Abstract
Additive Manufacturing (AM) has recently demonstrated significant medical progress. Due to advancements in materials and methodologies, various processes have been developed to cater to the medical sector's requirements, including bioprinting and 4D, 5D, and 6D printing. However, only a few studies have captured these emerging trends and their medical applications. Therefore, this overview presents an analysis of the advancements and achievements obtained in AM for the medical industry, focusing on the principal trends identified in the annual report of AM3DP.
Keywords: Additive manufacturing, Bioprinting, 3D printing, 4D printing, 5D printing, 6D printing
1. Introduction
Additive manufacturing (AM), implemented by Charles Hull in the 1980s [1], is usually referred to as three-dimensional (3D) printing, rapid prototyping, or free-form manufacturing [2]. It is considered an advanced digital manufacturing technique that directly creates a physical model of complex structures and geometries from CAD models by adding materials layer by layer [3]. Thus, it allows the creation of structures in a shorter period and with reduced costs compared to the traditional manufacturing method [4]. AM in product innovation can reduce lead times by up to 90% and manufacturing costs by up to 70% [5].
Between 2020 and 2022, a sequence shows that the area dedicated to medicine in AM remains at 22% compared to other fields where AM is used. This study discusses the importance of developing new trends and improving medical applications. The American Society of Mechanical Engineers (ASME) recently presented its Additive Manufacturing/3D Printing (AM3DP) Medical Annual Report [6], where significant participation of AM in some areas of the medical sector during the years 2020 and 2021 is exposed. This report shows that the development of prototypes and anatomical models are the areas with the most contributions, as shown in Table 1. Nonetheless, more specific areas such as design and fabrication of prosthesis [7], [8], [9], [10], [11], [12], [13], [14], [15], orthosis [16], [17], [18], [19], bone tissue reconstruction [4], [20], [21], [22], organ fabrication [23], [24], dental applications [25], [26], [27] and medical device manufacturing [28] have been considered in this research due to their great potential and development.
Table 1.
Contribution of additive manufacturing in different medical areas during 2020, 2021, and 2022 [6].
| Application area | 2020 | 2021 | 2022 |
|---|---|---|---|
| Prototypes | 62% | 62% | 66% |
| Anatomical models | 40% | 30% | 47% |
| Tooling and patterns | 27% | 28% | 31% |
| I do not use AM/3DP | 15% | 23% | 8% |
| Prosthesis | 29% | 21% | 29% |
| Surgical planning | 31% | 20% | 35% |
| Patient-matched devices | 34% | 18% | 26% |
| Biofabrication | 17% | 15% | 16% |
| Serialized devices | 11% | 13% | 12% |
| Dental | 16% | 10% | 23% |
| Active devices | 9% | 7% | 10% |
By the trends above, medical personnel must have at least some knowledge about the existing 3D printing technologies (3DP) to identify the process that aligns with their needs to solve a specific medical problem through AM. The solution might consider a 3D-printed product, an AM application, or a new methodology applied by staff to meet medical needs. Innovative answers are found, such as creating artificial organs, implants, orthoses, prostheses, new surgical planning, and instruments. Additionally, processes allow new ways of managing or controlling the interaction speeds of external agents with the body's patient, such as controlled medication dosage and improvement in the bone growth process with better combinations of materials and techniques that ensure compatibility. New AM areas are emerging to front-face problems whose limitations cannot be solved [29]. For this reason, the latest developments in this field are compiled in this document, including bioprinting and other new printing methodologies known as four-dimensional printing (4DP), five-dimensional printing (5DP), and six-dimensional printing (6DP). These could allow new points of focus and evolution, giving way to new applications that generate answers and needs to innovate in manufacturing methodologies through AM, creating a continuous growth cycle.
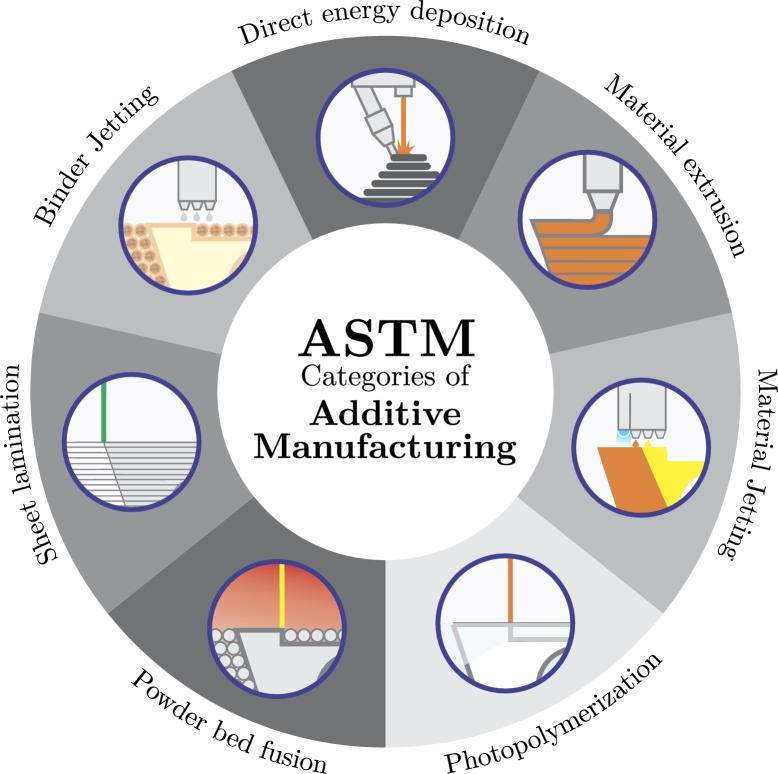
The article structure was an approach to AM processes used in medical applications in the last ten years. The interest area focuses on the future trends of AM in medical applications, giving rise to new technological advances from which medicine has been positively affected. These applications were selected due to a reformulation of the most common medical areas where AM is used, grouped into implants, prostheses, orthoses, surgical instruments, and prototypes, as shown in Fig. 1. Additionally, new trends were found, and due to their importance, they have been included and classified as bioprinting, 4DP, 5DP, and 6DP.
Figure 1.
ASTM categories of additive manufacturing.
The information was organized into sections with topics focused on the previously mentioned structure. Section 2 introduces AM applied in medicine and its classification, consisting of a general revision of the principal application. Each subsection has a structure organized with a short description of the method, types of materials applied, main medical applications, and future developments. Section 3 presents the applications and advantages of AM techniques in medicine. Section 4 introduces the recent tendencies of AM applied in healthcare. Finally, sections 5 and 6 are devoted to discussions and conclusions, respectively.
2. Additive manufacturing applied in medicine
AM techniques can be classified according to material deposition methods, energy sources, or operating principles [30]. However, according to the standards established by the International Organization for Standardization (ISO) and the American Society for Testing and Materials (ASTM), AM technologies can be classified into seven categories [31], presented in Fig. 1. These categories offer total freedom of design with the necessary adjustments for customization, optimizing the use of the material to be used, reducing residues, and at the same time, offering more speed in creating prototypes [32].
In the medical sector, the different AM processes are employed according to the type of material used, the degree of precision, and the complexity required. In the AM3D Printing annual medical report presented by ASME [6], it is reported that material extrusion was a popular category in the medical sector, with 55% in 2022, demonstrating a 4% increase compared to 2020 and 1% to 2021. However, in 2022, vat photopolymerization had a higher preference, with an acceptance of 57%, 17% more than in 2021 and 19% in 2020. The main reason is the increased variety of printing material unavailable before. Finally, powder bed fusion has 51% of acceptance in 2022, different from 41% in 2021 and 40% in 2020. The rest of the process also contributed to the medical sector, but their input is shorter compared to the AM procedures previously mentioned.
Nowadays, medical applications with engineering solutions are separated into multiple fields. AM has generated innovative ways to solve medical problems; implants, prosthesis, orthosis, surgical planning, and prototypes have been developed within them. Pharmaceutical and food applications are also under research efforts, but these are out of the scope of this article. The following subsections detail each AM technique according to its ASTM classification and highlight its main medical sector applications. The information is presented to understand the different processes and is oriented toward future trends in medical applications.
Other approaches have also been considered to illustrate the following sections. First, Mika Salmi wrote a review, “Additive Manufacturing Processes in Medical Applications,” oriented on the different categories of AM and materials used in medical and dental applications [33]. Also, Chunxu Li developed a review titled “Advances in Medical Applications of Additive Manufacturing,” which focus on pharmaceutics applications, devices, and implant medical oriented to the patient's diagnosis, highlighting the limitations to date of this technology [34].
2.1. Material extrusion (ME)
The process could be fed with different material forms, such as filament, pellets, or paste in a continuous form, which is heated using a coil above its melting temperature inside an extrusion room [35]. These are principally polymers, metals are a moderately viable option, and ceramics are the least used through this method [33]. The molten material is ejected through the extruder nozzle and deposited on the platform layer by layer in a steady stream. The main advantages AM techniques offer in this category are integrating different materials, cost-effective manufacturing, and ease of part scaling. However, the surface finish is irregular and has a low print resolution [36]. The applications with great potential within medicine for this category are implants (PEEK [37]; ATP powder [38]), prosthesis (Lignin biofilament; PLA [39]; PE-PP-ABS [40]), orthesis (ABS, PET, PC, PLA [41]), surgical instruments (ABS+ [42]; ABS [43]; TPU [44]) and other new trends. This category's most critical manufacturing technique is Fused Deposition Modeling (FDM) [45], [46], [47]. Related to this classification is the development of better control in the applications of the material used in 3DP. FDM is used to create fabrics like organic tissue, but some research exists on functional fabrics from structured inks to improve tissue engineering and allow new developments in the future [48].
On the other hand, there is an urgency to improve the organ printing process, which is continuously developing, following new ways to print organs in high demand. The main organs required are the liver, heart, and kidneys, but it is in its initial stages since there are still several complications related to the printing process and the materials used. However, as innovation has been going on, it will be possible to print not-so-complex, fully functional organs [49] quickly.
2.2. Powder bed fusion (PBF)
In this process, input material is used in powder form conformed by polymers, metals (the principal prime material), and ceramics (as the least used option) [33]. Thermal energy, mainly laser or electron beam, fuses powder particles layer by layer into a solid part. Manufacturers can benefit significantly from technologies in this category, considering that PBF manufacturing is relatively inexpensive, features high strength and rigidity in the manufactured parts, and has a high print resolution. However, one of the drawbacks is slow manufacturing and limited scalability [36]. The most typical applications are implants (Ti64, Co-Cr-Mo [50], - ceramic [51]) and prostheses (Co–Cr alloys, CP Ti, and Ti–6Al–4V [52]). Selective laser sintering (SLS) [53], [54], [55], [56], [57], electron beam fusion (EBM) [58], [59] and selective laser melting (SLM) [60], [61] belong to this category. Regarding the innovations based on this category, some areas need further development, such as 3DP of porous metallic structures used as implants in the body. Studies showed that the pores' size and shape influence the organism's cellular response, including the differentiation of osteoblasts and bone structure [62]. Additionally, in the pharmaceutical field, the development of sintering drug methods is considered, which has allowed the creation of release profiles that could be defined based on changes in the laser scanning speed [63].
2.3. Vat photopolymerization (VP)
For this methodology, the material used must be in a liquid state. Principal materials applied in this category are polymer, second place ceramics, and metals are not the best option for this process [33]. A liquid photopolymer is placed inside a vat or tank and cured selectively by a heat source, either by lasers or digital light projectors, due to their low-cost and very high resolution [64]. The main advantages in this category are the high speed of construction and excellent surface finish. However, 3D parts manufactured in this category have a short service life and deficient mechanical characteristics [36]. Its typical medical applications are manufacturing implants (resins [65]), orthosis (polyurethane acrylic [66]). Stereolithography (SLA) [67], [68], [69], digital light processing (DLP) [60], [70], [23], continuous liquid interface production (CLIP) [71], [72] and liquid crystal display (LCD) [73], [74] belong to this category of AM processes. In a matter of advances applied to this methodology, there is interaction development between equipment with sensors and biocompatible resins to generate response systems; this gives way to creating more complex systems, providing new fields and improvements in equipment response times [75]. About a prosthesis, components manufactured in bio-ceramics have been developed, which have shown excellent results with good biocompatibility with the organism for its application and excellent mechanical properties according to its application [76].
2.4. Binder jetting (BJ)
This process uses the material in powder form, which used to be polymers, such as the preferred alternative. Metals are used less often, and ceramics are much less [77]. A powder-based material and a binder that acts as a bonding agent between thin material layers form the 3D part. Its main advantages are low manufacturing cost, varied material options, and printing speed. However, it produces parts with limited mechanical properties [36]. Typical medical applications include the manufacture of implants (Diethylene Glycol-Ethylene Glycol, NP 316 SL [78]) and surgical instruments (Z151, PMMA [42]). Through this 3DP process, it has been possible to enter the pharmaceutical field, allowing the manufacture of controlled drugs; this means that the speed of elimination can be managed by modifying how it is available. The binder jetting has made it possible to create a drug's uniformity and manage a porous structure that offers rapid absorption, which means the physical form can be customized to control its dosing speed [79]. The article “Pharmaceutical application of powder-based binder jet 3DP process – A review” compiles results obtained in applying BJ in drug creation and modification. The results presented in this article show how It can be improved in engineering, cosmetic, pharmaceutical, and biomedical grounds through a new technology just starting to develop [80].
2.5. Material jetting (MJ)
The state of the material in this process must be liquid, with polymers being the predominant material in this category. Metals and ceramics are rarely used [33]. The process is similar to stereolithography, often compared to the standard 2D inkjet process. This process involves the selective deposition of a photopolymer and an initiator as a building material in droplets to form thin, cured layers that ultimately form the 3D part [81]. The resulting parts can be multi-material and have a good surface finish. However, they are usually limited to low-strength applications and require a highly controllable ink viscosity [36]. Potential applications include the fabrication of implants (ATZ ceramics [82]). While this process is used in various fields, it has been noted that its critical application lies in creating educational pieces for doctors to identify better a case concerning a patient [83].
