Abstract
Background
Paroxysmal nocturnal hemoglobinuria (PNH) is a rare hematologic disorder caused by uncontrolled terminal complement activation, which leads to intravascular hemolysis (IVH), thromboembolism (TE), renal failure, and premature mortality.
Methods
We performed a secondary analysis of data collected from patients enrolled in the Korean National PNH Registry to assess the relative importance of risk factors, specifically lactate dehydrogenase (LDH) and hemoglobin (Hb), in predicting the incidence of TE, impaired renal function, and death in complement inhibitor-naïve patients with PNH.
Results
Multivariate regression modeling indicated that LDH ≥ 1.5 × upper limit of normal (ULN), male sex, and pain were associated with increased risk of TE (P = 0.016, 0.045, and 0.033, respectively), hemoglobinuria and pain were associated with an increased risk of impaired renal function (P = 0.034 and 0.022, respectively), and TE was associated with an increased incidence of death (P < 0.001). Hb < 8 g/dL was not a predictor of TE, impaired renal function, or death in multivariate regression analyses. Standardized mortality ratio analysis indicated that LDH ≥ 1.5 × ULN (P < 0.001), Hb < 8 g/dL (P < 0.001), and Hb ≥ 8 g/dL (P = 0.004) were all risk factors for death; in contrast, patients with LDH < 1.5 × ULN had similar mortality to the general population.
Conclusion
In complement inhibitor-naïve patients with PNH, LDH ≥ 1.5 × ULN was a significant predictor of TE, and TE was a significant predictor of death. Hb was not a significant predictor of TE, impaired renal function, or death. Therefore, controlling IVH will improve clinical outcomes for patients with PNH.
Keywords: Paroxysmal Nocturnal Hemoglobinuria, Anemia, Hemolysis, Mortality
Graphical Abstract

INTRODUCTION
Paroxysmal nocturnal hemoglobinuria (PNH) is a rare, progressive, and life-threatening disease associated with uncontrolled terminal complement activity on blood cells, resulting in intravascular hemolysis (IVH), a risk of thromboembolism (TE) events, organ damage, and premature mortality.1,2,3,4,5,6,7 Patients with PNH experience debilitating clinical signs and symptoms, such as hemoglobinuria, abdominal and chest pain, anemia, and fatigue.1,4,5,8,9,10,11,12 The most common causes of death in patients with PNH are TE and renal failure, accounting for 40–67% and 8–18% of all PNH-related deaths, respectively.1,9,13,14
Lactate dehydrogenase (LDH) and hemoglobin (Hb) levels are two parameters used to monitor patients with PNH.15 Elevated levels of LDH in patients with PNH indicate IVH, with LDH ≥ 1.5 × upper limit of normal (ULN) associated with a significantly higher risk of TE.16,17 Low Hb levels indicate anemia, which can be caused by impaired erythropoiesis and hemolysis in the PNH population. However, it can be difficult to distinguish between these two contributors to anemia in patients with PNH without additional assessments of reticulocyte counts and LDH levels.1
Previously conducted retrospective studies, which included patients enrolled as part of the Korean National PNH Registry who had never received targeted treatment with a complement inhibitor, identified elevated LDH levels (≥ 1.5 × ULN) as associated with increased risk of TE and possibly mortality.14,17,18,19 However, the association between low Hb levels and mortality, renal function, or TE has not been studied within the PNH population. Previous studies have demonstrated a relationship between low Hb levels and poor renal function or TE in other conditions.20,21,22
Although IVH is an important contributor to anemia in PNH, there are limited real-world data on the relationship between LDH and Hb levels, and clinical outcomes in PNH. The primary objective of this study was to evaluate the key biomarkers, LDH and Hb, in predicting the incidence of TE, impaired renal function, and death in patients with PNH who had not been treated with complement inhibitors. Secondary objectives were to evaluate the relationship between LDH and Hb with long-term outcomes, and to estimate the standardized mortality ratio (SMR) of patients with PNH in comparison with an age- and sex-matched Korean population.
METHODS
Study design
This was a secondary analysis of a cohort study with data collected from the Korean National PNH Registry. Data were collected from patients with PNH enrolled in the registry prior to complement inhibitors being approved for use in South Korea (enrollment period: between July 2009 and November 2010). Although the patients were enrolled into the registry between 2009 and 2010, data from 1983–2009 were retrospectively collected using medical history data from before PNH diagnosis to registry enrollment.
Data source
The Korean National PNH Registry is a web-based system established by the Aplastic Anemia Working Party of the Korean Society of Hematology. There are nine participating institutions, which cover approximately 96% of the Korean PNH population. Patient data in the registry were captured using an electronic medical record form that collects patient demographics, medical history, and PNH-specific information, including erythrocyte and granulocyte clone size, symptoms and complications, laboratory values, treatment and, where applicable, cause of death.
