Abstract
The study aimed to identify prognostic factors for patients with acute lower gastrointestinal bleeding and to develop a high-accuracy prediction tool. The analysis included 8254 cases of acute hematochezia patients who were admitted urgently based on the judgment of emergency physicians or gastroenterology consultants (from the CODE BLUE J-study). Patients were randomly assigned to a derivation cohort and a validation cohort in a 2:1 ratio using a random number table. Assuming that factors present at the time of admission are involved in mortality within 30 days of admission, and adding management factors during hospitalization to the factors at the time of admission for mortality within 1 year, prognostic factors were established. Multivariate analysis was conducted, and scores were assigned to each factor using regression coefficients, summing these to measure the score. The newly created score (CACHEXIA score) became a tool capable of measuring both mortality within 30 days (ROC-AUC 0.93) and within 1 year (C-index, 0.88). The 1-year mortality rates for patients classified as low, medium, and high risk by the CACHEXIA score were 1.0%, 13.4%, and 54.3% respectively (all P < 0.001). After discharge, patients identified as high risk using our unique predictive score require ongoing observation.
Subject terms: Gastroenterology, Colonoscopy, Gastrointestinal diseases
Introduction
Patients with acute lower gastrointestinal bleeding (ALGIB) present with symptoms of hematochezia and sometimes require emergency hospitalization1–5. ALGIB can occasionally become severe and potentially fatal6–8. Gastrointestinal bleeding is categorized into upper gastrointestinal bleeding (AUGIB) and ALGIB, but ALGIB should be investigated separately from AUGIB. This is because, unlike AUGIB, there is no drug treatment such as acid secretion inhibitors for ALGIB, and epidemiological studies are crucial. Identifying prognostic factors for ALGIB could aid in its management.
Previous studies assessing the risk of long-term mortality (≥ 1 year) following hospitalization for ALGIB have been limited to a few single-center studies with small sample sizes (n < 500)9,10, and several variables potentially influencing mortality risk, such as vital signs, general condition, comorbidities, and in-hospital management, have not been evaluated2,10.
Furthermore, there is a need for careful follow-up of high-risk ALGIB patients, but a predictive scoring system that stratifies long-term mortality risk and identifies high-risk patients has not yet been established. Predictive scores for short-term mortality (30-day mortality or in-hospital mortality) have been reported in some previous studies5,7,11,12. However, the reported short-term mortality rates vary widely from 0.6 to 10.9%, lacking reliability7,11,12. Moreover, a study using a database of over 6000 patients based on the International Classification of Diseases codes found that factors such as admission vital signs and in-hospital rebleeding, which are involved in bleeding, were not considered significant risk factors for mortality11.
To address these issues, we conceived this study, believing that larger-scale, multicenter collaborative research is necessary. Computerized tomography (CT) and endoscopy are valuable modalities for diagnosing ALGIB, identifying the source of bleeding, and guiding treatment13–16. However, in previously reported large-scale studies, these data were insufficient. Considering this, we utilized nationwide data based on high diagnostic rates with adequately collected CT and endoscopy data to assess both short-term and long-term mortality rates. Additionally, since factors present at the time of hospital admission are expected to strongly influence short-term mortality, an ideal predictive tool would be one that can forecast short-term mortality at the time of admission. For long-term mortality, it is anticipated that factors present at the time of admission, as well as in-hospital management, contribute to mortality, hence an ideal predictive tool would be one that can estimate future mortality rates at discharge. Taking these elements into account, we developed a practical scoring system to determine short-term and long-term mortality risks and to stratify patients with high risk of death.
Methods
Study design, setting, and participants
This retrospective, multicenter, observational study was conducted between January 2010 and December 2019. It included patients with acute hematochezia who presented in an ambulatory setting and were urgently admitted to 49 hospitals across Japan. The decision for emergency admission was made by emergency physicians or gastroenterology consultants. The patient information gathered is reported as part of the CODE BLUE J-study (Colonic DivErticular Bleeding Leaders Update Evidence from a Multicenter Japanese Study)17,18. This study was conducted in accordance with the principles of the Declaration of Helsinki. As a retrospective study, the central institution (Tokyo Medical University) approved the use of an opt-out method, thereby waiving the necessity to obtain informed consent from patients. The central institution (Tokyo Medical University) has established a licensing committee/institutional review board for approving research involving human subjects. The research protocol was approved by the Tokyo Medical University Institutional Ethics Committee (T20190244). In this study, a single review by this ethics committee was applied and approved across all institutions (Supplementary Table 1).
This study aims to accurately predict mortality using clinical indicators present at the onset of the disease. Patients with ALGIB commonly visit the emergency department with acute hematochezia as their main complaint. “Hematochezia” refers to the discharge of red blood from the anus, which is predominantly indicative of lower gastrointestinal bleeding, but can also include cases of upper gastrointestinal bleeding. However, in actual clinical settings, it is not always possible to immediately determine the source of bleeding upon patient arrival. Therefore, to create a clinically relevant score, this study utilized data from patients presenting with hematochezia.
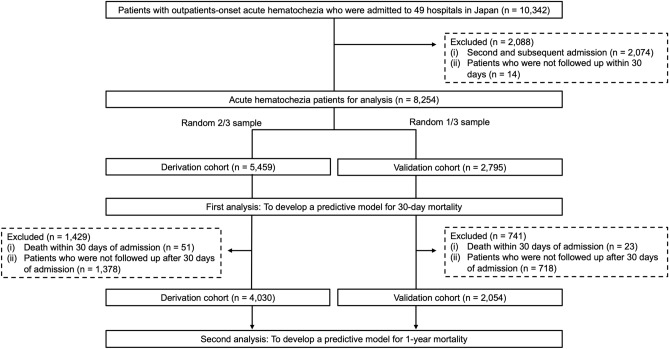
The remaining 8254 patients were divided using a random number table into a 2:1 ratio for a validation group to assess reliability and a derivation cohort to create a new score. In this study, short-term mortality was defined as death within 30 days, and long-term mortality as death within 1 year. All patients were used for predicting mortality within 30 days, the first part of our study. For the second part of our study, patients who died within 30 days of admission (n = 74) and those who were not followed up after 30 days of hospitalization (n = 2096) were excluded. The remaining 6084 cases were used for predicting 1-year mortality (Fig. 1).
Figure 1.
Flow chart.
Among patients with acute hematochezia in the original cohort (n = 10,342), we excluded patients with second or subsequent admissions (n = 2074) and patients who were not followed up within 30 days (n = 14). The remaining 8254 patients were divided using a random number table into a 2:1 ratio for a validation group to assess reliability and a derivation cohort to create novel scores. In this study, short-term mortality was defined as death within 30 days, and long-term mortality as death within 1 year. All patients were used for predicting mortality within 30 days, the first part of our study. For the second part of our study, patients who died within 30 days of hospitalization (n = 74) and patients who were not followed up after 30 days of admission (n = 2096) were eliminated, and the remaining 6084 patients were used to forecast 1-year mortality (Fig. 1). Follow-up was defined as cases with at least one hospital visit and examination performed as needed. The examination was conducted on patients with acute hematochezia (including AUIGB) rather than ALGIB because the main goal of this study was to properly predict mortality using clinical indicators at the time of onset.
Variables and outcomes
Data were collected from patient files and electronic endoscopic databases. In addition to in-hospital care data, baseline characteristics, including performance status (PS), vital signs, symptoms, initial laboratory findings, medications, and comorbidities, were collected. Comorbidities were collected to calculate the Charlson comorbidity index (CCI)19. In-hospital management included etiologies of bleeding sources (e.g., diverticular bleeding, colitis, ulcerative lesion, hemorrhoid, colorectal cancer, angioectasia, radiation proctitis, miscellaneous, and unknown origin) and in-hospital procedures (e.g., transfusion, endoscopic treatment, interventional radiology, and surgery). Endoscopic treatment was decided by the endoscopist following each hospital policy and included clipping, coagulation, band ligation, snare ligation, and hypertonic saline–epinephrine.