2.6. Directed energy deposition (DED)
In order to initiate this process, wire or powder must be utilized as the material process. Metals are predominantly used in this category, while ceramics are seldom used, and polymers cannot be processed through this method [33]. The utilization of thermal energy through either electron beams or lasers is implemented to melt materials onto the workpiece layer by layer. Consequently, these technologies can produce high-quality 3D parts. However, their usage is restricted to metal powders or wire source materials. Another crucial aspect to consider in DED is the balance between surface quality and printing speed [36]. Typical medical applications include the manufacture of implants (Titaniums Alloys [84]; Ti–6Al–4V alloy [85]). The leading technology in this category is laser-engineered net shaping (LENS) [86], [87]. Developments have been observed in manufacturing bone implants, as the process allows for products with properties that mimic the bone's characteristics. In this case, the degree of porosity influences the production of osteoblasts. However, it has been discovered that the material's compatibility can affect bone development. Applying a coating function through DEP can solve this issue while maintaining the structure's effectiveness. These advancements open the possibility of creating joint implants without cement, which was conventionally done [88], [89].
2.7. Sheet lamination (SL)
In this category, sheets are utilized as the primary material, typically composed of polymer or metal, except for ceramics, which are manufactured through a differing process. However, it is worth noting that this category has yet to make significant progress or yield significant contributions within the medical field [33]. The manufacturing process involves stacking several layers of composite material through laminates to produce a 3D object. These sheets are cut using either a knife or laser and are relatively easy to handle and cost-effective, making it a process of high quality. However, the finished product's quality mainly depends on the adhesive used and may require further processing [36]. Typical medical applications include the manufacture of prostheses (PLA, CFRC [90]) and surgical instruments (sheets [28]). Among the technological advancements highlighted in this category are Laminated object manufacturing (LOM) and ultrasonic additive manufacturing (UAM) [91]. One noteworthy application for this category is creating CT scan image representations, enabling more detailed and precise body scans. Obtaining these images involves taking layered scans of the body, which are then ordered and compiled to produce a 3D patient model, thereby improving diagnostic conditions [92].
3. Medical applications of AM techniques
Clinicians have effectively utilized various 3D printing technologies to cover various medical applications [93]. These include implants, prostheses, orthoses, surgical instruments, prototypes [94], and other applications unrelated to the overview aim. Models produced through AM technology are handy for surgeons performing preoperative procedures that improve surgery success rates [95]. One of the most significant benefits of employing AM in this area is developing better planning before proceeding to the operation room. This technology eases the process of studying the patient's condition, enabling efficient planning that reduces time and indirectly mitigates risks to the patient. The principal advantages of this technology include the ability to predict potential problems during the surgical process and prepare alternative procedures or specialist materials to address any potential complications [96]. This section focuses on the most critical medical applications where AM technology is essential.
3.1. Implants
Implants are permanently or temporarily grafted devices into the body to assist, improve, or maintain its function or contour [97]. Applying AM in medical materials for implants offers several advantages, including reducing response time to meet patient needs. Reducing waiting time is significant since there is a shortage of available organ transplants, and compatibility between the patient and the new organ is often low. AM also offers the versatility of creating customized implants based on the specific medical situation. Moreover, the production cost of customized implants is more accessible than previous methods, making it easier to attend to the needs of patients. Scaffold implants also support structures and join body parts when the bone has experienced a fracture or a fissure. AM in producing these implants allows the adaptation of the plate's shape according to the area's requirements before surgery and promotes bone healing [96].
In the medical industry, AM techniques are increasingly crucial for the application of medical implants, especially since they allow for the realization of defined graded pores and filigree structures [82]. Furthermore, implants can be made more functional by implementing and controlling the local release of therapeutic products [98]. Powder Bed Fusion (L-PBF) technologies are promising techniques for the fabrication of implants [99] with complex internal architectures with small strut sizes [100], and optimization of the printing parameters is crucial to obtain a better performance of the implants [101]. Additionally, these technologies allow for the combination of different materials to find the ideal combination of composites that matches the mechanical properties of the implants with the surrounding bone and tissue [102]. Although titanium alloys are the most commonly used materials due to their biocompatibility properties, super plasticity, and corrosion resistance, they have a high elastic modulus leading to bone resorption [103]. Implant fabrics of titanium can be structured with biometric pores to achieve an elastic modulus similar to cortical bone [104], improving osseointegration, providing better antibacterial properties, and preventing future infections [105]. Another challenge is using biomaterials that allow osseointegration and absorption in the body without causing side effects [55]. For this reason, one challenge is using biomaterials that allow osseointegration and absorption in the body without causing side effects [106].
Functional biometric networks are attracting the interest of researchers and surgeons for application in the design of bone implants [107]. Recently, L-PBF technologies have been used for the first time to produce Schwarz [108], gyroid, and diamond [107] unit cell networks to improve the mechanical properties required for their application. Additionally, it is possible to reduce the excessive weight of printed implants using the SLM technique by depositing titanium alloy coating layers on the surface using the arc ion plating (AIP) system [109]. Among the recent applications using AM techniques are the creation of Temporo Mandibular Joint (TMJ) implants [110] and the reconstruction of a bilateral ramus-condyle unit (RCU) defect [111] using the SLM technique. Finally, the manufacture of anatomical tracheal stents, based on ABS molds, was developed by FDM, and a chemical post-treatment using 100% acetone vapors was applied to improve the surface finish [47]. A device that applies UV treatment of porous implants to improve osseointegration while preserving their original topography and other morphological characteristics was also presented [112].
3.2. Prostheses
Prostheses are artificial devices designed to replace body parts that have been amputated or have not developed properly due to congenital anomalies [13], [12]. In some instances, surgical intervention may be required to replace damaged parts of the patient's body, such as heart valve [14], dental [113] and pelvic prostheses [114]. Prostheses are advantageous because they can be customized according to the patient's condition, allowing for tailor-made solutions to personal shortcomings and improving manageability and comfort for the user. With the advent of AM, patients can now easily access personalized prostheses and receive prompt solutions for their complications [96].
By 2050, the number of amputees in the U.S. alone will be approximately 4.4 million, highlighting the critical need for new manufacturing techniques to improve their performance [115]. While conventionally manufactured prostheses offer simple gross motor functions, AM techniques like fused filament fabrication and fused deposition of filament offer improved design and functionality that closely mirror limbs in the human body [116]. Soft tissue prostheses can also be produced through AM technologies, such as the ocular epithesis fabricated by Vat photopolymerization for maxillofacial rehabilitation. With shorter production and consultation times and improved personalized results, AM offers significant advantages over traditional manufacturing techniques in prosthetic devices [8].
3.3. Orthoses
Orthoses are external devices used to correct or improve the use of a body part, typically for individuals with difficulties functioning in their arms or legs due to deficiencies or deformities. Unlike surgical interventions, orthoses can be fitted and removed by the patient [117], allowing for a more patient-centered approach. The advantage of this ground lies in the ability to achieve an excellent coupling with the patient. When designing a custom orthosis, it is crucial to fit the product appropriately to the geometry of the area, especially in cases of existing anomalies or deformities [96].
AM allows for the development of more economical and accessible custom orthoses. The most commonly used technique for designing orthoses using AM is FDM, where the choice of materials plays an important role. The most commonly used materials for orthoses are thermoplastics, and carbon fiber [118]. A recent study has demonstrated that carbon fiber-reinforced polymer (CFRP) is more efficient and sustainable than traditional materials for ankle-foot orthoses [18].
3D printing (3DP) can eliminate several steps associated with traditional ankle-foot orthosis (AFO) fabrication methods and provide the design freedom to custom-fit orthoses to individual patients [119]. For hand orthoses, a study has shown that ABS material printed using FDM [17], resulted in better mechanical performance than PLA. Furthermore, selective laser sintering (SLS) has been implemented to fabricate an orthosis for patients with spastic paralysis, significantly increasing hand functionality [120].
3.4. Surgical instruments
This component plays a critical role in healthcare systems as it serves as a tool to prevent, diagnose, treat, and rehabilitate diseases safely and effectively [34]. Its primary purpose is to enable medical personnel to improve procedures through appropriate instrumentation [96]. Benefits of this include reduced patient exposure time during a medical intervention, improved accuracy, and reduced failures due to inadequate instrumentation. It is crucial to note the material used in its manufacture as it must not be carcinogenic, pyrogenic, toxic, allergic, or inflammatory [121].
One of the most significant advantages of AM technologies in manufacturing surgical instruments is their ability to produce efficient devices that meet the increasing demands within hospital operating rooms, all while being low in production cost [34] and manufacturing time [122]. Through AM, surgical instruments used in inguinal hernia surgery were designed without conventional stainless steel tools [123]. In a recent study, 3DP impression allowed the production of space support for intraesophageal sutures. These results achieved higher intraesophageal suture placement, thus allowing better intraesophageal resection [124].
Numerous studies have proposed new devices, such as an articulated mechanism that mimics the functionality of fingers, producing more excellent mechanical stability while avoiding gauze and allowing for efficient surgery [125], [126], [127]. There was also a proposal for a 3D-printed branched-over tube system combined with conventional endoscopes and instruments [128].
AM has also allowed the design of smart steerable needles, the first bipolar steerable forceps, and a 3D-printed device for trans-anal endoscopic surgical procedures that provide adequate working space without inflating the rectum [129]. In summary, AM technology has contributed significantly to the medical field, providing innovative solutions and improved patient outcomes.
3.5. Prototypes
This section aims to create designs for educational purposes and innovative proposals. It encompasses surgical planning and academic study applications for future doctors, generating prototypes that allow solving problems and reaching conclusions appropriate for medical complications that can occur in an operating room or for concerns due to a lack of understanding during the university stage [96].
In the field of surgery, medical personnel must be prepared for different situations. While some interventions are common and do not require a significant difference in the procedure carried out between patients, sometimes, there are medical complications that require better study during the preoperative period. A prototype uses a CT patient scan, an image processor for modeling, and a 3D printer to prepare a surgical procedure according to the needed [130].
For academic purposes, it has been found that complex systems can be challenging to visualize during the university stage. Among the many alternatives that promote assistance in studying are virtual models, three-dimensional anatomical models, and books with detailed and updated information. Another option available is printing 3D models of cases, which allows medical students to comprehend better the dimensions of models with real or proportional scales. Training with phantom models enables them to visualize different intern anatomic structures and the impression of complete, partial, or isolated systems of the areas of interest the student seeks to reinforce and understand [131].
4. Latest trends in AM applied to medicine
4.1. Bioprinting
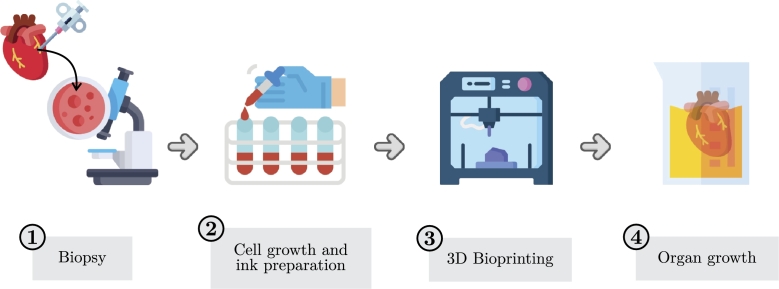
Bioprinting has experienced substantial growth and demonstrated remarkable potential for various biomedical applications [132]. Bioprinting allows for creation of complex 3D architectures incorporating living cells using bio-inks, such as stem cells, tissues, and organs suitable for human use [133]. This technology can eliminate the need for transplants from living donors or cadavers and even regenerate organs and tissues [132]. The process of bioprinting, illustrated in Fig. 2, begins with a biopsy of a real organ and employs technologies classified into four categories: extrusion-based, droplet-based, SLA-based, and laser-assisted [134], [135]. Although laser-based inkjet bioprinters are best suited for high resolution and precision positioning, extrusion-based, SLA-based, and droplet-based bioprinters enjoy more widespread use due to their cost-effectiveness and versatility [136], [137].
Figure 2.
Schematic illustration of the steps for 3D bioprinting.
While significant progress has been made in developing 3D bioprinting, several challenges remain, particularly in the context of living cells in printed structures [138], [139]. Bio-ink must be cytocompatible, constituting a critical barrier to practical and clinical progress in bioprinting [133]. Despite this, researchers and doctors continue to focus on future projections in the health area, with significant advances reported recently in skin modeling using 3D bioprinting methods compatible with human skin [140]. However, bioprinting applications in respiratory sciences have been relatively limited, with only a few studies developing tracheal grafts and lung-on-a-chip models [141], [142]. Bioprinting has proven incredibly useful in tissue engineering, regenerative medicine, and artificial organ printing. It is clear that 3D bioprinting has opened new frontiers in the biomedical field and can potentially bring about significant advances in modern medicine [143].
4.1.1. Applied bioprinting in tissue engineering/regenerative medicine
Human bones can heal and regenerate from minor injuries and fissures. However, this capacity is lost when bone defects or injuries exceed a critical size of 10 mm [144], [145], [146]. Approximately 2.2 million bone transplants are performed worldwide annually, resulting in a surgical cost of approximately $2.5 billion [30]. Thus, there is an increasing interest in studying new techniques and technologies to improve results and reduce costs. One such technique is tissue engineering (TE), where living cells are cultured and seeded into biocompatible scaffolds to fabricate implants that enable tissue regeneration [147]. It is important to note that advances in 3D bioprinting techniques have been instrumental in developing and evolving tissue engineering [133]. Biodegradable bone implants show promising potential for repairing bone defects, with the ideal implant degrading at a rate proportional to new bone formation [148]. However, optimal TE scaffold performance manufactured with AM technologies is still being pursued [30].
