Deciphering mechanisms of metabolic heterogeneity and plasticity in glioblastoma (GBM) provides new insight for the development of potentially effective therapies for this highly treatment-resistant disease. In the recent study by Liu et al.1, fatty acid-binding protein 7 (FABP7) was shown to elicit function through nuclear factor RXRα, activating a polyunsaturated fatty acid (PUFA)-mediated signaling pathway, which subsequently orchestrates stemness and invasion in GBM cells. Although elucidation of the mechanism and novel molecular targets in this study encourage the exploration of new therapeutic strategies, several aspects of the work deserve consideration.
First, the authors used an in vitro scratch assay as a model for invasion, a critical feature of GBM driving postoperative recurrence and decreased patient survival, and found increased FABP7 immunoreactivity in the closing gap of the scratch compared to the confluent region. However, this assay poorly emulates the physiological microenvironment of GBM. We, therefore, evaluated FABP7 expression in the Ivy-GAP database, based on histologically distinct anatomic features and laser microdissection of GBM surgical samples, and single-cell RNA-seq data2 consisting of tumor core and peritumoral infiltrating area. We found no increased expression of FABP7 in the infiltrating tumor region (Figure 1A and 1B). Therefore, the expression patterns of FABP7 should be more rigorously investigated in primary GBMs to assess its biological relevance.
Figure 1.
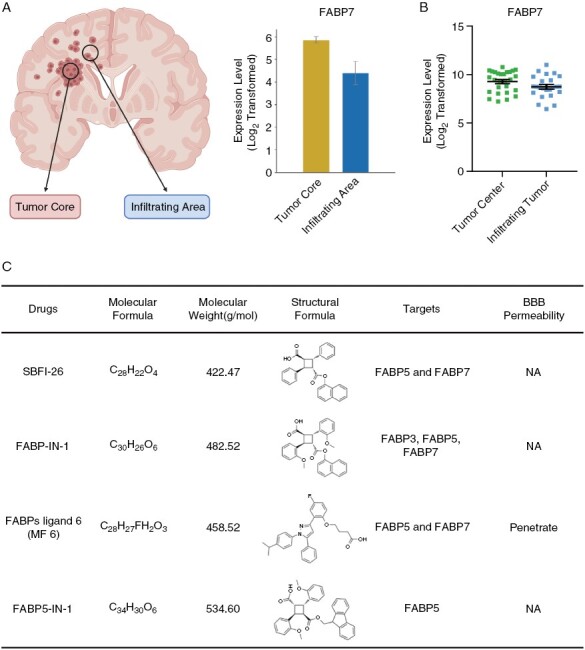
Expression levels of FABP7 based on different anatomic areas in GBM, and a summary of the characteristics of several FABP inhibitors. (A) Expression of FABP7 in tumor core and infiltrating area within GBM based on single-cell RNA-seq GBM data. (B) Expression of FABP7 in the tumor center and infiltrating tumor within GBM based on the Ivy-GAP database (https://glioblastoma.alleninstitute.org/). (C) A summary of physicochemical and biological properties of several FABPs inhibitors.
Second, the authors used SBFI-26 as an inhibitor for FABP7 to attenuate GSC invasion. However, SBFI-26 shows low specificity and targets both FABP5 and FABP7. FABP5 is also highly expressed in gliomas and enhances malignancy via activating NF-κB signaling.3 Therefore, the observed infiltration-suppressing effects of SBFI-26 might be mediated by FABP5. Furthermore, the ability of SBFI-26 to cross the blood–brain barrier (BBB) was not evaluated. MF 6, a novel FABP7 inhibitor shown to cross the BBB,4 may be more appropriate for in vivo validation. However, MF 6 and other FABP7 inhibitors are also not specific for FABP7 (Figure 1C). Thus, it is imperative to develop novel specific FABP7 inhibitors, and design therapeutics targeting this druggable molecule.
Third, using RXRα activation as the major consequence of FABP7 function does not fully explain the role of the PUFA-FABP7 axis in GBM progression. As major ligands of FABP7, PUFAs play multiple roles in tumorigenesis, including serving as an energy source, acting as a key component of membrane phospholipids, and mediating intracellular signaling via the NF-κB and PPAR networks. FABP7 has also been shown to promote nuclear lipid droplet formation, caveolae formation, and uptake of docosahexaenoic acid (DHA) which all contribute to the development of GBM. Moreover, previous studies have shown that the effect of FABP7 on glioma invasion is dependent on the proportion of arachidonic acid (AA) and DHA, with a high DHA:AA ratio inhibiting cell invasion, and a high AA:DHA ratio promoting cell invasion.5 Therefore, analyzing multiple outcomes of activating the PUFA-FABP7 axis and collecting DHA ratio data may help to define the role of FABP7 in GBM and therapeutic interventions.
Finally, several of the key findings in this study are based on experiments using the U87MG cell line. Because the growth patterns, function, and genetics of U87MG in culture deviate from primary GBM,6 we believe this cell line to be inappropriate for investigating the role of FABP7 in GSCs. Therefore, several GBM-derived primary cultures should be involved to assess the role of FABP7 in promoting GSC phenotypes.
