Abstract
Hydrological management in the use of peatland for agriculture is the backbone of its sustainability and a critical factor in climate change mitigation. This study evaluates the application of an integrated water management practice known as the “Water Management Trinity” (WMT), implemented since 1986 on a coconut plantation on the eastern coast of Sumatra, in relation to CO2 emissions and subsidence rates. The WMT integrates canals, dikes, and dams with water gates to regulate water levels for both coconut agronomy and the preservation of the peat soil. The WMT has successfully regulated and maintained an average yearly water table depth of −45 to −51 cm below the surface. The methodology involved a closed chamber method for measuring soil CO2 flux using a portable Infrared Gas Analyzer, conducted weekly over a six-month period to cover dry and rainy season at bi-modal climate condition. Subsidence measurements have been ongoing from 1986 to 2022. The results show bare peat soil has heterotrophic respiration CO2 emissions of 7.77 t C–CO2 ha−1 yr−1, while in coconut plantations 7.99 t C–CO2 ha−1 yr−1, similar to emissions in mineral soils. Autotrophic respiration leads to the overestimation of CO2 emissions on peatland and accounts for 212–424% of the total emissions. The cumulative subsidence from 1986 to 2022 is −56.3 cm, with a soil rise of +0.8 cm in 2022, indicating a flattening rate of subsidence. This is characterized by an increase in bulk density at the surface from 0.072 to 0.144 gr/cm3, with approximately 81% of the subsidence being due to compaction. The statistical analysis found no relationship between water table depth and CO2 emissions, indicating that water table depth cannot be used as a predictor for CO2 emissions. In summary, peatland agriculture has a promising future when managed sustainably using an integrated hydrological management system.
Keywords: Sustainable peatland management, Water management trinity, CO2 emission, Subsidence rates, Coconut agriculture
Highlights
-
•
The implementation of an integrated water management system in coconut agriculture minimizes CO2 emissions and subsidence.
-
•
Water table depth is not a predictor of peatland emissions due to the absence of correlation.
-
•
Peatland agriculture has a promising future when managed sustainably using an integrated hydrological management.
1. Introduction
Peatlands, which constitute approximately 3% of global land coverage [1], hold significant global importance [2,3]. In Indonesia, it comprises about 7% of the country's total land area [4]. Traditionally, ethnic groups such as the Bugis people in South Sulawesi, Banjarnese in South Kalimantan, and the Malay people in Riau Province have sustainably utilized peatlands for agriculture. They have successfully utilized peatland for rice fields, coconut, and rubber plantations [5,6], typically using only a portion of the peatlands influenced by river tides [7]. This suggests that predominantly shallow peatlands located adjacent to rivers or coastal areas have been traditionally utilized, due to the nutrient addition by floods and tides. Furthermore, the success of traditional agriculture practices on peatlands prompted the government to initiate large-scale agriculture projects in Central Kalimantan on Borneo Island. However, failing to realize that the success was contingent on specific local wisdom and management practices by understanding the characteristics of peatlands, especially those located near rivers or coastlines, the “Mega Rice Project (MRP)" was instituted by the President of Indonesia through Decree No. 82 of 1995, aiming to convert 1,457,100 ha of peatland for agricultural use; became a disaster [8]. The failure of the MRP resulted in widespread ecosystem degradation [9,10]. This oversight has been frequently cited as a point of criticism in the development of peatlands for agricultural purposes.
Fundamentally, the challenges surrounding peatland utilization originate from improper management and policies governing peatland use, which have led to peatland degradation [11]. Peatland excavation for canal development is a common practice designed to drain water, a requisite for optimal crop growth and the prevention of asphyxiation. The traditional practice developed drainage only on the edge of the peatland hydrological unit (PHU), hence it conserved the peat dome that serves as water reservoir and the core of the ecosystem. Under Indonesia Government Regulation No. 57 of 2016, the PHU serves as the main boundary for peatland management, constituting an interconnected ecosystem situated between two rivers, a river and the sea, and/or within a swamp. A peat dome, as a primary feature within a PHU, necessitates careful protection and effective water management to maintain ecosystem integrity and enable sustainable utilization. In the case of the MRP, the project involved excavating peat for canal development to facilitate drainage, including the excavation of the peat dome, which disrupted the hydrological function of the peatland [12,13]. Due to the lack of water management integration and damage to the peat dome, the peatland ecosystem within the project area experienced floods during the rainy season and droughts in the dry season, failing to meet its objective as productive agricultural land [14]. This situation was exacerbated during El Niño-induced droughts, leading to recurrent wildfires, further degrading the integrity of the peatland and the surrounding environment [15,16].
As efforts to restrain population growth had only limited success, the demand for land for habitation and economic activities intensifies. As of 2015, approximately 6.3 million hectares of Indonesia's peatlands, accounting for 47% of the country's peatland cover, were utilized for plantations, including both smallholder and industrial operations [4,17]. Given this scenario, the sustainable utilization of peatlands emerges as a critical factor for the nation's populace, environment, and economy. In the context of peatland, sustainable use implies the potential for sustainable utilization of the peat [18]. This could be translated seeks to enhance the socio-economic quality of life for those living within the peatland ecosystem, while concurrently safeguarding and preventing peatland degradation. At the same time, sustainable peatland practices could emerge from responsible management that integrates a sustainable approach into agricultural practices [19].
To evaluate the sustainability of peatland, access to comprehensive information regarding peatland use, carbon emissions, and proper water management is essential to mitigate degradation across the millions of hectares of peatland currently dedicated to agriculture and plantations. It is imperative that demand for commodities does not justify exploitative practices on peatlands without implementing sustainable strategies. Such neglect could detrimentally impact the ecosystem and the economy, causing devastation for peatland communities that could last for centuries [20]. In this context, sustainable application of peatland utilization can be achieved by applying integrated water management that will not repeat the same mistakes as the MRP. In the 1990s, following the failure of the MRP, Wösten et al. (1997) and Ritzema et al. (1998) introduced the concept of combining water drainage and conservation for peatland utilization in agriculture, wherein the water level is controlled for agronomic purposes and peat soil conservation [21,22]. This method, also known as ‘water stock’ management, redesigns the canal not as a drainage system but as a reservoir or stock, akin to an irrigation system [23]. Consequently, this technique of water conservation through reservoir canals or stock-based water management enables agricultural systems on peat soil to emulate pristine forests in preserving soil moisture. This is due to the year-round availability of water, which fosters stable agricultural production, thereby enhancing human welfare.
Despite ongoing discussion and innovation in the sustainable use of peatlands through integrated or hydrological water management, there remains a prevalent belief that sustainable agricultural practices on these lands are virtually unattainable due to the absence of fully developed methodologies [24]. Moreover, there is an underlying concern that the failures experienced with the MRP may recur, potentially compromising the peatland ecosystem. This is particularly concerning given that peatlands represent a substantial carbon stock, estimated to be between 13.6 and 40.6 Gigatons (Gt) [25]. The lessons learned from the unsuccessful MRP, primarily the absence of effective hydrological management, have been linked to various environmental issues such as carbon emissions and land subsidence, with Indonesian peatlands estimated to emit around 0.51 Gt CO2 annually due to agricultural drainage and degradation [26]. However, this estimation may be inflated as it extrapolates emission values across all of Indonesia [27]. In areas previously under the MRP, carbon emissions are primarily attributed to wildfires, exacerbated by the land being abandoned and unmanaged. These recurring wildfires, often linked to the dry season during El Niño events [28,29], burn and affect not only the biomass above the peat but also the peatland itself. Notably, during El Niño periods, wildfires emitted 0.13 Gt CO2 in 1997, 0.05 Gt CO2 in 2003/04, and 0.09 Gt CO2 in 2006 [30], figures that are significantly lower than the estimated annual emissions from peatlands. Concurrently, the conversion of ex-MRP areas for agricultural use could contribute between 0.09 and 0.22 Gt CO2 over the next 25 years [31]. Furthermore, the issue of subsidence, which involves peat compaction through physical and biological processes, is tied to inadequate water management [32]. It is estimated that each centimetre of peatland subsidence can release between 13.3 and 39.7 tons of CO2 per hectare per year [21]. Both subsidence and carbon emissions are intimately connected to water management practices; thus, enhancing water management to control and elevate the water table could significantly mitigate these emissions [33,34].
To address the challenges associated with peatland management and to emphasize the importance of water management, a critical threshold for the water table has been proposed: maintaining the level at −40 cm below the surface during droughts to protect against wildfires and at a natural level of 100 cm above the surface during the flood season, as highlighted in a case study focusing on the ex-MRP area [35]. This −40 cm threshold has been adopted by Indonesia's agricultural policies for peatlands. However, it tends to overlook the differences between managed agriculture and the restoration of degraded, unmanaged peatlands. For restoration efforts to meet this threshold, canal blocking is necessary to raise the water table, thereby reducing subsidence and CO2 emissions [36]. In contrast, water management in agriculture aims to prevent flooding and mitigate drought to ensure optimal conditions for crop growth, while also preserving the integrity of peatlands at both surface and deeper layers. While restoration involves canal blocking, agricultural management requires the use of dams with water gates to maintain the desired water table level throughout all seasons.
The application of hydrological management in the agricultural use of peatlands is urgent and pivotal for sustainability [37]. Therefore, research into existing agricultural practices on peatlands that employ integrated water management is necessary to develop methods and assess their impact on carbon emissions and subsidence rates. This is vital due to the limited understanding of carbon dynamics within peatland ecosystems [26]. The challenge is significant because the issues in managed agriculture on peatland are not only wildfires but also emissions from microbial activity that decomposes the peatland's organic matter upon exposure to oxygen, with the rate of decomposition often surpassing that of accumulation [38]. Consequently, our research question arises: What is the impact on CO2 emissions and subsidence rates of peatland agriculture that implements a sustainable approach through integrated water management?
To address this question, in the coastal regions of Riau Province, Indonesia, a controlled drainage system has been implemented for centuries as a means of managing water in peatland agriculture. Local communities have been cultivating coconut plantations on peatlands, utilizing the tides in riverine and coastal areas for nutrient enrichment and to transport the harvests [39]. This practice, which expanded into a large-scale operation in 1986, was designed to modernize the agricultural framework by establishing centralized processing industries and extending coconut cultivation both for smallholders and enterprises [40]. This evolution of local coconut farming methods into sustainable peatland usage has been guided by a deep understanding of the ecosystem. This led to the development of the “Water Management Trinity” (WMT), a controlled drainage system innovated locally, consisting of water gates, canals, and dikes. Operated since 1986 [41], the system effectively regulates the water supply and maintains optimal water table levels, exhibiting resilience even during dry periods and antedating the escalation of peatland sustainability concerns in the late 1990s.
Therefore, this research evaluates the impact of integrated water management in the WMT on CO2 emissions and subsidence rates in a coconut plantation along Riau's coast. The hypothesis is that the application of integrated water management, like the WMT, which regulates water table depth in peatland agriculture to maintain ideal water content, could minimize CO2 emissions and subsidence rates. Moreover, while there is extensive research on CO2 emissions from drained peatlands, degraded peatlands, rewetted wetlands, and pristine forests, studies focusing on CO2 emissions within agricultural systems—particularly those utilizing water management strategies for coconut crops—are limited. This deficiency in data signifies an important gap in scientific understanding that necessitates further research, especially in relation to the ongoing discussion regarding the sustainability of drainage-based agriculture.
