Summary
Background
Current approved chimeric antigen receptor (CAR) T-cell products are autologous cell therapies that are costly and poorly accessible to patients. We aimed to evaluate the safety and antitumor activity of a novel off-the-shelf anti-CD19 CAR-engineered allogeneic double-negative T cells (RJMty19) in patients with relapsed/refractory large B-cell lymphoma. We report the results from a first-in-human, open-label, single-dose, phase 1 study of allogeneic CD19-specific CAR double-negative T (CAR-DNT) cells.
Methods
Eligibility criteria included the presence of measurable lesions, at least 2 lines of prior immunochemotherapy, and an ECOG score of 0–1. We evaluated four dose levels (DL) of RJMty19 in a 3 + 3 dose-escalation scheme: 1 × 106, 3 × 106, 9 × 106 and 2 × 107 CAR-DNT cells per kilogram of body weight. All patients received lymphodepleting chemotherapy with fludarabine and cyclophosphamide. The primary endpoints were dose-limiting toxicities (DLTs), incidence of adverse events (AEs), and clinically significant laboratory abnormalities. Secondary endpoints included evaluation of standard cellular pharmacokinetic parameters, immunogenicity, objective response rates (ORR), and disease control rate (DCR) per Lugano 2014 criteria.
Findings
A total of 12 patients were enrolled between 22 July 2022 and 27 July 2023. Among these patients, 66% were classified as stage IV, 75% had an IPI score of 3 or higher, representing an intermediate risk or worse. The maximum tolerated dose was not reached because no DLT was observed. Four patient experienced grade 1 or 2 cytokine release syndrome and dizziness. The most common AEs were hematologic toxicities, including neutropenia (N = 12, 100%), leukopenia (N = 12, 100%), lymphopenia (N = 10, 83%), thrombocytopenia (N = 6, 50%), febrile neutropenia (N = 3, 25%), and anemia (N = 3, 25%). Seven subjects died till the cut-off date, five of them died of disease progression and two of them died of COVID 19. In all patients (N = 12), the ORR was 25% and CRR was 8.3%. DL1 and DL2 patients benefited less from the therapy (ORR: 17%, N = 1; DCR: 33%, N = 2). However, all DL3 patients achieved disease control (N = 3, 100%), and all DL4 patients achieved objective response (N = 3, 100%).
Interpretation
Our results demonstrate that CD19-CAR-DNT cells appear to be well tolerated with promising antitumor activity in LBCL patients. Further study of this product with a larger sample size is warranted. This phase 1 study is registered on clinicaltrials.gov (NCT05453669).
Funding
Wyze Biotech. Co., Ltd.
Keywords: Double-negative T cells, CD19-CAR-DNT cells, B-cell lymphoma, Safety, Antitumor activity
Research in context.
Evidence before this study
We searched PubMed for full-text clinical trials written in English published up to September 28, 2023, to identify papers on CAR-DNT cells. The search terms used were“DNT AND CAR”, and“double-negative T AND CAR”. The search revealed a scarcity of clinical reports of CAR-DNT cells except for 2 preclinical research which were published by Dr Li Zhang at University of Toronto.
Added value of this study
To the best of our knowledge, this is the first clinical trial to evaluate the safety and efficacy of CAR-DNT cells except for 2 preclinical research. Our results showed a good safety profile and preliminary activity of CD19-CAR-DNT cell therapy for patients with relapsed or refractory LBCL. The on-target, off-tumor toxicity of CD19-CAR-DNT cells observed in our study was manageable, suggesting that CAR-DNT cells could be an effective immunotherapeutic option for LBCL. Moreover, these results set the stage for a subsequent phase 2 study to further validate the efficacy and safety of CD19-CAR-DNT cells in LBCL.
Implications of all the available evidence
Our study provide support for the safety and efficacy of CD19-CAR-DNT cells, which warrant further large scale and multi-center clinical trials to evaluate it.
Introduction
Autologous CAR-T cell therapies have achieved unprecedented success over the past few decades particularly in hematological malignancies.1, 2, 3, 4, 5, 6, 7 Several CAR-T cell therapies have been approved for the treatment of B-cell non-Hodgkin lymphoma (NHL).7 However, autologous CAR-T cell therapy is extremely costly, have lengthy manufacturing times, product variability, and potential manufacturing failures that limit its widespread use in cancer treatment.8, 9, 10
One approach to overcome these barriers is to develop off-the-shelf adoptive cellular therapy. Double-negative T (DNT) cells are a subtype of mature T cells that express CD3 and either αβ+ or γδ+ T cell receptor (TCR), but not CD4, CD8 or CD56. TCRαβ+ DNT cells and TCRγδ+ DNT cells mediate comparable antileukemic activity.11,12 Allogeneic DNT cells fulfill the requirements for an off-the-shelf adoptive cellular therapy, with several advantages including scalability of expansion, donor-independent anticancer activity, cryopreservability, resistance to host versus graft (HvG) rejection, and no observed off-tumor toxicity.13
Preclinical studies have shown that CD19-CAR-DNT cells have a better safety profile and a lower probability of graft-versus-host disease (GvHD) compared to CD19-CAR-T cells.14 Moreover, CD19-CAR-DNT cells have comparable targeted tumor-killing abilities to CD19-CAR-T cells.14 Compared to DNT cells, there is a significant increase in tumor infiltration of CD19-CAR-DNT cells in xenograft models.14 In addition to CAR antigen recognition, LFA-1 and degranulation pathways play an important role in anti-tumor activity by CD19-CAR-DNT cells.14 Therefore, CD19-CAR-DNT cells can potentially be used to treat large B cell lymphoma (LBCL), the most prevalent lymphoma. Although the safety and antitumor activity of DNT cells in acute myeloid leukemia patients have been clinically validated,15 anti-tumor capabilities for CAR-engineered allogeneic DNT cells have never been evaluated clinically. Herein we report a first in human phase 1 clinical trial to explore the safety and antitumor activity of RJMty19, an anti-CD19 CAR-DNT cell product, in patients with relapsed/refractory (r/r) LBCL.
