Abstract
Over the past two decades, the surgical community has increasingly embraced robotic-assisted surgery (RAS) due to its potential to enhance accuracy and decrease surgical morbidity. Plastic surgery as a field has been historically slow to incorporate RAS, with lack of adequate training posing as one of the most commonly cited barriers. To date, robot technology has been utilized for various reconstructive procedures including flap elevation and inset, pedicle dissection, and microvascular anastomosis. As RAS continues to integrate within plastic surgery procedures, the need for a structured RAS curriculum designed for plastic surgery trainees is rising. This article delineates the essential components of a plastic surgery-specific RAS curriculum and outlines current training models and assessment tools utilized across surgical subspecialties to date.
Keywords: education, robotic surgery, surgical education, plastic surgery training
Long before robotic technology was applied to the field of plastic surgery, the development of robotics itself was driven by the introduction of two separate technological advances: telepresence and robots. Telepresence was pioneered in 1951 by Raymond Goertz who, while working for the Atomic Energy Commission (United States), designed the first teleoperated mechanical arm to handle hazardous radioactive material. The successful use of robots to perform repetitive tasks accurately occurred just 10 years later when, in 1961, George Devol and Joseph Engelberger developed Unimate (Unimation Inc., Danbury, CT), the first industrial robot, for General Motors. 1 2 These successful experiments soon led to robotics being integrated into other industrial applications around the world. The term “robot” was officially defined by the Robots Institute of America in 1979 as “a reprogrammable, multifunctional manipulator designed to move materials, parts, tools, or specialized devices through various programmed motions for the performance of a variety of tasks.” 1 By 1985, the first medical robot Puma 200 (Westinghouse Electric, Pittsburgh, PA) was utilized to perform stereotactic brain surgery. 3
Today, robots are a familiar part of contemporary medical practice, including applications in the field of plastic surgery. Hospitals are actively embracing robotic-assisted surgery (RAS) due to its potential to enhance speed and accuracy while decreasing morbidity in multiple settings. Specific to the field of plastic surgery, robotic technology has been incorporated into microsurgery and supermicrosurgery, head and neck reconstruction, breast and pelvic reconstruction, brachial plexus and peripheral nerve repair, and gender affirming surgery. 1 2 4
RAS has the potential to improve surgical outcomes for complex plastic surgery operations that require advanced technical dexterity and precision in hard-to-reach anatomical areas. Operations that required open surgical technique in the past, such as free-flap harvesting (e.g., deep inferior epigastric perforator [DIEP] flap harvest, latissimus dorsi [LD] flap harvest), have already been successfully adapted for RAS, offering patients a minimally invasive approach. 5 6 RAS DIEP flap harvest allows for long pedicle dissection (13–15 cm) with small fascial incisions (1–3 cm), which preserves abdominal wall integrity and lowers the risk of incisional bulging and herniation, which can be seen with abdominal based reconstruction. 6 7 8 9 LD flap harvest for breast reconstruction has historically required an incisional length anywhere from 15 to 45 cm for adequate exposure using conventional open techniques. 9 10 When harvested with robotic assistance, the LD flap incisional site is reduced to 4 or 5 cm—small enough to be hidden in a patient's axilla, which may be more favorable for the aesthetically conscious patient. 9 10 RAS flap harvest has even been shown to result in decreased postoperative pain, most likely related to reduced incisional morbidity, and shorter postoperative hospital stays. 5 10 11 Incorporating RAS can increase operative times up to 2.6 times longer, but as individual surgeons become more familiar with the RAS system, in conjunction with an increase in instruments made specifically for plastic surgery (e.g., robotic microsurgical tools, improved optical resolution), these increased operative times are expected to become commensurate with that of open approaches. 12
Motion scaling and tremor filtration in RAS technology are precision enhancers that provide advantage for the microsurgeon and trainee in technically demanding procedures. In motion scaling, the robotic arm simulates a surgeon's specific movement by a preset scaled factor (up or down). For example, in an 8:1 scaled setting, a motion of 8 mm made by the surgeon would be translated as a 1-mm motion by the robotic arm, allowing for more precise movements, especially useful in hard-to-reach areas. 13 14 15 The ability to scale down surgical motions to a ratio of 20:1 makes supermicrosurgical procedures on submillimeter lymphatic vessels and fascicle-oriented nerve reconstruction more accessible to the microsurgeon, as human limitations are augmented. 14 Tremor filtration improves surgical accuracy by registering the physiological tremor (ranges from 0.5 to 3.0 mm with an irregular frequency up to 30 Hz) of the operating surgeon on the master manipulator, which then filters out high-frequency components before transmitting the movement through the robotic arms. 14 The da Vinci robot system was also shown to eliminate hand dominance in surgical trainees across multiple specialties in both open and robotic surgery, uniquely allowing trainees to operate ambidextrously. 16
As more surgical fields adopt RAS technology, and as the public's interest in minimally invasive surgical options continues to expand, plastic surgeons must keep up with advancing technology. Plastic surgery as a field has historically been slow to embrace robotic surgery, with the most commonly cited barriers of RAS implementation being high cost, increased operating times, and a lack of adequate training. 17 As more robotic surgical platforms enter the market and create price competition, costs are expected to drop significantly, ameliorating this issue. The issues of long operating times and inadequate training, however, must be addressed through improved robotic education across the field of plastic surgery.
The concept of a “learning curve” in surgical training is used to denote the process of learning how to perform a new procedure efficiently and can be measured by decreased operative times for the same procedure. 18 Delineation of the learning curve in RAS highlights the importance of standardizing a robotic training curriculum that promotes earlier RAS training and enhanced robotic familiarity for plastic surgery trainees. The aim of this paper is to review the current landscape of robotic plastic surgery education and put forth a RAS training program for plastic surgery trainees.
Surgical Robots
Robotic surgery has a long history, beginning with the introduction of the Puma 200 (Westinghouse Electric, Pittsburgh, PA) for stereotactic computed tomography–guided brain biopsies in 1985. Surgical robots in the premillennial era have also included the PROBOT (Integrated Surgical Supplies Ltd., Mesa, AZ), ROBODOC (CUREXO Technology Corporation, Fremont, CA), AESOP (Computer Motion, Goleta, CA), and ZEUS (Computer Motion, Goleta, CA) systems. In the postmillenial era, the da Vinci Surgical System (Intuitive Surgical Inc., Sunnyvale, CA) has dominated the market. 3 The da Vinci is a robotic surgical system that has been commercially available in Europe since 1998 and was Food and Drug Administration approved for performing general laparoscopic procedures in 2000. 19 The da Vinci consists of a master console with a magnified, high-definition, three-dimensional view of the surgical field, a video platform/laparoscopic insufflator, and a patient-side cart with movable robotic arms. 20 Each arm holds detachable surgical tips through wristed technology, with each articulation allowing up to 6 degrees of freedom (3 degrees of translation, 3 degrees of rotation) and 90 degrees of articulation. EndoWrist technology therefore provides intuitive, hand-like rotation, with an additional degree of freedom given by the attached tool's functionality (e.g., cutting, grasping). 20 In 2003, the daVinci system was upgraded to include a fourth arm for optimal retraction, suction, and irrigation. 21 In 2006, the da Vinci S HD (second generation) added improved resolution, swifter instrument exchange, fewer cable connections, and extended reach instruments for multiquadrant access. 19 In 2009, the da Vinci Si HD added shared control capacity between dual consoles for training and surgeon collaboration, along with improvements to the user interface's digital integration and video resolution. In 2014, the da Vinci Xi (fourth generation) brought thinner, longer arms, added the capability of fluorescent imaging (Firefly), changed the setup to an overhead arrangement, and added an optional integrated operating table to allow for table motion and patient repositioning without having to undock the robot. 19 A lower-cost version of the Xi, the X, was released in 2017 with upgrades from Xi, but this model was criticized for its reduced versatility due to the installation of a side cart. 2 With each iteration, additional training has been necessary for surgeons and trainees alike to master the constantly changing technology needed to perform robotic surgery.
