Highlights
-
•
Impaired recovery of systolic and diastolic blood pressure after standing is associated with accelerated brain ageing, determined using the BrainPAD model.
-
•
No association was found between heart rate recovery and brain age acceleration.
-
•
Evaluation of the response of blood pressure to active standing may be an indicator of brain health, and accelerated brain ageing.
Keywords: Active stand, Cerebral autoregulation, Orthostatic hypotension, Brain aging
Abstract
Background
Impaired recovery of blood pressure (BP) in response to standing up is a prevalent condition in older individuals. We evaluated the relationship between the early recovery of hemodynamic responses to standing and brain health in adults over 50.
Methods
Participants from The Irish Longitudinal Study on Ageing (TILDA) (n=411; age 67.6 ± 7.3 years; 53.4 % women) performed an active stand challenge while blood pressure and heart rate were continuously monitored. The recovery of these parameters was determined as the slope of the BP and HR response, following the initial drop/rise after standing. We have previously reported a novel and validated measure of brain ageing using MRI data, which measures the difference between biological brain age and chronological age, providing a brain-predicted age difference (brainPAD) score.
Results
Slower recovery of systolic and diastolic BP was found to be significantly associated with higher brainPAD scores (i.e., biologically older brains), where a one-year increase in brainPAD was associated with a decrease of 0.02 mmHg/s and 0.01 mmHg/s in systolic and diastolic BP recovery, respectively, after standing. Heart rate (HR) recovery was not significantly associated with brainPAD score.
Conclusion
These results demonstrate that slower systolic and diastolic BP recovery in the early phase after standing is associated with accelerated brain aging in older individuals. This suggests that the BP response to standing, measured using beat-to-beat monitoring, has the potential to be used as a marker of accelerated brain aging, relying on a simple procedure and devices that are easily accessible.
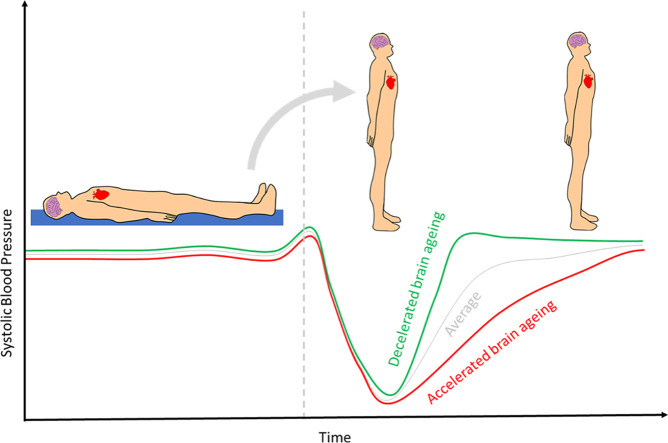
Graphical abstract
1. Introduction
After standing, systolic blood pressure (SBP) decreases to about 75% of its baseline value by 10 seconds, on average, with most of the deficit being recovered by 20 seconds. This has prompted attempts to characterise the early hemodynamic recovery after orthostasis, which is enabled by the use of continuous beat-to-beat monitoring devices. Initial orthostatic hypotension (OH) is commonly used to refer to a symptomatic decrease of ≥40 mmHg in SBP or ≥20 mmHg diastolic blood pressure (DBP) within 15 seconds of standing and has a prevalence of around 33 % in the over 50s [16]. Measures describing early orthostatic hemodynamic recovery, have been linked to outcomes including physical function (de [3,4,32,34]), global cognitive decline [30], depression [2], standing speed [35], mortality [29], frailty [28,34] and falls and ageing [15,21].
Stabilization of blood pressure (BP) after standing is mediated by the cardiovascular centre. This structure is located in the brain stem and receives information from several sensory receptors in the body and higher regions of the brain and regulates cardiac output and blood vessel diameter. Arterial baroreceptors (located primarily in the aortic arch and carotid artery sinuses) and cardiopulmonary baroreceptors (found in the atria and ventricles of the heart as well as the pulmonary circulation) are networked with areas in the cardiovascular centre and transmit real-time information about blood pressure [23]. Neuroimaging studies implicate a role of the thalamus, hypothalamus, insular cortex, anterior cingulate cortex, prefrontal cortex, amygdala and cerebellum in these functions ([23], [27]).
Ageing of the brain leads to structural changes in a region-specific and non-linear manner [8]. These alterations include brain atrophy, particularly a decrease in grey matter volume [13,41], increase of white matter hyperintensities (WMH) [18,24] and microbleeds [43,47]. Therefore, the regions implicated in autonomic control, including BP regulation, could be affected by brain ageing and it is plausible that accelerated brain ageing may cause impaired cerebral blood flow in response to postural transition.
The link between impaired orthostatic BP and brain health remains uncertain [6] with some studies reporting a lack of association with markers of brain health, such as WMHs [45] and cognitive tests [14], while others provide evidence for a connection [5,11,25,36].
The estimation of an individual's brain age, based on neuroimaging markers, can provide a more complete and subject-specific measure of their brain health. Brain-predicted age difference (BrainPAD) is a summary metric that reflects the difference between the predicted biological age of the brain and the chronological age of an individual [1]. BrainPAD model was created using machine learning in neuroimaging data, whereby grey matter density voxels from structural T1-weighted MRI data are used to predict chronological age [1]. Assuming that a healthy brain follows a trajectory of normal structural age-related changes, the alterations that deviate from that might be linked to an underlying problem, such as hypoperfusion, which can be associated with age-related disease or cognitive decline [8]. Brain age difference infers acceleration of brain ageing, where positive values indicate an older brain relative to an individual's years lived [1].
BrainPAD models based in brain structural analysis have previously been used to assess how ischemic heart disease and other vascular risk factors, such as arterial compliance and cardiac structure, are related to age-related decline of brain [42]. The use of brainPAD was proposed as it is summary objective metric of brain health, which has the potential to be used to communicate to patients the risk of brain impairment and promote interventive measures. The potential of BrainPAD as an objective marker of brain health, coupled with beat-to-beat monitoring of orthostatic BP, could further inform the association between brain health and impaired BP and heart rate (HR) regulation.