Methods
Study design and patients
We conducted an open-label, single-dose, phase 1 study of allogeneic CD19-CAR-DNT cells in patients with r/r LBCL at the Second Affiliated Hospital, School of Medicine, Zhejiang University. The study is registered on clinicaltrials.gov with the identifier NCT05453669.
Eligible participants age 18–75 years were recruited with r/r LBCL based on WHO 2016 criteria for diffuse large B-cell lymphoma (DLBCL), primary mediastinal large B-cell lymphoma (PMBCL), transformed follicular lymphoma (TFL), and high-grade B-cell lymphoma (HGBL). Inclusion criteria were: an Eastern Cooperative Oncology Group (ECOG) performance status of 0–2; an absolute neutrophil count of at least 1000 per μL; a platelet count of at least 30,000 per μL; with adequate organ function (alanine aminotransferase (ALT) ≤3 times the upper limit of normal [ULN], aspartate aminotransferase (AST) ≤3 times ULN, total bilirubin ≤1.5 times ULN, serum creatinine ≤1.5 times ULN); and CD19 positivity in tumor tissues by immunohistochemistry. Participants must have progressive or stable disease with best response to the most recent chemotherapy regimen, or disease progression or relapse within 12 months of autologous stem-cell transplantation with a prior chemotherapy regimen containing an anti-CD20 monoclonal antibody and an anthracycline. Participants were excluded if they had autologous stem cell transplantation within 2 months prior to screening, received prior CAR-T therapy or other gene-modified cell therapy, or had a life expectancy of less than 3 months. The full eligibility criteria are listed in the study protocol (Appendix).
The clinical protocol was reviewed and approved by the Clinical Research Ethics Committee of the Second Affiliated Hospital, School of Medicine, Zhejiang University. All patients provided written informed consent. The study was performed according to the Declaration of Helsinki version 2013 and Good Clinical Practice.
RJMty19 treatment
RJMty19, an anti-CD19 CAR-engineered allogeneic DNT cell product, was manufactured from peripheral blood mononuclear cells (PBMCs) of healthy donors. PBMCs were isolated by density gradient centrifugation using Ficoll (STEMCELL Technologies). Ex vivo expansion of DNT cells was performed in Wyze Biotech GMP facility. CD4+ and CD8+ T cells were depleted using RosetteSep® depletion kit according to the manufacturer's instruction (STEMCELL Technologies, Canada). The CD4+ and CD8+ T cell-depleted PBMCs was transduced using a lentiviral vector containing the anti-CD19 CAR and manufactured according to established Standard Operation Procedures (SOPs). The purity of CD4/CD8 negative T cells was more than 85% of CD3+ T cells before and after expansion. The CD19-CAR-DNT cells were cryopreserved in liquid nitrogen. Prior to RJMty19 infusion, participants were given lymphodepleting chemotherapy consisting of 500 mg/m2 intravenous cyclophosphamide and 25 mg/m2 intravenous fludarabine once per day on days −5, −4, and −3. On day 0, patients received a single intravenous infusion of RJMty19 at a designated dose. Dose escalation was based on the 3 + 3 dose-escalation scheme, including four cohorts: DL1 (1 × 106 CAR + cells/kg), DL2 (3 × 106 CAR + cells/kg), DL3 (9 × 106 CAR + cells/kg), and DL4 (2 × 107 CAR + cells/kg). Participants were hospitalized for 7–14 days after the RJMty19 infusion for monitoring. Disease assessments by Positron Emission Tomography/Computed Tomography (PET/CT) were obtained at 1 month and 3 months after infusion, and as clinically indicated. Surveillance using Computed Tomography (CT) and B-mode Ultrasound Imaging were obtained at 2 months after infusion and then every 3 months until month 24. Treatment responses were recorded per the Lugano 2014 Criteria.16 Serum cytokine concentrations, blood cell reconstitution, serum immunoglobulin concentrations, CD19 counts, and T cell subsets were analyzed before infusion and on days 1, 3, 5, 7, 10, 14, 21, and 28 after infusion, and then as clinically indicated. CAR-DNT cell concentrations in the blood were assessed using flow cytometry (CytoFLEX, BECKMAN). Serum cytokine concentrations were measured using Human Th1/Th2 Cytometric Beads Array kits. CAR copy number was monitored using qPCR (ABI 7500, Applied Biosystems).
All adverse events (AEs) and serious adverse events (SAEs) were monitored for up to 2 years. Cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS) were graded according to the American Society for Transplantation and Cellular Therapy consensus grading.17 All other adverse events and baseline laboratory values were graded using the National Cancer Institute's (NCI) Common Terminology Criteria for Adverse Events (CTCAE) version 5.0.
Outcomes
The primary endpoints were the safety of RJMty19 as determined by the incidence of dose-limiting toxicities (DLTs), the maximum tolerated dose (MTD, defined as the highest dose at which ≤1/6 subjects experienced DLTs), and the incidence of treatment-related adverse events (TRAEs). The secondary endpoints included objective response rate (ORR, defined as the proportion of patients who had a complete or partial response), disease control rate (DCR, defined as the proportion of patients who achieve stable disease, complete or partial response), progression-free survival (PFS), overall survival (OS), and cellular pharmacokinetic characteristics.