Current State of Robotic-Assisted Surgery and Training
Despite high initial costs, robotic technology has seen a significant increase in hospital system integration across the United States. The increased utilization of robotic surgical technology is due, in part, to the favorable public perception of robotic surgery, as well as the fact that many hospitals are looking to promote minimally invasive approaches. The da Vinci robot is currently the most popular surgical robot, with over 5,000 active systems performing more than one million surgeries annually. 22 RAS is used for a variety of procedures across many specialties, including obstetrics and gynecology, urology, and otolaryngology, as well as breast, endocrine, hepatobiliary, thoracic, colorectal, and general surgery. 2 Applications specific to the field of plastic surgery include breast reconstruction, microsurgery and supermicrosurgery, transoral robotic surgery, pelvic and abdominal reconstruction, nerve surgery, and gender affirmation surgery. 4
The rapid adoption of robotic surgery in the 2000s led to the development of virtual reality (VR) simulators and specific training curricula for learning the fundamental skills of robotic surgery (FSRS) in both surgical trainees and practicing surgeons seeking competency in robotic surgery. As the many da Vinci patents have expired between 2016 and 2022, the influx of competing robotic platforms is expected to reduce costs, making robotic surgical technology more accessible for both health care entities and residency training programs. 19
As RAS becomes more prevalent in plastic surgery, a standardized RAS training curriculum is necessary in order to ensure appropriate robotic training for plastic surgery residents. A comprehensive RAS training program should include three basic components: theoretical knowledge, simulation, and bedside assist and console management. 23 Alongside learning these technical skills, successful robotic surgery also requires mastery of multiple nontechnical skills (NTS). All of these components will be discussed in detail below.
Component 1: Theoretical Knowledge Acquisition
The first component of robotic surgery education takes the form of theoretical knowledge acquisition through self-paced online modules and didactic lectures that typically begin in the first year of training ( Fig. 1 ). There are multiple platforms that incorporate introductory, self-paced learning such as the Fundamentals of Robotic Surgery (FRS) and the FSRS. Knowledge acquisition is supplemented with hands-on introduction to the robotic system by either faculty or a robotic platform representative. Trainees should understand basic system components such as the console, remote manipulator arms, visualization towers, and robotic instruments, cables, and connectors. 24 Other didactic topics include how to troubleshoot common errors on the surgical robot, team coordination and communication, and situational awareness. 24
Fig. 1.
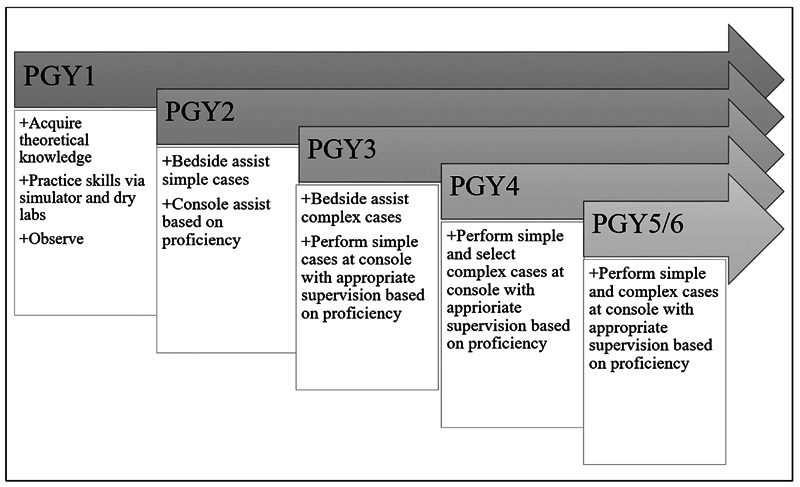
Robotic-assisted surgery trainee curriculum goals per postgraduate year (PGY).
Component 2: Basic Skill Acquisition through Simulation
The second component of RAS skill acquisition is active simulation, which provides an efficient and safe training platform that poses zero risk to patients. 25 The role of simulation is to closely emulate operating on the robotic system in order to achieve basic (manipulation of joystick, pedals, and camera) and advanced (dissection and suturing) gestural skills. 25 Alongside dedicated mentorship, structured simulation has been shown to reduce the number of operations needed in order to master a new operative procedure and therefore offers utility for busy surgical residents. 26 27 28 Many of the more commonly used robotic systems, such as DaVinci and SYMANI, have dedicated simulators on which skills can be acquired and practiced.
Dry and Wet Lab Simulation
Dry lab simulation allows trainees to operate with genuine robotic instruments on curated models that are designed for practice with robotic cutting, dissecting, and suturing. The use of dry lab models allows trainees to learn basic robotic techniques, gain confidence with the robotic system, and build the unique fine motor skills necessary for RAS. Dry lab simulation for robotic microanastomosis using artificial vessels has been validated and shown to be a cost-effective way to improve learner skill after as few as five sessions. 29 McDougall et al demonstrated that increased technical skill, reduced operating time, and more participation in RAS cases posttraining were associated with previous dry lab training. 30 However, dry lab simulation is limited by its ability to simulate the look and feel of operating on real human tissue and therefore should be utilized to gain general familiarity with the robotic system and development of basic psychomotor skills. 28
In wet laboratory simulation, cadaveric and animal models are incorporated, which are better suited for understanding tissue responsiveness to robotic force and acclimation to the lack of robotic haptic feedback. 28 Similar skills to dry lab simulation are practiced, with the added benefit of robotically handling tissue. Other wet lab applications include porcine models for robotic flap-harvest and mouse models for microanastomosis, which have been used in feasibility studies and could be a beneficial addition for plastic surgery trainee education when learning robotic flap harvest technique. 31 32 Cadaveric and animal models are more costly than artificial models, which may be a barrier for institutional integration. Additionally, a surgical robot is required for both dry and wet lab training, which can be difficult in terms of scheduling and accessibility, as hospitals often only own one to two surgical robots that are routinely in use in the operating room. One solution to this problem is to purchase a separate robotic system for the sole purpose of simulation and training. Another solution is the use of VR simulation.
