Abstract
Cone‐beam computed tomography (CBCT) system can provide real‐time 3D images and fluoroscopy images of the region of interest during the operation. Some systems can even offer augmented fluoroscopy and puncture guidance. The use of CBCT for interventional pulmonary procedures has grown significantly in recent years, and numerous clinical studies have confirmed the technology's efficacy and safety in the diagnosis, localization, and treatment of pulmonary nodules. In order to optimize and standardize the technical specifications of CBCT and guide its application in clinical practice, the consensus statement has been organized and written in a collaborative effort by the Professional Committee on Interventional Pulmonology of China Association for Promotion of Health Science and Technology.
Keywords: cone‐beam CT, diagnosis, localization, pulmonary nodule, treatment
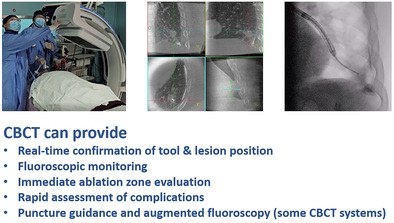
CBCT system plays a crucial role in interventional pulmonology procedures. It can help operators identifying the position of interventional tools and lesions, as well as monitoring operation area. These features facilitate accurate and efficient diagnosis, localization, and treatment of pulmonary nodules.
INTRODUCTION
With the popularization of low dose computed tomography (LDCT) scans, more and more pulmonary nodules are being detected. 1 Pulmonary nodules are an early manifestation of peripheral lung cancer. Due to the probability of malignancy, the diagnosis and treatment of pulmonary nodules have become the focus of clinicians. It is challenging to carry out interventional procedures on pulmonary nodules with traditional bronchoscopy swiftly and precisely when there are insufficient reliable navigation or verification techniques. Moreover, the risk of complications is relatively high when percutaneous transthoracic needle biopsy (PTNB) operations are performed under the guidance of traditional imaging methods. With the continuous improvement and development of technologies, more auxiliary guidance technologies have been iteratively updated, 2 , 3 including radial endobronchial ultrasound (R‐EBUS), 4 , 5 , 6 electromagnetic navigation bronchoscopy (ENB), 7 , 8 , 9 electromagnetic navigated PTNB, 10 , 11 virtual navigation bronchoscopy (VBN), 12 , 13 , 14 robotic bronchoscopy, 15 , 16 , 17 percutaneous puncture robotic system, 18 , 19 and the cone‐beam computed tomography (CBCT), 20 , 21 , 22 etc. Nonetheless, efficient and precise reach of pulmonary nodules with minimal invasiveness is still a challenging yet crucial task even with the arsenal of various navigation tools.
The capacity of CBCT has increased over decades of development since its first clinical application at the Mayo Clinic Biodynamics Research Laboratory in 1982. 23 A single CBCT imaging protocol may acquire up to 600 images in one C‐arm rotation. The acquired image series can be reconstructed into 3D stack of axial images, analogous to conventional multislice CT, to assist real‐time lesion localization and tool navigation in interventional procedures. As a relatively new technology in, many attempts have been made to integrate CBCT into routine protocols. Jin et al. were the first to publish their team's experience in applying CBCT‐guided PTNB on 71 patients in 2010 and found that CBCT guidance leads in better diagnostic accuracy (98.4%) and sensitivity (97.0%), as well as a shorter needle dwell time (8.7 ± 3.8 min vs. 10.9 ± 8.2 min). 24 In 2014, Hohenforst‐Schmidt et al. reported that the overall diagnostic yield was 70% for using bronchoscopy in conjunction with CBCT guidance. The diagnostic yield of CBCT‐guided biopsy is approximately twice that of conventional bronchoscopy for incidental solitary pulmonary nodules <2 cm. 20 In transbronchial or percutaneous transthoracic procedures, CBCT (with fixed C‐arm or mobile C‐arm) delineates positions of the target lesion and the tool in real time, and provides fluoroscopic monitoring during the procedure. 25 , 26 , 27 In ablation procedures, CBCT can also be used for treatment monitoring and evaluating ablation efficacy. 28 , 29 , 30 Some CBCT systems can provide augmented fluoroscopy to guide the tools to target lesions. 31 , 32 , 33 Based on the fixed CBCT system, the path from the puncture point on skin to target lesion can be planned and used for real‐time guidance during PTNB procedures. 34 Compared with conventional CT, the CBCT system provides 3D and fluoroscopic images in real time. When compared with fluoroscopy, CBCT may offer better visibility for pulmonary nodules that are difficult to detect with traditional fluoroscopy.