Mortality within 30 days of hospitalization was predicted using factors present at the time of admission, based on the assumption that these factors are strongly associated with death. For mortality within 1 year of hospitalization, it was assumed that in-hospital management factors also play a role in death, so these were added to the admission factors to predict mortality within one year. The list of predictive factors used is compiled in Supplementary Table 2. The cut-off values used were cited from papers on the prognosis and rebleeding of ALGIB and papers related to cachexia2,5,20–22. For the cut-off value of hematocrit, it was calculated using the cut-off value of hemoglobin used in this study and the normal mean corpuscular hemoglobin concentration.
Among the 6084 patients who were followed up for at least a year, the primary outcome was mortality within 1 year of admission, which was defined as death due to any reason after a month of admission until 365 days of admission. The secondary outcome was mortality within 30 days of admission, which was determined in the 8254 patients who were followed up for at least 30 days and who died for any reason within 30 days of admission; this period included the hospitalization period. Confirmation of death was determined from institutional medical records and death certificates, and death causes were categorized as hemorrhage-related or non-hemorrhage-related23. The cause of non-hemorrhage-related death was identified as a disease diagnosed by clinical examination, imaging studies, or autopsy.
Statistical analyses
All patients were divided into the derivation and the validation cohorts at a 2:1 ratio using a random number table. The proportions between the two groups were compared using the Mann–Whitney U test or Fisher’s exact tests. The statistical significance level was P < 0.05. A complete-case analysis approach was employed, utilizing all non-missing observations available in the relevant analyses. Table 1 and Supplementary Table 3 include details about the missing data.
Table 1.
Acute hematochezia patient characteristics in the validation and derivation cohorts.
| Characteristics | Patients, no. (%) | P value* | Missing values | |
|---|---|---|---|---|
| Derivation cohort (n = 5459) | Validation cohort (n = 2795) | |||
| Age ≥ 70 years | 3232 (59.2) | 1641 (58.7) | 0.670 | 0 |
| Sex (male) | 3300 (60.5) | 1673 (59.9) | 0.601 | 0 |
| BMI ≤ 18.5 | 651 (12.8) | 328 (12.6) | 0.773 | 185 (6.6) |
| Current drinker | 2202 (40.3) | 1103 (39.5) | 0.723 | 441 (15.8) |
| Current smoker | 910 (16.7) | 447 (16.0) | 0.774 | 387 (13.8) |
| Performance status ≥ 2 | 698 (12.8) | 357 (12.8) | 0.287 | 22 (0.8) |
| Hemodynamics | ||||
| Systolic blood pressure ≤ 100 mmHg | 739 (13.5) | 382 (13.7) | 0.892 | 46 (1.6) |
| Heart rate ≥ 100/min | 1087 (19.9) | 553 (19.8) | 0.884 | 54 (1.9) |
| Symptom | ||||
| Altered mental status | 371 (6.8) | 214 (7.7) | 0.160 | 1 (0.0) |
| Abdominal pain | 1004 (18.4) | 489 (17.5) | 0.319 | 4 (0.1) |
| Diarrhea | 607 (11.1) | 313 (11.2) | 0.941 | 6 (0.2) |
| Laboratory data | ||||
| Hemoglobin < 12 g/dL | 3157 (57.8) | 1608 (57.5) | 0.814 | 3 (0.1) |
| WBC > 10,000/mm3 | 1165 (21.3) | 607 (21.7) | 0.713 | 2 (0.1) |
| Platelet count < 150,000/mm3 | 857 (15.7) | 390 (14.0) | 0.038 | 3 (0.1) |
| Albumin level < 3.0 g/dL | 665 (12.2) | 310 (11.1) | 0.158 | 133 (4.8) |
| INR ≥ 1.5 | 444 (8.1) | 227 (8.1) | 0.966 | 351 (12.6) |
| Hematocrit ≤ 35% | 2939 (53.8) | 1517 (54.3) | 0.691 | 8 (0.3) |
| BUN ≥ 25 mg/dL | 1409 (25.8) | 665 (23.8) | 0.053 | 25 (0.9) |
| Creatinine ≥ 1.5 mg/dL | 669 (12.3) | 328 (11.7) | 0.544 | 28 (1.0) |
| CRP ≥ 1.0 mg/dL | 4236 (77.6) | 2198 (78.6) | 0.287 | 83 (3.0) |
| Home medication | ||||
| Low-dose aspirin | 1084 (19.9) | 549 (19.6) | 0.838 | 0 |
| Antiplatelet drug (non-aspirin) | 742 (13.6) | 398 (14.2) | 0.419 | 0 |
| Warfarin | 377 (6.9) | 202 (7.2) | 0.585 | 0 |
| Direct oral anticoagulant | 341 (6.2) | 158 (5.7) | 0.305 | 0 |
| NSAIDs | 637 (11.7) | 308 (11.0) | 0.401 | 0 |
| Acetaminophen | 130 (2.4) | 64 (2.3) | 0.818 | 0 |
| Corticosteroid | 290 (5.3) | 163 (5.8) | 0.332 | 0 |
| Comorbidity | ||||
| Previous diverticular bleeding | 804 (14.7) | 446 (16.0) | 0.145 | 1 (0.0) |
| Hypertension | 2941 (53.9) | 1522 (54.5) | 0.624 | 0 |
| Dyslipidemia | 1424 (26.1) | 708 (25.3) | 0.473 | 0 |
| Diabetes mellitus | 1032 (18.9) | 479 (17.1) | 0.051 | 0 |
| Diabetes complication | 173 (3.2) | 94 (3.4) | 0.646 | 0 |
| Hemiplegia | 149 (2.7) | 75 (2.7) | 0.943 | 1 (0.0) |
| Cerebrovascular disease | 775 (14.2) | 387 (13.8) | 0.688 | 0 |
| Chronic obstructive pulmonary disease | 161 (2.9) | 72 (2.6) | 0.362 | 0 |
| Dementia | 316 (5.8) | 149 (5.3) | 0.726 | 1 (0.0) |
| Collagen disease | 235 (4.3) | 110 (3.9) | 0.450 | 0 |
| Ischemic heart disease | 813 (14.9) | 422 (15.1) | 0.819 | 0 |
| Heart failure | 427 (7.8) | 232 (8.3) | 0.466 | 1 (0.0) |
| Previous peptic ulcer | 397 (7.3) | 179 (6.4) | 0.144 | 0 |
| Renal failure | 751 (13.8) | 368 (13.2) | 0.476 | 1 (0.0) |
| Peripheral arterial disease | 210 (3.8) | 111 (4.0) | 0.810 | 0 |
| Chronic hepatitis | 128 (2.3) | 58 (2.1) | 0.481 | 0 |
| Cirrhosis | 113 (2.1) | 57 (2.0) | 1.000 | 0 |
| Blood malignancy and nonmetastatic solid cancer† | 758 (13.9) | 384 (13.7) | 0.866 | 0 |
| Metastatic cancer | 140 (2.6) | 77 (2.8) | 0.611 | 0 |
| Diagnostic procedure | ||||
| CT | 3799 (69.6) | 2,018 (72.2) | 0.014 | 0 |
| Therapeutic procedures | ||||
| Endoscopic treatment | 1751 (32.1) | 896 (32.1) | 1.000 | 0 |
| Interventional radiology | 106 (1.9) | 53 (1.9) | 0.933 | 0 |
| Surgery | 90 (1.6) | 31 (1.1) | 0.053 | 0 |
| Blood transfusion during hospitalization | 1618 (29.6) | 829 (30.0) | 1.000 | 0 |
| The final diagnosis of hematochezia | ||||
| Colonic diverticular bleeding | 3137 (57.5) | 1672 (59.8) | 0.143 | 0 |
| Rectal ulcer | 152 (2.8) | 91 (3.3) | 0.242 | 0 |
| Angioectasia | 82 (1.5) | 34 (1.2) | 0.324 | 0 |
| Upper gastrointestinal bleeding | 109 (2.0) | 39 (1.4) | 0.054 | 0 |
| Small intestinal bleeding | 132 (2.4) | 64 (2.3) | 0.760 | 0 |
| Malignancy | 133 (2.4) | 54 (1.9) | 0.317 | 0 |
| Others | 382 (7.0) | 192 (6.9) | 0.855 | 0 |
| In-hospital outcomes | ||||
| Length of hospital stay ≥ 8 days | 2514 (46.1) | 1297 (46.4) | 0.436 | 0 |
| Rebleeding during hospitalization | 774 (14.2) | 378 (13.5) | 0.421 | 0 |
| Thromboembolism | 40 (0.7) | 15 (0.5) | 0.321 | 0 |
| Death within 30-day | 51 (0.9) | 23 (0.8) | 0.712 | 0 |
BMI body mass index, BUN blood urea nitrogen, CI confidence interval, CRP C-reactive protein, CT computed tomography, INR international normalized ratio, NSAIDs nonsteroidal anti-inflammatory drugs, WBC white blood cell count.
