Abstract
Introduction
This prospective, multicenter, randomized, double-masked pivotal phase 3 trial evaluated the efficacy and safety of the travoprost intracameral SE-implant (slow-eluting implant, the intended commercial product) and FE-implant (fast-eluting implant, included primarily for masking purposes) compared to twice-daily (BID) timolol ophthalmic solution, 0.5% in patients with open-angle glaucoma (OAG) or ocular hypertension (OHT).
Methods
The trial enrolled adult patients with OAG or OHT with an unmedicated mean diurnal intraocular pressure (IOP) of ≥ 21 and unmedicated IOP ≤ 36 mmHg at each diurnal timepoint (8 a.m., 10 a.m., and 4 p.m.) at baseline. The eligible eye of each patient was administered an SE-implant, an FE-implant or had a sham administration procedure. Patients who received an implant were provided placebo eye drops to be administered BID and patients who had the sham procedure were provided timolol eye drops to be administered BID. The primary efficacy endpoint, for which the study was powered, was mean change from baseline IOP at 8 a.m. and 10 a.m. at day 10, week 6, and month 3. Non-inferiority was achieved if the upper 95% confidence interval (CI) on the difference in IOP change from baseline (implant minus timolol) was < 1.5 mmHg at all six timepoints and < 1 mmHg at three or more timepoints. The key secondary endpoint was mean change from baseline IOP at 8 a.m. and 10 a.m. at month 12. Non-inferiority at month 12 was achieved if the upper 95% CI was < 1.5 mmHg at both timepoints. Safety outcomes included treatment-emergent adverse events (TEAEs) and ophthalmic assessments.
Results
A total of 590 patients were enrolled at 45 sites and randomized to one of three treatment groups: 197 SE-implant (the intended commercial product), 200 FE-implant, and 193 timolol. The SE-implant was non-inferior to timolol eye drops in IOP lowering over the first 3 months, and was also non-inferior to timolol at months 6, 9, and 12. The FE-implant was non-inferior to timolol over the first 3 months, and also at months 6 and 9. Of those patients who were on glaucoma medication at screening, a significantly greater proportion of patients in the SE- and FE-implant groups (83.5% and 78.7%, respectively) compared to the timolol group (23.9%) were on fewer topical glaucoma medications at month 12 compared to screening (P < 0.0001, chi-square test). TEAEs, mostly mild, were reported in the study eyes of 39.5% of patients in the SE-implant group, 34.0% of patients in the FE-implant group and 20.1% of patients in the timolol group.
Conclusions
The SE-travoprost intracameral implant demonstrated non-inferiority to timolol over 12 months whereas the FE-implant demonstrated non-inferiority over 9 months. Both implant models were safe and effective in IOP lowering in patients with OAG or OHT.
Trial Registration
ClinicalTrials.gov identifier, NCT03519386.
Keywords: iDose® TR, Intraocular pressure, Ocular drug delivery system, Ocular hypertension, Open-angle glaucoma, Travoprost intracameral implant
Key Summary Points
| Drug delivery systems such as the travoprost intracameral implant are being developed to address poor patient adherence with topical glaucoma medications. |
| Both the slow-eluting and fast-eluting travoprost intracameral implant provided clinically relevant intraocular pressure (IOP) reductions through 12 months in patients with open-angle glaucoma and ocular hypertension. |
| The IOP reductions with the slow-eluting travoprost intracameral implant were non-inferior to those produced by twice-daily instillations of timolol ophthalmic solution, 0.5% through 12 months; the IOP reductions with the fast-eluting implant were non-inferior to timolol through 9 months. |
| Topical glaucoma medication burden was substantially reduced in patients who had received travoprost intracameral implant 12 months following implant administration. |
| The intracameral implants had an acceptable safety profile. |
Introduction
Topical glaucoma medications are the predominant treatment option for lowering elevated intraocular pressure (IOP) in patients affected by open-angle glaucoma (OAG) or ocular hypertension (OHT). However, the typically asymptomatic and chronic nature of the disease, complex dosing regimens, difficulty with instillation, lack of belief in the necessity of treatment, and chronic side effects associated with topical glaucoma medications undermine patient adherence and quality of life [1–5]. Although non-adherence to glaucoma therapy is known to contribute to the progression of glaucoma [6–8], nearly half of patients with glaucoma discontinue therapy within 6 months [9].
Selective laser trabeculoplasty (SLT) has been proposed as a viable therapeutic option that can overcome the lack of patient adherence with topical glaucoma medications. While studies have shown that the IOP decrease after 360° treatment with SLT is comparable to that achieved with topical glaucoma medications [9, 10], its effect diminishes over time requiring additional intervention [11], the non-responder rate may be twice that compared to a topical prostaglandin analogue (PGA) [12], and complications, although uncommon, can include transient IOP spikes, anterior chamber inflammation, hyphema, choroidal effusion, macular edema, corneal haze, and shifts in refractive error [13]. The utility of SLT is limited in patients with physical constraints that prevent accurate placement of the laser treatment (e.g., head tremor, deeply recessed eyes, moderate to severe blepharospasm) [14], a patient population that would benefit greatly from not needing to administer topical medications.
Intracameral implants have also been developed as treatment modalities to address patient non-adherence with topical glaucoma medications. Two such implants have now been approved by the US Food and Drug Administration (FDA) for the reduction of elevated IOP in patients with OAG or OHT: the bimatoprost intracameral implant (Durysta®, Allergan Inc., Irvine, California, USA), an unanchored biodegradable implant designed to release bimatoprost for 3–4 months [15, 16], and the travoprost intracameral implant (iDose® TR; Glaukos Corporation, Aliso Viejo, California, USA) designed to release travoprost for up to 3 years [17].
The travoprost intracameral implant comprises a miniature biocompatible titanium reservoir (0.5 mm in diameter by 1.2 mm in length) and an anchor (0.6 mm in length) to secure the implant through the trabecular meshwork into the sclera at the iridocorneal angle. The reservoir holds 75 μg of a proprietary, preservative-free travoprost formulation, which is approximately 25,000 times more concentrated than the travoprost in travoprost ophthalmic solution, 0.004%. Held in place by a biocompatible titanium cap, the implant has a nanoporous ethylene–vinyl acetate (EVA) membrane which facilitates continuous, long-duration elution of therapeutic levels of travoprost directly into the anterior chamber (Fig. 1). The implant is pre-loaded into a sterile, single-dose inserter facilitating administration via a clear corneal incision.
Fig. 1.

Gonioscopic view of a travoprost intracameral implant anchored in the trabecular meshwork and sclera just anterior to the scleral spur, and oriented parallel to the iris
Two versions of the implant have been developed and evaluated in clinical trials: a slow-eluting implant, herein referred to as the SE-implant, and a fast-eluting implant, herein referred to as the FE-implant. The two implants differ in the thickness of the EVA membrane but are otherwise identical. In a phase 2b clinical trial, both implant models provided robust, sustained IOP-lowering, with 69% and 63% of patients in the SE-implant and FE-implant groups being well controlled on the same or fewer topical glaucoma medications 36 months following a single administration [17]. On the basis of the combination of a more favorable benefit-to-risk profile of the SE-implant over the FE-implant in the phase 2b trial and its enhanced manufacturability, the SE-implant was chosen as the commercial model and the FE-implant was maintained in trials for masking purposes.
The current phase 3 trial, conducted as part of the program to obtain US FDA approval, was designed and prospectively powered to demonstrate non-inferiority of the travoprost intracameral implant to timolol eye drops in IOP reduction over the first 3 months of the trial. After the phase 3 trial was initiated, but prior to the database lock and unmasking, a 12-month non-inferiority comparison to timolol was included as a secondary endpoint based on regulatory agency guidance; however, the trial was not prospectively powered for the non-inferiority comparison at 12 months. Furthermore, the trial was also designed to demonstrate an acceptable safety profile of the implant over a 12-month period, with a long-term safety extension ongoing through month 36.