An ideal TE scaffold should possess appropriate mechanical properties and a structure with high interconnected porosity to enhance protein absorption and bone tissue growth. These structure types promote biocompatibility, degradability, osteoconduction, osteoinduction, and permeability [30]. Ensuring cytocompatibility of the biotin is a fundamental prerequisite for effective bone tissue regeneration. Amorphous magnesium phosphate (AMP)-based bio-ink has been shown to form bone tissue in regenerative dentistry applications [149].
The 3D bioprinting technique is used to overcome vascularization challenges and effectively construct vascularized and osteogenetic tissues [150]. For example, 3D bioprinting has been used to fabricate liver tissue, mimicking the microstructure and microenvironment of the liver model [151]. In another significant achievement, tissue engineering was used to design the world's first synthetic trachea, resulting in the first human airway transplant. Researchers describe this achievement as “the end of the beginning of tissue engineering” [152]. The most crucial aspect of tissue engineering and regenerative medicine will be the development of innovative and more printable biomaterials for scaffolds, implants, and other products [133].
4.1.2. Artificial organs
According to statistics on organ donation, it has been reported that in the United States alone, approximately 104,000 patients are currently waiting for an organ transplant. Every day, about 17 people die while waiting for an organ transplant [153]. The organs in highest demand and priority for manufacturing are the liver, heart, and kidney [154]. The extensive waiting list for receptive organs is a cause of great concern and challenge in the medical sector. These problems have been a strong incentive to improve AM techniques and technologies in organ printing to achieve products that more closely mimic natural tissues [133].
AM uses different 3D printing techniques for organ fabrication, such as fused deposition modeling (FDM), which falls under the category of material extrusion and is commonly used due to its low cost and moderate flexibility [24]. However, the inkjet technique is also applicable. The successful fabrication of artificial organs using 3D printing technology depends on the appropriate selection of biomaterials with specific characteristics. Biocompatibility is the most crucial factor, as the material must not elicit an immune response or cause tissue rejection when implanted in the human body. This property ensures that the biomaterial can adequately respond in the human body during a specific situation [155]. In addition to biocompatibility, excellent mechanical properties are also essential for biomaterials, as they must possess mechanical stability and be harmless to function as a natural substitute [156]. Moreover, porosity is another critical factor that allows tissue growth and promotes specific interaction with the available surface area's target molecules, ensuring that the organ is functional and can perform its intended purpose [157].
3D bioprinting as a method for manufacturing artificial organs has managed to overcome certain drawbacks to meet the needs of patients [158]. This technology provides the opportunity to make implantable organs developed from the recipients' cells themselves, thereby reducing the likelihood of rejection of the manufactured organ [159]. Therefore, AM technology and bioprinting are considered appropriate technologies to meet the challenges of complex vascularized tissue manufacturers [160]. However, organ manufacturing has certain limitations, such as the lack of diversity of biomaterials [161] and limitations of 3D printers in speed and resolution. It is possible to achieve a resolution of 200 μm - 400 μm using microfilament extrusion, as inkjet-based prints only achieve a resolution of 20 μm to 100 μm. Furthermore, high shear stresses cause damage to the bio-ink cells [133]. Another important drawback is that long human trials are usually required before the transplantation of these organs, which can be risky in most cases.
4.2. 4D printing: definition, advantages and applications
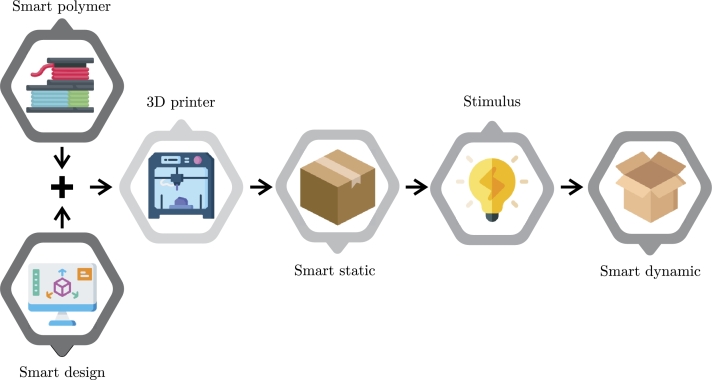
This method is a variant of 3D printing that incorporates smart materials (SM) capable of self-transforming when exposed to external stimuli, such as thermal, electrical, magnetic, or mechanical. A smart design is created that programs the behavior of SM following a particular stimulus. This design enables understanding how the product changes shape, making it possible to consider the printing process. The printed product has more than one shape, with defined characteristics that can be altered by a stimulus, changing its physical form or properties [162].”4D” refers to the time dimension, signifying that the product changes shape after printing due to the stimulus. Fig. 3 illustrates the schematic representation of the 4DP process; the 4DP process comprises five main factors: material, stimulus, AM printing process, modeling, and interaction mechanism [163].
Figure 3.
Schematic presenting the phases of 4D printing [164].
The product properties obtained using 4DP have numerous applications, but in medicine, the technique can help repair, regenerate, and replace diseased and damaged cells, tissues, and organs [165], [166]. This technique assumes that tissues and organs produced using 3D bioprinting should remodel and mature over time, like human body cells [167], to adjust their properties to achieve the required functionality [168]. 4DP has given AM more influence in the medical sector in recent years than 3D technology [169], [170]. This technique has changed lives, showing promising results in patient studies, as it provides opportunities to produce complex dynamic structures with high resolution, which are inaccessible through 3DP [171]. 4D-printed tissues and organs are expected to function naturally in the body after transplantation. Still, the proper selection of SM is required, just like cell-free printed structures such as prostheses or biomedical implants. However, this technology still requires technical advances in several disciplines in the long term, involving software, modeling, kinematics, and biochemistry [172].
Recent studies have shown promising results of 4D bioprinting applications for tissue creation, with the potential to mimic complex movements of native tissues in the body, such as the heart [173]. However, selecting appropriate bio-inks for bioprinting is a challenge since the viscosity of cell-loaded bio-inks must be low to avoid shear stress during the printing process and prevent cell damage [174], [175].
4.3. 5D printing: definition, advantages and applications
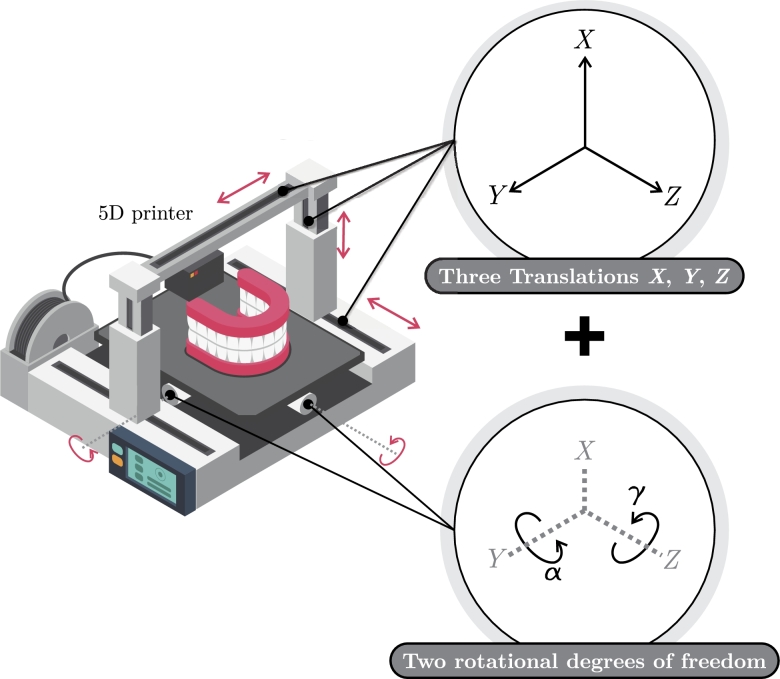
The concept of 5D printing is considered a recent development in 3D printing [176]. This technology presented the ability to print 3D objects with concave or curved shapes with high precision. Two additional axes were incorporated to achieve the printer process, namely the rotation of the printing platform and the extruder head, and this new methodology was termed 5DP [177]. 3D and 5DP techniques deploy similar technologies, such as using 3D CAD files and the same printing materials [178].
Standard 5DP procedures consist of three phases: pre-printing, printing, and post-printing, which must be validated and checked for model adherence to ensure the desired outcomes. Key considerations include requirements, model orientation, trajectory generation, printing process analysis, and digital model adherence [179]. A diagram of the 5D printer is presented in Fig. 4.
Figure 4.
Schematic of 5D printing.
There are numerous advantages to using 5DP technology, including printing curved or concave layers with high resolution and precision and an overall strength of approximately three to five times greater than 3D printed items. It is expected to overcome limitations in 3D and 4DP technologies [177]. For example, a concave lid is difficult to 3D print, as it requires numerous fillers, support, and a complex design, easily overcoming obstacles with 5DP [180]. 5DP can save up to 20% to 30% of printing materials for curved or concave surfaces. However, 5DP printing has disadvantages, namely the added expense of two more axes and the need for highly-skilled personnel to operate and maintain 5D printers [181].
Looking toward future developments in 5DP, exist the possibility of integrating machine learning (ML) and artificial intelligence (AI) to generate the intelligent use of materials to create multifunctional, ecological, and biocompatible components [182]. As a novel manufacturing process, 5D technology is set to overcome limitations in the medical sector [183], such as increasing the hardness of the manufactured material and precision required in dentistry and offering the potential to manufacture artificial bones with complex curves and exceptional strength for use in surgery [181]. In tissue engineering, 5PD can create bio-patches that identify specific infections through design or be used to deliver drugs [182]. The growth of 5DP in pharmaceutical applications is on the horizon, with potential growth within the next year [184]. Bioprinting applications demonstrate the superior results of 5DP technology, as bio-inks have cell encapsulation, which negatively affects the mechanic product printed properties, while 5DP produces quality products that maintain cellular properties [185]. Quality is important in orthopedic applications to achieve the desired objective of obtaining strong and complex properties [176].
4.4. 6D printing: definition, advantages and applications
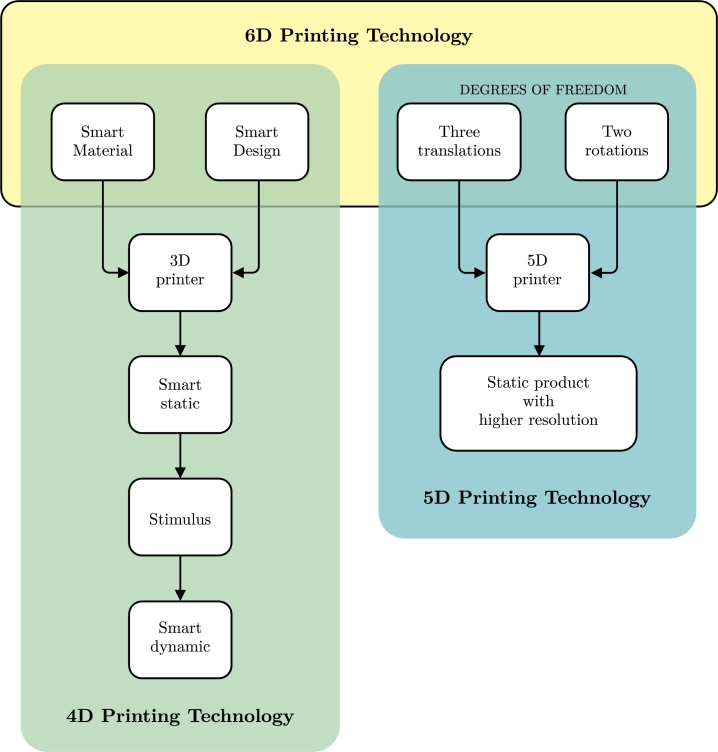
This innovative printing method is a result of merging 4DP and 5DP. It uses five degrees of freedom to produce the final object, requiring the designer to create a smart design that considers the properties and changes involving shape memory (SM). This material can modify its shape or properties in response to environmental stimuli [178]. Fig. 5 illustrates a schematic diagram explaining the 6DP process. The objects manufactured through this technique are expected to be stronger and of higher quality than those produced using previously mentioned methods, resulting in reduced printing time and material savings.
Figure 5.
6D printing as the combination of 4DP and 5DP.
Furthermore, printed structures or objects made using 6DP are expected to exhibit intelligent behavior with unique stimulus-response features. The intelligence of the materials can improve through the integration of computational optimization approaches [178]. Additionally, 6DP can support using single-phase polymers and nano-reinforced polymers as raw materials [186], [187], thus increasing the design complexity. As a result, the cost of the 6DP process will likely be high and contingent on the production batch size and automation level involved [178]. However, its main limitations include additional calibration, standardization requirements, and a higher setup cost. Similar to those involved in the 5DP process, skilled personnel are required for this printing technique [188].
One potential application of this technology in orthopedics is the production of “smart casts” that exert appropriate mechanical corrective loads in specific areas to maintain perfect fracture alignment while providing comfort to patients [189]. This behavior is achievable through SM, which can change shape, creating more space or narrowing it depending on the presence or absence of edema in the affected limb. Smart medical implants can treat complex medical cases using SM and the structural enhancements provided by printing with five degrees of freedom. Bone printing can achieve excellent results due to its ability to replicate the characteristic curve bone shape, while parts that need specific behavior modifications through stimulation can be printed with a specific SM [188]. In addition, printing bio-inks with 6DP can enhance their production process, using SM-based materials with better mechanical properties through 5DP, resulting in a higher quality of tissues with programmable behavior [185].
Table 2 presents a compilation of notable advancements that are considered representative due to their potential for future innovations in the respective areas where they are being developed. The table provides information on the application, printing process, materials used, and a brief summary of the findings from the referenced documents.
Table 2.
