Abstract
Background
During coronavirus disease 2019 (COVID-19) pandemic, several COVID-19 vaccines were licensed with fast-track procedures. Although these vaccines have demonstrated high immunogenicity, there has been concerns on the serious adverse events (AEs) following COVID-19 vaccination among adolescents. We aimed to analyze comparative safety of COVID-19 vaccination in adolescents.
Methods
In this pharmacovigilance study, we performed a disproportionality analysis using VigiBase, the World Health Organization’s global individual case safety report (ICSR) database. To compare serious AEs reported following COVID-19 vaccines vs. all other vaccines in adolescents aged 12–17 years, ICSRs following any vaccines on adolescents aged 12–17 years were included, defining cases as reports with the AEs of interest, with all other AEs as non-cases. The AEs of interest were myocarditis/pericarditis, multisystem inflammatory syndrome/Kawasaki disease (MIS/KD), anaphylaxis, Guillain-Barré syndrome (GBS), and immune thrombocytopenia (ITP). We conducted a disproportionality analysis to estimate reporting odds ratio (ROR) with 95% confidence interval (CI) for each AE of interest, adjusted for sex by using logistic regression.
Results
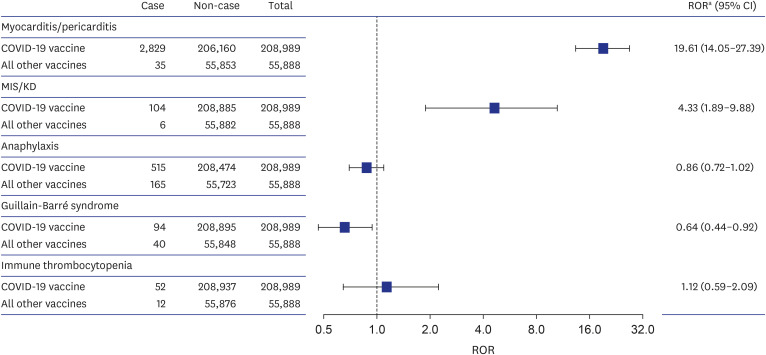
Of 99,735 AE reports after vaccination in adolescents, 80,018 reports were from COVID-19 vaccinated adolescents (52.9% females; 56.3% America). The AEs of interest were predominantly reported as serious AE (76.1%) with mRNA vaccines (99.4%). Generally, higher reporting odds for the AEs were identified following COVID-19 vaccination in adolescents; myocarditis/pericarditis (2,829 reports for the COVID-19 vaccine vs. 35 for all other vaccines, adjusted ROR [aROR], 19.61; 95% CI, 14.05–27.39), and MIS/KD (104 vs. 6, aROR, 4.33; 95% CI, 1.89–9.88). The reporting odds for anaphylaxis (515 vs. 165, aROR, 0.86; 95% CI, 0.72–1.02), GBS (94 vs. 40, aROR, 0.64; 95% CI, 0.44–0.92) and ITP (52 vs. 12, aROR, 1.12; 95% CI, 0.59–2.09) were not significantly higher following COVID-19 vaccination.
Conclusion
In this study, there were disproportionate reporting of immune-related AEs following COVID-19 vaccination. While awaiting definitive evidence, there is a need to closely monitor for any signs of immune-related AEs following COVID-19 vaccination among adolescents.
Keywords: COVID-19 Vaccine, Adolescents, Adverse Events, Disproportionality Analysis
Graphical Abstract
INTRODUCTION
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has infected over 760 million people and resulted in more than 6.9 million deaths worldwide, reported to the World Health Organization, as of June 2023.1 Owing to virus’s transmissibility and lethality, vaccination against SARS-CoV-2 is the most important public health intervention to mitigate from COVID-19 pandemic.
Several types of COVID-19 vaccines including inactivated vaccines, viral vector vaccines, subunit vaccines and mRNA vaccines have been developed. Pfizer-BioNTech vaccine (BNT162b2) was first authorized for emergency use in population aged ≥ 16 years on December 11, 2020 and expanded for adolescents aged 12–15 years on May 10, 2021.2,3 In the pre-authorization trials of BNT162b2 vaccine in adolescents, most of the reported adverse events (AEs) were mild-to-moderate localized reaction such as injection-site pain.4 Although the safety of COVID-19 vaccines had been assessed in the clinical trials, they were not powered to detect rare AEs and the safety findings may not be generalizable to entire adolescents.5 Moreover, post-authorization safety surveillance on the COVID-19 vaccines has been carried out mainly in adults and their safety information among adolescents in real-world setting is scarce.
Rare but serious AEs following COVID-19 vaccination in adolescents were recently described in several case reports. An increasing number of myocarditis/pericarditis and multisystem inflammatory syndrome (MIS), a severe inflammatory condition on multiple organ systems cases was reported following COVID-19 vaccination in adolescents and young adults.6,7,8,9,10 Subsequently, Food and Drug Administration (FDA) announced Adverse Events of Special Interest (AESIs) potentially associated with COVID-19 vaccines in pediatric population.11 Given the relative paucity of safety data, we conducted pharmacovigilance analysis to compare disproportionate reporting of the AESIs following COVID-19 vaccines with all other vaccines among adolescents in VigiBase.