Methods
Design
This was a prospective, randomized, double-masked trial conducted in 44 ophthalmology clinics in the USA and one in the Philippines. The trial was conducted in accordance with recognized international scientific and ethical standards, including but not limited to the International Council for Harmonisation (ICH) guideline for Good Clinical Practice (GCP), and the original principles embodied in the Declaration of Helsinki. Approval for the trial was obtained from an institutional review board (Western Institutional Review Board [20180735] for 43 of the sites in the USA and Wills Eye Hospital Institutional Review Board [18–763] for one site in the USA) or an independent ethics committee (St. Cabrini Medical Center-Asian Eye Institute Ethics Review Committee [2018–2023] for the single site in the Philippines). All patients provided written informed consent before undergoing any study-related change in their treatment or any study-related procedures. The trial was registered at ClinicalTrials.gov (NCT03519386).
Participants
At the screening visit, potential participants in the trial were required to be 18 years of age or older, provide written informed consent, and be willing and able to attend follow-up visits. Both eyes could be screened, but the prospective study eye was required to have a diagnosis of OAG (juvenile, primary, or including a pigmentary or pseudoexfoliative component) or OHT, be on zero to three topical glaucoma medications (this criterion was subsequently revised after the initiation of the trial to reduce the maximum number of topical glaucoma medications to two), have an IOP of ≥ 21 mmHg and ≤ 36 mmHg if not on glaucoma medication (there was no criterion in the eye that was being treated with glaucoma medications but the patient was required to discontinue use of these medications prior to the baseline visit), have a central corneal thickness of 440 to 620 μm, and have an open iridiocorneal angle (Shaffer grade ≥ 3) with normal anatomy and absence of angle abnormalities at the planned administration site. In addition, each eye was required to have a best corrected visual acuity (BCVA) of at least 20/80 Snellen.
At the baseline visit, the study eye was required to have a mean diurnal IOP (based on the average of the 8 a.m., 10 a.m., and 4 p.m. timepoints) of ≥ 21 mmHg, and the IOP was required to be ≤ 36 mmHg at each timepoint (8 a.m., 10 a.m., and 4 p.m.).
Exclusion criteria pertinent to the study eye included a cup-to-disc (C/D) ratio > 0.8, visual field mean deviation (MD) of − 12 dB or worse, visually significant cataract expected to require cataract surgery within the next 3 years, or pseudophakia with complicated cataract surgery that was performed within 90 days of the screening visit, prior argon laser trabeculoplasty or incisional glaucoma surgery, an iridotomy or laser trabeculoplasty (selective or micropulse) performed within 90 days of the screening visit, any history of or presence of active inflammation of the ocular structures, clinically significant corneal guttata or corneal dystrophy, or retinal or choroidal disorders. In addition, the patient could not be pregnant or planning a pregnancy, or have an immunodeficiency disorder or uncontrolled systemic disease. Patients with diabetes mellitus were not excluded from participation provided their condition was controlled and they were otherwise eligible; no additional precautions or testing (e.g., macular scans) was required.
The patient also was ineligible if the following occurred within the 30 days prior to the screening visit: use of an oral, intravenous, inhaled, or dermal (if applied to within 0.6 cm of the eye) steroid; use of a systemic carbonic anhydrase inhibitor (CAI); or a change in an existing systemic medication that could substantially affect IOP.
Procedures and Visits
At the screening visit, patients underwent the informed consent process, followed by collection of demographic data, as well as information regarding their medical/ocular history and concomitant medications. In addition, a manifest refraction was performed and Snellen visual acuity (VA) was measured, visual fields were measured via standard automated perimetry, slit lamp biomicroscopy was performed to assess the anterior ocular structures, IOP was measured via Goldmann tonometry at 8 a.m., 10 a.m., or 4 p.m., ultrasonic pachymetry was performed to measure central corneal thickness, gonioscopy was performed to assess the iridocorneal angle, and a dilated fundus exam was performed to assess the structures at the back of the eye, including nerve abnormalities and vertical C/D ratio.
Patients were required to meet all eligibility criteria at the screening visit and cease the use of their glaucoma medication(s), if applicable, prior to the baseline visit. The medication washout was 8 weeks for rho-kinase inhibitors, 4 weeks for β-blockers and PGAs, 3 weeks for α-agonists, 1 week for topical CAIs, and 5 days for miotics. Patients who were on no glaucoma medication could proceed directly to the baseline visit as early as the next day.
At the baseline visit, patients were questioned regarding any update to their medical/ocular history and concomitant medications, and the occurrence of potential adverse events. In addition, a pregnancy test was conducted on all women of childbearing potential, a manifest refraction was performed, and Early Treatment Diabetic Retinopathy Study (ETDRS) visual acuity was measured, a slit lamp exam was performed (including assessment of conjunctival hyperemia and iris color), specular microscopy was performed at selected sites to assess central corneal endothelial cell density, and IOP was measured via Goldmann tonometry at 8 a.m., 10 a.m., and 4 p.m..
Patients who met all entry criteria at this visit were designated a qualifying study eye by the investigator. If both eyes qualified, the right eye was designated as the study eye.
Following completion of the baseline visit, eligible patients were scheduled for the operative visit and dispensed a topical fluoroquinolone or broad-spectrum antibiotic eye drop for administration four times per day in the study eye for at least 1 day prior to surgery. Patients taking heparin were instructed to discontinue the medication 1 day prior to surgery.
On the day of surgery, patients were questioned regarding the occurrence of any adverse events since their baseline visit, and an additional drop of antibiotic was administered 30 min prior to surgery. Patients were randomized to treatment, and per randomized assignment, anesthesia (general, retrobulbar, peribulbar, or topical for implant patients; topical for sham procedure patients) was administered and the study eye was implanted with either an FE- or SE-implant or had the sham surgical procedure performed under aseptic conditions.
The implant procedure was performed with the patient’s head stabilized and turned away from the surgeon. A small amount of viscoelastic was placed on the cornea prior to positioning of the gonioprism to allow for clear visualization of the angle structures at the nasal implant location. A clear corneal incision of approximately 2.4 mm was created at the temporal limbus, and a cohesive viscoelastic was added to the anterior chamber to form the chamber and improve visualization of the angle. When ready for implantation, the sterile single-dose inserter was entered into the anterior chamber and advanced towards the angle where the implant was pressed through the trabecular meshwork with the anchor of the implant securely embedded in the sclera. After checking that the implant was fully anchored, the surgeon withdrew the inserter from the eye, and a high magnification examination was performed to confirm that the implant was properly positioned. Thereafter, the anterior chamber was irrigated and aspirated with balanced salt solution to remove all viscoelastic, and the anterior chamber inflated with saline solution, as needed, to achieve physiologic pressure.
The sham procedure was performed by placing the blunt hub of a sterile syringe against the anesthetized conjunctiva.
Following the procedure, patients were dispensed a topical non-steroidal anti-inflammatory eye drop to be used for 1 week, and instructed to continue with their topical antibiotic eye drops four times per day for 1 week.
Postoperatively, for the duration of the study, patients in the FE- and SE-implant groups were instructed to use topical placebo eye drops (artificial tears) twice daily (BID) in the study eye, while patients in the control group, following the sham surgical procedure, were instructed to instill topical timolol 0.5% solution BID in the study eye. Patients were to administer their eye drops at 8 a.m. and 8 p.m. daily, with the exception of study visits at which 8 a.m. IOP measurements were performed. At these visits, eye drops were administered after the 8 a.m. IOP measurement. Patients continued with their routine glaucoma treatment in their non-study eye.