Virtual Reality Surgical Simulation
The advancement of robotic surgery VR simulation has created the opportunity for trainees to practice RAS skills without the need for an actual robotic system. 33 Much like dry and wet lab training, VR simulators create the look and feel of operating on an actual robotic console, in which trainees practice psychomotor and basic procedural skills. VR surgical simulators available for purchase include the da Vinci Surgical Skills Stimulator (dVSS; Intuitive Surgical, Sunnyvale, CA), the DV-Trainer (Mimic Technologies Inc., Seattle, WA), the Robotix Mentor (Symbionix), and the new Ross II (Simulated Surgical System). 25 Simulators cost upward of $100,000, which can limit access for residency programs. The dVSS is the most widely utilized simulator among hospital institutions, and the DV-Trainer is the most robustly validated, displaying face, content, and construct validity. 19 25 34
Each VR system has compatible curriculum modules that introduce the learner to basic robotic skills and functionalities. Generally, VR curriculum includes a series of tasks such as instrument and camera control, robotic arm control, third-arm functionality, EndoWrist manipulation, needle handling, electrocautery, tissue handling, blunt dissection, vessel dissection, and knot tying. 33 Each task has varying levels of difficulty that the learner progresses through, and performance can be measured with assessments validated for VR robotic simulation.
Accessing a robotic simulator and VR training is expensive and can be cost-prohibitive. However, it is important to note that utilizing these technologies can potentially shorten the learning curve of robotic surgery training by achieving basic skills on low-fidelity, easily accessible, open and laparoscopic simulation, saving both time and money.
Transferability of Open, Laparoscopic, and Microsurgery Skills
There has been a rapid increase in robotic surgery over the past decade due to its advantages over open and laparoscopic surgery. 25 Numerous studies have assessed whether skills acquired through open or laparoscopic experience may transfer to and complement robotic-assisted operations, and if these skills can reduce the time needed on robotic simulators and, in effect, optimize training duration and cost-effectiveness. 25 35 Chahal et al conducted a systematic review that found trainees' open surgical skills successfully transferred to the robotic setting, with skill transfer measured by higher performance metrics in trainees with previous open surgery experience. 25 The authors attribute knowledge of anatomy and approaches for operating in a certain region as contributory to the successful skill transfer from open surgery. 25
Previous laparoscopic experience displayed conflicting evidence for robotic skill transfer, but did result in reduced robotic procedure time and positive skill transfer for advanced tasks in the initial phase of the learning curve. 25 35 Despite strong evidence of skill transfer, an understanding of laparoscopic surgery such as patient positioning, port placement, pneumoperitoneum creation, and management of complications are important aspects for performing RAS and should be incorporated into standardized training programs for well-rounded training. 24 Barnes et al reported that trainees find value in completing laparoscopic training prior to engaging with robotic surgery platforms, as it increases their comfort with hand motions, instrument manipulation, orientation relative to the camera, and understanding of surgical engagement. 36 As such, financially limited institutions could consider adding laparoscopic training to their current models, as this technology is relatively accessible and low-cost compared with robotic simulation training.
Microsurgery represents one of the most technically challenging fields of surgery, requiring significant dexterity and manual skill. Utilizing the robotic platform to learn microanastomotic technique may be of use to surgical trainees, which in conventional microsurgery represents one of the longest learning curves to master. 29 37 In a study conducted by Van Mulken et al, robotic-assisted microvascular anastomoses had a steeper learning curve slope when compared with anastomoses performed with conventional microsurgery, indicating a more rapid improvement and thus, skill acquisition. 11 Karamanoukian et al found no significant difference between increased operative times and technical errors in fully trained microvascular surgeons and midlevel surgical trainees while performing robotic-assisted microvascular anastomosis. 38 While Karamanoukian's study suggests there is no transfer of microsurgical skill for RAS, it also indicates that previous microsurgical training is not required to learn how to perform robotic-assisted microvascular anastomosis, with the potential for trainees to gain microsurgical robotic experience even earlier in their training. 38
Familiarizing oneself with the practice of microscopic surgery has been shown to be useful when learning robotic surgery. 39 This parallel is due to the similarity between gestures and ergonomics of the two techniques, which both involve working in a reduced workspace under binocular vision. That is, working under magnification, either under loupes or through an operative microscope, is analogous to working at the console of a da Vinci robot, suggesting that this practice can help operators become more accustomed to robotic surgery.
In summary, open, laparoscopic, and robotic surgery are all useful adjuncts when learning RAS and should be integrated into RAS training in a specialty-specific approach.
Component 3: Bedside Assist and Console Training
RAS mastery requires extensive preparation prior to performing surgery at the console. Preconsole training should ensure that trainees have a foundational knowledge of the robotic system and have participated in an adequate number of RAS cases as the bedside assistant. 23 As the bedside assistant, performance of critical tasks such as trocar placement, robot docking, efficient instrument exchange, lavage, and troubleshooting develop both technical and operational RAS skills. Bedside assisting also portends experience in active, direct communication with the console surgeon, as the bedside assist is often the sole person at the patient's side during the case and maintains visualization of the global operating field. Current literature sites an appropriate range of 5 to 20 cases as bedside assistant, most often starting in the second year of training, before progressing to console surgeon. 23
Dual-Console Training
Dual-console setups can be incorporated as a valuable training adjunct as trainees begin learning how to operate at the console. 24 The da Vinci Si and Xi systems introduced the dual-console platform, which allows two surgeons to operate at two different console systems independently. The da Vinci Xi can transfer instruments from one console to the other with the press of a button, which encourages active skill teaching and demonstration during robotic cases. 40 The platform has the added capability of applying an emergency stop to one of the consoles during a procedure, if necessary. Dual-console technology allows trainees to safely practice and perform RAS under close supervision, while the attending can at the same time use her console for real-time feedback and instruction. 40
Numerous studies cite that for trainees, the most challenging progression in RAS training is that of advancing from bedside assistant to console surgeon. 41 42 Dual-console technology may augment the leap from bedside to console and create opportunity for both integrated teaching and active assessment of the surgical trainee.
After achieving sufficient bedside assist experience, the resident transitions to operating at the robotic console as lead surgeon, typically in the third or fourth year of training. Through console training, the resident develops a holistic understanding of the interactions between the patient, console surgeon, bedside assistant, and supporting team members, and sees how these components relate to the success of the operation. 24 The timing for bedside to console transition is variable, with most residents stating that this transition is dependent on faculty comfortability with both RAS themselves and trainee skill. 41 Another challenge for navigating this transition is that the trainee usually has to find her own replacement for bedside assist. 41 However, this role is typically filled by a surgical technologist, as it is imperative for residents to get adequate exposure operating at the console in order to be prepared for future practice, especially as RAS becomes more integrated into the field of plastic surgery.
Existing Technical Curricula
Robotic surgery does not currently have a designated gold-standard curriculum for surgical residents and robotically naïve surgeons. The need for a standardized curriculum is mounting, as an increasing number of surgical specialties are expanding operations to incorporate robotic surgery. As outlined above, robotic surgery for the novice typically starts with a theoretical introduction of the basic principles of robotic surgery and familiarization with the function of the various components of the robotic system ( Fig. 1 ). 43 Bedside and console training typically follow. A comprehensive training curriculum that results in robotic surgical proficiency does not yet exist outside of the individualized apprenticeship model. However, multiple professionally supported and well-validated robotic surgery courses are available for the purpose of initial accreditation before progressing to bedside training, which we will outline below ( Table 1 ).