2. Materials and methods
2.1. The context of study area
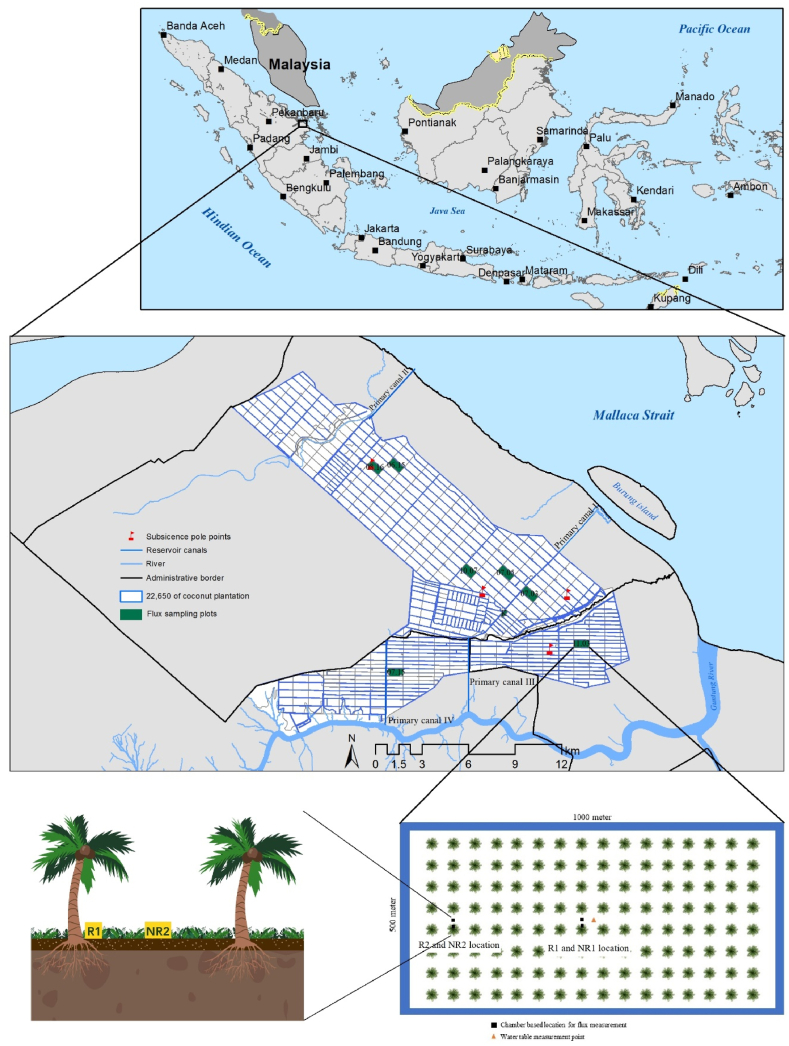
The research was conducted on a 22,650-ha coconut plantation in Pulau Burung District, Indragiri Hilir Regency of Riau Province (latitude 108o 30′ 02″ to 108o 45′ 02″ and north longitude 1o 12′ 32″ to 1o 18’ 30”), situated within the Kampar River-Gaung River PHU ecosystem that encompasses 710,823 ha (Fig. 1). This plantation, located in coastal tropical peatland, features varying peat depths, ranging from 2 m at the coastal or river boundaries to 11 m at the peat dome. Data from 2012 to 2022 indicate that the average annual rainfall in Indragiri Hilir is 1875 mm [42,43]. The climate follows a bimodal pattern with two rainfall peaks in April and November, typical for Sumatra, and experiences the longest dry spell during El Niño [44]. The elevation of the plantation ranges from 1.8 m to 6.2 m above sea level [41].
Fig. 1.
Map of the study area showing the locations of planting block samples, subsidence pole locations, and the placement of R and NR plot illustrations.
Indragiri Hilir serves as a leading coconut producer in Indonesia, contributing to 10.6% of the country's coconut production [45]. Historically, the development of coconut agriculture in the region dates back to the 1800s, aligning with the establishment of the East India Company in Singapore in 1819 [46]. Initially, coconuts were grown for personal use and traded on a limited scale. By the late 1800s, due to both civil war and conflicts with colonialism, the Banjarnese and Bugis people migrated to what is now Indragiri Hilir Regency. The Bugis, hailing from Sulawesi, a center for coconut cultivation, and the Banjarnese, with their expertise in wetland management from South Kalimantan, jointly contributed to the development of coconut agriculture on peatland. Given that 79% of the area is peatland [47], water management has been pivotal. The people of Indragiri Hilir have employed controlled canals with water gates, or ‘parit,’ to regulate water during the dry season by harnessing tidal conditions, prevent flooding during the rainy season and high tides, and facilitate the water transport of coconut harvests. Consequently, Indragiri Hilir, located nearest to Singapore on the east coast of Sumatra, evolved into a significant center for the coconut trade, a status it maintains. As of 2019, there were approximately 80,264 coconut farmers in Indragiri Hilir managing a collective plantation area of 261,232 ha [42]. Moreover, the 2022 population of Pulau Burung District was recorded at 10,645, with a community highly dependent on the coconut supply chain, encompassing those working in the coconut processing industry as well as farmers.
The 22,650-ha coconut plantation, managed by the Sambu Group since 1986, is an enterprise plantation that has been developed in conjunction with thousands of hectares of Perkebunan Inti Rakyat (Nucleus Estate Smallholder; NES) as per Indonesian Presidential Instruction No. 1 of 1986. This development, paired with a processing industry, aims to shorten the supply chain and ensure market stability to support sustainability. This plantation was selected for research due to its integrated water management system known as the “Water Management Trinity” (WMT), which was designed based on local wisdom, recognizing the importance of keeping the peatland consistently wet and moist from the outset. The ‘trinity’ refers to three key water management constructions — canals, dikes, and dams with water gates — that work together to maintain the peat's moisture level. The canals serve as water reservoirs rather than conventional drainage systems. The WMT is a closed system, designed to prevent influence by sea tides and flooding during high tides, and to regulate water input from rainfall, thus preventing flooding and drought in coconut plantations on peatland. It comprises primary, secondary, and tertiary canals that segment the area into 50-ha planting blocks (1000 × 500 m). The primary canal dimensions are 25 to 25 m wide and 5 m deep, the secondary canals are 8–15 m wide and 4 m deep, and the tertiary canals range from 4 to 6 m wide and 4 m deep. Secondary and tertiary canals mainly act as channels to the primary canal, providing the main access to the planting plots and facilitating the transport of plants, fertilizers, fuel, and building materials. Due to the challenges of constructing and maintaining roads on peat soil, water transport is the preferred method. Each primary canal is equipped with overflow gates to regulate water levels within the system; however, overflow gates alone are insufficient to maintain the water table due to variations in elevation. Consequently, the secondary canals use adjustable gates, functioning as barrages and sluice gates, before channeling water into the primary canal. In compliance with government regulations mandating a 40-cm water table, the WMT adjusts water levels throughout the rainy and dry seasons to meet this requirement.
2.2. Carbon flux measurement
CO2 emissions from peatlands in this study were assessed using a chamber-based method designed to measure soil CO2 flux. The research utilized the closed-chamber technique to accurately quantify diffuse CO2 flux through the soil. The closed chamber is a sealed container of known dimensions, measuring 30 cm × 30 cm x 30 cm (Fig. 2A and B). It was placed face-down on the soil to effectively trap the gas, allowing for subsequent measurement of the gas inside the chamber. To prevent air leakage during measurement, a 30 × 30 cm iron chamber base, shaped like a "U" and functioning as a water container, was filled with water to ensure airtight conditions. Additionally, the chamber base was inserted approximately 5 cm into the soil. This base was filled with water to create airtight conditions. In order to ensure accuracy and prevent overestimation of soil CO2 flux, the chamber, constructed from acrylic [48], was insulated with aluminum. This insulation was critical in minimizing temperature fluctuations within the chamber. The chamber was also equipped with both a thermometer and a fan to facilitate the thorough mixing of air inside. Air from the chamber was circulated to an infrared gas analyzer (IRGA), specifically a model ZFP9GC11 from Fuji Electric, Tokyo, Japan. Measurements were recorded at intervals of 0, 3, and 6 min to stabilize CO2 gas flow in IRGA, which was then examined using linear regression. Prior to the use of the IRGA for measurement, it was calibrated using soda lime and standardized CO2 gas to ensure precision. Additionally, before conducting the measurements on the field, the plots were cleared of weeds and the chamber base was installed permanently to maintain consistency in the measurement setup.
Fig. 2.
(A) Closed chamber design, featuring (a) a 30 × 30 cm chamber equipped with [1] a thermometer [2], a mini fan for air mixing inside the chamber [3], a hose system connecting to the IRGA sensor for on-site measurement [4], pressure compensation; and (b) a chamber base with [5] a water container to prevent gas leakage during measurement. (B) Shows the condition during measurement. (C) and (D) depict intercropping and monocropping, with the chamber base permanently positioned next to the basal root and between coconut trees during measurement.
The 22,650-ha coconut plantation is segmented into 50-ha planting blocks, locally referred to as “persil”. Each block is bordered by a canal and maintains a density of 173 coconut trees per hectare [41]. For this research, seven planting blocks were strategically selected based on several criteria: accessibility to the location, peat depth, and land cover (Fig. 1). Accessibility was a critical factor, considering the challenges posed by the unpaved, muddy roads that frequently become impassable during rainy periods, making boat transport a necessary yet costly alternative in certain conditions. For assessing peat depth, data collected by the Sambu Group in 2015 was utilized. This data was gathered using a soil auger at 440 points spaced 1 km apart and was later processed using the kriging method for interpolation [49]. The land cover within the plantation varied, including both coconut monocrops and intercrops. The grass understory is part of a cover crop managed for soil fertility purposes using a nutrient cycling method. It is cut (weeded) manually every 4 months and left to decompose naturally to provide nutrients to the coconut trees. The cover crop species in the plantation are dominated by Nephrolepis sp., followed by Dicliptera chinensis, Sideria sp., Stenochlaena palustris, Blumea balsamifera, Broadleaf signalgrass, Ficus nitida, Cryptolepis, Pteris sp., and Miconia mirabilis.
The variety of coconut planted across these blocks is PB 121 (MYD x WAT). In monocrop areas, the planting design follows an equilateral triangle configuration with a specified spacing of 8 m between trees. However, in practice, this distance often varies, typically ranging from 6.10 to 10.45 m [50]. In contrast, the intercropping layout for pineapple and coconut involves three rows of coconut trees, each spaced 8 m apart. This is followed by a 16-m gap designated for pineapple cultivation, and then another set of three rows of coconut trees. This intercropping design is considered an optimal agricultural method and is not limited to just younger coconut plants [51].
The measurement design was structured to distinguish between root-based and peat-based emissions, with the aim of avoiding overestimation that could result from including root respiration in the total CO2 emission calculations. This necessitates the separation of accurate CO2 emissions from peatland, distinguishing them from coconut root respiration and the associated rhizosphere activities during photosynthesis (RA) and the heterotrophic respiration (RH) of soil microorganisms and biota that decompose peat organic material. To fulfill this objective, each of the seven sampling locations was equipped with two measurement points located at the center of the planting block, roughly 250 m away from the nearest canal. Each point was outfitted with a permanently placed chamber base.
The first chamber base, positioned adjacent to the basal root of a coconut tree, was designated for measuring total soil respiration (RS), capturing both the autotrophic component, particularly the root respiration of the coconut tree, and the soil's heterotrophic component. This setup was labeled as [R-1 (root)]. The second chamber, situated between two coconut trees at a distance of approximately 3.5–4 m from them, was aimed at measuring the heterotrophic respiration of the coconut tree (RH-COCONUT), specifically excluding root respiration. This was marked as [NR-1 (non-root)]. Simultaneously, to assess the heterotrophic respiration from only peat soil, uncontaminated by fine root and weed activities, CO2 flux measurements were conducted on a dedicated bare peat land area, labeled as the control plot [K (RH-PEAT)]. Additionally, to calculate RA, the following equation was used:
| RA = RS − RH-PEAT | (1) |
Soil CO2 flux measurements were conducted over a six-month period from August 27, 2021, to February 28, 2022. These measurements were conducted weekly for each block, between 08:00 a.m. and 16:00 p.m. local time (GMT +7). The scheduling of these measurements was influenced by logistical challenges, such as the inaccessibility of very muddy roads and limitations in the use of pong-pong motorboats, as well as prevailing rainy conditions.
Initially, 14 measurement points were established across seven planting blocks for this study. After two months of observation, it was observed that coconut productivity was notably higher near the canals than at the center of the planting blocks. As a result, additional chamber bases were installed at extra measurement points near the canals in planting blocks 07.03, 07.15, and 10.07. These additional sites were labeled as [R-2] and [NR-2], increasing the total number of measurement points to 21. The purpose of these additional points was to assess the impact of increased productivity on soil CO2 flux.
Concurrently with the soil CO2 flux measurements, data on various environmental parameters were collected, including water table depth, soil temperature, and soil water content. The water table depth was manually measured during each soil CO2 flux measurement session using 4-m-long, perforated 2.5-inch PVC dip-wells, positioned adjacent to the chamber base. For a broader perspective, these readings were complemented by the company's routine bi-weekly water table observation from 106 points across the plantations, as required by the Ministry of Environment and Forestry Regulation No. 15 of 2017. It should be noted that the water levels from the specific measurement points and the company's regular measurements may vary due to weather fluctuations and elevation differences. Soil temperature was gauged using a soil thermometer at a depth of 10 cm. Soil water content was also recorded during each measurement session.