Follow-up visits were scheduled at day 1–2, day 10, weeks 4 and 6, and months 3, 6, 9, and 12. Diurnal (8 a.m., 10 a.m., and 4 p.m.) IOP measurements were taken at day 10, week 6, and months 3 and 12. An IOP measurement at a single time of day (8 a.m., 10 a.m., or 4 p.m.) was taken at day 1–2, week 4, and months 6 and 9. Each time IOP was measured, two measurements were taken and recorded unless they differed by more than 2 mmHg, in which case a third measurement was taken and recorded. The IOP for each measurement timepoint was the mean of two or the median of the three measurements. Additional assessments at all visits included questioning the patient for any treatment-emergent adverse events (TEAEs) or changes in concomitant medications, measurement of VA (pinhole at day 1–2 and day 10; corrected at weeks 4 and 6, and months 3, 6, 9 and 12), slit lamp biomicroscopy (including assessment of conjunctival hyperemia in comparison to color photographs, and iris color at all visits except for day 1–2). At selected visits, the following additional assessments were performed: visual fields at months 6 and 12, specular microscopy at months 3 and 12, ophthalmoscopy at months 3, 6, 9, and 12 (including C/D measurement at months 6 and 12, and under dilation at month 12), pachymetry at month 12, and gonioscopy at day 10, weeks 4 and 6, and months 3, 6, and 12.
If a postoperative increase in IOP was observed within the first 2 days following surgery, a paracentesis was performed as necessary to remove any retained viscoelastic and to reduce IOP, or a glaucoma medication could be prescribed or administered. If an increase in IOP was observed at day 3 or later and the IOP was > 22mmHg, IOP was rechecked within 7 days. A topical glaucoma medication, preferably a CAI, was administered or prescribed if the rechecked IOP was > 25 mmHg, or if the rechecked IOP was > 22 mmHg but ≤ 25 mmHg and the IOP reduction from baseline was < 20%.
Masking
Several procedures were implemented in this trial to maintain masking. First, two models of the implant, which were identical in appearance, were evaluated despite the intent to seek regulatory approval for only the SE-implant. Second, patients randomized to the timolol eye drop group underwent a sham surgical procedure. Third, patients randomized to the implant groups received placebo eye drops. Furthermore, all surgical kits were packaged alike, in boxes labeled only with the study number and unique kit number, and all bottles of eye drops were identical in size, cap, and bottle color and packaged in identical kit boxes with masked labeling. Importantly, IOP was measured using a two-person technique with one person viewing through the oculars of the biomicroscope and a second person masked to treatment assignment recording the measurement. Finally, the implants were placed at the 10 o’clock position (left eye) or 2 o’clock position (right eye) to reduce visibility to the casual observer. Therefore, both the study staff performing certain measures (i.e., IOP readings, assessment of symptomology) and the patient remained masked as to the identity of the assigned treatment.
Efficacy and Safety Outcomes Measures
The key efficacy outcome measure was the time-matched change from baseline in IOP in the study eye.
Safety parameters included intra- and postoperative TEAEs, BCVA, findings from slit lamp biomicroscopy, gonioscopy, ophthalmoscopy (including C/D ratio), pachymetry, visual field evaluation, specular microscopy, conjunctival hyperemia assessment, and iris color assessment. General guidelines for reporting TEAEs included a ≥ 30% reduction from baseline for corneal endothelial cell density (that was confirmed at a follow-up visit), a ≥ 10-letter reduction from baseline for BCVA, a 2-grade worsening from baseline for conjunctival hyperemia, an IOP increase of ≥ 10 mmHg from baseline, and a ≥ 2.5 dB worsening from baseline in visual field mean deviation (that was confirmed at a follow-up visit).
Statistical Analysis
The primary efficacy objective for the trial was to demonstrate that the mean changes from baseline in diurnal IOP in the study eye for the SE-implant group, intended for regulatory approval, as well for the FE-implant, utilized primarily for masking purposes, were non-inferior over the first 3 months of the trial to the mean change from baseline in diurnal IOP in the timolol group at 8 a.m. and 10 a.m. at each of day 10, week 6, and month 3 visits (six timepoints). The key secondary efficacy objective, which was included on the basis of regulatory agency guidance after the trial was initiated but prior to database lock and unmasking, was to demonstrate that the mean change from baseline in diurnal IOP in the study eye of the implant groups was non-inferior to the mean change from baseline in diurnal IOP in the timolol group at 8 a.m. and 10 a.m. at month 12. An additional secondary efficacy objective was to demonstrate that the mean change from baseline in IOP in implant groups was non-inferior to the mean change from time-matched baseline in IOP in the timolol group at months 6 and 9.
For the primary efficacy endpoint, non-inferiority of the implant to timolol was established if the upper limit of the two-sided 95% confidence interval (CI) for the difference in the mean of change from baseline in IOP was < 1.5 mmHg at each of the six post-baseline timepoints and was < 1 mmHg at half or more of the six post-baseline timepoints. For the key secondary efficacy endpoint, non-inferiority of the implant to timolol was established if the upper limit of the two-sided 95% confidence interval for the difference in mean change from baseline in IOP was < 1.5 mmHg at the 8 a.m. and 10 a.m. timepoints at month 12.
The sample size for the trial was based on the primary efficacy endpoint (i.e., six IOP post-baseline timepoints collected at 8 a.m. and 10 a.m. at day 10, week 6, and month 3). A total of 186 patients per group were needed to reach 85% power to demonstrate non-inferiority of at least one implant group over the timolol group using a 1.5 mmHg margin at all six timepoints and a 1.0 mmHg margin at three or more of the six timepoints. The calculations assumed a true mean IOP difference of zero, standard deviations (SD) of 3.0 mmHg for the timolol control group and 4.0 mmHg for the implant groups, normal distributions for the IOP measurements at the six timepoints through month 3, and one-sided t tests to test for non-inferiority at α = 0.025.
To control the overall type I error at 0.05 level for comparing the two implant groups to timolol, a fixed sequence hierarchical testing procedure was used whereby the SE-implant group was compared to timolol first at a two-sided alpha level of 0.05. If the non-inferiority criteria were met for the SE-implant group versus timolol, the FE-implant group was then compared to timolol at two-sided alpha level of 0.05. The primary endpoint was tested first, and if non-inferiority to timolol was established for both implant groups, the key secondary endpoint was then tested; comparing the SE-implant group versus timolol first and if the non-inferiority criteria were met, then comparing the FE-implant group versus timolol. Type I error was not controlled for testing non-inferiority of other secondary endpoints.
An analysis of covariance (ANCOVA) model with change from baseline in IOP at the given visit and timepoint as the response, treatment as a main effect factor, and time-matched baseline IOP as a covariate was applied for each timepoint of each visit separately. Point estimates (least squares [LS] mean) and corresponding 95% CIs were calculated for the difference between implant groups versus timolol group (i.e., implant group minus timolol group).
The primary analysis was performed for the intent-to-treat (ITT) population with the worse-half imputation method to handle missing/intercurrent events at each visit and timepoint separately. In addition, use of additional topical glaucoma medication was accounted for at the visit level.
For the primary analysis of the primary and secondary efficacy endpoints, missing IOP data for patients who had intercurrent events (e.g., patient dropout due to a TEAE or lack of efficacy, use of additional glaucoma medications) were imputed using a multiple imputation (Monte Carlo Markov chain, MCMC) method on the worse half of patients (i.e., for each treatment group and timepoint, the worse half of patients were those whose IOP reductions from baseline were less than the median IOP reduction for that treatment groups and timepoint). Missing IOP data for patients who did not have an intercurrent event (e.g., those who dropped out for reasons unrelated to treatment) were imputed using multiple imputation techniques (MCMC) from the randomized treatment group with each timepoint within each visit modeled separately.
Additional analyses were performed on the proportion of patients in each treatment group on no additional glaucoma medications at each visit, the proportion of patients who were well controlled on the same or fewer topical glaucoma medications at month 12 compared to screening, and of those patients who were on glaucoma medication at screening, the proportion who were well controlled on fewer topical glaucoma medications at month 12 compared to screening.
The ITT population, used for all efficacy analyses, included all patients who were randomized and allocated patients according to their original treatment assignment regardless of the actual treatment received.
Safety analyses were performed on the safety population which included all patients who were randomized and received at least one dose of study treatment and allocated patients according to the actual treatment received.
Statistical analyses were performed using SAS (Statistical Analysis Software, SAS Institute, Inc., Cary, North Carolina, US) version 9.4 or higher.
