations closely mirrored maternal Delivery plasma concentrations, with the choline intervention (vs. control) yielding higher TMAO concentrations (p = .04). Contrary to the maternal compartment, the choline intervention did not result in significant differences in the lipid-soluble choline metabolome for any metabolite (p > .65).
3.6 |. Deuterated-choline metabolome response to intervention across pregnancy
The provision of methyl-d9-choline allowed the tracking of the deuterated-choline moiety and its incorporation into the choline metabolome in all sample matrices, including maternal plasma (Table 5) and maternal urine across study visits (Table S3), as well as the placenta and newborn cord plasma (Table S4). The data collectively reveal both the complexity of choline dynamics across pregnancy (violating steady state assumptions), in different measured compartments, as well as the complexity of designing and interpreting stable isotope investigations across different dietary regimens that influence endogenous pool sizes. The volume of deuterated data precludes complete summary of all findings; below, we detail observations that provide insight into dynamics observed in our total metabolite observations detailed above.
TABLE 5.
Plasma choline metabolite enrichments and enrichment ratios for the control and intervention groups at Visit 2 through delivery1
| Variables | Visit 2 (gestation week 20–24) |
Visit 3 (gestation week 28–32) |
Delivery |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | Intervention | p-Value | Control | Intervention | p-Value | Control | Intervention | p-Value | |
| Enrichment % | |||||||||
| d9-Choline | 1.87a [1.59, 2.19] | 1.85a [1.67, 2.05] | .87 | 2.73a [1.93, 3.85] | 2.08a [1.73, 2.48] | .38 | 2.13a [1.81, 2.50] | 1.81a [1.19, 2.76] | .06 |
| d3-Choline | 0.56a [0.47, 0.64] | 0.74a [0.63, 0.85] | .01 | 0.74b [0.64, 0.85] | 0.86b [0.73, 0.98] | .13 | 0.87c [0.74, 0.99] | 0.92b [0.75, 1.08] | .60 |
| d9-Betaine | 2.45a [2.06, 2.92] | 2.77a [2.47, 3.11] | .14 | 3.28a [2.47, 4.35] | 2.70a [2.20, 3.32] | .37 | 2.57a [2.18, 3.01] | 3.32a [1.61, 4.14] | .25 |
| d3-Betaine | 0.49a [0.41, 0.57] | 0.62a [0.52, 0.72] | .11 | 0.65a,b [0.57, 0.74] | 0.75a [0.66, 0.84] | .11 | 0.76b [0.66, 0.86] | 0.79a [0.66, 0.92] | .73 |
| d6-Dimethylglycine | 9.63a [6.71, 12.6] | 11.0a [9.53, 12.5] | .37 | 10.2a [8.29, 12.1] | 10.0a [8.29, 11.7] | .87 | 8.80a [7.57, 10.0] | 8.68a [6.69, 10.7] | .91 |
| d3-Dimethylglycine | 0.45a [0.33, 0.58] | 0.48a [0.37, 0.59] | .70 | 0.63b [0.48, 0.77] | 0.66b [0.53, 0.80] | .69 | 0.67b [0.49, 0.84] | 0.75b [0.56, 0.94] | .51 |
| d9-TMAO | 1.00a [0.67, 1.50] | 8.59a [6.38, 11.6] | <.0001 | 1.05a [0.78, 1.42] | 9.87b [4.15, 23.5] | <.0001 | 1.29a [0.94, 1.78] | 21.5a,b [3.06, 150] | .10 |
