Abstract
Receptor dimerization is a crucial intermediate step in activation of signaling by receptor tyrosine kinases (RTKs). However, dimerization of the RTK Neu (also designated ErbB-2, HER-2, and p185neu), while necessary, is not sufficient for signaling. Earlier work in our laboratory had shown that introduction of an ectopic cysteine into the Neu juxtamembrane domain induces Neu dimerization but not signaling. Since Neu signaling does require dimerization, we hypothesized that there are additional constraints that govern signaling ability. With the importance of the interreceptor cross-phosphorylation reaction, a likely constraint was the relative geometry of receptors within the dimer. We have tested this possibility by constructing a consecutive series of cysteine substitutions in the Neu juxtamembrane domain in order to force dimerization along a series of interreceptor faces. Within the group that dimerized constitutively, a subset had transforming activity. The substitutions in this subset all mapped to the same face of a predicted alpha helix, the most likely conformation for the intramembrane domain. Furthermore, this face of interaction aligns with the projected Neu* V664E substitution and with a predicted amphipathic interface in the Neu juxtamembrane domain. We propose that these results identify an RTK dimer interface and that dimerization of this RTK induces an extended contact between juxtamembrane and intramembrane alpha helices.
Ligand-induced oligomerization of receptor tyrosine kinases (RTKs) is required for signal transduction. This conclusion is supported by the findings that hormone binding stimulates receptor dimerization, dimerization correlates with increased receptor phosphorylation, and oligomeric receptors are associated with higher levels of kinase activity than receptor monomers (14, 58, 59). Additionally, reagents that induce dimerization can activate RTKs. For example, the RTK Neu is activated by bivalent, but not monovalent, antibodies that cross-link the extracellular domain (57). Finally, kinase-defective RTKs dominantly inhibit signaling by wild-type receptors (39). These and other studies have reinforced the model that receptor dimerization provides an essential link between ligand binding and receptor activation (26, 27, 42).
Identifying the molecular interactions in the hormone-receptor (H-R) complex is essential for understanding the mechanism of receptor activation and may lead to development of new classes of RTK inhibitors. Knowledge of these contacts is limited by the paucity of crystallographic data. The crystal structure of the extracellular domain of the cytokine family receptor human growth hormone (hGH) receptor has provided a model of ligand-induced receptor dimerization. The hGH binds to its receptor with a 1:2 ligand-receptor stoichiometry, with hGH apparently forming first a high-affinity interaction with one receptor and then a lower-affinity interaction with a second receptor. The binding of the first H-R complex to the second receptor is thought to be stabilized by receptor-receptor (R-R) interactions along the extracellular juxtamembrane domain that extend into the transmembrane domain of the full-length receptor (18). The crystal structure of the vascular endothelial growth factor in complex with a minimal binding domain of the RTK Flt-1 revealed that the ligand and its receptor form a 2:2 H-R complex (55). However, the limited portion of the receptor analyzed did not extend beyond the ligand binding domain to include residues that might be involved in R-R interactions.
The lack of structural information concerning R-R contacts has hampered determination of the mechanism by which hormone binding regulates RTK dimerization and signaling. A current working model for members of the epidermal growth factor (EGF) receptor (EGFR) (ErbB) family of RTKs is that the active H-R complex consists of an H2-R2 tetramer produced by dimerization of 1:1 H-R complexes formed first (31). Joining of these complexes to form H2-R2 tetramers is driven by the intrinsic bivalency of EGF family hormones and is enhanced by membrane anchoring (52). Although dimerization is initiated by H-R contacts, there is compelling evidence for the existence and importance of R-R contacts in RTK oligomers as well. The first evidence was that an activated form of the avian EGFR homolog, v-ErbB, bears an amino-terminal truncation that deletes the hormone binding domain. Further studies have shown that truncation of other RTKs (Neu, Kit, Ros, Met, Ret, and Trk) leads to hormone-independent signaling, and in some cases this has been shown to be accompanied by dimerization (5, 20, 23, 37, 38, 51, 59). These studies established that ligand binding is not necessary for receptor activation. The gain-of-function phenotype caused by truncations further suggests that the extracellular domain inhibits dimerization until ligand binding occurs.
Genetic analysis of RTK R-R contacts has led to preliminary models for how dimerization leads to receptor signaling. An important model system for this work has been the RTK encoded by the neu/erbB-2/HER-2 gene (2, 28, 47). Neu is a member of the EGFR subfamily of RTKs (4, 16, 56). Neu has no known ligand, but it can be activated by interaction with other EGFR family members (30, 48; reviewed in references 19, 28, and 40). neu was first identified as a rat oncogene predisposing the animals to tumors of the nervous system. The first oncogenic allele of neu, neu*, encodes a single amino acid substitution in the predicted transmembrane domain of its product, Neu* (3), replacing Val with Glu at amino acid position 664. This substitution causes increased Neu* dimerization, Neu* tyrosine kinase activity, and Neu* turnover rate (5, 49, 54), suggesting that the mutation mimics normal activation by a yet-unidentified ligand.
Most models for function of the neu* mutation are based upon the assumption that the transmembrane domain is an alpha helix and that the glutamic acid is protonated in the lipid environment. These conclusions are supported by pH titration and spectroscopic analysis of reconstituted transmembrane domains (45). Molecular modeling suggested two related packing models, in which the carboxyl group of one receptor forms hydrogen bonds with the backbone carbonyl of A661 or, alternatively, forms a bidentate hydrogen bond with the corresponding glutamic acid on the second receptor (50). In another model, based upon molecular dynamics simulation, the receptors are in a nonsymmetric complex, with one transmembrane domain partly wrapped around the other (22).
While some of these models emphasize focal interreceptor contacts, other results hint that the normal intramembrane contact face may be extended. For example, the dimerization interface of glycophorin A (GpA) requires extended interaction of seven amino acids (33). Dimerization or transforming activity of glutamic acids in the Neu transmembrane domain is greatly enhanced with introduction of two glutamic acids, and this is most favorable when they are spaced as a heptad repeat, which would place them on the same face of an alpha helix (7, 12). The degree to which the transmembrane domain contributes to normal signaling is uncertain. Transmembrane domains are not absolutely required for dimerization of Neu and ErbB-3, but replacement of the ErbB-3 transmembrane domain with fibroblast growth factor receptor transmembrane sequences or a glycophosphatidylinositol anchor impaired formation of heterodimers with Neu (52).
Previously, our laboratory developed a system for defining the constraints on dimerization and activation of Neu by using substitution of ectopic cysteines to induce disulfide-linked dimers in vivo (10). Changing alanine to cysteine at position 653 (A653C) in the extracellular juxtamembrane domain induced Neu dimerization in vivo but not signaling. The same substitution in cis with the activating V664E substitution in the transmembrane domain did not interfere with transforming activity. Interestingly, this A653C/V664E double mutant showed elevated disulfide-mediated dimerization of Neu* relative to A653C and V664E single mutants, showing that the accumulation of disulfide-linked dimers acts as an in vivo cross-link that reflects the rate at which dimerization is induced at other locations. Analogous results were later obtained with an ectopic Cys introduced into the EGFR juxtamembrane domain: this mutation failed to activate EGFR signaling but stabilized EGF-induced dimers (46).
The ability of a disulfide cross-link to form at the juxtamembrane domains of both Neu and the EGFR implies that the juxtamembrane domains of these receptors are closely apposed in the receptor dimer. This observation is consistent with a substantial body of evidence indicating that V664E works by inducing an intramembrane R-R contact nearby (34, 45, 50). These results support the model that the signal created by hormone binding to EGF family RTKs, which results in dimerization of the extracellular domains, is communicated to the intracellular domain through the formation of juxtamembrane and intramembrane R-R contacts.