Results
Disposition
A total of 954 patients were screened, of which 364 patients (38.2%) were not randomized because of failure at screening or baseline. Of the 590 eligible patients that were randomized, 197 were randomized to the SE-implant group, 200 to the FE-implant group, and 193 to the timolol group.
A total of 565 of 590 randomized patients (95.8%) completed the month 12 visit, and 22 patients discontinued prior to month 12, primarily as a result of withdrawal of consent (seven patients, 1.2%). Five patients (0.8%; one in the FE-implant group and four in the timolol group) discontinued prematurely because of non-ocular serious adverse events resulting in death, and five patients (0.8%; one in the SE-implant, two in the FE-implant, and two in the timolol groups) discontinued prematurely because of TEAEs.
Demographic and Baseline Ocular Characteristics
Demography and baseline ocular characteristics of the patient population are presented in Tables 1 and 2, respectively. Baseline characteristics were well balanced among the treatment groups regarding demography, as well as study eye characteristics.
Table 1.
Demography (intent-to-treat population)
| SE-implant (N = 197) | FE-implant (N = 200) | Timolol (N = 193) | Total (N = 590) | |
|---|---|---|---|---|
| Age (years) | ||||
| Mean (SD) | 63.2 (12.6) | 63.8 (11.5) | 63.8 (11.4) | 63.6 (11.8) |
| Min, max | 24, 89 | 22, 86 | 28, 94 | 22, 94 |
| Sex, n (%) | ||||
| Male | 98 (49.7) | 91 (45.5) | 85 (44.0) | 274 (46.4) |
| Female | 99 (50.3) | 109 (54.5) | 108 (56.0) | 316 (53.6) |
| Race, n (%) | ||||
| White | 120 (60.9) | 143 (71.5) | 128 (66.3) | 391 (66.3) |
| Black or African American | 50 (25.4) | 38 (19.0) | 41 (21.2) | 129 (21.9) |
| Asian | 19 (9.6) | 15 (7.5) | 16 (8.3) | 50 (8.5) |
| Native Hawaiian or Pacific Islander | 0 | 1 (0.5) | 0 | 1 (0.2) |
| American Indian or Alaska Native | 0 | 0 | 2 (1.0) | 2 (0.3) |
| Other or unknown | 8 (4.1) | 3 (1.5) | 6 (3.1) | 17 (2.9) |
| Ethnicity, n (%) | ||||
| Hispanic or Latino | 15 (7.6) | 9 (4.5) | 10 (5.2) | 34 (5.8) |
| Not Hispanic or Latino | 179 (90.9) | 191 (95.5) | 180 (93.3) | 550 (93.2) |
| Unknown | 3 (1.5) | 0 | 3 (1.6) | 6 (1.0) |
FE-implant fast-eluting travoprost intracameral implant, ITT intent-to-treat, SD standard deviation, SE-implant slow-eluting travoprost intracameral implant
Table 2.
Baseline study eye characteristics (intent-to-treat population)
| SE-implant (N = 197) | FE-implant (N = 200) | Timolol (N = 193) | Total (N = 590) | |
|---|---|---|---|---|
| Diagnosis | ||||
| Open-angle glaucoma (OAG)a | 170 (86.3) | 175 (87.5) | 167 (86.5) | 512 (86.8) |
| Ocular hypertension (OHT) | 27 (13.7) | 25 (12.5) | 26 (13.5) | 78 (13.2) |
| Number of glaucoma medication classes used at screening, n (%) | ||||
| 0 | 54 (27.4) | 43 (21.5) | 46 (23.8) | 143 (24.2) |
| 1 | 99 (50.3) | 116 (58.0) | 100 (51.8) | 315 (53.4) |
| 2 | 38 (19.3) | 37 (18.5) | 41 (21.2) | 116 (19.7) |
| 3 | 6 (3.0) | 4 (2.0) | 6 (3.1) | 16 (2.7) |
| Screening IOP (mmHg) | ||||
| Mean (SD) | 19.77 (4.46) | 19.52 (4.50) | 19.67 (4.35) | 19.66 (4.43) |
| Mean (SD) IOP (mmHg) at baseline | ||||
| 8 a.m. | 24.37 (3.25) | 24.72 (3.40) | 24.72 (3.51) | 24.60 (3.39) |
| 10 a.m. | 24.09 (3.32) | 24.27 (3.27) | 24.03 (3.10) | 24.13 (3.23) |
| 4 p.m. | 23.59 (3.15) | 23.57 (3.14) | 23.61 (3.17) | 23.59 (3.15) |
| Iris color, n (%) | ||||
| Blue | 41 (20.8) | 57 (28.5) | 55 (28.5) | 153 (25.9) |
| Brown | 122 (61.9) | 109 (54.5) | 106 (54.9) | 337 (57.1) |
| Green | 7 (3.6) | 7 (3.5) | 7 (3.6) | 21 (3.6) |
| Hazel | 27 (13.7) | 26 (13.0) | 25 (13.0) | 78 (13.2) |
| Other | 0 | 1 (0.5) | 0 | 1 (0.2) |
FE-implant fast-eluting travoprost intracameral implant, IOP intraocular pressure, OAG open-angle glaucoma, OHT ocular hypertension, SD standard deviation, SE-implant slow-eluting travoprost intracameral implant
aOpen-angle glaucoma included juvenile, primary, pigmentary, and pseudoexfoliative
Efficacy
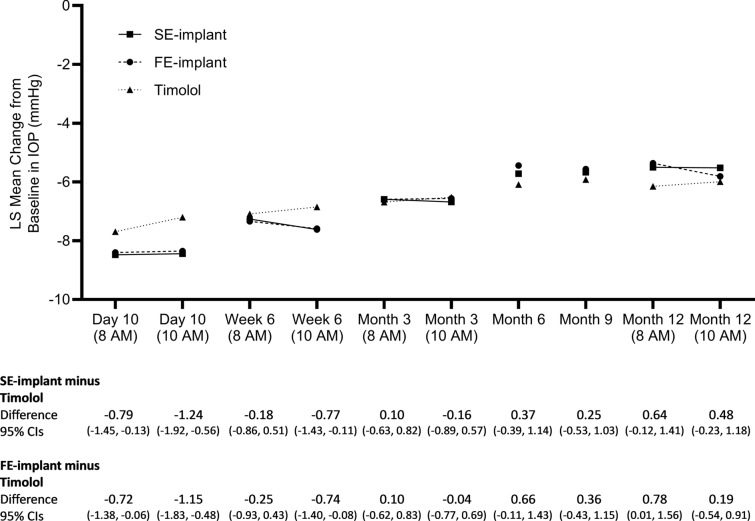
The trial met the primary efficacy endpoint. Both the SE- and FE-implant groups were statistically and clinically non-inferior to timolol as the upper limit of the 95% CI of the difference between the implant groups and the timolol group was < 1.5 mmHg and also < 1 mmHg for all six timepoints (i.e., 8 a.m. and 10 a.m. at day 10, week 6, and month 3) (Fig. 2). Notably, the SE-implant was also superior to timolol at three of the six timepoints.
Fig. 2.
LS mean change from baseline in IOP at the 8 a.m. and 10 a.m. measurement timepoints at day 10, week 6, month 3 and month 12, and at the 8 a.m., 10 a.m., or 4 p.m. measurement timepoint at month 6 and 9. Data below the plot indicate the LS means difference and upper and lower 95% CIs in the IOP change from baseline (in mmHg) between the implant group and the timolol group at each timepoint and visit. CIs confidence intervals, FE-implant fast-eluting travoprost intracameral implant, IOP intraocular pressure, LS mean least squares mean, SE-implant slow-eluting travoprost intracameral implant
The trial also met the key secondary efficacy endpoint for the SE-implant. The criterion for statistical non-inferiority to timolol was met for the SE-implant as the upper limit of the 95% CI of the difference between the SE-implant group and the timolol group was < 1.5 mmHg at both the 8 a.m. and 10 a.m. timepoints at month 12.