| d3-TMAO2 | 1.10a [0.00, 2.46] | 2.06a,b [0.00, 4.22] | .42 | 0.28a [0.17, 0.38] | 1.96a [0.24, 3.68] | .046 | 0.36a [0.17, 0.55] | 0.19b [0.05, 0.34] | .15 |
| d9-PC | 1.27a [1.15, 1.38] | 1.20a [1.06, 1.33] | .39 | 1.45a [1.25, 1.65] | 1.15a [0.95, 1.35] | .03 | 1.33a [1.12, 1.53] | 1.03a [0.81, 1.26] | .06 |
| d3-PC | 0.67a [0.59, 0.77] | 0.95a [0.81, 1.13] | .002 | 0.79b [0.68, 0.92] | 1.00a [0.82, 1.23] | .07 | 0.88a,b [0.76, 1.03] | 1.04a [0.76, 1.44] | .77 |
| d9-Sphingomyelin | 1.40a [1.21, 1.58] | 1.37a [1.11, 1.62] | .85 | 1.62a [1.40, 1.85] | 1.33a [1.13, 1.53] | .04 | 1.55a [1.33, 1.76] | 1.20a [0.95, 1.46] | .04 |
| d9-LysoPC | 1.29a [1.21, 1.39] | 1.23a [1.09, 1.40] | .33 | 1.49a [1.37, 1.63] | 1.26a [0.98, 1.62] | .046 | 1.36a [1.16, 1.59] | 1.18a [0.88, 1.60] | .15 |
| Enrichment ratios | |||||||||
| d9-Betaine:d9-Choline | 1.33a [1.18, 1.47] | 1.50a [1.40, 1.60] | .04 | 1.27a [1.17, 1.38] | 1.30b [1.18, 1.42] | .71 | 1.21a [1.17, 1.25] | 1.36a,b [1.28, 1.45] | .002 |
| d9-Betaine:d9-PC | 1.90a [1.72, 2.09] | 2.35a [2.16, 2.55] | .001 | 2.20a [1.79, 2.69] | 2.49a [2.13, 2.91] | .20 | 1.94a [1.79, 2.11] | 3.28a [1.81, 5.96] | .60 |
| d9-PC:d9-Choline | 0.71a [0.64, 0.78] | 0.65a [0.61, 0.68] | .11 | 0.68a [0.52, 0.90] | 0.58a [0.46, 0.74] | .41 | 0.64a [0.58, 0.70] | 0.88a [0.51, 1.51] | .95 |
| d9-Sphingomyelin:d9-PC | 1.10a [0.99, 1.21] | 1.13a [1.00, 1.26] | .70 | 1.14a [1.01, 1.27] | 1.22a [1.04, 1.41] | .43 | 1.18a [1.08, 1.29] | 1.15a [0.94, 1.36] | .75 |
| d6-Dimethylglycine:d9-Betaine | 3.83a [3.50, 4.18] | 4.00a [3.70, 4.31] | .40 | 3.55a [2.93, 4.30] | 3.75a [3.39, 4.14] | .35 | 3.53a [3.18, 3.92] | 8.39a [2.33, 30.2] | .45 |
| d3-PC:d9-PC | 0.53a [0.48, 0.58] | 0.79a [0.72, 0.87] | <.0001 | 0.55a [0.50, 0.60] | 0.91b [0.81, 1.03] | <.0001 | 0.68b [0.61, 0.75] | 1.04c [0.91, 1.19] | <.0001 |
| d3-Choline:d3-PC | 0.82a [0.78, 0.87] | 0.78a [0.76, 0.81] | .14 | 0.94b [0.89, 1.00] | 0.88b [0.80, 0.97] | .17 | 0.99a,b [0.94, 1.04] | 1.26a,b [0.75, 2.11] | .73 |
| d3-Betaine:d3-Choline | 0.88a [0.85, 0.92] | 0.83a [0.81, 0.86] | .01 | 0.89a [0.85, 0.92] | 0.89a [0.84, 0.94] | .92 | 0.89a [0.84, 0.93] | 0.89a [0.83, 0.95] | .95 |
Abbreviations: PC, phosphatidylcholine; TMAO, trimethylamine N-oxide.
Values are Mean [95% CI]. The intervention group (n = 15) received 550 mg of choline from choline chloride (500 mg unlabeled choline + 50 mg deuterium-labeled d9-choline), while the control group (n = 15) received 25 mg of d9-choline only. Repeated measures mixed models with random intercepts and slopes and heterogenous autoregressive covariance structures were employed followed by the Tukey-Kramer post-hoc test. A time × intervention interaction term was used to obtain p-value for the intervention effect at each time point. Superscript letters within a row indicate significant difference (p < .05) among visits within each group.
