INTRODUCTION
Patients presenting with obtundation or coma showing restricted diffusion in the bilateral basal ganglia on magnetic resonance imaging (MRI) are often reflexively diagnosed with hypoxic ischemic encephalopathy (HIE). Recognizing an alternate, newly defined pathological process may provide patients with earlier treatment options and improved outcomes. Cerebellar, hippocampal, and basal nuclei transient edema with restricted diffusion (CHANTER) syndrome presents similarly, often in opioid abuse settings.
In this study, we present three cases of CHANTER syndrome at our institution: three women between the ages of 23 and 60 years who exhibited decreased levels of consciousness. Two presented after overdose events, whereas one presented after what was initially believed to be HIE secondary to respiratory distress. MRI revealed distinct features of CHANTER syndrome.
Imaging can distinguish between CHANTER syndrome and other similar processes. In contrast to HIE, which typically carries a poor prognosis, CHANTER syndrome responds well to early medical and surgical interventions for acute obstructive hydrocephalus. These interventions might not be considered under the false premise of irreversible brain injury [1,2]. Therefore, the early recognition of CHANTER syndrome can significantly decrease patient morbidity and mortality. This is particularly important in the context of the opioid epidemics that have plagued our community [3].
Case 1
A 23-year-old female was found unresponsive following an unknown dose of the opioid agonist oxycodone-acetaminophen. Urine drug screening (UDS) was positive for multiple narcotics, and she was treated with intravenous naloxone, which led to a subsequent improvement in her mental status. On the second day, the patient experienced a cardiac arrest and was subsequently intubated.
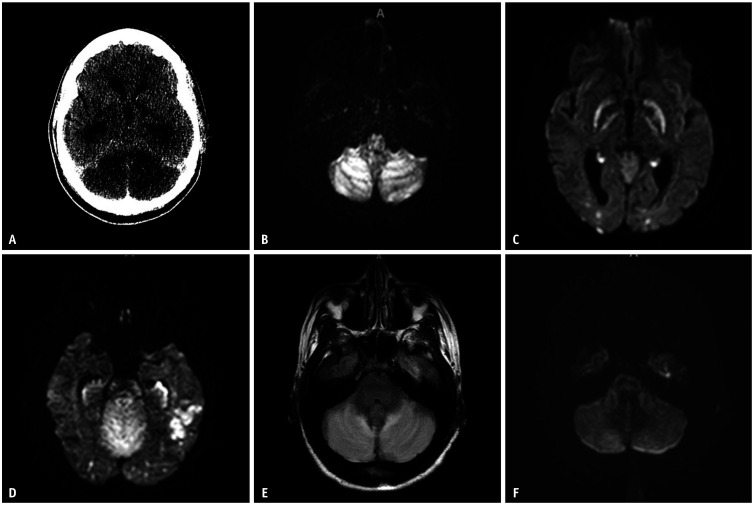
Initial computed tomography (CT) revealed multifocal hypoattenuation in the bilateral basal ganglia and cerebellar hemispheres. The mass effect from the posterior fossa resulted in effacement of the fourth ventricle, leading to upstream hydrocephalus (Fig. 1A).
Fig. 1. Selective images from Case 1. A: Initial head CT depicts bilateral cerebellar hypodensities. B-D: Subsequent MRI DWI images on the following day show restricted diffusion in the bilateral cerebellar hemispheres (B), bilateral globus pallida (C), and bilateral hippocampi and occipital lobes, with asymmetric restricted diffusion in the left temporal lobe (D). E: FLAIR sequence showed increased signal in the bilateral cerebellar hemispheres. F: Follow up MRI DWI obtained three days after the initial presentation shows near-reversal of restricted diffusion in the bilateral cerebellar hemispheres. CT = computed tomography, MRI = magnetic resonance imaging, DWI = diffusion-weighted imaging, FLAIR = fluid attenuated inversion recovery.
The patient underwent a suboccipital craniotomy and external ventricular drain (EVD) placement. Initial MRI revealed multifocal areas of restricted diffusion on diffusion-weighted imaging (DWI) involving the bilateral cerebellar hemispheres, hippocampi, basal ganglia, and cortical and subcortical white matter in the left temporal and bilateral parieto-occipital lobes (Fig. 1B-D), along with cerebellar edema (Fig. 1E). Repeat MRI two days later demonstrated resolution of the diffusion in the cerebellum (Fig. 1F). After five days, the patient was successfully extubated and weaned to room air. The EVD was removed ten days after placement. She was started on levetiracetam, an antiepileptic drug, with plans to follow up with neurology and addiction medicine outpatients.
Case 2
A 60-year-old female presented with encephalopathy, hypertensive emergency, and worsening hypoxic respiratory failure that required intubation. Her family reported that she complained of severe headaches requiring multiple narcotics. The patient was initially treated with intravenous fluids and empirical antibiotics.