A summary of applications for latest AM technologies.
| Latest Trend | Application | Method | Material | Reference |
|---|---|---|---|---|
| Bioprinting |
Implant |
ME |
Collagen |
Lee et al. (2019) create engineer components of the human heart at various scales, from capillaries to the entire organ [190]. |
| Irgacure PEGDA Sodium alginate |
Lan li et al. (2021) repair of long segmental bone defects in situ [191]. |
|||
| Ecoflex-0030 PDMS-1700 |
Zhou et al. (2021) present a high printing methodology accuracy in situ by a superimposed magnetic field for internal organs [192]. |
|||
| ME/PBF |
PLA PA 12 |
Capel et al. (2019) proposed a methodology to generate reproducible and scalable tissue-engineered primary human muscle [193]. |
||
| Tissue reconstruction |
ME |
Sodium alginate Gelatine Fibrinogen |
Dai et al. (2017) proposed a method to fabricate self-assembled multicellular heterogeneous brain tumor fibers to study their behavior with an organic matrix [194]. |
|
| Alginate PVA |
Luo et al. (2017) produce porous scaffolds to promote water absorption and manipulate mechanical properties [195]. |
|||
| Gelatin Glycerol |
Rodriguez et al. (2017) develop a material system to provide structural support during reconstruction process to soft tissue [196]. |
|||
| Ambulatory procedures | ME | Nanocellulose | Rees et al. (2015) Create wound dressing with antibacterial properties [197]. and porous structure | |
| 4DP |
Implants |
ME |
PLA |
Lin et al. (2021) developed a patient-specific absorbable left atrial appendage occluder (LAAO) that can match the tissue deformation of the left atrial appendage (LAA) [198]. |
| Smart Material Development |
Castro et al. (2017) developed multifunctional smart materials applied in bioprinting [173]. |
|||
| BDE PBE DA Graphene nanoplatelets |
Cui et al. (2019) built a brain model of near-infrared light (NIR) to evaluate the capacity for controllable 4D transformation and the feasibility of photothermal stimulation for modulating neural stem cell behaviors [199]. |
|||
| VP |
PCL |
Zarek et al. (2017) fabricated a printable shape memory endoluminal device with a series of medical imaging modalities [200]. |
||
| Prototype |
ME |
DBBM BDM n-butylamine |
López et al. (2018) developed large-scale structured elements with prebuilt orientation to increase the work they can do in a robotic application [201]. |
|
| Tissue reconstruction |
VP |
SOEA Acetone BTMP |
Miao et al. (2016) tested soybean oil epoxidized acrylate how a novel and renewable liquid resin to biomedical scaffolds highly biocompatible [202]. |
|
| GO Carbon Porous Nanocookies 4-HBA PU-EO-PO Irgacure 819 |
Fang et al. (2020) proposed a process to induce magnetoelectric conversion for growth factor release and cell stimulation for enhanced neuronal cell activation and proliferation in vitro and in vivo [203] | |||
| 5DP |
Implant/ Tissue reconstruction |
ME |
Alginate |
Foresti et al. (2020) developed devices with a low impact on cell death nano-laden with fluorescent particles applied in scaffolds and high-resolution self-dissolving incorporating nanoparticles and interacting organ physiology where it will be used [179]. |
| Prototypes | VP | ND | Gillaspie et al. (2016) show a procedure to analyze a patient state with a complex thoracic tumor scan and print for surgical planning [204]. | |
| 6DP | Implants | Propose projection for future applications | Haleem et al. (2018) propose a method to create implants with high resolution due to the use of multi-degree of freedom and a printing biomaterial that reply body system organic behavior [176]. | |
| Legend | PEGDA (Poly(ethylene glycol) diacrylate), PDMS (Polydimethylsiloxane), PLA (Polylatic Acid), PA-12 (Polyamide-12), PBE (poly(propylene glycol) bis(2-aminopropyl) ether), DA (decylamine), BDE (Bisphenol A diglycidyl ether), PCL (Polycaprolactone), DBBM (diacrylate 1,4-bis-[4-(6-acryloyloxyhexyloxy)benzoyloxy]-2-methylbenzene), BDM (2-benzyl-2-(dimethylamino)-4-morpholinobutyrophenone), SOEA (soybean oil epoxidized acrylate), BTMP (bis(2,4,6-trimethylbenzoyl)-phenylphosphineoxide), ND (No defined) | |||
5. Discussions
This overview aims to gather information on the latest trends in AM and its applications in the medical field, where diverse outcomes have been obtained. Through literature research, the present document has been made within the updated information on bioprinting, 4DP, 5DP, and 6DP. However, it is worth noting that, to date, there have been limited substantial contributions towards using 5DP and 6DP technologies in medical settings, with only minor exceptions. The present section has a double purpose. The first is to address the different perspectives when discussing the aforementioned technologies; and the second is to discuss the direction of new trends. It is important to mention that the authors have not considered certain fields in the medical areas. The pharmaceutical sector focuses on developing medicines with complex geometries and materials with multiple release profiles. The AM processes used for drug fabrication are mentioned in this document. Most printed drugs product depends on the material selected, its characteristics, and how it is administered to the body [205]. Research in food engineering has focused on control applications, with future proposals for 4DP, 5DP, and 6DP technologies [185]. The main objective of using AM for food production is to manipulate the structural design of food, which can impact nutrition and texture, particularly for individuals with special dietary requirements due to medical conditions [206]. Although these fields were not the principal focus of the article, they are mentioned, given their importance and relationship to medical practices, as they involve interaction with the human body.
5.1. Multi-dimensional, multi-axis, and multi-material vs. 4DP, 5DP and 6DP
During the development of the bibliographical foundation of the document, using the methodology with the chosen search criteria, it was discovered that some authors had differing opinions on how particular technologies were addressed. In certain documents, terms such as “multi-axis”, “multi-dimensional”, and “multi-material” were used when referring to 4DP, 5DP, and 6DP technologies [207]. It must be clarified that a significant scientific faction adopted the “X” DP terminology. However, it is not a correct term because the final product is not printed using 4, 5, or 6 dimensions. Instead, it is printed with three dimensions, such as all-manufactured products. The confusion arises because printers use axis systems that provide various degrees of freedom (DOF). A traditional 3D printer has only three DOF since it has three axes: X, Y, and Z. Technological advancements have led to printers with more DOF, with models featuring four, five, or even six DOF. Notably, multi-dimensional can refer to any process involving more than two DOF. This can refer to anything with multiple movement dimensions, which should be considered during the search process.
Concerning the usage of SM technology, multi-material processes present a similar problem when dealing with terminology. While 4DP involves using SM to create an intelligent print that can change shape with time based on external stimulation, so-called multi-material processes may require explicit clarification as they tend to generate confusion. A 4DP process can be classified as multi-material, but not the other way around due to multi-material processes related to the number of materials utilized during the printing process, whether traditional or SM, and the demand for at least two materials. For example, a process with a multi-material (2 or more) and multi-axis/multi-dimensional (2 axes or more) system can generate products. However, it can still be a widely known 3D commercial product since it may feature two traditional materials and create with 3 DOF.
Nevertheless, information collected for the present manuscript was based on the nomenclature that has become widely accepted in the scientific domain. While 4DP, 5DP, and 6DP are non-technical names, they are widely recognized as such, so for developing a new trends section, they will be treated as such. While the technical terminology was sparsely based, concerning references compared to commercial terminology, readers may seek multi-axis or multi-dimensional alternatives to refer to the DOF. At the same time, multi-material can be used to uncover mixtures of traditional materials with SMs leading to shape-changing products.
5.2. Projections and new trends
The latest advancements in AM have demonstrated high effectiveness as potential solutions to the current limitations in medical interventions. Extensive research has been conducted, providing opportunities for future applications based on the outcomes of ongoing experiments. Recent trends have shown an increased interest in enhancing medical procedures with the emergence of new technologies. Noteworthy efforts are being made to develop materials that generate new studies, leading to the discovery of novel applications.
5DP and 6DP technologies have contributed little due to their relative novelty. However, they are being utilized to develop methodologies and approaches that meet medical requirements as per the need. It must be underscored that innovation's raw material component holds great significance, particularly in areas such as implants and tissue engineering. Developing an impression material analogous to the body's requirements concerning biocompatibility, mechanical resistance, and response to stimuli is the most challenging aspect of these research areas.
Therefore, 2020 and 2021 witnessed an increased interest in the ME fields, while new materials for VP were developed, resulting in a surge in their usage in 2022, far surpassing the primary one in subsequent years. The increase was confirmed by expanding the bibliographic base, as the most abundant content was focused on preparing and creating biocompatible matter for use in organisms.
6. Conclusions
Over the last decade, AM has experienced significant growth in various areas, particularly in the production of complex parts utilized in the medical field. AM has advantages over traditional manufacturing processes, including the reduction of material and manufacturing time, as well as the ability to create parts with different strengths and functionality. Although 3D printing is considered a mature process due to its many years of development, there are still limitations that scientists and doctors are trying to address by incorporating new printing techniques and materials. These new proposals have led to emerging technologies such as bioprinting, 4DP, 5DP, and 6DP for new applications.
One of AM's most significant achievements has been the incorporation of 4DP. This process allows organs and implants to react similarly to the human body by using shape memory materials (SM) that respond to external stimuli. Despite significant progress in 4DP, SM is still limited to responding to only a few stimuli. Consequently, bioactive structures that mimic human tissues have not yet been achieved. However, ongoing projects aim to create new alternatives to achieve such similarities. Meanwhile, efforts are being made to improve bio-ink advancements to enhance the material's interaction with the human body, improving coupling.
Technologies such as 5DP and 6DP are still in their early stages of development for medical applications. Nevertheless, efforts are focused on creating solutions systems for real problems. The reviewed documentation underscores the advances in prostheses, implants, and orthoses are increasingly promising and result in optimally created complex products. Therefore, it is vital to develop bibliographic reviews to visualize the development of each field and measure and highlight the growth of the process, its qualities, applications, and effectiveness.
CRediT authorship contribution statement
Jorge L. Amaya-Rivas: Writing – review & editing, Investigation, Conceptualization, Methodology, Writing – original draft. Bryan S. Perero: Methodology, Writing – review & editing, Conceptualization, Visualization. Carlos G. Helguero: Visualization, Writing – review & editing, Conceptualization. Jorge L. Hurel: Writing – review & editing, Validation, Supervision, Methodology, Writing – original draft. Juan M. Peralta: Writing – review & editing, Writing – original draft. Francisca A. Flores: Validation, Writing – review & editing. José D. Alvarado: Writing – original draft.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Jorge L. Amaya-Rivas, Email: jlamaya@espol.edu.ec.
Bryan S. Perero, Email: bperero@espol.edu.ec.
Carlos G. Helguero, Email: chelguer@espol.edu.ec.
Jorge L. Hurel, Email: jhurel@espol.edu.ec.
Juan M. Peralta, Email: jperal@espol.edu.ec.
Francisca A. Flores, Email: ffloresn@espol.edu.ec.
José D. Alvarado, Email: jodaalva@espol.edu.ec.
Data availability
No data was used for the research described in the article.