Despite the ever‐growing capacities and diverse applications of CBCT, the relevant procedures and precautions for using CBCT to assist in interventional operations on pulmonary nodules have not yet been standardized. Therefore, the Professional Committee on Interventional Pulmonology of China Association for Promotion of Health Science and Technology organized experts in relevant fields to formulate this technical consensus. Due to the lack of high‐level clinical evidence, this consensus only represents the general recommendations of the expert group based on clinical experience and relevant CBCT literature, without grading the level of evidence and strength of recommendations. This consensus will be further updated as more evidence emerges in the future.
INDICATIONS AND CONTRAINDICATIONS
Indications
Indications for CBCT‐guided pulmonary nodule biopsy
(1) Indeterminate pulmonary nodules greater than 8 mm in diameter, require a biopsy to be clarified. (2) Rebiopsy for recurrent or progressive lesion to guide following treatment. 35
In transbronchial pulmonary nodule biopsy, CBCT allows accurate biopsy of the target lesion by providing real‐time 3D images and fluoroscopy to identify the relationship between tools and target lesion. CBCT is useful for pulmonary nodules that are difficult to perform biopsy using conventional bronchoscopy, particularly those that are hard to detect with fluoroscopy. CBCT is also invaluable for special procedures such as peripheral transbronchial needle aspiration (TBNA), cryobiopsy, and bronchoscopic transparenchymal nodule access (BTPNA). 21 , 36 , 37 CBCT can also be used in PTNB to guide puncture, to confirm needle tip position and to detect complications. CBCT is suitable for pulmonary nodules that are tricky for PTNB (e.g., nodules which are adjacent to pulmonary bulla, to interlobar fissures, to big vessels and other tissues or pulmonary nodules with long puncture paths in the lungs), and its precise guide was found to minimize the risk of complications caused by repeated adjustments of the needle tip position. 22
Indications for CBCT‐guided pulmonary nodule localization
Pulmonary nodules with malignant tendency that are proposed for thoracoscopic sublobar resection and meet one of the following criteria: (1) Single or multiple nodules; less than 1.0 cm in diameter or more than 1.5 cm from the pleura. (2) Radiographic findings are pure ground‐glass opacity nodules or subsolid nodules. (3) Nodules that are difficult to localize intraoperatively by direct visualization or by tactile localization. 38 , 39
CBCT can provide a fluoroscopic and 3D image during localization and assist in confirming the position of the placed markers. CBCT can also guide and monitor the needle puncture when performing percutaneous transthoracic pulmonary nodule localization. Furthermore, compared with conventional CT, CBCT occupies less space and is suitable for one‐stop pulmonary nodule localization and resection in the operating room, hence minimizes preoperative time and repetitive patient transfers.
Indications for CBCT‐guided pulmonary nodule ablation therapy
Curative ablation is recommended as the primary treatment or retreatment after recurrence for single or multiple malignant pulmonary nodules with maximal diameters ≤3 cm, without distal metastasis and in patients not suitable for surgery or who refuse to undergo surgery. Also, pulmonary tumor with ≤3 metastatic lesions in unilateral lung or ≤5 in bilateral lungs. 40 The indication for palliative ablation is more flexible, such as size and number of lesions may exceed the criteria for curative ablation. 35 In pulmonary nodule ablation, CBCT can be used to precisely localize the ablation electrode and its relationship with the lesion. It can also help in monitoring the ablation procedure, evaluating the ablation zone and checking for complications. In transbronchial pulmonary nodule ablation, CBCT can ensure that the ablation location and ablation zone meet the therapeutic needs. When performing transthoracic pulmonary nodule ablation, it can also guide the puncture of needle electrode, which is especially suitable for nodules with high operation risk.
Contraindications
Contraindications for transbronchial pulmonary nodule biopsy
(1) Uncorrectable coagulation disorders or platelet counts <60 × 109/L. (2) Unstable hemodynamic conditions. (3) Severe respiratory failure (arterial partial pressure of oxygen <60 mmHg after aggressive oxygen therapy). (4) Within 4 weeks after acute myocardial infarction. (5) Massive hemoptysis. (6) Pregnancy. (7) Other high risk conditions including malignant cardiac arrhythmia, severe cardiopulmonary insufficiency, intracranial hypertension, severe pulmonary hypertension, extreme systemic failure and severe psychiatric disorders. 41
Contraindications for transthoracic pulmonary nodule biopsy
(1) Significant infectious lesions, large vessels, or vital organs lie in the puncture path. (2) Severe emphysema, pulmonary bulla, pulmonary fibrosis, single lung (anatomically or functionally) or FEV1 ≤ 35% of predicted value. (3) Pulmonary vascular disease (pulmonary hypertension, pulmonary aneurysms, arteriovenous malformations, etc.). (4) Patient unable to cooperate with examination (maintain position and hold breath). (5) Patients in poor general condition and with significant vital organ insufficiency. (6) Uncorrectable coagulation disorder. (7) Patients on mechanical ventilation. 42
Contraindications for localization of pulmonary nodules
There are no specific contraindications for pulmonary nodule localization except for patients with contraindications for transbronchial or transthoracic interventions, or patients who cannot tolerate following operations. Iodic agent such as indocyanine green (ICG) should not be used in patients with an iodine allergy.