The finding that A653C induces dimerization without activating signaling was contrary to the common belief that dimerization is sufficient for receptor activation. However, other evidence indicates that RTK dimerization, while necessary, is not sufficient for signaling. We found that dimerization of Neu can be induced by introducing a dimerization domain from GpA or by moving a Neu* dimerization motif to a different location within the transmembrane domain. In both cases, the resulting receptor dimers lacked transforming activity (7). These results showed that additional constraints govern the formation of productive receptor dimers with signaling activity. Such constraints might include the ability of two receptor molecules to closely pack together, steric hindrance by the extracellular domain, conformational regulation, or the geometry of interreceptor contacts.
We hypothesized that A653C dimers lack transforming activity because the receptors are not brought together in a productive configuration. For example, the interreceptor disulfide bond may align the receptors along an improper face of interaction, or it may initiate a contact that is too distant from other critical contact sites to nucleate a productive interface. Alternatively, this mutation may simply interfere with a conformational regulation or disrupt a receptor structure required for signaling. In order to determine whether Cys substitutions at other juxtamembrane locations can induce signaling by Neu, we have used site-directed mutagenesis to individually replace a series of juxtamembrane amino acids with Cys. A subset of juxtamembrane Cys substitutions was found to have transforming activity. These data identify a productive interreceptor interface and suggest a primarily helical structure for the interface. We propose that this interface reflects the physiological receptor dimer interface, the first to be identified for any RTK.
MATERIALS AND METHODS
Cell culture.
COS-7, NIH 3T3, and FR3T3 cells were grown in Dulbecco-Vogt modified Eagle’s medium supplemented with 10% calf serum (CS) under an atmosphere of 5% CO2 at 37°C. Ψ2 cells were grown in Dulbecco-Vogt modified Eagle’s medium supplemented with 10% fetal bovine serum (FBS).
Antibodies.
The following antibodies were used: anti-Neu antibodies 7.16.4 (21) (Ab-4; Oncogene Science, Cambridge, Mass.) and SC284 (Santa Cruz Biotechnology, Santa Cruz, Calif.), antiphosphotyrosine antibodies PY20 (Transduction Laboratories, Lexington, Ky.) and 4G10 (Upstate Biotechnology Incorporated, Lake Placid, N.Y.), anti-Shc antibody S14630 (Transduction Labs, Lexington, Ky.), and the secondary antibodies horseradish peroxidase-linked donkey anti-rabbit immunoglobulin (for SC284 and anti-Shc) and horseradish peroxidase-linked sheep anti-mouse immunoglobulin (for 4G10) (Amersham, Arlington Heights, Ill.). The normal mouse serum was purchased from Pierce.
Plasmids.
Wild-type pSRα- and pDOL-neu, neu*, neuC653, and neu*C653 plasmids were described previously (5, 7–9). Mutations were introduced by PCR amplification. Two outside primers spanned the NdeI and BglI sites in neu, while inside forward and reverse mutagenic primers introduced mutations. Two fragments were amplified with one of each of the outside or inside primers. The amplified fragments were then annealed, extended, and reamplified to create a full-length fragment containing NdeI and BglI sites. The NdeI-BglI fragment was first cloned into SRα-neu with a three-part ligation containing BglI-EcoRI and EcoRI-NdeI fragments from wild-type SRα-neu. These constructs were recloned into pDOL-neu with a three-part ligation containing the mutant NdeI-BglI fragment and the BglI-Sal and Sal-NdeI fragments from wild-type pDOL-neu.
The synthetic oligonucleotides used for the outside primers had the following sequences: forward, 5′-GGA AGT ACC CGG ATG AGG AGG G-3′, and reverse, 5′-CCA GCT GTA CTG TGG ATG TCA GG-3′. The inside primers had the following sequences: Cys-652, forward, 5′-GCA GAG CAG TGC GCC AGC CC-3′, and reverse, 5′-GGG CTG GCG CAC TGC TCT GC-3′; Cys-654, forward, 5′-CAG AGA GCC TGC CCG GTG-3′, and reverse, 5′-CAC CGG GCA GGC TCT CTG-3′; Cys-655, forward, 5′-AGA GCC AGC TGC GTG ACA-3′, and reverse, 5′-TGT CAC GCA GCT GGC TCT-3′; Cys-656, forward, 5′-GCC AGC CCG TGT ACA TTC-3′, and reverse, 5′-GAA TGT ACA CGG GCT GGC-3′; Cys-657, forward, 5′-AGC CCG GTG TGT TTC ATC-3′, and reverse, 5′-GAT GAA ACA CAC CGG GCT-3′; Cys-658, forward, 5′-GGT GAC ATG CAT CAT TGC-3′, and reverse, 5′-GCA ATG ATG CAT GTC ACC-3′; Cys-659, forward, 5′-GGT GAC ATT CTG CAT TGC-3′, and reverse, 5′-GCA ATG CAG AAT GTC ACC-3′; Cys-660, forward, 5′-CCC GGT GAC ATT CAT CTG TGC AAC TG-3′, and reverse, 5′-CAG TTG CAC AGA TGA ATG TCA CCG GG-3′; Ala insertion between positions 658 and 659, forward, 5′-GGT GAC ATT CGC GAT CAT TGC-3′, and reverse, 5′-GCA ATG ATC GCG AAT GTC ACC-3′; Cys-656/Ala insertion, forward, 5′-GCC AGC CCG TGT ACA TTC GCG ATC ATT GC-3′, and reverse, 5′-GCA ATG ATC GCG AAT GTA CAC GGG CTG GC-3′; deleted Ile-659, forward, 5′-GGT GAC ATT CAT TGC-3′, and reverse, 5′-GCA ATG AAT GTC ACC-3′; Cys-655/ΔIle-659, forward, 5′-CGT GAC ATT CAT TGC-3′, and reverse, 5′-GCA ATG AAT GTC ACG-3′; and Cys-657/ΔIle-659, forward, 5′-GGT GTG TTT CAT TGC-3′, and reverse, 5′-GCA ATG AAA CAC ACC-3′.
COS cell expression.
SRα plasmids were introduced into COS-7 cells by transfection with DEAE-dextran and chloroquine as described previously (24), except that calcium- and magnesium-free phosphate-buffered saline (PBS), rather than Tris-saline, was used. Cells were labeled beginning 24 h after transfection. Procedures for metabolic labeling with [35S]Cys, immunoprecipitation, and gel electrophoresis have been described previously (11, 29, 47). Immunoprecipitations were done in radioimmunoprecipitation assay (RIPA) buffer consisting of 10 mM NaPO4 (pH 7.2), 1% Triton X-100, 0.1% sodium dodecyl sulfate, 1% sodium deoxycholate, 150 mM NaCl, 1% aprotinin, 2 mM EDTA, 50 mM NaF, and 1 mM Na orthovanadate. Antibodies were precipitated with protein A–Sepharose CL-4B (Pharmacia, Piscataway, N.J.) which was presoaked in PBS, preadsorbed with unlabeled parental cell lysates for 1 h, and then washed with RIPA buffer.
Production of stable cell lines expressing pDOL-neu plasmids.
Viral particles containing the pDOL-Cys-neu constructs were produced by transfecting the pDOL plasmids into Ψ2 cells as described previously (9) and then splitting a 100-mm-diameter dish of cells 1:3 and selecting in 10% FBS with 900 μg of Geneticin (Gibco BRL, Grand Island, N.Y.) per ml. After colonies formed, they were pooled. Virus was harvested in 10 ml (100-mm-diameter dish) of 2% FBS after 2 days and frozen at −80°C in aliquots.