Lower limit of the confidence intervals for visit 2 were negative and thus reported as zero.
Consistent with our findings that the choline intervention resulted in higher maternal plasma betaine and dimethylglycine levels, the choline intervention group exhibited significantly elevated enrichments of d3-choline and d3-PC relative to the control group at Visit 2 (p = .01 and p = .002, respectively) and a trend towards higher enrichments at Visit 3 (p = .13 and p = .07, respectively). These differences are indicative of greater utilization of choline-derived methyl groups to fuel the PEMT pathway of PC biosynthesis, which produces d3-PC that is capable of being hydrolyzed by phospholipase D to generate d3-choline. The effect of the choline intervention occurred in the context of increasing PEMT activity across pregnancy, as evidenced by the control group exhibiting increased d3-choline and d3-PC enrichments at Visit 3 (p = .000005 and p = .03, respectively) and Delivery (p = .000001 and p = .09, respectively), relative to Visit 2. In the choline intervention group, changes across study visits were less evident for d3-PC, but d3-choline was increased at Visit 3 (p = .004) and Delivery (p = .006), relative to Visit 2. Despite this clear evidence of increased use of choline as a methyl donor, no significant impacts of the choline intervention were observed at any study visit for plasma d9-betaine, d3-betaine, d6-dimethylglycine or d3-dimethylglycine, relative to the control group (p > .10), suggesting plasma pools of these methyl metabolites are poor indicators of tissue-specific metabolism. One intriguing observation, however, was the increase in d3-dimethlylglycine enrichments at Visit 3 and Delivery, relative to Visit 2 in both study groups (p < .05), suggestive of an intake-independent, trimester-specific increase in choline derived from d3-PC hydrolysis as a methyl donor. Plasma enrichments of d9-PC, d9-sphingomyelin, and d9-LysoPC were lower in the choline intervention group (vs. control) at Visit 3 (p < .05, all metabolites) and Delivery (p = .06, p = .04, and p = .15, respectively), consistent with increased total plasma pool sizes of these compounds and increased d9-choline dilution in hepatic choline pools. Lastly, the deuterated TMAO pattern proved informative. The choline intervention led to substantially greater d9-TMAO enrichments at Visit 2 and Visit 3 (p < .0001), consistent with our intervention design (co-ingestion of the d9-choline and high dose choline supplement) and increased TMA generation when luminal choline content is high. Despite no dietary ingestion of d3-choline, d3-TMAO was detectable, and higher in the choline intervention group at Visit 3 (p = .046) relative to the control group, consistent with enterohepatic recycling of d3-PC and exposure of the d3-choline moiety to the intestinal microbiota.
4 |. DISCUSSION
This randomized controlled trial conducted in free-living pregnant persons investigated the effect of prenatal choline supplementation on choline and its metabolome in biological samples obtained across the second and third trimesters of pregnancy. Throughout the trial, all participants consumed self-selected diets estimated to provide median intakes of ~353 mg total choline/d for the choline intervention group and ~377 mg total choline/d for the control group.