We hypothesised that accelerated physiological ageing of the brain is associated with a poorer peripheral hemodynamic BP recovery in response to an orthostatic challenge. The recovery of SBP, DBP and HR were measured in participants during an active stand challenge and the associations between these parameters and BrainPAD score were determined.
2. Methods
2.1. Sample
The Irish Longitudinal Study on Ageing (TILDA) is a cohort study of community-dwelling older adults (aged ≥ 50 years) in Ireland, which collects data on their health, financial and social circumstances and investigates the interaction between these measures [12]. The study commenced in 2009 and waves of data collection have taken place every two years since. For the purposes of this study, cross-sectional data from wave 3 (2014-2015), which was the first wave at which a sub sample of participants had MRI brain scans, was used.
The sub sample of participants had a detailed home-based computer assisted personal interview (CAPI) assessment during which information of self-reported health, social and economic variables were collected. A health assessment (full details can be accessed elsewhere [10,22]) was also carried out at a dedicated centre by trained nurses, during which measures of peripheral and central hemodynamics were determined. The multiparametric MRI brain scans took place at a nearby teaching hospital.
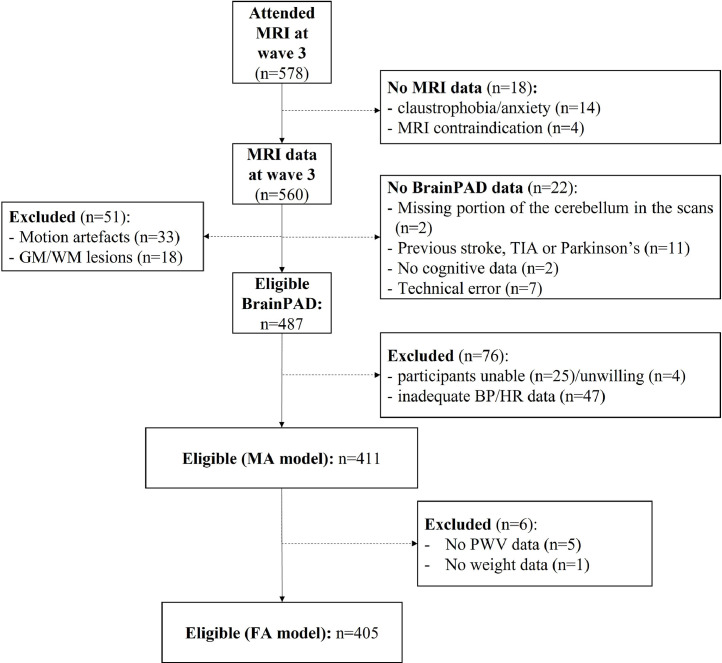
In total, 578 participants agreed to undergo MRI scans. 18 participants did not provide data (due to claustrophobia/anxiety (n=14) or MRI contraindication (n=4). A further 73 participants were excluded from BrainPAD calculation due to motion artifacts (n=33), grey matter/white matter lesions (n=18), missing portion of the cerebellum in the scans (n=2), previous stroke, transient ischemic attack, or Parkinson's disease (n=11), those without cognitive data (n=2) and due to technical errors (n=7). The final sample size with a BrainPAD score was 487 individuals (Fig. 1). The MRI examination was performed 62 ± 40 days after the health assessment.
Fig. 1.
Flowchart of the sample selection. Abbreviations: MRI: Magnetic Resonance Imaging; BrainPAD: Brain-Predicted Age Difference; GM: gray matter; WM: white matter; TIA: transient ischemic attack; BP: blood pressure; HR: heart rate; PWV: pulse wave velocity; MA: minimally adjusted; FA: fully adjusted.
2.2. Standard protocol approvals, registration, and patient consent
TILDA is compliant with the Declaration of Helsinki and was granted ethical approval by the Health Sciences Research Ethics Committee at Trinity College Dublin (Dublin, Ireland) [12]. Written informed consent was obtained from participants. Individuals were invited in the first instance using a random sampling approach previously described [49]. Additional ethics approval was received for the MRI sub-study from the St James's Hospital/Adelaide and Meath Hospital, inc. National Children's Hospital, Tallaght Research Ethic Committee (Dublin, Ireland). Those attending for MRI were also required to complete an additional MRI-specific consent form. Scanning was performed at the National Centre for Advanced Medical Imaging (CAMI) at St. James's Hospital, Dublin, Ireland.
2.3. Active stand protocol
Participants were required to lie in a supine position without speaking or moving for at least 10 minutes before standing up unassisted as quickly as possible after a countdown from 5 seconds. Over the course of the challenge, three markers of orthostatic hemodynamics were measured continuously: SBP, DBP and HR. A digital photoplethysmograph on the middle finger of the left hand (Finometer MIDI device, Finapres Medical Systems BV, Amsterdam, The Netherlands) facilitated beat-to-beat tracking of SBP, DBP and HR. This device also allowed for the time when the stand was initiated to be identified using a built in height correction unit [35]. A sling supported the participants’ left hand at approximately heart level. The room in which the tests were performed was kept at a temperature between 21°C and 23°.
2.4. MRI data collection and BrainPAD estimation
Structural T1-weighted scans were acquired using a 3T system (Achieva, Philips Medical Systems, The Netherlands) and a 32-channel head coil. A three-dimensional magnetization-prepared rapid gradient echo (MP-RAGE) sequence was used, with the following scan parameters: FOV [mm]: 240×240×162; voxel size [mm] = 0.8 × 0.8 × 0.9; SENSE factor: 2; TR: 6.7 ms; TE: 3.1 ms; flip angle: 8°. The process for generating the BrainPAD scores for this cohort is documented in detail elsewhere [1]. Briefly, a diverse training set of T1-weighted MRI neuroimaging data from multi-site studies utilising different equipment and scanning parameters was processed [20]. This sample (n=1359) was then used to develop a model for prediction of brain age from structural scans based on voxel-wise densities in grey matter (GM) density values. The model was previously used to determine brain-age in three independent datasets including TILDA, showing a significant chronological age prediction and a robust negative correlation with specific domains of cognitive function [1].