References
- 1.Hull C.W. 1984. Apparatus for production of three-dimensional objects by stereolithography. United States Patent, Appl., No. 638905, Filed. [Google Scholar]
- 2.Rezvani Ghomi E., Khosravi F., Neisiany R.E., Singh S., Ramakrishna S. Future of additive manufacturing in healthcare. Curr. Opin. Biomed. Eng. 2021;17 [Google Scholar]
- 3.Kumar R., Kumar M., Chohan J.S. The role of additive manufacturing for biomedical applications: a critical review. J. Manuf. Process. 2021;64:828–850. [Google Scholar]
- 4.Charbonnier B., Hadida M., Marchat D. Additive manufacturing pertaining to bone: hopes, reality and future challenges for clinical applications. Acta Biomater. 2021;121:1–28. doi: 10.1016/j.actbio.2020.11.039. [DOI] [PubMed] [Google Scholar]
- 5.Hajare D.M., Gajbhiye T.S. Additive manufacturing (3d printing): recent progress on advancement of materials and challenges. 1st International Conference on Physics of Materials and TechnologyMater. Today Proc. 2022;58:736–743. [Google Scholar]
- 6.December 2022. A. S. of Mechanical Engineers (ASME), “Additive manufacturing 3d printing year in review 2022,” AM Medical, New York, NY, Report of AM contributions to medical field. [Google Scholar]
- 7.Jha M.K., Gupta S., Chaudhary V., Gupta P. Material selection for biomedical application in additive manufacturing using topsis approach. Mater. Today Proc. 2022 [Google Scholar]
- 8.Miechowicz S., Wojnarowska W., Majkut S., Trybulec J., Pijanka D., Piecuch T., Sochacki M., Kudasik T. Method of designing and manufacturing craniofacial soft tissue prostheses using additive manufacturing: a case study. Biocybern. Biomed. Eng. 2021;41(2):854–865. [Google Scholar]
- 9.Leite M., Soares B., Lopes V., Santos S., Silva M.T. Design for personalized medicine in orthotics and prosthetics. 29th CIRP Design Conference 2019, 08-10 May 2019, Póvoa de Varzim, PortgalProc. CIRP. 2019;84:457–461. [Google Scholar]
- 10.Kalyan Chakravathy Y., Prasad Paladagu R., Sai Ram Gopisetti N., Dinesh Reddy D., Srinath A. Prototyping evaluation of prosthetic leg: a case study. International Conference on Advances in Materials, Manufacturing and Applied SciencesMater. Today Proc. 2019;16:463–469. [Google Scholar]
- 11.an Jin Y., Plott J., Chen R., Wensman J., Shih A. Additive manufacturing of custom orthoses and prostheses – a review. cIRP 25th Design Conference Innovative Product CreationProc. CIRP. 2015;36:199–204. [Google Scholar]
- 12.Rochlitz B., Pammer D., Kiss R. Functionality and load-bearing analysis of 3d-printed prosthetic feet. Mater. Today Proc.; 19 - 22 September 2017, Trieste, Italy; 2018. pp. 26566–26571. [Google Scholar]
- 13.Neethan R., Nidershan S., Mugilgeethan V., Tharsika T., Anburuvel A. A study of three-dimensional (3-d) printed prosthetic upper limb models in local context. Mater. Today Proc. 2020;23:8–11. advanced Materials for Clean Energy and Health Applications (AMCEHA), University of Jaffna, Jafna, Sri Lanka, 6-8 February, 2019. [Google Scholar]
- 14.Coulter F.B., Schaffner M., Faber J.A., Rafsanjani A., Smith R., Appa H., Zilla P., Bezuidenhout D., Studart A.R. Bioinspired heart valve prosthesis made by silicone additive manufacturing. Matter. 2019;1(1):266–279. [Google Scholar]
- 15.Ackland D., Robinson D., Lee P.V.S., Dimitroulis G. Design and clinical outcome of a novel 3d-printed prosthetic joint replacement for the human temporomandibular joint. Clin. Biomech. 2018;56:52–60. doi: 10.1016/j.clinbiomech.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 16.Totah D., Kovalenko I., Saez M., Barton K. Manufacturing choices for ankle-foot orthoses: a multi-objective optimization. 3rd CIRP Conference on BioManufacturingProc. CIRP. 2017;65:145–150. [Google Scholar]
- 17.Lokesh Kumar M., Dhanush Babu R., Sherwin Robert R., Veezhinathan M. Evaluation of 3d printed customizable hand orthosis for forearm fractures based on finite element modelling. Mater. Today Proc. 2022 [Google Scholar]
- 18.Ali M.H., Smagulov Z., Otepbergenov T. Finite element analysis of the cfrp-based 3d printed ankle-foot orthosis. 5th International Conference on Computer Science and Computational Intelligence 2020Proc. Comput. Sci. 2021;179:55–62. [Google Scholar]
- 19.Dal Maso A., Cosmi F. 3d-printed ankle-foot orthosis: a design method. 35th Danubia Adria Symposium on Advances in Experimental MechanicsMater. Today Proc. 2019;12:252–261. [Google Scholar]
- 20.Barba D., Alabort E., Reed R. Synthetic bone: design by additive manufacturing. Acta Biomater. 2019;97:637–656. doi: 10.1016/j.actbio.2019.07.049. [DOI] [PubMed] [Google Scholar]
- 21.Zheng J., Zhao H., Ouyang Z., Zhou X., Kang J., Yang C., Sun C., Xiong M., Fu M., Jin D., Wang L., Li D., Li Q. Additively-manufactured peek/ha porous scaffolds with excellent osteogenesis for bone tissue repairing. Composites, Part B, Eng. 2022;232 [Google Scholar]
- 22.Subramaniyan M., Eswaran P., Appusamy A., Srimannarayana Raju P., Rahini V., Madhumitha T., Thisha R. A survey on applications of additive manufacturing techniques in tissue engineering. 2nd International Conference on Materials, Manufacturing, and Machining for Industry 4.0Mater. Today Proc. 2021;45:8036–8040. [Google Scholar]
- 23.Mao Q., Wang Y., Li Y., Juengpanich S., Li W., Chen M., Yin J., Fu J., Cai X. Fabrication of liver microtissue with liver decellularized extracellular matrix (decm) bioink by digital light processing (dlp) bioprinting. Mater. Sci. Eng. C. 2020;109 doi: 10.1016/j.msec.2020.110625. [DOI] [PubMed] [Google Scholar]
- 24.Javaid M., Haleem A. 3d printed tissue and organ using additive manufacturing: an overview. Clin. Epidemiol. Glob. Health. 2020;8(2):586–594. [Google Scholar]
- 25.Javaid M., Haleem A. Current status and applications of additive manufacturing in dentistry: a literature-based review. J. Oral Biol. Craniofac. Res. 2019;9(3):179–185. doi: 10.1016/j.jobcr.2019.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang Tu C., Tsai P.-I., Chen S.-Y., Kuo M.Y.-P., Sun J.-S., Chang J.Z.-C. 3d laser-printed porous ti6al4v dental implants for compromised bone support. J. Formos. Med. Assoc. 2020;119(1, Part 3):420–429. doi: 10.1016/j.jfma.2019.07.023. [DOI] [PubMed] [Google Scholar]
- 27.Wally Z.J., Haque A.M., Feteira A., Claeyssens F., Goodall R., Reilly G.C. Selective laser melting processed ti6al4v lattices with graded porosities for dental applications. J. Mech. Behav. Biomed. Mater. 2019;90:20–29. doi: 10.1016/j.jmbbm.2018.08.047. [DOI] [PubMed] [Google Scholar]
- 28.Culmone C., Smit G., Breedveld P. Additive manufacturing of medical instruments: a state-of-the-art review. Addit. Manuf. 2019;27:461–473. [Google Scholar]
- 29.Zhonghua S. Patient-specific 3d-printed models in pediatric congenital heart disease. Children. 2023;10(2):319. doi: 10.3390/children10020319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qu H. Additive manufacturing for bone tissue engineering scaffolds. Mater. Today Commun. 2020;24 [Google Scholar]
- 31.Astm I. Astm52900-15 standard terminology for additive manufacturing—general principles—terminology, vol. 3. ASTM Int. 2015;3(4):5. West Conshohocken, PA. [Google Scholar]
- 32.Daminabo S., Goel S., Grammatikos S., Nezhad H., Thakur V. Fused deposition modeling-based additive manufacturing (3d printing): techniques for polymer material systems. Mater. Today Chem. 2020;16 [Google Scholar]
- 33.Salmi M. Additive manufacturing processes in medical applications. Materials. 2021;14:191. doi: 10.3390/ma14010191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li C., Pisignano D., Zhao Y., Xue J. Advances in medical applications of additive manufacturing. Engineering. 2020;6(11):1222–1231. [Google Scholar]
- 35.Haryńska A., Kucinska-Lipka J., Sulowska A., Gubanska I., Kostrzewa M., Janik H. Medical-grade pcl based polyurethane system for fdm 3d printing—characterization and fabrication. Materials. 2019;12(6):887. doi: 10.3390/ma12060887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dommati H., Ray S.S., Wang J.-C., Chen S.-S. A comprehensive review of recent developments in 3d printing technique for ceramic membrane fabrication for water purification. RSC Adv. 2019;9:16869–16883. doi: 10.1039/c9ra00872a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Honigmann P., Sharma N., Okolo B., Popp U., Msallem B., Thieringer F.M. Patient-specific surgical implants made of 3d printed peek: material, technology, and scope of surgical application. BioMed Res. Int. 2018;2018 doi: 10.1155/2018/4520636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Igawa K., Mochizuki M., Sugimori O., Shimizu K., Yamazawa K., Kawaguchi H., Nakamura K., Takato T., Nishimura R., Suzuki S., Anzai M., Chung U.-I., Sasaki N. Tailor-made tricalcium phosphate bone implant directly fabricated by a three-dimensional ink-jet printer. J. Artif. Organs. 2006;9(4):234–240. doi: 10.1007/s10047-006-0347-y. [DOI] [PubMed] [Google Scholar]
- 39.Rivet I., Dialami N., Cervera M., Chiumenti M., Valverde Q. Mechanical analysis and optimized performance of g-code driven material extrusion components. Addit. Manuf. 2023;61 [Google Scholar]
- 40.Rezaie F., Farshbaf M., Dahri M., Masjedi M., Maleki R., Amini F., Wirth J., Moharamzadeh K., Weber F.E., Tayebi L. 3d printing of dental prostheses: current and emerging applications. J. Compos. Sci. 2023;7(2) doi: 10.3390/jcs7020080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sakib-Uz-Zaman C., Khondoker M.A.H. Polymer-based additive manufacturing for orthotic and prosthetic devices: industry outlook in Canada. Polymers. 2023;15(6) doi: 10.3390/polym15061506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Salmi M. Possibilities of preoperative medical models made by 3d printing or additive manufacturing. J. Med. Eng. 2016;2016:6. doi: 10.1155/2016/6191526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shen S., Wang H., Xue Y., Yuan L., Zhou X., Zhao Z., Dong E., Liu B., Liu W., Cromeens B., Adler B., Besner G., Xu R.X. Freeform fabrication of tissue-simulating phantom for potential use of surgical planning in conjoined twins separation surgery. Sci. Rep. 2017;7(1) doi: 10.1038/s41598-017-08579-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang J.-J., Ren J.-A., Wang G.-F., Li Z.-A., Wu X.-W., Ren H.-J., Liu S. 3d-printed “fistula stent” designed for management of enterocutaneous fistula: an advanced strategy. World J. Gastroenterol. 2017;23(41):7489–7494. doi: 10.3748/wjg.v23.i41.7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cailleaux S., Sanchez-Ballester N.M., Gueche Y.A., Bataille B., Soulairol I. Fused deposition modeling (fdm), the new asset for the production of tailored medicines. J. Control. Release. 2021;330:821–841. doi: 10.1016/j.jconrel.2020.10.056. [DOI] [PubMed] [Google Scholar]
- 46.Shanmugam V., Das O., Babu K., Marimuthu U., Veerasimman A., Johnson D.J., Neisiany R.E., Hedenqvist M.S., Ramakrishna S., Berto F. Fatigue behaviour of fdm-3d printed polymers, polymeric composites and architected cellular materials. Int. J. Fatigue. 2021;143 [Google Scholar]
- 47.Colpani A., Fiorentino A., Ceretti E. Design and fabrication of customized tracheal stents by additive manufacturing. 23rd International Conference on Material FormingProc. Manuf. 2020;47:1029–1035. [Google Scholar]
- 48.Wang P., Sun Y., Li D., Ma Z., Zhang B., Diao L., Liu H. Extrusion-based 3d co-printing: printing material design and novel workflow for fabricating patterned heterogeneous tissue structures. Mater. Des. 2023;227 [Google Scholar]
- 49.Shinkar K., Rhode K. Could 3d extrusion bioprinting serve to be a real alternative to organ transplantation in the future? Ann. 3D Printed Med. 2022;7 [Google Scholar]
- 50.Lowther M., Louth S., Davey A., Hussain A., Ginestra P., Carter L., Eisenstein N., Grover L., Cox S. Clinical, industrial, and research perspectives on powder bed fusion additively manufactured metal implants. Addit. Manuf. 2019;28:565–584. [Google Scholar]
- 51.Wilkes J., Hagedorn Y., Meiners W., Wissenbach K. Additive manufacturing of zro-alo ceramic components by selective laser melting. Rapid Prototyping J. 2013;19(1):51–57. [Google Scholar]
- 52.Ma K., Chen H., Shen Y., Guo Y., Li W., Wang Y., Zhang Y., Sun Y. Feasibility study and material selection for powder-bed fusion process in printing of denture clasps. Comput. Biol. Med. 2023;157 doi: 10.1016/j.compbiomed.2023.106772. [DOI] [PubMed] [Google Scholar]
- 53.Ren Y., Dong P., Zeng Y., Yang T., Huang H., Chen J. Effect of heat treatment on properties of al-mg-sc-zr alloy printed by selective laser melting. Appl. Surf. Sci. 2022;574 [Google Scholar]
- 54.Chiu K.-Y., Chen K.-K., Wang Y.-H., Lin F.-H., Huang J.-Y. Formability of fe-doped bioglass scaffold via selective laser sintering. Ceram. Int. 2020;46(10, Part B):16510–16517. [Google Scholar]