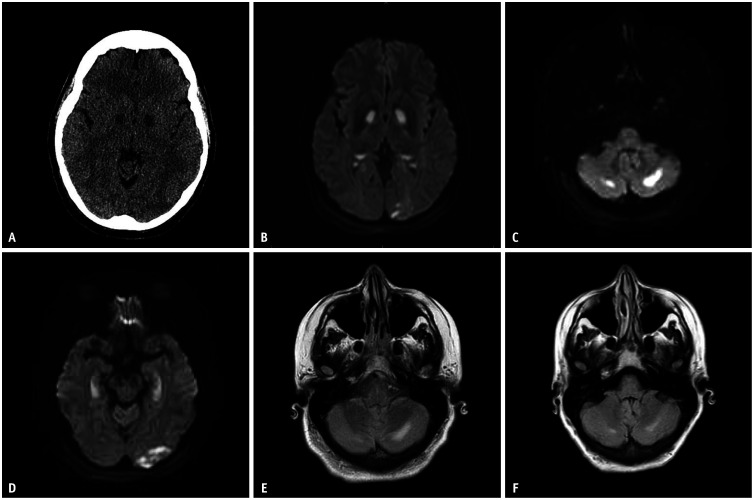
Initial head CT showed symmetric foci of hypoattenuation within the globi pallidi (Fig. 2A). Subsequent MRI showed symmetric areas of T2/fluid attenuated inversion recovery (FLAIR) hyperintensity and restricted diffusion involving the bilateral globus pallidus (Fig. 2B) and hippocampi. Additionally, MRI revealed asymmetrical involvement of the cerebellum, cerebral cortex, and subcortical and deep white matter (Fig. 2C-E). Repeat MRI two weeks later demonstrated varying degrees of improvement in the FLAIR signal changes (Fig. 2F).
Fig. 2. Selective images from Case 2. A: Initial head CT depicts bilateral basal ganglia hypodensities. B-D: Subsequent MRI DWI images on the following day reveal restricted diffusion in the bilateral basal ganglia, with asymmetric restricted diffusion in the left occipital lobe (B), bilateral cerebellar hemispheres (C), and bilateral hippocampi (D). E, F: There is a bilateral increased FLAIR signal in the cerebellar hemispheres (E), which shows partial resolution on follow up MRI two weeks later (F). CT = computed tomography, MRI = magnetic resonance imaging, DWI = diffusion-weighted imaging, FLAIR = fluid attenuated inversion recovery.
Post-extubation, the patient experienced recurrent encephalopathic episodes. Subsequently, the patient underwent percutaneous endoscopic gastrostomy tube placement and was discharged to a rehabilitation facility.
Case 3
A 55-year-old female was unresponsive to treatment for generalized convulsions and hypertension. The patient was intubated to protect her airway. UDS was positive for cannabis and multiple narcotics, and the patient was treated with naloxone, which resulted minimal improvement in mentation.
Initial CT of the head revealed chronic changes without any acute findings. Five days later, brain MRI showed multifocal areas of restricted diffusion involving the bilateral cerebellar hemispheres and hippocampi, posterior body of the right caudate nucleus, and small areas of the left caudate nucleus, with an associated corresponding T2/FLAIR hyperintense signal. Subsequent MRI one month later showed resolution of the DWI changes. FLAIR signal changes in the cerebellum were persistent, but resolved in the hippocampus.
The patient underwent a tracheostomy and PEG placement. Eventually, her tracheostomy was decannulated, and she began to tolerate a pureed diet. The patient was then transferred for inpatient rehabilitation.
DISCUSSION
All three patients presented with variable symmetrical diffusion abnormalities in the globus pallidus. One patient had asymmetrical cerebellar diffusion changes, whereas the other two demonstrated symmetrical involvement. Additionally, two of our patients showed asymmetrical cortical and white matter involvement. None of the three patients had vascular disease, as confirmed by CT angiograms. All patients showed varying degrees of improvement on follow-up MRI, with cases 2 and 3 demonstrating near-complete resolution of their initial findings.
CHANTER is an emerging diagnosis with a poorly understood pathophysiology. Acute management typically involves opioid reversal with naloxone, an opioid antagonist. Initial CT findings revealing acute cerebellar edema with secondary obstructive hydrocephalus are managed either medically with mannitol and hypertonic saline or surgically with temporary ventricular shunting and decompressive craniectomy [4,5]. MRI commonly demonstrates diffusion restriction in the cerebellar cortices and hippocampi symmetrically and in the bilateral basal ganglia asymmetrically, with variable cerebral cortical and white matter involvement. Although diffusion changes are reversible, FLAIR reversibility varies from negligible to complete resolution in different locations, suggesting that many of these cases are also associated with hypoxic injury. Its etiology is believed to be mitochondrial injury caused by opiates and other toxic agents, compounded with hypoxia. Notably, neurons in the hippocampus and cerebellum are more vulnerable to opioids [6,7]. Imaging manifestations can vary, and obstructive hydrocephalus may not develop in all patients, depending on the severity and duration of cerebellar involvement.