*Bold values indicate P < 0.05.
†Blood malignancy was included with the comorbidity of leukemia and lymphoma.
Cox regression analysis was determined based on 8–10 events per predictor variable24,25. The logistic regression model was employed to calculate crude odds ratios (ORs), adjusted ORs, and 95% confidence intervals (CIs) for 30-day mortality. Model selection for multivariable analyses of 30-day mortality was conducted using the stepwise method (P-value to enter = 0.10 and P-value to stay = 0.05). The final model was identified based on the Akaike information criterion, and the goodness of fit was examined using the Hosmer–Lemeshow test26. Accordingly, the predictor weight was calculated based on model coefficients. Each point value in the scoring system was assigned to each rounded coefficient value. The area under the receiver operating characteristic curve (ROC-AUC) data were used to evaluate the score’s discriminatory power. The validation cohort was used to evaluate the validity of prediction scores. Based on the number of score points, patients were divided into three groups, and the mortality of each group was compared using Fisher’s exact test with Bonferroni correction. We compared the scores developed in this study with three previously reported ALGIB scores (Sengupta, Oakland, and NOBLADS) and incorporated the Charlson Comorbidity Index (CCI) as a common measure for mortality prediction to evaluate the prognostic utility of comorbidities1,2,12,19. We compared the ROC-AUC using the DeLong test27. The specifics of each score are shown in Supplementary Table 4.
We created a scoring system for predicting 1-year mortality following admission using baseline characteristics and in-hospital management factors. In the univariate analysis, the Cox proportional hazards model was used to assess the predictors of 1-year mortality. This analysis was used to produce crude hazard ratios (HRs) and 95% CIs. Model selection, the final model, the weight of the predictor, and the value of scoring system points were determined employing the same steps as the first analysis. The accuracy of the predictive model for 1-year mortality was assessed by the c-statistic using Harrell’s technique28. The mortality of each group was compared using the Kaplan–Meier method and the Cox proportional hazards model. Subjects were separated into three groups depending on the scores. Each risk factor for 1-year mortality was broken down into three distinct categories in the subgroup analysis to analyze the relationships with long-term death using the Cox proportional hazards model. We followed the TRIPOD statement for reporting this clinical prediction model study29. R version 4.2.2 was used to conduct all statistical tests.
Results
Predictive factors for 30-day mortality and predictive score
The analysis included 8254 patients (median [interquartile range] age, 74 [63–82] years; 4973 [60.2%] men). Except for platelet counts of < 150,000/mm3 and the use of computed tomography for diagnosis, the baseline characteristics of the derivation (n = 5459) and validation (n = 2795) cohorts were similar (Table 1).
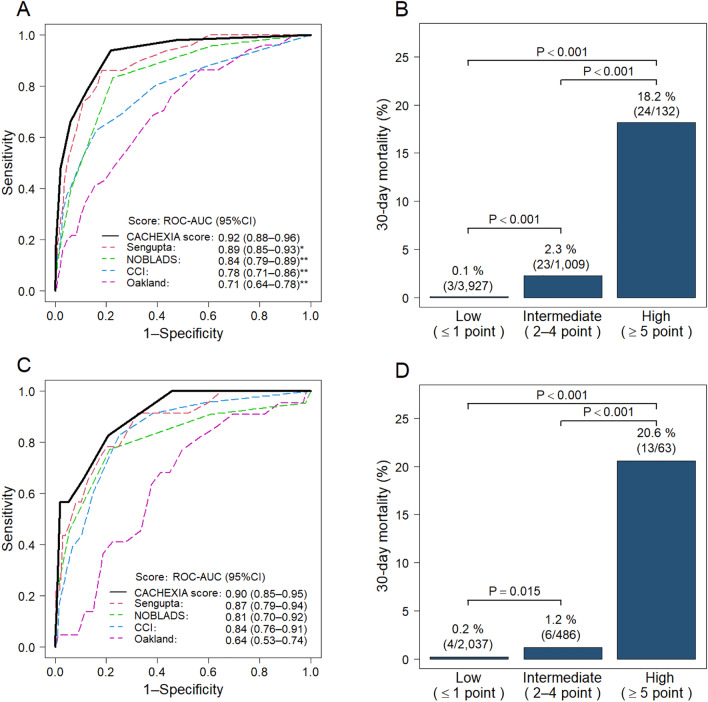
In the derivation cohort, 51 (0.9%) of 5459 patients died within 30 days; 7 (0.1%) patients died of bleeding-related causes (Supplementary Table 5). The univariate analysis revealed 26 baseline parameters linked to 30-day mortality. Multivariate logistic regression analysis identified the following six factors as risk factors for 30-day mortality: performance status (PS) ≥ 2, albumin level < 3.0 g/dL, blood urea nitrogen (BUN) ≥ 25 mg/dL, C-reactive protein (CRP) ≥ 1.0 mg/dL, comorbid metastatic cancer, and cirrhosis (Table 2). Based on the coefficients from the multivariate analysis, we created a novel weighted score for predicting 30-day mortality (maximum 11 points) utilizing these six factors (Table 2). For the derivation cohort, the ROC-AUC of the new score was 0.92 (95% CI 0.88–0.96), which was significantly higher than existing clinical risk scores (ROC-AUC: Sengupta, 0.89; NOBLADS, 0.84; CCI, 0.78; Oakland, 0.71) (Fig. 2A). Based on the novel score, patients in the derivation cohort were categorized into low-score ≤ 1 (n = 3927), medium-score 2–4 (n = 1009), and high-score ≥ 5 (n = 132) groups. The 30-day mortality rates for the low-, medium-, and high-score groups were 0.1% (n = 5), 2.3% (n = 23), and 18.2% (n = 24), respectively (P < 0.001 for each comparison) (Fig. 2B).
Table 2.