Contraindications for ablation of pulmonary nodules
(1) Patients with uncorrectable coagulation disorders, platelet counts <50 × 109/L or anticoagulant or antiplatelet drug withdrawal for less than 5–7 days. (2) Severe cardiopulmonary abnormalities. (3) Extensive extrapulmonary metastases of malignant tumors with an expected survival less than 6 months. (4) The Eastern Cooperative Oncology Group (ECOG) performance status (PS) scale >3. (5) Patients with severe infections, severe dysfunction of vital organs, poor general condition, severe psychiatric disorders, and other conditions with a high risk of complications, or conditions that are not suitable for transbronchial or transthoracic interventions. 40 , 43 In addition, radiofrequency ablation is contraindicated in those with pacemakers and metallic implants in the chest. Transthoracic ablation is contraindicated if significant infectious lesions, large vessels, or vital organs lie in the puncture path.
EQUIPMENT AND INSTRUMENTS
CBCT system
A CBCT system consists of an x‐ray generator (high voltage generator, x‐ray tube), a digital imaging system (e.g., flat panel detector), a mechanical system, a computer control system, an image processing system, and ancillary systems. By rotating the gantry for nearly 200 degrees around the patient, CBCT acquires up to 600 images using imaging protocols ranging from 3 to 30 s. The acquired 2D scans are reconstructed into axial 3D volumetric images (similar to a conventional multislice helical CT) based on the Feldkamp algorithm at the post‐processing workstation. 44 CBCT offers a wide field of view, high acquisition rate, and real time imaging capacity. The reconstructed 3D volume accurately depicts 3D anatomical structures and tools placed, thus facilitating interventional workflow in navigation and lesion localization.
CBCT systems can be divided into fixed and mobile systems. For fixed CBCT systems, they can be subdivided into ceiling‐system, floor‐system, biplane‐system, and robotic‐system (more suitable for hybrid operating room). The ceiling‐ and the robotic‐ CBCT systems require less space, and allow the tracheoscopist to maneuver at the side of the patient's head. Therefore ceiling‐ and robotic‐systems see more uses in respiratory interventions. 21
Existing mobile CBCT systems can also offer 3D imaging (Cios Spin, Siemens Healthineers; Vision RFD 3D, Ziehm Imaging). Mobile CBCT has a smaller field of view, longer scanning time (approximately 30–60 s), lacks augmented fluoroscopy and automatic needle planning to assist percutaneous transthoracic puncture; nonetheless, mobile CBCT still demonstrates the capacity to assist pulmonary nodule diagnosis and treatment. In addition, mobile CBCT system has advantages of mobility, less space occupancy, and low cost; these characteristics make mobile CBCT more applicable in operating rooms with restricted conditions. 45
Bronchoscopy
All types of bronchoscopes can be used with CBCT. Commonly used bronchoscopes include: ultrathin bronchoscope (common products with 2.8–3.0 mm outer diameter and 1.2–1.7 mm working channel), thin bronchoscope (common products with 4.0–4.2 mm outer diameter and 2.0 mm working channel), and therapeutic bronchoscope (common products with 5.9 mm outer diameter and 2.8–3.0 mm working channel). 46
Navigation tools and devices
R‐EBUS is used to obtain cross‐sectional images perpendicular to the ultrasound probe by scanning in a circular pattern. It can assist localizing and assessing lesion, as well as tissues adjacent to lesions. R‐EBUS probes with an outer diameter of 1.4 and 1.7 mm are commonly used. ENB, VBN, or robotic bronchoscopy can also be used to help operator reach the target lesion in complex bronchial structures, improving success rate as well as reducing the procedure time and radiation exposure. When performing transthoracic procedure, different navigation systems or robot‐assisted transthoracic puncture systems can be used conjointly with CBCT, as appropriate, to guide the needle insertion.
Biopsy tools
According to the size of the lesion, malignant tendency, and the required specimen volume, the operator can choose different biopsy tools appropriately. Transbronchial biopsies are usually performed with guide sheath sets with an outer diameter of 1.95 and 2.55 mm (containing biopsy forceps with an outer diameter of 1.5 and 1.9 mm, cytology brushes with an outer diameter of 1.4 and 1.8 mm). Transbronchial cryobiopsy and peripheral TBNA can also be performed with either a flexible cryoprobe (typically 1.1–1.9 mm in outer diameter) or a flexible aspiration needle (18–22 G, typically around 1.5–2.0 mm in outer diameter).