Table 1. Assessment scales for competency in robotic surgery training.
| Author | Setting | Outcome | Domains | Scoring | |
|---|---|---|---|---|---|
| GEARS | Goh et al | Simulation drills | Robotic technical performance | Depth perception Bimanual skill Efficiency Force control Autonomy Robot control |
Max total score = 35 Expert = 29.8 ± 0.4 Intermediate = 24 ± 2.8 Novice = 16 ± 3 |
| R-OSATS | Siddiqui et al | Proctored dry lab exam (five inanimate drills) | Robotic surgical competence | Accuracy Force/tissue handling Dexterity Efficiency |
Max score per drill = 20 Minimum threshold score per drill = 13 |
| SARMS | Selber et al | Dry lab microvascular anastomosis | Robotic microsurgical technical performance | Dexterity Visuospatial ability Operative flow Camera movement Depth perception Wrist articulation Communication Atraumatic needle handling Atraumatic tissue handling Overall performance |
Each item scaled from 1 (novice) to 5 (expert) Overall performance scaled from 1 (poor) to 5 (excellent) |
| RAS - NOTECHS | Schreyer et al | Robotic operating room live | Nontechnical skills of robotic surgical team | Leadership and management Teamwork and cooperation Problem-solving and decision-making Situational awareness |
Each item scaled 1–8 1 = “consistently compromises patient safety and teamwork” 8 = “consistently enhances patient safety and teamwork” Baseline competence per item = 6 |
| ICARS | Raison et al | Robotic operating room live | Nontechnical skills of robotic console surgeon | Communication and team skills Leadership Decision-making Situational awareness Stress and distractors Overall Score |
Each item scaled 1 (unacceptable) to 5 (excellent) Overall score scaled 1 (unacceptable) to 5 (excellent) |
Abbreviations: ICARS, Interpersonal and Cognitive Assessment for Robotic Surgery; RAS-NOTECHS, Robotic-Assisted Surgery, Nontechnical Skills; R-OSATS, Robotic-Objective Structure Assessment of Technical Skill.
Fundamentals of Robotic Surgery
The FRS course was developed by a team of expert robotic surgeons spanning 11 specialties alongside the US Department of Defense and the Minimally Invasive Robotics Association. 43 FRS is meant to be broad, so regardless of both robotic system and learner surgical specialty, the curriculum provides a foundation that can be applied across robotic platforms. Training serves as a basic introduction to robotic surgery, with virtual modules that focus on the key skills and requirements for the robotic surgeon. The modules are as follows: introduction to surgical robotic systems, didactic instruction on robotic surgery systems and psychomotor skills curriculum, and nontechnical instruction on team coordination, effective communication, and situational awareness. FRS was validated in a 2020 multi-institutional trial of 33 robotic surgery experts and 123 inexperienced surgical trainees who showed marked improvement in completion time and errors committed during five basic robotics tasks after undergoing FRS training. 44 To date, FRS is the only robotic surgery curriculum that has been launched for public use, with free access online at frsurgery.org. The FRS group is in the process of developing specialty-based accreditation schemes in order to provide trainees with specialty-specific skills.
Fundamental Skills of Robotic Surgery
The FSRS course is a simulation-based course developed by Roswell Cancer Institute in Buffalo, NY. 45 The curriculum consists of 16 tasks divided into four modules developed by experts in robotic surgery and a VR simulation engineer. Modules consist of basic console orientation, psychomotor skills, basic surgical skills, and intermediate surgical skills based on validated tasks for the Fundamentals of Laparoscopic Surgery curriculum. An evaluation is performed at the end of each module, and an assessment of overall robotic skills gained upon completion is assigned based on the Robotics Skills Assessment Score. The curriculum utilizes the Robotic Surgical Simulator, a validated VR simulator that retails between $95,000 and $125,000. 46 A randomized, multisite study validated the FSRS curriculum's face, content, and construct validity. 47
Basic Skills Training Curriculum
The Basic Skills Training Curriculum (BSTC) course was developed at the University of Toronto in order to teach basic robotic skills across several specialties over the course of 4 weeks. 48 Week 1 consists of completing online modules, including the first module of the FRS training mentioned above. Weeks 2 to 4 involve hands-on practice sessions with the dVSS. These sessions involve camera navigation, third-arm functionality, instrument clutching, EndoWrist manipulation of objects, cautery, needle handling and driving, and dissection. Proficiency is tested at the end of 4 weeks, measuring time to completion and number of errors committed during standardized ring transfer and needle passing tasks. BSTC was validated during a pilot study of 37 trainees who displayed significant improvement on standardized assessments after undergoing BSTC training. 48
European Association of Urology Robotic Urology Section Fellowship Curriculum
The European Association of Urology Robotic Urology Section (ERUS) course is a urology-specific, 3-month course taken at an approved “host center” developed by the European Association of Urology. 49 The training starts with an online theoretical training module followed by simulation and observation in dry and wet labs and a VR simulation over a period of 1 week. The trainee then begins hands-on training, performing live surgery starting with observation and bedside assistance. Next, the trainee completes module training for robotic-assisted radical prostatectomy under attending supervision. At the end of the 12-week training period, trainees are evaluated for proficiency by their mentors using the Global Evaluative Assessment of Robotic Skills (GEARS) and Nontechnical Skills for Surgeons (NOTSS). ERUS was validated in 2019 in a pilot study with a single trainee, and there are plans in place for future validation with a larger sample size. 49
Existing Robotic Surgery Curricula
Standardized, validated assessment tools are necessary in order to track skill acquisition as the trainee progresses through the robotic surgery curriculum. Although time to complete a task is often used, this is not necessarily indicative of skill acquisition and mastery of technique. 50 Validated evaluation scales for robotic surgery skill acquisition should include observation of fundamental movements and behaviors with minimal errors that display an adequate understanding of the technique. Here we outline multiple validated assessment tools commonly used for robotic surgery training.
Global Evaluative Assessment of Robotic Skills
The GEARS course was adapted from the Global Assessment of Laparoscopic Skills created by Vassiliou et al in 2005 to evaluate performance in laparoscopic surgery. 51 52 GEARS includes four domains (depth perception, bimanual skill, efficiency, and autonomy) with the addition of robotics-specific parameters, robotic control, and force control. 50 GEARS was shown to successfully differentiate between individuals with various levels of robotic surgery experience (novice, intermediate, and expert) in a cross-sectional study conducted by Sánchez et al and can therefore be used to track skill acquisition over time. 50
Robotic-Objective Structure Assessment of Technical Skills
The Robotic-Objective Structure Assessment of Technical Skills course utilizes five standardized simulation drills that measure robotic skill competency. Three exercises focus on general robotic skills (dexterity, needle manipulation, and suturing) in a dry lab setting and can be applied to trainees across specialties. Each task is scored in the following categories: (1) accuracy, (2) force/tissue handling, (3) dexterity, and (4) efficiency. Siddiqui et al at Duke University established a proposed minimum passing score of 14 out of 20 per drill after comparing trainees to experienced robotic surgeons. 53
Structured Assessment of Robotic Microsurgical Skills
The Structured Assessment of Robotic Microsurgical Skills (SARMS) course adapts the Structured Assessment of Microsurgical Skills to include parameters relevant to robotic surgery. Microsurgical skills assessment includes dexterity, visuospatial ability, operative flow, and judgement. Robotic skills are assessed by camera movement, depth perception, wrist articulation, and atraumatic needle and tissue handling. Selber et al at the University of Texas MD Anderson Cancer Center conducted a validation for the SARMS that showed excellent interrater reliability for each skill area. 39
Existing Nontechnical Curricula
The role of NTS in robotic surgery has been shown to be critical for successful performance. A study of 146 surgical adverse events found that after inexperience/incompetence, the five most common contributing factors were communication breakdown, excessive workload/lack of staffing, lack of supervision, fatigue, and interruptions/distractions. To address these challenges, assessment tools specifically measuring NTS in robotic surgery were developed.