2.3. Subsidence measurement
Subsidence rates on the area has been systematically monitored since 1986, with the initial data collection commencing in 1987. This data was acquired using four iron poles, which were embedded into the peat soil down to the mineral layer (comprising over 60% clay) and distributed evenly (Fig. 1). Embedding these poles to such a depth was essential to ensure stabilization and to prevent any vertical movement. While the number of poles used for this purpose is relatively limited, a factor attributed to the initial installation not being designed with future research needs in mind, these poles have nevertheless provided valuable long-term data on subsidence. This long-term dataset offers insights into the subsidence trends over an extended period, despite the limited number of measurement points.
2.4. Soil sampling
Soil samples were collected from blocks 07.03, 07.05, 07.15, 10.07, and 11.03 during CO2 flux measurement periods. Alongside this, bulk density measurements were conducted at various soil depths, specifically 0–5 cm, 5–15 cm, 15–25 cm, 25–35 cm, 35–50 cm, and 50–70 cm. These measurements were carried out at approximately the same planting block locations, with minor adjustments, where Ochs et al. (1992) had previously conducted bulk density measurements in November 1986 [41]. By adopting this methodological approach, it was possible to analyze changes in bulk density over time and assess its contribution to subsidence. The soil samples were subsequently tested at IPB University in Bogor, Indonesia.
2.5. Analysis
The measurement obtained CO2 gas concentration (ppm v), followed by linear regression to determine the dC/dT value. The CO2 flux was calculated from dC/dT using the following equation:
| (2) |
where:
= flux (g C–CO2/m2/min)
= density of CO2 under the standard condition (1.96 x 103 g/m3)
= volume (m3) and the bottom area of the chamber (m2), respectively
= change in CO2 concentration in the chamber during the period (ppm/min)
T = temperature inside the chamber (oC)
= conversion factor for CO2 to C ()
To transform six months of weekly data into annual projections, the weekly measurement points were initially validated through linear regression analysis. This process involved omitting outliers that were attributed to factors like sensor variability during high temperatures or human errors. The six-month measurement period provided 384 data points (n = 384) for R-1 and R-2, NR-1 and NR-2, out of which 31 were excluded. For the control plot K, there were 25 data points (n = 25), resulting in 378 processed data points (N = 378) in total.
The calculation of annual CO2 emissions was performed using the linear regression method. This regression utilized {(xi, yj)} data pairs from each plot, where ‘xi’ represents the cumulative number of days from the first measurement to each subsequent measurement, and ‘yj’ denotes the cumulative daily flux at these measurements, with newer data being progressively accumulated over time. Therefore, the computation of annual flux (in tons per hectare per year) is not a straightforward multiplication but involves accumulation to derive a linear function y = mx + b, where all R2 coefficients are >0.99. Furthermore, the data underwent statistical analysis, including linear regression and Analysis of Variance (ANOVA), to examine the relationship between soil CO2 flux and water table depth. This analysis aimed to provide a more comprehensive understanding of the factors influencing CO2 emissions in the studied peatlands.
3. Result
3.1. Soil characteristics and water management results
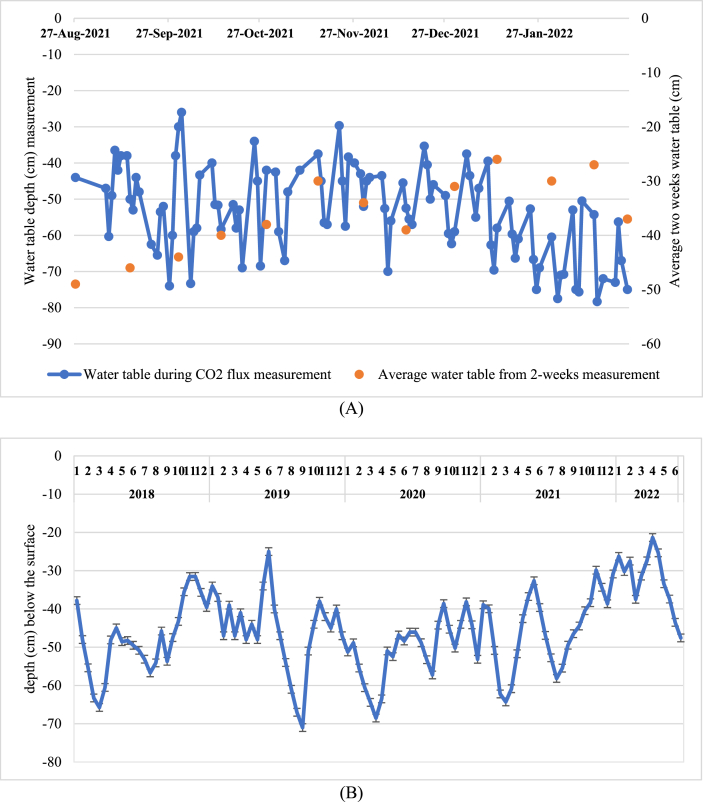
Chemical characteristics of peat soil are displayed in Table 1. The soil properties are characterized as sapric or well-decomposed, with a color of 7.5 YR 5/6 (strong brown). The soil pH ranges from 3.5 to 4, which is at the upper level of the range for peat's pH, typically between 2.3 and 4.5 [52]. The levels of Calcium (Ca), Magnesium (Mg), Potassium (K), and Natrium (Na) fall within the expected ranges: 0.01–6.0% for Ca, 0.01–1.5% for Mg, 0.001–0.8% for K, and 0.02–5.0% for Na [53]. The water table level during the measurement period is shown in Fig. 3A. During the measurement, the water table in R and NR plots ranged from −20 to −86 cm below the surface, with an average of −54 cm. This variability was observed during both the rainy and dry seasons, as designed in this research. With those water table, the average water content % dry basis in R plots (R-1 and R-2) and NR plots (NR-1 and NR-2) is 279 ± 68% and 293 ± 76%, respectively. Bi-weekly measurements by the Sambu Group from 2018 to 2022 indicated a range of −20 to −79 cm (Fig. 3B), which average ranged from −45 to −51 cm below the surface, these figures reflect the variability influenced by rainfall levels, water regulation, and evapotranspiration rates ranging from 2.1 to 2.4 mm/day. The variance between the minimum and maximum water table levels can be attributed to factors like rainfall and terrain slope. As water naturally flows to lower elevations due to gravity, the plantation utilizes secondary canals to equalize water levels across all areas. Although the water table depth may fluctuate depending on seasonal variations and rainfall levels, the WMT effectively manages the water level to maintain standards suitable for both agronomic and peat preservation purposes. These regulated water table depths offer practical solutions for agronomic activities while ensuring high soil moisture retention.
Table 1.
Chemical characteristics of peat soil.
| Location | Label | pH 1:5 |
Ash |
Dry Ashing |
Dry Ashing |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| H2O | Ca |
Mg |
K |
Na |
Fe |
Cu |
Zn |
Mn |
|||
| … (%) … | … … …. (%) … … …. | ….. (ppm) ….. | |||||||||
| K1 | 3.84 | 13.61 | 0.72 | 0.04 | 0.01 | 0.02 | 1746.33 | 15.58 | 47.74 | 53.86 | |
| 07.03 | R | 3.93 | 1.64 | 0.15 | 0.15 | 0.02 | 0.03 | 365.84 | 26.94 | 50.04 | 44.60 |
| NR | 3.52 | 2.55 | 0.08 | 0.08 | 0.02 | 0.02 | 350.94 | 16.25 | 22.31 | 41.79 | |
| 07.05 | R | 4.13 | 3.43 | 0.13 | 0.18 | 0.02 | 0.03 | 449.07 | 17.66 | 21.91 | 45.22 |
| NR | 3.85 | 2.16 | 0.45 | 0.07 | 0.01 | 0.02 | 435.97 | 15.00 | 22.27 | 19.60 | |
| 07.15 | R | 3.97 | 2.67 | 0.15 | 0.17 | 0.03 | 0.02 | 457.20 | 79.29 | 35.38 | 87.62 |
| NR | 4.05 | 3.42 | 0.10 | 0.11 | 0.03 | 0.03 | 342.55 | 16.53 | 26.24 | 51.06 | |
| 10.07 | R | 3.78 | 2.91 | 0.36 | 0.23 | 0.02 | 0.03 | 506.53 | 69.55 | 45.38 | 90.73 |
| NR | 3.72 | 1.89 | 0.35 | 0.07 | 0.01 | 0.03 | 265.15 | 15.20 | 22.75 | 42.19 | |
| 11.03 | R | 3.98 | 4.33 | 0.41 | 0.09 | 0.02 | 0.03 | 679.81 | 19.09 | 56.81 | 42.49 |
| NR | 4.16 | 4.27 | 0.44 | 0.07 | 0.01 | 0.02 | 450.84 | 24.20 | 38.73 | 62.08 | |
Fig. 3.
(A) Variation in water table depth during measurements, and (B) the average water table depth from 2018 to mid-2022 from two-weekly measurements.
3.2. Soil CO2 fluxes
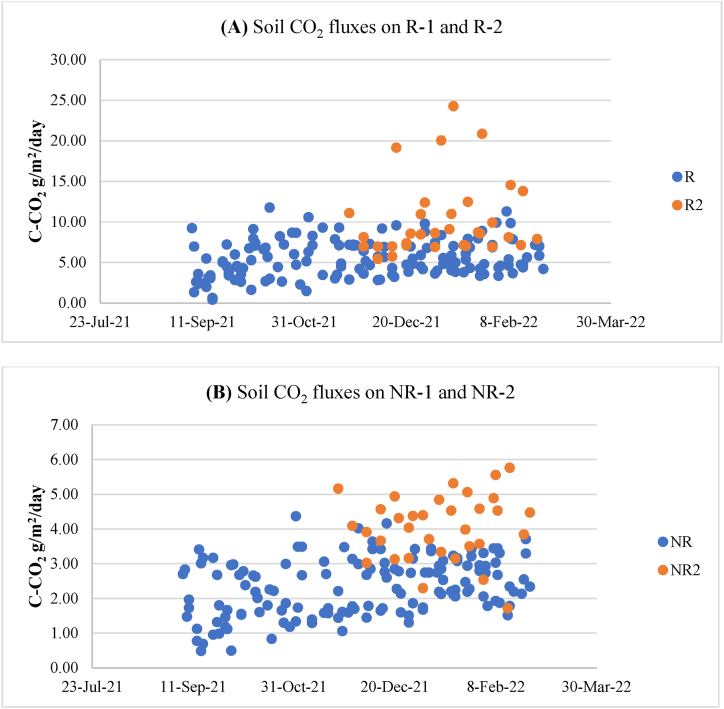
The distribution of soil CO2 fluxes is presented in Fig. 4, with the annual cumulative CO2 flux for each plot detailed in Table 2. The results show that R-1 and R-2, which represent RS or soil flux affected by coconut tree respiration, ranged from 0.41 to 5.14 g C–CO2 sq-m−1 day−1 for R-1 and 1.71–5.76 g C–CO2 sq-m−1 day−1 for R-2. The annual cumulative CO2 emissions ranged from 12.42 to 28.78 t C–CO2 ha−1 yr−1 with an average of 20.07 t C–CO2 ha−1 yr−1 for R-1, and from 27.22 to 44.18 t C–CO2 ha−1 yr−1 with an average of 33.74 t C–CO2 ha−1 yr−1 for R-2. These findings suggest that the productivity of coconut trees influences the CO2 flux in the peat soil from the photosynthesis process. The increased CO2 flux in R-2 plots is likely due to enhanced nutrient content at the edge of the planting block, resulting from surface runoff during rainy periods. Additionally, Table 2 shows that monocrops show slightly higher CO2 emissions than intercrops.
Fig. 4.
Distribution of daily soil CO2 flux per square meter for R plots (A) and NR plots (B).
Table 2.