Predictors of 30-day mortality by logistic regression model (derivation cohort, n = 5459).
| Characteristics | Crude odds ratio (95% CI) | P value* | Adjusted odds ratio (95% CI) | Coefficient (95% CI) | P value* | Score points |
|---|---|---|---|---|---|---|
| Age ≥ 70 years | 2.25 (1.18–4.32) | 0.014 | ||||
| Sex (male) | 1.20 (0.68–2.14) | 0.533 | ||||
| BMI ≤ 18.5 | 2.16 (1.09–4.27) | 0.027 | ||||
| Current drinker | 0.42 (0.21–0.84) | 0.014 | ||||
| Current smoker | 1.35 (0.66–2.75) | 0.412 | ||||
| Performance status ≥ 2 | 9.97 (5.67–17.51) | < 0.001 | 5.54 (2.54–12.05) | 1.71 (0.93–2.49) | < 0.001 | 2 |
| Hemodynamics | ||||||
| Systolic blood pressure ≤ 100 mmHg | 4.86 (2.78–8.50) | < 0.001 | ||||
| Heart rate ≥ 100/min | 1.08 (0.55–2.11) | 0.825 | ||||
| Symptom | ||||||
| Altered mental status | 2.71 (1.26–5.82) | 0.011 | ||||
| Abdominal pain | 1.00 (0.48–2.06) | 0.992 | ||||
| Diarrhea | 0.67 (0.32–1.44) | 0.306 | ||||
| Laboratory data | ||||||
| Hemoglobin < 12 g/dL | 3.43 (1.67–7.06) | < 0.001 | ||||
| WBC > 10,000/mm3 | 2.83 (1.62–4.94) | < 0.001 | ||||
| Platelet count < 150,000/mm3 | 2.72 (1.51–4.88) | < 0.001 | ||||
| Albumin level < 3.0 g/dL | 20.52 (10.85–38.82) | < 0.001 | 5.45 (2.23–13.31) | 1.70 (0.80–2.59) | < 0.001 | 2 |
| INR ≥ 1.5 | 2.81 (1.43–5.52) | 0.003 | ||||
| Hematocrit ≤ 35% | 3.13 (1.60–6.12) | < 0.001 | ||||
| BUN ≥ 25 mg/dL | 5.38 (2.99–9.49) | < 0.001 | 2.57 (1.19–5.57) | 0.95 (0.17–1.72) | 0.016 | 1 |
| Creatinine ≥ 1.5 mg/dL | 3.62 (2.01–6.52) | < 0.001 | ||||
| CRP ≥ 1.0 mg/dL | 10.42 (5.53–19.62) | < 0.001 | 3.35 (1.41–7.92) | 1.21 (0.35–2.07) | 0.006 | 1 |
| Home medication | ||||||
| Low-dose aspirin | 1.39 (0.74–2.61) | 0.313 | ||||
| Antiplatelet drug (non-aspirin) | 0.85 (0.36–1.99) | 0.702 | ||||
| Warfarin | 2.17 (0.97–4.84) | 0.060 | ||||
| Direct oral anticoagulant | 0.61 (0.15–2.52) | 0.495 | ||||
| NSAIDs | 2.13 (1.03–4.40) | 0.041 | ||||
| Acetaminophen | 1.68 (0.41–7.00) | 0.474 | ||||
| Corticosteroid | 1.14 (0.35–3.60) | 0.855 | ||||
| Comorbidity | ||||||
| Previous diverticular bleeding | 0.23 (0.06–0.97) | 0.044 | ||||
| Hypertension | 0.82 (0.47–1.43) | 0.485 | ||||
| Dyslipidemia | 0.69 (0.34–1.38) | 0.292 | ||||
| Diabetes mellitus | 1.32 (0.69–2.54) | 0.398 | ||||
| Diabetes complication | 1.93 (0.59–6.25) | 0.275 | ||||
| Hemiplegia | 3.97 (1.55–10.14) | 0.004 | ||||
| Cerebrovascular disease | 1.87 (0.98–3.59) | 0.059 | ||||
| Chronic obstructive pulmonary disease | 3.66 (1.44–9.34) | 0.007 | ||||
| Dementia | 3.08 (1.44–6.60) | 0.004 | ||||
| Collagen disease | 1.39 (0.43–4.51) | 0.579 | ||||
| Ischemic heart disease | 0.91 (0.41–2.02) | 0.814 | ||||
| Heart failure | 2.56 (1.24–5.29) | 0.011 | ||||
| Previous peptic ulcer | 1.39 (0.55–3.52) | 0.486 | ||||
| Renal failure | 2.17 (1.15–4.08) | 0.017 | ||||
| Peripheral arterial disease | 2.76 (1.09–7.02) | 0.033 | ||||
| Chronic hepatitis | 1.71 (0.41–7.11) | 0.460 | ||||
| Cirrhosis | 5.33 (2.08–13.69) | < 0.001 | 4.72 (1.44–15.49) | 1.55 (0.36–2.74) | 0.011 | 2 |
| Blood malignancy and nonmetastatic solid cancer† | 6.13 (3.52–10.68) | < 0.001 | ||||
| Metastatic cancer | 14.23 (7.40–27.36) | < 0.001 | 10.84 (4.56–25.94) | 2.38 (1.51–3.26) | < 0.001 | 3 |
BMI body mass index, BUN blood urea nitrogen, CI confidence interval, CRP C-reactive protein, INR international normalized ratio, NSAIDs nonsteroidal anti-inflammatory drugs, WBC white blood cell count.
*Bold values indicate P < 0.05.
†Blood malignancy was included with the comorbidity of leukemia and lymphoma.
Figure 2.
Predictive ability of the CACHEXIA score for 30-day mortality. (A) Comparison of scoring methods in the derivation cohort (n = 5459). (B) 30-day death rates by risk category for the derivation cohort (n = 5459). (C) Comparison of scoring systems in the validation cohort (n = 2795). (D) 30-day mortality rates by risk category in the validation cohort (n = 2795). P values were determined using Fisher’s exact test with Bonferroni correction (B,D). *P value was 0.003, **P value was < 0.001. ROC-AUC receiver operator characteristic curves of the area under the curve, CCI Charlson comorbidity index.
In the validation cohort, the ROC-AUC for the new score was 0.90 (95% CI 0.85–0.95) (Fig. 2C). Patients in low-, medium-, and high-score groups had 30-day mortality rates of 0.2% (n = 4), 1.2% (n = 6), and 20.6% (n = 13), respectively. The mortality rates were significantly higher in the medium-score group compared with the low-score group and in the high-score group compared with the low- and medium-score groups (low vs. medium score, P = 0.003; high vs. medium/low scores, P < 0.001) (Fig. 2D). The calibration plot of actual probability compared with the predicted probability of 30-day mortality showed an intercept of − 0.03, a slope of 0.90, and a mean absolute prediction error of 0.005, suggesting acceptable calibration (Supplementary Fig. 1A).
Predictive factors for 1-year mortality and predictive score
Mortality within 1 year was assessed in 6084 cases after excluding the 2170 cases (Fig. 1). The characteristics and in-hospital care of the derivation and validation cohorts were similar, except for five factors: platelet count < 150,000/mm3, diabetes mellitus, the use of CT for diagnosis, surgery for hemostasis, and bleeding due to cancer (Supplementary Table 3). In the derivation cohort, 163 (3.0%) of 4030 patients died within 1 year. Univariate analysis showed that 34 factors were associated with 1-year mortality among the baseline characteristics and in-hospital management data. The Cox proportional hazards model showed 10 risk factors for 1-year mortality, including PS ≥ 2, albumin level < 3.0 g/dL, BUN ≥ 25 mg/dL, CRP ≥ 1.0 mg/dL, comorbid metastatic cancer, cirrhosis, body mass index (BMI) < 18.5, blood transfusion during hospitalization, blood malignancy and solid cancer, and bleeding from malignancy (hematochezia due to cancer) (Table 3). Based on the coefficients from the multivariate analysis, we created a novel weighted score for predicting 1-year mortality (maximum 17 points) (Table 3).
Table 3.