Statistical analysis
The sample size for this study was based on 3 + 3 design principles to determine the MTD. Up to six participants were estimated to be enrolled at each dose level. All participants who received the RJMty19 infusion were included in the safety and antitumor activity analyses. Descriptive analyses utilized medians and means as appropriate. Group comparisons were performed by a two-tailed Wilcoxon ranked sum test. PFS and OS were analyzed using the Kaplan–Meier method. P values < 0.05 were considered statistically significant. Analyses were conducted using R statistical software version 4.3.1.
Role of the funding source
The clinical trial funding source reviewed the final manuscript but had no influence on the study design, the collection, analysis, interpretation of data, the writing of the report, or the decision to submit the paper for publication.
Results
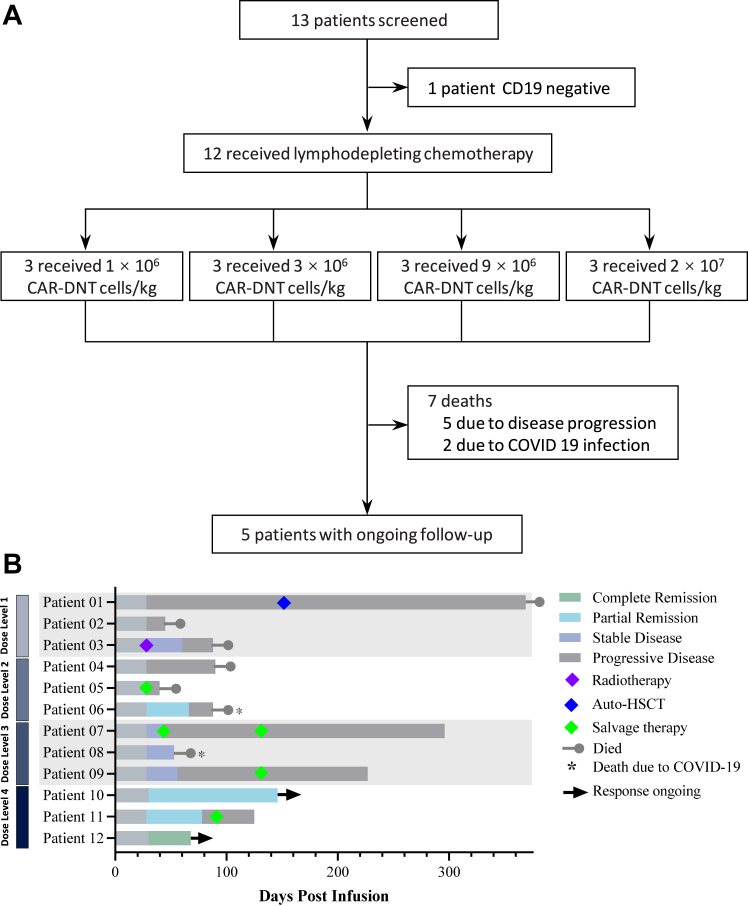
Between 22 July 2022 and 27 July 2023, 13 participants were screened and 12 were enrolled, including 11 with DLBCL and one with high-grade B-cell lymphoma (HGBL) (Fig. 1, Table 1). Participant demographics and clinical characteristics are shown in Table 1 and Supplementary Table S1. All participants who received RJMty19 were followed up until September 28, 2023, or death, with a median follow-up time of 89 days (interquartile range [IQR]: 45–369 days).
Fig. 1.
Study profile and Swimmer's plot. (A) Study profile. CAR, chimeric antigen receptor; DNT, Double negative T cell (B) Swimmer's plot (N = 12), in which each bar represents individual subject as designated.
Table 1.
Baseline characteristics of patients.
| All (N = 12) | DL1 (N = 3) | DL2 (N = 3) | DL3 (N = 3) | DL4 (N = 3) | |
|---|---|---|---|---|---|
| Age, years | 65 (45–74) | 60 (55–74) | 58 (52–65) | 50 (45–67) | 66 (65–67) |
| Sex | |||||
| Female | 6 (50%) | 1 (33%) | 3 (100%) | 2 (67%) | 0 (0%) |
| Male | 6 (50%) | 2 (67%) | 0 (0%) | 1 (33%) | 3 (100%) |
| Time from diagnosis, months | 13 (6–96) | 13 (11–21) | 8 (7–12) | 28 (21–35) | 6 (6–96) |
| Disease—No. (%) | |||||
| DLBCL | 11 (92) | 3 (100%) | 2 (67%) | 3 (100%) | 3 (100%) |
| HGBL | 1 (8%) | 0 (0%) | 1 (33%) | 0 (0%) | 0 (0%) |
| Disease Stage—No. (%) | |||||
| Ⅱ | 2 (17%) | 0 (0%) | 0 (0%) | 2 (67%) | 0 (0%) |
| Ⅲ | 2 (17%) | 1 (33%) | 1 (33%) | 0 (0%) | 0 (0%) |
| Ⅳ | 8 (66%) | 2 (67%) | 2 (67%) | 1 (33%) | 3 (100%) |
| ≥3 IPI score—No. (%) | 9 (75%) | 2 (67%) | 2 (67%) | 2 (67%) | 3 (100%) |
| Blood LDH increased—No. (%) | 9 (75%) | 2 (67%) | 2 (67%) | 2 (67%) | 3 (100%) |
| Prior therapies—No. (%) | |||||
| ≥3 prior lines of therapy | 9 (75%) | 3 (100%) | 2 (67%) | 2 (67%) | 2 (67%) |
| BTK inhibitors ± combinations | 5 (42%) | 2 (67%) | 1 (33%) | 0 (0%) | 2 (67%) |
| Lenalidomide | 4 (33%) | 2 (67%) | 0 (0%) | 2 (67%) | 0 (0%) |
| Venetoclax | 1 (8%) | 1 (33%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Refractory status at study entry | |||||
| Complete response | 5 (42%) | 2 (67%) | 0 (0%) | 2 (67%) | 1 (33%) |
| Partial response | 4 (33%) | 1 (33%) | 1 (33%) | 1 (33%) | 1 (33%) |
| Stable disease | 2 (17%) | 0 (0%) | 1 (33%) | 0 (0%) | 1 (33%) |
| Progressive disease | 1 (8%) | 0 (0%) | 1 (33%) | 0 (0%) | 0 (0%) |
Note: Data are median (IQR) or No (%), DL, Dose Level.