Study population
The study population has been described previously and included patients with a confirmed diagnosis of PNH by flow cytometry. In patients diagnosed before the establishment of flow cytometry, a positive Ham or sucrose-lysis test was used to confirm PNH.14,23 Only patients from the full data set with known LDH and Hb levels were included in the present analysis. None of the patients included in the study had received complement inhibitor treatment.
Patients were stratified by LDH level at diagnosis, with IVH defined as LDH ≥ 1.5 × ULN, and lowest level of Hb within 2 months of diagnosis. Hb levels were stratified as Hb < 8 g/dL and Hb ≥ 8 g/dL.23 Classic PNH was defined as patients with clinical evidence of IVH, but no evidence of other bone marrow disorder.14 PNH-cytopenia was defined as patients with PNH in the setting of other bone marrow disorder (with or without evidence of IVH). Subclinical PNH was defined as patients with neither clinical nor biochemical evidence of IVH.
Outcomes
Outcomes assessed included TE events, impaired renal function, and mortality. TE included venous and arterial events that occurred 6 months before diagnosis of PNH and after diagnosis of PNH. TE events occurring before PNH diagnosis were included in the risk factor and mortality analyses because these events were necessary to establish the PNH diagnosis. Impaired renal function was defined as acute renal failure or estimated glomerular filtration rate less than 60 mL/min/1.73 m2 at diagnosis.
Statistical analyses
Patient demographics and clinical characteristics were summarized using descriptive statistics. Univariate and multivariate regression modeling tested the extent to which risk factors including LDH ≥ 1.5 × ULN and Hb < 8 g/dL predict TE, impaired renal function, and death. SMRs were calculated as a ratio of observed deaths in the PNH population to expected deaths of age- and sex-matched individuals in the general Korean population for the same follow-up period studied. This period ranged from 1983 to 2009, the year when the first patient in the cohort was diagnosed with PNH and the end of follow-up, respectively. Korean National Mortality rates were provided for each year in the relevant period from Statistics Korea (www.kostat.go.kr), as previously described.14 Number and percent of missing data were reported; no imputation was made for missing data in the original data source.
Ethics statement
This study was conducted in accordance with the Declaration of Helsinki, as well as local regulations for human subject research. The study protocol was reviewed and approved by the Institutional Review Board of participating institutions and of The Catholic University of Korea (KC21ZSDE0735). Informed consent was waived by the board.
RESULTS
Patient characteristics
In total, 301 patients were included in the study, the median (interquartile range, IQR) age at diagnosis was 37.0 (27.0–50.2) years and 48.8% (n = 147) were female; median (IQR) follow-up since diagnosis was 6.6 (3.2–11.2) years (Table 1). Demographics and baseline characteristics for the 217 patients with known LDH and Hb levels were comparable to the full cohort.
Table 1. Patient demographics stratified by levels of LDH and Hb and TE event pre- and post-PNH diagnosis date.
| Characteristics | Full study cohort (N = 301) | Patients with known LDH and Hb levels (n = 217) | Stratified by LDH | Stratified by Hb | Stratified by TE event timing in relation to diagnosisa | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| < 1.5 × ULN (n = 52) | ≥ 1.5 × ULN (n = 165) | P value | ≥ 8 g/dL (n = 106) | < 8 g/dL (n = 111) | P value | Pre-diagnosisb (n = 7) | Post-diagnosisa (n = 43) | ||||