Plasma choline and its metabolome were highly responsive to prenatal supplemental choline (550 vs. 25 mg choline/d), despite Visits 1–3 relying on fasted plasma samples and measuring metabolite concentrations in the context of progression through pregnancy and its associated diluting effects due to plasma volume expansion. Water soluble metabolites were most significantly responsive to the choline intervention relative to the control, exhibiting higher circulating concentrations of free choline (1.2–1.4-times), betaine (1.6–1.8-times), and dimethylglycine (1.4–1.6-times) at the measured study time points. The elevated plasma betaine concentrations were remarkably consistent at Study Visit 2, Visit 3 and Delivery in response to the choline intervention, despite increasing plasma volume expansion, suggesting homeostatic efforts to maintain plasma betaine ~17 μmol/L and indicating that plasma betaine could serve as a sensitive indicator (or biomarker) of supplemental choline intake, specifically in the form of a choline salt (as opposed to PC). Plasma TMAO was also responsive to choline supplementation, though unlike plasma betaine, concentrations increased variably across study visits (2.4–6.7-times), and may be indicative of interactions between the increased choline intake and pregnancy-related changes in microbiome composition or hepatic FMO3 activity. Notably, no significant differences were detected between groups in urinary choline or betaine, and only a modest increase in urinary DMG (1.4–1.6-times) at Visits 2–3 was observed; the non-statistically significant differential in urinary choline between the intervention and control group (~10.5 mg choline/g creatinine; total 24 h creatinine mean, 0.9 g) represents <2% of the total supplemental dose. Whether urinary metabolite increases with intervention represent changes in the free fraction of metabolites in urine, or metabolites found within cells or extracellular vesicles requires future investigation. Such findings indicate that the substantial increase in plasma choline and betaine in response to the supplemental choline dose of 550 mg/d did not exceed the metabolic capacity of cells or the renal threshold.
The plasma lipid-soluble choline metabolome was less significantly responsive to the choline intervention, a perhaps unsurprising finding given their determination by not only choline intakes but also lipoprotein metabolism and its associated variation during pregnancy. Nevertheless, consistent trends across the lipid-soluble metabolites point towards an effect of choline supplementation on maintaining elevated plasma concentrations towards the end of pregnancy, likely due to choline facilitating increased hepatic VLDL synthesis and export.
The longitudinal change across the last two-thirds of pregnancy was relatively similar in both groups and consistent with prior reports16–18 with a few notable exceptions. First, a decline in circulating betaine, relative to Visit 1, was observed in the control group only, likely due to an increased demand for choline-derived methyl groups and the impact of plasma volume expansion. Second, it was notable that the Delivery concentrations for the lipid soluble metabolome (PC, SM, lysoPC) consistently decreased towards Visit 1 values in the control group; an observation that was not observed in the choline intervention group. PC, SM and lysoPC are constituents of all lipoproteins and PC is particularly required for hepatic VLDL biosynthesis28 and the export of triacylglyercol from maternal liver into circulation. Importantly, hyperlipoproteinemia during the second half of pregnancy is a well-recognized feature of normal pregnancy,29,30 and helps to ensure an adequate supply of lipids (fatty acids, phospholipids, and cholesterol) to the developing fetus. In the present study, the control group’s intake of choline appeared insufficient to support the metabolic demands of pregnancy as it relates to lipoprotein synthesis, exhibiting Delivery PC concentrations nearly identical (1.02-times) to Visit 1 concentrations. Conversely, the choline intervention increased (~1.15 times) plasma PC levels at Visit 3 and Delivery relative to Visit 1. Likewise and concurrently, higher LDL-C was also observed in the choline intervention group at Visit 3 (Table S1). Notably, habitual dietary choline intakes by the control group were numerically higher than nationally representative data for U.S. pregnant individuals (~360 vs. ~320 mg/d21,22), suggesting that similar differences between the choline intervention cohort and the general population would be expected.
The decline in PC across the last third of pregnancy in the control group transpired with supplemental choline intakes (as choline chloride) of 25 mg/d, an amount historically used in choline-containing prenatal vitamin formulations, suggesting this level of supplementation is not enough to meet the demands of pregnancy. Conversely, findings from our previous controlled feeding study that provided 380 mg choline/d from the diet and either 100 or 550 mg supplemental choline (as choline chloride) did not detect differences between the choline intake groups in PC concentrations,3 suggesting that daily intakes of 100 mg supplemental choline/d may be enough to support PC and VLDL biosynthesis during the last third of pregnancy. This latter controlled feeding trial, however, still demonstrated cognitive benefits from the higher choline intervention dose (Caudill et al. 2018), suggesting that the capacity to support PC and VLDL biosynthesis is likely not a primary mechanism through which supplemental choline improves infant cognitive outcomes.