METHODS
Study design and data source
In this pharmacovigilance study, we analyzed individual case safety reports (ICSRs) on the AESIs reported following COVID-19 vaccines or all other vaccines in the VigiBase until July 2023. VigiBase, managed by Uppsala Monitoring Centre (UMC, Uppsala, Sweden), is the largest database of its kind in the world. It contains over 30 million safety reports of suspected AEs and medicines from more than 150 countries since 1967. Health professionals, patients and some pharmaceutical companies report suspected AEs of medicines to their national pharmacovigilance centre. Then, the ICSRs are sent to VigiBase after the reports are reviewed and analyzed locally. The VigiBase can be made available to anyone with a health profession degree with the possibility of a fee being charged. The de-identified ICSRs contain detailed information on the demographics (age group, sex, region), drug information (indication of use, route of administration), AEs (seriousness, outcome, time to onset) and administrative data (type and source of report).12 The reported drugs are categorized as ‘suspected’ (potentially related to the AE without explicit drug interaction causing it), ‘interacting’ (if the AE is suspected due to drug interaction between two or more drugs), or ‘concomitant’ (drugs used simultaneously without being suspected as the cause of the AE by the reporter).13 Based on this informative database, numerous pharmacovigilance studies have been extensively conducted.14,15,16
Data extraction and selection
From all ICSRs related to vaccination in VigiBase up until July 2023, we extracted those listed COVID-19 or other vaccines reported from age group between 12 and 17 years. For COVID-19 vaccine-related reports, we further restricted to those reported after December 11, 2020, the first date of FDA approval of COVID-19 vaccine for emergency use. Also, for all other vaccines-related reports, we included only reports after January 30, 2020 to reflect the declaration of a Public Health Emergency of International Concern (PHEIC) which facilitates an enhanced surveillance network.17 We included reports recorded as “suspected” on the reported drug information to improve the validity of included reports for analysis. For the follow-up reports with additional information submitted after an initial report, only the latest reported data were included. Also, we excluded reports with missing data on the covariates used in our analysis (i.e., unknown demographic data, missing preferred terms code data, etc.). The detailed information on the report selection is summarized in Supplementary Fig. 1.
Vaccination
As the Anatomical Therapeutic Chemical (ATC) codes for COVID-19 vaccines were not available, we used VigiBase’s drug record number to identify AE reports for COVID-19 vaccines. The types of COVID-19 vaccine included in this study were mRNA, viral vector, inactivated, and subunit vaccines. For comparator, we used ATC code ‘J07’ to identify AE reports for all other vaccines.
AESI
We selected myocarditis/pericarditis, multisystem inflammatory syndrome/Kawasaki disease (MIS/KD), anaphylaxis, Guillain-Barré syndrome (GBS), immune thrombocytopenia (ITP) for our study after reviewing not only case reports but also Center for Disease Control’s review report, FDA’s safety monitoring protocol which selected potential AESIs with following considerations; Events that are related to severity of COVID-19 that could possibly be related to vaccine failure/immunogenicity, events that are potentially linked to new platforms or adjuvants, events that are potentially specific to certain population of interest, recommendations from other surveillance systems, and serious events that have been studied with other vaccinations.7,11,18,19,20,21 The AEs were identified using Standardised MedDRA Queries which are validated, pre-determined sets of Medical Dictionary for Regulatory Activities terms based on extensive review, analysis, and expert discussion.22 The detailed Preferred Terms used for defining the AESIs are listed in Supplementary Table 1.
Disproportionality analysis
We performed a disproportionality analysis (i.e., case/non-case analysis) to assess comparative reporting of the AEs between COVID-19 vaccine and all other vaccines in adolescents. This analysis aims to generate hypothesis for confirmatory studies by providing a statistical relationship between drug of interest and AE.23 It is similar to a case-control study, comparing proportion of AE of interest in a group of interest with that of a control group. If the proportion is greater in the group exposed to drug of interest than group not exposed to that drug, then there is a disproportionality for the drug-AE pair. In our analysis, cases included reports with the 5 AESIs, with all other AEs as non-cases. Thereafter, proportion of AE reports on COVID-19 vaccine and all other vaccines within the cases and non-cases were compared.
Statistical analysis
Descriptive analysis was used to summarize the report characteristics. Categorical variables were presented using numbers and proportions (%) and continuous variables using median and interquartile range (IQR). Reporting odds ratio (ROR) and 95% confidence interval (CI) were calculated to estimate the extent to which AESI is associated with COVID-19 vaccines vs. all other vaccines among adolescents. ROR is a ratio, similar to an odds ratio in case-control study and is equivalent to exposure odds among reported cases of interest over exposure odds among reported non-cases. We used logistic regression model to estimate the RORs adjusted for sex. Disproportionate reporting was defined as a lower boundary of the 95% CI of ROR greater than 1 according to UMC’s criteria.24 Furthermore, we conducted sensitivity analysis to increase the validity of the study and evaluate the consistency of main findings. To account for the Weber effect of new vaccines,25 we compared reporting of AESI between COVID-19 vaccinated adolescents (12–17 years) with COVID-19 vaccinated adults (≥ 18 years).
Data management and analysis were conducted using SAS 9.4 version software program (SAS Inc., Cary, NC, USA), and statistical significance was defined as two-sided P < 0.05.
Ethics statement
The Institutional Review Board (IRB) of Sungkyunkwan University approved the study (IRB No. SKKU 2023-02-038); the board waived the requirement for obtaining informed consent as this study used anonymized administrative data.
RESULTS
Study population and baseline characteristics of reports
There was a total of 99,735 reports after vaccination among adolescents aged 12–17 years recorded in the VigiBase by July 2023. Of these, 80,018 were reports on COVID-19 vaccines and 19,717 were on all other vaccines. Among COVID-19 vaccine reports, AEs were reported more frequently in women (52.9%), mainly in Americas (56.3%), and with mRNA vaccines (95.2%). As for all other vaccines, AEs were mostly from women (56.3%), largely in Americas (57.5%), and with viral vaccines (58.5%) (Table 1).
Table 1. Characteristics of the individual case safety reports following vaccination among adolescents (12–17 years) retrieved from World Health Organization VigiBase.
| Characteristics | COVID-19 vaccination | All other vaccinationa | |
|---|---|---|---|
| No. of reports | 80,018 | 19,717 | |
| Sex | |||
| Male | 37,706 (47.1) | 8,606 (43.7) | |
| Female | 42,312 (52.9) | 11,111 (56.3) | |
| Region | |||
| African | 2,319 (2.9) | 1,551 (7.8) | |
| Americas | 45,023 (56.3) | 11,330 (57.5) | |
| South-East Asia | 706 (0.9) | 40 (0.2) | |
| Europe | 19,544 (24.4) | 4,706 (23.9) | |
| Eastern Mediterranean | 1,398 (1.8) | 27 (0.1) | |
| Western Pacific | 11,028 (13.8) | 2,063 (10.5) | |
| Type of report | |||
| Spontaneous | 77,482 (96.8) | 19,447 (98.6) | |
| Report from study | 1,202 (1.5) | 184 (0.9) | |
| Others | 1,334 (1.7) | 85 (0.5) | |
| Notifier | |||
| Physician | 9,571 (11.9) | 2,459 (12.5) | |
| Pharmacist | 3,248 (4.1) | 569 (2.9) | |
| Other health professional | 10,044 (12.6) | 3,109 (15.8) | |
| Others | 57,155 (71.4) | 13,580 (68.8) | |
| Type of vaccine | |||
| mRNA | 76,200 (95.2) | - | |
| Viral vector | 2,315 (2.9) | - | |
| Inactivated | 883 (1.1) | - | |
| Subunit | 250 (0.3) | - | |
| Unknown | 370 (0.5) | 0 (0) | |
| Viral | - | 11,541 (58.5) | |
| Bacterial | - | 7,613 (38.6) | |
| Vira-bacterial combined | - | 554 (2.8) | |
| Other | - | 9 (0.1) | |
Values are presented as number (%).