Dishes (100-mm diameter) containing FR3T3 cells were infected as described in the table footnotes. The cells were selected for G418 resistance with 0.5 mg of Geneticin per ml. G418-resistant colonies were pooled and screened for expression.
Analysis of transforming ability by focus assay.
FR3T3 cells were infected with the mutant pDOL-neu plasmids. Two days after infection, the cells were divided into two groups: no selection (5% CS) and selection for G418 resistance with 0.5 mg of Geneticin per ml in 10% CS. Cells were incubated as described above for 14 days, with the medium changed every 3 days, and then were stained with crystal violet (Sigma). The foci on the unselected plates and the colonies on the selected plates were counted and tabulated.
Immunoprecipitation and immunoblotting.
The polyclonal FR3T3 cell lines were starved overnight in 0.1% fetal CS and then washed in PBS on ice and lysed in CHAPS lysis buffer {50 mM Tris HCl [pH 8.0], 50 mM NaCl, 0.7% 3-[(3-cholamidopropyl)-dimethylammino]-1-propanesulfonate [CHAPS], 10 mM NaF, 10 mM sodium orthophosphate, 2 mM EDTA, 1 mM sodium orthovanadate, 10 μg of leupeptin per ml, and 10 μg of aprotinin per ml}. Lysates were cleared by centrifugation at 4°C for 15 min. Two hundred micrograms of protein was immunoprecipitated with 1 μg of anti-Neu SC284 and then electrophoresed, transferred to nitrocellulose, blocked in 5% bovine serum albumin in Tris-buffered saline with 0.5% Tween 20, and detected by immunoblotting with either anti-Neu SC284 (1:100) or antiphosphotyrosine 4G10 (1:1,000). Similarly, 2 mg of protein was immunoprecipitated with 10 μg of anti-Shc (preabsorbed to protein A-Sepharose) and then blotted with anti-Neu SC284.
RESULTS
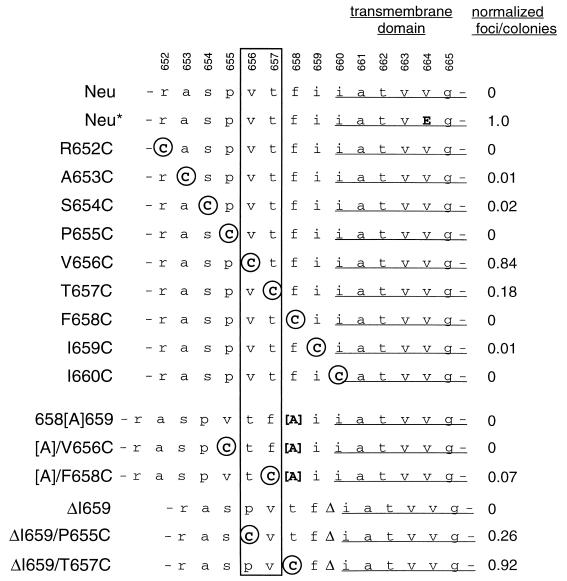
Introduction of ectopic cysteines is a powerful functional mapping tool that has been used to identify dimer interfaces in signal transduction by bacterial aspartate receptors and erythropoietin receptors (13, 35, 53). Since the novel interreceptor disulfide bonds induced by unpaired cysteines enforce dimerization at different points of contact, this approach permits identification of the subset of receptor dimers that have constitutive activity. We produced a series of nine consecutive Cys substitutions from position 652 to position 660 to determine whether enforced apposition of any of these residues would lead to dimers with signaling activity (Fig. 1).
FIG. 1.
Directed mutations in Neu. The Neu sequence (3) is shown at the top, with amino acid positions within the Neu polypeptide indicated. Mutant Neu proteins are designated with the original amino acid, amino acid position, and then the engineered replacement. Substituted Cys residues are shown circled in uppercase, other amino acid substitutions and insertions are shown in uppercase, and wild-type amino acids are shown in lowercase. Brackets flank Ala insertions; Δ denotes a deletion. The focus activities of mutants, normalized to 1.0 for Neu*, are from Tables 1 and 2. The box indicates the positions of the strongest activating Cys substitutions (see text).
Dimerization of Neu-cysteine mutants.
To determine whether the substitutions induce dimerization, these alleles were expressed in COS cells, and immunoprecipitated Neu was resolved by electrophoresis under nonreducing conditions (Fig. 2). As shown previously (8, 54), wild-type Neu runs as monomers under these conditions (Fig. 2A, neu). The neu* mutation V664E induces moderate dimerization, which is sensitive to reducing agents (Fig. 2B). This presumably reflects occasional spontaneous disulfide bonding involving the Cys-rich extracellular domains under conditions in which V664E drives abundant formation of noncovalent dimers (54). Directed mutations that did not introduce additional Cys residues, 658[A]659 and ΔI659, yielded proteins that did not dimerize (Fig. 2A). In contrast, with one exception, the receptors with Cys substitutions all dimerized as well as or better than Neu*. These dimers were disrupted by reducing agents, verifying that they are mediated by disulfide bonds (Fig. 2B).
FIG. 2.
Dimerization of Neu mutants. COS-7 cells in 100-mm-diameter dishes were transfected with 10 μg of plasmid DNA harboring the neu mutants described in Fig. 1 and then labeled in 2 ml of 100-μCi/ml [35S]Cys-Met for 24 h. Cells were washed in 10 ml of PBS containing 0.9 mM CaCl2, 0.5 mM MgCl2, and 10 mM iodoacetamide and then lysed in 1.5 ml of phosphate-buffered RIPA buffer containing iodoacetamide (10 mM), phenylmethylsulfonyl fluoride (PMSF) (1 mM), Na3VO4 (1 mM), and aprotinin (1%). Iodoacetamide was included in buffers to prevent formation of disulfide bonds after lysis. Lysates were immunoprecipitated with the rat Neu-specific 7.16.4 antibody. Samples were analyzed under nonreducing (A) and reducing (B) conditions on 4 to 12% acrylamide–0.19 to 0.56% bisacrylamide gradient gels. The fluorographed gels were exposed to preflashed film at −80°C for 6 days.
Of the nine consecutive Cys substitution mutants tested, only the I660C mutant failed to dimerize (Fig. 2A). I660C is the most carboxyl terminal of the substitutions tested and is located within the predicted transmembrane domain based upon hydropathy plotting. This suggests that the I660C mutant failed to dimerize because the mutation is to an intramembrane amino acid, and the intramembrane environment does not support formation of disulfide bonds (for example, because of the unavailability of protein disulfide isomerase). This is consistent with the behavior caused by another directed substitution, V664C, which is predicted to lie well within the transmembrane domain and produced a protein that also failed to dimerize (data not shown). The lack of I660C dimerization supports the prediction that position 660 is located just within the intramembrane domain and suggests Cys scanning as a simple approach for localizing junctions of transmembrane and extracellular domains.
Transformation of rat fibroblasts by Neu-cysteine mutants.
We next determined whether the mutant proteins induce cell transformation. FR3T3 rat fibroblasts were infected with recombinant retroviruses encoding the mutants and assayed for focus formation (Table 1). Only a subset of substitution mutants had focus-inducing activity, verifying that dimerization is not sufficient for transformation. Two mutants, V656C and T657C, had strong focus-inducing activity. Three others, A653C, S654C, and I659C had weaker transforming activity that was still above background. The R652C, P655C, F658C, and I660C mutants did not induce foci in these experiments. The weak transforming activity demonstrated by A653C contrasts with its lack of activity in our previous study (8). This probably reflects our change from the use of NIH 3T3 cells to the use of rat FR3T3 cells for focus assays. The lower background of spontaneous focus formation in the rat cells makes them more sensitive indicators for detecting weak focus activity.