Robotic-Assisted Surgery, Nontechnical Skills
The Robotic-Assisted Surgery, Nontechnical Skills (RAS-NOTECHS) course was developed using the Delphi approach for operating room personnel (surgeons, nurses, and anesthetists) at two different academic urology departments. 54 Adapted from the validated NOTECHS II used in conventional surgery, RAS-NOTECHS assesses nontechnical behaviors in the operating room specific to robotic surgery challenges. Fifteen expert-approved, robotic-specific behavioral markers that apply to multidisciplinary RAS teams were added to the NOTECHS II assessment (12 markers pertaining to the surgeon, 9 pertaining to nursing, and 5 pertaining to anesthesia). The assessment includes four behavioral dimensions: leadership and management, teamwork and cooperation, problem-solving and decision-making, and situational awareness. In a study conducted by Schreyer et al, RAS experts established content validation of the RAS-NOTECHS; however, further work is needed to establish overall construct and criterion validity. 54
Interpersonal and Cognitive Assessment for Robotic Surgery
The Interpersonal and Cognitive Assessment for Robotic Surgery (ICARS) course was developed as a measure of nontechnical behavioral skills specifically required in robotic surgery. 55 NTS behaviors were chosen from robotic theatre observation and interviews with robotic surgeons. ICARS is used to evaluate the surgeon both at the bedside and the console. NTS include communication and team skills, leadership, decision-making, situational awareness, stress, and distractors. ICARS was validated in a simulated operating room environment consisting of four scripted scenarios, each with increasing difficulty. ICARS was found to successfully distinguish between individuals with varying levels of robotics experience and to be equivalent to the gold standard, NOTSS, on Bland–Altman analysis. 55
It is widely known that robotic surgery presents unique challenges regarding operative flow between surgeon and operating room staff. Unlike in traditional surgery, where all members of the surgical team are centered around the operating table and often facing one another, the robotic surgeon operates at a console stationed away from the patient. Due to the immersive nature of operating at the console, the surgeon cannot see the patient or robot and therefore relies on the operating room team to ensure a sterile surgical field, prevent robotic arm collisions, and inform her of relevant events outside of her field of vision. Without visual cues and feedback, uncertainty regarding whom the surgeon is addressing is also more likely unless there is explicit, targeted communication.
Reduced face-to-face interaction makes the efficiency and overall success of the operation dependent on strong communication and NTS, including situational awareness, decision-making capacity, leadership, and teamwork. 54 Studies have shown a link between a lack of NTS and increased technical errors, operative disruptions, and worse teamwork, with failures in communication being the second most common cause of surgical incidents. 54 As such, in order to track proficiency of skill acquisition in a field so reliant on NTS, these behaviors must be evaluated alongside technical skills so that opportunities for learning and improvement can occur. To date, there are two validated, reliable assessments to gauge robotic-specific NTS, ICARS and RAS-NOTECHS, described above. Assessing and incorporating NTS development during RAS training is a key pillar to future success as a robotic surgeon and team. Despite validated assessments for measuring NTS competence in RAS, formal instruction on developing these skills is lacking in many published curricula and is an area that should be investigated and incorporated into future training. 24
Learning Curve
A learning curve illustrates the rate of learning during new skill acquisition. The curve is steepest at the beginning, while new knowledge is rapidly integrated, and reaches a plateau once maximum efficiency is attained. White et al conducted a 4-year prospective study of 168 patients in order to denote the learning curve for transoral robotic surgery for head and neck reconstruction at a single institution. 56 Patients were divided into four consecutive groups with 42 patients each, based on surgery date. Over time, there was a significant decrease in robotic docking operative duration, with a 47% reduction in overall operative time between groups 1 and 4. The efficiency plateau began to emerge after the 110th transoral robotics case. 56
Selber et al investigated the learning curve for microsurgical robotic surgery with 10 plastic surgeons that each performed five recorded robotic microvascular anastomoses. 37 Each session was evaluated using the SARMS assessment tool. Overall performance and indicative skill improved for all surgeons over the course of the five sessions, with an average reduction in operating time from 30.1 to 18.9 minutes. A univariate mixed-model analysis showed a cumulative increase in skill proficiency with additional sessions, with atraumatic needle handling and atraumatic tissue handling improving the quickest. 37
In 2006, Patel proposed a 20-case minimum in general surgery training with competency acquisition starting with robotic docking and setup, progressing to assistance with console and surgery, and then performing parts of procedures or entire procedures independently. 57 Current literature describing the learning curve for RAS naïve surgeons and trainees is surgery-specific, and thus less helpful for defining basic RAS competency case requirements. As such, more studies are needed in order to further denote the learning curves for plastic surgery-specific RAS so that an effective RAS training protocol can be implemented into plastic surgery training.
A 2017 review article by Mazzon et al outlining learning curves for robotic urologic surgeries suggests that patient outcomes and surgeon comfort continue to improve with ongoing practice in robotic surgery beyond the current minimum of 50 cases required by the Accreditation Council for Graduate Medical Education (ACGME). 58 For robotic-assisted laparoscopic prostatectomy, operative time, blood loss, early continence, patency, and positive surgical margin rate generally plateaued after 50 to 250 cases. For robotic-assisted radical cystectomy and robotic-assisted partial nephrectomy, the learning curve plateaus at an earlier point, after anywhere between 20 and 100 cases depending on the study and outcomes measured. However, some studies demonstrated have continued improvement well beyond 100 cases. In recognition of this need for additional experience, the ACGME is raising its minimum requirements from 50 to 80 RAS cases for 2021 graduates. 24
There is not currently a standard minimum requirement for robotic surgical cases established by the ACGME for integrated plastic surgery trainees. A survey conducted by Jimenez et al in 2022 reported that 89.7% of plastic surgery residents and practicing plastic surgeons believed that RAS should be implemented into future plastic surgery training, with many respondents citing cost, increased operative times, and inadequate training as barriers to RAS implementation. The areas in which RAS was believed to be most valuable in plastic surgery include microsurgical anastomosis/reconstruction (81.8%), abdominal wall reconstruction (74.4%), breast reconstruction (62.1%), and head and neck reconstruction (83%) ( Fig. 2 ). 17 With the rapid growth of RAS in both general surgery and surgical subspecialties, a set standard case requirement to denote basic RAS competency for plastic surgery trainees needs to be defined.