2.5. Covariate measurements
Resting BP was measured using an automated digital sphygmomanometer (Omron, Kyoto, Japan). Cases where SBP>=140 mmHg and/or DBP>=90 mmHg were classified as hypertension. Pulse wave velocity (m/s) was determined as the time that a pulse takes to travel from the carotid to the femoral artery, which was measured using a Vicorder device (SMT medical GmbH & Co. KG, Wuerzburg, Germany), divided by the distance between the sternal notch and the center of the femoral cuff. Height was measured to the nearest 0.01m (Seca 240 Stadiometer, Seca Ltd, Birmingham, UK) and weight to the nearest 0.1 kg (Seca 861 Electronic Scales, Seca Ltd, Birmingham, UK). Medication use was reported by the participants, and it was classified using the Anatomical Therapeutic Chemical (ATC) classification codes as antidepressant (ATC code: N06A) and antihypertensive (ATC code: C02, C03, C07, C08 and C09). Smoking status (never, past, current), education level, cardiac diseases (binary variable for any of heart attack, heart failure, angina, stroke, diabetes mellitus, transient ischemic attack, or heart murmur) and number of comorbidities (including chronic pain, cataracts, glaucoma, age macular degeneration, lung disease, asthma, arthritis, osteoporosis, cancer, Parkinson's disease, ulcer, varicose ulcer, liver disease, thyroid problems, kidney disease and anemia) were also collated. Transition time was calculated according to a previously reported algorithm based on changes in the height correction data during the active stand, which allows to determine when the stand started and ended [35].
2.6. Statistical analysis
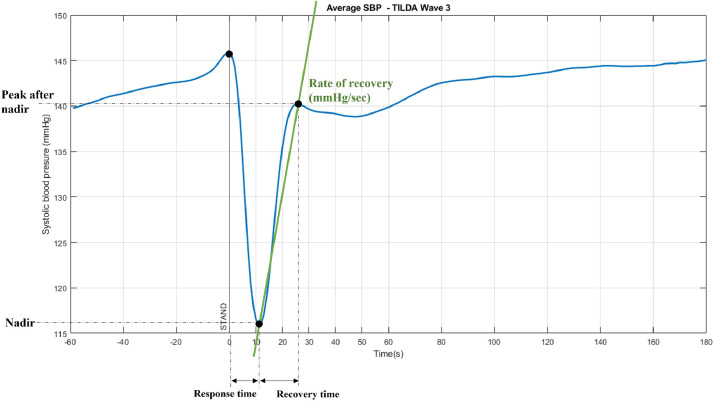
Each hemodynamic measure was processed using MATLAB (R2023a, The MathWorks, Inc., MA, USA). Signals from the Finometer device (i.e. SBP, DBP, HR) were recorded at 200Hz, from which beat-to-beat data (non-uniform time scale) were obtained. The beat-to-beat data were then interpolated to obtain 1 Hz signals (uniform time scale). All data were smoothed using an 11th order median filter followed by a 10-point moving average filter. The time of standing was considered as the moment at which the participant initiated the change of position, identified with the height correction unit of the Finometer. The recovery of SBP and DBP was described as the slope measured between the point where BP reaches the nadir and where it peaks after that (the overshoot, in most cases) [38] (Fig. 2). In the case of HR, the recovery rate consists in the slope between the point where HR reaches its peak, after standing, and the next nadir. A faster recovery was defined as a greater absolute value of BPrecovery rate and HRrecovery rate, where BPrecovery rate is positive and HRrecovery rate is negative.
Fig. 2.
Calculation of the recovery rate after standing. The recovery rate of SBP was determined as rate of increase in SBP from the nadir until the next peak post standing, where greater positive values mean that the recovery of SBP occurred faster. The same calculations were completed for the recovery rate of DBP and HR. In the case of HR recovery, the slope is negative and greater absolute values mean that the recovery is faster. Abbreviations: SBP: systolic blood pressure.
Statistical analysis was performed by fitting linear regression models to the data using STATA (v15.1, StataCorp, TX, USA). Two models were fitted for each of the hemodynamic recovery measures as a dependent variable and BrainPAD as an independent variable: one model adjusting for age and sex only, the other adjusting for age, sex, transition time, cardiovascular disease, hypertension, medications (antihypertensive and antidepressant), weight, height, pulse wave velocity as well as smoking status. The results were interpreted using the unstandardised regression coefficient for BrainPAD which represents the expected change in the recovery for each additional year of brain ageing.
3. Results
3.1. Study participants
In total 411 participants had complete orthostatic BP/HR and BrainPAD data (Fig. 1). The cohort description is summarised in the Table 1.
Table 1.
Sample description of the cohort
| Participant characteristic | Minimally adjusted models (n=411) | |
|---|---|---|
| Age [years], mean ± SD | 67.6 ± 7.3 | |
| Females, % (n) | 53.4 (219/411) | |
| Weight [kg], mean ± SD | 76.3 ± 14.0 | |
| Height [cm], mean ± SD | 165.6 ± 9.4 | |
| Transition time [s], mean ± SD | 7.3 ± 2.9 | |
| MOCA score, mean ± SD | 25.9 ± 3.0 | |
| CES-D score, mean ± SD | 2.7 ± 3.4 | |
| Education level, % (n) | Primary/none | 18.3 (75/411) |
| Secondary | 37.2 (153/411) | |
| Third/higher | 44.5 (183/411) | |
| Smoking, % (n) | Never | 50.4 (207/411) |
| Past | 43.1 (177/411) | |
| Current | 6.6 (27/411) | |
| Antidepressant use, % (n) | 6.1 (25/411) | |
| Antihypertensive use % (n) | 38.4 (158/411) | |
| One or more cardiac condition, % (n) | 14.1 (58/411) | |
| Number of comorbidities, mean ± SD | 1.2 ± 1.3 | |
| Seated SBP [mmHg], mean ± SD | 133.8 ± 18.7 | |
| Seated DBP [mmHg], mean ± SD | 80.0 ± 9.9 | |
| Seated HR [bpm], mean ± SD | 67.8 ± 11.1 | |
| Pulse wave velocity [m/sec], mean ± SD | 10.7 ± 2.0 | |
| Baseline supine SBP* [mmHg], mean ± SD | 142.5 ± 21.5 | |
| Baseline supine DBP* [mmHg], mean ± SD | 76.1 ± 10.0 | |
| Baseline supine HR* [mmHg], mean ± SD | 64.8 ± 9.4 | |
| SBP drop (in relation to the baseline) [mmHg], mean ± SD | -27.0 ± 15.8 | |
| DBP drop (in relation to the baseline) [mmHg], mean ± SD | -15.6 ± 9.2 | |
| HR increase (in relation to the baseline) [mmHg], mean ± SD | 15.3 ± 6.5 | |
| SBP recovery time [s], mean ± SD | 17.7 ± 10.5 | |
| DBP recovery time [s], mean ± SD | 20.5 ± 10.6 | |
| HR recovery time [s], mean ± SD | 16.8 ± 8.4 | |
| SBP recovery rate [mmHg/s], mean ± SD | 1.96 ± 0.96 | |
| DBP recovery rate [mmHg/s], mean ± SD | 1.1 ± 0.5 | |
| HR recovery rate [bpm/s], mean ± SD | -0.7 ± 0.6 | |
| BrainPAD score [years], mean ± SD | -7.4 ± 7.3 | |
* The baseline values of SBP, DBP and HR were calculated as the average of the values measured from the 60s to the 30s before standing [16].