Predictors of 1-year mortality by Cox proportional hazards model (derivation cohort, n = 4030).
| Characteristics | Crude hazard ratio (95% CI) | P value* | Adjusted hazard ratio (95% CI) | Coefficient (95% CI) | P value* | Score points |
|---|---|---|---|---|---|---|
| Age ≥ 70 years | 1.98 (1.39–2.84) | < 0.001 | ||||
| Sex (male) | 1.17 (0.85–1.61) | 0.347 | ||||
| BMI ≤ 18.5 | 4.01 (2.89–5.55) | < 0.001 | 1.80 (1.21–2.68) | 0.59 (1.89–0.99) | 0.004 | 1 |
| Current drinker | 0.75 (0.53–1.06) | 0.102 | ||||
| Current smoker | 0.69 (0.43–1.12) | 0.136 | ||||
| Performance status ≥ 2 | 4.29 (3.09–5.96) | < 0.001 | 2.22 (1.49–3.31) | 0.80 (0.40–1.20) | < 0.001 | 2 |
| Hemodynamics | ||||||
| Systolic blood pressure ≤ 100 mmHg | 2.92 (2.08–4.09) | < 0.001 | ||||
| Heart rate ≥ 100/min | 1.61 (1.14–2.27) | 0.007 | ||||
| Symptom | ||||||
| Altered mental status | 1.18 (0.64–2.18) | 0.588 | ||||
| Abdominal pain | 0.81 (0.53–1.23) | 0.319 | ||||
| Diarrhea | 1.17 (0.70–1.95) | 0.562 | ||||
| Laboratory data | ||||||
| Hemoglobin < 12 g/dL | 3.91 (2.55–6.00) | < 0.001 | ||||
| WBC > 10,000/mm3 | 1.26 (0.88–1.80) | 0.210 | ||||
| Platelet count < 150,000/mm3 | 2.66 (1.92–3.69) | < 0.001 | ||||
| Albumin level > 3.0 g/dL | 6.77 (4.95–9.26) | < 0.001 | 2.03 (1.33–3.09) | 0.71 (0.28–1.13) | 0.001 | 1 |
| INR ≥ 1.5 | 1.99 (1.33–2.98) | < 0.001 | ||||
| Hematocrit ≤ 35% | 3.61 (2.44–5.33) | < 0.001 | ||||
| BUN ≥ 25 mg/dL | 3.24 (2.38–4.40) | < 0.001 | 1.90 (1.32–2.73) | 0.64 (0.28–1.01) | 0.001 | 1 |
| Creatinine ≥ 1.5 mg/dL | 3.35 (2.42–4.64) | < 0.001 | ||||
| CRP ≥ 1.0 mg/dL | 3.08 (2.27–4.19) | < 0.001 | 1.70 (1.15–2.51) | 0.53 (0.14–0.92) | 0.008 | 1 |
| Home medication | ||||||
| Low-dose aspirin | 1.03 (0.71–1.49) | 0.884 | ||||
| Antiplatelet drug (non-aspirin) | 0.96 (0.62–1.49) | 0.842 | ||||
| Warfarin | 1.16 (0.68–1.97) | 0.584 | ||||
| Direct oral anticoagulant | 1.29 (0.73–2.27) | 0.381 | ||||
| NSAIDs | 2.03 (1.35–3.04) | < 0.001 | ||||
| Acetaminophen | 2.32 (1.19–4.55) | 0.014 | ||||
| Corticosteroid | 2.02 (1.25–3.25) | 0.004 | ||||
| Comorbidity | ||||||
| Previous diverticular bleeding | 0.34 (0.18–0.67) | < 0.001 | ||||
| Hypertension | 0.72 (0.53–0.98) | 0.038 | ||||
| Dyslipidemia | 0.77 (0.53–1.10) | 0.148 | ||||
| Diabetes mellitus | 1.26 (0.89–1.81) | 0.197 | ||||
| Diabetes complication | 1.57 (0.80–3.08) | 0.187 | ||||
| Hemiplegia | 1.47 (0.60–3.57) | 0.399 | ||||
| Cerebrovascular disease | 1.16 (0.73–1.70) | 0.611 | ||||
| Chronic obstructive pulmonary disease | 1.73 (0.88–3.39) | 0.110 | ||||
| Dementia | 2.72 (1.65–4.49) | < 0.001 | ||||
| Collagen disease | 1.61 (0.91–2.83) | 0.102 | ||||
| Ischemic heart disease | 0.81 (0.52–1.27) | 0.354 | ||||
| Heart failure | 2.40 (1.62–3.57) | < 0.001 | ||||
| Previous peptic ulcer | 1.48 (0.91–2.41) | 0.119 | ||||
| Renal failure | 2.16 (1.53–3.04) | < 0.001 | ||||
| Peripheral arterial disease | 1.49 (0.79–2.83) | 0.219 | ||||
| Chronic hepatitis | 1.74 (0.85–3.54) | 0.128 | ||||
| Cirrhosis | 5.64 (3.41–9.32) | < 0.001 | 4.44 (2.46–8.01) | 1.49 (0.90–2.08) | < 0.001 | 3 |
| Blood malignancy and nonmetastatic solid cancer† | 7.82 (5.72–10.68) | < 0.001 | 3.03 (1.97–4.66) | 1.11 (0.68–1.54) | < 0.001 | 2 |
| Metastatic cancer | 14.96 (10.58–21.16) | < 0.001 | 4.48 (2.75–7.31) | 1.50 (1.01–1.99) | < 0.001 | 3 |
| Diagnostic procedure | ||||||
| CT | 0.80 (0.58–1.09) | 0.161 | ||||
| Therapeutic procedures | ||||||
| Endoscopic treatment | 0.77 (0.53–1.12) | 0.171 | ||||
| Interventional radiology | 1.03 (0.33–3.21) | 0.965 | ||||
| Surgery | 2.34 (1.10–4.99) | 0.028 | ||||
| Blood transfusion during hospitalization | 3.51 (2.58–4.77) | < 0.001 | 1.92 (1.37–2.82) | 0.65 (0.27–1.04) | 0.001 | 1 |
| The final diagnosis of hematochezia | ||||||
| Colonic diverticular bleeding | 0.34 (0.25–0.48) | < 0.001 | ||||
| Rectal ulcer | 5.43 (3.19–9.24) | < 0.001 | ||||
| Angioectasia | 2.23 (0.99–5.05) | 0.054 | ||||
| Upper gastrointestinal bleeding | 3.59 (1.89–6.80) | < 0.001 | ||||
| Small intestinal bleeding | 2.24 (1.10–4.56) | 0.026 | ||||
| Malignancy | 6.43 (4.17–9.92) | < 0.001 | 2.61 (1.56–4.36) | 0.96 (0.45–1.47) | < 0.001 | 2 |
| Others | 2.61 (1.71–4.00) | < 0.001 | ||||
| In-hospital outcomes | ||||||
| Length of hospital stay ≥ 8 days | 2.43 (1.74–3.38) | < 0.001 | ||||
| Rebleeding during hospitalization | 1.01 (0.65–1.57) | 0.956 | ||||
| Thromboembolism | 4.54 (2.01–10.27) | < 0.001 | ||||
BMI body mass index, BUN blood urea nitrogen, CI confidence interval, CRP C-reactive protein, CT computed tomography, INR international normalized ratio, NSAIDs nonsteroidal anti-inflammatory drugs, WBC white blood cell count.
*Bold values indicate P < 0.05.
†Blood malignancy was included with the comorbidity of leukemia and lymphoma.