Transthoracic pulmonary nodule biopsies can be performed with aspiration needles or cutting biopsy needles. Most aspiration needles are 20–22G Chiba needles. Cutting needles often used are 18–20G semi‐automatic or fully automatic biopsy needles with biopsy guns. They are mostly used in combination with coaxial needles (slightly thicker than biopsy needles by 1G) to minimize the puncture damage and the risk of needle tract implantation.
Localization medicine and instruments
Commonly used biological dyes include iodine oil, methylene blue, and indocyanine green. Metallic markers such as hook wire and spring coils can be used. For indocyanine green localization, a near‐infrared fluorescence thoracoscope should be used in combination.
Treatment devices and instruments
These include rigid or flexible radiofrequency ablation electrodes, microwave ablation electrodes, cryoablation electrodes, coablation electrodes (e.g., combined liquid nitrogen cryoablation and radiofrequency heating), and their corresponding devices.
PREOPERATIVE PREPARATION
Operating room and personnel set‐up
Criteria for setting up CBCT‐equipped operating rooms can be referenced from DSA interventional operating room standards. Radiation shielding, room temperature, and humidity must be maintained accordingly. The operating room should provide adequate space for CBCT and other medical devices (e.g., ablation devices, anesthesia machines, etc.). Therefore, it is recommended that the operating room with a mobile CBCT system has a minimum area of 30 m2, plus 2 m2 of space reserved for CBCT equipment. An operation room with a fixed CBCT system should have no less than 45 m2 of clean area. The C‐arm and operating bed should be set up along the long axis of the operating room. The size of operating room varies with the equipment size and motion range and should be adjusted according to practical needs. The decontamination level and corresponding system of the operating room must meet the criteria of the operation to be performed.
The team for performing CBCT‐guided transthoracic/transbronchial operation consists of operators, assistants, and radiologists who have received professional training, possessed relevant qualifications and certificates. An anesthetic unit is required for patients who undergo general anesthesia.
Preoperative evaluation
All patients should receive preoperative tests, including routine blood, coagulation function, blood biochemistry, infectious disease screening and electrocardiogram, and so on. For elderly patients or those who have comorbid underlying cardiac or pulmonary disease, cardiac ultrasound and pulmonary function examination should be performed. Patients with severe diseases should have cross‐consultation with experts in the relevant departments to evaluate the pros and cons of the operation. Thin‐slice CT scan is required to delineate the location, size, and shape of the target lesion, as well as its location relative to the surrounding bronchus, blood vessels, organs and other tissues. A preliminary plan of the operation path and operation position are also made accordingly. 41 , 43 , 47 Patients who are scheduled for general anesthesia should be evaluated by anesthesiologists; patients who are scheduled for local anesthesia should have their cooperation ability assessed (e.g., breath‐holding ability, position keeping ability). If ablation is to be performed without pathology assessment, the treatment plan should be evaluated in a multidisciplinary discussion (at least including medical, surgical, and medical imaging department), and the patient should be informed of the potential risks and benefits. 48 For patients who are scheduled for curative ablation, relevant examinations (e.g., abdominal ultrasound imaging and CT, bone scan, ultrasound imaging of superficial lymph nodes, cranial enhanced MRI, etc.) should be performed for staging. PET/CT examination is also recommended to exclude distal metastasis if possible.
Patient preparation
(1) Patients and their families should be fully informed of purpose, method, cost, and risks of the operation. Patients and their families should sign the consent form. (2) Patient should abstain from solid food for 4 h and liquids for 2 h before local anesthesia, or abstain from solid food for 8 h and liquids for 2 h before general anesthesia. 41 , 49 (3) Anticoagulant therapy and/or antiplatelet drug should be withdrawn within 5–7 days before the operation. (4) Venous access should be established. (5) Preoperative patient education and psychological counseling. 40 If the operation is to be performed under local anesthesia, the patient should be trained in breath‐holding preoperatively to ensure that the CBCT acquisition will not be affected by respiratory movements.
STANDARD PROCEDURE
CBCT‐guided transbronchial pulmonary nodule biopsy
CBCT‐guided bronchoscopy is commonly performed under general anesthesia and endotracheal intubation. Patients are asked to lie in a supine position on a special examination bed for CBCT. For lesions in the lower or dorsal lobes, to avoid a hypostatic effect or pulmonary atelectasis that may impede diagnosis, a pillow can be placed under the lesion side back to elevate the target lobe. The ventilator is usually set at volume control mode. Tidal volume is set at 8–10 mL/kg (ideal bodyweight). Positive end‐expiratory pressure (PEEP) is maintained at 8–15 cmH2O to ensure the bronchioles are fully open during the operation. The inspired oxygen concentration is kept as low as possible while meeting the oxygen saturation requirement (>94%). Once the artificial airway is set, the C‐arm is adjusted to keep the lesion at image center. A preoperative CBCT is performed in a high spatial resolution imaging protocol, and the generated 3D volume is used to confirm that the lesion can be observed using the current imaging protocol. Patients must hold respirations during CBCT scanning to minimize motion artifacts. The adjustable pressure limiting (APL) valve of anesthetic machine is adjusted to maintain appropriate intra‐airway pressure (usually set to 10–20 cmH2O) to avoid atelectasis or diaphragm elevation during image acquisition.