| Demographics | |||||||||||
| Median (IQR) age at diagnosis, yr | 37.0 (27.0–50.2) | 37.0 (27.0–50.0) | 39.0 (27.0–52.0) | 37.0 (26.8–48.0) | 0.700 | 37.0 (25.8–51.0) | 36.0 (27.0–46.0) | 0.872 | 59.0 (41.5–66.0) | 33.5 (24.5–50.5) | |
| Female, n (%) | 147 (48.8) | 109 (50.2) | 29 (55.8) | 80 (48.5) | 0.418 | 55 (51.9) | 54 (48.6) | 0.781 | 2 (28.6) | 16 (37.2) | |
| Median (IQR) follow-up, years | 6.6 (3.2–11.2) | 6.5 (3.1–11.0) | 4.6 (1.9–6.7) | 7.1 (3.4–11.8) | 0.001 | 7.0 (3.5–11.7) | 5.4 (2.4–9.4) | 0.046 | 5.4 (1.9–8.0) | 7.1 (3.4–12.8) | |
| Concomitant treatment, n (%) | |||||||||||
| Corticosteroids | 233 (77.4) | 171 (78.8) | 32 (61.5) | 139 (84.2) | < 0.001 | 82 (77.4) | 89 (80.2) | 0.732 | 6 (85.7) | 40 (93.0) | |
| RBC transfusion | 178 (59.1) | 131 (60.4) | 33 (63.5) | 98 (59.4) | 0.719 | 50 (47.2) | 81 (73.0) | < 0.001 | 4 (57.1) | 37 (86.0) | |
| NSAIDs | 66 (21.9) | 51 (23.5) | 5 (9.6) | 46 (27.9) | 0.012 | 21 (19.8) | 30 (27.0) | 0.274 | 3 (42.9) | 22 (51.2) | |
| Immunosuppressive treatment | 62 (20.6) | 45 (20.7) | 10 (19.2) | 35 (21.2) | 0.912 | 18 (17.0) | 27 (24.3) | 0.244 | 0 (0.0) | 17 (39.5) | |
| Anticoagulants | 44 (14.6) | 35 (16.1) | 1 (1.9) | 34 (20.6) | < 0.001 | 19 (17.9) | 16 (14.4) | 0.604 | 5 (71.4) | 23 (53.5) | |
| Opioids | 39 (13.0) | 28 (12.9) | 2 (3.8) | 26 (15.8) | 0.031 | 9 (8.5) | 19 (17.1) | 0.091 | 0 (0.0) | 13 (30.2) | |
| Bone marrow transplant | 37 (12.3) | 26 (12.0) | 7 (13.5) | 19 (11.5) | 0.895 | 13 (12.3) | 13 (11.7) | 1.000 | 0 (0.0) | 10 (23.3) | |
| Median (IQR) PNH clone sizes (%) | |||||||||||
| Granulocyte | 48.8 (23.7–77.8) | 49.2 (25.6–76.6) | 21.8 (6.0–45.0) | 56.8 (32.5–81.7) | < 0.001 | 45.2 (24.6–73.0) | 56.0 (29.0–79.6) | 0.154 | 46.4 (41.8–75.9) | 53.5 (29.8–86.0) | |
| RBC | 27.9 (9.0–51.2) | 27.0 (7.9–50.5) | 7.5 (1.0–27.1) | 33.0 (13.6–54.8) | < 0.001 | 27.5 (10.7–58.9) | 24.5 (4.7–46.4) | 0.126 | 58.6 (38.9–68.0) | 27.0 (8.3–45.0) | |
| PNH subgroups, n (%) | |||||||||||
| Classic PNH | 157 (52.2) | 115 (53.0) | 27 (51.9) | 88 (53.3) | 0.324 | 62 (58.5) | 53 (47.7) | 0.135 | 6 (85.7) | 20 (46.5) | |
| PNH-cytopenia | 141 (46.8) | 101 (46.5) | 24 (46.2) | 77 (46.7) | 44 (41.5) | 57 (51.4) | 1 (14.3) | 22 (51.2) | |||
| Subclinical PNH | 3 (1.0) | 1 (0.5) | 1 (1.9) | 0 (0.0) | 0 (0.0) | 1 (0.9) | 0 (0.0) | 1 (2.3) | |||
| LDH (× ULN) | |||||||||||
| Median (IQR) fold above ULN | 4.0 (1.7–7.5) | 3.9 (1.7–7.5) | - | - | - | 3.9 (2.0–6.8) | 4.1 (1.4–7.9) | 0.881 | 3.0 (2.6–8.8) | 4.9 (2.7–8.8) | |
| < 1.5 × ULN | 53 (17.6) | 52 (24.0) | - | - | - | 22 (20.8) | 30 (27.0) | 0.356 | 0 (0.0) | 1 (2.3) | |
| ≥ 1.5 × ULN | 171 (56.8) | 165 (76.0) | - | - | - | 84 (79.2) | 81 (73.0) | 6 (85.7) | 34 (79.1) | ||
| Hb (g/dL) | |||||||||||
| Median (IQR) | 7.8 (6.3–9.7) | 7.8 (6.3–9.7) | 7.1 (6.2–10.5) | 8.0 (6.3–9.3) | 0.908 | - | - | - | 8.0 (6.9–10.0) | 7.8 (6.6–8.9) | |
| ≥ 8 g/dL | 134 (44.5) | 106 (48.8) | 22 (42.3) | 84 (50.9) | 0.356 | - | - | - | 4 (57.1) | 20 (46.5) | |
| < 8 g/dL | 139 (46.2) | 111 (51.2) | 30 (57.7) | 81 (49.1) | - | - | - | 3 (42.9) | 20 (46.5) | ||
Bold indicates statistical significance at the 5% level.
Hb = hemoglobin, IQR = interquartile range, LDH = lactate dehydrogenase, NSAID = non-steroidal anti-inflammatory drug, PNH = paroxysmal nocturnal hemoglobinuria, RBC = red blood cell, TE = thromboembolism, ULN = upper limit of normal.
aA patient who experienced a TE event both pre- and post-diagnosis was included in the ‘Pre-diagnosis’ group and excluded from the ‘Post-diagnosis’ group.
bTime from TE event to PNH diagnosis ranged from 0 to 125 days.