Abbreviations: SD: standard deviation; MOCA: Montreal Cognitive Assessment; CES-D: Center for Epidemiologic Studies Depression Scale; SBP: systolic blood pressure; DBP: diastolic blood pressure; HR: heart rate; BrainPAD: Brain-Predicted Age Difference.
The average age of the sample was 67.6 ± 7.3 years and 53.4 % were female. Medication use was prevalent with 38.4 % and 6.1 % using antihypertensives and antidepressants, respectively; 14.1 % had at least one cardiovascular condition. Average BrainPAD score was -7.4 ± 7.3 years, (i.e. the predicted age based on the MRI data was 7.4 years less than chronological age) ranging from -29.3 to +22.9 years.
3.2. Association between BrainPAD and hemodynamic recovery responses
The recovery rate of orthostatic SBP and DBP was associated with the extent of brain ageing) where an increase in the brainPAD score (accelerated brain ageing) is associated with a decrease in the SBP and DBP recovery rate.
Each additional year of BrainPAD was associated with a -0.02 mmHg/s (95 % CI: -0.03 – 0.00, P<.05) recovery rate of SBP in the minimally and fully adjusted models (Supplementary Tables S1 and S2). The relationship between BrainPAD and DBP recovery rate was similar, with a one year increase in predicted brain age relative to chronological age being associated with an estimated -0.01 mmHg/s (95 % CI: -0.02– 0.00, P<0.01) DBP recovery rate for both minimally and fully adjusted models.
No association was found between the HR recovery rate and the BrainPAD for both the age and sex adjusted model (β = 0.01 bpm/s, 95 % CI: 0.00 – 0.01, P=0.2151) and the fully adjusted model (β = 0.00 bpm/s, 95 % CI: 0.00 – 0.01, P=0.3845).
4. Discussion
4.1. Summary
In this study, we observed that accelerated brain ageing, estimated from structural MRI, was associated with slower recovery of BP after standing. Each year increase in the predicted brain age in relation to the chronological age of an individual (brainPAD), is associated with a reduction in BP recovery rate by 0.02 mmHg/s (SBP) and 0.01 mmHg/s (DBP). HR recovery rate after standing was not associated with brain ageing.
4.2. Previous literature and significance
The relationship between orthostatic hemodynamics and brain health, assessed by structural evaluation, has been previously studied, using different markers and methodologies.
The measure used to evaluate the recovery of BP, following the initial drop that occurs after standing, was the recovery response rate, or the speed of BP increase. This measure was previously used to assess early hemodynamic responses after standing [38] and it can be useful for assessing the effectiveness of the autonomic system in recovering from the BP drop.
Brain health was assessed in this study by using a BrainPAD model, which predicts the difference between biological and chronological age of the brain, based on the GM density [1]. In a normal brain, grey matter decreases with time, and therefore this appears to be a reliable indicator of brain ageing [13,48]. Moreover, the age-related heterogeneity of GM changes [13,41] is represented in BrainPAD, as the GM voxels have different weights on the brain-age prediction [1]. On the other hand, WMH, which have shown to be associated with OH [5,25,45], are not included in the model.
The BrainPAD model used in this study was developed using machine learning on multi-site data (training cohort, n=1359 healthy adults, mean age 40.04±17.78 years, age range = 18.00 - 88.36 years; 63 % females), to ensure generalizability. This was then tested with three independent cohorts (testing data), including the Dokuz Eylül University dataset, the Cognitive Reserve/ReferenceAbility NeuralNetwork study and TILDA, which were from different countries (Turkey, United States of America and Ireland, respectively) and had broad age ranges (47.56–93.51 years, 19–80 years and 50–88 years, respectively). It showed negative correlations with several measures of cognitive performance, including general cognitive status, semantic verbal fluency, processing speed, visual attention, and cognitive flexibility [1]. Since then, BrainPAD was also used in another study using the Alzheimer's Disease Neuroimaging Initiative (ADNI) cohort, where it was related to the integrity of subcortical structures [39,40].
Even though the model used in this study is relatively simple, comparing to recent models available using multimodal data [7] or deep learning [46], which can achieve higher accuracy, it has proven to be generalizable (applicable to samples using different scanning devices and protocols) and it provides comparable accuracy to other models in the literature which were also validated in independent samples [1]. BrainPAD is therefore a well-validated summary metric that reflects general structural changes in the brain associated with ageing and it is related with cognitive performance.
Associations between slower BP responses and brain health markers were previously observed in a cross-sectional study where sustained OH at 20s and 30s post-stand, when coupled with supine hypertension, was associated with lower cognition and executive function [17].