TABLE 1.
Focus formation induced by Cys substitution mutantsa
| Virus | Expt 1
|
Expt 2
|
||||
|---|---|---|---|---|---|---|
| No. of foci | No. of colonies | Ratio (foci/colonies) | No. of foci | No. of colonies | Ratio (foci/colonies) | |
| neu | 0 | 175 | 0 | 0 | 558 | 0 |
| neu* | 438 | 552 | 0.79 | 835 | 1,152 | 0.72 |
| R652C | 0 | 645 | 0 | 0 | 38 | 0 |
| A653C | 2 | 340 | 0.01 | 15 | 1,282 | 0.01 |
| S654C | 2 | 528 | 0.004 | 30 | 1,208 | 0.02 |
| P655C | 0 | 902 | 0 | 0 | 730 | 0 |
| V656C | 102 | 155 | 0.66 | 268 | 438 | 0.61 |
| T657C | 90 | 580 | 0.16 | 138 | 1,115 | 0.12 |
| F658C | 0 | 992 | 0 | 2 | 1,570 | 0.001 |
| I659C | 10 | 815 | 0.01 | 18 | 1,268 | 0.01 |
| I660C | 0 | 462 | 0 | 0 | 1,298 | 0 |
FR3T3 cells were infected with pDOL-neu virus. Dishes (100-mm diameter) were incubated at 37°C in 3 ml of virus stock containing 1 μg of Polybrene per ml for 2 h. Two days after infection, the cells were divided into two groups: no selection (5% CS) and selection for G418 resistance with 0.5 mg of Geneticin per ml in 10% CS. Both sets of cells were incubated at 37°C for 10 or 14 days (experiments 1 and 2, respectively), with the medium changed every 3 days, and then were stained with crystal violet (Sigma). The foci on the unselected plates and the colonies on the G418-selected plates were tabulated.
The lack of transforming activity of several mutants might have a number of minor technical explanations, including poor expression, inappropriate transport, and kinase defects. However, all mutants tested were expressed well (Fig. 1). The mutant proteins were transported to the cell surface, since transfected cell lines show ample surface expression when assayed by immunofluorescence with anti-Neu antibody (6a). Finally, the mutants were functional in immune complex kinase assays, indicating that the receptors are active enzymatically (data not shown).
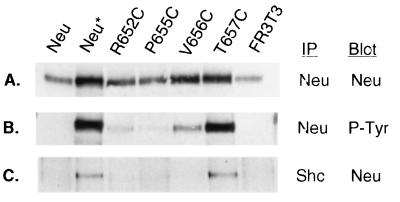
We next chose a subset of Cys substitution alleles to determine whether transformation correlates with other parameters associated with Neu-mediated transformation, specifically the levels of receptor tyrosine phosphorylation and association with downstream substrates, such as Shc (17). Lysates from polyclonal FR3T3 cell lines were immunoprecipitated with anti-Neu or anti-Shc and then immunoblotted with anti-Neu or antiphosphotyrosine (Fig. 3). While Neu was expressed at slightly varying levels among these cell lines, the amount of receptor phosphorylation relative to receptor expression was dramatically higher in cell lines expressing either Neu* or the transforming Cys substitutions V656C and T657C (Fig. 3A and B). Furthermore, in the two cases where Neu was highly phosphorylated, Neu* and T657C, Neu associated with immunoprecipitated Shc (Fig. 3C).
FIG. 3.
Cys mutant expression, tyrosine phosphorylation, and association with Shc. FR3T3 cell lines established from the focus assays were assayed to determine the levels of receptor tyrosine phosphorylation relative to expression. Two hundred micrograms of lysate was immunoprecipitated (IP) with anti-Neu and then electrophoresed on an 8% acrylamide (37.5:1 acrylamide/bisacrylamide ratio) gel, transferred to nitrocellulose, and immunoblotted with either anti-Neu SC284 (A) or antiphosphotyrosine 4G10 (B). Two milligrams each of the same lysates was immunoprecipitated with anti-Shc, processed as described above, and immunoblotted with anti-Neu SC284 (C).
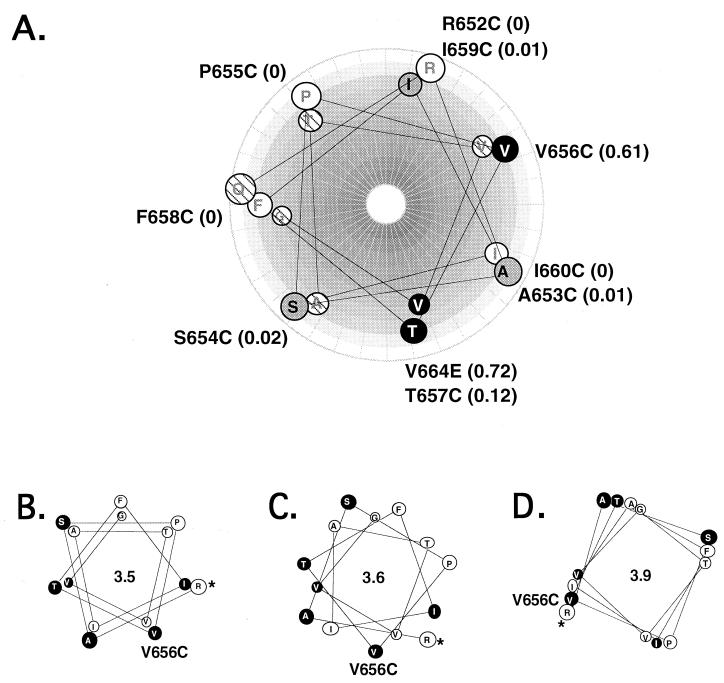
Our working model was that the Neu transmembrane domain is an alpha helix and that in a receptor dimer these helices pair to form an extended interface. The consecutive Cys substitutions would symmetrically rotate these helices relative to one another in the dimer. If there is only a single functional dimer interface, then the sites of productive Cys substitutions should show helical symmetry, with activating substitutions being located along a specific face. Hence, we next determined whether the positions of transformation-competent Cys substitutions are associated with a specific face of a predicted alpha helix. Cys residues in each mutant were aligned along helical-wheel representations of the juxtamembrane and transmembrane domains (Fig. 4A). Ectopic cysteines of the strongest transforming mutants, V656C and T657C, and the weaker transforming mutants, A653C, S654C, and I659C, are all located on the same side of the predicted alpha helix. The nontransforming mutants, R652C, P655C, and F658C, are grouped on the opposite face. Only a single substitution positioned on the active face, I660C, led to a protein with no transforming activity. However, the I660C mutant does not dimerize (Fig. 2A) and so would not be expected to have transforming activity. This result supports our assumption that transformation is mediated by disulfide-mediated dimerization, rather than conformational or other effects (Fig. 4A). Thus, these observations suggest that the juxtamembrane domain is helical and that a productive receptor dimer has a specific symmetric face of interaction.
FIG. 4.