Prenatal choline supplementation can support PC biosynthesis by providing choline that can be incorporated directly into the PC molecule by the CDP-choline pathway. Alternatively, the choline molecule can be “catabolized” such that its methyl groups are incorporated into the PC molecule by the PEMT pathway. The total concentrations of the oxidative choline derivatives (i.e., betaine and DMG), and the enrichments of d3-PC and d3-choline, suggest that prenatal choline supplementation improved the supply of methyl groups for use by the PEMT pathway. PC molecules produced by the PEMT are enriched in long chain unsaturated fatty acids, including docosahexaenoic acid (DHA), as compared to PC molecules produced by the CDP-choline pathway.31 Like choline, DHA is critical for optimal growth, development and maturation of the newborn brain.32,33 In previous work conducted in third trimester pregnant participants, biomagnification of PEMT-PC was observed in cord blood as compared to maternal blood3 indicating preferential uptake of PEMT-PC compared to CDP-PC by the developing fetus. In the present study, we did not detect biomagnification of PEMT-PC in the newborn compartment, possibly because prenatal choline supplementation was administered two times longer than in the previous study3 and PEMT-PC and/or DHA34 was not limited. However, we did detect a higher ratio of d3-PC:d9-PC in cord plasma that mirrored findings in maternal blood, and is consistent with accrual of d3-PC in the fetal compartment.
Very few differences were detected in the enrichment data between the choline intervention and control groups despite relatively substantial differences in total pool sizes. One explanation for these null findings relates to our study design and the administration of the d9-choline tracer. Although we attempted to account for an expected two times greater total choline intake in the choline intervention arm (as compared to the control), we did not adjust for relative differences in the total free or water-soluble choline intakes and the subsequent dilution of the orally administered d9-choline in the unlabeled d0-choline pool. For example, the control group consumed ~100 mg of water-soluble choline/d, with ~25% as d9-choline (~75 mg from diet and 25 mg as d9-choline). In contrast, the intervention group consumed ~625 mg, with 8% as d9-choline (~75 mg from diet, 500 mg as d0-choline and 50 mg as d9-choline). Thus, even though our design doubled the intake of d9-choline (50 mg vs. 25 mg in the intervention vs. control arm), an additional 3X dilution factor remains when considering from the perspective of water-soluble choline, which predominantly enters the portal vein for delivery to liver. This is conservative still, given that the intervention group gets a significant bolus of water-soluble choline all at once with the label, and the control group sees a small quantity of d9-choline alongside minimal water-soluble choline (depending on timing of consuming the supplement relative to a meal). As such, the lack of group differences in the enrichment percent in the oxidative choline derivatives, which increase in response to a water-soluble choline load, likely reflects markedly greater oxidation of choline in the intervention (vs control) group. Future studies may choose to further adjust study doses accounting for water-versus lipid-soluble choline intakes, or provide the same dose to both arms and rely on dilution to make inferences about choline metabolism.
Newborns exhibited approximately three times higher free choline in cord plasma as compared to maternal plasma, which is consistent with previous reports.9,16,19,35 Prenatal choline supplementation did not further increase newborn plasma choline concentrations suggesting that mechanisms are in place to ensure sufficient transfer of this metabolite to the development fetus. However, this single, steady-state measure is unlikely to adequately capture the dynamics of choline flux in the fetal compartment. Newborns born to choline supplemented mothers, however, did exhibit higher circulating betaine, a methyl donor, and borderline higher dimethylglycine, which contributes glycine for glutathione synthesis in human development and is required for numerous other metabolic processes.35 Functional consequences of the neonate entering the postnatal environment with higher betaine and methyl donor status should be explored in larger studies, including at-risk clinical populations.
In sum, the maternal plasma choline metabolome is highly responsive to prenatal choline supplementation with betaine concentrations being most sensitive. The disparate plasma PC concentration trends between the choline intervention and control groups of the present study is a novel contribution to the field, and strongly indicates that habitual choline intakes, even in this well-nourished, high socioeconomic status population, are inadequate to meet the choline demands of pregnancy. The greater enrichment of d3-PC and d3-choline, along with elevated PC and LDL-C, in the choline intervention (vs. control) arm supports the notion that choline chloride supplementation facilitates greater PEMT activity (and synthesis of PC molecules enriched in long-chain unsaturated fatty acids) by bolstering methyl group supply.