Longitudinal studies also demonstrated this connection [30,50]. OH at 40s and 110s post-stand was linked to a decrease in cognitive performance in a 4-year follow-up study in our research group, which was more marked in individuals with co-existing supine hypertension [30]. In a different population, OH, measured 1, 2 and 3 minutes after standing, increased the risk of developing dementia in a period of 25 years, particularly in individuals lacking a compensatory increase in heart rate [50]. One of the explanations proposed for these findings is that episodes of transient hypoperfusion could lead to damage of some brain regions. In fact, hypoperfusion, identified using MRI scans, was shown to be associated with accelerated cognitive decline after a 10-year follow-up [51]. Faster changes in BP (within seconds or minutes) result in increased changes in cerebral blood flow (CBF), specially at very high frequencies, where the mechanism of autoregulation is more ineffective. However, at a frequency of approximately 0.05 Hz (corresponding to a period of 20 seconds), the mechanism of autoregulation is relatively improved and major changes in BP are slightly dampened in CBF [6]. Therefore, the pathophysiological mechanisms involved are not yet clear. Nevertheless, the findings from this study support the idea that brain health is related to the efficiency of blood pressure regulation.
HR recovery is another measure commonly used to characterise the hemodynamic response to active stand. After standing, the sudden drop in BP detected by the baroreceptors, leads to a decrease in parasympathetic and increase in sympathetic stimulation by the cardiovascular centre in the brain, which results in a rising heart rate. In the present study, no significant relationship was found between the recovery rate of HR and brain ageing.
Results from the Framingham Heart Study Third Generation have shown that decreased orthostatic change in mean arterial pressure was associated with reduced brain volume in older participants and in those with stiffer arteries [9]. Arterial stiffness was also proved to be a risk factor for cognitive decline [26]. Loss of compliance of the aorta and carotid arteries exposes the brain to high pulsatility, which can lead to tissue damage [19] and contributes to the increase of SBP and decrease of DBP through early reflection of the pulse wave [37]. On the other hand, increased arterial stiffness is known to attenuate cardiovagal baroreflex sensitivity, impairing the regulation of BP [33]. Due to these effects, PWV and hypertension were also included as covariates on the fully adjusted models.
Although associations were found between BrainPAD and the recovery rate of BP, the causal direction of this relationship remains unclear. Brain ageing affects regions implicated in BP control, which reflects on poor BP recovery. On the other hand, an initially impaired regulation of BP could lead to repeated transient hypoperfusion in the brain, potentially causing tissue damage in several regions over time. Even though the connection between the speed of BP recovery after standing and cerebral perfusion is not certain [6], if impairment of SBP and DBP stabilization in the early phase of standing does contribute for accelerated brain ageing, it allows a possibility of clinical intervention, in order to avoid further damage. However, these events might occur in a cyclic manner, leading to mixed effects and making it difficult to determine the origin of the impairment.
4.3. Strengths and limitations
There are several strengths to this analysis. The sample size is large and well characterised, allowing to adjust for several confounders. Continuous beat-to-beat BP and HR were assessed simultaneously, which allowed for the detection of changes that cannot be detected using common oscillometric devices. Standing time was calculated objectively using a height sensor.
Limitations associated with BrainPAD, include the fact that this model uses exclusively voxel-wise GM density data and does not consider other neuroimaging data which may be informative about brain ageing, such as WM volumes and microstructural integrity, functional connectivity data and brain vascular function [1,31].
Future work using a topographical approach, such as Voxel-Based Morphometry (VBM) or including other neuroimaging markers of brain vascular function, which are part of the TILDA dataset, could contribute to understanding the relationship between brain health and impaired hemodynamics. More recent brain-age prediction models, which allow for better neuroanatomical interpretation [44], could also help in investigating this association.
Longitudinal work would help clarifying the direction of the potential causal pathways implicated and the outcomes. Studies following young and healthy individuals to old age would also be especially useful, since this would allow for the identification of early disturbances in the brain and/or in the circulatory system.
5. Conclusion
It was observed that individuals with biologically older brains show a slower recovery of SBP and DBP after standing. Future longitudinal analysis are necessary to assess the direction of causality between impaired BP response to standing and brain health.
Availability of data
The data collected for this study is sensitive, requests for access should be directed to The Irish Longitudinal Study on Ageing (TILDA), Trinity Central, 152-160 Pearse St, Dublin 2, D02 R590, Republic of Ireland (email: tilda@tcd.ie).
Sponsor's role
The funding sources had no part in the design, methodology, participant recruitment, data collection and analysis, or preparation of this paper.
CRediT authorship contribution statement
Morgana A. Shirsath: Investigation, Methodology, Software, Visualization, Writing – original draft, Writing – review & editing. John D. O'Connor: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Visualization, Project administration, Writing – original draft, Writing – review & editing, Validation. Rory Boyle: Conceptualization, Data curation, Formal analysis, Methodology, Software, Writing – review & editing, Validation. Louise Newman: Conceptualization, Data curation, Formal analysis, Methodology, Software, Writing – review & editing, Validation. Silvin P. Knight: Conceptualization, Data curation, Methodology, Project administration, Supervision, Writing – review & editing, Validation. Belinda Hernandez: Conceptualization, Data curation, Formal analysis, Methodology, Writing – review & editing, Validation. Robert Whelan: Conceptualization, Methodology, Supervision, Writing – review & editing. James F. Meaney: Supervision, Writing – review & editing. Rose Anne Kenny: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
The authors would like to acknowledge the continued commitment and cooperation of the TILDA participants and research team.
Funding
This research was funded by the Health Research Board (HRB) [HRA-PHR-2014-667]. The Irish Longitudinal Study on Ageing is funded by Irish Life, The Atlantic Philanthropies and the Department of Health in Ireland. The National Centre for Advanced Medical Imaging (CAMI) is grant-funded by the HRB. One author (SPK) acknowledges funding from Science Foundation Ireland (SFI) [18/FRL/6188].
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cccb.2024.100212.