Helical-wheel representations of Neu juxtamembrane domain. A portion of the Neu juxtamembrane-transmembrane domain (Q651 through G665) was positioned on predicted alpha helices. In this and subsequent figures the projection looks down into the helix, with the helix rotating clockwise towards the carboxyl terminus. (A) Alpha helix with pitch of 3.5 residues/turn, beginning with Q651. Wild-type amino acids are marked on the helix, and cysteine or glutamic acid substitutions are marked outside the helix. White circles, nontransforming substitutions; grey circles, intermediate substitutions; black circles, strongly transforming substitutions. Hatched residues were not tested. Relative focus activities are shown in parentheses. (B, C, and D) Helical wheels plotted at three different pitches. Relative locations of residues with the helical plot at a pitch of 3.5 residues/turn (B), 3.6 residues/turn (C), or 3.9 residues/turn (D) are shown. For orientation, the position of V656 is marked. Filled circles mark positions of substitutions with strong or intermediate transforming activity. Asterisks mark the amino terminus of the projection at R652.
Second-site mutations.
Although we hypothesized that these cysteine substitutions are transforming because they induce a specific R-R geometry, an alternative explanation is that certain substitutions create or disrupt some other structure involved in receptor activation. If the most important feature of the active complex is projection of Cys onto a specific face with helical structure, then it should be possible to predictably induce or interfere with activity based upon the projected helical face of ectopic cysteines. Thus, we constructed a series of insertion and deletion mutants that contain a Cys substitution shifted from a nontransforming position to a predicted transforming position or vice versa.
The positions of the strongest activating Cys substitutions (positions 656 and 657) are boxed in Fig. 1. Insertion of an amino acid between positions 658 and 659 would be predicted to rotate membrane-distal amino acids. This would inactivate transformation by V656C by moving that Cys off the active face to the position originally occupied by the transformation-incompetent substitution P655C. Consistent with this prediction, the double mutant [A]/V656C, with Ala inserted between residues 658 and 659 and V656C, had no transforming activity (Table 2), despite the facts that this protein dimerizes (Fig. 2A) and that V656C alone has strong transforming activity. In further support of our model, the double mutant [A]/F658C, with F658C now moved into a position consonant with activity, has acquired transforming activity (Table 2).
TABLE 2.
Focus formation by Cys substitution mutants with insertions and deletionsa
| Virus | Expt 1
|
Expt 2
|
Expt 3
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| No. of foci | No. of colonies | Ratio (foci/ colonies) | No. of foci | No. of colonies | Ratio (foci/ colonies) | No. of foci | No. of colonies | Ratio (foci/ colonies) | |
| neu | 0 | 175 | 0 | 0 | 558 | 0 | 0 | 96 | 0 |
| neu* | 438 | 552 | 0.79 | 835 | 1,152 | 0.72 | 87 | 205 | 0.42 |
| 658[A]659 | 0 | 320 | 0 | 0 | 588 | 0 | |||
| [A]/V656C | 0 | 775 | 0 | 0 | 906 | 0 | |||
| [A]/F658C | 9 | 187 | 0.05 | 13 | 270 | 0.05 | |||
| ΔI659 | 0 | 525 | 0 | 0 | 678 | 0 | |||
| ΔI659/P655C | 172 | 768 | 0.22 | 95 | 542 | 0.18 | |||
| ΔI659/T657C | 798 | 1,100 | 0.72 | 468 | 705 | 0.66 | 41 | 105 | 0.39 |
FR3T3 cells were infected with pDOL-neu virus and then selected and processed as described in Table 1, footnote a, for experiments 1 and 2. Cells in experiment 3 were incubated at 37°C for 10 days before scoring.
We used a similar approach to determine whether deletion of a residue between Cys substitutions and the transmembrane domain would also modulate Neu transforming activity in a predictable way. A mutant with a deletion of the isoleucine at position 659 (ΔI659) has no transforming activity (Table 2). This deletion would be predicted to rotate amino acid 655 to the position normally occupied by 656 (Fig. 1). Indeed, the double mutant ΔI659/P655C has transforming activity, in contrast to P655C alone (Table 2).
A final double mutant, ΔI659/T657C, did not behave as predicted, however. The transforming activity of ΔI659/T657C was even higher than that of T657C (Table 2). This result indicates that there may be additional sequence specificity involved in the R-R interaction at this position and suggests that the sequence of the region around position 657 which was retained in the deletion mutant may be influential in directing specific helical packing.
DISCUSSION
We introduced a series of single Cys substitutions in the Neu extracellular juxtamembrane domain in order to induce constitutive receptor dimerization. These substitutions induced signaling when they were located on a specific face of a predicted alpha helix. The sole Cys substitution on this face that did not induce transformation also did not induce dimerization, indicating that transforming activity is associated with dimerization and not some other structural feature. The validity of the predicted contact face was tested by second-site amino acid insertions and deletions. Three of the four double mutants tested behaved as predicted, suggesting that the major factor in determining transforming activity is the face onto which the ectopic Cys projects.
V664E activation.
With our finding that seven of eight transformation-inducing substitutions place Cys on a specific predicted helical face, it is noteworthy that this face aligns well with that predicted for the Neu* V664E substitution, while none of the nontransforming Cys mutants that dimerize have substitutions on this predicted face (Fig. 4A). Most evidence for the mechanism of activation of Neu* indicates that the novel Glu side chains are protonated and bind directly to the opposite receptor. Because the face of interaction predicted by the glutamic acid-induced dimerization is the same as that predicted by Cys scanning, this alignment supports the model that a transmembrane alpha-helix extends out of the membrane and participates in R-R contacts that facilitate dimerization and signaling. The results are also consistent with our earlier finding that dimerization of Neu-GpA chimeras did not activate signaling (7), since the GpA motifs in those receptors would be predicted to pack along the nonpermissive face identified here (1, 36).
Juxtamembrane alpha helix.
These results favor the existence of a specific interreceptor contact face that is most compatible with signaling. This contact face is distributed along a helix, consistent with theoretical models and spectroscopic data that predict a helical structure for single membrane-spanning peptides (32, 45). Such a structure for Neu is further supported by the finding that heptad spacing of introduced Glu residues is optimal for transforming activity (12). The transmembrane helix extends out of the transmembrane domain into the extracellular domain, since active Cys substitutions in the juxtamembrane domain align with the face of V664E.
Helical pitch and nature of packing.
The helical structures depicted in Fig. 4A and 4B are based on a periodicity of 3.5 residues per turn, which is typical of a left-handed coiled coil (15). Clustering of active Cys substitutions is also compatible with a parallel interaction of alpha helices with a periodicity of 3.6 residues per turn, which is typical of free alpha helices (Fig. 4C). However, an extended right-handed interaction, which would yield a periodicity of 3.9 residues per turn (Fig. 4D), would not be consistent with our model, since with this pitch the transforming cysteines would be distributed around the entire helix. Thus, we suggest that the putative dimer interface consists of left-handed coiled-coil or parallel helical interactions.
These data support the model that dimerization is sufficient for transformation provided that it occurs along a specific face of interaction. However, we observed a polarity in the efficiency of focus formation, suggesting additional constraints beyond the rotational orientation of the ectopic Cys residues: V656C and T657C had substantially greater transforming activity than A653C and S654C, which are on the same face but more distal to the membrane (Table 1). One interpretation for this finding is based on the idea that the face of interaction is extended and is primarily located within the transmembrane domain. The ability of a disulfide cross-link to lever the appropriate intramembrane interaction, or at least to increase the local concentration of compatible faces, would decrease as the distance from the membrane increases. Alternatively, the juxtamembrane alpha-helical structure may be destabilized or terminated with distance from the membrane, so that a dimer with a cross-link far from the membrane may allow more movement of the transmembrane domains relative to each other, reducing the likelihood of optimal packing.
Spontaneous mutations that activate Neu.