- 55.Gayer C., Ritter J., Bullemer M., Grom S., Jauer L., Meiners W., Pfister A., Reinauer F., Vučak M., Wissenbach K., Fischer H., Poprawe R., Schleifenbaum J.H. Development of a solvent-free polylactide/calcium carbonate composite for selective laser sintering of bone tissue engineering scaffolds. Mater. Sci. Eng. C. 2019;101:660–673. doi: 10.1016/j.msec.2019.03.101. [DOI] [PubMed] [Google Scholar]
- 56.Tortorici M., Gayer C., Torchio A., Cho S., Schleifenbaum J.H., Petersen A. Inner strut morphology is the key parameter in producing highly porous and mechanically stable poly(e-caprolactone) scaffolds via selective laser sintering. Mater. Sci. Eng. C. 2021;123 doi: 10.1016/j.msec.2021.111986. [DOI] [PubMed] [Google Scholar]
- 57.Zhao Z., Li J., Wei Y., Yu T. Design and properties of graded polyamide12/hydroxyapatite scaffolds based on primitive lattices using selective laser sintering. J. Mech. Behav. Biomed. Mater. 2022;126 doi: 10.1016/j.jmbbm.2021.105052. [DOI] [PubMed] [Google Scholar]
- 58.Moiduddin K., Darwish S., Al-Ahmari A., ElWatidy S., Mohammad A., Ameen W. Structural and mechanical characterization of custom design cranial implant created using additive manufacturing. Electron. J. Biotechnol. 2017;29:22–31. [Google Scholar]
- 59.Surmeneva M.A., Khrapov D., Prosolov K., Kozadayeva M., Koptyug A., Volkova A., Paveleva A., Surmenev R.A. The influence of chemical etching on porous structure and mechanical properties of the ti6al4v functionally graded porous scaffolds fabricated by ebm. Mater. Chem. Phys. 2022;275 [Google Scholar]
- 60.Li J., Li Z., Shi Y., Wang H., Li R., Tu J., Jin G. In vitro and in vivo comparisons of the porous ti6al4v alloys fabricated by the selective laser melting technique and a new sintering technique. J. Mech. Behav. Biomed. Mater. 2019;91:149–158. doi: 10.1016/j.jmbbm.2018.12.007. [DOI] [PubMed] [Google Scholar]
- 61.Singla A.K., Banerjee M., Sharma A., Singh J., Bansal A., Gupta M.K., Khanna N., Shahi A., Goyal D.K. Selective laser melting of ti6al4v alloy: process parameters, defects and post-treatments. J. Manuf. Process. 2021;64:161–187. [Google Scholar]
- 62.Lee Y., Jung A., Heo S., Gweon B., Lim D. Influences of surface topography of porous titanium scaffolds manufactured by powder bed fusion on osteogenesis. J. Mater. Res. Technol. 2023;23:2784–2797. [Google Scholar]
- 63.Trenfield S.J., Xu X., Goyanes A., Rowland M., Wilsdon D., Gaisford S., Basit A.W. Releasing fast and slow: non-destructive prediction of density and drug release from sls 3d printed tablets using nir spectroscopy. Int. J. Pharm. X. 2023;5 doi: 10.1016/j.ijpx.2022.100148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xu X., Awad A., Robles-Martinez P., Gaisford S., Goyanes A., Basit A.W. Vat photopolymerization 3d printing for advanced drug delivery and medical device applications. J. Control. Release. 2021;329:743–757. doi: 10.1016/j.jconrel.2020.10.008. [DOI] [PubMed] [Google Scholar]
- 65.Tahayeri A., Morgan M., Fugolin A.P., Bompolaki D., Athirasala A., Pfeifer C.S., Ferracane J.L., Bertassoni L.E. 3d printed versus conventionally cured provisional crown and bridge dental materials. Dent. Mater. 2018;34(2):192–200. doi: 10.1016/j.dental.2017.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhao Y., Zhong J., Wang Y., Chen Q., Yin J., Wang J., Zhao H., Li Y., Gong H., Huang W. Photocurable and elastic polyurethane based on polyether glycol with adjustable hardness for 3d printing customized flatfoot orthosis. Biomater. Sci. 2023;11:1692–1703. doi: 10.1039/d2bm01538b. [DOI] [PubMed] [Google Scholar]
- 67.Guillaume O., Geven M.A., Varjas V., Varga P., Gehweiler D., Stadelmann V.A., Smidt T., Zeiter S., Sprecher C., Bos R.R., Grijpma D.W., Alini M., Yuan H., Richards G.R., Tang T., Qin L., Yuxiao L., Jiang P., Eglin D. Orbital floor repair using patient specific osteoinductive implant made by stereolithography. Biomaterials. 2020;233 doi: 10.1016/j.biomaterials.2019.119721. [DOI] [PubMed] [Google Scholar]
- 68.Kumar M., Ghosh S., Kumar V., Sharma V., Roy P. Tribo-mechanical and biological characterization of pegda/bioceramics composites fabricated using stereolithography. J. Manuf. Process. 2022;77:301–312. [Google Scholar]
- 69.Chen F., Wu Y.-R., Wu J.-M., Zhu H., Chen S., Hua S.-B., He Z.-X., Liu C.-Y., Xiao J., Shi Y.-S. Preparation and characterization of zro2-al2o3 bioceramics by stereolithography technology for dental restorations. Addit. Manuf. 2021;44 [Google Scholar]
- 70.Li Y., Mao Q., Yin J., Wang Y., Fu J., Huang Y. Theoretical prediction and experimental validation of the digital light processing (dlp) working curve for photocurable materials. Addit. Manuf. 2021;37 [Google Scholar]
- 71.Guldberg R.E., K. G. Deformation and fatigue of tough 3d printed elastomer scaffolds processed by fused deposition modeling and continuous liquid interface production. J. Mech. Behav. Biomed. Mater. 2017;75:1–13. doi: 10.1016/j.jmbbm.2017.06.038. [DOI] [PubMed] [Google Scholar]
- 72.Xueyong D., Bingxue H., Rui H., Liling C., Yingying T., Canhui L., Zhenming C., Wei Z., Ximu Z. 3d printing of robust and biocompatible poly (ethylene glycol) diacrylate/nano-hydroxyapatite composites via continuous liquid interface production. J. Phys. Chem. B. 2021;9:1315–1324. doi: 10.1039/d0tb02182b. [DOI] [PubMed] [Google Scholar]
- 73.Mele M., Campana G. Advancing towards sustainability in liquid crystal display 3d printing via adaptive slicing. Sustain. Prod. Consump. 2022;30:488–505. [Google Scholar]
- 74.Xenikakis I., Tsongas K., Tzimtzimis E.K., Zacharis C.K., Theodoroula N., Kalogianni E.P., Demiri E., Vizirianakis I.S., Tzetzis D., Fatouros D.G. Fabrication of hollow microneedles using liquid crystal display (lcd) vat polymerization 3d printing technology for transdermal macromolecular delivery. Int. J. Pharm. 2021;597 doi: 10.1016/j.ijpharm.2021.120303. [DOI] [PubMed] [Google Scholar]
- 75.Bucciarelli A., Paolelli X., De Vitis E., Selicato N., Gervaso F., Gigli G., Moroni L., Polini A. Vat photopolymerization 3d printing optimization of high aspect ratio structures for additive manufacturing of chips towards biomedical applications. Addit. Manuf. 2022;60 [Google Scholar]
- 76.Zhang L., Liu H., Yao H., Zeng Y., Chen J. Preparation, microstructure, and properties of zro2(3y)/al2o3 bioceramics for 3d printing of all-ceramic dental implants by vat photopolymerization. Chin. J. Mech. Eng. Addit. Manuf. Front. 2022;1(2) [Google Scholar]
- 77.Zhou Z., Lennon A., Buchanan F., McCarthy H.O., Dunne N. Binder jetting additive manufacturing of hydroxyapatite powders: effects of adhesives on geometrical accuracy and green compressive strength. Addit. Manuf. 2020;36 [Google Scholar]
- 78.Elliott A., AlSalihi S., Merriman A.L., Basti M.M. Infiltration of nanoparticles into porous binder jet printed parts. Am. J. Eng. Appl. Sci. 2016;9(1):128–133. [Google Scholar]
- 79.Kozakiewicz-Latała M., Nartowski K., Dominik A., Malec K., Gołkowska A., Złocińska A., Rusińska M., Szymczyk-Ziółkowska P., Ziółkowski G., Górniak A., Karolewicz B. Binder jetting 3d printing of challenging medicines: from low dose tablets to hydrophobic molecules. Eur. J. Pharm. Biopharm. 2022;170:144–159. doi: 10.1016/j.ejpb.2021.11.001. [DOI] [PubMed] [Google Scholar]
- 80.Sen K., Mehta T., Sansare S., Sharifi L., Ma A., B. C. Pharmaceutical applications of powder-based binder jet 3d printing process – a review. Adv. Drug Deliv. Rev. 2021;177 doi: 10.1016/j.addr.2021.113943. [DOI] [PubMed] [Google Scholar]
- 81.Sturm L.D., Albakri M.I., Tarazaga P.A., Williams C.B. In situ monitoring of material jetting additive manufacturing process via impedance based measurements. Addit. Manuf. 2019;28:456–463. [Google Scholar]
- 82.Schwarzer E., Holtzhausen S., Scheithauer U., Ortmann C., Oberbach T., Moritz T., Michaelis A. Process development for additive manufacturing of functionally graded alumina toughened zirconia components intended for medical implant application. J. Eur. Ceram. Soc. 2019;39(2):522–530. [Google Scholar]
- 83.Tyagi S., Yadav A., Deshmukh S. Review on mechanical characterization of 3d printed parts created using material jetting process. Mater. Today Proc. 2022;51:1012–1016. [Google Scholar]
- 84.Tuomi J.T., Björkstrand R.V., Pernu M.L., Salmi M.V.J., Huotilainen E.I., Wolff J.E.H., Vallittu P.K., Mäkitie A.A. In vitro cytotoxicity and surface topography evaluation of additive manufacturing titanium implant materials. J. Mater. Sci., Mater. Med. 2017;28(3):53. doi: 10.1007/s10856-017-5863-1. [DOI] [PubMed] [Google Scholar]
- 85.Ramakrishnaiah R., Al kheraif A.A., Mohammad A., Divakar D.D., Kotha S.B., Celur S.L., Hashem M.I., Vallittu P.K., Rehman I.U. Preliminary fabrication and characterization of electron beam melted ti–6al–4v customized dental implant. Saudi J. Biol. Sci. 2017;24(4):787–796. doi: 10.1016/j.sjbs.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sahasrabudhe H., Bandyopadhyay A. In situ reactive multi-material ti6al4v-calcium phosphate-nitride coatings for bio-tribological applications. J. Mech. Behav. Biomed. Mater. 2018;85:1–11. doi: 10.1016/j.jmbbm.2018.05.020. [DOI] [PubMed] [Google Scholar]
- 87.Heer B., Zhang Y., Bandyopadhyay A. Additive manufacturing of alumina-silica reinforced ti6al4v for articulating surfaces of load-bearing implants. Ceram. Int. 2021;47(13):18875–18885. [Google Scholar]
- 88.Ryu D.J., Ban H.Y., Jung E.Y., Sonn C., Hong D.H., Ahmad S., Gweon B., Lim D., Wang J.H. Osteo-compatibility of 3d titanium porous coating applied by direct energy deposition (ded) for a cementless total knee arthroplasty implant: in vitro and in vivo study. J. Clin. Med. 2020;9(2) doi: 10.3390/jcm9020478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ryu D., Jung A., Ban H., Kwak T., Shin E., Gweon B., Lim D., Wang J. Enhanced osseointegration through direct energy deposition porous coating for cementless orthopedic implant fixation. Sci. Rep. 2021;11(22317) doi: 10.1038/s41598-021-01739-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Paz-González J.A., Velasco-Santos C., Villarreal-Gómez L.J., Alcudia-Zacarias E., Olivas-Sarabia A., Cota-Leal M.A., Flores-López L.Z., Gochi-Ponce Y. Structural composite based on 3d printing polylactic acid/carbon fiber laminates (pla/cfrc) as an alternative material for femoral stem prosthesis. J. Mech. Behav. Biomed. Mater. 2023;138 doi: 10.1016/j.jmbbm.2022.105632. [DOI] [PubMed] [Google Scholar]
- 91.Kumar M. Bhuvanesh, Sathiya P. Methods and materials for additive manufacturing: a critical review on advancements and challenges. Thin-Walled Struct. 2021;159 [Google Scholar]
- 92.Jahnke P., Schwarz S., Ziegert M., Schwarz F., Hamm B., Scheel M. Paper-based 3d printing of anthropomorphic ct phantoms: feasibility of two construction techniques. Eur. Radiol. 2019;29:1384–1390. doi: 10.1007/s00330-018-5654-1. [DOI] [PubMed] [Google Scholar]
- 93.Durfee W.K., Iaizzo P.A. In: Engineering in Medicine. Iaizzo P.A., editor. Academic Press; 2019. Chapter 21 - medical applications of 3d printing; pp. 527–543. [Google Scholar]
- 94.Tamayo J.A., Riascos M., Vargas C.A., Baena L.M. Additive manufacturing of ti6al4v alloy via electron beam melting for the development of implants for the biomedical industry. Heliyon. 2021;7(5) doi: 10.1016/j.heliyon.2021.e06892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rakesh Reddy R., Jaya Prakash K., Koteswari S., Ud A., Srinivasa Prasad B., Narayan Y. Additive manufacturing of a human mandible and finite element analysis of dental implant for prosthodontic applications. International Conference on Advances in Materials Research - 2019Mater. Today Proc. 2021;45:3028–3035. [Google Scholar]
- 96.Javaid M., Haleem A. Additive manufacturing applications in medical cases: a literature based review. Alexandria J. Med. 2018;54(4):411–422. [Google Scholar]
- 97.Kholgh Eshkalak S., Rezvani Ghomi E., Dai Y., Choudhury D., Ramakrishna S. The role of three-dimensional printing in healthcare and medicine. Mater. Des. 2020;194 [Google Scholar]
- 98.Burton H.E., Eisenstein N.M., Lawless B.M., Jamshidi P., Segarra M.A., Addison O., Shepherd D.E., Attallah M.M., Grover L.M., Cox S.C. The design of additively manufactured lattices to increase the functionality of medical implants. Mater. Sci. Eng. C. 2019;94:901–908. doi: 10.1016/j.msec.2018.10.052. [DOI] [PubMed] [Google Scholar]
- 99.Sing S.L., An J., Yeong W.Y., Wiria F.E. Laser and electron-beam powder-bed additive manufacturing of metallic implants: a review on processes, materials and designs. J. Orthop. Res. 2016;34(3):369–385. doi: 10.1002/jor.23075. [DOI] [PubMed] [Google Scholar]
- 100.Van Bael S., Kerckhofs G., Moesen M., Pyka G., Schrooten J., Kruth J.-P. Micro-ct-based improvement of geometrical and mechanical controllability of selective laser melted ti6al4v porous structures. Mater. Sci. Eng. A. 2011;528(24):7423–7431. [Google Scholar]