For CBCT that are equipped with augmented fluoroscopy functionality, the user can mark the lesions on the reconstructed 3D volumes, and fuse marked lesions onto subsequent fluoroscopic images for guidance using vendor provided softwares (iGuide toolbox, Siemens Healthineers; OncoSuite, Philips; ASSIST, GE Healthcare).
The bronchoscope is advanced towards the lesion as far as possible after routine white light bronchoscopy has been performed and secretion in airway has been cleared by suction. The operator can choose to use ENB, VBN, fluoroscopy, augmented fluoroscopy, and other navigation tools to reach the target lesion. R‐EBUS probe is then recommended to be used for preliminary confirmation of reaching the target lesion, as well as investigating the maximum cross‐section of the lesion and observing the positional relationship between the lesion and adjacent bronchus, blood vessels and other structures.
After preliminary reaching the lesion, the bronchoscope and the interventional tools in the working channel (which can be via a biopsy tool, guide sheath, locatable wire, or R‐EBUS probe) are properly fixed using a bronchoscope fixator, then a CBCT scan is again performed. The spatial position relationship between the guide or biopsy tool and the target lesion can be confirmed by the generated coronal, axial and sagittal images, and appropriate adjustments can be made according to the scanning results. If necessary, another CBCT scan can be performed for arrival confirmation after adjustment. If the operator fails to reach a satisfactory position after three adjustments, using other navigation or biopsy methods should be considered. After the tool is confirmed to be positioned at lesion site (tool‐in‐lesion), it is recommended to save a fluoroscopic image at the end of expiratory phase as a reference image for the subsequent interventional procedure.
Biopsy is performed subsequently after confirming tool‐in‐lesion. The operator can take the biopsy under fluoroscopy at the end of the expiratory phase referring to the fluoroscopic image saved previously. The required volume of specimen and the selection of biopsy tools are comprehensively determined by the operator according to the biopsy safety, efficiency, and lesion characteristics. Rapid on‐site evaluation (ROSE) can be performed to assess the eligibility of biopsy samples, to form a preliminary diagnosis and to guide the further biopsy.
Fluoroscopy can be used to quickly exclude pneumothorax once the biopsy is finished. The bronchoscope and guide sheath can be kept in situ for one minute to prevent bleeding. In cases of hemorrhage, iced saline and hemostatic drugs can be locally applied. Balloon compression and interventional operation may be carried out for patients with severe hemorrhage. If no hemorrhage is present, the patient is then transferred to a ward for further observation.
CBCT‐guided transbronchial pulmonary nodule localization
Transbronchial pulmonary nodule localization and video‐assisted thoracic surgery (VATS) are often performed as a one‐stop operation in a hybrid operation room, where patients are placed under general anesthesia and tracheal intubation. Patients lie in a supine position for preoperative CBCT scanning. Using navigation techniques such as ENB, a guide sheath or locatable wire is advanced close to the lesion site (≤15‐mm) or to the desired multipoint localization position. Subsequently, a second CBCT scan is carried out to verify the desired position has been reached. The tool position may be adjusted according to the CBCT image. Single or multipoint localization is carried out based on the location of the lesion, CT bronchial signals, and the intended surgical approach.
A pulmonary nodule may be localized using biological dyes or metallic markers after guide sheath is positioned at desired site. Staining: 0.5–1.0 mL diluted methylene or indocyanine green is delivered through the guide sheath, followed by 20 mL of air to push the remaining dye in the guide sheath lumen into the lesion. The guide sheath remains in situ for a minute for observing reversal flow of dye. Metallic marker: guide wire or biopsy forceps may be used to deliver metallic markers through guide sheath to the target site. 35
A CBCT scan is carried out following localization in order to verify the relative location relationship between markers and lesions and to evaluate possible complications such as bleeding and pneumothorax. According to the marker locations, VATS surgery is subsequently carried out.
CBCT‐guided transbronchial pulmonary nodule ablation
General anesthesia and tracheal intubation are recommended prerequisite for performing transbronchial pulmonary nodule ablation. The guide sheath or the tip of the bronchoscope are advanced to the lesion site using ENB or other navigation techniques (the procedure is similar to that delineated in step 1 to step 3, “CBCT‐guided transbronchial pulmonary nodule positioning” section).