COVID-19 = coronavirus infectious disease 2019.
aAll other vaccines include papillomavirus vaccine (5,825), meningococcal vaccine (5,321), influenza vaccine (2,401), pertussis vaccine (1,627), hepatitis vaccine (1,052), measles vaccine (1,020), bacterial and viral vaccines, combined (554), varicella zoster vaccine (451), tetanus vaccine (381), poliomyelitis vaccine (381), yellow fever vaccine (185), pneumococcal vaccine (175), encephalitis vaccines (91), other viral vaccines (79), rabies vaccine (49), cholera vaccine (36), haemophilus influenza B vaccine (36), typhoid vaccine (24), tuberculosis vaccine (10), rubella vaccine (6), diphtheria vaccine (4), anthrax vaccine (2), rotavirus diarrhea vaccine (1), and other vaccines (6).
Of the 3,594 cases of AESI after COVID-19 vaccination, 76.1% were serious AEs, with the proportion being the highest for MIS/KD (98.1%), followed by GBS (92.5%), myocarditis/pericarditis (79.1%), ITP (76.9%) and anaphylaxis (52.0%). Commonly, AESIs were chiefly reported after receiving mRNA vaccines (Table 2).
Table 2. Characteristics of the adverse events of special interest for coronavirus disease 2019 vaccines among adolescents (12–17 years) retrieved from World Health Organization VigiBase.
| Characteristics | Total (N = 3,594) | Myocarditis/Pericarditis (n = 2,829) | Multisystem inflammatory syndrome/Kawasaki disease (n = 104) | Anaphylaxis (n = 515) | Guillain-Barré syndrome (n = 94) | Immune thrombocytopenia (n = 52) | |
|---|---|---|---|---|---|---|---|
| Outcome | |||||||
| Recovered | 1,104 (30.7) | 759 (26.8) | 49 (47.1) | 281 (54.6) | 10 (10.6) | 5 (9.6) | |
| Recovered with sequelae | 23 (0.6) | 18 (0.6) | 1 (1.0) | 4 (0.8) | 0 (0) | 0 (0) | |
| Recovering | 370 (10.3) | 338 (12.0) | 3 (2.9) | 21 (4.0) | 4 (4.2) | 4 (7.7) | |
| Not recovered | 366 (10.2) | 343 (12.1) | 2 (1.9) | 8 (1.6) | 7 (7.5) | 6 (11.5) | |
| Death | 6 (0.2) | 4 (0.1) | 0 (0) | 2 (0.4) | 0 (0) | 0 (0) | |
| Unknown | 1,725 (48.0) | 1,367 (48.4) | 49 (47.1) | 199 (38.6) | 73 (77.7) | 37 (71.2) | |
| Seriousnessa | |||||||
| Yes | 2,735 (76.1) | 2,238 (79.1) | 102 (98.1) | 268 (52.0) | 87 (92.5) | 40 (76.9) | |
| No | 859 (23.9) | 591 (20.9) | 2 (1.9) | 247 (48.0) | 7 (7.5) | 12 (23.1) | |
| Type of vaccine | |||||||
| mRNA | 3,568 (99.4) | 2,817 (99.6) | 104 (100) | 505 (98.0) | 90 (95.7) | 52 (100.0) | |
| Viral vector | 5 (0.1) | 2 (0.1) | 0 (0) | 3 (0.6) | 0 (0) | 0 (0) | |
| Inactivated | 8 (0.2) | 0 (0) | 0 (0) | 4 (0.8) | 4 (4.3) | 0 (0) | |
| Subunit | 1 (0.0) | 0 (0) | 0 (0) | 1 (0.2) | 0 (0) | 0 (0) | |
| Unknown | 12 (0.3) | 10 (0.3) | 0 (0) | 2 (0.4) | 0 (0) | 0 (0) | |
The characteristics of Adverse Event of Special Interest are analyzed from 208,989 coronavirus disease 2019 vaccine-adverse event pairs in the World Health Organization VigiBase between December 2020 and July 2023.
aFatal, life-threatening, requiring hospitalization, resulting in significant disability/incapacity, and other medically important conditions.