At diagnosis, 52.2% of patients (n = 157) were categorized as having classic PNH, 46.8% (n = 141) as having PNH-cytopenia, and 1.0% (n = 3) had subclinical PNH. In addition to the diagnosis of PNH, bone marrow disorders were reported in 47.8% of patients either before or after diagnosis of PNH. Of these, 41.5% of patients had a history of aplastic anemia and 6.3% of patients had a history of myelodysplastic syndrome. The most frequently reported symptoms or complications related to PNH were hemoglobinuria (56.8%), pain (55.8%), and abdominal pain (47.2%). To note, general pain consisted of myalgia, and excluded abdominal pain. In total, 50 patients (16.6%) experienced 74 TE events, which occurred at both venous and arterial sites (Supplementary Table 1). Most patients (43 [86.0%]) experienced TE events after PNH diagnosis (64 events [86.5%]). Of the 10 events (13.5%) that occurred in 7 patients (14.0%) pre-diagnosis of PNH, 9 (90%) took place within 43 days of the diagnosis. The event that took place 125 days pre-diagnosis was followed by an event 36 days pre-diagnosis in the same patient. Among the 43 patients (14.3%) from the full data set who died during the study, the most common cause of death was sepsis/infection (14 patients [32.6%]) (Supplementary Table 2).
Among 217 patients with recorded LDH levels at diagnosis, 24.0% (n = 52) had LDH < 1.5 × ULN and 76.0% (n = 165) had LDH ≥ 1.5 × ULN. When stratified by LDH status, incidence of TE (P < 0.001), death (P = 0.019), hemoglobinuria (P = 0.046), abdominal pain (P = 0.006), and pain (P = 0.020) were higher in patients with LDH ≥ 1.5 × ULN compared with those with LDH < 1.5 × ULN (Fig. 1A). A significantly greater proportion of patients with LDH ≥ 1.5 × ULN had use of concomitant treatment than patients who had LDH < 1.5 × ULN, including corticosteroids (P < 0.001), non-steroidal anti-inflammatory drugs (P = 0.012), anticoagulants (P < 0.001), and opioids (P = 0.031) (Table 1).
Fig. 1. Signs and symptoms related to paroxysmal nocturnal hemoglobinuria and clinical outcomes stratified by levels of (A) LDH and (B) Hb.
Hb = hemoglobin, LDH = lactate dehydrogenase, TE = thromboembolism, ULN = upper limit of normal.
*P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001.
Among the 217 patients with recorded Hb levels at diagnosis, 48.8% (n = 106) had Hb ≥ 8 g/dL and 51.2% (n = 111) had Hb < 8 g/dL. The proportion of patients with LDH ≥ 1.5 × ULN was similar between patients with Hb ≥ 8 g/dL and Hb < 8 g/dL (79.2% vs 73.0%, respectively). When stratified by Hb status, no significant differences in clinical outcomes (mortality [P = 0.287], impaired renal function [P = 0.815], and TE [P = 0.846]) were observed in patients with Hb < 8 g/dL compared with Hb ≥ 8 g/dL (Fig. 1B). The only characteristic that differed between groups was use of red blood cell transfusion from diagnosis to follow-up, which was more frequent among patients with Hb < 8 g/dL than those with Hb ≥ 8 g/dL (P < 0.001).
Regression analyses
During the follow-up period and among the 217 patients with known LDH and Hb levels, TE was reported in 18.0% (n = 39), impaired renal function in 15.2% (n = 33), and 13.4% (n = 29) died. Univariate regression analyses indicated that in patients with PNH, LDH ≥ 1.5 × ULN (P = 0.008), male sex (P = 0.040), abdominal pain (P = 0.005), dyspnea (P = 0.005), and pain (P = 0.002) were associated with an increased risk of TE. In addition, hemoglobinuria (P = 0.002), abdominal pain (P = 0.001), dyspnea (P = 0.002), and pain (P = 0.001) were associated with an increased risk of impaired renal function. TE (P < 0.001), impaired renal function (P = 0.014), LDH ≥ 1.5 × ULN (P = 0.035), and abdominal pain (P = 0.007) were associated with an increased incidence of death.
Multivariate regression modeling indicated that in patients with PNH, LDH ≥ 1.5 × ULN (P = 0.016), male sex (P = 0.045), and pain (P = 0.033) were associated with increased risk of TE. Hemoglobinuria (P = 0.034) and pain (P = 0.022) were associated with an increased risk of impaired renal function. TE was associated with an increased incidence of death (P < 0.001) (Table 2).