A flexible ablation electrode is inserted into the lesion site through the guide sheath or bronchoscope. The electrode and bronchoscope are then properly fixed using a bronchoscope fixator. A CBCT scan is performed to confirm the electrode is in the desired position.
The guide sheath and bronchoscope are retracted for 2–3‐cm under fluoroscopic monitoring while the electrode remains in situ to prevent collateral damage during ablation. The bronchoscope, guide sheath, and electrode are remained fixed throughout ablation. Ablation parameters may be referenced from device vendors and may vary with different ablation electrodes, purpose of ablation (curative treatment or tumor reduction), size of pulmonary nodules, and surrounding tissues.
The vital signs of the patient are monitored during ablation. For ablations that are performed under local anesthe, respiration, pain, cough, and hemoptysis should also be monitored. CBCT scans may be performed during ablation to assess electrode position, ablation zone, and exclude potential complications (for example, hemorrhage and pneumothorax).
A post‐ablation CBCT scan is again performed to preliminarily assess the ablation efficacy. To accomplish the goal of complete ablation, the ablation zone should encompass the target lesion and the surrounding normal lung tissue (5–10 mm). It is possible to perform two ablation if the ablation zone is not ideal. Complications such as hemorrhage and pneumothorax should also be checked after ablation. A patient in a stable condition can be transferred to the ward for further observation, and a chest CT will be re‐examined 1 day later.
CBCT‐guided transthoracic needle biopsy
-
1
Transthoracic pulmonary nodule biopsy is often carried out under local anesthesia. Depending on the location of the lesion, the patient is placed in a position that is convenient for puncture and easy to maintain. A preoperative CBCT baseline scan of the lesion area is performed to identify the lesion location and to ensure that the nodule is visible under the current scanning protocol. The patient is asked to hold their breath to reduce respiration‐induced motion artifacts.
-
2
Puncture direction and entry point can be determined by CBCT guidance, a manual method, or by other guiding techniques such as electromagnetic navigation system and transthoracic interventional robotics. 10 , 18 CBCT‐guided puncture planning: Some CBCT can guide the operator to puncture the needle according to the planned path. After preoperative CBCT is performed, the desired entry point and target lesion can be manually marked on the reconstructed 3D volumes using matched software (for example, Needle Guidance, Siemens; XperGuide, Phillip), thus the planned puncture path is formed and the penetration depth is determined. The planned puncture path should avoid scapula, ribs, and vessels. The marked sites are then displayed through augmented fluoroscopy to assist puncture. The CBCT will then automatically adjust its C‐arm and instruct operator to make fine adjustments, aligning the lesion, entry point, and x‐ray tube such that they form bull's eye view in augmented fluoroscopy. Once C‐arm reaches the planned angle, the laser mark is projected towards the lesion, assisting operator in determining the puncture entry point and direction.
-
3
After sterilizing and applying local anesthesia around the puncture site, the needle is advanced towards the nodule with its tip and tail maintaining at the center of laser cross mark. The patient is asked to keep a steady and regular breathing. The C‐arm may be rotated to view the puncture procedure from different angles to ensure accurate puncture along the planned path to the target lesion. A CBCT scan is performed after the needle tip reaches the target nodule, and the needle may be repositioned accordingly based on the CBCT scan.
-
4
A biopsy is performed if the needle is confirmed to be in the desired location. The exact biopsy site varies with nodule characteristics: for larger nodules, necrotic regions within the nodule should be avoided; for nodules with cavities, tissue samples should be extracted from solid tissue. Coaxial technique can be used to minimize the puncture damage and the risk of needle tract implantation incurred from repetitive puncture attempts. For smaller nodules where cutting biopsy has a high risk of complications, fine needle aspiration may be applied. 32
-
5
A CBCT scan is again performed after biopsy to exclude complications such as hemorrhage or pneumothorax. If the patient is in a stable condition they may be transferred to the ward for further observation. Patients are taught to avoid events that may elevate intrathoracic pressure such as coughing, breath‐holding, and heavy lifting.
CBCT‐guided transthoracic pulmonary nodule localization
Transthoracic pulmonary nodule localization is often performed under local anesthesia. It may also be carried out as part of one‐stop localization and VATS, with patients placed under general anesthesia. A preoperative CBCT scan is performed and a reasonable puncture path is planned. A needle is then advanced according to the planned angle and penetration depth. A fluoroscopy and CBCT scan can be performed during the procedure to adjust the puncture direction and confirm the arrival of needle tip at the lesion site (≤10 mm) (operation procedure is similar to that delineated in step 1 to step 3, “CBCT‐guided transthoracic pulmonary nodule biopsy” section).