AESI
Myocarditis/Pericarditis
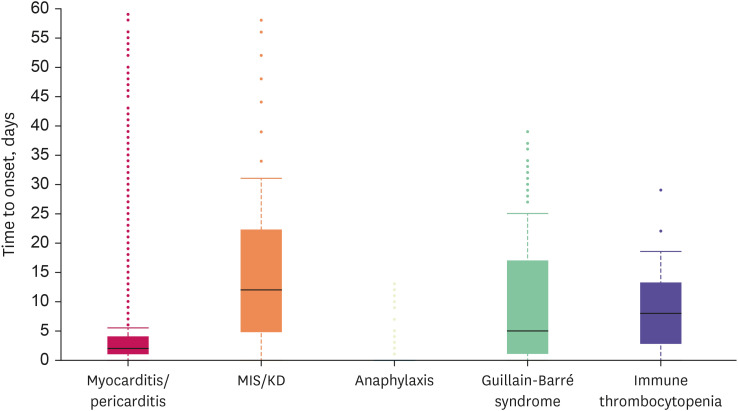
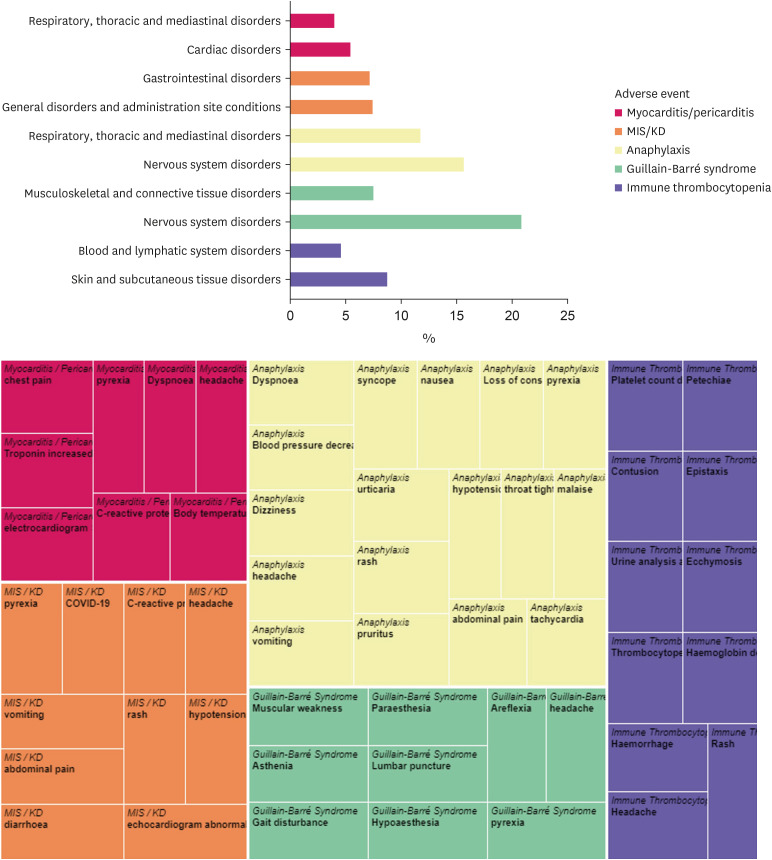
We identified 2,829 cases (1.3%) and 35 cases (0.18%) of myocarditis/pericarditis reported for COVID-19 vaccines and all other vaccines, respectively. Compared with all other vaccines, COVID-19 vaccine was associated with an increased risk of reporting myocarditis/pericarditis (adjusted ROR [aROR], 19.61; 95% CI, 14.05–27.39) and the risk was higher in male vs. female (aROR, 5.15; 95% CI, 4.67–5.67) (Fig. 1, Supplementary Table 2). The reported median time to onset of myocarditis/pericarditis was 2 days (IQR, 1–4) in COVID-19 vaccines (Fig. 2). The most frequent MedDRA SOC category reported together with myocarditis/pericarditis for COVID-19 vaccines was ‘cardiac disorders,’ and the most frequent reported symptom was ‘chest pain’ (Fig. 3).
Fig. 1. Reporting odds of myocarditis/pericarditis, MIS/KD, anaphylaxis, Guillain-Barré syndrome, and immune thrombocytopenia for COVID-19 vaccines among adolescents (12–17 years) in World Health Organization VigiBase.
ROR = reporting odds ratio, CI = confidence interval, COVID-19 = coronavirus disease 2019, MIS = multisystem inflammatory syndrome, KD = Kawasaki disease.
aROR estimated using a logistic regression model adjusted for sex.
Fig. 2. Time to onset of myocarditis/pericarditis, MIS/KD, anaphylaxis, Guillain-Barré syndrome, and immune thrombocytopenia after coronavirus disease 2019 vaccination among adolescents (12–17 years), recorded in World Health Organization VigiBase.
MIS = multisystem inflammatory syndrome, KD = Kawasaki disease.
Fig. 3. Distribution of the adverse events, according to system Organ Class and preferred Term, reported together with myocarditis/pericarditis, MIS/KD, anaphylaxis, Guillain-Barré syndrome, and immune thrombocytopenia in World Health Organization VigiBase.
MIS = multisystem inflammatory syndrome, KD = Kawasaki disease.
MIS/KD
There were 104 cases (0.05%) and 6 cases (0.03%) of MIS/KD reported for COVID-19 vaccines and all other vaccines, respectively. Compared with all other vaccines, COVID-19 vaccine was associated with an increased risk of reporting MIS/KD (aROR, 4.33; 95% CI, 1.89–9.88), with higher risk in male (aROR, 1.72; 95% CI, 1.16–2.56) (Fig. 1, Supplementary Table 2). The reported median time to onset of MIS/KD was 10 days (IQR, 3–22) in COVID-19 vaccines (Fig. 2). Concerning MIS/KD, ‘gastrointestinal disorders’ were frequently reported together, and the most frequent symptom was pyrexia (Fig. 3).
Anaphylaxis
Increased reporting odds was not observed for anaphylaxis after COVID-19 vaccination vs. all other vaccination (aROR, 0.86; 95% CI, 0.72–1.02) (Fig. 1). Typically, anaphylaxis was reported on the date of vaccination (Fig. 2). ‘Nervous system disorders’ were the most frequently reported with anaphylaxis, and dyspnea was the most frequently reported symptom (Fig. 3).
GBS
There was no higher reporting observed for GBS after COVID-19 vaccination compared to all other vaccination (aROR, 0.64; 95% CI, 0.44–0.92) (Fig. 1). The median time to onset of GBS was 5 days (IQR, 1–17) in COVID-19 vaccinated adolescents (Fig. 2). The most frequent MedDRA SOC category reported together with GBS after COVID-19 vaccination was ‘nervous system disorders,’ and the most frequent reported symptom was ‘muscular weakness’ (Fig. 3).
ITP
Likewise, COVID-19 vaccine was not associated with an increased risk of reporting ITP (aROR, 1.12; 95% CI, 0.59–2.09) compared with all other vaccines (Fig. 1). The 50% of the COVID-19 vaccinated adolescent patients developed ITP within 10 days (IQR, 2–15). ‘Skin and subcutaneous tissue disorders’ were reported frequently together with ITP for COVID-19 vaccines, and the most frequent symptom was ‘platelet count decrease’ (Fig. 3).
Sensitivity analysis
When compared with COVID-19 vaccinated adults (≥ 18 years), most of AESIs were highly reported in COVID-19 vaccinated adolescents (Supplementary Table 3).