Supplementary Material
ACKNOWLEDGEMENTS
Funded by Balchem Corporation; the Cornell Institute of Biotechnology’s Center for Advanced Technology (CAT) grant through New York State Division of Science, Technology, and Innovation (NYSTAR); and the National Institute of Food and Agriculture U.S. Department of Agriculture (NIFA/USDA), HATCH under accession number 1013729. Salary for KCK was provided from a National Institute of Health Training Grant (T32HD087137). ST was partially supported by Chiang Mai University [CMU-8392(10)/COE65].
Funding information
Balchem Corporation; Cornell Institute of Biotechnology’s Center for Advanced Technology (CAT); National Institute of Food and Agriculture U.S. Department of Agriculture (NIFA/USDA), Grant/Award Number: 1013729; National Institute of Health Training, Grant/Award Number: T32HD087137; Chiang Mai University, Grant/Award Number: CMU-8392(10)/COE65
Abbreviations:
- DHA
docosahexaenoic acid
- FAs
fatty acids
- GW
gestational week
- LysoPC
lysophosphatidylcholine
- PC
phosphatidylcholine
- PEMT
phosphatidylethanolamine N-methyltransferase
- TMAO
trimethylamine N-oxide
Footnotes
DISCLOSURES
The authors report no conflict of interests. Funders had no role in the study design, data collection, or the analysis and interpretation of the data.
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of the article at the publisher’s website.
REFERENCES
- 1.Caudill MA. Pre- and postnatal health: evidence of increased choline needs. J Am Diet Assoc 2010;110(8):1198–1206. doi: 10.1016/j.jada.2010.05.009 [DOI] [PubMed] [Google Scholar]
- 2.Yan J, Jiang X, West AA, et al. Maternal choline intake modulates maternal and fetal biomarkers of choline metabolism in humans. Am J Clin Nutr 2012;95(5):1060–1071. doi: 10.3945/ajcn.111.022772 [DOI] [PubMed] [Google Scholar]
- 3.Yan J, Jiang X, West AA, et al. Pregnancy alters choline dynamics: results of a randomized trial using stable isotope methodology in pregnant and nonpregnant women. Am J Clin Nutr 2013;98(6):1459–1467. doi: 10.3945/ajcn.113.066092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jiang X, West AA, Caudill MA. Maternal choline supplementation: a nutritional approach for improving offspring health? Trends Endocrinol Metab 2014;25(5):263–273. doi: 10.1016/j.tem.2014.02.001 [DOI] [PubMed] [Google Scholar]
- 5.Korsmo HW, Jiang X, Caudill MA. Choline: exploring the growing science on its benefits for moms and babies. Nutrients 2019;11(8):E1823. doi: 10.3390/nu11081823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caudill MA, Strupp BJ, Muscalu L, Nevins JEH, Canfield RL. Maternal choline supplementation during the third trimester of pregnancy improves infant information processing speed: a randomized, double-blind, controlled feeding study. FASEB J 2018;32(4):2172–2180. doi: 10.1096/fj.201700692RR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zeisel SH, Mar MH, Zhou Z, da Costa KA. Pregnancy and lactation are associated with diminished concentrations of choline and its metabolites in rat liver. J Nutr 1995;125(12):3049–3054. doi: 10.1093/jn/125.12.3049 [DOI] [PubMed] [Google Scholar]
- 8.Gwee MC, Sim MK. Free choline concentration and cephalin-N-methyltransferase activity in the maternal and foetal liver and placenta of pregnant rats. Clin Exp Pharmacol Physiol 1978;5(6):649–653. doi: 10.1111/j.1440-1681.1978.tb00721.x [DOI] [PubMed] [Google Scholar]
- 9.Zeisel SH, Epstein MF, Wurtman RJ. Elevated choline concentration in neonatal plasma. Life Sci 1980;26(21):1827–1831. doi: 10.1016/0024-3205(80)90585-8 [DOI] [PubMed] [Google Scholar]