Appendix. Supplementary materials
References
- 1.Boyle Rory, Jollans Lee, Rueda-Delgado Laura M, Rizzo Rossella, Yener Görsev G, McMorrow Jason P, Knight Silvin P, Carey Daniel, Robertson Ian H, Emek-Savaş Derya D. Brain-predicted age difference score is related to specific cognitive functions: a multi-site replication analysis. Brain ImAging Behav. 2021;15(1):327–345. doi: 10.1007/s11682-020-00260-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Briggs Robert, Carey Daniel, Claffey Paul, McNicholas Triona, Newman Louise, Nolan Hugh, Kennelly Sean P., Kenny Rose Anne. The association between frontal lobe perfusion and depressive symptoms in later life. British J. Psychiat. 2019;214(4):230–236. doi: 10.1192/bjp.2018.288. [DOI] [PubMed] [Google Scholar]
- 3.Briggs Robert, Donoghue Orna A, Carey Daniel, O'Connell Matthew D.L., Newman Louise, Kenny Rose Anne. What is the relationship between orthostatic blood pressure and spatiotemporal gait in later life? J. Am. Geriatr. Soc. 2020:16379. doi: 10.1111/jgs.16379. Marchjgs. [DOI] [PubMed] [Google Scholar]
- 4.Bruïne Eline S.de, Reijnierse Esmee M., Trappenburg Marijke C., Pasma Jantsje H., de Vries Oscar J., Meskers Carel G.M.M., Maier Andrea B. Diminished dynamic physical performance is associated with orthostatic hypotension in geriatric outpatients. J. Geriatr. Phys. Ther. 2018;42(3):E28–E34. doi: 10.1519/JPT.0000000000000186. [DOI] [PubMed] [Google Scholar]
- 5.Buckley Anne, Carey Daniel, Meaney James M, Kenny RoseAnne, Harbison Joseph. Is there an association between orthostatic hypotension and cerebral white matter hyperintensities in older people? The irish longitudinal study on ageing. JRSM. Cardiovasc. Dis. 2020;9(January) doi: 10.1177/2048004020954628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Claassen Jurgen A H R, Thijssen Dick H J, Panerai Ronney B, Faraci Frank M. Regulation of cerebral blood flow in humans: physiology and clinical implications of autoregulation. Physiol. Rev. 2021 doi: 10.1152/physrev.00022.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cole James H. Multimodality neuroimaging brain-age in Uk biobank: relationship to biomedical, lifestyle, and cognitive factors. Neurobiol. Aging. 2020;92:34–42. doi: 10.1016/j.neurobiolaging.2020.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cole James H, Franke Katja. Predicting age using neuroimaging: innovative brain ageing biomarkers. Trends. Neurosci. 2017;40(12):681–690. doi: 10.1016/j.tins.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 9.Cooper Leroy L., Himali Jayandra J., Torjesen Alyssa, Tsao Connie W., Beiser Alexa, Hamburg Naomi M., DeCarli Charles, et al. Inter-relations of orthostatic blood pressure change, Aortic stiffness, and brain structure and function in young adults. J. Am. Heart. Assoc. 2017;6(8):1–9. doi: 10.1161/JAHA.117.006206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cronin Hilary, O'Regan Clare, Finucane Ciaran, Kearney Patricia, Kenny Rose Anne. Health and aging: development of the Irish longitudinal study on ageing health assessment. J. Am. Geriatr. Soc. 2013;61:S269–S278. doi: 10.1111/jgs.12197. [DOI] [PubMed] [Google Scholar]
- 11.Cui Yi, Zhang Hua, Zhao Yingxin, Sun Shangwen, Chai Qiang, Gong Gary, Liu Zhendong. Home-measured orthostatic hypotension associated with cerebral small vessel disease in a community-based older population. Hypertension Research. 2020 doi: 10.1038/s41440-020-0429-x. March. [DOI] [PubMed] [Google Scholar]
- 12.Donoghue Orna A, McGarrigle Christine A, Foley Margaret, Fagan Andrew, Meaney James, Kenny Rose Anne. Cohort profile update: the irish longitudinal study on ageing (TILDA) Int. J. Epidemiol. 2018;47(August):1398. doi: 10.1093/ije/dyy163. 1398l. [DOI] [PubMed] [Google Scholar]
- 13.Farokhian Farnaz, Yang Chunlan, Beheshti Iman, Matsuda Hiroshi, Wu Shuicai. Age-related gray and white matter changes in normal adult brains. Aging Dis. 2017;8(6):899–909. doi: 10.14336/AD.2017.0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feeney Joanne, O'Leary Neil, Kenny Rose Anne. Impaired orthostatic blood pressure recovery and cognitive performance at two-year follow up in older adults: the irish longitudinal study on ageing. Clinical Autonomic Research. 2016;26(2):127–133. doi: 10.1007/s10286-016-0340-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finucane Ciarán, O'Connell Matthew D.L., Donoghue Orna, Richardson Kathryn, Savva George M., Kenny Rose Anne. Impaired orthostatic blood pressure recovery is associated with unexplained and injurious falls. J. Am. Geriatr. Soc. 2017;65(3):474–482. doi: 10.1111/jgs.14563. [DOI] [PubMed] [Google Scholar]
- 16.Ciarán Finucane, O'Connell Matthew D.L., Fan Chie Wei, Savva George M., Soraghan Christopher J., Nolan Hugh, Cronin Hilary, Kenny Rose Anne. Age-Related normative changes in phasic orthostatic blood pressure in a large population study: findings from the irish longitudinal study on ageing (TILDA) Circulation. 2014;130(20):1780–1789. doi: 10.1161/CIRCULATIONAHA.114.009831. [DOI] [PubMed] [Google Scholar]
- 17.Frewen John, Finucane Ciaran, Savva George M, Boyle Gerard, Kenny Rose Anne. Orthostatic hypotension is associated with lower cognitive performance in adults aged 50 plus with supine hypertension. J. Gerontol. a Biol. Sci. Med. Sci. 2014;69(7):878–885. doi: 10.1093/gerona/glt171. [DOI] [PubMed] [Google Scholar]