DISCUSSION
In this worldwide observational study, we assessed the safety of COVID-19 vaccination in adolescents, using validated pharmacovigilance method. We found higher reporting of myocarditis/pericarditis and MIS/KD after COVID-19 vaccination compared to all other vaccination. The 5 AESIs were not reported in the clinical trials of BNT162b2 and mRNA-1273 vaccines in adolescents.4,26 This is likely due to the small population size of the clinical trial to detect rare AEs. Based on our results, close monitoring for these rare but serious inflammatory reactions after COVID-19 vaccination among adolescents until definitive causal relationship can be established.
Myocarditis/pericarditis have been reported not only as severe AEs after COVID-19 vaccination, but also as serious complication from COVID-19 infection, especially in male adolescents.27,28 According to analysis of the Vaccine Adverse Event Reporting System data up until June 11, 2021, disproportionate reporting of myocarditis/pericarditis was higher in male vs. female and 12–24 years vs. ≥ 25 years.29 In Danish population-based study, the incidence of myopericarditis among 12–17 years who were vaccinated with BNT162b2 was higher in male vs. female (97 vs. 16 per million persons).30 Consistently, our findings highlight higher risk of reporting myocarditis/pericarditis after COVID-19 vaccination in male adolescents. Also, we found higher reporting of MIS/KD following COVID-19 vaccination in adolescents, with higher risk among male adolescents. Our result is in line with the previous finding on the higher reporting rate of hyperinflammatory syndrome in COVID-19 vaccinated male vs. female adolescents (2.4 vs. 0.5 per million doses, P = 0.039).31
Overall, there was no increased risk of reporting anaphylaxis, GBS and ITP following COVID-19 vaccination in our analysis. In previous study, COVID-19 vaccine did not specifically increase the risk of anaphylaxis.32 One thing to note is that women were more likely to report anaphylaxis after COVID-19-vaccination in our analysis, which is consistent with the existing study on post-vaccination anaphylaxis, indicating a need for caution especially among women.33 Like our result, neither the ChAdOx1 nCoV-19 nor mRNA-based vaccines were associated with higher risk of reporting GBS vs. influenza vaccines according to previous pharmacovigilance study using VigiBase (IC025 = −1.84, ROR025 = 0.11; IC025 = −1.86, ROR025 = 0.06, respectively).34 Based on analysis of EudraVigilance, UK MHRA and Health-Infobase databases, there was no association between ITP and BNT162b2 vaccine.35 Additionally, in a study analyzing VAERS data, 77 cases were reported among 35 million individuals vaccinated solely with mRNA vaccines. This incidence aligns with the background rate of ITP.36 However, all the studies were conducted among adults, and the risk of ITP with COVID vaccination in pediatrics remains unknown.
The association between COVID-19 vaccination and myocarditis/pericarditis has yet been clearly understood, but several hypotheses could be considered. First, higher incidence of myocarditis/pericarditis may exist in the circumstances related to higher immune reactivity. In previous studies on the association between myocarditis/pericarditis and COVID-19 vaccine, the risk was more pronounced among younger patients and after second dose.7,14 This may be related to more active adaptive immune response in younger individuals, which may bring about increase of CD4+ T cells, leading to heart-specific autoimmunity.37 Second, the immune system might recognize the mRNA in vaccine as an antigen, activating pro-inflammatory cascades and immunological pathway in the heart. Another mechanism is derived from molecular analogy between the spike protein of SARS-CoV-2 and cardiac self-antigen. Antibodies combined to SARS-CoV-2 spike glycoproteins would cross-react with structurally resembling human protein sequences, developing myocardial inflammation.38 These mechanisms can be influenced by genetic background, sex and age.39 The male predominance in myocarditis/pericarditis following COVID-19 vaccination is related to sex hormone. Testosterone is thought to contribute to inhibition of anti-inflammatory cells and engagement in promoting Th-1 immune response, whereas estrogen has inhibitory effects on pro-inflammatory T cells, inducing a decrease of cell-mediated immune response.40 Despite unclear pathophysiology of MIS/KD, possible pathogenesis is as follows: SARS-CoV-2 infection and BNT162b2 vaccination have been shown to induce receptor-binding protein (RBD) antibodies, which were identified in patients with MIS/KD.41 It was suggested that immune reactions to exposure to SARS-CoV-2 RBD antibodies by SARS-CoV-2 infection or COVID-19 vaccination shape the manifestation of MIS. But such possible pathophysiology must be interpreted carefully because it is possible that SARS-CoV-2 infection around COVID-19 vaccination may have led to MIS/KD and incorrectly attributed to vaccination.42,43
Though no disproportionate reporting of anaphylaxis, GBS and ITP after COVID-19 vaccination compared with all other vaccination, potential mechanisms of these 3 AEs following COVID-19 vaccination are being raised. Hypersensitivity reactions like anaphylaxis following COVID-19 vaccination can arise from vaccine components including active immunizing antigen, adjuvant, etc. An IgE-mediated systemic response to the excipients in COVID-19 vaccine could involve in the occurrence of the anaphylaxis.44,45 According to experimental studies, it has been demonstrated that polyethylene glycol that are included in COVID-19 mRNA vaccine to transport and stabilize the mRNA might trigger severe allergic reactions.46,47 Regarding GBS after COVID-19 vaccination, there is a potential pathogenesis in adenovirus-vector vaccine. Cross-reactivity between antibodies induced by adenovirus-vector vaccine and glycoproteins on the peripheral nerves may theoretically lead to GBS.48 Still, it is necessary to clarify this possible immunopathological mechanism through multiple studies. Literature reviews of ITP after COVID-19 vaccination suggest the following plausible mechanism. Antibodies produced in response to COVID-19 vaccination may target platelets and megakaryocyte. This interaction can lead to opsonization, triggering apoptosis in the adhered platelets, subsequently resulting in the clearance of antibody-coated platelets by the spleen.49 Another possibility is that enhancement of macrophage-mediated clearance or impaired platelet production as a part of inflammatory response to COVID-19 vaccination may result in exacerbation of pre-existing, subclinical ITP.50
To our knowledge, this is the first pharmacovigilance study exploring comparative reporting of AEs after COVID-19 vaccination among adolescents, along with some strengths. First, we used the most extensive collection of data regarding side effects of drugs. The VigiBase covers more than 90% of the world’s population which provides opportunity to explore rare AEs at worldwide scale. Second, our study provided real-world evidence on the safety of COVID-19 vaccination in adolescents. Most of the safety information about COVID-19 vaccination in adolescents have come from case reports or clinical trials conducted under controlled setting.