No dose-limiting toxicities were reported, thus the MTD was not identified. Four participants (33%) had fever after RJMty19 infusion with onset 0–5 days post-infusion, all resolved with anti-pyretic treatments or prophylactic antibiotics. Of the 4 participants with fever, one experienced grade 2 CRS with fever, hypotension and chills, which improved with IV fluids. Three of the 4 participants experienced grade 1 CRS with fever only. ICANS was not observed in any participant. The only neurotoxicity was dizziness in one participant observed 2 days after the infusion. Although RJMty19 was derived from allogeneic healthy donors, no SAEs or no signs of GvHD were observed.
The most common ≥3 grade AEs were hematological abnormalities, including neutropenia (N = 12,100%), leukopenia (N = 12,100%), lymphopenia (N = 10, 83%), thrombocytopenia (N = 6, 50%), febrile neutropenia (N = 3, 25%) and anemia (N = 4, 33%) (Table 2). Six out of 12 patients still had grade 2 neutropenia at 28 days after RJMty19 infusion despite the addition of G-CSF. Additionally, one participant experienced grade 2 urinary tract infection at 3 days after infusion. One participant experienced a grade 3 shingles at 38 days after infusion who was not treated with antiviral prophylaxis before or after the infusion due to impaired renal disfunction.
Table 2.
Adverse events.
| AEs | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|---|
| Hematologic AEs | ||||
| Anemia | 0 (0%) | 0 (0%) | 4 (33%) | 0 (0%) |
| Febrile neutropenia | 0 (0%) | 0 (0%) | 3 (25%) | 0 (0%) |
| Lymphocyte count decreased | 0 (0%) | 0 (0%) | 1 (8%) | 9 (75%) |
| Neutrophil count decreased | 0 (0%) | 0 (0%) | 1 (8%) | 11 (92%) |
| Platelet count decreased | 0 (0%) | 1 (8%) | 1 (8%) | 5 (42%) |
| White blood cell decreased | 0 (0%) | 0 (0%) | 2 (17%) | 10 (83%) |
| Immune system disorders | ||||
| CRS | 3 (25%) | 1 (8%) | 0 (0%) | 0 (0%) |
| Hypogammaglobulinemia | 5 (42%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Nervous system disorders | ||||
| Dizziness | 1 (8%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Metabolism and nutrition disorders | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Hyperuricemia | 2 (17%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Hypoalbuminemia | 0 (0%) | 1 (8%) | 0 (0%) | 0 (0%) |
| Musculoskeletal and connective tissue disorders | ||||
| Back pain | 1 (8%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Pain in extremity | 2 (17%) | 1 (8%) | 0 (0%) | 0 (0%) |
| Respiratory, thoracic and mediastinal disorders | ||||
| Cough | 3 (25%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Dyspnea | 1 (8%) | 0 (0%) | 0 (0%) | 0 (0%) |
| General disorders and administration site conditions | ||||
| Chills | 1 (8%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Edema limbs | 2 (17%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Fatigue | 1 (8%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Fever | 3 (25%) | 1 (8%) | 0 (0%) | 0 (0%) |
| Pain | 4 (33%) | 1 (8%) | 0 (0%) | 0 (0%) |
| Gastrointestinal disorders | ||||
| Abdominal pain | 1 (8%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Flatulence | 1 (8%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Nausea | 3 (25%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Vomiting | 1 (8%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Infections and infestations | ||||
| Urinary tract infection | 0 (0%) | 1 (8%) | 0 (0%) | 0 (0%) |
| Vascular disorders | ||||
| Hypotension | 1 (8%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Cardiac disorders/Respiratory, thoracic and mediastinal disorders | ||||
| Chest tightness | 1 (8%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Investigations | ||||
| Blood lactate dehydrogenase increased | 6 (50%) | 0 (0%) | 0 (0%) | 0 (0%) |
Note: Data are No (%); All AEs except for CRS and ICANS were graded according to NCI CTCAE 5.0; CRS and ICANS was graded according to the criteria of American Society for Transplantation and Cellular Therapy consensus grading. Abbreviations: CRS, cytokine release syndrome; ICANS, immune effector cell-associated neurotoxicity syndrome. All adverse events regardless of relationship with CAR-DNT cell treatment, which occurred within 30 days post-infusion in the 12 patients, are shown in the table.