At months 6 and 9 (visits at which IOP was measured at 8 a.m., 10 a.m., or 4 p.m. and used time-matched baseline), non-inferiority to timolol was demonstrated for both implant groups using the same definition as used for month 12, as the upper limit of the 95% CI of the difference between the implant groups and the timolol group was < 1.5 mmHg.
In more detail, during the initial 3-month primary efficacy period, LS mean IOP changes from baseline ranged from − 6.6 to − 8.5 mmHg in the SE-implant group, from − 6.6 to − 8.4 mmHg in the FE implant group, from − 6.5 to − 7.7 mmHg in the timolol group across the six timepoints (Fig. 2).
At months 6 and 9, the LS mean IOP changes from baseline were − 5.7 and − 5.7 mmHg in the SE-implant group, − 5.4 and − 5.6 mmHg in the FE-implant group, respectively, and − 6.1 and − 5.9 mmHg in the timolol group (Fig. 2).
Finally, at the month 12 8 a.m. and 10 a.m. timepoints, respectively, the LS mean IOP changes from baseline were − 5.5 and − 5.5 mmHg in the SE-implant group, − 5.4 and − 5.8 mmHg in the FE-implant group, and − 6.2 and − 6.0 mmHg in the timolol group (Fig. 2).
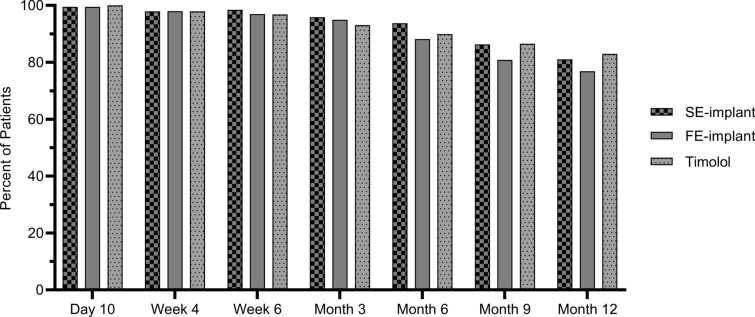
The clinical relevance of the IOP-lowering efficacy achieved by the implants was also evaluated by an analysis of the percentage of patients completely free of topical glaucoma medications. Most patients across all three treatment groups required no additional glaucoma medication in the study eye for up to 12 months post-baseline (Fig. 3), with similar percentages at all visits in the SE-implant group compared to the timolol group, and in the FE-implant group compared to the timolol group (P > 0.05, Fisher’s exact test). At month 12, 81.1% of patients in the SE-implant group, 76.9% of patients in the FE-implant group, and 83.0% of patients in the timolol group were not using additional glaucoma medication.
Fig. 3.
The proportion of patients who did not require additional topical glaucoma medications was similar in the SE-implant group and in the FE-implant group compared to the timolol group at each visit (P > 0.05, Fisher’s exact test). FE-implant fast-eluting travoprost intracameral implant, SE-implant slow-eluting travoprost intracameral implant
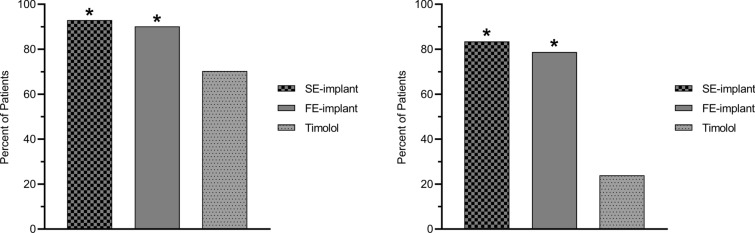
Furthermore, at the month 12 visit, a significantly greater percentage of patients in the SE-implant group (93.0%) and in the FE-implant group (90.2%) compared with the timolol group (70.3%) were on the same or fewer topical glaucoma medications compared to screening (SE-implant versus timolol, P < 0.0001; FE-implant versus timolol, P < 0.0001; chi-square test) (Fig. 4). In addition, of those patients who were on glaucoma medication at screening, a significantly greater percentage of patients in the SE-implant group (83.5%) and in the FE-implant group (78.7%) compared with the timolol group (23.9%) were on fewer topical glaucoma medications at month 12 compared to screening (SE-implant versus timolol, P < 0.0001; FE-implant versus timolol, P < 0.0001; chi-square test) (Fig. 4).
Fig. 4.
The proportion of patients on the same or fewer topical glaucoma medications (at left) and of those who were on glaucoma medication at screening, the proportion of patients who were on fewer topical glaucoma medications (at right) at month 12 compared to screening. In both analyses, the proportion of patients in the SE-implant group and in the FE-implant group was significantly greater than the proportion of patients in the timolol group (*P < 0.0001, chi-square test comparing each implant group to timolol). FE-implant fast-eluting travoprost intracameral implant, SE-implant slow-eluting travoprost intracameral implant
Adverse Events
A summary of TEAEs is presented in Table 3, and study eye TEAEs in 3% or greater of patients in any treatment group are presented in Table 4.
Table 3.
Summary of treatment-emergent adverse events (safety population)
| SE-implant (N = 195) | FE-implant (N = 200) | Timolol (N = 194) | |
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| TEAEs | |||
| Study eye | 77 (39.5) | 68 (34.0) | 39 (20.1) |
| Non-ocular or non-study eye | 54 (27.7) | 65 (32.5) | 54 (27.8) |
| Related to study treatment | |||
| Study eye | 41 (21.0) | 35 (17.5) | 5 (2.6) |
| Non-ocular or non-study eyea | 1 (0.5) | 1 (0.5) | 0 |
| Maximum severity of severe | |||
| Study eye | 6 (3.1) | 4 (2.0) | 0 |
| Non-ocular or non-study eye | 9 (4.6) | 11 (5.5) | 8 (4.1) |
| TEAEs leading to study discontinuation | |||
| Study eyeb | 3 (1.5) | 5 (2.5) | 2 (1.0) |
| Non-ocular or non-study eyec | 0 | 2 (1.0) | 0 |
| Serious TEAEs | |||
| Study eyed | 3 (1.5) | 1 (0.5) | 0 |
| Non-ocular or non-study eye | 9 (4.6) | 13 (6.5) | 10 (5.2) |
| Deathse | 0 | 2 (1.0) | 4 (2.1) |
COVID-19 Coronavirus disease 2019, FE-implant fast-eluting travoprost intracameral implant, IOP intraocular pressure, SE-implant slow-eluting travoprost intracameral implant, TEAEs treatment-emergent adverse events
aNon-ocular or non-study eye TEAEs related to treatment included headache in the FE-implant group and rhinorrhea in the SE-implant group
bStudy eye TEAEs leading to discontinuation included one serious adverse event of endophthalmitis
cNon-ocular TEAEs leading to discontinuation included benign brain tumor and hip fracture, both in the FE-implant group
dStudy eye serious TEAEs included endophthalmitis, retinal detachment, and increased IOP in the SE-implant group, and increased IOP in the FE-implant group
eDeaths included abdominal aortic aneurysm and metastatic lung cancer in the FE-implant group and two deaths due to COVID-19, one due to cardiopulmonary arrest, one due to breast cancer in the timolol group
Table 4.