CO2 flux in each sampling plots of coconut plantation.
| Planting block | Land cover | Peat depth (meter) | Peat type | Annual soil CO2 flux (t C–CO2 ha−1 yr−1) |
|||
|---|---|---|---|---|---|---|---|
| R-1 | NR-1 | R-2 | NR-2 | ||||
| 08.16 | intercrop | 2–3 | sapric | 17.62 | 8.83 | – | – |
| 11.03 | intercrop | 5–6 | sapric | 17.62 | 8.83 | – | – |
| 07.03 | monocrop | 3–4 | sapric | 12.42 | 5.55 | 29.82 | 16.06 |
| 07.15 | monocrop | 3–4 | sapric | 23.33 | 8.78 | 27.22 | 11.32 |
| 07.05 | monocrop | 5–6 | sapric | 28.78 | 8.19 | – | – |
| 06.15 | monocrop | 5–6 | sapric | 18.43 | 7.84 | – | – |
| 10.07 | monocrop | 5–6 | sapric | 23.12 | 7.97 | 44.18 | 13.63 |
| Control plot | Bare peat | 4–5 | sapric | – | 7.77 | – | – |
| Average (without control plot) | 20.07 | 7.99 | 33.74 | 13.67 | |||
For NR-1 and NR-2, representing RPEAT or soil flux not affected by coconut tree respiration, the range was 0.48–4.37 g C–CO2 sq-m−1 day−1 for NR-1 and 1.71–5.76 g C–CO2 sq-m−1 day−1 for NR-2. The annual cumulative CO2 emissions ranged from 5.55 to 8.83 t C–CO2 ha−1 yr−1 with an average of 7.99 t C–CO2 ha−1 yr−1 for NR-1, and from 11.32 to 16.06 t C–CO2 ha−1 yr−1 with an average of 13.67 t C–CO2 ha−1 yr−1 for NR-2. The control plot (K), designated as RH-PEAT, recorded 7.77 t C–CO2 ha−1 yr−1. The CO2 flux in NR-1 was 2.8% higher compared to the control plot. This finding suggests that RH-PEAT, representing the CO2 emission from peatland soil, is similar to RH-COCONUT. The minimal influence of microbial activity, soil biota, and possibly fine coconut roots seeking nutrients, litter, and weeds in plots distant from coconut trees indicates that root respiration significantly affects peat soil CO2 fluxes. These results imply that, for accurate measurements to define RH-PEAT and RH influenced by vegetation, identifying bare peatland areas may be more effective than using the trenching root technique. Some studies have demonstrated that defining RH-PEAT using trenching can lead to higher CO2 flux measurements compared to RH obtained between two trees [54]. Moreover, with the obtained RS and RH, we can calculate Autotrophic respiration (RA). RA in coconut plantations contributes significantly to the RH-PEAT, adding 212% in R-1 and up to 424% in R-2. Several factors can affect this contribution from vegetation respiration. Notably, in these coconut plantations, no synthetic fertilizers are applied, which could potentially minimize RH, both RH-COCONUT and RH-PEAT. For example, in palm oil plantations of 9 years with high doses of synthetic fertilizer application (74–174 kg of N per hectare annually, 7–9 kg rock phosphate, and 239–311 kg of KCl), root respiration added only 75% to RH-PEAT [54]. Jamili et al. (2021) found root respiration contributing an additional 117% to RH-PEAT [55].
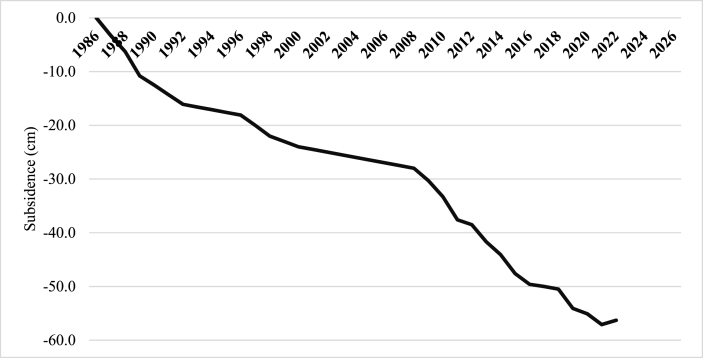
3.3. Subsidence
The analysis of subsidence data spanning thirty-five years (Fig. 5) reveals a cumulative subsidence of −56.3 cm from 1986 to 2022, corresponding to an annual rate of −1.56 cm yr−1. Elevated subsidence rates observed in the first three years of development were due to rapid consolidation and compaction [41]. In 2009, 2010, 2011, and 2019, higher rates were attributed to the dry seasons of El Niño. Interestingly, in 2022, the plantation experienced a bounce-back effect, halting subsidence and even showing a positive change of +0.8 cm, indicating no subsidence. The graphic also suggests a flattening rate of subsidence, implying that consolidation and compaction have reached their limit and that regulated water table management could minimize subsidence to the lowest possible level. This indicates that the peat soil has stabilized, supporting the potential for long-term agricultural utilization.
Fig. 5.
Cumulative subsidence recorded from 1986 to 2022.
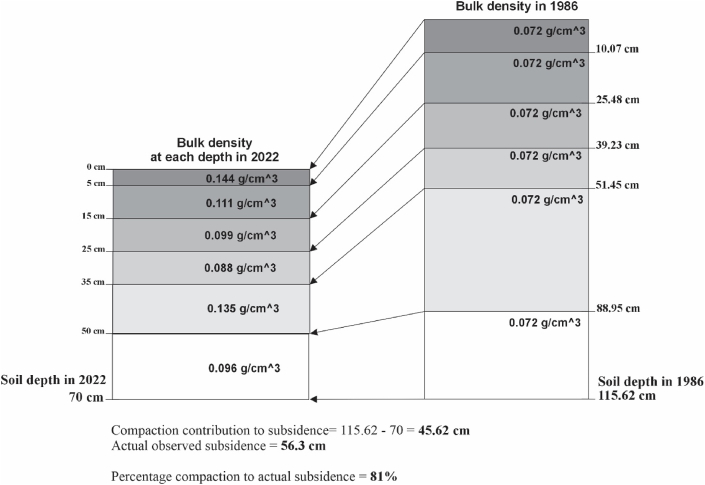
In 1986, the initial condition before the development of the coconut plantation exhibited a bulk density of 0.072 g/cm3 at a depth of 0–20 cm [41]. This was characterized by fibric material with organic forest components such as wood and branches. The surface bulk density is also representative of the bulk density at every depth, as the depth bulk density cannot be higher than the surface peat bulk density due to the saturated water table. Recent measurements taken from approximately the same location as the initial measurements with minor adjustment revealed a bulk density of 0.144 g/cm3. However, variations in bulk density at a depth of 0–20 cm were observed across different plantation blocks, ranging from 0.10 to 0.20 g/cm3, with an average density of 0.144 ± 0.029 g/cm3. This implies that over three decades of coconut plantation cultivation, the bulk density or mass of peatland in the same volume has approximately doubled. This indicates that the same volume of soil now contains more mass, suggesting that the surface of the peat layer has become compacted, or that the peat has undergone shrinkage, as represented by subsidence.
3.4. Relationship with water table depth
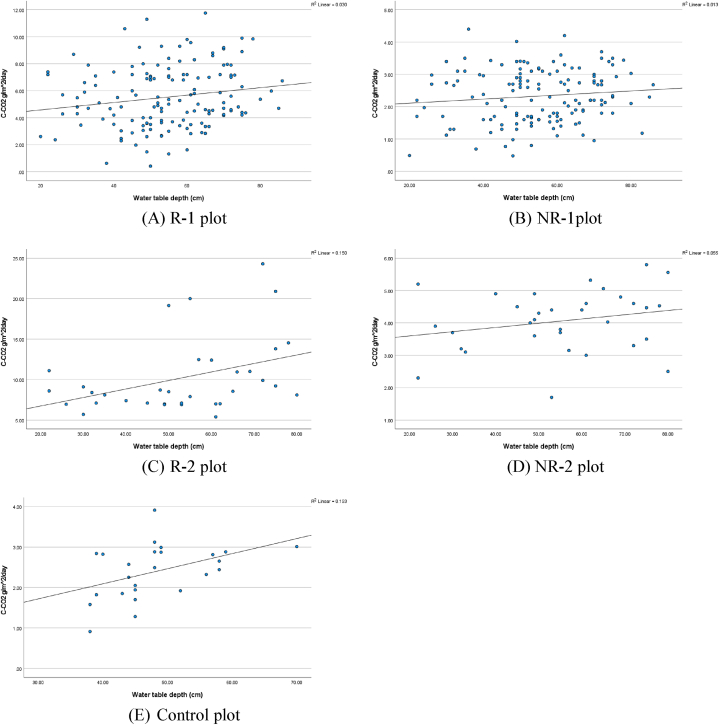
Understanding the relationship or correlation between CO2 emissions and environmental parameters is essential for comprehending the underlying mechanisms. Specifically, in the context of CO2 emissions and subsidence on peatlands, the depth of the water table is a pivotal factor in controlling their magnitudes. Generally, deeper water tables from the surface are associated with higher emissions and subsidence, though these rates vary across different land use types, as indicated by Carlson et al. (2015) [56]. Contrary to these general observations, the study found no significant relationship between the water table depth and soil CO2 flux, as evidenced in both R-1 (n = 144) and NR-1 (n = 142) plots, with R2 coefficients of 0.030 (p-value = 0.037) and 0.013 (p-value = 0.181), respectively (Fig. 7). This pattern persisted in R-2 (n = 34) and NR-2 (n = 33) plots, which exhibited R2 coefficients of 0.150 (p = 0.024) and 0.055 (p-value = 0.187), respectively. Even in the control plot K (n = 25), the regression analysis yielded an R2 coefficient of 0.193 with a p-value of 0.028. These low R2 values indicate that the depth of the water table does not significantly explain the variations in CO2 emissions, regardless of the statistical significance of the variable. The graphical analysis further supports this conclusion, showing that the distribution of data is random and lacks any discernible pattern that would suggest the water table depth as a reliable predictor of CO2 emissions. Therefore, due to its very low R2 value, the depth of the water table should not be considered a reliable predictor for CO2 emissions.
Fig. 7.
Statistical analysis of linear regression for each plot.
4. Discussions
4.1. Redefining peatland CO2 emission
Limited publications concerning CO2 emission in coconut plantations on peatlands were available at the time of this study, necessitating comparisons to CO2 emissions from palm oil plantations. Khasanah and van Noordwijk (2019) reported mixed coconut emissions using subsidence data, making direct comparisons challenging [57]. CO2 emissions in 7-year-old palm oil plantations have been reported at 28.4 t CO2 ha−1 yr−1 [58]. In 15-year-old highly fertilized palm oil plantations with applied dolomite and NPK fertilizer, CO2 flux reached approximately 46 ± 30 t CO2 ha−1 yr−1 [59]. These higher emissions in palm oil should be contextualized by considering the crop's higher productivity and the role of fertilizers in accelerating photosynthesis and microbial activity in the soil. For instance, 6-year-old palm oil plantations exhibited higher emissions compared to 15-year-olds, with 44.7 and 47.8 t CO2 ha−1 yr−1, respectively [60]. In the same 6-year-old palm oil plantations, CO2 emissions could reach 66 ± 25 t CO2 ha−1 yr−1, characterized by varying soil characteristics due to different drainage durations [61]. In Acacia plantations, designed for rapid biomass production, CO2 emissions ranged from 80 to 94 t CO2 ha−1 yr−1, influenced by diurnal temperature fluctuations [62]. Similarly, in settled agriculture, emissions are comparable to those from Acacia plantations, with CO2 emissions reported at 77 t CO2 ha−1 yr−1 [63]. These comparisons highlight the complexity of CO2 emission assessments on peatlands, emphasizing the need to consider various factors, including the contribution of root respiration and the type of vegetation present.
This result raises a pertinent question: should the term ‘peatland emission’ refer solely to heterotrophic respiration from peat soil (RH-PEAT or RH-COCONUT), or should it encompass the total soil respiration (RS), which also includes autotrophic respiration (RA) varying with productivity?
It was found that plants capture and release CO2 through their respiration, and this process must be incorporated into CO2 emission analyses, as it could potentially result in higher emissions than existing estimates [64]. Additionally, higher gross primary production (GPP) results in increased CO2 emissions on peatland, particularly in younger, more productive crops [65]. Additionally, understanding the biophysical characteristics of peatland, particularly the level of peat decomposition, can elucidate patterns related to CO2 emissions. Decomposition in peat, or oxidation, involves the breakdown of peat material by soil microorganisms and biota, producing CO2, H2O, nutrients, and transforming the peat soil into a more sapric state [66]. This process results in less organic peat material to decompose, thereby reducing CO2 emissions from soil microorganisms and biota. Interestingly, coconut plantations exhibit similar emission numbers to those from mineral soils. CO2 emissions on Andisol soil in Bogor, Indonesia, cultivated for tea and bare soil, were reported at 15.6 and 7.32 t C–CO2 ha−1 yr−1, respectively [67]. In Acrisol soil with young (1-year-old) and mature (12-year-old) palm oil plantations, CO2 emissions were 8.03 and 11.73 t CO2 ha−1 yr−1, respectively [68].