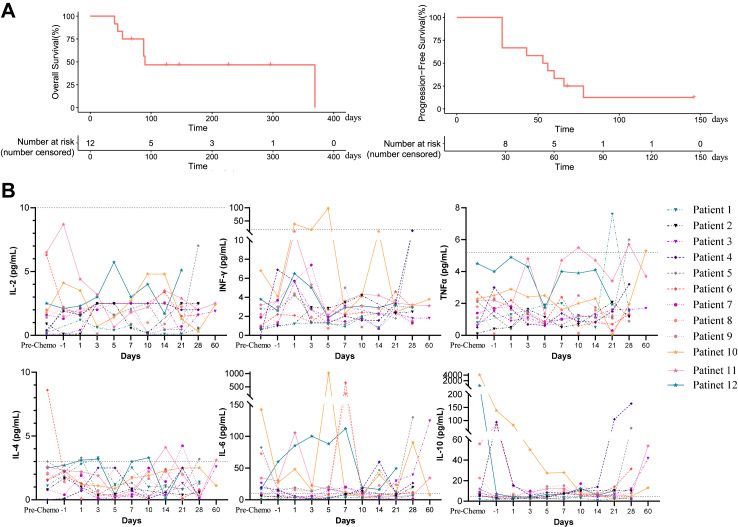
Four of the 12 participants achieved response to treatment. Remarkably, at the highest dose of 2 × 107 CAR + cells/kg (DL4), one of 3 participants achieved complete remission, and 2 of 3 participants achieved partial remission while all participants who received CD19-CAR-DNT cells at dose level 3 (DL3) achieved disease control. However, participants who received DL1 and DL2 benefited less from the therapy (ORR: 17%, N = 1; DCR: 33%, N = 2) (Fig. 1B). Patient 12, a 67-year-old male with high tumor burden (tumor mass = 115.8 × 99.6 mm), was enrolled into the DL4 group. He achieved complete remission assessed by PET/CT 1 month after treatment. As displayed in the Kaplan–Meier plot, median duration of remission has not yet reached at the time of the interim analysis. The estimated median OS was not reached (95% CI: 93-NE) (Fig. 2A). Seven participants died due to primary disease progression (N = 5) and COVID-19 infection (N = 2). Comparing antitumor activity at different dose levels of CD19-CAR-DNT cells, participants were further analyzed. Patients who received CD19-CAR-DNT cells at higher-dose cohorts (≥9 × 106 CAR + cells/kg, N = 6) had better survival compared with the lower-dose cohorts (≤3 × 106 CAR + cells/kg, N = 6) (Supplementary Figure S1). Taken together, the data suggests that RJMty19 acts in a dose-dependent manner.
Fig. 2.
Patient's survival and kinetics of cytokines. (A) Kaplan–Meier curves showing overall survival and progression-free survival for all enrolled patients. (B) Cytokine levels in the peripheral blood of each patient were detected using CBA assays. The gray dashed line represents the upper limit of normal.
Serum levels of IL-2, TNFα, IFNγ, IL-10, IL-6, and IL-10 were measured at baseline and several timepoints during the follow-up period (Fig. 2B). In the non-remission group, the median maximum fold-change from Day-1 was 3.94 (IQR: 1.58–16.43), 2.86 (IQR: 1.67–5.57), 3.07(IQR: 1.06–16.00) for IL6, IFNγ and TNFα respectively. In the remission group, the median maximum fold-change from Day-1 was 20.54 (IQR: 1.87–160.52), 2.72 (IQR: 2.09–23.86), 1.81 (IQR: 1.23–2.59) for IL6, IFNγ and TNFα respectively. There is no obvious difference in IL6 (P = 0.61), IFNγ (P = 0.73) and TNFα levels (P = 0.99) between remission group and non-remission group.
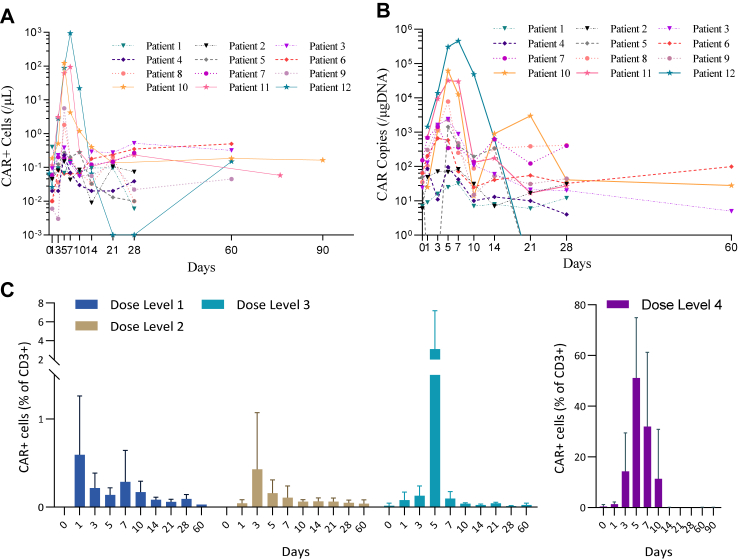
CD19-CAR-DNT cell expansion and CAR copy numbers are summarized in Fig. 3. The Tmax of CAR-DNT cells was between Day 3 and Day 7, with a median number of 0.52 cells/μL (IQR: 0.15–936.39). Similarly, the Tmax of CAR copy number occurred between Day 3 and Day 7, with a median of 2339 copies/μg DNA (IQR: 32–458,856). CAR-DNT cells amplified in vivo in a dose-dependent manner. The median absolute accounts of peak CAR-DNT cells in DL1, DL2, DL3, and DL4 cohorts were 0.40, 0.28, 1.81, and 122.20 cells/μL respectively. The median values of peak CAR copy number in these cohorts were 83, 662, 2339, and 62,085 copies/μg DNA respectively (Supplementary Figure S2). Accordingly, the proportion of CAR-DNT cells in CD3+ T cells reached 71%, 28%, 63% in DL4 cohorts (N = 3) respectively at Cmax time point (Fig. 3C). Significant DNT cell amplification was obtained in vivo for DL3 and DL4, with a median peak proportion of CD3+ T cells of 23% (IQR: 16%–27%) and 86% (IQR: 58%–95%) respectively (Supplementary Figure S3e). Interestingly, endogenous CD8+ T cells were better expanded in vivo than endogenous CD4+ T cells (Supplementary Figure S3f).