Fig. 2.
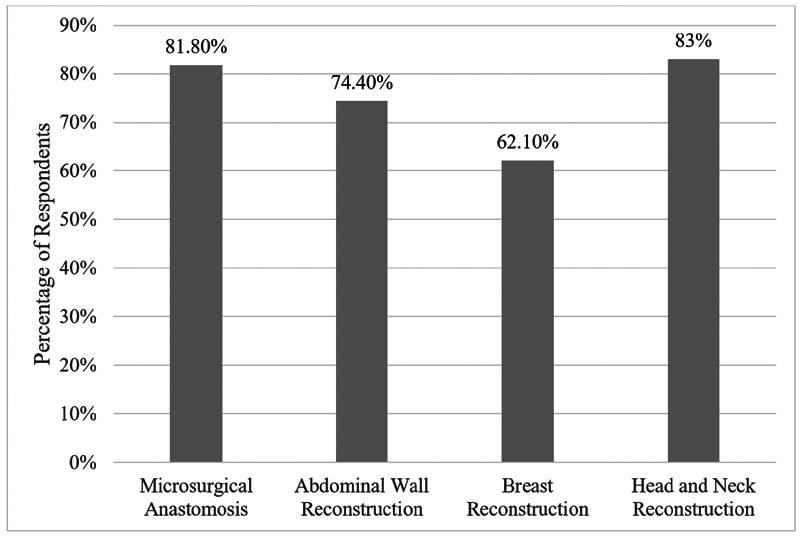
Proportion of plastic surgery trainees considering different types of robotic-assisted plastic surgery procedures as safe and valuable.
Training Pathways and Implications for Robotic Skill Acquisition
Plastic surgery training began as an offshoot from full general surgery training. Over the years, the ties between general and plastic surgery have loosened to the point that most plastic surgery programs have become integrated, including less and less general surgery. The consequence of this is that fewer plastic surgery trainees have any experience in robotic surgery during residency. This makes training for specific robotic plastic surgery procedures more difficult because the resident must be trained on the robot from the ground up for a specific procedure, rather than with graduated responsibility, as in general surgery or urology training. Most independent trainees find themselves less prepared for plastic surgery practice than integrated trainees, where plastic surgery is taught more consistently through all six years.
For robotic surgery, there is an exception. Since independent residents partake in robotic training during residency, they are easier to teach and more readily learn robotic plastic surgery procedures due to the foundation of skills acquired during general surgery training. This places independent trainees at an advantage when it comes to learning robotic plastic surgery. The senior author doubts that this will alter the training pathway choice for many graduates coming out of medical school, but it may. At the least, it is an unanticipated advantage to independent residencies that they may more easily train robotic plastic surgeons.
Conclusion
RAS has become increasingly popular in recent years due to its favorable public perception and the fact that many hospitals are looking to promote minimally invasive surgery. As RAS continues to take hold in plastic surgery, the need for a standardized robotic training curriculum for trainees continues to rise. Multiple studies have shown that trainees display improvements in technical performance and confidence level after completing a structured robotic surgery skills training program. 59 60 We therefore propose that an RAS training curriculum specific to plastic surgery should maintain the four basic tenants of current robotic training: theoretical knowledge, simulation, bedside assist, and console operating, with adaptations included within each tenant for specific plastic surgery robotic skills and procedures ( Table 2 ).
Table 2. Essential knowledge and skill acquisition for competency in robotic-assisted surgery.
| Theoretical knowledge |
|---|
| Robotic console, platform, and functions |
| Design and function of robotic instruments |
| Robotic software interface |
| Common system errors and solutions |
| Optimal patient positioning |
| Technical skills |
| Maneuver patient cart and dock to port |
| Perform planned and unplanned docking |
| Convert to open surgery |
| Introduce and exchange robotic instruments |
| Handle robotic instruments and camera from console |
| Demonstrate proper handling of tissue and dissection |
| Demonstrate robotic suturing and knot tying |
| Nontechnical skills |
| Understand team members and roles |
| Define operating room workflow |
| Communicate effectively during robotic operations |
| Train new/existing team members |
For robotic-assisted plastic surgery procedures that have proven clinical feasibility, steps and necessary skills to complete these operations should be outlined by experienced faculty and then incorporated into dry, wet, and VR simulation training.
Institutional variability in RAS procedures is a limiting factor in standardized bedside and console training, as the trainee is limited to the robotic-assisted plastic surgery procedures performed at her respective institution. A solution to address this variability could include a centralized RAS boot camp for plastic surgery trainees, modeled after the ERUS Robotic Fellowship Program in which trainees travel to a designated host institution for dry, wet, and VR simulation practice followed by active participation in RAS cases under the supervision of faculty. Creating a centralized RAS curriculum for plastic surgery trainees substantiates the notion that the field of plastic surgery will be able to keep up with the demand for minimally invasive options in the future and remain up to date with the technological innovations taking hold in surgery today.
Funding Statement
Funding None
Footnotes
Conflict of Interest None declared.