Therefore, the term ‘CO2 emission from peatland’ should ideally represent RH from peat soil alone, without the influence of RA. Alternatively, it could be calculated based on the vegetated area and the peat soil only. Using this methodology, the actual CO2 emissions from coconut plantations can be calculated. Assuming an optimal radius of primary roots is 2 m from the stem, with a coconut tree diameter of 20 cm [69], and a tree density of 173 trees per hectare [41], the area influenced by root emissions (RA + RH-COCONUT) is calculated as 0.24 ha, while the area affected only by RH-COCONUT covers 0.76 ha. Consequently, the true CO2 emission in the coconut plantation is calculated to be 11.28 t C–CO2 ha−1 yr−1.
4.2. Subsidence and CO2 emission
The subsidence result in coconut plantation is considerably lower than the cumulative subsidence observed over 18 years in palm oil plantations and 5 years in Acacia plantations, which were reported at 212 cm and 142 cm, respectively [34]. Compared to the average subsidence rate of 4.2–4.3 cm yr−1 recorded over nine years (2007–2016) in Acacia plantations [70], the annual subsidence rate in coconut plantations is notably lower. Specifically, in the first year of development, subsidence was recorded at only 3.3 cm. Ochs et al. (1992) attribute this low rate to the compaction procedure implemented to stabilize the medium for coconut growth, as uncompacted peat yielded a much higher subsidence of 13.5 cm in the first year [41]. This contrasts markedly with findings from other studies, which suggest that the first year of peat development can lead to subsidence levels of 50–75 cm [34,71].
These findings reinforce the hypothesis that the WMT contributes to a lower subsidence rate in coconut plantations. The reservoirs in the plantation canals ensure the maintenance of water supply within the peat soil, both in the deep layer and the interlayer, thereby enabling the peat to retain its original form. To support interpretation, an analysis comparing current bulk density to initial bulk density is essential. Subsidence in peat soil is characterized by changes in bulk density, although this concept is still not fully proven due to limited long-term observation of peat soil changes. Bulk density refers to the mass per unit volume of soil [72]. Physical processes such as compaction and shrinkage tend to increase bulk density, whereas peat decomposition leads to the humification of peat organic matter into a well-decomposed state. Changes in bulk density are a natural process, and the conversion of forests to plantations has been observed to alter the initial bulk density from 0.112 to 0.168 g/cm3 [73,74]. The assessment of measured bulk density at various depths is pivotal in determining the role of compaction in subsidence. The data, as shown in Fig. 6, indicate an increase in mass within the same volume (cm3) compared to the initial bulk density recorded in 1986. This increase in bulk density has been identified as a contributing factor to 45.62 cm of subsidence. Within this measure, 10.48 cm of subsidence is attributed to processes such as decomposition and/or other factors not analyzed in this study.
Fig. 6.
Changes in bulk density at various depths to identify contributors to subsidence.
Utilizing the methodology of Hooijer et al. (2012), which involves calculating subsidence contributions by comparing initial and final bulk densities, it is observed that 92% of subsidence is due to peat decomposition [34]. Hooijer et al. (2012) noted significant subsidence rates of 5 cm yr−1 in palm oil and Acacia plantations, associated with low surface peat soil bulk densities ranging from 0.087 to 0.089 g/cm3. This observation aligns with the findings of Evans et al. (2019), who reported that a low bulk density of 0.087–0.089 g/cm3 resulted in higher annual subsidence rates between 4.2 and 4.3 cm year−1 [70]. Supporting these results, Othman et al. (2011) reported that increased bulk densities are linked to reduced subsidence rates [75]. Their research revealed that an initial bulk density of 0.09 g/cm3 led to subsidence rates of 3.54 and 2.77 cm yr−1 over a 16-year period, with final bulk densities of 0.15 and 0.21 g/cm3, respectively. These studies collectively support the conclusion drawn from the current data, which indicates that the primary cause of subsidence in peatlands is attributed to the process of compaction.
The result suggests that subsidence, resulting from compaction which consequently increases bulk density, leads to negligible emissions from subsidence. Contrary to expectations, the annual rate of subsidence did not align with the predictive equation proposed by Wösten and Ritzema (2002), which primarily considers water table depth as the main determinant of subsidence rate [71]. Additionally, the correlation between subsidence and CO2 emissions remains inconclusive, despite the recommendations by Hooijer et al. (2012), to use a subsidence-based approach for estimating carbon loss in tropical peatlands [34].
4.3. Redefining the relationship between water table and CO2 emissions
The statistic results can be logically attributed to the fact that the WMT maintained moist conditions in the peat soil throughout the six-month measurement period. This consistency in soil water content levels renders the application of water table depth as a determinant factor for CO2 emissions less relevant in scenarios where water levels are controlled. The canals, serving as reservoirs, help maintain high water levels, which consequently reduce CO2 emissions from the peat soil. This finding supports Gusmayanti et al. (2019), who found no significant relationship between CO2 flux and water table depth (R = −0.01, p-value = 0.587), due to maintained water table depths of 64 ± 16 cm (Site 1) and 54 ± 20 cm (Site 2) [76]. However, this contrasts with earlier studies by Couwenberg et al. (2010) & Hooijer et al. (2010), which suggested that a 10-cm decrease in water table depth could lead to an increase in emissions of 9.0–9.1 t CO2 ha−1 yr−1 [77,78]. Hooijer et al. (2010) based their findings on a small sample size (n = 8) from five previous publications and reported a relatively high R2 value of 0.71 [78]. Carlson et al. (2015), analyzing data from 8 studies using closed chamber method, obtained an R2 value of 0.56 [56]. Their interpretation suggested that at a water table depth of 70 cm, the total respiration (RS) is 20 t CO2 ha−1 yr−1. They also noted that even when peatland is inundated (water table depth of zero), it does not equate to zero emissions. Furthermore, they found that water table depth accounts for only 45% of the variation in net carbon loss, with the remaining variation explained by factors unrelated to water table depth, such as vegetation age and fertilizer application. More recent studies, such as Prananto et al. (2020), which considered a larger pool of data, proposed that a 10-cm decrease in water table level in plantations resulted in an emission increase of 5.1 t CO2 ha−1 yr−1 [79]. However, these studies reported an R2 value of 0.07 (p = 0.0206) with a sample size of n = 63. Despite these findings indicating that the water table depth is not a strong predictor, Prananto et al. (2020) still concluded that the water table depth still influences the CO2 emission rate on peatlands.
The prevailing conclusion that lowering the water table significantly increases CO2 emissions is supported mainly through literature review, despite an insignificant R2 value that close to zero. Lowering the water table in agricultural contexts initiates the decomposition of peat organic soil by soil microorganisms and biota, converting organic material into CO2, H2O, and nutrients. In sapric peat soil, where organic matter is already well-decomposed, there is limited organic material left for decomposition, leading to reduced activities of soil microorganisms due to a lack of food. This research suggests that while the water table should not be considered a primary predictor of emissions, heterotrophic respiration (RH-COCONUT) only contributes to 40% of total respiration, which is significantly less than the 82–92% estimated by previous studies [34,56]. This indicates that the decomposition rate on peatland declines as the humification level increases. Further research is needed to investigate these matters.
Limited to examining the relationship between the water table and CO2 emissions, this study highlights the need to analyze diverse factors influencing CO2 emissions on peatlands. Soil CO2 flux is a dynamic process, heavily influenced by environmental conditions. For instance, an increase in soil nutrient content, such as from fertilizer application, can stimulate biological activities, leading to enhanced soil respiration. Melling et al. (2005) found that emissions from pristine forests were higher than those from younger palm oil and sago plantations [80]. Marwanto and Agus (2014) noted the absence of a relationship between CO2 emission and both soil and air temperature [59]. Carlson et al. (2015) indicated that peat depth and time since draining are not significant predictors, while vegetation age is a strong predictor due to the rate of root respiration [56]. Swails et al. (2022) observed that soil respiration on peatland is linked to soil temperature [81]. This research also determined that water content, with an average water content during the six-month research period of 273% ± 79%, is not a significant predictor of CO2 emission estimation (R2 = 0.000, p value = 0.773). Therefore, with no single definitive predictor for CO2 emissions from peat soil, comprehensive measurement including complete environmental parameters and fire risk is necessary to elucidate the patterns of peatland emissions, which are not solely related to water table depth.
4.4. Implication to peatland agriculture and its management
The challenges of agricultural utilization of peatlands are not confined to tropical regions but are a global concern. In Europe, where agriculture has been integral to civilization for centuries, optimally managed peatlands are among the most fertile lands [82,83]. However, these same agricultural activities have led to the degradation of 50% of peatland ecosystems in Europe [84]. In tropical regions, the use of peatlands for agriculture is often viewed with pessimism, largely due to their potential to damage peat ecosystems and their propensity to generate higher carbon emissions than non-peat soils [24]. This negative outlook is particularly pronounced in the case of industrial-scale agriculture on peatlands.
Peatland agriculture, when conducted responsibly, can be a sustainable and eco-friendly option, as demonstrated by the success of coconut plantations. This achievement is rooted in centuries of accumulated knowledge and cultural practices, coupled with the implementation of integrated water management in wetlands. For generations, people have utilized peatlands influenced by tides for coconut cultivation. They constructed ‘parit’ or small canals to define planting blocks and facilitate coconut transport during high tides [39]. These canals are connected to rivers and are equipped with water gates to prevent flooding during spring tides, leading the region to be known as “Negeri Seribu Parit” or the land of a thousand canals [85]. The centuries-old tradition of coconut cultivation on peatland continues to this day. This knowledge and technology have become integral to peatland utilization for agriculture. In essence, this study posits that peatland agriculture is feasible if it adheres to the key principles of responsible peatland management and the application of integrated water management on a larger scale. The importance of integrated water management goes beyond controlling the water table level; it plays a pivotal role in creating reservoirs, mitigating flood risks during the rainy season, reducing fire hazards in dry conditions, supporting transportation systems, and, most importantly, serving as a foundational element for the agronomic system of agriculture itself.
It is essential to recognize that the primary goal of water management in peatland agriculture is minimizing problems associated with peatland use while maximizing productivity. Uncontrolled drainage, which functions as a canal, can lead to peatland degradation and render it unproductive, as observed in the MRP case. However, applying water management strategies similar to those used in natural forest regimes or for restoration purposes is not advisable for agricultural lands. Additionally, water table depths above the surface, which indicate flooding, can lead to significant disruptions. Therefore, effective water management is essential to mitigate these risks and regulate the water table for optimal productivity. Conditions in agricultural peatlands differ significantly from those in forested intact peatlands, which exhibit high variability in water table depth and often experience flooding during heavy rainy seasons. Brady (1997) observed that water table depths in forested peatlands in South Sumatra and Riau vary significantly, ranging from −85 to −180 cm below the surface during the dry season, to 35 cm above the surface, indicating flooding [86]. This variability in water table depth, influenced by the season, affects the interlayer of peat and is catalyzed by soil microorganisms and biota decomposing peat organic material. Even with litter sourced from forest trees, the bulk density at a depth of 0–20 cm varies from 0.10 to 0.19 g/cm3, higher than the initial conditions in coconut plantations with a bulk density of 0.072 g/cm3. In forested peatlands in Sarawak, Malaysia, water table levels range from −52.1 cm below the surface to 11.2 cm above the surface [87], with an intermediate to high level of decomposition and bulk densities ranging from 0.10 to 0.14 g/cm3. Similarly, Takahashi et al. (2002) measured long-term water table levels in a forest from 1994 to 2002 and found that the lowest water table reached −80 cm during El Niño, while the highest water table was approximately 25 cm above the surface, indicating flooding [88].
The conditions of intact forested peatlands, characterized by high variability in water table depth and flooding during the rainy season, are not suitable models for agricultural land. In these forested areas, an increase in water table depth, even to the point of flooding up to 50 cm above the surface, can lead to increased CH4 emissions [89]. Deshmukh et al. (2021) also observed that in degraded forested peatlands adjacent to industrial plantations, there was no significant correlation between CO2 emissions and water table depth, a pattern that also applied to CH4 emissions [89]. The increase in CH4 emissions with rising water table depth, up to 20 cm above the surface, is a phenomenon specific to forested peatlands [90]. Hirano et al. (2012) identified a relationship between decreasing water table depth and ecosystem respiration (RA), indicating that CO2 emissions were not primarily from peat soil decomposition processes [91]. This significant effect of decreasing water table depth on CO2 emissions was observed only in forested areas. A similar pattern was noted in Brunei Darussalam, where soil CO2 flux trends followed water table changes in forest environments, ranging from −40 cm below the surface to 20 cm above it [92]. However, this study did not distinguish between RA contribution and heterotrophic respiration (RH), nor did it address CH4 emissions during flooded conditions. It is also noteworthy that the critical threshold of −40 cm below the surface, as proposed by Wösten et al. (2008), is appropriate only for unmanaged, degraded peatlands requiring restoration or rewetting [35]. In such environments, flooding can occur up to 100 cm above the surface, making this threshold less applicable to agricultural peatlands.