Fig. 3.
Pharmacokinetics of RJMty19. CAR-DNT cell kinetics were assessed by flow cytometry (A) and quantitative PCR (B) at different timepoints. Higher proportions of CAR+ cells in CD3+ T cells were seen in patients who received higher doses of CAR-DNT cell therapy (C). Note: Data are median (IQR) or No (%), DL, Dose Level.
Discussion
The results from our first-in-human, open-label, single-dose, phase 1 study demonstrate the feasibility of using off-the-shelf anti-CD19 CAR-DNT cells in the treatment of relapsed and refractory LBCL. We found that response rate was dose-dependent, with DL4 cohort of CAR-DNT demonstrating 100% ORR. There were no serious AEs related to the treatment during this interim analysis.
Previous studies report a strong association between autologous robust CAR-T cell expansion and the development of CRS and ICANS.18,19 However, this response was not observed following RJMty19 infusion as no ≥ grade 3 CRS and ICANS were observed in this study. Furthermore, GvHD and DLTs were also not observed in this study. These results are similar to other off-the-shelf cell therapy products.20,21 For example, of the 19 patients treated with NKX019, a CAR-engineered allogeneic NK cell product, the ≥ grade 3 AEs were hematologic toxicity (84%) and infection (5%). No DLTs, neurotoxicity, or GvHD were reported in patients with NKX019 treatment.20 Although the underlying mechanism still needs to be elucidated, the lower CRS severity from CD19-CAR-DNT therapy may be due to different cytokine profiles than conventional CD4+ CD8+ CART. As described above, all ≥ grade 3 AEs were hematological toxicity in patients with RJMty19 treatment most likely due to lymphodepleting chemotherapy rather than the anti-CD19 CAR-DNT infusion. Overall, allogenic CAR-DNT cell therapy show a manageable safety profile that may be superior to conventional CART.
Notably, the dose-dependent response was observed in DL4 cohort (N = 3), which achieved a 100% ORR and a 33% complete remission rate (CRR). In particular, patient 12 had a stage IV non-germinal center B cell like DLBCL with MYC and BCL6 translocation, IPI score of 4, and disseminated tumor burden, with the largest target lesion reaching 115.8 × 99.6 mm. He achieved CR on day 30 after receiving RJMty19 infusion and maintain the CR status till the cut-off date. Objective remission rates in B-cell lymphoma patients range from 44 to 91% and CRR from 28 to 68% based on 10 independent autologous CAR-T clinical studies.7 Thus, our allogenic product had promising antitumor activity at the high-dose level. Our preclinical data shows that multiple CAR-DNT infusions significantly enhanced tumor growth inhibition compared to single administrations in NOG mice, indicating that multiple-CAR-DNT cell infusions may be necessary to achieve better antitumor activity.22 The above data suggest that participants can benefit from high dose level of CD19-CAR-DNT cells. However large-scale and multi-center designed clinical trials are needed to further evaluate CAR-DNT cells in the future.
It is well known that expansion of autologous CAR-T in vivo is significantly associated with clinical response.6 Similarly, RJMty19 is amplified in vivo in a dose-dependent manner.20,21 The mean absolute account of peak CAR-DNT cells in DL4 cohort was 384.17 cells/μL, which was comparable to the autologous CAR-T therapy.6,23 All these data suggest that high-dose regimen might be necessary for allogenic CAR-DNT therapies to achieve a better outcome. Interestingly, we observed that CD4/CD8 ratio in the peripheral blood of patients continued to decrease after RJMty19 infusion (Supplementary Figure S3), indicating that the endogenous CD8+ cells in the patient expanded more than CD4+ cells. This contrasts with CAR-αβ T cell therapy where CD4+ T cells were better expanded in vivo after infusion.24 Many studies had revealed that some cytokines, such as IFNγ and TNFα, play important roles in the anti-tumor activity with autologous CAR-T cell therapy.25,26 However, in our study, no significant cytokine releases were found, indicating that CAR-DNT cells holds a different anti-tumor mechanism(s) from conventional autologous CAR-T cells. One potential mechanism from preclinical studies suggest that LFA-1 and perforin-granzyme B degranulation pathways are involved in CD19-CAR-DNT-mediated targeting of B-ALL.14
There are several limitations inherent to this single-center phase 1 study, in particular the comparatively small number of participants and the short follow up time for this interim analysis. Since most participants had DLBCL, these results may be difficult to interpret for all refractory LBCLs. However, this first in human clinical trial provides an early indication of the potential for allogeneic CD19-CAR-DNT therapy use in relapsing and refractory LBCLs. Unlike genetic knockout of TCR α constant chain on the surface of conventional CD4+ and CD8+ T cell to evade the GvHD and HvG, preclinical studies show that DNTs are resistant to an alloreactive T cell response without any genetic modification. In murine models, DNTs can suppress alloreactive T cells by acquiring allo-MHC (major histocompatibility complex) via trogocytosis and using the acquired MHC to recognize alloreactive conventional T cells to target them in a Fas-FasL–dependent manner.27,28 In addition, human TCRαβ+ DNTs can suppress conventional T cells by impairing their metabolic reprograming.29,30 Further investigations are needed to fully understand the molecular mechanisms by which human DNTs evade HvG rejection.