- 10.Welsch F Studies on accumulation and metabolic fate of (N-Me3h)choline in human term placenta fragments. Biochem Pharmacol 1976;25(9):1021–1030. doi: 10.1016/0006-2952(76)90490-1 [DOI] [PubMed] [Google Scholar]
- 11.Baumgartner HK, Trinder KM, Galimanis CE, et al. Characterization of choline transporters in the human placenta over gestation. Placenta 2015;36(12):1362–1369. doi: 10.1016/j.placenta.2015.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sweiry JH, Page KR, Dacke CG, Abramovich DR, Yudilevich DL. Evidence of saturable uptake mechanisms at maternal and fetal sides of the perfused human placenta by rapid paired-tracer dilution: studies with calcium and choline. J Dev Physiol 1986;8(6):435–445. [PubMed] [Google Scholar]
- 13.Kwan STC, King JH, Yan J, et al. Maternal choline supplementation during murine pregnancy modulates placental markers of inflammation, apoptosis and vascularization in a fetal sex-dependent manner. Placenta 2017;53:57–65. doi: 10.1016/j.placenta.2017.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garner SC, Mar MH, Zeisel SH. Choline distribution and metabolism in pregnant rats and fetuses are influenced by the choline content of the maternal diet. J Nutr 1995;125(11):2851–2858. doi: 10.1093/jn/125.11.2851 [DOI] [PubMed] [Google Scholar]
- 15.Meck WH, Williams CL. Metabolic imprinting of choline by its availability during gestation: implications for memory and attentional processing across the lifespan. Neurosci Biobehav Rev 2003;27(4):385–399. doi: 10.1016/s0149-7634(03)00069-1 [DOI] [PubMed] [Google Scholar]
- 16.Ozarda Ilcol Y, Uncu G, Ulus IH. Free and phospholipid-bound choline concentrations in serum during pregnancy, after delivery and in newborns. Arch Physiol Biochem 2002;110(5):393–399. doi: 10.1076/apab.110.5.393.11832 [DOI] [PubMed] [Google Scholar]
- 17.Velzing-Aarts FV, Holm PI, Fokkema MR, van der Dijs FP, Ueland PM, Muskiet FA. Plasma choline and betaine and their relation to plasma homocysteine in normal pregnancy. Am J Clin Nutr 2005;81(6):1383–1389. doi: 10.1093/ajcn/81.6.1383 [DOI] [PubMed] [Google Scholar]
- 18.Fernàndez-Roig S, Cavallé-Busquets P, Fernandez-Ballart JD, et al. Low folate status enhances pregnancy changes in plasma betaine and dimethylglycine concentrations and the association between betaine and homocysteine. Am J Clin Nutr 2013;97(6):1252–1259. doi: 10.3945/ajcn.112.054189 [DOI] [PubMed] [Google Scholar]
- 19.McMahon KE, Farrell PM. Measurement of free choline concentrations in maternal and neonatal blood by micropyrolysis gas chromatography. Clin Chim Acta 1985;149(1):1–12. doi: 10.1016/0009-8981(85)90267-0 [DOI] [PubMed] [Google Scholar]
- 20.Lewis ED, Subhan FB, Bell RC, et al. Estimation of choline intake from 24 h dietary intake recalls and contribution of egg and milk consumption to intake among pregnant and lactating women in Alberta. Br J Nutr 2014;112(1):112–121. doi: 10.1017/S0007114514000555 [DOI] [PubMed] [Google Scholar]
- 21.Wallace TC, Fulgoni VL. Assessment of total choline intakes in the United States. J Am Coll Nutr 2016;35(2):108–112. doi: 10.1080/07315724.2015.1080127 [DOI] [PubMed] [Google Scholar]