Expression of Neu in transgenic mouse mammary glands induces tumors that often harbor oncogenic mutations in Neu (25). These mutations encode small deletions in the juxtamembrane region (43). Most of the deletions result in elimination of single Cys residues that might normally participate in intrareceptor disulfide bonds. These unpaired Cys residues would be free to form interreceptor disulfide bonds, which could lead to constitutive dimerization and transformation. This seems to be the case, since engineered elimination of individual cysteines in the juxtamembrane domain of wild-type Neu at positions 635, 639, and 647 resulted in weak transforming alleles. The transforming activity of two mutant alleles, one with a deletion and one with a Cys-to-Ser substitution, was dependent on disulfide-mediated interactions, since 2-mercaptoethanol inhibited the transforming activity of the mutants (44). These results with mutations that unpair Cys residues complement the work described here. They are consonant with our data in demonstrating that unpaired Cys residues can induce transformation. However, helical-wheel predictions based on the locations of these Cys residues show that they project circumferentially around a helix (not shown), so the results are not compatible with the rotational specificity that we have observed. Since the affected sites are membrane distal to those investigated here, this may simply reflect a more extreme version of the polarity discussed above. Alternatively, the activating deletions may do more than uncover cysteines, since they lie within a Cys-rich domain that may function separately from the juxtamembrane domain.
Helical packing and normal signaling.
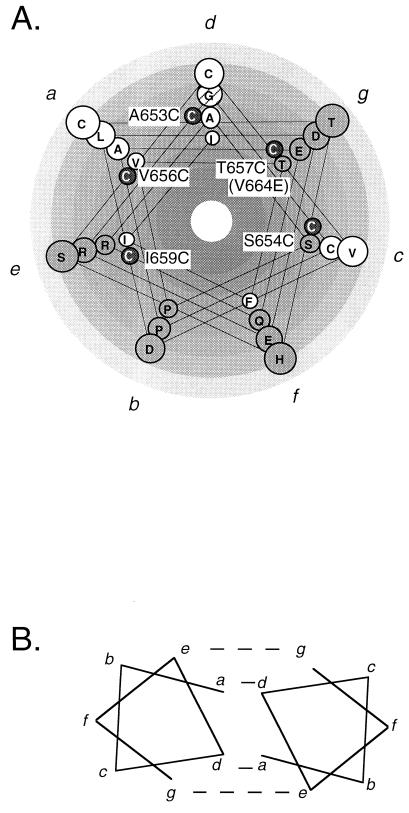
If a specific helix-helix packing is required for constitutive signaling induced by Cys substitutions, then this packing may be important for signaling by the wild-type receptor. The juxtamembrane domain sequence has a heptad repeat pattern typical of alpha helices that interact in an aqueous environment (Fig. 5A) (15). Helical-wheel analysis reveals two hydrophobic columns that align with the face of active Cys substitutions. In contrast, columns of hydrophilic residues align with the nonpermissive face. Thus, the apolar columns represent logical helical-packing interfaces.
FIG. 5.
Hydrophobicity plot of Neu juxtamembrane domain. (A) Neu juxtamembrane and transmembrane sequences (amino acids C635 to I660) were plotted on a helical wheel (at 3.5 residues/turn) and colored according to the theoretical side chain hydropathy scales (41). Open circles, hydrophobic residues; hatched circle, Gly; grey circles, hydrophilic residues. C, transforming Cys substitutions; a, b, c, d, e, f, and g, positions for amino acids in models shown in panel B and Fig. 6. (B) Heptads designated a through g (carboxyl terminus towards amino terminus) can pack as depicted into a coiled coil of alpha helices. a and d residues tend to be hydrophobic; e and g are charged and form ionic bonds (15).
The face of interaction between soluble alpha-helices often consists of two interacting apolar residues at positions a and d (Fig. 5B). This interaction is stabilized by ionic interactions across positions e and g (15). In Neu, these requirements are met by heptad repeats in the region spanning residues 643 to 656 (Fig. 6). The apolar residues are A649 and V656 for position a and A653 for position d. The ionic interactions across positions e and g may be formed by the basic residues R645 and R652 at position e interacting with the acidic residues D643 and E650 at position g (Fig. 6). Hence, we predict that this contact face, the same predicted by the Cys substitutions, is involved in dimerization of Neu. Acidic and basic residues in these complementary positions are found in Neu, but not other ErbB family members, and may explain the unusually strong ligand-independent signaling activity of this receptor.
FIG. 6.
Predicted coiled-coil interaction of parallel helices mediated by heptad repeats. Predicted juxtamembrane interactions between a and d and between e and g are marked with dashed lines. Interactions between two consecutive heptads, indicated above and below positions a through e, are plotted on a 3.5-residue/turn helix.
The striking alignment of this predicted face of interaction in the juxtamembrane domain with the activating V664E substitution and the face that is permissive for disulfide-induced signaling suggests that a juxtamembrane helix extends out as far as position 653 and is involved in juxtamembrane dimerization. Although this region includes prolines at positions 648 and 655, prolines are occasionally found in alpha helices, where they are associated with kinks in the helical axis (6, 60).
A final question is why there should be any specificity in the positioning of receptors in the dimer. The simplest model is that receptor signaling is activated through a cross-phosphorylation reaction within the dimer. The geometry of receptor interaction will determine the degree to which the receptors can pack closely and the position of the kinase domain on one receptor molecule relative to the phosphorylation sites on the other. Thus, these contacts may be instrumental in determining the selection of cross-phosphorylation sites, which in turn are involved in regulation of catalytic activity and coupling to downstream pathways.
Despite the overwhelming clinical importance of RTKs, including Neu, in cancer and cardiovascular disease, attempts to develop antagonists for hormones that activate these receptors have met with limited success. One difficulty is that these large peptide hormones probably bind through extended contact faces that may be difficult to block with small molecules. In contrast, the putative interreceptor contact face that we have identified comprises a compact structure that we anticipate will be absolutely required for receptor activation. This interface will make an ideal target for development of therapeutics. The Cys mapping approach for identification of receptor interfaces should be directly applicable to other EGF family receptor kinases and to other groups within the RTK superfamily.
ACKNOWLEDGMENTS
We thank Jonathan Mc-Menamin-Balano for the analysis of cell surface expression by immunofluorescence. Steve Smith, Frank Jones, and Michael DiGiovanna contributed valuable suggestions for the manuscript.
This work was supported by Public Health Service grant R01CA45708 from the National Cancer Institute.
REFERENCES
- 1.Adams P D, Engelman D M, Brunger A T. Improved prediction for the structure of the dimeric transmembrane domain of glycophorin A obtained through global searching. Proteins. 1996;26:257–261. doi: 10.1002/(SICI)1097-0134(199611)26:3<257::AID-PROT2>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 2.Akiyama T, Sudo C, Ogawara H, Toyoshima K, Yamamoto T. The product of the human c-erbB-2 gene: a 185-kilodalton glycoprotein with tyrosine kinase activity. Science. 1986;232:1644–1646. doi: 10.1126/science.3012781. [DOI] [PubMed] [Google Scholar]
- 3.Bargmann C I, Hung M-C, Weinberg R A. Multiple independent activations of the neu oncogene by a point mutation altering the transmembrane domain of p185. Cell. 1986;45:649–657. doi: 10.1016/0092-8674(86)90779-8. [DOI] [PubMed] [Google Scholar]
- 4.Bargmann C I, Hung M C, Weinberg R A. The neu oncogene encodes an epidermal growth factor receptor-related protein. Nature. 1986;310:226–230. doi: 10.1038/319226a0. [DOI] [PubMed] [Google Scholar]
- 5.Bargmann C I, Weinberg R A. Oncogenic activation of the neu-encoded receptor protein by point mutation and deletion. EMBO J. 1988;7:2043–2052. doi: 10.1002/j.1460-2075.1988.tb03044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barlow D, Thornton J. Helix geometry in proteins. J Mol Biol. 1988;201:601–619. doi: 10.1016/0022-2836(88)90641-9. [DOI] [PubMed] [Google Scholar]
- 6a.Burke, C., and J. McMenamin-Balano. Unpublished data.