The primary differential diagnosis is HIE, in which symmetrical brain involvement is observed. Imaging differences include more extensive, frequent, and symmetrical involvement of the cerebral cortices, thalami, and basal ganglia structures, such as the caudate and lentiform nuclei. Additionally, HIE exhibits less frequent involvement of the cerebellum and hippocampus [8]. Opioid-associated amnestic syndrome presents with a milder course, featuring only symmetrical hippocampal involvement [9]. Posterior reversible encephalopathy (PRES) may occasionally present with brainstem and cerebellar edema but typically does not involve the hippocampus and lacks diffusion changes [10]. Heroin-associated spongiform leukoencephalopathy can exhibit a similar clinical presentation but manifests more white matter involvement than cortical lesions with inconsistent diffusion changes [11,12].
In conclusion, CHANTER is one facet within the spectrum of opioids and related toxin-induced encephalopathies, characterized by distinctive imaging findings. Recognizing CHANTER is crucial for radiologists, given its overall favorable prognosis compared with other non-reversible encephalopathies when recognized early.
Acknowledgments
We would like to thank Dr. Houman Sotoudeh for his guidance in preparing this manuscript and Dr. Veeranjaneyulu Prattipati for providing cases to include in the manuscript.
Footnotes
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
- Conceptualization: Siddhartha Gaddamanugu.
- Funding acquisition: Siddhartha Gaddamanugu.
- Project administration: Siddhartha Gaddamanugu.
- Resources: Siddhartha Gaddamanugu.
- Visualization: Siddhartha Gaddamanugu, Renu Pandit.
- Writing—original draft: all authors.
- Writing—review & editing: all authors.
Funding Statement: None
References
- 1.Mallikarjun KS, Parsons MS, Nigogosyan Z, Goyal MS, Eldaya RW. Neuroimaging findings in CHANTER syndrome: a case series. AJNR Am J Neuroradiol. 2022;43:1136–1141. doi: 10.3174/ajnr.A7569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Howard RS, Holmes PA, Koutroumanidis MA. Hypoxic-ischaemic brain injury. Pract Neurol. 2011;11:4–18. doi: 10.1136/jnnp.2010.235218. [DOI] [PubMed] [Google Scholar]
- 3.Niles JK, Gudin J, Radcliff J, Kaufman HW. The opioid epidemic within the COVID-19 pandemic: drug testing in 2020. Popul Health Manag. 2021;24(S1):S43–S51. doi: 10.1089/pop.2020.0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jasne AS, Alsherbini KH, Smith MS, Pandhi A, Vagal A, Kanter D. Cerebellar hippocampal and basal nuclei transient edema with restricted diffusion (CHANTER) syndrome. Neurocrit Care. 2019;31:288–296. doi: 10.1007/s12028-018-00666-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou YMJ, Shah M, Fayngersh A. A case of cerebellar hippocampal and basal nuclei transient edema with restricted diffusion syndrome with poor clinical outcome. Cureus. 2022;14:e22767. doi: 10.7759/cureus.22767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barash JA, Kofke WA. Connecting the dots: an association between opioids and acute hippocampal injury. Neurocase. 2018;24:124–131. doi: 10.1080/13554794.2018.1475572. [DOI] [PubMed] [Google Scholar]
- 7.Schadrack J, Willoch F, Platzer S, Bartenstein P, Mahal B, Dworzak D, et al. Opioid receptors in the human cerebellum: evidence from [11C]diprenorphine PET, mRNA expression and autoradiography. Neuroreport. 1999;10:619–624. doi: 10.1097/00001756-199902250-00032. [DOI] [PubMed] [Google Scholar]
- 8.Huang BY, Castillo M. Hypoxic-ischemic brain injury: imaging findings from birth to adulthood. Radiographics. 2008;28:417–439. doi: 10.1148/rg.282075066. quiz 617. [DOI] [PubMed] [Google Scholar]
- 9.Barash JA, Whitledge J, Watson CJ, Boyle K, Lim C, Lev MH, et al. Opioid-associated amnestic syndrome: description of the syndrome and validation of a proposed definition. J Neurol Sci. 2020;417:117048. doi: 10.1016/j.jns.2020.117048. [DOI] [PubMed] [Google Scholar]
- 10.Grossbach AJ, Abel TJ, Hodis B, Wassef SN, Greenlee JD. Hypertensive posterior reversible encephalopathy syndrome causing posterior fossa edema and hydrocephalus. J Clin Neurosci. 2014;21:207–211. doi: 10.1016/j.jocn.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 11.Keogh CF, Andrews GT, Spacey SD, Forkheim KE, Graeb DA. Neuroimaging features of heroin inhalation toxicity: “chasing the dragon.”. AJR Am J Roentgenol. 2003;180:847–850. doi: 10.2214/ajr.180.3.1800847. [DOI] [PubMed] [Google Scholar]
- 12.Alshamam MS, Sumbly V, Nso N, Saliaj M, Gurung DO. Heroin-induced leukoencephalopathy. Cureus. 2021;13:e13093. doi: 10.7759/cureus.13093. [DOI] [PMC free article] [PubMed] [Google Scholar]