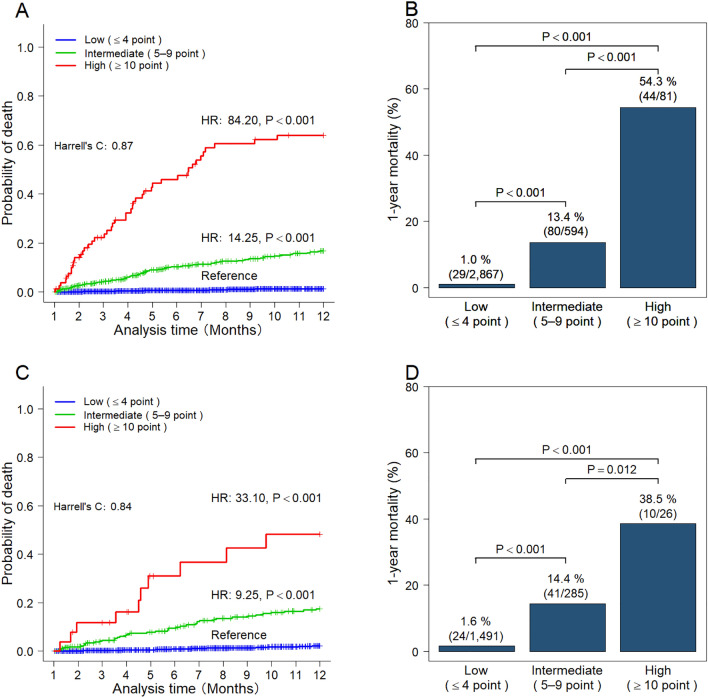
In the derivation cohort, the c-statistic for the novel score was 0.87 (95% CI 0.84–0.90). Patients were categorized into low-score ≤ 4 (n = 2867), medium-score 5–9 (n = 594), and high-score ≥ 10 (n = 81) groups. A log-rank test revealed that patients in the high-score group had significantly higher probabilities of death compared with patients in the low-score group (HR 84.20; 95% CI 52.6–137.9; P < 0.001) and the medium-score group (HR 14.25; 95% CI 9.32–21.8; P < 0.001) (Fig. 3A). The 1-year mortality rates for the low-, medium-, and high-score groups were 1.0%, 13.4%, and 54.3%, respectively (all: P < 0.001) (Fig. 3B).
Figure 3.
Predictive ability of the CACHEXIA score for 1-year mortality. (A) Cumulative probability of mortality according to risk category in the derivation cohort (n = 4030). (B) One-year mortality rates by risk category in the derivation cohort (n = 4030). (C) The cumulative death probability according to risk category in the validation cohort (n = 2054). (D) One-year mortality rates by risk category in the validation cohort (n = 2054). P values were calculated using the Cox proportional hazards model (A,C) and Fisher’s exact test with Bonferroni correction (B,D). Cases with missing data were excluded from the full case analysis (B,D). ROC-AUC receiver operator characteristic curves of the area under the curve, CCI Charlson comorbidity index.
The c-statistic for novel scores in the validation cohort was 0.84 (95% CI 0.80–0.89). Compared to the low-score group, the high-score group (HR 33.10; 95% CI 15.8–69.4; P < 0.001) and the intermediate-score group (HR 9.25; 95% CI 5.6–15.3; P < 0.001) had significantly higher probabilities of death (Fig. 3C). The 1-year mortality rates for the low-, medium-, and high-score groups were 1.6%, 14.4%, and 38.5%, respectively (low vs. intermediate and low vs. high: P < 0.001; intermediate vs. high: P = 0.012) (Fig. 3D). The calibration plot of actual probability compared with the predicted probability of 1-year survival showed a mean absolute prediction error of 0.006 (Supplementary Fig. 1B).
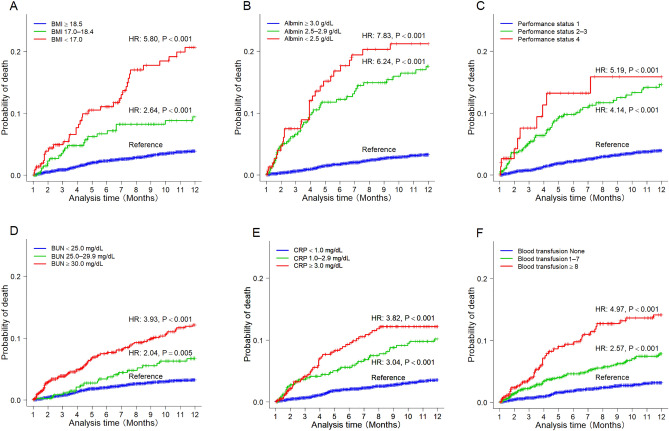
Group analysis was carried out to investigate the association between 1-year mortality and various factors, which were split into three categories. In the Cox proportional hazards model, a decrease in BMI (< 17.0, 17.0–18.4, and ≥ 18.5) and albumin levels (< 2.5, 2.5–2.9, and ≥ 3.0 g/dL) significantly increased the probability of 1-year mortality (all: P < 0.001) (Fig. 4A,B). In contrast, increases in PS (1, 2, 3, and 4), BUN (< 25.0, 25.0–29.9, > 30.0 mg/dL), CRP (< 1.0, 1.0–2.9, and > 3.0 mg/dL), and the amount of blood transfusion (none, 1–7 units, and 8 units) significantly increased the probability of 1-year mortality (all: P < 0.001) (Fig. 4C–F).
Figure 4.
Cumulative probability of mortality according to risk factors using the Kaplan–Meier method. (A) BMI (< 17.0, 17.0–18.4, and ≥ 18.5). (B) Albumin level (< 2.5, 2.5–2.9, and ≥ 3.0 g/dL). (C) Performance status (1, 2–3, and 4). (D) BUN (< 25.0, 25.0–29.9, and > 30.0 mg/dL). (E) CRP (< 1.0, 1.0–2.9, and ≥ 3.0 mg/dL). (F) Amount of blood transfusion (none, 1–7 units, and ≥ 8 units). P values were calculated using the Cox proportional hazards model. BMI body mass index, BUN blood urea nitrogen, CRP C-reactive protein.
Discussion
To calculate long-term mortality risks, we analyzed information from a multicenter trial that included 8254 patients with acute hematochezia who required emergency hospitalization. The 30-day and 1-year mortality rates were low, at 0.9% and 3.0%, respectively. We created a highly accurate (c-index 0.87) long-term prognostic scoring system called the CACHEXIA score (Cancer including metastasis tumor, blood tumor, and bleeding from tumor, Albumin, Cirrhosis, High PS, EXtremely thin (i.e., low BMI), Increased CRP and BUN, Anemia (i.e., blood transfusion)). Each predictor was assigned a score based on adjusted regression coefficients, and risk was stratified by summing these scores. The 1-year mortality rates for patients with low-, medium-, and high-risk CACHEXIA scores were 1.0%, 13.4%, and 54.3%, respectively. A few of these variables also helped forecast the short-term prognosis. Our results demonstrate that cachexia-related factors rather than bleeding-related factors are highly correlated with prognosis in patients with acute hematochezia. The innovative predictive score facilitates accurate stratification of the high-risk group despite the low mortality rate of patients with acute hematochezia.
Aoki et al.9 (n = 342) and Arroja et al.10 (n = 364) reported mortality rates of 4.2% and 2.2% 1 year post-hospitalization, respectively, for the long-term prognosis of ALGIB, which are comparable to that of the present study (3.0%). Thus, results of previous studies were validated by our large cohort. Regarding short-term outcomes (death within 30 days or in-hospital mortality), previously reported risk factors include age, comorbid illnesses, hypoalbuminemia, and low BMI, which, apart from age, do not contradict the results of this study5,7,11,30. Strate et al., based on an analysis of over 200,000 cases using ICD codes, pointed out that age, comorbidities, and bleeding while hospitalized for other diseases are strongly correlated with in-hospital mortality. In contrast, they noted that diverticular bleeding, a common cause of severe bleeding, is not associated with systemic illness and therefore does not contribute to mortality risk11. The short-term mortality rate (0.9%) in this study was lower than the mortality rate in a study conducted by Sengupta et al. (10.9%). However, predictors of mortality help explain this disparity. Metastatic tumors and cirrhosis accounted for 12.3% and 6.6% of patients in the previous analysis, respectively, which were higher than the rates in the present study (2.6% and 2.1%, respectively). Thus, the prevalence of prognostic indicators may have a significant impact on the mortality rate.