Table 2. Regression analyses showing predictive risk factors for TE, impaired renal function, and death in patients with PNHa .
| Characteristics | Univariate | Multivariate | |||
|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | ||
| TE regression | |||||
| LDH ≥ 1.5 × ULN | 15.26 (2.04–114.10) | 0.008 | 12.21 (1.61–92.86) | 0.016 | |
| Hb < 8 g/dL | 1.14 (0.57–2.29) | 0.710 | 1.14 (0.53–2.42) | 0.743 | |
| PNH-cytopenia | 0.96 (0.48–1.92) | 0.906 | 0.88 (0.41–1.87) | 0.745 | |
| Male sex | 2.12 (1.03–4.35) | 0.040 | 2.19 (1.02–4.72) | 0.045 | |
| Abdominal pain | 2.88 (1.37–6.06) | 0.005 | - | - | |
| Dyspnea | 2.78 (1.36–5.68) | 0.005 | 1.90 (0.86–4.19) | 0.112 | |
| Pain | 3.90 (1.64–9.31) | 0.002 | 2.79 (1.09–7.17) | 0.033 | |
| Impaired renal function regression | |||||
| LDH ≥ 1.5 × ULN | 2.56 (0.86–7.66) | 0.093 | 1.73 (0.54–5.55) | 0.360 | |
| Hb < 8 g/dL | 1.17 (0.56–2.47) | 0.672 | 1.37 (0.60–3.11) | 0.451 | |
| PNH-cytopenia | 0.51 (0.23–1.11) | 0.091 | 0.54 (0.23–1.25) | 0.148 | |
| Hemoglobinuria | 4.70 (1.74–12.72) | 0.002 | 3.13 (1.09–8.96) | 0.034 | |
| Abdominal pain | 4.06 (1.74–9.48) | 0.001 | - | - | |
| Dyspnea | 3.49 (1.60–7.65) | 0.002 | 2.24 (0.96–5.21) | 0.062 | |
| Pain | 6.22 (2.10–18.42) | 0.001 | 3.86 (1.22–12.24) | 0.022 | |
| Mortality regression | |||||
| LDH ≥ 1.5 × ULN | 4.89 (1.12–21.31) | 0.035 | 2.85 (0.57–14.17) | 0.201 | |
| Hb < 8 g/dL | 1.67 (0.75–3.73) | 0.210 | 1.45 (0.57–3.65) | 0.432 | |
| PNH-cytopenia | 2.03 (0.91–4.52) | 0.085 | 2.27 (0.89–5.78) | 0.086 | |
| TE | 8.83 (3.77–20.70) | < 0.001 | 6.76 (2.64–17.33) | < 0.001 | |
| Impaired renal function | 3.08 (1.26–7.53) | 0.014 | 2.58 (0.83–8.04) | 0.103 | |
| Abdominal pain | 3.25 (1.37–7.71) | 0.007 | 2.50 (0.93–6.75) | 0.070 | |
| Hemoglobinuria | 0.71 (0.32–1.56) | 0.394 | 0.36 (0.13–1.01) | 0.052 | |
CI = confidence interval, Hb = hemoglobin, LDH = lactate dehydrogenase, OR = odds ratio, PNH = paroxysmal nocturnal hemoglobinuria, TE = thromboembolism, ULN = upper limit of normal.
aPNH-cytopenia refers to PNH in the setting of other specified bone marrow disorder + subclinical PNH. Number of patients with known LDH and Hb status = 217; number of patients with TE = 39; number of patients with impaired renal function = 33; number of deaths = 29; Bold indicates statistical significance at the 5% level.
Hb < 8 g/dL was not a predictor of TE, impaired renal function, or death in patients with PNH in any of the univariate or multivariate analyses.
SMR
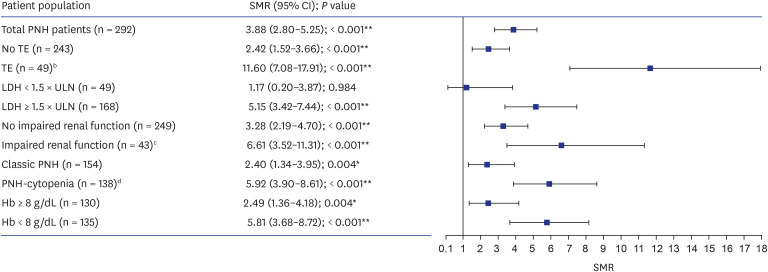
In total, nine patients from the PNH population were excluded from SMR calculation owing to missing age or sex data. When compared with the age- and sex-matched general Korean population, SMRs indicated that a diagnosis of PNH increased the risk of death by 3.9-fold (Fig. 2); TE (11.6-fold increase; P < 0.001) was associated with the greatest risk of death among patients with PNH. SMR analysis indicated that LDH ≥ 1.5 × ULN (5.2-fold increase; P < 0.001), Hb < 8 g/dL (5.8-fold increase; P < 0.001), and Hb ≥ 8 g/dL (2.5-fold increase; P = 0.004) were risk factors for death compared with the general population. Patients with LDH < 1.5 × ULN were the only subset of patients with a mortality similar to that of the age- and sex-matched population (SMR = 1.17; P = 0.984).
Fig. 2. SMR of the PNH population compared with age- and sex-matched general Korean population.a .