- 18.Garnier-Crussard Antoine, Bougacha Salma, Wirth Miranka, André Claire, Delarue Marion, Landeau Brigitte, Mézenge Florence, et al. White matter hyperintensities across the adult lifespan: relation to age, Aβ load, and cognition. Alzheimer's Research & Therapy. 2020;12(1):127. doi: 10.1186/s13195-020-00669-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Henskens Léon H G, Kroon Abraham A, Oostenbrugge Robert J Van, Gronenschild Ed H B M, Fuss-Lejeune Monique M J J, Hofman Paul A M, Lodder Jan, Leeuw Peter W De. Increased aortic pulse wave velocity is associated with silent cerebral small-vessel disease in hypertensive patients. Hypertension. 2008;52(6):1120–1126. doi: 10.1161/HYPERTENSIONAHA.108.119024. [DOI] [PubMed] [Google Scholar]
- 20.Jollans Lee, Boyle Rory, Artiges Eric, Banaschewski Tobias, Desrivières Sylvane, Grigis Antoine, Martinot Jean-Luc, Paus Tomáš, Smolka Michael N, Walter Henrik. Quantifying performance of machine learning methods for neuroimaging data. Neuroimage. 2019;199:351–365. doi: 10.1016/j.neuroimage.2019.05.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawaguchi T., Uyama O., Konishi M., Nishiyama T., Iida T. Orthostatic hypotension in elderly persons during passive standing: a comparison with young persons. J. Gerontol. - Series A Bio. Sci. Med. Sci. 2001;56(5):273–280. doi: 10.1093/gerona/56.5.M273. [DOI] [PubMed] [Google Scholar]
- 22.Kenny Rose Anne, Coen Robert F, Frewen John, Donoghue Orna A, Cronin Hilary, Savva George M. Normative values of cognitive and physical function in older adults: findings from the irish longitudinal study on ageing. J. Am. Geriatr. Soc. 2013;61:S279–S290. doi: 10.1111/jgs.12195. [DOI] [PubMed] [Google Scholar]
- 23.Kimmerly Derek S. A review of human neuroimaging investigations involved with central autonomic regulation of baroreflex-mediated cardiovascular control. Autono. Neurosci. 2017;207(November 2016):10–21. doi: 10.1016/j.autneu.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 24.Leeuw F-E de, de Groot J C, Achten E, Oudkerk M, Ramos L M P, Heijboer R, Hofman A, Jolles J, van Gijn J, Breteler M M B. Prevalence of cerebral white matter lesions in elderly people: a population based magnetic resonance imaging study. The rotterdam scan study. J Neurol, Neurosurg Psychiat. 2001;70(1) doi: 10.1136/jnnp.70.1.9. 9 LP –14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lim E., Oh Y., Kim J., Lee K. Orthostatic and supine blood pressures are associated with white matter hyperintensities in Parkinson disease. J. Neurol. Sci. 2013;333:e147. doi: 10.1016/j.jns.2013.07.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Qian, Fang Jinghuan, Cui Chaohua, Dong Shuju, Gao Lijie, Bao Jiajia, Li Yanbo, Ma Mengmeng, Chen Ning, He Li. Association of aortic stiffness and cognitive decline: a systematic review and meta-analysis. Front. Aging Neurosci. 2021;13:322. doi: 10.3389/fnagi.2021.680205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Looze Céline De, Williamson Wilby, Hirst Rebecca, O'Connor John, Knight Silvin, McCrory Cathal, Carey Daniel, Kenny Rose-Anne Anne Rose-anne. Impaired orthostatic heart rate recovery is associated with smaller thalamic volume: results from the irish longitudinal study on aging (TILDA) Hum. Brain Mapp. 2020;January(April):1–9. doi: 10.1002/hbm.25022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maguire Fiachra, Romero-Ortuno Roman, O'Connor John D, Reilly Richard B, Knight Silvin P, Kenny Rose-Anne. One-dimensional statistical parametric mapping identifies impaired orthostatic cerebrovascular and cardiovascular response in frailty index. J. Gerontology. 2021;76(5):885–892. doi: 10.1093/gerona/glaa315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCrory Cathal, Berkman Lisa F., Nolan Hugh, O'Leary Neil, Foley Margaret, Kenny Rose Anne. Speed of heart rate recovery in response to orthostatic challenge. Circ. Res. 2016;119(5):666–675. doi: 10.1161/CIRCRESAHA.116.308577. [DOI] [PubMed] [Google Scholar]
- 30.McNicholas Triona, Tobin Katy, Carey Daniel, O'Callaghan Susan, Kenny Rose Anne. Is baseline orthostatic hypotension associated with a decline in global cognitive performance at 4-year follow-up? Data from TILDA (The Irish Longitudinal Study on Ageing) J. Am. Heart. Assoc. 2018;7(19) doi: 10.1161/jaha.118.008976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Millar Peter R, Gordon Brian A, Luckett Patrick H, Benzinger Tammie L S, Cruchaga Carlos, Fagan Anne M, Hassenstab Jason J, Perrin Richard J, Schindler Suzanne E, Allegri Ricardo F. Multimodal brain age estimates relate to alzheimer disease biomarkers and cognition in early stages: a cross-sectional observational study. Elife. 2023;12:e81869. doi: 10.7554/eLife.81869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mol Arjen, Reijnierse Esmee M., Trappenburg Marijke C., Wezel Richard J.A.van, Maier Andrea B., Meskers Carel G.M. Rapid systolic blood pressure changes after standing up associate with impaired physical performance in geriatric outpatients. J. Am. Heart. Assoc. 2018;7(21):1–10. doi: 10.1161/JAHA.118.010060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Monahan K D, Dinenno F A, Seals D R, Clevenger C M, Desouza C A, Tanaka H. Age-associated changes in cardiovagal baroreflex sensitivity are related to central arterial compliance. Am. J. Physiol. Heart. Circ. Physiol. 2001;281(1):H284–H289. doi: 10.1152/ajpheart.2001.281.1.H284. [DOI] [PubMed] [Google Scholar]
- 34.O'Connell Matthew DL, Savva George M., Finucane Ciarán, Romero-Ortuno Roman, Fan Chie Wei, Kenny Rose Anne. Impairments in hemodynamic responses to orthostasis associated with frailty: results from the irish longitudinal study on ageing (TILDA) J. Am. Geriatr. Soc. 2018;66(8):1475–1483. doi: 10.1111/jgs.15327. [DOI] [PubMed] [Google Scholar]