However, there were some limitations in this study and our findings should be interpreted with caution. First, as VigiBase is based on passively collected reports and the spontaneous reports have inherent limitation of under reporting, reporting bias may exist that may have influenced our results. Second, there have been extensive media attention on the COVID-19 vaccines and discussion about safety of COVID-19 vaccines which might trigger higher reporting of specific AEs such as MIS/KD. Furthermore, COVID-19 pandemic has had a global impact, and since then, the system of monitoring and reporting AEs has changed significantly, leading to more active reporting of AEs after vaccination. Therefore, to mitigate probability of overestimation and ensure comparability, we compared reports from COVID-19 vaccination with those from all other vaccination after PHEIC and conducted sensitivity analysis comparing reports within COVID-19 vaccinated groups. Third, although immune reactivity by dose could influence the risk of AE, we could not assess the dose effect of COVID-19 vaccination due to lack of data on the dose number. Fourth, there may discrepancies in the medical resources for reporting of AESIs or availability of COVID-19 vaccines, which may have resulted in significant proportion of the reports from the United States and Europe in our study. In addition, as information on the comorbidities is not available in VigiBase, adjustment for analysis was limited. Lastly, as we could not assess the history of SARS-CoV-2 infection, uncertainty remains regarding whether AEs such as MIS/KD were directly due to COVID-19 vaccination or the result of SARS-CoV-2 infection. In other words, our findings could not determine causal association between AESI and COVID-19 vaccination in adolescents, rather would raise hypotheses for further definitive studies.
In conclusion, our pharmacovigilance study has observed some evidence of safety concerns regarding COVID-19 vaccination in adolescents at real-world settings. Our findings could contribute to understanding safety profile of COVID-19 vaccination. Although the benefits of COVID-19 vaccination outweigh the risks, adequate monitoring for AEs is necessary when administering COVID-19 vaccine in adolescents. Furthermore, further studies are required to elucidate causal association between AESI and COVID-19 vaccination in adolescents.
Footnotes
Funding: This research was supported by the Ministry of Food and Drug Safety of South Korea (grant numbers 21153MFDS607 and 22183MFDS433). Also supported by the Sungkyunkwan University and the BK21 FOUR (Graduate School Innovation) funded by the Ministry of Education (MOE, Korea) and National Research Foundation of Korea (NRF). The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the manuscript. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Disclosure: The authors declare no competing interests. Dr. Shin received grants from the Ministry of Food and Drug Safety, the National Research Foundation of Korea, and Pharmaceutical Companies, including Pfizer, Celltrion, and SK bioscience. No other relationships or activities have influenced the submitted work. No other relationships or activities have influenced the submitted work.
Data Availability Statement: The data that support the findings of this study are available from Uppsala Monitoring Centre, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of Uppsala Monitoring Centre.
- Conceptualization: Kim DH, Kim JH, Shin JY.
- Data curation: Kim DH, Kim JH, Oh IS.
- Formal analysis: Kim DH, Kim JH, Oh IS, Shin JY.
- Funding acquisition: Shin JY.
- Investigation: Kim DH, Kim JH, Oh IS, Shin JY.
- Visualization: Kim DH, Kim JH.
- Writing - original draft: Kim DH, Kim JH.
- Writing - review & editing: Kim DH, Kim JH, Choe YJ, Choe SA, Shin JY.
SUPPLEMENTARY MATERIALS
List of MedDRA Preferred Terms for 5 adverse events MedDRA Classification Version 24.1
Reporting odds of myocarditis/pericarditis, MIS/KD, anaphylaxis, Guillain-Barré syndrome and immune thrombocytopenia for coronavirus disease 2019 vaccines among adolescents (12–17 years) in WHO VigiBase according to sex within WHO VigiBase
Reporting odds of myocarditis/pericarditis, MIS/KD anaphylaxis, Guillain-Barré syndrome and immune thrombocytopenia for COVID-19 vaccines among adolescents (12–17 years) in WHO VigiBase when compared with COVID-19 vaccinated adults (≥ 18 years) within WHO VigiBase
Flow chart of Individual case safety report inclusion and exclusion within WHO VigiBase.
References
- 1.World Health Organization. WHO coronavirus (COVID-19) dashboard. [Updated 2023]. [Accessed June 20, 2023]. https://covid19.who.int/
- 2.Food and Drug Administration (US) Pfizer-BioNTech COVID-19 vaccine emergency use authorization review memorandum. [Updated 2020]. [Accessed June 20, 2023]. https://www.fda.gov/media/144416/download .
- 3.Food and Drug Administration (US) Pfizer-BioNTech COVID-19 vaccine EUA amendment review memorandum. [Updated 2021]. [Accessed June 20, 2023]. https://www.fda.gov/media/148542/download .
- 4.Frenck RW, Jr, Klein NP, Kitchin N, Gurtman A, Absalon J, Lockhart S, et al. Safety, immunogenicity, and efficacy of the BNT162b2 COVID-19 vaccine in adolescents. N Engl J Med. 2021;385(3):239–250. doi: 10.1056/NEJMoa2107456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu W, Tang J, Chen C, Wang C, Wen W, Cheng Y, et al. Safety and efficacy of the COVID-19 vaccine in children and/or adolescents: a meta-analysis. J Infect. 2022;84(5):722–746. doi: 10.1016/j.jinf.2022.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim HW, Jenista ER, Wendell DC, Azevedo CF, Campbell MJ, Darty SN, et al. Patients with acute myocarditis following mRNA COVID-19 vaccination. JAMA Cardiol. 2021;6(10):1196–1201. doi: 10.1001/jamacardio.2021.2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Foltran D, Delmas C, Flumian C, De Paoli P, Salvo F, Gautier S, et al. Myocarditis and pericarditis in adolescents after first and second doses of mRNA COVID-19 vaccines. Eur Heart J Qual Care Clin Outcomes. 2022;8(2):99–103. doi: 10.1093/ehjqcco/qcab090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim D, Choi JH, Jang JY, So O, Cho E, Choi H, et al. A case report for myopericarditis after BNT162b2 COVID-19 mRNA vaccination in a Korean young male. J Korean Med Sci. 2021;36(39):e277. doi: 10.3346/jkms.2021.36.e277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yousaf AR, Cortese MM, Taylor AW, Broder KR, Oster ME, Wong JM, et al. Reported cases of multisystem inflammatory syndrome in children aged 12-20 years in the USA who received a COVID-19 vaccine, December, 2020, through August, 2021: a surveillance investigation. Lancet Child Adolesc Health. 2022;6(5):303–312. doi: 10.1016/S2352-4642(22)00028-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kest H, Kaushik A, DeBruin W, Colletti M, Goldberg D. Multisystem inflammatory syndrome in children (MIS-C) associated with 2019 novel coronavirus (SARS-CoV-2) infection. Case Rep Pediatr. 2020;2020:8875987. doi: 10.1155/2020/8875987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Food and Drug Administration. Assessment of Risk of Safety Outcomes Following COVID-19 Vaccination. [Updated 2021]. [Accessed June 20, 2023]. https://www.bestinitiative.org/wp-content/uploads/2021/04/COVID-19-Vaccine-Safety-Inferential-Draft-Master-Protocol.pdf .