Study eye treatment-emergent adverse events in 3% or greater of patients in any treatment group (safety population)
| MedDRA SOC PT |
SE-implant (N = 195) | FE-implant (N = 200) | Timolol (N = 194) |
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| Patients with any TEAEs in the study eye | 77 (39.5) | 68 (34.0) | 39 (20.1) |
| Eye disorders | |||
| Cataract | 7 (3.6) | 3 (1.5) | 3 (1.5) |
| Dry eye | 7 (3.6) | 6 (3.0) | 3 (1.5) |
| Iritis | 12 (6.2) | 1 (0.5) | 0 |
| Ocular hyperemia | 5 (2.6) | 9 (4.5) | 0 |
| Punctate keratitis | 3 (1.5) | 6 (3.0) | 5 (2.6) |
| Visual acuity reduced | 9 (4.6) | 2 (1.0) | 1 (0.5) |
| Investigations | |||
| Intraocular pressure increased | 9 (4.6) | 15 (7.5) | 4 (2.1) |
| Nervous system disorders | |||
| Visual field defect | 6 (3.1) | 6 (3.0) | 2 (1.0) |
Adverse events verbatim terms were coded using MedDRA (version 21.0, English) to the appropriate SOC and PT
FE-implant fast-eluting travoprost intracameral implant, MedDRA Medical Dictionary for Regulatory Activities, PT preferred term, SE-implant slow-eluting travoprost intracameral implant, SOC system organ class, TEAE treatment-emergent adverse event
The majority of patients with study eye TEAEs experienced mild, transient TEAEs, with severe TEAEs occurring in six patients in the SE-implant group (3.1%) and four in the FE-implant group (2.0%). The most commonly reported severe ocular TEAE was increased IOP in four patients (two patients in each of the implant groups) followed by TEAEs of visual acuity reduced, in three patients (two in the SE-implant group and one in the FE-implant group). Both events were transient for the majority of cases, and most often related to the administration procedure.
Four of the reported severe TEAEs (two of IOP increased, one of retinal detachment, and one of endophthalmitis) were also considered serious adverse events (Table 4).
Endothelial Cell Density
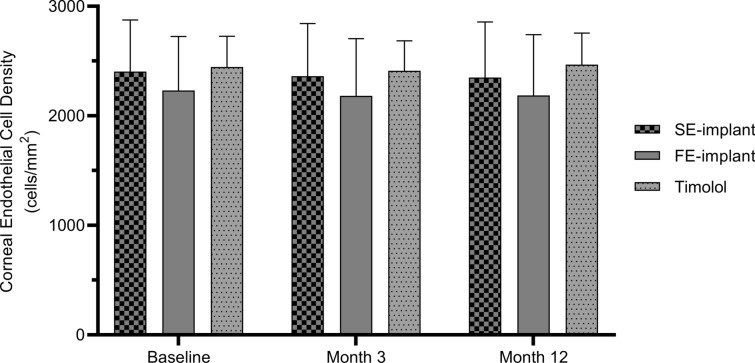
Mean endothelial cell density, measured in a subset of eyes (66 of the total 590), by visit is shown in Fig. 5.
Fig. 5.
Mean (+ SD) central corneal endothelial cell density at baseline, month 3, and month 12. FE-implant fast-eluting travoprost intracameral implant, SD standard deviation, SE-implant slow-eluting travoprost intracameral implant
At baseline, mean (SD) endothelial cell density was 2403.95 (472.25) cells/mm2, 2230.55 (493.90) cells/mm2, and 2445.49 (282.23) cells/mm2 in the SE-implant, FE-implant, and timolol groups, respectively. No patient in the SE-implant group exceeded the pre-defined endothelial cell loss threshold of ≥ 30% and the more stringent ≥ 20% from baseline.
Central Corneal Thickness
At baseline, mean (SD) corneal thickness in study eyes was 550.3 (35.7), 554.0 (36.3), and 552.5 (36.4) µm in the SE-implant FE-implant, and timolol groups, respectively. There was no clinically meaningful change from baseline in any treatment group at month 12; mean (SD) changes were − 4.5 (15.3), − 5.1 (19.6), and 0.8 (13.3) µm in the SE-implant, FE-implant, and timolol groups, respectively.
Visual Field
At baseline, mean (SD) visual field MD in study eyes was − 1.72 (2.86) dB, − 1.91 (2.93) dB, and − 1.87 (3.03) dB in the SE-implant, FE-implant, and timolol groups, respectively. There was no clinically meaningful change in visual field MD in any treatment group. At month 12, the mean (SD) changes in visual field MD from baseline were − 0.59 (2.45) dB in the SE-implant group, − 0.37 (3.04) dB in the FE-implant group, and − 0.32 (2.75) dB in the timolol group.
Visual Acuity
Visual acuity by visit is shown in Fig. 6.
Fig. 6.
Best corrected visual acuity (number of ETDRS letters, + SD) at baseline, weeks 4 and 6, and months 3, 6, 9, and 12. ETDRS Early Treatment Diabetic Retinopathy Study, FE-implant fast-eluting travoprost intracameral implant, SD standard deviation, SE-implant slow-eluting travoprost intracameral implant
At baseline, mean (SD) BCVA values (ETDRS letters read) in study eyes were 83.6 (5.1), 83.2 (5.1), and 82.4 (5.8) in the SE-implant, FE-implant, and timolol groups, respectively. There was no clinically meaningful change in BCVA over the course of 12 months.
Conjunctival Hyperemia
Mean conjunctival hyperemia scores were generally very low in all groups at all timepoints during the study with mean values at or below 0.4 on a scale ranging from 0 (normal) to 3 (severe) (Fig. 7).
Fig. 7.
Mean conjunctival hyperemia score (+ SD) at baseline, and each post-baseline visit. Conjunctival hyperemia was measured at the slit-lamp against a photographic scale with grades of 0 (normal), 0.5 (trace), 1 (mild), 2 (moderate), and 3 (severe). FE-implant fast-eluting travoprost intracameral implant, SD standard deviation, SE-implant slow-eluting travoprost intracameral implant
Most patients had normal or trace (scores of 0 or 0.5) conjunctival hyperemia scores at baseline (SE-implant, 90.3%; FE-implant, 91.0%; timolol, 91.8%). As a result of the surgical procedure, the percentage of implant patients with normal or trace hyperemia decreased slightly in the earlier postoperative visits and was lowest at day 10 for the SE-implant group (83.6%) and at week 4 for the FE-implant group (83.2%). However, at month 12, 88.3% of patients in the SE-implant group, 89.8% of patients in the FE-implant group, and 96.2% of patients in the timolol group were back to baseline with normal or trace conjunctival hyperemia. Severe hyperemia (score of 3) was observed in only two patients during the 12-month period, both in the FE-implant group, with one at day 10 and one at week 4.
No patient in any treatment group was reported to have an increase in iris pigmentation, nor was any patient observed to have periorbital fat atrophy during the 12-month period.
Discussion
This phase 3 clinical trial demonstrated that the SE-implant, intended for regulatory approval, produced IOP-lowering efficacy in patients with OAG or OHT that was non-inferior to that produced by BID administration of timolol 0.5% over the 12-month period. The FE-implant, used in the trial primarily for study masking purposes, was non-inferior to timolol over the first 9 months.
Both implant models produced statistically significant and clinically relevant IOP reductions from baseline over the entire 12 months, and address the problem of poor patient adherence to topical glaucoma medications, the key challenge related to glaucoma management [18, 19]. IOP reductions with the to-be-marketed SE-implant ranged from 6.6 to 8.5 mmHg at the primary efficacy endpoint (8 a.m. and 10 a.m. at day 10, week 6, and month 3) used for application of regulatory approval in the USA. At months 6 and 9, when IOP was measured at 8 a.m., 10 a.m., or 4 p.m., IOP reductions were 5.7 mmHg at both visits, while at month 12, when IOP was measured at 8 a.m. and 10 a.m., IOP reductions were 5.5 mmHg at both timepoints, with non-inferiority of the SE-implant to timolol being demonstrated over the entire 12 months.
Topical medication burden was substantially reduced in patients in the SE-implant group, with 81.1% of patients in the SE-implant group completely free of topical glaucoma medications 12 months following administration of their implant. In contrast, in the Artemis-1 and Artemis-2 studies conducted for the approval of the 10 μg bimatoprost intracameral implant, three implants were administered over a period of 12 months with the third implant administered at month 8. Four months after this last implant administration, 81.8% and 84.3% of implant patients did not receive additional glaucoma medications [15, 16]. The results for the patients treated with timolol were relatively similar across the trials (83.0% in our trial, and 86.4% and 84.1% in the two bimatoprost implant trials [15, 16]), validating the comparison.