CI = confidence interval, Hb = hemoglobin, LDH = lactate dehydrogenase, PNH = paroxysmal nocturnal hemoglobinuria, SMR = standardized mortality ratio, TE = thromboembolism, ULN = upper limit of normal.
aPatients with missing age/sex are excluded from calculation of SMR (nine patients).
bOne patient with TE had missing age/sex.
cOne patient with impaired renal function had missing age/sex.
dThree patients with classic PNH and six patients with PNH-cytopenia had missing age/sex.
*P ≤ 0.01, **P ≤ 0.001.
DISCUSSION
In this analysis of patients with PNH from the Korean National PNH Registry who had not been treated with complement inhibitors, multivariate regression analyses showed that LDH ≥ 1.5 × ULN was a significant predictor of TE, and TE was a significant predictor of death. Hb < 8 g/dL was not predictive of TE, impaired renal function, or death in any of the univariate or multivariate regression analyses. When compared with the age- and sex-matched general Korean population, SMR analyses indicated that LDH ≥ 1.5 × ULN and anemia, irrespective of Hb level, were risk factors for death in this PNH population; in contrast, patients with LDH < 1.5 × ULN had a life expectancy similar to that of the general Korean population. These findings suggest that controlling IVH in PNH will reduce the incidence of TE and death.24,25
This study expands on previous work by Jang et al.14 by including predictors of impaired renal function and TE in the analyses, in addition to mortality. The previous study analyzed the same patient population; however, the current study also evaluated the importance of Hb within the PNH population. Predictors of mortality from the current study are broadly consistent with those of the previous findings from Jang et al.14 In the current study, impaired renal function and PNH-cytopenia were not significant predictors of mortality in multivariate analyses, likely owing to the current data set being restricted to patients with known LDH and Hb statuses. Interestingly, the proportion of patients with LDH ≥ 1.5 × ULN was similar between patients with Hb < 8 g/dL and Hb ≥ 8 g/dL, and the difference in the incidence of TE between these Hb level stratifications was also non-significant, indicating that it is not the level of Hb that predicts TE, but rather LDH ≥ 1.5 × ULN and thus IVH. Significant predictors of impaired renal function were hemoglobinuria and pain (from the multivariate analysis), while predictors of TE were LDH ≥ 1.5 × ULN, male sex, and pain, which is in line with other reports.26,27
SMR analyses showed a similar trend to the previous study.14 As patients with high LDH had higher mortality, and patients with low LDH had a similar mortality compared with the general population, the data provide further evidence that high LDH indicative of IVH was a significant risk factor for death in patients with PNH. Although the SMR analyses indicated that anemia, irrespective of Hb level, was associated with an increased risk of death, the SMR analyses do not adjust for covariates such as LDH ≥ 1.5 × ULN. Thus it is likely that the elevated LDH is driving the increased risk of death in patients with anemia when compared with the general population.
There are several limitations of this study. The medical records used were collected in real-world clinical practice and some of the data could be missing or incomplete, which may produce biased estimates. The registry is restricted to Korean patients with PNH, and therefore results may not be generalizable to the wider PNH population. However, this database was selected because it covers approximately 96% of patients with PNH in Korea. Lastly, the data were collected more than 15 years ago and may not be reflective of current clinical practice.
In conclusion, evaluating LDH and Hb in predicting long-term outcomes in patients with PNH is crucial for the clinical management of PNH. It is known that IVH is one of the primary clinical manifestations of PNH, as indicated by LDH ≥ 1.5 × ULN, shown here to be strongly associated with TE, and TE to be strongly associated with death in patients with PNH. Hb was not a significant predictor of TE, impaired renal function, or death. Therefore, controlling IVH will improve life-threatening clinical outcomes for patients with PNH (Supplementary Data 1).
ACKNOWLEDGMENTS
The authors would like to thank the patient advocate for reviewing the plain language summary of this article.
Footnotes
Funding: This study was sponsored by Alexion, AstraZeneca Rare Disease. Medical writing assistance was provided by Jon Carthy, PhD, Rebecca Spencer Martín, MSci, and Rebecca Hornby, PhD, of Oxford PharmaGenesis, Oxford, UK, with funding from Alexion, AstraZeneca Rare Disease (2022).
Disclosure: Jun Ho Jang has no conflicts of interest to declare. Jin Seok Kim has received fees from Alexion, AstraZeneca Rare Disease. Cindy Thiow Koon Lim is an employee of IQVIA. Nora J. Kleinman is an employee of IQVIA. Karl-Johan Myren is an employee of and shareholder in Alexion, AstraZeneca Rare Disease. Alice Wang was an employee of and shareholder in Alexion, AstraZeneca Rare Disease at the time of study completion. Yogesh Patel is an employee of and shareholder in Alexion, AstraZeneca Rare Disease. Jong Wook Lee has received grants and fees from Alexion, AstraZeneca Rare Disease, and has served as a member of an advisory board for Alexion, AstraZeneca Rare Disease.
- Conceptualization: Lee JW.
- Data curation: Jang JH, Kim JS, Lim CTK, Kleinman NJ, Myren KJ, Wang A, Patel Y, Lee JW.