Pulmonary nodules can be marked using biological dyes or metallic markers after the coaxial needle is positioned at the target site. Staining: After pulling out the stylet and confirming that the needle is not in the blood vessel by gently withdrawing the plunger of the syringe, diluted dyes such as indocyanine green or methylene blue are slowly injected. The needle is then withdrawn to about 1 cm below the pleura to inject the dye again. Metallic markers: metallic markers are sent though coaxial needle. If a hook wire is used for localization, its tail should be fixed at the skin. In case a microcoil is used, its tail can be fixed inside lung or outside of visceral pleura. 50
CBCT acquisitions are performed again to confirm the relative positional relationship between localization markers and lesions. Complications such as hemorrhage or pneumothorax are also evaluated. Subsequent this, VATS is performed according to the distribution area of biological dyes or the location of metallic markers.
CBCT‐guided transthoracic pulmonary nodule ablation
Transthoracic pulmonary nodule ablation can be performed under either general or local anesthesia, or local anesthesia with moderate sedation. After placing the patient in a position that is convenient for puncture and easy to maintain depending on the location of the lesion, a preoperative CBCT scan is performed and an appropriate puncture path is planned. To reduce motion artifacts incurred from respiration during CBCT scanning, patients under general anesthesia should have the ventilator paused with airway pressure maintained at a constant and appropriate level, and those who are under local anesthesia are asked to hold their breath.
An ablation needle can be punctured towards the lesion under CBCT guidance or other methods. Once the needle tip reaches the target site, a CBCT scan is again performed to confirm the postional relationship between the needle tip and the lesion (operation procedure is similar to that delineated in step 2 to step 3, “CBCT guided transthoracic pulmonary nodule biopsy” section). The patient is asked to maintain steady and regular breathing and in a fixed body position during the puncture procedure and subsequent ablation.
Ablation is started after the needle is confirmed to be in the desired position. Ablation parameters may be referenced from device manufactures and may vary with different ablation electrodes, purpose of ablation (curative treatment or tumor reduction), size of pulmonary nodules, and surrounding tissues.
The vital signs of the patient are monitored during ablation. For ablations that are performed under local anesthesia, respiration, pain, cough, and hemoptysis should also be monitored. CBCT scans may be performed during ablation to confirm that the ablation needle remains in position, and to check for complications like hemorrhage or pneumothorax.
After ablation, another CBCT scan is performed to preliminarily assess ablation efficacy and complications. A successful ablation must include the whole lesion and its surrounding lung tissue (5–10 mm). Additional ablations may be carried out if the acquired ablation zone does not meet the treatment needs. Complications such as hemorrhage and pneumothorax should also be checked after ablation. If the patient is in a stable condition they can be transferred to the ward for further observation, and a chest CT will be re‐examined 1 day later.
COMPLICATIONS AND MANAGEMENT
Pneumothorax
Pneumothorax is a common complication, and some patients who receive a transthoracic puncture may also develop subcutaneous emphysema. 51 Transbronchial biopsy has a relatively lower pneumothorax risk. While pneumothorax usually manifests within one hour of the procedure, it may also occur after 24‐h in some of the population. 52 Observation with/without inhaling oxygen via nasal cannula is the treatment choice for pneumothorax with minor or no significant symptoms. In cases of severe pneumothorax (exceeds 30% of pulmonary field) or worsening symptoms (chest pain, tachypnea, sweating, paleness, etc.), chest tube suction or closed drainage of the thoracic cavity should be applied in addition to oxygen therapy. The chest tube may be checked a day or two after placement and can be removed if air is absorbed. 53
Hemorrhage and hemoptysis
Hemorrhage and hemostasis are common complications and usually self‐limited. Special treatment is not necessary in cases of minor hemorrhage. 54 Hemorrhage which occurs during an ablation procedure may be remedied directly with ablation itself. In cases of severe bleeding and hemostasis, the patient should lie in a lateral position with the bleeding side down to keep the airways unobstructed. Trachea intubation should be performed if necessary. 55 If circumstances require it, hemostatic medications, transfusions, interventional therapy, or even surgery can be applied. 56 Thoracic puncture and drainage should be performed in cases of massive hemothorax, and antibiotics are recommended to prevent secondary infection.