Furthermore, in our trial, 93.0% of patients in the SE-implant group were well controlled (based on protocol-specified criteria for postoperative IOP management) on the same or fewer topical medications 12 months following implant administration compared to screening. The 93.0% value from this phase 3 trial corroborates results of a completed 3-year phase 2b trial in which 92% of patients in the SE-implant group were well controlled on the same or fewer topical medications 12 months following implant administration compared to screening [17]. In the phase 2b trial, at 3 years 69% of the patients in the SE-implant group were well controlled on the same or fewer topical medications compared to screening; a similar 3-year evaluation will be available from this ongoing phase 3 trial.
In addition, in the current trial, of those patients who were on glaucoma medication at screening, 83.5% of patients in the SE-implant group versus only 23.9% of patients in the timolol group were well controlled on fewer topical medications at month 12 compared to screening. Reduction in topical glaucoma medication burden has a significant impact on the quality of life of patients with glaucoma [20–22].
Both implant groups had an acceptable safety profile, with the majority of ocular TEAEs being mild. One implant patient had endophthalmitis which resolved with treatment. There were no clinically meaningful differences between treatment groups in BCVA or visual field outcomes. There was no evidence of iris hyperpigmentation or periorbital fat atrophy, side effects often associated with the use of topical travoprost [23–26]. Remarkably, the degree of conjunctival hyperemia in the implant groups was very low and, despite the surgical intervention associated with the implant administration procedure, was less than that reported for topically administered travoprost [27]. In addition, TEAEs of ocular or conjunctival hyperemia were 2.6% in the SE-implant group, substantially less than the 30–50% reported with topically administered travoprost [24, 27]. Thus, by delivering travoprost directly into the anterior chamber, the travoprost intracameral implants reduced or eliminated many of the side effects associated with topical PGAs.
One limitation of the current study is that IOP measurements at months 6 and 9 were not collected at 8 a.m. and 10 a.m. to allow for more direct comparison to other visits. However, IOP was measured at 8 a.m., 10 a.m., or 4 p.m. and compared to time-matched baseline. A second limitation is that the non-inferiority analyses for the month 6 and 9 visits were not prospectively defined. However, the analyses used the same definition as for month 12. A third limitation of the study is that the trial was not prospectively powered for the 6, 9, and 12-month IOP efficacy evaluations.
The strength of the study is its 12-month duration allowing for the analysis of the long-term efficacy and safety of the travoprost intracameral implant, including that for iris pigmentation changes and periorbital fat atrophy which may not be noticeable for several months [28–30], and the long-term rate of conjunctival hyperemia. Another strength of the study was the ability to evaluate long-term reduction of the topical glaucoma medication burden.
Conclusion
The study showed that the SE-implant was non-inferior in IOP reduction to timolol ophthalmic solution, 0.5% BID over a 12-month period and demonstrated a reduction in long-term topical glaucoma medication burden. The FE-implant was non-inferior to timolol over 9 months. Both models of the travoprost intracameral implant were well tolerated and safe for use in patients with OAG or OHT.
Acknowledgements
Medical Writing, Editorial, and Other Assistance.
The authors acknowledge David Bardin, PhD of Glaukos Corporation for graphing the figures.
Authorship.
Authorship of this manuscript is in accordance with the guidelines of the International Committee of Medical Journal Editors.
Author Contributions
Research design: Long V. Doan, Kerry G. Stephens, David Applegate, and L. Jay Katz. Data acquisition: Steven R. Sarkisian Jr, Robert E. Ang, Andy M. Lee, John P. Berdahl, Sebastian B. Heersink, and James H. Burden. Data analysis and interpretation: Dale W. Usner, Angela C. Kothe, David Applegate, L. Jay Katz, and Tomas Navratil. Manuscript preparation: Angela C. Kothe, David Applegate, Dale W. Usner, and Tomas Navratil. Manuscript review: Steven R. Sarkisian Jr., Robert E. Ang, Andy M. Lee, John P. Berdahl, Sebastian B. Heersink, James H. Burden, Long V. Doan, Kerry G. Stephens, David Applegate, Angela C. Kothe, Dale W. Usner, L. Jay Katz, and Tomas Navratil.
Funding
Wholly funded by Glaukos Corporation (Aliso Viejo, CA), including the journal’s Rapid Service Fee.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of Interest
Steven R. Sarkisian Jr. has received research funding from Glaukos, AbbVie, Allysta Pharmaceuticals, Elios Vision, iStar Medical, Ocular Therapeutix, and Sight Sciences; consulting fees from Glaukos, Alcon, AbbVie, Bausch & Lomb, Beaver-Visitec, Icare USA, Ocular Science, and Sight Sciences; payment/honoraria for lectures/speaker’s bureau from Aerie Pharmaceuticals, Alcon, AbbVie, and Bausch & Lomb; and owns stock/stock options in Ocular Science and Sight Sciences. Robert E. Ang has received research funding from Glaukos and honoraria for lectures/speaker’s bureau from Glaukos. Andy M. Lee has received research funding from Glaukos and Elios Vision; consulting fees from Glaukos; and payment/honoraria for lectures/speaker’s bureau from Elios Vision. John P. Berdahl has received consulting fees from Alcon, Bausch & Lomb, Elios Vision, Glaukos, Imprimis, Sight Sciences, ViaLase, and Johnson & Johnson. Sebatian B. Heersink has received research funding from Glaukos, Alcon Research, Allergan, Sight Sciences, EyePoint Pharmaceuticals, Sun Pharmaceuticals, AbbVie, Oyster Point Pharma, Bausch & Lomb, Cloudbreak, Combangio, Genentech, Johnson & Johnson, Ivantis, Kala Pharmaceuticals, Lenstec, Lenz, Nicox Ophthalmics, Noveome and Ocular Therapeutix; consulting fees from Carl Zeiss Meditec Cataract Technology, Bausch & Lomb, and Rayner Intraocular Lenses; and payment/honoraria for lectures/speaker’s bureau from Iridex Corporation. James H. Burden has received research funding from Glaukos. Long V. Doan, Kerry G. Stephens, David Applegate, Angela C. Kothe, Dale W. Usner, L. Jay Katz, and Tomas Navratil are employees of Glaukos Corporation and may have received stock and/or stock options.
Ethical Approval
Human subjects were included in this trial. The trial was undertaken in accordance with the tenets of the Declaration of Helsinki, and with approval of the relevant institutional review board/independent ethics committee (Western Institutional Review Board, Puyallup, Washington [reference number 20180735] or Wills Eye Hospital Institutional Review Board, Philadelphia, Pennsylvania [reference number 18–763] for sites in the USA, and St. Cabrini Medical Center – Asian Eye Institute Ethics Review Committee, Makati City, Philippines [reference number 2018–023] for the single site in the Philippines). All subjects provided informed consent to participate in the study.
Footnotes
Prior Presentation: This manuscript is based on work that was presented in part at the 2023 annual meeting of the Association for Vision and Ophthalmology (April 23–27, 2023, New Orleans, LA) [Katz LJ, Sarkisian SE, Voskanyan LA, et al. Results of the prospective, randomized, controlled, multicenter phase 3 trials of the travoprost intraocular implant versus topical timolol. Invest Ophthalmol Vis Sci. 2023;64:4296]; the 10th World Glaucoma Congress (June 28 to July 1, 2023; Rome, Italy); and the 41st congress of the European Society of Cataract and Refractive Surgery (September 8–12, 2023; Vienna, Austria).