- 101.Velu R., Calais T., Jayakumar A., Raspall F. A comprehensive review on bio-nanomaterials for medical implants and feasibility studies on fabrication of such implants by additive manufacturing technique. Materials. 2019;13(1):92. doi: 10.3390/ma13010092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sharma A., Oh M.C., Kim J.-T., Srivastava A.K., Ahn B. Investigation of electrochemical corrosion behavior of additive manufactured ti–6al–4v alloy for medical implants in different electrolytes. J. Alloys Compd. 2020;830 [Google Scholar]
- 103.Depboylu F.N., Yasa E., Poyraz Özgür, Minguella-Canela J., Korkusuz F., De los Santos López M.A. Titanium based bone implants production using laser powder bed fusion technology. J. Mater. Res. Technol. 2022;17:1408–1426. [Google Scholar]
- 104.Manakari V., Parande G., Gupta M. Selective laser melting of magnesium and magnesium alloy powders: a review. Metals. 2016;7(1):2. [Google Scholar]
- 105.Zadpoor A.A. Additively manufactured porous metallic biomaterials. J. Phys. Chem. B. 2019;7(26):4088–4117. doi: 10.1039/c9tb00420c. [DOI] [PubMed] [Google Scholar]
- 106.Albrektsson T., Chrcanovic B., Jacobsson M., Wennerberg A. Osseointegration of implants: a biological and clinical overview. JSM Dent. Surg. 2017;2(3) [Google Scholar]
- 107.Soro N., Brodie E.G., Abdal-hay A., Alali A.Q., Kent D., Dargusch M.S. Additive manufacturing of biomimetic titanium-tantalum lattices for biomedical implant applications. Mater. Des. 2022 [Google Scholar]
- 108.Soro N., Attar H., Brodie E., Veidt M., Molotnikov A., Dargusch M.S. Evaluation of the mechanical compatibility of additively manufactured porous ti–25ta alloy for load-bearing implant applications. J. Mech. Behav. Biomed. Mater. 2019;97:149–158. doi: 10.1016/j.jmbbm.2019.05.019. [DOI] [PubMed] [Google Scholar]
- 109.Ni J., Liu F., Yang G., Lee G.-H., Chung S.-M., Lee I.-S., Chen C. 3d-printed ti6al4v femoral component of knee: improvements in wear and biological properties by aip tin and ticrn coating. J. Mater. Res. Technol. 2021;14:2322–2332. [Google Scholar]
- 110.Sharma M., Soni M. Direct metal laser sintering of ti6al4v alloy for patient-specific temporo mandibular joint prosthesis and implant. 2nd International Conference on Future Learning Aspects of Mechanical EngineeringMater. Today Proc. 2021;38:333–339. [Google Scholar]
- 111.Bohlouli H.F.F.B.M., Khojasteh A. Reconstruction of bilateral ramus-condyle unit defect using custom titanium prosthesis with preservation of both condyles. J. Mech. Behav. Biomed. Mater. 2021;124 doi: 10.1016/j.jmbbm.2021.104765. [DOI] [PubMed] [Google Scholar]
- 112.Yin C., Zhang T., Wei Q., Cai H., Cheng Y., Tian Y., Leng H., Wang C., Feng S., Liu Z. Surface treatment of 3d printed porous ti6al4v implants by ultraviolet photofunctionalization for improved osseointegration. Bioact. Mater. 2022;7:26–38. doi: 10.1016/j.bioactmat.2021.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Blesslin Sheeba T., Albert Raj A., Ravikumar D., Sheeba Rani S., Vijayakumar P., Subbiah R. Analysis of silica based prosthetic human teeth using 3d printing machine for dental assistance. International Conference on Advances in Materials Research - 2019Mater. Today Proc. 2021;45:2440–2443. 2019. [Google Scholar]
- 114.Iqbal T., Wang L., Li D., Dong E., Fan H., Fu J., Hu C. A general multi-objective topology optimization methodology developed for customized design of pelvic prostheses. Med. Eng. Phys. 2019;69:8–16. doi: 10.1016/j.medengphy.2019.06.008. [DOI] [PubMed] [Google Scholar]
- 115.Ziegler-Graham K., MacKenzie E.J., Ephraim P.L., Travison T.G., Brookmeyer R. Estimating the prevalence of limb loss in the United States: 2005 to 2050. Arch. Phys. Med. Rehabil. 2008;89(3):422–429. doi: 10.1016/j.apmr.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 116.Manero A., Smith P., Sparkman J., Dombrowski M., Courbin D., Kester A., Womack I., Chi A. Implementation of 3d printing technology in the field of prosthetics: past, present, and future. Int. J. Environ. Res. Public Health. 2019;16(9):1641. doi: 10.3390/ijerph16091641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.2015. https://humantechpando.com/whats-the-difference-between-orthotics-and-prosthetics/ H.T. Prosthetics and Orthotics, “What's the difference between orthotics and prosthetics,” [Online]. Available:
- 118.Patel P., Gohil P. Custom orthotics development process based on additive manufacturing. Mater. Today Proc. 2022 [Google Scholar]
- 119.Wojciechowski E., Chang A.Y., Balassone D., Ford J., Tegan L., Cheng Little L., Little D., Menezes M.P., Hogan S., Burns J. Feasibility of designing, manufacturing and delivering 3d printed ankle-foot orthoses: a systematic review. J. Foot Ankle Res. 2019;12(11):1–12. doi: 10.1186/s13047-019-0321-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Toth L., Schiffer A., Nyitrai M., Pentek A., Told R., Maroti P. Developing an anti-spastic orthosis for daily home-use of stroke patients using smart memory alloys and 3d printing technologies. Mater. Des. 2020;195 [Google Scholar]
- 121.Niveditha R., Saranya R., Vishnu T., Chamundeswari K. Comparative study on structural enhancement of polymer based medical devices using additive manufacturing technology and pop (plating on plastics) Mater. Today Proc. 2022 [Google Scholar]
- 122.Kaleev A., Kashapov L., Kashapov N., Kashapov R. Application of reverse engineering in the medical industry. IOP Conf. Ser., Mater. Sci. Eng. 2017;240(1) IOP Publishing Ltd. [Google Scholar]
- 123.George M., Hawes H., Aroom K., Gill B.S., Love J. Inguinal hernia repair using 3d printed surgical instruments in the cadaveric model: a feasibility study. Global Surg. 2017;3(2):1–4. [Google Scholar]
- 124.Steinemann D.C., Müller P.C., Apitz M., Nickel F., Kenngott H.G., Müller-Stich B.P., Linke G.R. An ad hoc three dimensionally printed tool facilitates intraesophageal suturing in experimental surgery. J. Surg. Res. 2018;223:87–93. doi: 10.1016/j.jss.2017.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sakes A., Hovland K., Smit G., Geraedts J., Breedveld P. Design of a novel three-dimensional-printed two degrees-of-freedom steerable electrosurgical grasper for minimally invasive surgery. J. Med. Devices. 2018;12(1) [Google Scholar]
- 126.Dikici S., Aldemir Dikici B., Eser H., Gezgin E., Başer Ö., Şahin S., Yılmaz B., Oflaz H. Development of a 2-dof uterine manipulator with led illumination system as a new transvaginal uterus amputation device for gynecological surgeries. Minim. Invasive Ther. Allied Technol. 2018;27(3):177–185. doi: 10.1080/13645706.2017.1341927. [DOI] [PubMed] [Google Scholar]
- 127.Sahlabadi M., Jezler K., Hutapea P. Smart Materials, Adaptive Structures and Intelligent Systems, vol. 58264. American Society of Mechanical Engineers; 2017. Novel steerable smart needle with a built-in recovery mechanism for multiple actuations. [Google Scholar]
- 128.Roppenecker D.B., Pfaff A., Coy J.A., Lueth T.C. 2013 IEEE/RSJ International Conference on Intelligent Robots and Systems. IEEE; 2013. Multi arm snake-like robot kinematics; pp. 5040–5045. [Google Scholar]
- 129.García J.I.R., Velasco J.M.S., Suárez M.V., Pereira A.C., Sosa V., Rodríguez J.L.C. Design engineering in surgery. How to design, test and market surgical devices made with 3 d printing? Cir. Esp. 2018;96(4):198–204. doi: 10.1016/j.ciresp.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 130.Yasli M., Dabbagh S., Tasoglu S., Aydin S. Additive manufacturing and three-dimensional printing in obstetrics and gynecology: a comprehensive review. Arch. Gynecol. Obstet. 2023 doi: 10.1007/s00404-023-06912-1. [DOI] [PubMed] [Google Scholar]
- 131.Agung N., Nadhif M., Irdam G., Mochtar C. The role of 3d-printed phantoms and devices for organ-specified appliances in urology. Int. J. Bioprint. 2021;7(2) doi: 10.18063/ijb.v7i2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Trenfield S.J., Awad A., Madla C.M., Hatton G.B., Firth J., Goyanes A., Gaisford S., Basit A.W. Shaping the future: recent advances of 3d printing in drug delivery and healthcare. Expert Opin. Drug Deliv. 2019;16(10):1081–1094. doi: 10.1080/17425247.2019.1660318. [DOI] [PubMed] [Google Scholar]
- 133.Rezvani Ghomi E., Khosravi F., Neisiany R.E., Singh S., Ramakrishna S. Future of additive manufacturing in healthcare. Curr. Opin. Biomed. Eng. 2021;17 [Google Scholar]
- 134.Donderwinkel I., Van Hest J.C., Cameron N.R. Bio-inks for 3d bioprinting: recent advances and future prospects. Polym. Chem. 2017;8(31):4451–4471. [Google Scholar]
- 135.Hakobyan D., Kerouredan O., Remy M., Dusserre N., Medina C., Devillard R., Fricain J.-C., Oliveira H. 3D Bioprinting. Springer; 2020. Laser-assisted bioprinting for bone repair; pp. 135–144. [DOI] [PubMed] [Google Scholar]
- 136.Jana S., Lerman A. Bioprinting a cardiac valve. Biotechnol. Adv. 2015;33(8):1503–1521. doi: 10.1016/j.biotechadv.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 137.Ke D., Niu C., Yang X. Evolution of 3d bioprinting-from the perspectives of bioprinting companies. Bioprinting. 2022;25 [Google Scholar]
- 138.Derakhshanfar S., Mbeleck R., Xu K., Zhang X., Zhong W., Xing M. 3d bioprinting for biomedical devices and tissue engineering: a review of recent trends and advances. Bioactiv. Mater. 2018;3(2):144–156. doi: 10.1016/j.bioactmat.2017.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Jakus A.E. In: 3D Printing in Orthopaedic Surgery. Dipaola M., Wodajo F.M., Elsevier, editors. 2019. Chapter 1 - an introduction to 3d printing—past, present, and future promise; pp. 1–15. [Google Scholar]
- 140.Singh M., Jonnalagadda S. Advances in bioprinting using additive manufacturing. Eur. J. Pharm. Sci. 2020;143 doi: 10.1016/j.ejps.2019.105167. [DOI] [PubMed] [Google Scholar]
- 141.Bock S., Rades T., Rantanen J., Scherließ R. Additive manufacturing in respiratory sciences - current applications and future prospects. Adv. Drug Deliv. Rev. 2022 doi: 10.1016/j.addr.2022.114341. [DOI] [PubMed] [Google Scholar]
- 142.Galliger Z., Vogt C.D., Panoskaltsis-Mortari A. 3d bioprinting for lungs and hollow organs. Transl. Res. 2019;211:19–34. doi: 10.1016/j.trsl.2019.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Barros A.S., Costa A., Sarmento B. Building three-dimensional lung models for studying pharmacokinetics of inhaled drugs. Adv. Drug Deliv. Rev. 2021;170:386–395. doi: 10.1016/j.addr.2020.09.008. [DOI] [PubMed] [Google Scholar]
- 144.Heuijerjans A., Wilson W., Ito K., van Donkelaar C. The critical size of focal articular cartilage defects is associated with strains in the collagen fibers. Clin. Biomech. 2017;50:40–46. doi: 10.1016/j.clinbiomech.2017.09.015. [DOI] [PubMed] [Google Scholar]
- 145.El-Rashidy A.A., Roether J.A., Harhaus L., Kneser U., Boccaccini A.R. Regenerating bone with bioactive glass scaffolds: a review of in vivo studies in bone defect models. Acta Biomater. 2017;62:1–28. doi: 10.1016/j.actbio.2017.08.030. [DOI] [PubMed] [Google Scholar]
- 146.Du X., Fu S., Zhu Y. 3d printing of ceramic-based scaffolds for bone tissue engineering: an overview. J. Phys. Chem. B. 2018;6(27):4397–4412. doi: 10.1039/c8tb00677f. [DOI] [PubMed] [Google Scholar]
- 147.Sheoran A.J., Kumar H., Arora P.K., Moona G. Bio-medical applications of additive manufacturing: a review. 30th International Conference on Flexible Automation and Intelligent Manufacturing (FAIM2021)Proc. Manuf. 2020;51:663–670. [Google Scholar]
- 148.Lai Y., Cao H., Wang X., Chen S., Zhang M., Wang N., Yao Z., Dai Y., Xie X., Zhang P., et al. Porous composite scaffold incorporating osteogenic phytomolecule icariin for promoting skeletal regeneration in challenging osteonecrotic bone in rabbits. Biomaterials. 2018;153:1–13. doi: 10.1016/j.biomaterials.2017.10.025. [DOI] [PubMed] [Google Scholar]
- 149.Dubey N., Ferreira J.A., Malda J., Bhaduri S.B., Bottino M.C. Extracellular matrix/amorphous magnesium phosphate bioink for 3d bioprinting of craniomaxillofacial bone tissue. ACS Appl. Mater. Interfaces. 2020;12(21):23752–23763. doi: 10.1021/acsami.0c05311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Shao L., Gao Q., Xie C., Fu J., Xiang M., He Y. Directly coaxial 3d bioprinting of large-scale vascularized tissue constructs. Biofabrication. 2020;12(3) doi: 10.1088/1758-5090/ab7e76. [DOI] [PubMed] [Google Scholar]
- 151.Yu C., You S., Zhu W., Sun B., Chen S. Emerging Digital Micromirror Device Based Systems and Applications XII, vol, 11294. International Society for Optics and Photonics; 2020. Dmd-based rapid 3d bioprinting for precision tissue engineering and regenerative medicine. [Google Scholar]
- 152.Macchiarini P., Jungebluth P., Go T., Asnaghi M.A., Rees L.E., Cogan T.A., Dodson A., Martorell J., Bellini S., Parnigotto P.P., et al. Clinical transplantation of a tissue-engineered airway. Lancet. 2008;372(9655):2023–2030. doi: 10.1016/S0140-6736(08)61598-6. [DOI] [PubMed] [Google Scholar]