The findings of this study indicate that the application of integrated water management in agricultural utilization of peatlands can effectively minimize CO2 emissions and subsidence. Integrated water management fosters undisturbed anoxic conditions in the deep layer (a zone that is always inundated) and fluctuates between oxic and anoxic conditions in the interlayer (the zone between the lowest and highest water table depths), which reduces CO2 emissions from peat soil [93]. The application of this management approach in coconut agriculture on peatlands alters the physical characteristics of the peatland, leading to a reduction in CO2 emissions and subsidence. Changes in bulk density, resulting in denser peat per volume and thus subsidence, contribute to peat humification but do not act as a predictor for CO2 emissions, as the peat remains present. CO2 emission is a part of the respiration of soil microorganisms and biota, known as heterotrophic respiration in peat soil. In conditions where root respiration and litter supply are absent, as in well-decomposed peat, the CO2 emissions from RH are significantly lower compared to those from mineral soil emissions.
To address the ongoing debate between environmental and socio-economic sustainability in the use of peatlands for agriculture, the focus should not solely be on the water table but on the water content condition of the peat soil during climate variability, including rainy and dry seasons and evapotranspiration. The peat soil should maintain field capacity, avoiding conditions of being flooded or drying below field capacity. The water content, as a result of water table regulation, is a vital factor in this scenario. Integrated water management such as the WMT ensures that the peat soil remains consistently moist, with moisture levels recorded at 279 and 293%, indicative of a low bulk density due to its sapric characteristics [94]. With this level of moisture and degree of humification, the risk of wildfires can be minimized, while simultaneously supporting coconut productivity.
The application of integrated water management in peatland agriculture not only enhances environmental sustainability but also increases social and economic value, thereby improving the welfare of people in the region as part of sustainable development. This research deviates from mainstream perspectives, largely due to the analysis of changes in peatland characteristics, which have been minimally examined in previous studies. The findings are corroborated by the persistence of traditional peatland agriculture, which continues to thrive beyond the estimated lifespan of peatland disappearance as projected by Wösten and Ritzema (2001) [95]. For instance, in this study, with a peat depth of ∼300 cm, the estimated lifespan due to subsidence should be 192 years. However, with well-decomposed peatland and the application of water management, future subsidence could be near zero due to stabilized peat soil, potentially extending the lifespan of peatland indefinitely.
In summary, peatland agriculture holds a promising future if managed sustainably, countering current skepticism. The findings suggest that with sustainable peatland management, the relationship between water table depth and soil CO2 flux, as well as subsidence, can be reconsidered. This concept is not limited to coconut plantations; research indicates that industrial pulp (Acacia crassicarpa) plantations can also achieve a carbon-negative status with proper water management [96]. A similar, newer approach to integrated water management, termed eco-management, focuses on ‘water stock’ management planning to preserve peat and its biodiversity [23]. A fundamental aspect of this approach involves transforming existing drainage systems or conventional water management, which typically remove water from peat, into reservoirs or stock-based water management systems. In practical terms, this entails controlling water levels using water gates, akin to an irrigation system, as a fundamental part of responsible peatland management. This approach presents a viable opportunity for responsible private equity to implement sustainable practices, emphasizing integrated water management, which supports the sustainability of soil and agriculture in peatland usage.
5. Conclusion
The Water Management Trinity (WMT) exemplifies the practical application of integrated water management on peatland for agricultural purposes, specifically in the context of coconut agriculture on the tropical peatlands along the east coast of Sumatra. This system effectively transforms drainage canals in peatlands into controlled reservoirs, yielding multiple benefits for agronomic purposes, transportation, flood control, and fire prevention, particularly during dry seasons. In this study, the WMT, as an integrated water management system, has successfully maintained acceptable water table levels through the use of permanent watergates.
The research concludes that the implementation of the WMT as a water management system can significantly mitigate high CO2 emissions and subsidence rates in peatland agriculture. The findings indicate that the water table should not be regarded as a primary predictor for the estimation of CO2 emissions and subsidence in peatland agriculture; rather, it may be considered a supplementary dataset parameter. The WMT presents a viable opportunity for sustainable peatland agricultural practices, effectively preserving peatland integrity, supporting sustainable development, and contributing to climate change mitigation.
Funding
This research received partial funding from the Allan Robertson Grants, awarded by the International Peatland Society in 2021.
CRediT authorship contribution statement
Nurul Ihsan Fawzi: Writing – review & editing, Writing – original draft, Visualization, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Basuki Sumawinata: Validation, Supervision, Software, Methodology, Formal analysis, Data curation, Conceptualization, Funding acquisition, Investigation, Writing – review & editing. Suwardi: Writing – review & editing, Writing – original draft, Validation, Supervision, Software, Methodology, Formal analysis, Data curation, Conceptualization, Writing – review & editing, Writing – original draft, Validation, Supervision, Software, Methodology, Formal analysis, Data curation, Conceptualization. Annisa Noyara Rahmasary: Writing – original draft, Supervision, Resources, Project administration, Methodology, Funding acquisition, Conceptualization, Software. Ika Zahara Qurani: Writing – original draft, Supervision, Resources, Project administration, Methodology, Funding acquisition. Raihan Garin Naufaldary: Resources, Project administration, Methodology, Investigation, Formal analysis, Data curation. Ratu Nabillah: Writing – review & editing, Visualization, Validation, Software. Heru Bagus Palunggono: Writing – review & editing, Validation, Supervision, Methodology. Budi Mulyanto: Writing – review & editing, Validation, Supervision, Methodology.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
We express our sincere gratitude to the local communities who supported our research. Special thanks go to the staff of Sambu Group, particularly those from the Research and Advisory division, who provided invaluable assistance in the field.
References
- 1.Xu J., Morris P.J., Liu J., Holden J., Peatmap Refining estimates of global peatland distribution based on a meta-analysis. Catena. 2018;160:134–140. doi: 10.1016/j.catena.2017.09.010. [DOI] [Google Scholar]
- 2.Ribeiro K., Pacheco F.S., Ferreira J.W., de Sousa-Neto E.R., Hastie A., Krieger Filho G.C., Alvalá P.C., Forti M.C., Ometto J.P. Tropical peatlands and their contribution to the global carbon cycle and climate change. Glob. Change Biol. 2021;27:489–505. doi: 10.1111/gcb.15408. [DOI] [PubMed] [Google Scholar]
- 3.Strack M., Davidson S.J., Hirano T., Dunn C. The potential of peatlands as nature-based climate solutions. Curr. Clim. Change Rep. 2022;8:71–82. doi: 10.1007/s40641-022-00183-9. [DOI] [Google Scholar]
- 4.Anda M., Ritung S., Suryani E., Sukarman, Hikmat M., Yatno E., Mulyani A., Subandiono R.E., Suratman Husnain. Revisiting tropical peatlands in Indonesia: semi-detailed mapping, extent and depth distribution assessment. Geoderma. 2021;402 doi: 10.1016/j.geoderma.2021.115235. [DOI] [Google Scholar]
- 5.Limin S.H., Jentha, Ermiasi Y. History of the development of tropical peatland in Central Kalimantan, Indonesia. Tropics. 2007;16:291–301. doi: 10.3759/tropics.16.291. [DOI] [Google Scholar]
- 6.Suwardi B. Mulyanto, Sumawinata B., Sandrawatu A. The history of peatland management in Indonesia. Jurnal Ilmiah Pertanian Gakuryoku. 2005;11:120–126. [Google Scholar]
- 7.Furukawa H. Kyoto University Press; Japan: 1994. Coastal Wetlands of Indonesia: Environment, Subsistence and Exploitation. [Google Scholar]
- 8.Limin S.H., Yuprin A.D., Tampung N.S., Yunsiska E. In: Restoration of Tropical Peatlands. Wösten J.H.M., Rieley J.O., Page S.E., editors. Alterra; Wageningen: 2008. The key to successful management of peatland in Central Kalimantan is correct government policy; pp. 200–209. [Google Scholar]
- 9.Hoscilo A., Page S.E., Tansey K.J., Rieley J.O., Hoscilo A., Page S.E., Tansey K.J., Rieley J.O. Effect of repeated fires on land-cover change on peatland in Southern Central Kalimantan, Indonesia, from 1973 to 2005. Int. J. Wildland Fire. 2011;20:578–588. doi: 10.1071/WF10029. [DOI] [Google Scholar]
- 10.Medrilzam M., Smith C., Aziz A.A., Herbohn J., Dargusch P. Smallholder farmers and the dynamics of degradation of peatland ecosystems in Central Kalimantan, Indonesia. Ecol. Econ. 2017;136:101–113. doi: 10.1016/j.ecolecon.2017.02.017. [DOI] [Google Scholar]
- 11.Dohong A., Aziz A.A., Dargusch P. A review of the drivers of tropical peatland degradation in South-East Asia. Land Use Pol. 2017;69:349–360. doi: 10.1016/j.landusepol.2017.09.035. [DOI] [Google Scholar]
- 12.Daryono H. Potency, problems, policy and peatland management needed for sustainable peat swamp forest. Jurnal Analisis Kebijakan Kehutanan. 2009;6:71–101. doi: 10.20886/jakk.2009.6.2.%25p. [DOI] [Google Scholar]
- 13.Novitasari N., Sujono J., Harto S., Maas A., Jayadi R. AIP Conference Proceedings 1977. 2018. Restoration of peat dome in ex-Mega Rice Project area in Central Kalimantan. 040008, doi:10.1063/1.5042978. [Google Scholar]
- 14.Novitasari N., Sujono J., Harto S., Maas A., Jayadi R. Drought index for peatland wildfire management in Central Kalimantan, Indonesia during El Niño phenomenon. J. Disaster Res. 2019;14:939–948. doi: 10.20965/jdr.2019.p0939. [DOI] [Google Scholar]
- 15.Hayasakaand H., Putra E.I. Reassessment of peatland fires in Central Kalimantan. IOP Conf. Ser. Earth Environ. Sci. 2022;959 doi: 10.1088/1755-1315/959/1/012053. [DOI] [Google Scholar]