- 35.O'Connor John D., O'Connell Matthew D.L., Nolan Hugh, Newman Louise, Knight Silvin P., Kenny Rose Anne. Impact of standing speed on the peripheral and central hemodynamic response to orthostasis. Hypertension. 2020;75(2):524–531. doi: 10.1161/HYPERTENSIONAHA.119.14040. [DOI] [PubMed] [Google Scholar]
- 36.O'Hare Celia, Kenny Rose-Anne, Aizenstein Howard, Boudreau Robert, Newman Anne, Launer Lenore, Satterfield Suzanne, Yaffe Kristine, Rosano Caterina, Study Health A B C. Cognitive status, gray matter atrophy, and lower orthostatic blood pressure in older adults. Journal of Alzheimer's Disease: JAD. 2017;57(4):1239–1250. doi: 10.3233/JAD-161228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O'Rourke Michael F, Hashimoto Junichiro. Mechanical Factors in Arterial Aging: A Clinical Perspective. J. Am. Coll. Cardiol. 2007;50(1):1–13. doi: 10.1016/j.jacc.2006.12.050. [DOI] [PubMed] [Google Scholar]
- 38.Pérez-Denia Laura, Claffey Paul, Byrne Lisa, Rice Ciara, Kenny Rose Anne, Finucane Ciarán. Increased multimorbidity is associated with impaired cerebral and peripheral hemodynamic stabilization during active standing. J. Am. Geriatr. Soc. 2022;70(7):1973–1986. doi: 10.1111/jgs.17810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Plini Emanuele R G, Melnychuk M C, Harkin A, Dahl M J, McAuslan M, Kühn S, Boyle R T, et al. Dietary tyrosine intake (FFQ) is associated with locus coeruleus, attention and grey matter maintenance: an MRI structural study on 398 healthy individuals of the berlin aging study-II. J. Nutr. Health Aging. 2023 doi: 10.1007/s12603-023-2005-y. [DOI] [PubMed] [Google Scholar]
- 40.Plini Emanuele R G, O'Hanlon Erik, Boyle Rory, Sibilia Francesca, Rikhye Gaia, Kenney Joanne, Whelan Robert, Melnychuk Michael C, Robertson Ian H, Dockree Paul M. Examining the role of the noradrenergic locus coeruleus for predicting attention and brain maintenance in healthy old age and disease: An MRI structural study for the Alzheimer's disease neuroimaging initiative. Cells. 2021 doi: 10.3390/cells10071829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ramanoël Stephen, Hoyau Elena, Kauffmann Louise, Renard Félix, Pichat Cédric, Boudiaf Naïla, Krainik Alexandre, Jaillard Assia, Baciu Monica. Gray matter volume and cognitive performance during normal aging. a voxel-based morphometry study. Front. Aging Neurosci. 2018;10:235. doi: 10.3389/fnagi.2018.00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rauseo Elisa, Salih Ahmed, Raisi-Estabragh Zahra, Aung Nay, Khanderia Neha, Slabaugh Gregory G, Marshall Charles R, et al. Ischemic heart disease and vascular risk factors are associated with accelerated brain aging. JACC. 2023;16(7):905–915. doi: 10.1016/j.jcmg.2023.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Romero José Rafael, Preis Sarah R, Beiser Alexa, DeCarli Charles, Viswanathan Anand, Martinez-Ramirez Sergi, Kase Carlos S, Wolf Philip A, Seshadri Sudha. Risk factors, stroke prevention treatments, and prevalence of cerebral microbleeds in the framingham heart study. Stroke. 2014;45(5):1492–1494. doi: 10.1161/STROKEAHA.114.004130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sihag Saurabh, Mateos Gonzalo, McMillan Corey, Ribeiro Alejandro. Explainable brain age prediction using CoVariance neural networks. ArXiv. 2023 [Google Scholar]
- 45.Soennesyn Hogne, Nilsen Dennis W., Oppedal Ketil, Greve Ole Jacob, Beyer Mona K., Aarsland Dag. Relationship between orthostatic hypotension and white matter hyperintensity load in older patients with mild dementia. PLoS. One. 2012;7(12):8–13. doi: 10.1371/journal.pone.0052196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tanveer M, Ganaie M A, Beheshti Iman, Goel Tripti, Ahmad Nehal, Lai Kuan-Ting, Huang Kaizhu, Zhang Yu-Dong, Del Ser Javier, Lin Chin-Teng. Deep learning for brain age estimation: a systematic review. Information Fusion. 2023;96:130–143. doi: 10.1016/j.inffus.2023.03.007. [DOI] [Google Scholar]
- 47.Vernooij M W, Lugt Aad van der, Ikram Mohammad Arfan, Wielopolski P A, Niessen W J, Hofman Albert, Krestin G P, Breteler M M B. Prevalence and risk factors of cerebral microbleeds: the rotterdam scan study. Neurology. 2008;70(14):1208–1214. doi: 10.1212/01.wnl.0000307750.41970.d9. [DOI] [PubMed] [Google Scholar]
- 48.Vinke Elisabeth J, Groot Marius de, Venkatraghavan Vikram, Klein Stefan, Niessen Wiro J, Arfan Ikram M, Vernooij Meike W. Trajectories of imaging markers in brain aging: the rotterdam study. Neurobiol. Aging. 2018;71:32–40. doi: 10.1016/j.neurobiolaging.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 49.Whelan Brendan J., Savva George M. Design and methodology of the irish longitudinal study on ageing. J. Am. Geriatr. Soc. 2013;61(SUPPL2):S265–S268. doi: 10.1111/jgs.12199. [DOI] [PubMed] [Google Scholar]
- 50.Wolters Frank J., Mattace-Raso Francesco U.S.S, Koudstaal Peter J., Arfan Ikram Albert Hofman, M., Brain Heart, Connection Collaborative Orthostatic Hypotension and the long-term risk of dementia: a population-based study. PLoS. Med. 2016;13(10):1–15. doi: 10.1371/journal.pmed.1002143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wolters Frank J, Zonneveld Hazel I, Hofman Albert, Lugt Aad van der, Koudstaal Peter J, Vernooij Meike W, Arfan Ikram M. Cerebral perfusion and the risk of dementia: a population-based study. Circulation. 2017;136(8):719–728. doi: 10.1161/CIRCULATIONAHA.117.027448. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data collected for this study is sensitive, requests for access should be directed to The Irish Longitudinal Study on Ageing (TILDA), Trinity Central, 152-160 Pearse St, Dublin 2, D02 R590, Republic of Ireland (email: tilda@tcd.ie).