References
- 1.Stringham J, Ashkenazy N, Galor A, Wellik SR. Barriers to glaucoma medication compliance among veterans: dry eye symptoms and anxiety disorders. Eye Contact Lens. 2018;44(1):50–54. doi: 10.1097/ICL.0000000000000301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robin AL, Covert D. Does adjunctive glaucoma therapy affect adherence to the initial primary therapy? Ophthalmology. 2005;112(5):863–868. doi: 10.1016/j.ophtha.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 3.Rees G, Leong O, Crowston JG, Lamoureux EL. Intentional and unintentional nonadherence to ocular hypotensive treatment in patients with glaucoma. Ophthalmology. 2010;117(5):903–908. doi: 10.1016/j.ophtha.2009.10.038. [DOI] [PubMed] [Google Scholar]
- 4.Kolko M, Gazzard G, Baudouin C, et al. Impact of glaucoma medications on the ocular surface and how ocular surface disease can influence glaucoma treatment. Ocul Surf. 2023;29:456–468. doi: 10.1016/j.jtos.2023.05.012. [DOI] [PubMed] [Google Scholar]
- 5.Bedrood S, Berdahl J, Sheybani A, Singh IP. Alternatives to topical glaucoma medication for glaucoma management. Clin Ophthalmol. 2023;17:3899–3913. doi: 10.2147/OPTH.S439457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Newman-Casey PA, Niziol LM, Gillespie BW, et al. The association between medication adherence and visual field progression in the Collaborative Initial Glaucoma Treatment Study. Ophthalmology. 2020;127:477–483. doi: 10.1016/j.ophtha.2019.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shu YH, Wu J, Luong T, et al. Topical medication adherence and visual field progression in open-angle glaucoma: analysis of a large US health care system. J Glaucoma. 2021;30(12):1047–1055. doi: 10.1097/IJG.0000000000001943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ranjala AP, Prager AJ, Park MS, Tanna AP. Association of the effectiveness of eye drop self-instillation and glaucoma progression. J Glaucoma. 2022;31(3):156–159. doi: 10.1097/IJG.0000000000001982. [DOI] [PubMed] [Google Scholar]
- 9.Nordstrom BL, Friedman DS, Mozaffari E, et al. Persistence and adherence with topical glaucoma therapy. Am J Ophthalmol. 2005;140(4):598–606. doi: 10.1016/j.ajo.2005.04.051. [DOI] [PubMed] [Google Scholar]
- 10.Gazzard G, Konstantakopoulou E, Garway-Heath D, et al. Laser in Glaucoma and Ocular Hypertension (LiGHT) Trial: six-year results of primary selective laser trabeculoplasty versus eye drops for the treatment of glaucoma and ocular hypertension. Ophthalmology. 2023;130(2):139–151. doi: 10.1016/j.ophtha.2022.09.009. [DOI] [PubMed] [Google Scholar]
- 11.Katz LJ, Steinmann WC, Kabir A, et al. Selective laser trabeculoplasty versus medical therapy as initial treatment of glaucoma: a prospective, randomized trial. J Glaucoma. 2012;21(7):460–468. doi: 10.1097/IJG.0b013e318218287f. [DOI] [PubMed] [Google Scholar]
- 12.Guo Y, Ioannidou A, Jute P. Selective laser trabeculoplasty: a review of repeatability. Ann Eye Sci. 2019;4:20. doi: 10.21037/aes.2019.05.01. [DOI] [Google Scholar]
- 13.Nagar M, Ogunyomade A, O'Brart DP, Howes F, Marshall J. A randomised, prospective study comparing selective laser trabeculoplasty with latanoprost for the control of intraocular pressure in ocular hypertension and open angle glaucoma. Br J Ophthalmol. 2005;89(11):1413–1417. doi: 10.1136/bjo.2004.052795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Song J. Complications of selective laser trabeculoplasty: a review. Clin Ophthalmol. 2016;10:137–143. doi: 10.2147/OPTH.S84996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clement CI. Initial treatment: prostaglandin analog or selective laser trabeculoplasty. J Curr Glaucoma Pract. 2012;6(3):99–103. doi: 10.5005/jp-journals-10008-1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bacharach J, Tatham A, Ferguson G, et al. Phase 3, randomized, 20-month study of the efficacy and safety of bimatoprost implant in patients with open-angle glaucoma and ocular hypertension (ARTEMIS 2) Drugs. 2021;81(17):2017–2033. doi: 10.1007/s40265-021-01624-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Medeiros FA, Walters TR, Kolko M, et al. Phase 3, randomized, 20-month study of bimatoprost implant in open-angle glaucoma and ocular hypertension (ARTEMIS 1) Ophthalmology. 2020;127(12):1627–1641. doi: 10.1016/j.ophtha.2020.06.018. [DOI] [PubMed] [Google Scholar]
- 18.Berdahl JP, Sarkisian SR, Ang RE, et al. Efficacy and safety of the travoprost intraocular implant in reducing topical IOP-lowering medication burden in patients with open-angle glaucoma or ocular hypertension. Drugs. 2024;84:83–97. doi: 10.1007/s40265-023-01973-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Budenz DL. A clinician's guide to the assessment and management of nonadherence in glaucoma. Ophthalmology. 2009;116(11 Suppl):S43–S47. doi: 10.1016/j.ophtha.2009.06.022. [DOI] [PubMed] [Google Scholar]
- 20.Moore SG, Richter G, Modjtahedi BS. Factors affecting glaucoma medication adherence and interventions to improve adherence: a narrative review. Ophthalmol Ther. 2023;12:2863–2880. doi: 10.1007/s40123-023-00797-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balkrishnan R, Bond JB, Byerly WG, Camacho FT, Anderson RT. Medication-related predictors of health-related quality of life in glaucoma patients enrolled in a Medicare health maintenance organization. Am J Geriatr Pharmacother. 2003;1(2):75–81. doi: 10.1016/S1543-5946(03)90003-1. [DOI] [PubMed] [Google Scholar]
- 22.Skalicky SE, Goldberg I, McCluskey P. Ocular surface disease and quality of life in patients with glaucoma. Am J Ophthalmol. 2012;153(1):1–9. doi: 10.1016/j.ajo.2011.05.033. [DOI] [PubMed] [Google Scholar]
- 23.Quaranta L, Riva I, Gerardi C, et al. Quality of life in glaucoma: a review of the literature. Adv Ther. 2016;33:959–981. doi: 10.1007/s12325-016-0333-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Netland PA, Landry T, Sullivan EK, et al. Travoprost compared with latanoprost and timolol in patients with open-angle glaucoma or ocular hypertension. Am JOphthalmol. 2001;132:472–484. doi: 10.1016/S0002-9394(01)01177-1. [DOI] [PubMed] [Google Scholar]
- 25.Goldberg I, Cunha-Vaz J, Jakobsen JE, et al. Comparison of topical travoprost eye drops given once daily and timolol 0.5% given twice daily in patients with open-angle glaucoma or ocular hypertension. J Glaucoma. 2001;10(5):414–421. doi: 10.1097/00061198-200110000-00010. [DOI] [PubMed] [Google Scholar]
- 26.Patradul C, Tantisevi V, Manassakorn A. Factors related to prostaglandin-associated periorbitopathy in glaucoma patients. Asia Pac J Ophthalmol (Phila) 2017;6(3):238–242. doi: 10.22608/APO.2016108. [DOI] [PubMed] [Google Scholar]
- 27.Kucukevcilioglu M, Bayer A, Uysal Y, Altinsoy HI. Prostaglandin associated periorbitopathy in patients using bimatoprost, latanoprost and travoprost. Clin Exper Ophthalmol. 2013;42(2):126–131. doi: 10.1111/ceo.12163. [DOI] [PubMed] [Google Scholar]
- 28.Parrish RK, Palmberg P, Sheu WP, for the XLT Study Group A comparison of latanoprost, bimatoprost, and travoprost in patients with elevated intraocular pressure: a 12-week, randomized, masked-evaluator multicenter study. Am J Ophthalmol. 2003;135(5):688–703. doi: 10.1016/S0002-9394(03)00098-9. [DOI] [PubMed] [Google Scholar]
- 29.Wistrand PJ, Stjernschantz J, Olsson K. The incidence and time-course of latanoprost-induced iridial pigmentation as a function of eye color. Surv Ophthalmol. 1997;41(2):S129–S138. doi: 10.1016/S0039-6257(97)80020-3. [DOI] [PubMed] [Google Scholar]
- 30.Maruyama K, Shirato S, Tsuchisaka A. Incidence of deepening of the upper eyelid sulcus after topical use of travoprost ophthalmic solution in Japanese. J Glaucoma. 2014;23(3):160–163. doi: 10.1097/IJG.0b013e31826a7e09. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.