- 7.Burke C L, Lemmon M A, Coren B A, Engelman D M, Stern D F. Dimerization of the p185neu transmembrane domain is necessary but not sufficient for transformation. Oncogene. 1997;14:687–696. doi: 10.1038/sj.onc.1200873. [DOI] [PubMed] [Google Scholar]
- 8.Cao H, Bangalore L, Bormann B J, Stern D F. An extra cysteine proximal to the transmembrane domain induces differential dimerization of p185neu and p185neu*. J Biol Chem. 1992;267:20489–20492. [PubMed] [Google Scholar]
- 9.Cao H, Bangalore L, Bormann B J, Stern D F. A subdomain in the transmembrane domain is necessary for p185neu* activation. EMBO J. 1992;11:923–932. doi: 10.1002/j.1460-2075.1992.tb05131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cao H, Bangalore L, Dompe C, Bormann B J, Stern D F. An extra cysteine proximal to the transmembrane domain induces differential dimerization of p185neu and p185neu*. J Biol Chem. 1992;267:20489–20492. [PubMed] [Google Scholar]
- 11.Cao H, Decker S, Stern D F. TPA inhibits the tyrosine kinase activity of the neu protein in vivo and in vitro. Oncogene. 1991;6:705–711. [PubMed] [Google Scholar]
- 12.Chen L I, Webster M K, Meyer A N, Donoghue D J. Transmembrane domain sequence requirements for activation of the p185c-neu receptor tyrosine kinase. J Cell Biol. 1997;137:619–631. doi: 10.1083/jcb.137.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chervitz S A, Lin C M, Falke J J. Transmembrane signaling by the aspartate receptor: engineered disulfides reveal static regions of the subunit interface. Biochemistry. 1995;34:9722–9733. doi: 10.1021/bi00030a010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cochet C, Kashles O, Chambaz E M, Borello I, King C R, Schlessinger J. Demonstration of epidermal growth factor-induced receptor dimerization in living cells using a chemical covalent cross-linking agent. J Biol Chem. 1988;263:3290–3295. [PubMed] [Google Scholar]
- 15.Cohen C, Parry D A D. Alpha-helical coiled-coils and bundles: how to design an alpha-helical protein. Proteins. 1990;7:1–15. doi: 10.1002/prot.340070102. [DOI] [PubMed] [Google Scholar]
- 16.Coussens L, Yang-Feng T L, Liao Y-C, Chen E, Gray A, McGrath J, Seeburg P H, Libermann T A, Schlessinger J, Francke U, Levinson A, Ullrich A. Tyrosine kinase receptor with extensive homology to EGF receptor shares chromosomal location with neu oncogene. Science. 1985;230:1132–1139. doi: 10.1126/science.2999974. [DOI] [PubMed] [Google Scholar]
- 17.Dankort D L, Wang Z, Blackmore V, Moran M F, Muller W J. Distinct tyrosine autophosphorylation sites negatively and positively modulate Neu-mediated transformation. Mol Cell Biol. 1997;17:5410–5425. doi: 10.1128/mcb.17.9.5410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Vos A M, Ultsch M, Kossiakoff A A. Human growth hormone and extracellular domain of its receptor: crystal structure of the complex. Science. 1992;255:306–312. doi: 10.1126/science.1549776. [DOI] [PubMed] [Google Scholar]
- 19.Dougall W C, Qian X, Peterson N C, Miller M J, Samanta A, Greene M I. The neu-oncogene: signal transduction pathways, transformation mechanisms and evolving therapies. Oncogene. 1994;9:2109–2123. [PubMed] [Google Scholar]
- 20.Downward J, Yarden Y, Mayes E, Scrace G, Totty N, Stockwell P, Ullrich A, Schlessinger J, Waterfield M D. Close similarity of epidermal growth factor receptor and v-erbB oncogene protein sequences. Nature. 1984;307:521–527. doi: 10.1038/307521a0. [DOI] [PubMed] [Google Scholar]
- 21.Drebin J A, Stern D F, Link V C, Weinberg R A, Greene M I. Monoclonal antibodies recognize a cell-surface antigen associated with an activated cellular oncogene. Nature. 1984;312:545–548. doi: 10.1038/312545a0. [DOI] [PubMed] [Google Scholar]
- 22.Garnier N, Genest D, Duneau J P, Genest M. Molecular modeling of c-erbB2 receptor dimerization: coiled-coil structure of wild and oncogenic transmembrane domains—stabilization by interhelical hydrogen bonds in the oncogenic form. Biopoly. 1997;42:157–168. doi: 10.1002/(SICI)1097-0282(199708)42:2<157::AID-BIP5>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 23.Giordiano S, Di Renzo M F, Narsimahan R P, Cooper C S, Rosa C, Comoglio P M. Biosynthesis of the protein encoded by the c-met proto-oncogene. Oncogene. 1989;4:1383–1388. [PubMed] [Google Scholar]
- 24.Guan J-L, Rose J K. Conversion of a secretory protein into a transmembrane protein results in its transport to the Golgi complex but not the cell surface. Cell. 1984;37:779–787. doi: 10.1016/0092-8674(84)90413-6. [DOI] [PubMed] [Google Scholar]
- 25.Guy C T, Webster M A, Schaller M, Parsons T J, Cardiff R D, Muller W J. Expression of the neu protooncogene in the mammary epithelium of transgenic mice induces metastatic disease. Proc Natl Acad Sci USA. 1992;89:10578–10582. doi: 10.1073/pnas.89.22.10578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heldin C-H, Ostman A. Ligand-induced dimerization of growth factor receptors: variations on the theme. Cytokine Growth Factor Rev. 1996;7:33–40. doi: 10.1016/1359-6101(96)00002-0. [DOI] [PubMed] [Google Scholar]
- 27.Heldin C H. Dimerization of cell surface receptors in signal transduction. Cell. 1995;80:213–223. doi: 10.1016/0092-8674(95)90404-2. [DOI] [PubMed] [Google Scholar]
- 28.Hynes N E, Stern D F. The biology of erbB-2/neu/HER-2 and its role in cancer. Biochim Biophys Acta Rev Cancer. 1994;1198:165–184. doi: 10.1016/0304-419x(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 29.Kamps M P, Sefton B M. Identification of multiple novel polypeptide substrates of the v-src, v-yes, v-fps, v-ros, and v-erb-B oncogenic tyrosine protein kinases utilizing antisera against phosphotyrosine. Oncogene. 1988;2:305–315. [PubMed] [Google Scholar]
- 30.Kokai Y, Meyers J N, Wada T, Brown V I, LeVea C M, Davis J G, Dobashi K, Greene M I. Synergistic interaction of p185 c-neu and the EGF receptor leads to transformation of rodent fibroblasts. Cell. 1989;58:287–292. doi: 10.1016/0092-8674(89)90843-x. [DOI] [PubMed] [Google Scholar]
- 31.Lemmon M A, Bu Z, Ladbury J E, Zhou M, Pinchasi D, Lax I, Engelman D, Schlessinger J. Two EGF molecules contribute additively to stabilization of the EGFR dimer. EMBO J. 1997;16:281–294. doi: 10.1093/emboj/16.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lemmon M A, Engelman D M. Helix-helix interactions inside lipid bilayers. Curr Opin Struct Biol. 1992;2:511–518. [Google Scholar]
- 33.Lemmon M A, Flanagan J M, Hunt J F, Adair B D, Bormann B-J, Dempsey C E, Engelman D M. Glycophorin A dimerization is driven by specific interactions between transmembrane α-helices. J Biol Chem. 1992;267:7683–7689. [PubMed] [Google Scholar]