In conclusion, this first in human phase 1 clinical study demonstrates the safety and anti-tumor activity of RJMty19 in patients with r/r LBCL. RJMty19 is derived from healthy donors and does not require gene editing of the endogenous TCR, which decreases manufacturing costs and complexity with the additional benefits associated with allogeneic cell therapy. Our data shows that RJMty19 is well tolerated with no serious AEs, and superior antitumor activity at the higher doses. Thus, CD19-CAR-DNT cell immunotherapy is a promising new treatment for relapsing and refractory B cell lymphoma. Longer follow up is needed to determine the safety and efficacy of antitumor activity after RJMty19 infusions. Further validation of anti-CD19 CAR-DNT treatment effects is warranted in a multi-center clinical trial.
Contributors
XX, HL, XQ, PC, and XL performed clinical study visits, and collected the data. DW performed experiments and wrote the manuscript of preclinic studies. GS performed and analyzed the clinic data, and wrote the manuscript. YC performed the quality control of the products and performed PK/PD studies. LY contributed to the study design, edited and reviewed the manuscript, and interpreted the result. WQ designed the study, analyzed the data, and wrote and reviewed the manuscript. All authors reviewed and approved the final version of the manuscript. LY and QW accessed and verified the underlying data in the study.
Data sharing statement
The study protocol and individual patient data are provided in the appendix. Any request for data sharing should be directed to the corresponding author via qianwb@zju.edu.cn. Any data (e.g., individual patient data or imaging data) that can be shared will need approval from the Institutional Review Board of the Second Affiliated Hospital, School of Medicine, Zhejiang University. These data will be available 6 months after the publication of the article. All data shared will be de-identified.
Declaration of interests
DW, GS, YC and LY are employed by Wyze Biotech Co., Ltd, Zhongshan, Guangdong, China. DW and LY are inventors of several DNT cell technology-related patents and intellectual properties. HL, XQ, PC, XL, WQ declare no competing interests.
Acknowledgements
This study was funded by Wyze Biotech Co., Ltd, Zhongshan, Guangdong, China. We thank the patients who volunteered to participate in this study, their families and caregivers, the physicians and nurses who gave clinical care, and the staff who coordinated the clinical study.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2024.102516.
Contributor Information
Liming Yang, Email: lyang@wyzebiotech.com.
Wenbin Qian, Email: qianwb@zju.edu.cn.
Appendix A. Supplementary data
References
- 1.Larson R.C., Maus M.V. Recent advances and discoveries in the mechanisms and functions of CAR T cells. Nat Rev Cancer. 2021;21(3):145–161. doi: 10.1038/s41568-020-00323-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hong M., Clubb J.D., Chen Y.Y. Engineering CAR-T cells for next-generation cancer therapy. Cancer Cell. 2020;38(4):473–488. doi: 10.1016/j.ccell.2020.07.005. [DOI] [PubMed] [Google Scholar]
- 3.June C.H., O'Connor R.S., Kawalekar O.U., Ghassemi S., Milone M.C. CAR T cell immunotherapy for human cancer. Science. 2018;359(6382):1361–1365. doi: 10.1126/science.aar6711. [DOI] [PubMed] [Google Scholar]
- 4.Schuster S.J., Tam C.S., Borchmann P., et al. Long-term clinical outcomes of tisagenlecleucel in patients with relapsed or refractory aggressive B-cell lymphomas (JULIET): a multicenter, open-label, single-arm, phase 2 study. Lancet Oncol. 2021;22(10):1403–1415. doi: 10.1016/S1470-2045(21)00375-2. [DOI] [PubMed] [Google Scholar]
- 5.Munshi N.C., Anderson L.D., Shah N., et al. Idecabtagene vicleucel in relapsed and refractory multiple myeloma. N Engl J Med. 2021;384(8):705–716. doi: 10.1056/NEJMoa2024850. [DOI] [PubMed] [Google Scholar]
- 6.Neelapu S.S., Locke F.L., Bartlett N.L., et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377(26):2531–2544. doi: 10.1056/NEJMoa1707447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cappell K.M., Kochenderfer J.N. Long-term outcomes following CAR T cell therapy: what we know so far. Nat Rev Clin Oncol. 2023;20(6):359–371. doi: 10.1038/s41571-023-00754-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao J., Lin Q., Song Y., Liu D. Universal CARs, universal T cells, and universal CAR T cells. J Hematol Oncol. 2018;11(1):132. doi: 10.1186/s13045-018-0677-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lyman G.H., Nguyen A., Snyder S., Gitlin M., Chung K.C. Economic evaluation of chimeric antigen receptor T-cell therapy by site of care among patients with relapsed or refractory large B-cell lymphoma. JAMA Netw Open. 2020;3(4):e202072. doi: 10.1001/jamanetworkopen.2020.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Graham C., Jozwik A., Pepper A., Benjamin R. Allogeneic CAR-T cells: more than ease of access? Cells. 2018;7(10):155. doi: 10.3390/cells7100155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Z.X., Yang L., Young K.J., DuTemple B., Zhang L. Identification of a previously unknown antigen-specific regulatory T cell and its mechanism of suppression. Nat Med. 2000;6(7):782–789. doi: 10.1038/77513. [DOI] [PubMed] [Google Scholar]