- Formal analysis: Lim CTK, Kleinman NJ.
- Investigation: Jang JH, Kim JS, Lim CTK, Kleinman NJ, Myren KJ, Wang A, Patel Y, Lee JW.
- Writing - original draft: Jang JH, Kim JS, Lim CTK, Kleinman NJ, Myren KJ, Wang A, Patel Y, Lee JW.
- Writing - review & editing: Jang JH, Kim JS, Lim CTK, Kleinman NJ, Myren KJ, Wang A, Patel Y, Lee JW.
SUPPLEMENTARY MATERIALS
Plain language summary
Location and timing of TE events stratified by pre- or post-PNH diagnosis date
Causes of death throughout the study
References
- 1.Parker C, Omine M, Richards S, Nishimura J, Bessler M, Ware R, et al. Diagnosis and management of paroxysmal nocturnal hemoglobinuria. Blood. 2005;106(12):3699–3709. doi: 10.1182/blood-2005-04-1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hillmen P, Lewis SM, Bessler M, Luzzatto L, Dacie JV. Natural history of paroxysmal nocturnal hemoglobinuria. N Engl J Med. 1995;333(19):1253–1258. doi: 10.1056/NEJM199511093331904. [DOI] [PubMed] [Google Scholar]
- 3.Kulasekararaj AG, Brodsky RA, Nishimura JI, Patriquin CJ, Schrezenmeier H. The importance of terminal complement inhibition in paroxysmal nocturnal hemoglobinuria. Ther Adv Hematol. 2022;13:20406207221091046. doi: 10.1177/20406207221091046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi HJ, Lee SN. A clinical study on paroxysmal nocturnal hemoglobinuria in Korea. Ewha Med J. 1995;18(3):189–197. [Google Scholar]
- 5.Choi CW, Jang JH, Kim JS, Jo DY, Lee JH, Kim SH, et al. Efficacy of eculizumab in paroxysmal nocturnal hemoglobinuria patients with or without aplastic anemia: prospective study of a Korean PNH cohort. Blood Res. 2017;52(3):207–211. doi: 10.5045/br.2017.52.3.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim JS, Jang JH, Jo DY, Ahn SY, Yoon SS, Lee JH, et al. Long-term efficacy and safety of eculizumab in patients with paroxysmal nocturnal hemoglobinuria and high disease burden: real-world data from Korea. J Korean Med Sci. 2023;38(41):e328. doi: 10.3346/jkms.2023.38.e328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim YD, Yang JW, Choi JW, Kim BR, Yu JM, Kim YS, et al. Hemolytic crisis and acute kidney injury in patient with paroxysmal nocturnal hemoglobinuria in Korea – case report and review of literature. Korean J Nephrol. 2009;28:236–242. [Google Scholar]
- 8.Rother RP, Bell L, Hillmen P, Gladwin MT. The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin: a novel mechanism of human disease. JAMA. 2005;293(13):1653–1662. doi: 10.1001/jama.293.13.1653. [DOI] [PubMed] [Google Scholar]
- 9.Nishimura JI, Kanakura Y, Ware RE, Shichishima T, Nakakuma H, Ninomiya H, et al. Clinical course and flow cytometric analysis of paroxysmal nocturnal hemoglobinuria in the United States and Japan. Medicine (Baltimore) 2004;83(3):193–207. doi: 10.1097/01.md.0000126763.68170.46. [DOI] [PubMed] [Google Scholar]
- 10.Schrezenmeier H, Muus P, Socié G, Szer J, Urbano-Ispizua A, Maciejewski JP, et al. Baseline characteristics and disease burden in patients in the International Paroxysmal Nocturnal Hemoglobinuria Registry. Haematologica. 2014;99(5):922–929. doi: 10.3324/haematol.2013.093161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Socié G, Mary JY, de Gramont A, Rio B, Leporrier M, Rose C, et al. Paroxysmal nocturnal haemoglobinuria: long-term follow-up and prognostic factors. Lancet. 1996;348(9027):573–577. doi: 10.1016/s0140-6736(95)12360-1. [DOI] [PubMed] [Google Scholar]
- 12.Kim JS, Lee JW, Kim BK, Lee JH, Chung J. The use of the complement inhibitor eculizumab (Soliris®) for treating Korean patients with paroxysmal nocturnal hemoglobinuria. Korean J Hematol. 2010;45(4):269–274. doi: 10.5045/kjh.2010.45.4.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hillmen P, Muus P, Dührsen U, Risitano AM, Schubert J, Luzzatto L, et al. Effect of the complement inhibitor eculizumab on thromboembolism in patients with paroxysmal nocturnal hemoglobinuria. Blood. 2007;110(12):4123–4128. doi: 10.1182/blood-2007-06-095646. [DOI] [PubMed] [Google Scholar]