- 153.Resources H., Administration S. Organ donation statistics. https://www.organdonor.gov/learn/organ-donation-statistics [Online]. Available:
- 154.Bejoy A.M., Makkithaya K.N., Hunakunti B.B., Hegde A., Krishnamurthy K., Sarkar A., Lobo C.F., Keshav D., G D., S D.D., Mascarenhas S., Chakrabarti S., Kalepu S.R.R.D., Paul B., Mazumder N. An insight on advances and applications of 3d bioprinting: a review. Bioprinting. 2021;24:176. [Google Scholar]
- 155.Zhang B., Montgomery M., Chamberlain M.D., Ogawa S., Korolj A., Pahnke A., Wells L.A., Masse S., Kim J., Reis L., et al. Biodegradable scaffold with built-in vasculature for organ-on-a-chip engineering and direct surgical anastomosis. Nat. Mater. 2016;15(6):669–678. doi: 10.1038/nmat4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hangge P., Pershad Y., Witting A.A., Albadawi H., Oklu R. Three-dimensional (3d) printing and its applications for aortic diseases. Cardiovasc. Diagn. Ther. 2018;8(Suppl 1):S19. doi: 10.21037/cdt.2017.10.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zhou W.Y., Lee S.H., Wang M., Cheung W.L., Ip W.Y. Selective laser sintering of porous tissue engineering scaffolds from poly (l-lactide)/carbonated hydroxyapatite nanocomposite microspheres. J. Mater. Sci., Mater. Med. 2008;19(7):2535–2540. doi: 10.1007/s10856-007-3089-3. [DOI] [PubMed] [Google Scholar]
- 158.Koffler J. The future of biomimetic 3d printing. J. 3D Print. Med. 2019;3(2):63–65. [Google Scholar]
- 159.Munoz-Abraham A.S., Rodriguez-Davalos M.I., Bertacco A., Wengerter B., Geibel J.P., Mulligan D.C. 3d printing of organs for transplantation: where are we and where are we heading? Curr. Transplant. Rep. 2016;3(1):93–99. [Google Scholar]
- 160.Datta P., Ayan B., Ozbolat I.T. Bioprinting for vascular and vascularized tissue biofabrication. Acta Biomater. 2017;51:1–20. doi: 10.1016/j.actbio.2017.01.035. [DOI] [PubMed] [Google Scholar]
- 161.Guvendiren M., Molde J., Soares R.M., Kohn J. Designing biomaterials for 3d printing. ACS Biomater. Sci. Eng. 2016;2(10):1679–1693. doi: 10.1021/acsbiomaterials.6b00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Muehlenfeld C., Roberts S.A. 3D and 4D Printing in Biomedical Applications. 2018. 3d/4d printing in additive manufacturing: process engineering and novel excipients. [Google Scholar]
- 163.Ahmed A., Arya S., Gupta V., Furukawa H., Khosla A. 4d printing: fundamentals, materials, applications and challenges. Polymer. 2021;228 [Google Scholar]
- 164.Trenfield S.J., Awad A., Madla C.M., Hatton G.B., Firth J., Goyanes A., Gaisford S., Basit A.W. Shaping the future: recent advances of 3d printing in drug delivery and healthcare. Expert Opin. Drug Deliv. 2019;16(10):1081–1094. doi: 10.1080/17425247.2019.1660318. [DOI] [PubMed] [Google Scholar]
- 165.Amukarimi S., Mozafari M. 4d bioprinting of tissues and organs. Bioprinting. 2021;23 [Google Scholar]
- 166.Mitchell A., Lafont U., Hołyńska M., Semprimoschnig C. Additive manufacturing — a review of 4d printing and future applications. Addit. Manuf. 2018;24:606–626. https://www.sciencedirect.com/science/article/pii/S2214860417304013 [Online]. Available: [Google Scholar]
- 167.Gao B., Yang Q., Zhao X., Jin G., Ma Y., Xu F. 4d bioprinting for biomedical applications. Trends Biotechnol. 2016;34(9):746–756. doi: 10.1016/j.tibtech.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 168.Li Y.-C., Zhang Y.S., Akpek A., Shin S.R., Khademhosseini A. 4d bioprinting: the next-generation technology for biofabrication enabled by stimuli-responsive materials. Biofabrication. 2016;9(1) doi: 10.1088/1758-5090/9/1/012001. [DOI] [PubMed] [Google Scholar]
- 169.Jiang R., Kleer R., Piller F.T. Predicting the future of additive manufacturing: a delphi study on economic and societal implications of 3d printing for 2030. Technol. Forecast. Soc. Change. 2017;117:84–97. [Google Scholar]
- 170.Tibbits S. 4d printing: multi-material shape change. Archit. Des. 2014;84(1):116–121. [Google Scholar]
- 171.Ionov L. 4d biofabrication: materials, methods, and applications. Adv. Healthc. Mater. 2018;7(17) doi: 10.1002/adhm.201800412. [DOI] [PubMed] [Google Scholar]
- 172.Sonatkar J., Kandasubramanian B., Ismail S.O. 4d printing: pragmatic progression in biofabrication. Eur. Polym. J. 2022;169 [Google Scholar]
- 173.Castro N., Meinert C., Levett P., Hutmacher D. Current developments in multifunctional smart materials for 3d/4d bioprinting. Curr. Opin. Biomed. Eng. 2017;2:67–75. [Google Scholar]
- 174.Gungor-Ozkerim P.S., Inci I., Zhang Y.S., Khademhosseini A., Dokmeci M.R. Bioinks for 3d bioprinting: an overview. Biomater. Sci. 2018;6(5):915–946. doi: 10.1039/c7bm00765e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Chen Y., Xiong X., Liu X., Cui R., Wang C., Zhao G., Zhi W., Lu M., Duan K., Weng J., et al. 3d bioprinting of shear-thinning hybrid bioinks with excellent bioactivity derived from gellan/alginate and thixotropic magnesium phosphate-based gels. J. Phys. Chem. B. 2020;8(25):5500–5514. doi: 10.1039/d0tb00060d. [DOI] [PubMed] [Google Scholar]
- 176.Haleem A., Javaid M., Vaishya R. 5d printing and its expected applications in orthopaedics. J. Clin. Orthop. Trauma. 2019;10(4):809–810. doi: 10.1016/j.jcot.2018.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Cheng Y., Fu Y., Ma L., Yap P.L., Losic D., Wang H., Zhang Y. Rheology of edible food inks from 2d/3d/4d printing, and its role in future 5d/6d printing. Food Hydrocoll. 2022;132 [Google Scholar]
- 178.Stelios G.I.K., A. P. Additive manufacturing for effective smart structures: the idea of 6d printing. J. Compos. Sci. 2021;5(119):1–11. [Google Scholar]
- 179.Foresti R., Rossi S., Pinelli S., Alinovi R., Sciancalepore C., Delmonte N., Selleri S., Cafarra C., Raposio E., Macaluso G., Macaluso C., Freyrie A., Miragoli M., Perini P. In-vivo vascular application via ultra-fast bioprinting for future 5d personalised nanomedicine. Sci. Rep. 2020;3205 doi: 10.1038/s41598-020-60196-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Nahata S., Ozdoganlar O.B. Feasibility of metal additive manufacturing for fabricating custom surgical instrumentation for hip and knee implants. Proc. Manuf. 2019;34:772–779. [Google Scholar]
- 181.Anas S., Khan M.Y., Rafey M., Faheem K. Concept of 5d printing technology and its applicability in the healthcare industry. International Conference on Applied Research and Engineering 2021Mater. Today Proc. 2022;56:1726–1732. [Google Scholar]
- 182.Pugliese R., Regondi S. Artificial intelligence-empowered 3d and 4d printing technologies toward smarter biomedical materials and approaches. Polymers. 2022;14(14) doi: 10.3390/polym14142794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Reddy P.R., Devi P.A. Review on the advancements of additive manufacturing-4d and 5d printing. Int. J. Mech. Prod. Eng. Res. Dev. 2018;8(4):397–402. [Google Scholar]
- 184.Rahman M., Almalki W., Alghamdi S., Alharbi K., Khalilullah H., Akhter M.H., Keshari A., Sharma N., Singh T., Soni K., Hafeez A., Beg S. Three ‘d's: design approach, dimensional printing, and drug delivery systems as promising tools in healthcare applications. Drug Discov. Today. 2021;26(11):2726–2733. doi: 10.1016/j.drudis.2021.06.016. [DOI] [PubMed] [Google Scholar]
- 185.Ghazal A., Zhang M., Mujumdar A., Ghamry M. Progress in 4d/5d/6d printing of foods: applications and r&d opportunities. Crit. Rev. Food Sci. Nutr. 2022;63:1–24. doi: 10.1080/10408398.2022.2045896. [DOI] [PubMed] [Google Scholar]
- 186.Yu X., Cheng H., Zhang M., Zhao Y., Qu L., Shi G. Graphene-based smart materials. Nat. Rev. Mater. 2017;2(9):1–13. [Google Scholar]
- 187.M.A. Kaleem, Manufacturing graphene based polymer matrix composites (gpmcs) via 3d printing (additive manufacturing): a review, 2019.
- 188.Georgantzinos S.K., Giannopoulos G.I., Bakalis P.A. Additive manufacturing for effective smart structures: the idea of 6d printing. J. Compos. Sci. 2021;5(5) [Google Scholar]
- 189.N. K., K. K., Vasiliadis A.V. From three-dimensional (3d)- to 6d-printing technology in orthopedics: science fiction or scientific reality? J. Funct. Biomater. 2022;13(3):1–6. doi: 10.3390/jfb13030101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Lee A., Hudson A., Shiwarski D., Tashman J., Hinton T., Yerneni S., Bliley J., Campbell P., Feinberg A. 3d bioprinting of collagen to rebuild components of the human heart. Science. 2019;365(6452):482–487. doi: 10.1126/science.aav9051. [DOI] [PubMed] [Google Scholar]
- 191.Li L., Shi J., Ma K., Jin J., Wang P., Liang H., Cao Y., Wang X., Jiang Q. Robotic in situ 3d bio-printing technology for repairing large segmental bone defects. J. Adv. Res. 2021;30:75–84. doi: 10.1016/j.jare.2020.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Ferromagnetic soft catheter robots for minimally invasive bioprinting, Nature communications. [DOI] [PMC free article] [PubMed]
- 193.Capel A.J., Rimington R.P., Fleming J.W., Player D.J., Baker L.A., Turner M.C., Jones J.M., Martin N.R., Ferguson R.A., Mudera V.C., et al. Scalable 3d printed molds for human tissue engineered skeletal muscle. Front. Bioeng. Biotechnol. 2019;7:20. doi: 10.3389/fbioe.2019.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Dai X., Liu L., Ouyang J., Li X., Zhang X., Lan Q., Xu T. Coaxial 3d bioprinting of self-assembled multicellular heterogeneous tumor fibers. Sci. Rep. 2017;7:1457. doi: 10.1038/s41598-017-01581-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Luo Y., Luo G., Gelinsky M., Huang P., Ruan C. 3d bioprinting scaffold using alginate/polyvinyl alcohol bioinks. Mater. Lett. 2017;189:295–298. [Google Scholar]
- 196.Rodriguez M.J., Brown J., Giordano J., Lin S.J., Omenetto F.G., Kaplan D.L. Silk based bioinks for soft tissue reconstruction using 3-dimensional (3d) printing with in vitro and in vivo assessments. Biomaterials. 2017;117:105–115. doi: 10.1016/j.biomaterials.2016.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Rees A., Powell L., Chinga-Carrasco G., Gethin D., Syverud K., Hill K., Thomas D. 3d bioprinting of carboxymethylated-periodate oxidized nanocellulose constructs for wound dressing applications. BioMed Res. Int. 2015;2015 doi: 10.1155/2015/925757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Lin C., Liu L., Liu Y., Leng J. 4d printing of bioinspired absorbable left atrial appendage occluders: a proof-of-concept study. ACS Appl. Mater. Interfaces. 2021;13:12668–12678. doi: 10.1021/acsami.0c17192. [DOI] [PubMed] [Google Scholar]
- 199.Cui H., Miao S., Esworthy T., Lee S.-J., Zhou X., Hann S.Y., Webster T.J., Harris B.T., Zhang L.G. A novel near-infrared light responsive 4d printed nanoarchitecture with dynamically and remotely controllable transformation. Nano Res. 2019;12(6):1381–1388. doi: 10.1007/s12274-019-2340-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Zarek M., Mansour N., Shapira S., Cohn D. 4d printing of shape memory-based personalized endoluminal medical devices. Macromol. Rapid Commun. 2017;38(2) doi: 10.1002/marc.201600628. [DOI] [PubMed] [Google Scholar]
- 201.López-Valdeolivas M., Liu D., Broer D.J., Sánchez-Somolinos C. 4d printed actuators with soft-robotic functions. Macromol. Rapid Commun. 2018;39(5) doi: 10.1002/marc.201700710. [DOI] [PubMed] [Google Scholar]
- 202.Miao S., Zhu W., Castro N.J., Nowicki M., Zhou X., Cui H., Fisher J.P., Zhang L.G. 4d printing smart biomedical scaffolds with novel soybean oil epoxidized acrylate. Sci. Rep. 2016;6(1) doi: 10.1038/srep27226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Fang J.-H., Hsu H.-H., Hsu R.-S., Peng C.-K., Lu Y.-J., Chen Y.-Y., Chen S.-Y., Hu S.-H. 4d printing of stretchable nanocookie@ conduit material hosting biocues and magnetoelectric stimulation for neurite sprouting. NPG Asia Mater. 2020;12(1):1–16. [Google Scholar]
- 204.Gillaspie E.A., Matsumoto J.S., Morris N.E., Downey R.J., Shen K.R., Allen M.S., Blackmon S.H. From 3-dimensional printing to 5-dimensional printing: enhancing thoracic surgical planning and resection of complex tumors. Ann. Thorac. Surg. 2016;101(5):1958–1962. doi: 10.1016/j.athoracsur.2015.12.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Mancilla-De-la Cruz J., Rodriguez-Salvador M., An J., Chua C.K. Three-dimensional printing technologies for drug delivery applications: processes, materials, and effects. Int. J. Bioprint. 2022;8(4):622. doi: 10.18063/ijb.v8i4.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Lee A.Y., Pant A., Pojchanun K., Lee C.P., An J., Hashimoto M., Tan U.-X., Leo C.H., Wong G., Chua C.K., Zhang Y. Three-dimensional printing of food foams stabilized by hydrocolloids for hydration in dysphagia. Int. J. Bioprint. 2021;7(4):393. doi: 10.18063/ijb.v7i4.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.An J., Leong K.F. Multi-material and multi-dimensional 3d printing for biomedical materials and devices. Biomed. Mater. Devices. 2022 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.