References
- 1.Leal Ghezzi T, Campos Corleta O. 30 years of robotic surgery. World J Surg. 2016;40(10):2550–2557. doi: 10.1007/s00268-016-3543-9. [DOI] [PubMed] [Google Scholar]
- 2.Maza G, Sharma A. Past, present, and future of robotic surgery. Otolaryngol Clin North Am. 2020;53(06):935–941. doi: 10.1016/j.otc.2020.07.005. [DOI] [PubMed] [Google Scholar]
- 3.Ismail I, Wolff S, Gronfier A, Mutter D, Swanström L L. A cost evaluation methodology for surgical technologies. Surg Endosc. 2015;29(08):2423–2432. doi: 10.1007/s00464-014-3929-4. [DOI] [PubMed] [Google Scholar]
- 4.Hassanein A H, Mailey B A, Dobke M K. Robot-assisted plastic surgery. Clin Plast Surg. 2012;39(04):419–424. doi: 10.1016/j.cps.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 5.Bishop S N, Asaad M, Liu J et al. Robotic harvest of the deep inferior epigastric perforator flap for breast reconstruction: a case series. Plast Reconstr Surg. 2022;149(05):1073–1077. doi: 10.1097/PRS.0000000000008988. [DOI] [PubMed] [Google Scholar]
- 6.Lee M J, Won J, Song S Y et al. Clinical outcomes following robotic versus conventional DIEP flap in breast reconstruction: a retrospective matched study. Front Oncol. 2022;12:989231. doi: 10.3389/fonc.2022.989231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Selber J C. The robotic DIEP flap. Plast Reconstr Surg. 2020;145(02):340–343. doi: 10.1097/PRS.0000000000006529. [DOI] [PubMed] [Google Scholar]
- 8.Gundlapalli V S, Ogunleye A A, Scott K et al. Robotic-assisted deep inferior epigastric artery perforator flap abdominal harvest for breast reconstruction: a case report. Microsurgery. 2018;38(06):702–705. doi: 10.1002/micr.30297. [DOI] [PubMed] [Google Scholar]
- 9.Clemens M W, Kronowitz S, Selber J C. Robotic-assisted latissimus dorsi harvest in delayed-immediate breast reconstruction. Semin Plast Surg. 2014;28(01):20–25. doi: 10.1055/s-0034-1368163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Selber J C. Robotic latissimus dorsi muscle harvest. Plast Reconstr Surg. 2011;128(02):88e–90e. doi: 10.1097/PRS.0b013e31821ef25d. [DOI] [PubMed] [Google Scholar]
- 11.MicroSurgical Robot Research Group . van Mulken T JM, Wolfs J AGN, Qiu S S et al. One-year outcomes of the first human trial on robot-assisted lymphaticovenous anastomosis for breast cancer–related lymphedema. Plast Reconstr Surg. 2022;149(01):151–161. doi: 10.1097/PRS.0000000000008670. [DOI] [PubMed] [Google Scholar]
- 12.Li R A, Jensen J, Bowersox J C. Microvascular anastomoses performed in rats using a microsurgical telemanipulator. Comput Aided Surg. 2000;5(05):326–332. doi: 10.1002/1097-0150(2000)5:5<326::AID-IGS2>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 13.Chang E I, Skoracki R J, Chang D W. Lymphovenous anastomosis bypass surgery. Semin Plast Surg. 2018;32(01):22–27. doi: 10.1055/s-0038-1636510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.MicroSurgical Robot Research Group . van Mulken T JM, Schols R M, Scharmga A MJ et al. First-in-human robotic supermicrosurgery using a dedicated microsurgical robot for treating breast cancer-related lymphedema: a randomized pilot trial. Nat Commun. 2020;11(01):757. doi: 10.1038/s41467-019-14188-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamamoto T, Yamamoto N, Kageyama T et al. Supermicrosurgery for oncologic reconstructions. Glob Health Med. 2020;2(01):18–23. doi: 10.35772/ghm.2019.01019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Badalato G M, Shapiro E, Rothberg M B et al. The da Vinci robot system eliminates multispecialty surgical trainees' hand dominance in open and robotic surgical settings. JSLS. 2014;18(03):e2014.00399. doi: 10.4293/JSLS.2014.00399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jimenez C, Stanton E, Sung C, Wong A K. Does plastic surgery need a rewiring? A survey and systematic review on robotic-assisted surgery. JPRAS Open. 2022;33:76–91. doi: 10.1016/j.jpra.2022.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin C Y, Liu Y C, Chen M C, Chiang F F. Learning curve and surgical outcome of robotic assisted colorectal surgery with ERAS program. Sci Rep. 2022;12(01):20566. doi: 10.1038/s41598-022-24665-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zaman M F, Buchholz N, Bach C. Robotic surgery and its application in urology: a journey through time. EMJ Uro. 2021:72–82. [Google Scholar]
- 20.Bodner J, Augustin F, Wykypiel Het al. The da Vinci robotic system for general surgical applications: a critical interim appraisal Swiss Med Wkly 2005135(45-46):674–678. [DOI] [PubMed] [Google Scholar]
- 21.Esposito M P, Ilbeigi P, Ahmed M, Lanteri V. Use of fourth arm in da Vinci robot-assisted extraperitoneal laparoscopic prostatectomy: novel technique. Urology. 2005;66(03):649–652. doi: 10.1016/j.urology.2005.03.061. [DOI] [PubMed] [Google Scholar]
- 22.Cepolina F, Razzoli R P. An introductory review of robotically assisted surgical systems. Int J Med Robot. 2022;18(04):e2409. doi: 10.1002/rcs.2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lagrange F, Fiard G, Larose C, Eschwege P, Hubert J. Role and training of the bedside surgeon in robotic surgery: a survey among French urologists-in-training. Res Rep Urol. 2022;14:17–22. doi: 10.2147/RRU.S344369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang R S, Ambani S N. Robotic surgery training: current trends and future directions. Urol Clin North Am. 2021;48(01):137–146. doi: 10.1016/j.ucl.2020.09.014. [DOI] [PubMed] [Google Scholar]
- 25.Chahal B, Aydın A, Amin M SA et al. Transfer of open and laparoscopic skills to robotic surgery: a systematic review. J Robot Surg. 2022 doi: 10.1007/s11701-022-01492-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boone B A, Zenati M, Hogg M E et al. Assessment of quality outcomes for robotic pancreaticoduodenectomy: identification of the learning curve. JAMA Surg. 2015;150(05):416–422. doi: 10.1001/jamasurg.2015.17. [DOI] [PubMed] [Google Scholar]
- 27.Rice M K, Hodges J C, Bellon J et al. Association of mentorship and a formal robotic proficiency skills curriculum with subsequent generations' learning curve and safety for robotic pancreaticoduodenectomy. JAMA Surg. 2020;155(07):607–615. doi: 10.1001/jamasurg.2020.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thornblade L W, Fong Y. Simulation-based training in robotic surgery: contemporary and future methods. J Laparoendosc Adv Surg Tech A. 2021;31(05):556–560. doi: 10.1089/lap.2021.0082. [DOI] [PubMed] [Google Scholar]
- 29.Selber J C, Chang E I, Liu J et al. Tracking the learning curve in microsurgical skill acquisition. Plast Reconstr Surg. 2012;130(04):550e–557e. doi: 10.1097/PRS.0b013e318262f14a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McDougall E M, Corica F A, Chou D S et al. Short-term impact of a robot-assisted laparoscopic prostatectomy ‘mini-residency’ experience on postgraduate urologists' practice patterns. Int J Med Robot. 2006;2(01):70–74. doi: 10.1002/rcs.71. [DOI] [PubMed] [Google Scholar]
- 31.van Mulken T JM, Schols R M, Qiu S S et al. Robotic (super) microsurgery: feasibility of a new master-slave platform in an in vivo animal model and future directions. J Surg Oncol. 2018;118(05):826–831. doi: 10.1002/jso.25195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Katz R D, Rosson G D, Taylor J A, Singh N K. Robotics in microsurgery: use of a surgical robot to perform a free flap in a pig. Microsurgery. 2005;25(07):566–569. doi: 10.1002/micr.20160. [DOI] [PubMed] [Google Scholar]