- 14.Jang JH, Kim JS, Yoon SS, Lee JH, Kim YK, Jo DY, et al. Predictive factors of mortality in population of patients with paroxysmal nocturnal hemoglobinuria (PNH): results from a Korean PNH registry. J Korean Med Sci. 2016;31(2):214–221. doi: 10.3346/jkms.2016.31.2.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brodsky RA, Lee JW, Nishimura JI, Szer J. Lactate dehydrogenase versus haemoglobin: which one is the better marker in paroxysmal nocturnal haemoglobinuria? Br J Haematol. 2022;196(2):264–265. doi: 10.1111/bjh.17860. [DOI] [PubMed] [Google Scholar]
- 16.Brodsky RA. Paroxysmal nocturnal hemoglobinuria. Blood. 2014;124(18):2804–2811. doi: 10.1182/blood-2014-02-522128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee JW, Jang JH, Kim JS, Yoon SS, Lee JH, Kim YK, et al. Clinical signs and symptoms associated with increased risk for thrombosis in patients with paroxysmal nocturnal hemoglobinuria from a Korean Registry. Int J Hematol. 2013;97(6):749–757. doi: 10.1007/s12185-013-1346-4. [DOI] [PubMed] [Google Scholar]
- 18.Kim JS, Lee JW, Yoon SS, Lee JH, Jo DY, Jang JH, et al. Association between elevated hemolysis at diagnosis and early mortality and risk of thrombosis in paroxysmal nocturnal hemoglobinuria (PNH) patients with cytopenia. Blood. 2010;116(21):4241. [Google Scholar]
- 19.Lee JW, Jang JH, Kim JS, Yoon SS, Lee JH, Kim YK, et al. Uncontrolled complement activation and the resulting chronic hemolysis as measured by LDH serum level at diagnosis as predictor of thrombotic complications and mortality in a large cohort of patients with paroxysmal nocturnal hemoglobinuria (PNH) Blood. 2011;118(21):3166. [Google Scholar]
- 20.Astor BC, Muntner P, Levin A, Eustace JA, Coresh J. Association of kidney function with anemia: the Third National Health and Nutrition Examination Survey (1988-1994) Arch Intern Med. 2002;162(12):1401–1408. doi: 10.1001/archinte.162.12.1401. [DOI] [PubMed] [Google Scholar]
- 21.Westenbrink BD, Alings M, Connolly SJ, Eikelboom J, Ezekowitz MD, Oldgren J, et al. Anemia predicts thromboembolic events, bleeding complications and mortality in patients with atrial fibrillation: insights from the RE-LY trial. J Thromb Haemost. 2015;13(5):699–707. doi: 10.1111/jth.12874. [DOI] [PubMed] [Google Scholar]
- 22.Chi G, Gibson CM, Hernandez AF, Hull RD, Kazmi SHA, Younes A, et al. Association of anemia with venous thromboembolism in acutely ill hospitalized patients: an APEX trial substudy. Am J Med. 2018;131(8):972.e1–972.e7. doi: 10.1016/j.amjmed.2018.03.031. [DOI] [PubMed] [Google Scholar]
- 23.Kim JS, Jang JH, Yoon SS, Lee JH, Kim YK, Jo DY, et al. Distinct subgroups of paroxysmal nocturnal hemoglobinuria (PNH) with cytopenia: results from South Korean National PNH Registry. Ann Hematol. 2016;95(1):125–133. doi: 10.1007/s00277-015-2511-z. [DOI] [PubMed] [Google Scholar]
- 24.Höchsmann B, de Fontbrune FS, Lee JW, Kulagin AD, Hillmen P, Wilson A, et al. Effect of eculizumab treatment in patients with paroxysmal nocturnal hemoglobinuria with or without high disease activity: real-world findings from the International Paroxysmal Nocturnal Hemoglobinuria Registry. Eur J Haematol. 2022;109(3):197–204. doi: 10.1111/ejh.13773. [DOI] [PubMed] [Google Scholar]
- 25.de Latour RH, Hill A, Füreder W, Piatek C, Griffin M, Ogawa M, et al. Ravulizumab reduces the risk of thrombosis in adult patients with paroxysmal nocturnal hemoglobinuria and high disease activity: 2-year data from a phase 3, open-label study. Hemasphere. 2021;5(Suppl 2):109–110. [Google Scholar]
- 26.Boehme AK, Pamboukian SV, George JF, Dillon C, Levitan EB, Griffin R, et al. Predictors of thromboembolic events in patients with ventricular assist device. ASAIO J. 2015;61(6):640–647. doi: 10.1097/MAT.0000000000000284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gizzi M, Oberic L, Massard C, Poterie A, Le Teuff G, Loriot Y, et al. Predicting and preventing thromboembolic events in patients receiving cisplatin-based chemotherapy for germ cell tumours. Eur J Cancer. 2016;69:151–157. doi: 10.1016/j.ejca.2016.10.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Plain language summary
Location and timing of TE events stratified by pre- or post-PNH diagnosis date
Causes of death throughout the study