- 34.Lofts F J, Hurst H C, Sternberg M J E, Gullick W J. Specific short transmembrane sequences can inhibit transformation by the mutant neu growth factor receptor in vitro and in vivo. Oncogene. 1993;8:2813–2820. [PubMed] [Google Scholar]
- 35.Lynch B A, Koshland D E. Disulfide cross-linking studies of the transmembrane regions of the aspartate sensory receptor of Escherichia coli. Proc Natl Acad Sci USA. 1991;88:10402–10406. doi: 10.1073/pnas.88.23.10402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.MacKenzie K R, Prestegard J H, Engelman D M. A transmembrane helix dimer: structure and implications. Science. 1997;276:131–133. doi: 10.1126/science.276.5309.131. [DOI] [PubMed] [Google Scholar]
- 37.Martin-Zanca D, Hughes S H, Barbacid M. A human oncogene formed by the fusion of truncated tropomyosin and protein-tyrosine kinase sequences. Nature. 1986;319:743–748. doi: 10.1038/319743a0. [DOI] [PubMed] [Google Scholar]
- 38.Matsushime H, Wang L-H, Shibuya M. Human c-ros-1 gene homologous to the v-ros sequence of UR2 sarcoma virus encodes for a transmembrane receptor-like molecule. Mol Cell Biol. 1986;6:3000–3004. doi: 10.1128/mcb.6.8.3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Redemann N, Holzmann B, von Ruden T, Wagner E F, Schlessinger J, Ullrich A. Antioncogenic activity of signalling-defective epidermal growth factor receptor mutants. Mol Cell Biol. 1992;12:491–498. doi: 10.1128/mcb.12.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Riese D J, II, Stern D F. Specificity within the EGF/ErbB receptor family signaling network. Bioessays. 1998;20:41–48. doi: 10.1002/(SICI)1521-1878(199801)20:1<41::AID-BIES7>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 41.Roseman M A. Hydropathy of polar amino acid side-chains is markedly reduced by flanking peptide bonds. Mol Biol. 1988;200:513–522. doi: 10.1016/0022-2836(88)90540-2. [DOI] [PubMed] [Google Scholar]
- 42.Schlessinger J. Signal transduction by allosteric receptor oligomerization. Trends Biochem Sci. 1988;13:443–447. doi: 10.1016/0968-0004(88)90219-8. [DOI] [PubMed] [Google Scholar]
- 43.Siegel P M, Dankort D L, Hardy W R, Muller W J. Novel activating mutations in the neu proto-oncogene involved in induction of mammary tumors. Mol Cell Biol. 1994;14:7068–7077. doi: 10.1128/mcb.14.11.7068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Siegel P M, Muller W J. Mutations affecting conserved cysteine residues within the extracellular domain of Neu promote receptor dimerization and activation. Proc Natl Acad Sci USA. 1996;93:8878–8883. doi: 10.1073/pnas.93.17.8878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith S O, Smith C S, Bormann B J. Strong hydrogen bonding interactions involving a buried glutamic acid in the transmembrane sequence of the neu/erbB-2 receptor. Nat Struct Biol. 1996;3:252–258. doi: 10.1038/nsb0396-252. [DOI] [PubMed] [Google Scholar]
- 46.Sorokin A, Lemmon M A, Ullrich A, Schlessinger J. Stabilization of an active dimeric form of the epidermal growth factor receptor by introduction of an inter-receptor disulfide bond. J Biol Chem. 1994;269:9752–9759. [PubMed] [Google Scholar]
- 47.Stern D F, Heffernan P A, Weinberg R A. p185, a product of the neu proto-oncogene, is a receptorlike protein associated with tyrosine kinase activity. Mol Cell Biol. 1986;6:1729–1740. doi: 10.1128/mcb.6.5.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stern D F, Kamps M P. EGF-stimulated tyrosine phosphorylation of p185neu: a potential model for receptor interactions. EMBO J. 1988;7:995–1001. doi: 10.1002/j.1460-2075.1988.tb02906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stern D F, Kamps M P, Cao H. Oncogenic activation of p185neu stimulates tyrosine phosphorylation in vivo. Mol Cell Biol. 1988;8:3969–3973. doi: 10.1128/mcb.8.9.3969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sternberg M J E, Gullick W J. Neu receptor dimerization. Nature. 1989;339:587. doi: 10.1038/339587a0. [DOI] [PubMed] [Google Scholar]
- 51.Takahshi M, Cooper G M. ret transforming gene encodes a fusion protein homologous to tyrosine kinases. J Biol Chem. 1987;263:3400–3447. doi: 10.1128/mcb.7.4.1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tzahar E, Pinkas-Kramarski R, Moyer J D, Klapper L N, Alroy I, Levkowitz G, Shelly M, Henis S, Eisenstein M, Ratzkin B J, Sela M, Andrews G C, Yarden Y. Bivalence of EGF-like ligands drives the ErbB signaling network. EMBO J. 1997;16:4938–4950. doi: 10.1093/emboj/16.16.4938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Watowich S S, Hilton D J, Lodish H P. Activation and inhibition of erythropoietin receptor function: role of receptor dimerization. Mol Cell Biol. 1994;14:3535–3549. doi: 10.1128/mcb.14.6.3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weiner D B, Liu J, Cohen J A, Williams W V, Greene M I. A point mutation in the neu oncogene mimics ligand induction of receptor aggregation. Nature. 1989;339:230–231. doi: 10.1038/339230a0. [DOI] [PubMed] [Google Scholar]
- 55.Wiesmann C, Fuh G, Christinger H W, Eigenbrot C, Wells J A, deVos A M. Crystal structure at 1.7Å resolution of VEGF in complex with domain 2 of the Flt-1 receptor. Cell. 1997;91:695–704. doi: 10.1016/s0092-8674(00)80456-0. [DOI] [PubMed] [Google Scholar]
- 56.Yamamoto T, Ikawa S, Akiyama T, Semba K, Nomura N, Miyajima N, Saito T, Toyoshima K. Similarity of protein encoded by the human c-erb-B-2 gene to epidermal growth factor receptor. Nature. 1986;319:230–234. doi: 10.1038/319230a0. [DOI] [PubMed] [Google Scholar]
- 57.Yarden Y. Agonistic antibodies stimulate the kinase encoded by the neu protooncogene in living cells but the oncogenic mutant is constitutively active. Proc Natl Acad Sci USA. 1990;87:2569–2573. doi: 10.1073/pnas.87.7.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yarden Y, Schlessinger J. Epidermal growth factor induces rapid, reversible aggregation of the purified epidermal growth factor receptor. Biochemistry. 1987;26:1443–1451. doi: 10.1021/bi00379a035. [DOI] [PubMed] [Google Scholar]
- 59.Yarden Y, Schlessinger J. Self-phosphorylation of epidermal growth factor receptor: evidence for a model of intermolecular allosteric activation. Biochemistry. 1987;26:1434–1442. doi: 10.1021/bi00379a034. [DOI] [PubMed] [Google Scholar]
- 60.Yun R H, Anderson A, Hermans J. Proline in α-helix: stability and conformation studied by dynamics stimulation. Proteins. 1991;10:219–228. doi: 10.1002/prot.340100306. [DOI] [PubMed] [Google Scholar]