We recognize the importance of the knowledge gap Liu et al. sought to fill by expanding the available data on an FABP7-based GBM targeting therapy. We also believe in emphasizing some of the areas where additional experiments may more strongly implicate FABP7 as a viable druggable target yielding improved GBM patient survival.
Contributor Information
Yanfei Sun, Department of Neurosurgery, Qilu Hospital, Cheeloo College of Medicine and Institute of Brain and Brain-Inspired Science, Shandong University, Jinan, China; Jinan Microecological Biomedicine Shandong Laboratory and Shandong Key Laboratory of Brain Function Remodeling, Jinan, China.
Guangjing Mu, Department of Neurosurgery, Qilu Hospital, Cheeloo College of Medicine and Institute of Brain and Brain-Inspired Science, Shandong University, Jinan, China; Jinan Microecological Biomedicine Shandong Laboratory and Shandong Key Laboratory of Brain Function Remodeling, Jinan, China.
Zhiwei Xue, Department of Neurosurgery, Qilu Hospital, Cheeloo College of Medicine and Institute of Brain and Brain-Inspired Science, Shandong University, Jinan, China; Jinan Microecological Biomedicine Shandong Laboratory and Shandong Key Laboratory of Brain Function Remodeling, Jinan, China.
Shuai Wang, Departments of Neurosurgery, NYU Grossman School of Medicine, New York, New York, USA.
Xingang Li, Department of Neurosurgery, Qilu Hospital, Cheeloo College of Medicine and Institute of Brain and Brain-Inspired Science, Shandong University, Jinan, China; Jinan Microecological Biomedicine Shandong Laboratory and Shandong Key Laboratory of Brain Function Remodeling, Jinan, China.
Shilei Ni, Department of Neurosurgery, Qilu Hospital, Cheeloo College of Medicine and Institute of Brain and Brain-Inspired Science, Shandong University, Jinan, China; Jinan Microecological Biomedicine Shandong Laboratory and Shandong Key Laboratory of Brain Function Remodeling, Jinan, China.
Mingzhi Han, Department of Neurosurgery, Qilu Hospital, Cheeloo College of Medicine and Institute of Brain and Brain-Inspired Science, Shandong University, Jinan, China; Jinan Microecological Biomedicine Shandong Laboratory and Shandong Key Laboratory of Brain Function Remodeling, Jinan, China; Medical Integration and Practice Center, Cheeloo College of Medicine, Shandong University, Jinan, China.
Conflict of interest statement
None declared.
Consent for publication
All authors have agreed to publish this manuscript.
Funding
This work was supported by the Natural Science Foundation of China (82103277, 82111530202, 82172740), Shandong Foundation for Taishan Scholars (tsqn202211041, tstp20230656), the Research Project of Jinan Microecological Biomedicine Shandong Laboratory (JNL-2022041C, JNL-2023004C), the Shandong Excellent Young Scientists Fund Program (2022HWYQ-035), the Shandong Provincial Natural Science Foundation (ZR2021QH030, ZR2022ZD17), and the Qilu Young Scholar Program of Shandong University, China.
Author contributions
Y.S., S.W., and M.H. designed the project. Y.S., G.M., and Z.X. wrote the manuscript. M.H., X.L., and S.N. supervised the project. All authors contributed to the article and approved the submitted version.
Ethics statement
All experimental procedures were approved by the institutional research ethics committee of Shandong University.
Data Availability
All data generated or analyzed during this study are included in this article.
References
- 1. Liu RZ, Choi WS, Jain S, et al. Stationary-to-migratory transition in glioblastoma stem-like cells driven by a FABP7-RXRalpha neurogenic pathway. Neuro Oncol. 2023;25(12):2177–2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Darmanis S, Sloan SA, Croote D, et al. Single-cell RNA-Seq analysis of infiltrating neoplastic cells at the migrating front of human glioblastoma. Cell Rep. 2017;21(5):1399–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang Y, Wahafu A, Wu W, et al. FABP5 enhances malignancies of lower-grade gliomas via canonical activation of NF-kappaB signaling. J Cell Mol Med. 2021;25(9):4487–4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cheng A, Jia W, Kawahata I, Fukunaga K.. A novel fatty acid-binding protein 5 and 7 inhibitor ameliorates oligodendrocyte injury in multiple sclerosis mouse models. EBioMedicine. 2021;72:103582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Elsherbiny ME, Emara M, Godbout R.. Interaction of brain fatty acid-binding protein with the polyunsaturated fatty acid environment as a potential determinant of poor prognosis in malignant glioma. Prog Lipid Res. 2013;52(4):562–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Allen M, Bjerke M, Edlund H, Nelander S, Westermark B.. Origin of the U87MG glioma cell line: good news and bad news. Sci Transl Med. 2016;8(354):354re353. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article.