- 22.Bailey RL, Pac SG, Fulgoni VL, Reidy KC, Catalano PM. Estimation of total usual dietary intakes of pregnant women in the United States. JAMA Netw Open 2019;2(6):e195967. doi: 10.1001/jamanetworkopen.2019.5967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holm PI, Ueland PM, Kvalheim G, Lien EA. Determination of choline, betaine, and dimethylglycine in plasma by a high-throughput method based on normal-phase chromatography-tandem mass spectrometry. Clin Chem 2003;49(2):286–294. doi: 10.1373/49.2.286 [DOI] [PubMed] [Google Scholar]
- 24.Koc H, Mar M-H, Ranasinghe A, Swenberg JA, Zeisel SH. Quantitation of choline and its metabolites in tissues and foods by liquid chromatography/electrospray ionization-isotope dilution mass spectrometry. Anal Chem 2002;74(18):4734–4740. doi: 10.1021/ac025624x [DOI] [PubMed] [Google Scholar]
- 25.Ganz AB, Shields K, Fomin VG, et al. Genetic impairments in folate enzymes increase dependence on dietary choline for phosphatidylcholine production at the expense of betaine synthesis. FASEB J 2016;30(10):3321–3333. doi: 10.1096/fj.201500138RR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klatt KC, McDougall MQ, Malysheva OV, Brenna JT, Roberson MS, Caudill MA. Reproductive state and choline intake influence enrichment of plasma lysophosphatidylcholine-DHA: a post hoc analysis of a controlled feeding trial. Br J Nutr 2019;122(11):1221–1229. doi: 10.1017/S0007114519002009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olsson U Confidence intervals for the mean of a log-normal distribution. J Stat Educ 2005;13(1). doi: 10.1080/10691898.2005.11910638 [DOI] [Google Scholar]
- 28.Yao ZM, Vance DE. The active synthesis of phosphatidylcholine is required for very low density lipoprotein secretion from rat hepatocytes. J Biol Chem 1988;263(6):2998–3004. [PubMed] [Google Scholar]
- 29.Knopp RH, Bergelin RO, Wahl PW, Walden CE, Chapman M, Irvine S. Population-based lipoprotein lipid reference values for pregnant women compared to nonpregnant women classified by sex hormone usage. Am J Obstet Gynecol 1982;143(6):626–637. doi: 10.1016/0002-9378(82)90107-7 [DOI] [PubMed] [Google Scholar]
- 30.Darmady JM, Postle AD. Lipid metabolism in pregnancy. Br J Obstet Gynaecol 1982;89(3):211–215. doi: 10.1111/j.1471-0528.1982.tb03616.x [DOI] [PubMed] [Google Scholar]
- 31.DeLong CJ, Shen YJ, Thomas MJ, Cui Z. Molecular distinction of phosphatidylcholine synthesis between the CDP-choline pathway and phosphatidylethanolamine methylation pathway. J Biol Chem 1999;274(42):29683–29688. doi: 10.1074/jbc.274.42.29683 [DOI] [PubMed] [Google Scholar]
- 32.Koletzko B, Cetin I, Brenna JT, et al. Dietary fat intakes for pregnant and lactating women. Br J Nutr 2007;98(5):873–877. doi: 10.1017/S0007114507764747 [DOI] [PubMed] [Google Scholar]
- 33.Brenna JT. Animal studies of the functional consequences of suboptimal polyunsaturated fatty acid status during pregnancy, lactation and early post-natal life. Matern Child Nutr 2011;7(Suppl 2):59–79. doi: 10.1111/j.1740-8709.2011.00301.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luxwolda MF, Kuipers RS, Sango WS, Kwesigabo G, Dijck-Brouwer DAJ, Muskiet FAJ. A maternal erythrocyte DHA content of approximately 6 g% is the DHA status at which intrauterine DHA biomagnifications turns into bioattenuation and postnatal infant DHA equilibrium is reached. Eur J Nutr 2012;51(6):665–675. doi: 10.1007/s00394-011-0245-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Friesen RW, Novak EM, Hasman D, Innis SM. Relationship of dimethylglycine, choline, and betaine with oxoproline in plasma of pregnant women and their newborn infants. J Nutr 2007;137(12):2641–2646. doi: 10.1093/jn/137.12.2641 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