Risk factors identified in our study included many diseases with poor progressive prognosis, such as malignant disease and cirrhosis. Notably, the findings showed that cachexia, a poor prognostic condition, had a significant impact on the long-term prognosis of patients with hematochezia. Cachexia is a debilitating condition characterized by poor nutritional status, weight loss, and decreased BMI as objective indicators31,32. Furthermore, increased catabolism, cancer treatment resistance, and elevated PS are associated with refractory cancer cachexia33. Cancer cachexia is associated with systemic inflammation, and CRP levels correlate with shorter survival in patients with advanced cancer22. Therefore, cachexia is closely associated with unfavorable prognostic variables found in the current study, including high PS, low albumin level, high CRP, and low BMI.
The CACHEXIA score developed in our study is a long-term prognostic score encompassing short-term prognostic factors. This score is used to predict long-term prognosis in patients with acute hematochezia. Furthermore, this new score has been shown to be a more accurate predictive tool for short-term mortality than other ALGIB prognostic scores. Previously, Nagata et al. reported that patients with gastrointestinal bleeding have a poorer long-term prognosis than patients with non-gastrointestinal bleeding34. Stratified data on long-term prognosis are scarce, and prognostication after gastrointestinal bleeding is essential in managing patients with multiple diseases. Patients highly at risk of death may refuse invasive cancer treatments and receive more comprehensive medical care. Patients with high scores are expected to have a poor prognosis and may be candidates for mortality prevention strategies. Pharmacological therapies, nutrition therapy, exercise, and psychosocial interventions are vital in preventing the advancement of cachexia35–38. Conversely, even with repeated bleeding, patients with low scores would be expected to have a good prognosis and may be candidates for interventions against bleeding.
The strength of this study was its ability to assess long-term results despite the copious amounts of data gathered during the medical record survey. However, this study had several limitations. First, this research was retrospective and subject to selection bias. For instance, our investigation was limited to cases of hematochezia with emergency admissions. Assuming that patients with life-threatening lower gastrointestinal bleeding are hospitalized emergently, we focused on patients requiring emergency admission. However, admission criteria vary by country and facility, reducing external validity. The Oakland score is widely used globally as a severity score for lower gastrointestinal bleeding and is specified in UK guidelines as a criterion for hospitalization, suggesting its widespread use in many countries and facilities. In our study, we could not confirm that the Oakland score was used as a criterion for hospitalization. Instead, we used the ROC-AUC to compare the CACHEXIA score with the Oakland score, examining which is more suitable for predicting mortality. Additionally, our study did not include a comparison with cases manageable on an outpatient basis, leaving doubts about the appropriateness of using the CACHEXIA score for all cases of hematochezia. In fact, all chronic diseases included in the CACHEXIA score variables are associated with a high 1-year mortality rate. Therefore, the CACHEXIA score might not be a prognostic tool specialized for gastrointestinal bleeding. Second, all participating institutions were Japanese institutions with a sizable number of beds and access to endoscopy. As a result, the generalizability of our scores in different situations, including nonemergency hospitals and hospitals in other countries, should be confirmed. Thirdly, in this study, 2096 cases were lost to follow-up from the 1-year cohort. With ALGIB cases, there tends to be a cessation of follow-up visits once the hemorrhage stops and the anemia improves, as patients become asymptomatic. This is considered one of the reasons why long-term prognosis studies of lower gastrointestinal bleeding are difficult. To resolve such issues, retrospective studies have their limitations. Therefore, it is desirable to conduct prospective studies to carry out more rigorous and high-quality investigations.
In conclusion, our nationwide long-term data indicate the prognosis of patients with acute hematochezia is related more closely to chronic diseases and associated cachexia than to bleeding severity, diagnosis, and treatment. Although the overall mortality rate was low, based on our novel predictive score, high-risk patients should be followed up at the medical facility even after discharge from ALGIB hospitalization.
Supplementary Information
Acknowledgements
The authors thank Takuya Shimamura, Takuya Matsunaga, Yoko Oshikiri, and Naohiko Gunji for their assistance with data collection and analysis. The authors would also like to thank Enago (www.enago.jp) for the manuscript review and editing support.
Author contributions
N.N. was the principal investigator of this study. N.N. and N.T. designed and conducted the study and interpreted the data. N.T. mainly wrote the article. K.Kobayashi, A.Yamauchi, A.Yamada, J.O., T.I., T.A., Y.Sato, T.Kishino, N.I., T.S., M.M., A.T., K.M., K.Kinjo, S.Fujimori, T.U., H.S., S.S., T.N., J.H., T.F., Y.Kinjo, A.M., S.K., T.M., R.G., H.F., Y.F., T.H., Y.T., K.Narimatsu, N.M., K.Nagaike, T.Kinjo, Y.S., S.Funakoshi, K.K., T.M., Y.Komaki, K.M., K.W., and M.K. collected and interpreted the data. E.S. performed the statistical analysis. N.N., A.T., and M.F. provided corrections and advice on preparing the article.
Funding
The funding was provided by Koseikan Institutional Research Grant (grant number: 22-01-01), The Ministry of Health, Labour, and Welfare, Japan (19HB1003), Japan Society for the Promotion of Science KAKENHI Grants (JP17K09365 and JP20K08366), Smoking Research Foundation, Takeda Science Foundation, Tokyo Medical University Cancer Research Foundation, Tokyo Medical University Research Foundation, Grants-in-Aid for Research from the National Center for Global Health and Medicine (29-2001, 29-2004, 19A1011, 19A1022, 19A-2015, 29-1025, and 30-1020).
Data availability
Access to the data supporting the results of this study will be requested and reviewed with the principal investigator of this study through the corresponding author. The data are not available to the public due to privacy and ethical restrictions.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-55889-7.