- 12.Lee J., Minden M.D., Chen W.C., et al. Allogeneic human double negative T cells as a novel immunotherapy for acute myeloid leukemia and its underlying mechanisms. Clin Cancer Res. 2018;24(2):370–382. doi: 10.1158/1078-0432.CCR-17-2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee J.B., Kang H., Fang L., D'Souza C., Adeyi O., Zhang L. Developing allogeneic double-negative T cells as a novel off-the-shelf adoptive cellular therapy for cancer. Clin Cancer Res. 2019;25(7):2241–2253. doi: 10.1158/1078-0432.CCR-18-2291. [DOI] [PubMed] [Google Scholar]
- 14.Vasic D., Lee J.B., Leung Y., et al. Allogeneic double-negative CAR-T cells inhibit tumor growth without off-tumor toxicities. Sci Immunol. 2022;7(70) doi: 10.1126/sciimmunol.abl3642. [DOI] [PubMed] [Google Scholar]
- 15.Tang B., Lee J.B., Cheng S., et al. Allogeneic double-negative T cell therapy for relapsed acute myeloid leukemia patients post allogeneic hematopoietic stem cell transplantation: a first-in-human phase I study. Am J Hematol. 2022;97(7):E264–E267. doi: 10.1002/ajh.26564. [DOI] [PubMed] [Google Scholar]
- 16.Cheson B.D., Fisher R.I., Barrington S.F., et al. Recommendations for initial evaluation, staging, and response assessment of hodgkin and non-hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32(27):3059–3067. doi: 10.1200/JCO.2013.54.8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee D.W., Santomasso B.D., Locke F.L., et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant. 2019;25(4):625–638. doi: 10.1016/j.bbmt.2018.12.758. [DOI] [PubMed] [Google Scholar]
- 18.Jain M.D., Miklos D.B., Jacobson C.A., et al. Axicabtagene ciloleucel in combination with the 4–1BB agonist utomilumab in patients with relapsed/refractory large B-cell lymphoma: phase 1 results from ZUMA-11. Clin Cancer Res. 2023;29(20):4118–4127. doi: 10.1158/1078-0432.CCR-23-0916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Locke F.L., Rossi J.M., Neelapu S.S., et al. Tumor burden, inflammation, and product attributes determine outcomes of axicabtagene ciloleucel in large B-cell lymphoma. Blood Adv. 2020;4(19):4898–4911. doi: 10.1182/bloodadvances.2020002394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dickinson M., Hamad N., Bryant C.E., et al. First in human data of NKX019, an allogeneic CAR NK for the treatment of relapsed/refractory (R/R) B-cell malignancies. Hematol Oncol. 2023;41(S2):526–527. [Google Scholar]
- 21.Neelapu S.S., Stevens D.A., Hamadani M., et al. A phase 1 study of ADI-001: anti-CD20 CAR-engineered allogeneic gamma Delta1 (γδ) T cells in adults with B-cell malignancies. Blood. 2022;140(Supplement 1):4617–4619. [Google Scholar]
- 22.Yang L., Wang D., Xu S., et al. Abstract 5510: preclinical study of allogeneic CD19-CAR-DNT cells as an off-the-shelf immunotherapy drug for NHL. Cancer Res. 2022;82 [Google Scholar]
- 23.Westin J.R., Oluwole O.O., Kersten M.J., et al. Survival with axicabtagene ciloleucel in large B-cell lymphoma. N Engl J Med. 2023;389(2):148–157. doi: 10.1056/NEJMoa2301665. [DOI] [PubMed] [Google Scholar]
- 24.Zhang D.K.Y., Adu-Berchie K., Iyer S., et al. Enhancing CAR-T cell functionality in a patient-specific manner. Nat Commun. 2023;14(1):506. doi: 10.1038/s41467-023-36126-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang D., Li X., Qiu L., et al. Detecting 24 kinds of cytokines via flow cytometry aimplex kit is an effective way to monitor CRS after CAR-T cells infusion. Blood. 2020;136(Supplement 1):50–51. [Google Scholar]
- 26.Cosenza M., Sacchi S., Pozzi S. Cytokine release syndrome associated with T-cell-based therapies for hematological malignancies: pathophysiology, clinical presentation, and treatment. Int J Mol Sci. 2021;22(14):7652. doi: 10.3390/ijms22147652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ford M.S., Young K.J., Zhang Z., et al. The immune regulatory function of lymphoproliferative double negative T cells in vitro and in vivo. J Exp Med. 2002;196(2):261–267. doi: 10.1084/jem.20020029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ford McIntyre M.S., Young K.J., Gao J., et al. Cutting edge: in vivo trogocytosis as a mechanism of double negative regulatory T cell-mediated antigen-specific suppression. J Immunol. 2008;181(4):2271–2275. doi: 10.4049/jimmunol.181.4.2271. [DOI] [PubMed] [Google Scholar]
- 29.Haug T., Aigner M., Peuser M.M., et al. Human double-negative regulatory T-cells induce a metabolic and functional switch in effector T-cells by suppressing mTOR activity. Front Immunol. 2019;10:883. doi: 10.3389/fimmu.2019.00883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Achita P., Dervovic D., Ly D., et al. Infusion of ex-vivo expanded human TCR-alphabeta(+) double-negative regulatory T cells delays onset of xenogeneic graft-versus-host disease. Clin Exp Immunol. 2018;193(3):386–399. doi: 10.1111/cei.13145. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.