- 16.Putra E., Hayasaka H., Takahashi H., Usup A. Recent peat fire activity in the Mega Rice Project area, Central Kalimantan, Indonesia. J. Disaster Res. 2008;3 doi: 10.20965/jdr.2008.p0334. [DOI] [Google Scholar]
- 17.Miettinen J., Shi C., Liew S.C. Land cover distribution in the peatlands of Peninsular Malaysia, Sumatra and Borneo in 2015 with changes since 1990. Glob. Ecol. Conserv. 2016;6:67–78. doi: 10.1016/j.gecco.2016.02.004. [DOI] [Google Scholar]
- 18.Nursyamsi D., Noor M., Maftu’ah E. In: Tropical Peatland Ecosystems. Osaki M., Tsuji N., editors. Springer Japan; Tokyo: 2016. Peatland management for sustainable agriculture; pp. 493–511. [DOI] [Google Scholar]
- 19.Clarke D., Rieley J. sixth ed. International Peatland Society, Jyväskylä; Finland: 2019. Strategy for Responsible Peatland Management. [Google Scholar]
- 20.Uda S.K., Hein L., Sumarga E. Towards sustainable management of Indonesian tropical peatlands. Wetl. Ecol. Manag. 2017;25:683–701. doi: 10.1007/s11273-017-9544-0. [DOI] [Google Scholar]
- 21.Wösten J.H.M., Ismail A.B., van Wijk A.L.M. Peat subsidence and its practical implications: a case study in Malaysia. Geoderma. 1997;78:25–36. doi: 10.1016/S0016-7061(97)00013-X. [DOI] [Google Scholar]
- 22.Ritzema H.P., Mat Hassan AbdM., Moens R.P. A new approach to water management of tropical peatlands: a case study from Malaysia. Irrigat. Drain. Syst. 1998;12:123–139. doi: 10.1023/A:1005976928479. [DOI] [Google Scholar]
- 23.Kato T., Osaki M., Tsuji N., Silsigia S. In: Tropical Peatland Eco-Management. Osaki M., Tsuji N., Foead N., Rieley J., editors. Springer; Singapore: 2021. Principles of eco-management in a large-scale ecosystem of tropical peatland; pp. 63–86. [DOI] [Google Scholar]
- 24.Wijedasa L.S., Jauhiainen J., Könönen M., Lampela M., Vasander H., Leblanc M.-C., Evers S., Smith T.E.L., Yule C.M., Varkkey H., Lupascu M., Parish F., Singleton I., Clements G.R., Aziz S.A., Harrison M.E., Cheyne S., Anshari G.Z., Meijaard E., Goldstein J.E., Waldron S., Hergoualc’h K., Dommain R., Frolking S., Evans C.D., Posa M.R.C., Glaser P.H., Suryadiputra N., Lubis R., Santika T., Padfield R., Kurnianto S., Hadisiswoyo P., Lim T.W., Page S.E., Gauci V., Van Der Meer P.J., Buckland H., Garnier F., Samuel M.K., Choo L.N.L.K., O’Reilly P., Warren M., Suksuwan S., Sumarga E., Jain A., Laurance W.F., Couwenberg J., Joosten H., Vernimmen R., Hooijer A., Malins C., Cochrane M.A., Perumal B., Siegert F., Peh K.S.-H., Comeau L.-P., Verchot L., Harvey C.F., Cobb A., Jaafar Z., Wösten H., Manuri S., Müller M., Giesen W., Phelps J., Yong D.L., Silvius M., Wedeux B.M.M., Hoyt A., Osaki M., Hirano T., Takahashi H., Kohyama T.S., Haraguchi A., Nugroho N.P., Coomes D.A., Quoi L.P., Dohong A., Gunawan H., Gaveau D.L.A., Langner A., Lim F.K.S., Edwards D.P., Giam X., Van Der Werf G., Carmenta R., Verwer C.C., Gibson L., Gandois L., Graham L.L.B., Regalino J., Wich S.A., Rieley J., Kettridge N., Brown C., Pirard R., Moore S., Capilla B.R., Ballhorn U., Ho H.C., Hoscilo A., Lohberger S., Evans T.A., Yulianti N., Blackham G., Onrizal, Husson S., Murdiyarso D., Pangala S., Cole L.E.S., Tacconi L., Segah H., Tonoto P., Lee J.S.H., Schmilewski G., Wulffraat S., Putra E.I., Cattau M.E., Clymo R.S., Morrison R., Mujahid A., Miettinen J., Liew S.C., Valpola S., Wilson D., D’Arcy L., Gerding M., Sundari S., Thornton S.A., Kalisz B., Chapman S.J., Su A.S.M., Basuki I., Itoh M., Traeholt C., Sloan S., Sayok A.K., Andersen R. Denial of long-term issues with agriculture on tropical peatlands will have devastating consequences. Glob. Change Biol. 2017;23:977–982. doi: 10.1111/gcb.13516. [DOI] [PubMed] [Google Scholar]
- 25.Warren M., Hergoualc’h K., Kauffman J.B., Murdiyarso D., Kolka R. An appraisal of Indonesia’s immense peat carbon stock using national peatland maps: uncertainties and potential losses from conversion. Carbon Bal. Manag. 2017;12:12. doi: 10.1186/s13021-017-0080-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rieley J.O., Wüst R.A.J., Jauhiainen J., Page S.E., Wösten H., Hooijer A., Siegert F., Limin S.H., Vasander H., Stanhlhut M. In: Peatlands and Climate Change. Strack M., editor. International Peat Society; Jyväskylä: 2008. Tropical peatlands: carbon stores, carbon gas emissions and contribution to climate change processes; pp. 148–180. [Google Scholar]
- 27.Sumawinata B. In: Wise Use of Tropical Peatland. Sudarsono R., Hatano T., Inoue S., Limin G., Djajakirana Suwardi, editors. Bogor Agricultural University & Hokkaido University; Bogor, Indonesia: 2009. Darmawan, Current issues of tropical peatland in Indonesia; pp. 1–10. [Google Scholar]
- 28.Fanin T., van der Werf G.R. Precipitation–fire linkages in Indonesia (1997–2015) Biogeosciences. 2017;14:3995–4008. doi: 10.5194/bg-14-3995-2017. [DOI] [Google Scholar]
- 29.Yulianti N., Hayasaka H. Recent active fires under El Niño conditions in Kalimantan, Indonesia. Am. J. Plant Sci. 2013;4:685–696. doi: 10.4236/ajps.2013.43A087. [DOI] [Google Scholar]
- 30.Putra E., Hayasaka H. JAFSE Annual Sympoium 2009; Tokyo: 2009. Carbon emission from severe peat fires in Mega Rice Project area, Indonesia; pp. 1–2. [Google Scholar]
- 31.Dohong A., Aziz A.A., Dargusch P. Carbon emissions from oil palm development on deep peat soil in Central Kalimantan Indonesia. Anthropocene. 2018;22:31–39. doi: 10.1016/j.ancene.2018.04.004. [DOI] [Google Scholar]
- 32.Page S., Mishra S., Agus F., Anshari G., Dargie G., Evers S., Jauhiainen J., Jaya A., Jovani-Sancho A.J., Laurén A., Sjögersten S., Suspense I.A., Wijedasa L.S., Evans C.D. Anthropogenic impacts on lowland tropical peatland biogeochemistry. Nat. Rev. Earth Environ. 2022;3:426–443. doi: 10.1038/s43017-022-00289-6. [DOI] [Google Scholar]
- 33.Astiani D., Burhanuddin B., Gusmayanti E., Widiastuti T., Taherzadeh M.J. Enhancing water levels of degraded, bare, tropical peatland in West Kalimantan, Indonesia: impacts on CO2 emission from soil respiration. Biodiversitas J. Biol. Divers. 2018;19:472–477. doi: 10.13057/biodiv/d190221. [DOI] [Google Scholar]
- 34.Hooijer A., Page S., Jauhiainen J., Lee W.A., Lu X.X., Idris A., Anshari G. Subsidence and carbon loss in drained tropical peatlands. Biogeosciences. 2012;9:1053–1071. doi: 10.5194/bg-9-1053-2012. [DOI] [Google Scholar]
- 35.Wösten J.H.M., Clymans E., Page S.E., Rieley J.O., Limin S.H. Peat–water interrelationships in a tropical peatland ecosystem in Southeast Asia. Catena. 2008;73:212–224. doi: 10.1016/j.catena.2007.07.010. [DOI] [Google Scholar]
- 36.Ritzema H., Limin S., Kusin K., Jauhiainen J., Wösten H. Canal blocking strategies for hydrological restoration of degraded tropical peatlands in Central Kalimantan, Indonesia. Catena. 2014;114:11–20. doi: 10.1016/j.catena.2013.10.009. [DOI] [Google Scholar]
- 37.Wijedasa L.S., Page S.E., Evans C.D., Osaki M. Time for responsible peatland agriculture. Science. 2016;354:562. doi: 10.1126/science.aal1794. [DOI] [PubMed] [Google Scholar]
- 38.Hirano T., Kusin K., Limin S., Osaki M. Carbon dioxide emissions through oxidative peat decomposition on a burnt tropical peatland. Glob. Change Biol. 2014;20:555–565. doi: 10.1111/gcb.12296. [DOI] [PubMed] [Google Scholar]
- 39.Sutarta E.S. Water management in tidal swampy areas for coconut cultivation. Coconut Res. Dev. J. 1990;6 doi: 10.37833/cord.v6i02.241. 34–34. [DOI] [Google Scholar]
- 40.Akmal A., Warto W., Sariyatun S. The rapid growth of coconut estates in Indragiri Hilir 1980s – 1990s. Jurnal Sejarah Citra Lekha. 2021;5:121–134. [Google Scholar]
- 41.Ochs R., Bengy A.D., Bonneau X. Établissement d’une cocoteraie en tourbe profonde. Oleagineux. 1992;47:9–22. [Google Scholar]
- 42.Indragiri Hilir Central Bureau of Statistics . Indragiri Hilir Regency in Figures 2023. Indragiri Hilir Central Bureau of Statistics; Tembilahan: 2023. [Google Scholar]
- 43.Indragiri Hilir Central Bureau of Statistics . Precipitation by Month (Mm), 2019-2022) 2023. https://inhilkab.bps.go.id/indicator/151/77/1/curah-hujan-menurut-bulan.html (Accessed 9 November 2023) [Google Scholar]
- 44.Muharomah R., Setiawan B.I. Identification of climate trends and patterns in South Sumatra. Agro Sur. 2022;36:79–87. doi: 10.29244/j.agromet.36.2.79-87. [DOI] [Google Scholar]
- 45.Indragiri Hilir Central Bureau of Statistics . Production and average production per hectare of coconuts plants in Indragiri Hilir Regency 2015. 2021. https://inhilkab.bps.go.id/statictable/2016/08/31/130/produksi-rata-rata-produksi-per-ha-kelapa-dalam-di-kabupaten-indragiri-hilir-2015.html (Accessed 7 April 2023) [Google Scholar]
- 46.Cerepak P.J. Establishing the intimate link: 20th century tropical agriculture and the establishment of the coconut zone. J. Marit. Stud. Natl. Integr. 2020;4:1–11. doi: 10.14710/jmsni.v4i1.8026. [DOI] [Google Scholar]
- 47.Mubekti Studi pewilayahan dalam rangka pengelolaan lahan gambut berkelanjutan di Provinsi Riau. Jurnal Sains Dan Teknologi Indonesia. 2011;13:88–94. [Google Scholar]
- 48.Zaman M., Kleineidam K., Bakken L., Berendt J., Bracken C., Butterbach-Bahl K., Cai Z., Chang S.X., Clough T., Dawar K., Ding W.X., Dörsch P., dos Reis Martins M., Eckhardt C., Fiedler S., Frosch T., Goopy J., Görres C.-M., Gupta A., Henjes S., Hofmann M.E.G., Horn M.A., Jahangir M.M.R., Jansen-Willems A., Lenhart K., Heng L., Lewicka-Szczebak D., Lucic G., Merbold L., Mohn J., Molstad L., Moser G., Murphy P., Sanz-Cobena A., Šimek M., Urquiaga S., Well R., Wrage-Mönnig N., Zaman S., Zhang J., Müller C. In: Measuring Emission of Agricultural Greenhouse Gases and Developing Mitigation Options Using Nuclear and Related Techniques: Applications of Nuclear Techniques for GHGs. Zaman M., Heng L., Müller C., editors. Springer International Publishing; Cham: 2021. Methodology for measuring greenhouse gas emissions from agricultural soils using non-isotopic techniques; pp. 11–108. [DOI] [Google Scholar]
- 49.Sambu Group . Sambu Group, Indragiri Hilir; 2015. Inventarisasi karakteristik lahan gambut areal perkebunan kelapa dan nanas PT RSUP di Kabupaten Indragiri Hilir Provinsi Riau. (Unpublished report) [Google Scholar]
- 50.Philippe R., Jourdan C. Centre de coopération internationale en recherche agronomique pour le développement (Cirad); Paris: 1998. Mission to the RSUP Pulau Burung Coconut Plantation (Sumatra/Indonesia): 3rd November to 14th December 1997. [Google Scholar]
- 51.Fangren P., Baolong H., Juhana T. Productivity and nutrient cycling in an agroforestry ecosystem for interplant of pineapple and coconut. J. For. Res. 1999;10:163–167. doi: 10.1007/BF02855424. [DOI] [Google Scholar]
- 52.Page S., Rieley J. In: The Wetland Book: II: Distribution, Description, and Conservation. Finlayson C.M., Milton G.R., Prentice R.C., Davidson N.C., editors. Springer Netherlands; Dordrecht: 2018. Tropical peat swamp forests of Southeast Asia; pp. 1753–1761. [DOI] [Google Scholar]
- 53.Andriesse J.P. Food and Agriculture Organization of the United Nations; Rome: 1988. Nature and Management of Tropical Peat Soils. [Google Scholar]