- 12.Uppsala Monitoring Center. VigiBase. [Updated 2023]. [Accessed June 20, 2023]. https://www.who-umc.org/vigibase/vigibase/
- 13.Strandell J, Wahlin S. Pharmacodynamic and pharmacokinetic drug interactions reported to VigiBase, the WHO global individual case safety report database. Eur J Clin Pharmacol. 2011;67(6):633–641. doi: 10.1007/s00228-010-0979-y. [DOI] [PubMed] [Google Scholar]
- 14.Chouchana L, Blet A, Al-Khalaf M, Kafil TS, Nair G, Robblee J, et al. Features of inflammatory heart reactions following mRNA COVID-19 vaccination at a global level. Clin Pharmacol Ther. 2022;111(3):605–613. doi: 10.1002/cpt.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park J, Kim D, Song TJ. A disproportionality analysis for association of systemic capillary leak syndrome with COVID-19 vaccination using the world health organization pharmacovigilance database. Vaccines (Basel) 2022;10(6):835. doi: 10.3390/vaccines10060835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Merino D, Gérard AO, Van Obberghen EK, Ben Othman N, Ettore E, Giordana B, et al. COVID-19 vaccine-associated transient global amnesia: a disproportionality analysis of the WHO safety database. Front Pharmacol. 2022;13:909412. doi: 10.3389/fphar.2022.909412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilder-Smith A, Osman S. Public health emergencies of international concern: a historic overview. J Travel Med. 2020;27(8):taaa227. doi: 10.1093/jtm/taaa227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shimabukuro T, Nair N. Allergic reactions including anaphylaxis after receipt of the first dose of Pfizer-BioNTech COVID-19 vaccine. JAMA. 2021;325(8):780–781. doi: 10.1001/jama.2021.0600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.CDC COVID-19 Response Team; Food and Drug Administration. Allergic reactions including anaphylaxis after receipt of the first dose of Moderna COVID-19 vaccine - United States, December 21, 2020-January 10, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(4):125–129. doi: 10.15585/mmwr.mm7004e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.CDC COVID-19 Response Team; Food and Drug Administration. Allergic reactions including anaphylaxis after receipt of the first dose of Pfizer-BioNTech COVID-19 vaccine - United States, December 14-23, 2020. MMWR Morb Mortal Wkly Rep. 2021;70(2):46–51. doi: 10.15585/mmwr.mm7002e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention (US) Selected adverse events reported after COVID-19 vaccination. [Updated 2023]. [Accessed June 20, 2023]. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/adverse-events.html .
- 22.MedDRA. Standardised MedDRA queries. [Updated 2023]. [Accessed June 20, 2023]. https://www.meddra.org/standardised-meddra-queries .
- 23.Renoud L, Khouri C, Revol B, Lepelley M, Perez J, Roustit M, et al. Association of facial paralysis with mRNA COVID-19 vaccines: a disproportionality analysis using the world health organization pharmacovigilance database. JAMA Intern Med. 2021;181(9):1243–1245. doi: 10.1001/jamainternmed.2021.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.European Medicines Agency. Screening for Adverse Reactions in EudraVigilance (EMA/849944/2016) Amsterdam, The Netherlands: European Medicines Agency; 2016. [Google Scholar]
- 25.Hoffman KB, Dimbil M, Erdman CB, Tatonetti NP, Overstreet BM. The Weber effect and the United States Food and Drug Administration’s Adverse Event Reporting System (FAERS): analysis of sixty-two drugs approved from 2006 to 2010. Drug Saf. 2014;37(4):283–294. doi: 10.1007/s40264-014-0150-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ali K, Berman G, Zhou H, Deng W, Faughnan V, Coronado-Voges M, et al. Evaluation of mRNA-1273 SARS-CoV-2 vaccine in adolescents. N Engl J Med. 2021;385(24):2241–2251. doi: 10.1056/NEJMoa2109522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diaz GA, Parsons GT, Gering SK, Meier AR, Hutchinson IV, Robicsek A. Myocarditis and pericarditis after vaccination for COVID-19. JAMA. 2021;326(12):1210–1212. doi: 10.1001/jama.2021.13443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patone M, Mei XW, Handunnetthi L, Dixon S, Zaccardi F, Shankar-Hari M, et al. Risks of myocarditis, pericarditis, and cardiac arrhythmias associated with COVID-19 vaccination or SARS-CoV-2 infection. Nat Med. 2022;28(2):410–422. doi: 10.1038/s41591-021-01630-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Centers for Disease Control and Prevention (US) Advisory Committee on Immunization Practices (ACIP): coronavirus disease 2019 (COVID-19) vaccines. [Updated 2021]. [Accessed June 20, 2023]. https://www.cdc.gov/vaccines/acip/meetings/slides-2021-06.html .