- 33.SAGES Robotic Task Force . Chen R, Rodrigues Armijo P, Krause C, Siu K C, Oleynikov D. A comprehensive review of robotic surgery curriculum and training for residents, fellows, and postgraduate surgical education. Surg Endosc. 2020;34(01):361–367. doi: 10.1007/s00464-019-06775-1. [DOI] [PubMed] [Google Scholar]
- 34.Puliatti S, Mazzone E, Dell'Oglio P. Training in robot-assisted surgery. Curr Opin Urol. 2020;30(01):65–72. doi: 10.1097/MOU.0000000000000687. [DOI] [PubMed] [Google Scholar]
- 35.Sundelin M O, Paltved C, Kingo P S, Kjölhede H, Jensen J B. The transferability of laparoscopic and open surgical skills to robotic surgery. Adv Simul (Lond) 2022;7(01):26. doi: 10.1186/s41077-022-00223-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barnes K E, Brian R, Greenberg A L et al. Beyond watching: harnessing laparoscopy to increase medical students' engagement with robotic procedures. Am J Surg. 2023;S0002-9610(23):92–2. doi: 10.1016/j.amjsurg.2023.02.024. [DOI] [PubMed] [Google Scholar]
- 37.Alrasheed T, Liu J, Hanasono M M, Butler C E, Selber J C. Robotic microsurgery: validating an assessment tool and plotting the learning curve. Plast Reconstr Surg. 2014;134(04):794–803. doi: 10.1097/PRS.0000000000000550. [DOI] [PubMed] [Google Scholar]
- 38.Karamanoukian R L, Bui T, McConnell M P, Evans G RD, Karamanoukian H L. Transfer of training in robotic-assisted microvascular surgery. Ann Plast Surg. 2006;57(06):662–665. doi: 10.1097/01.sap.0000229245.36218.25. [DOI] [PubMed] [Google Scholar]
- 39.Selber J C, Alrasheed T. Robotic microsurgical training and evaluation. Semin Plast Surg. 2014;28(01):5–10. doi: 10.1055/s-0034-1368161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bolger J C, Broe M P, Zarog M A et al. Initial experience with a dual-console robotic-assisted platform for training in colorectal surgery. Tech Coloproctol. 2017;21(09):721–727. doi: 10.1007/s10151-017-1687-8. [DOI] [PubMed] [Google Scholar]
- 41.Zhao B, Hollandsworth H M, Lee A M et al. Making the jump: a qualitative analysis on the transition from bedside assistant to console surgeon in robotic surgery training. J Surg Educ. 2020;77(02):461–471. doi: 10.1016/j.jsurg.2019.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shaw R D, Eid M A, Bleicher J et al. Current barriers in robotic surgery training for general surgery residents. J Surg Educ. 2022;79(03):606–613. doi: 10.1016/j.jsurg.2021.11.005. [DOI] [PubMed] [Google Scholar]
- 43.Smith R, Patel V, Satava R. Fundamentals of robotic surgery: a course of basic robotic surgery skills based upon a 14-society consensus template of outcomes measures and curriculum development. Int J Med Robot. 2014;10(03):379–384. doi: 10.1002/rcs.1559. [DOI] [PubMed] [Google Scholar]
- 44.Satava R M, Stefanidis D, Levy J S et al. Proving the effectiveness of the fundamentals of robotic surgery (FRS) skills curriculum: a single-blinded, multispecialty, multi-institutional randomized control trial. Ann Surg. 2020;272(02):384–392. doi: 10.1097/SLA.0000000000003220. [DOI] [PubMed] [Google Scholar]
- 45.Chowriappa A J, Shi Y, Raza S J et al. Development and validation of a composite scoring system for robot-assisted surgical training–the Robotic Skills Assessment Score. J Surg Res. 2013;185(02):561–569. doi: 10.1016/j.jss.2013.06.054. [DOI] [PubMed] [Google Scholar]
- 46.Members Of The Society Of Urologic Robotic Surgeons . Lallas C D, Davis J W. Robotic surgery training with commercially available simulation systems in 2011: a current review and practice pattern survey from the society of urologic robotic surgeons. J Endourol. 2012;26(03):283–293. doi: 10.1089/end.2011.0371. [DOI] [PubMed] [Google Scholar]
- 47.Stegemann A P, Ahmed K, Syed J R et al. Fundamental skills of robotic surgery: a multi-institutional randomized controlled trial for validation of a simulation-based curriculum. Urology. 2013;81(04):767–774. doi: 10.1016/j.urology.2012.12.033. [DOI] [PubMed] [Google Scholar]
- 48.Foell K, Finelli A, Yasufuku Ket al. Robotic surgery basic skills training: evaluation of a pilot multidisciplinary simulation-based curriculum Can Urol Assoc J 20137(11-12):430–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Volpe A, Ahmed K, Dasgupta P et al. Pilot validation study of the european association of urology robotic training curriculum. Eur Urol. 2015;68(02):292–299. doi: 10.1016/j.eururo.2014.10.025. [DOI] [PubMed] [Google Scholar]
- 50.Sánchez R, Rodríguez O, Rosciano J et al. Robotic surgery training: construct validity of Global Evaluative Assessment of Robotic Skills (GEARS) J Robot Surg. 2016;10(03):227–231. doi: 10.1007/s11701-016-0572-1. [DOI] [PubMed] [Google Scholar]
- 51.Vassiliou M C, Feldman L S, Andrew C G et al. A global assessment tool for evaluation of intraoperative laparoscopic skills. Am J Surg. 2005;190(01):107–113. doi: 10.1016/j.amjsurg.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 52.Goh A C, Goldfarb D W, Sander J C, Miles B J, Dunkin B J. Global evaluative assessment of robotic skills: validation of a clinical assessment tool to measure robotic surgical skills. J Urol. 2012;187(01):247–252. doi: 10.1016/j.juro.2011.09.032. [DOI] [PubMed] [Google Scholar]
- 53.Siddiqui N Y, Tarr M E, Geller E J et al. Establishing benchmarks for minimum competence with dry lab robotic surgery drills. J Minim Invasive Gynecol. 2016;23(04):633–638. doi: 10.1016/j.jmig.2016.03.014. [DOI] [PubMed] [Google Scholar]
- 54.Schreyer J, Koch A, Herlemann A et al. RAS-NOTECHS: validity and reliability of a tool for measuring non-technical skills in robotic-assisted surgery settings. Surg Endosc. 2022;36(03):1916–1926. doi: 10.1007/s00464-021-08474-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Raison N, Ahmed K, Abe T et al. Cognitive training for technical and non-technical skills in robotic surgery: a randomised controlled trial. BJU Int. 2018;122(06):1075–1081. doi: 10.1111/bju.14376. [DOI] [PubMed] [Google Scholar]
- 56.White H N, Frederick J, Zimmerman T, Carroll W R, Magnuson J S. Learning curve for transoral robotic surgery: a 4-year analysis. JAMA Otolaryngol Head Neck Surg. 2013;139(06):564–567. doi: 10.1001/jamaoto.2013.3007. [DOI] [PubMed] [Google Scholar]
- 57.Patel V R. Essential elements to the establishment and design of a successful robotic surgery programme. Int J Med Robot. 2006;2(01):28–35. doi: 10.1002/rcs.77. [DOI] [PubMed] [Google Scholar]
- 58.Mazzon G, Sridhar A, Busuttil G et al. Learning curves for robotic surgery: a review of the recent literature. Curr Urol Rep. 2017;18(11):89. doi: 10.1007/s11934-017-0738-z. [DOI] [PubMed] [Google Scholar]
- 59.Nathan A, Patel S, Georgi M et al. Virtual classroom proficiency-based progression for robotic surgery training (VROBOT): a randomised, prospective, cross-over, effectiveness study. J Robot Surg. 2023;17(02):629–635. doi: 10.1007/s11701-022-01467-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Waters P S, Flynn J, Larach J T et al. Fellowship training in robotic colorectal surgery within the current hospital setting: an achievable goal? ANZ J Surg. 2021;91(11):2337–2344. doi: 10.1111/ans.16677. [DOI] [PubMed] [Google Scholar]