References
- 1.Oakland K, et al. Derivation and validation of a novel risk score for safe discharge after acute lower gastrointestinal bleeding: A modelling study. Lancet Gastroenterol. Hepatol. 2017;2:635–643. doi: 10.1016/S2468-1253(17)30150-4. [DOI] [PubMed] [Google Scholar]
- 2.Aoki T, et al. Development and validation of a risk scoring system for severe acute lower gastrointestinal bleeding. Clin. Gastroenterol. Hepatol. 2016;14:1562–1570. doi: 10.1016/j.cgh.2016.05.042. [DOI] [PubMed] [Google Scholar]
- 3.Strate LL, Orav EJ, Syngal S. Early predictors of severity in acute lower intestinal tract bleeding. Arch. Intern. Med. 2003;163:838–843. doi: 10.1001/archinte.163.7.838. [DOI] [PubMed] [Google Scholar]
- 4.Velayos FS, et al. Early predictors of severe lower gastrointestinal bleeding and adverse outcomes: A prospective study. Clin. Gastroenterol. Hepatol. 2004;2:485–490. doi: 10.1016/S1542-3565(04)00167-3. [DOI] [PubMed] [Google Scholar]
- 5.Niikura R, et al. Factors affecting in-hospital mortality in patients with lower gastrointestinal tract bleeding: A retrospective study using a national database in Japan. J. Gastroenterol. 2015;50:533–540. doi: 10.1007/s00535-014-0994-3. [DOI] [PubMed] [Google Scholar]
- 6.Oakland K, et al. Acute lower GI bleeding in the UK: Patient characteristics, interventions and outcomes in the first nationwide audit. Gut. 2018;67:654–662. doi: 10.1136/gutjnl-2016-313428. [DOI] [PubMed] [Google Scholar]
- 7.Strate LL, Ayanian JZ, Kotler G, Syngal S. Risk factors for mortality in lower intestinal bleeding. Clin. Gastroenterol. Hepatol. 2008;6:1004–1010. doi: 10.1016/j.cgh.2008.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kollef MH, O’Brien JD, Zuckerman GR, Shannon W. BLEED: A classification tool to predict outcomes in patients with acute upper and lower gastrointestinal hemorrhage. Crit. Care Med. 1997;25:1125–1132. doi: 10.1097/00003246-199707000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Aoki T, et al. Recurrence and mortality among patients hospitalized for acute lower gastrointestinal bleeding. Clin. Gastroenterol. Hepatol. 2015;13:488–494. doi: 10.1016/j.cgh.2014.06.023. [DOI] [PubMed] [Google Scholar]
- 10.Arroja B, et al. Acute lower gastrointestinal bleeding management in Portugal: A multicentric prospective 1-year survey. Eur. J. Gastroenterol. Hepatol. 2011;23:317–322. doi: 10.1097/MEG.0b013e328344ccb5. [DOI] [PubMed] [Google Scholar]
- 11.Sengupta N, Tapper EB. Derivation and internal validation of a clinical prediction tool for 30-day mortality in lower gastrointestinal bleeding. Am. J. Med. 2017;130:e1–e8. doi: 10.1016/j.amjmed.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 12.Devani K, et al. Trends in hospitalization, mortality, and timing of colonoscopy in patients with acute lower gastrointestinal bleeding. Endosc. Int. Open. 2021;9:E777–E789. doi: 10.1055/a-1352-3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nagata N, et al. Role of urgent contrast-enhanced multidetector computed tomography for acute lower gastrointestinal bleeding in patients undergoing early colonoscopy. J. Gastroenterol. 2015;50:1162–1172. doi: 10.1007/s00535-015-1069-9. [DOI] [PubMed] [Google Scholar]
- 14.Obana T, et al. Prospective evaluation of contrast-enhanced computed tomography for the detection of colonic diverticular bleeding. Dig. Dis. Sci. 2013;58:1985–1990. doi: 10.1007/s10620-013-2629-6. [DOI] [PubMed] [Google Scholar]
- 15.Strate LL, Syngal S. Predictors of utilization of early colonoscopy vs radiography for severe lower intestinal bleeding. Gastrointest. Endosc. 2005;61:46–52. doi: 10.1016/S0016-5107(04)02227-8. [DOI] [PubMed] [Google Scholar]
- 16.García-Blázquez V, et al. Accuracy of CT angiography in the diagnosis of acute gastrointestinal bleeding: Systematic review and meta-analysis. Eur. Radiol. 2013;23:1181–1190. doi: 10.1007/s00330-012-2721-x. [DOI] [PubMed] [Google Scholar]
- 17.Nagata N, et al. Identifying bleeding etiologies by endoscopy affected outcomes in 10,342 cases with hematochezia: CODE BLUE-J study. Am. J. Gastroenterol. 2021 doi: 10.14309/ajg.0000000000001413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagata N, et al. Nationwide large-scale data of acute lower gastrointestinal bleeding in Japan uncover detailed etiologies and relevant outcomes: CODE BLUE J-study. MedRxiv. 2021;1:35. [Google Scholar]
- 19.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 20.Nagata N, et al. Safety and effectiveness of early colonoscopy in management of acute lower gastrointestinal bleeding on the basis of propensity score matching analysis. Clin. Gastroenterol. Hepatol. 2016;14:558–564. doi: 10.1016/j.cgh.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 21.Gobinet-Suguro M, et al. Treatment strategies for reducing early and late recurrence of colonic diverticular bleeding based on stigmata of recent hemorrhage: A large multicenter study. Gastrointest. Endosc. 2022;95:1210–1222. doi: 10.1016/j.gie.2021.12.023. [DOI] [PubMed] [Google Scholar]
- 22.Deans C, Wigmore SJ. Systemic inflammation, cachexia and prognosis in patients with cancer. Curr. Opin. Clin. Nutr. Metab. Care. 2005;8:265–269. doi: 10.1097/01.mco.0000165004.93707.88. [DOI] [PubMed] [Google Scholar]
- 23.Sung JJY, et al. Causes of mortality in patients with peptic ulcer bleeding: A prospective cohort study of 10,428 cases. Am. J. Gastroenterol. 2010;105:84–89. doi: 10.1038/ajg.2009.507. [DOI] [PubMed] [Google Scholar]
- 24.Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR. A simulation study of the number of events per variable in logistic regression analysis. J. Clin. Epidemiol. 1996;49:1373–1379. doi: 10.1016/S0895-4356(96)00236-3. [DOI] [PubMed] [Google Scholar]
- 25.Cepeda MS, Boston R, Farrar JT, Strom BL. Comparison of logistic regression versus propensity score when the number of events is low and there are multiple confounders. Am. J. Epidemiol. 2003;158:280–287. doi: 10.1093/aje/kwg115. [DOI] [PubMed] [Google Scholar]
- 26.Hosmer DW, Lemeshow S, Cook ED. Applied Logistic Regression. 2. Wiley; 2000. [Google Scholar]
- 27.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics. 1988;44:837–845. doi: 10.2307/2531595. [DOI] [PubMed] [Google Scholar]
- 28.Harrell FE, Lee KL, Mark DB. Multivariable prognostic models: Issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat. Med. 1996;15:361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 29.Moons KGM, et al. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): Explanation and elaboration. Ann. Intern. Med. 2015;162:W1–W73. doi: 10.7326/M14-0698. [DOI] [PubMed] [Google Scholar]
- 30.Radaelli F, et al. Clinical management and patient outcomes of acute lower gastrointestinal bleeding. A multicenter, prospective, cohort study. Dig. Liver Dis. 2021;53:1141–1147. doi: 10.1016/j.dld.2021.01.002. [DOI] [PubMed] [Google Scholar]
- 31.Bozzetti F, Mariani L. Defining and classifying cancer cachexia: A proposal by the SCRINIO Working Group. J. Parenter. Enteral Nutr. 2009;33:361–367. doi: 10.1177/0148607108325076. [DOI] [PubMed] [Google Scholar]
- 32.Bennani-Baiti N, Walsh D. What is cancer anorexia–cachexia syndrome? A historical perspective. J. R. Coll. Phys. Edinb. 2009;39:257–262. [PubMed] [Google Scholar]
- 33.Fearon K, et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011;12:489–495. doi: 10.1016/S1470-2045(10)70218-7. [DOI] [PubMed] [Google Scholar]
- 34.Nagata N, et al. Acute severe gastrointestinal tract bleeding is associated with an increased risk of thromboembolism and death. Clin. Gastroenterol. Hepatol. 2017;15:1882–1889. doi: 10.1016/j.cgh.2017.06.028. [DOI] [PubMed] [Google Scholar]
- 35.Arends J, et al. Cancer cachexia in adult patients: ESMO clinical practice guidelines. ESMO Open. 2021;6:100092. doi: 10.1016/j.esmoop.2021.100092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Borg JJ, Anker SD, Rosano G, Serracino-Inglott A, Strasser F. Multimodal management as requirement for the clinical use of anticachexia drugs—A regulatory and a clinical perspective. Curr. Opin. Support. Palliat. Care. 2015;9:333–345. doi: 10.1097/SPC.0000000000000176. [DOI] [PubMed] [Google Scholar]
- 37.Fearon K, Arends J, Baracos V. Understanding the mechanisms and treatment options in cancer cachexia. Nat. Rev. Clin. Oncol. 2013;10:90–99. doi: 10.1038/nrclinonc.2012.209. [DOI] [PubMed] [Google Scholar]
- 38.Bland KA, et al. Quality of life and symptom burden improve in patients attending a multidisciplinary clinical service for cancer cachexia: A retrospective observational review. J. Pain Symptom Manag. 2021;62:e164–e176. doi: 10.1016/j.jpainsymman.2021.02.034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Access to the data supporting the results of this study will be requested and reviewed with the principal investigator of this study through the corresponding author. The data are not available to the public due to privacy and ethical restrictions.