- 30.Nygaard U, Holm M, Bohnstedt C, Chai Q, Schmidt LS, Hartling UB, et al. Population-based incidence of myopericarditis after COVID-19 vaccination in Danish adolescents. Pediatr Infect Dis J. 2022;41(1):e25–e28. doi: 10.1097/INF.0000000000003389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ouldali N, Bagheri H, Salvo F, Antona D, Pariente A, Leblanc C, et al. Hyper inflammatory syndrome following COVID-19 mRNA vaccine in children: a national post-authorization pharmacovigilance study. Lancet Reg Health Eur. 2022;17:100393. doi: 10.1016/j.lanepe.2022.100393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim MS, Jung SY, Ahn JG, Park SJ, Shoenfeld Y, Kronbichler A, et al. Comparative safety of mRNA COVID-19 vaccines to influenza vaccines: a pharmacovigilance analysis using WHO international database. J Med Virol. 2022;94(3):1085–1095. doi: 10.1002/jmv.27424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McNeil MM, Weintraub ES, Duffy J, Sukumaran L, Jacobsen SJ, Klein NP, et al. Risk of anaphylaxis after vaccination in children and adults. J Allergy Clin Immunol. 2016;137(3):868–878. doi: 10.1016/j.jaci.2015.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim JE, Park J, Min YG, Hong YH, Song TJ. Associations of Guillain-Barré syndrome with coronavirus disease 2019 vaccination: disproportionality analysis using the World Health Organization pharmacovigilance database. J Peripher Nerv Syst. 2022;27(3):206–214. doi: 10.1111/jns.12507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Al-Samkari H. COVID-19 vaccination and immune thrombocytopenia: cause for vigilance, but not panic. Res Pract Thromb Haemost. 2023;7(1):100039. doi: 10.1016/j.rpth.2023.100039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee EJ, Beltrami-Moreira M, Al-Samkari H, Cuker A, DiRaimo J, Gernsheimer T, et al. SARS-CoV-2 vaccination and ITP in patients with de novo or preexisting ITP. Blood. 2022;139(10):1564–1574. doi: 10.1182/blood.2021013411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vdovenko D, Eriksson U. Regulatory role of CD4+ T cells in myocarditis. J Immunol Res. 2018;2018:4396351. doi: 10.1155/2018/4396351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vojdani A, Kharrazian D. Potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases. Clin Immunol. 2020;217:108480. doi: 10.1016/j.clim.2020.108480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heymans S, Cooper LT. Myocarditis after COVID-19 mRNA vaccination: clinical observations and potential mechanisms. Nat Rev Cardiol. 2022;19(2):75–77. doi: 10.1038/s41569-021-00662-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heymans S, Eriksson U, Lehtonen J, Cooper LT., Jr The quest for new approaches in myocarditis and inflammatory cardiomyopathy. J Am Coll Cardiol. 2016;68(21):2348–2364. doi: 10.1016/j.jacc.2016.09.937. [DOI] [PubMed] [Google Scholar]
- 41.Trougakos IP, Terpos E, Zirou C, Sklirou AD, Apostolakou F, Gumeni S, et al. Comparative kinetics of SARS-CoV-2 anti-spike protein RBD IgGs and neutralizing antibodies in convalescent and naïve recipients of the BNT162b2 mRNA vaccine versus COVID-19 patients. BMC Med. 2021;19(1):208. doi: 10.1186/s12916-021-02090-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jain E, Donowitz JR, Aarons E, Marshall BC, Miller MP. Multisystem inflammatory syndrome in children after SARS-CoV-2 vaccination. Emerg Infect Dis. 2022;28(5):990–993. doi: 10.3201/eid2805.212418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iyengar KP, Nune A, Ish P, Botchu R, Shashidhara MK, Jain VK. Multisystem inflammatory syndrome after SARS-CoV-2 vaccination (MIS-V), to interpret with caution. Postgrad Med J. 2022;98(e2):e91. doi: 10.1136/postgradmedj-2021-140869. [DOI] [PubMed] [Google Scholar]
- 44.Laisuan W. COVID-19 Vaccine anaphylaxis: current evidence and future approaches. Front Allergy. 2021;2:801322. doi: 10.3389/falgy.2021.801322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McNeil MM, DeStefano F. Vaccine-associated hypersensitivity. J Allergy Clin Immunol. 2018;141(2):463–472. doi: 10.1016/j.jaci.2017.12.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sellaturay P, Nasser S, Ewan P. Polyethylene glycol-induced systemic allergic reactions (anaphylaxis) J Allergy Clin Immunol Pract. 2021;9(2):670–675. doi: 10.1016/j.jaip.2020.09.029. [DOI] [PubMed] [Google Scholar]
- 47.Cabanillas B, Akdis CA, Novak N. Allergic reactions to the first COVID-19 vaccine: a potential role of polyethylene glycol? Allergy. 2021;76(6):1617–1618. doi: 10.1111/all.14711. [DOI] [PubMed] [Google Scholar]
- 48.Allen CM, Ramsamy S, Tarr AW, Tighe PJ, Irving WL, Tanasescu R, et al. Guillain-Barré syndrome variant occurring after SARS-CoV-2 vaccination. Ann Neurol. 2021;90(2):315–318. doi: 10.1002/ana.26144. [DOI] [PubMed] [Google Scholar]
- 49.Kim G, Choi EJ, Park HS, Lee JH, Lee JH, Lee KH. A case report of immune thrombocytopenia after ChAdOx1 nCoV-19 vaccination. J Korean Med Sci. 2021;36(43):e306. doi: 10.3346/jkms.2021.36.e306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sivaramakrishnan P, Mishra M. Vaccination-associated immune thrombocytopenia possibly due to ChAdOx1 nCoV-19 (Covishield) coronavirus vaccine. BMJ Case Rep. 2022;15(3):e249237. doi: 10.1136/bcr-2022-249237. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of MedDRA Preferred Terms for 5 adverse events MedDRA Classification Version 24.1
Reporting odds of myocarditis/pericarditis, MIS/KD, anaphylaxis, Guillain-Barré syndrome and immune thrombocytopenia for coronavirus disease 2019 vaccines among adolescents (12–17 years) in WHO VigiBase according to sex within WHO VigiBase
Reporting odds of myocarditis/pericarditis, MIS/KD anaphylaxis, Guillain-Barré syndrome and immune thrombocytopenia for COVID-19 vaccines among adolescents (12–17 years) in WHO VigiBase when compared with COVID-19 vaccinated adults (≥ 18 years) within WHO VigiBase
Flow chart of Individual case safety report inclusion and exclusion within WHO VigiBase.