- 54.Ishikura K., Hirano T., Okimoto Y., Hirata R., Kiew F., Melling L., Aeries E.B., Lo K.S., Musin K.K., Waili J.W., Wong G.X., Ishii Y. Soil carbon dioxide emissions due to oxidative peat decomposition in an oil palm plantation on tropical peat. Agric. Ecosyst. Environ. 2018;254:202–212. doi: 10.1016/j.agee.2017.11.025. [DOI] [Google Scholar]
- 55.Jamili M.J., Nugroho B., Sumawinata B., Anwar S. Dynamics of CO2 fluxes from oil palm plantations on peatland. J. Nat. Resour. Environ. Manag. 2021;11:430–441. [Google Scholar]
- 56.Carlson K.M., Goodman L.K., May-Tobin C.C. Modeling relationships between water table depth and peat soil carbon loss in Southeast Asian plantations. Environ. Res. Lett. 2015;10 doi: 10.1088/1748-9326/10/7/074006. [DOI] [Google Scholar]
- 57.Khasanah N., van Noordwijk M. Subsidence and carbon dioxide emissions in a smallholder peatland mosaic in Sumatra, Indonesia. Mitig. Adapt. Strategies Glob. Change. 2019;24:147–163. doi: 10.1007/s11027-018-9803-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Comeau L.-P., Hergoualc’h K., Smith J.U., Verchot L. Working Paper 110. CIFOR; Bogor, Jawa Barat, Indonesia: 2013. Conversion of intact peat swamp forest to oil palm plantation: effects on soil CO2 fluxes in Jambi, Sumatra. [Google Scholar]
- 59.Marwanto S., Agus F. Is CO2 flux from oil palm plantations on peatland controlled by soil moisture and/or soil and air temperatures? Mitig. Adapt. Strategies Glob. Change. 2014;19:809–819. doi: 10.1007/s11027-013-9518-3. [DOI] [Google Scholar]
- 60.Dariah A., Marwanto S., Agus F. Root- and peat-based CO2 emissions from oil palm plantations. Mitig. Adapt. Strategies Glob. Change. 2014;19:831–843. doi: 10.1007/s11027-013-9515-6. [DOI] [Google Scholar]
- 61.Husnain H., Wigena I.G.P., Dariah A., Marwanto S., Setyanto P., Agus F. CO2 emissions from tropical drained peat in Sumatra, Indonesia. Mitig. Adapt. Strategies Glob. Change. 2014;19:845–862. doi: 10.1007/s11027-014-9550-y. [DOI] [Google Scholar]
- 62.Jauhiainen J., Hooijer A., Page S.E. Carbon dioxide emissions from an Acacia plantation on peatland in Sumatra, Indonesia. Biogeosciences. 2012;9:617–630. doi: 10.5194/bg-9-617-2012. [DOI] [Google Scholar]
- 63.Ali M., Taylor D., Inubushi K. Effects of environmental variations on CO2 efflux from a tropical peatland in eastern Sumatra. Wetlands. 2006;26:612. doi: 10.1672/0277-5212(2006)26[612:EOEVOC]2.0.CO;2. [DOI] [Google Scholar]
- 64.Huntingford C., Atkin O.K., Martinez-de la Torre A., Mercado L.M., Heskel M.A., Harper A.B., Bloomfield K.J., O'Sullivan O.S., Reich P.B., Wythers K.R., Butler E.E., Chen M., Griffin K.L., Meir P., Tjoelker M.G., Turnbull M.H., Sitch S., Wiltshire A., Malhi Y. Implications of improved representations of plant respiration in a changing climate. Nat. Commun. 2017;8:1602. doi: 10.1038/s41467-017-01774-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kiew F., Hirata R., Hirano T., Xhuan W.G., Aries E.B., Kemudang K., Wenceslaus J., San L.K., Melling L. Carbon dioxide balance of an oil palm plantation established on tropical peat. Agric. For. Meteorol. 2020;295 doi: 10.1016/j.agrformet.2020.108189. [DOI] [Google Scholar]
- 66.Bot A., José B. Food and Agriculture Organization of the United Nations; Rome: 2005. The Importance of Soil Organic Matter: Key to Drought-Resistant Soil and Sustained Food Production. [Google Scholar]
- 67.Hendri J., Sumawinata B., Baskoro D.P.T. CO2 flux from tropical land uses on Andisol in West Java, Indonesia. J. Trop. Soils. 2015;19:121–130. doi: 10.5400/jts.2014.v19i3.121-130. [DOI] [Google Scholar]
- 68.Meijide A., de la Rua C., Guillaume T., Röll A., Hassler E., Stiegler C., Tjoa A., June T., Corre M.D., Veldkamp E., Knohl A. Measured greenhouse gas budgets challenge emission savings from palm-oil biodiesel. Nat. Commun. 2020;11:1089. doi: 10.1038/s41467-020-14852-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mohan Kumar B., Jose S. Phenotypic plasticity of roots in mixed tree species agroforestry systems: review with examples from peninsular India. Agrofor. Syst. 2018;92:59–69. doi: 10.1007/s10457-016-0012-2. [DOI] [Google Scholar]
- 70.Evans C.D., Williamson J.M., Kacaribu F., Irawan D., Suardiwerianto Y., Hidayat M.F., Laurén A., Page S.E. Rates and spatial variability of peat subsidence in Acacia plantation and forest landscapes in Sumatra, Indonesia. Geoderma. 2019;338:410–421. doi: 10.1016/j.geoderma.2018.12.028. [DOI] [Google Scholar]
- 71.Wösten H., Ritzema H. Peatlands for People: Natural Resource Functions and Sustainable Management. BPPT/IPA; Jakarta: 2002. Challenges in land and water management for peatland development in Sarawak; pp. 51–55. [Google Scholar]
- 72.Grossman R.B., Reinsch T.G. Methods of Soil Analysis. John Wiley & Sons, Ltd; 2002. Bulk density and linear extensibility; pp. 201–228. [DOI] [Google Scholar]
- 73.Firdaus M., Gandaseca S., Ahmed O., Majid N.M.Ab. Effect of converting secondary tropical peat swamp forest into oil palm plantation on selected peat soil physical properties. Am. J. Environ. Sci. 2010;6:402–405. doi: 10.3844/ajessp.2010.402.405. [DOI] [Google Scholar]
- 74.Junedi H., Armanto M.E., Bernas S.M., Imanudin M.S. Changes to some physical properties due to conversion of secondary forest of peat into oil palm plantation. Sriwijaya J. Environ. 2017;2:76–80. doi: 10.22135/sje.2017.2.3.76-80. [DOI] [Google Scholar]
- 75.Othman H., Mohammed A.T., Darus F.M., Harun M.H., Zambri M.P. Best management practice for oil palm cultivation on peat: ground water-table maintenance in relation to peat subsidence and estimation of CO2 emissions at Sessang, Sarawak. J. Oil Palm Res. 2011;23:1078–1086. [Google Scholar]
- 76.Gusmayanti E., Anshari G.Z., Pramulya M., Ruliyansyah A. CO2 fluxes from drained tropical peatland used for oil palm plantation in relation to peat characteristics and crop age after planting. Biodiversitas J. Biol. Divers. 2019;20 doi: 10.13057/biodiv/d200622. [DOI] [Google Scholar]
- 77.Couwenberg J., Dommain R., Joosten H. Greenhouse gas fluxes from tropical peatlands in south-east Asia. Glob. Change Biol. 2010;16:1715–1732. doi: 10.1111/j.1365-2486.2009.02016.x. [DOI] [Google Scholar]
- 78.Hooijer A., Page S., Canadell J.G., Silvius M., Kwadijk J., Wösten H., Jauhiainen J. Current and future CO2 emissions from drained peatlands in Southeast Asia. Biogeosciences. 2010;7:1505–1514. doi: 10.5194/bg-7-1505-2010. [DOI] [Google Scholar]
- 79.Prananto J.A., Minasny B., Comeau L.-P., Rudiyanto R., Grace P. Drainage increases CO2 and N2O emissions from tropical peat soils. Glob. Change Biol. 2020;26:4583–4600. doi: 10.1111/gcb.15147. [DOI] [PubMed] [Google Scholar]
- 80.Melling L., Hatano R., Goh K.J. Soil CO2 flux from three ecosystems in tropical peatland of Sarawak, Malaysia. Tellus B. 2005;57:1–11. doi: 10.3402/tellusb.v57i1.16772. [DOI] [Google Scholar]
- 81.Swails E.E., Ardón M., Krauss K.W., Peralta A.L., Emanuel R.E., Helton A.M., Morse J.L., Gutenberg L., Cormier N., Shoch D., Settlemyer S., Soderholm E., Boutin B.P., Peoples C., Ward S. Response of soil respiration to changes in soil temperature and water table level in drained and restored peatlands of the southeastern United States. Carbon Bal. Manag. 2022;17:18. doi: 10.1186/s13021-022-00219-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Everett K.R. Pedogenesis and Soil Taxonomy: the Soil Orders. first ed. Elsevier Science Publisher; Amsterdam: 1983. Histosols; pp. 1–54. [Google Scholar]
- 83.Joosten H., Clarke D. International Peat Society & International Mire Conservation Group; Saarijärvi: 2002. Wise Use of Mires and Peatlands: Background and Principles Including a Framework for Decision-Making. [Google Scholar]
- 84.Tanneberger F., Appulo L., Ewert S., Lakner S., Ó Brolcháin N., Peters J., Wichtmann W. The power of nature-based solutions: how peatlands can help us to achieve key EU sustainability objectives. Advanced Sustainable Systems. 2021;5:1–10. doi: 10.1002/adsu.202000146. [DOI] [Google Scholar]
- 85.Jamaluddin J. Tradisi dan modal kultural etnis bugis di Riau dan Jambi. Kontekstualita. 2018;33:126–141. doi: 10.30631/kontekstualita.v35i02.80. [DOI] [Google Scholar]
- 86.Brady M.A. University of British Columbia; Indonesia: 1997. Organic Matter Dynamics of Coastal Peat Deposits in Sumatra. [Google Scholar]
- 87.Busman N.A., Melling L., Goh K.J., Imran Y., Sangok F.E., Watanabe A. Soil CO2 and CH4 fluxes from different forest types in tropical peat swamp forest. Sci. Total Environ. 2023;858 doi: 10.1016/j.scitotenv.2022.159973. [DOI] [PubMed] [Google Scholar]
- 88.Takahashi H., Usup A., Hayasaka H., Limin S.H. Hokkaido University and Indonesian Institute of Sciences; Bali: 2002. Estimation of Ground Water Level in a Peat Swamp Forest as an Index of Peat/forest Fire; pp. 311–314. [Google Scholar]
- 89.Deshmukh C.S., Julius D., Desai A.R., Asyhari A., Page S.E., Nardi N., Susanto A.P., Nurholis N., Hendrizal M., Kurnianto S., Suardiwerianto Y., Salam Y.W., Agus F., Astiani D., Sabiham S., Gauci V., Evans C.D. Conservation slows down emission increase from a tropical peatland in Indonesia. Nat. Geosci. 2021;14:484–490. doi: 10.1038/s41561-021-00785-2. [DOI] [Google Scholar]
- 90.Jovani Sancho A.J., O’Reilly P., Anshari G., Chong X.Y., Crout N., Evans C., Evers S., Gan J., Gibbins C., Gusmayanti E., Jamaludin J., Jaya A., Page S., Yosep, Upton C., Wilson P., Sjögersten S. 2023. CH4 and N2O emissions from smallholder agricultural systems on tropical peatlands in Southeast Asia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hirano T., Segah H., Kusin K., Limin S., Takahashi H., Osaki M. Effects of disturbances on the carbon balance of tropical peat swamp forests. Glob. Change Biol. 2012;18:3410–3422. doi: 10.1111/j.1365-2486.2012.02793.x. [DOI] [Google Scholar]
- 92.Hoyt A.M., Gandois L., Eri J., Kai F.M., Harvey C.F., Cobb A.R. CO2 emissions from an undrained tropical peatland: interacting influences of temperature, shading and water table depth. Glob. Change Biol. 2019;25:2885–2899. doi: 10.1111/gcb.14702. [DOI] [PubMed] [Google Scholar]
- 93.Liu L., Chen H., Tian J. Varied response of carbon dioxide emissions to warming in oxic, anoxic and transitional soil layers in a drained peatland. Commun. Earth Environ. 2022;3:1–12. doi: 10.1038/s43247-022-00651-y. [DOI] [Google Scholar]
- 94.Andriesse J.P. Royal Tropical Institute, Department of Agricultural Research; Amsterdam: 1974. Tropical Lowland Peats in South-East Asia. [Google Scholar]
- 95.Wösten H., Ritzema H. Land and water management options for peatland development in Sarawak, Malaysia. Int. Peat J. 2001;11:59–66. [Google Scholar]
- 96.Sumawinata B., Djajakirana G., Suwardi Darmawan. IPB Press; Bogor, Indonesia: 2014. Carbon Dynamics in Tropical Peatland Planted Forests: One-Year Research Findings in Sumatra, Indonesia. [Google Scholar]