Pleural reaction
Pleural reaction can be mainly attributed to vagus nerve stimulation during transthoracic procedures; in some cases, it is tied to insufficient anesthesia or highly stressed patients. Common manifestations include paleness, profuse sweating, chest tightness, palpitations, dizziness, and syncope. 57 Most patients experience minor symptoms that do not require treatment. Patients with severe symptoms may experience sweating, blood pressure drop, or even shock and syncope. Operations must be ceased immediately, and epinephrine or glucose solution should be applied in conjunction with oxygen inhalation and vital signs monitoring. 58
Post‐ablation syndrome
Around one‐third of patients experience post‐ablation syndrome which is primarily attributed to absorption of necrotic tissue and released inflammatory factors. 59 The syndrome often lasts three to five days, with fever, fatigue, malaise, nausea, and vomiting as common clinical manifestations. Symptomatic treatment is sufficient in most scenarios, with nonsteroidal anti‐inflammatory drugs and low‐dose glucocorticoids as alternatives if necessary.
Lung infection
Older patients or those with severe pulmonary diseases such as chronic obstructive pulmonary disease and interstitial lung disease have a higher risk of secondary lung infection after ablation. Prophylactic use of antibiotics is permissible one day before ablation and the last three days after ablation. Lung infection should be suspected if the body temperature still exceeds 38.5°C five days after ablation. 43 For infected patients, anti‐infection treatment plan should be timely adjusted according to etiological test results and drug susceptibility results of blood, pus, sputum, or alveolar lavage fluid.
Other complications
Transthoracic intervention induced systemic air embolism can be attributed to biopsy of cavitated lesions, cough, and positive pressure ventilation, and so on. Despite its low incidence rate, arterial systemic air embolism can cause severe results including shock, cardiac arrest, and hemiplegic paralysis, and so on. 34 Once systemic air embolism occurs, high concentration oxygen therapy or even hyperbaric oxygen therapy should be actively administered. 60 , 61 Other rare complications include needle tract implantation, cardiac tamponade, bronchopleural fistula, and peripheral nerve injury, and so on. 62
CLINICAL APPLICATION RECOMMENDATIONS
Value of CBCT in transbronchial pulmonary nodules interventions
We recommend the use of CBCT in pulmonary nodules that are difficult to reach with traditional navigation techniques and in some special operations requiring high precision.
We recommend the routine use of CBCT in transbronchial pulmonary nodule ablation to ensure that the position of the ablation electrode and the ablation zone meet the therapeutic needs.
During transbronchial interventions for pulmonary nodules, the fluoroscopy or augmented fluoroscopy function of the CBCT system can be utilized to guide the bronchoscope and tools to the target nodule. Reconstructed 3D image of CBCT can also be used to confirm the position of the intervention tool. While ENB, VBN, R‐EBUS, fluoroscopy, and many other techniques are available to assist in guiding the bronchoscope to the target lesion, only a handful of these aforementioned techniques allow for real time confirmation of the positional relationship between target lesion and intervention tool. For example, R‐EBUS is susceptible to the influence of hemorrhage and atelectasis, which may result in “false‐positive” images and may mislead the operator to an undesired location. 52 In 2D fluoroscopy, the tool and the lesion may overlap spatially on the projected image while they are apart in 3D space, and the fluoroscopy may erroneously indicate that the tool has reached the target position. In addition, small pulmonary nodules, pure ground‐glass opacity nodules, and pulmonary nodules obscured by bony structures or close to some tissue structures (such as the mediastinum and diaphragm) are often invisible under fluoroscopy, which makes transbronchial interventions on these nodules more difficult. CBCT systems not only provide fluoroscopic imaging of the target area, but some CBCT systems can also offer augmented fluoroscopy to mark the pulmonary nodules that are hard to detect using conventional fluoroscopy, and then project these marks on the fluoroscopic image to guide operations. In addition, CBCT can also scan the lesion area in real time and generate a 3D image during the procedures. The 3D image generated by CBCT is less affected by the density or location of the nodules than fluoroscopy, and it is also easier to detect complications such as pneumothorax and atelectasis than fluoroscopy. 52 The 3D imaging capacity of CBCT enable operators to assess the position of the tool in real time, thus ensuring accurate intervention. When the position of the tool approaches or deviates from the pulmonary nodule, the operator can refer to the CBCT images and adjust the path of the bronchoscope and tool accordingly. Park et al. concluded that the reach of “tool‐in‐lesion” via CBCT before biopsy was highly correlated with a diagnostic result (aOR 53.31, p = 0.001). 63 For incidental isolated pulmonary nodules that are smaller than 2 cm, a study in 2014 showed that the diagnostic yield of transbronchial biopsy performed with CBCT was approximately twice that of conventional bronchoscopy. 20 In transbronchial nodule ablation, CBCT not only allows for accurate ablation of the target lesion, but also provides intraoperative ablation zone monitoring and complication detection. Chan et al. 29 performed transbronchial ablation in 25 patients with peripheral lung cancer with lesions <3 cm in combination of ENB and CBCT for guidance. All ablations were successful in reaching the desired ablation zone, without any recurrence during a 1‐year follow‐up.
CBCT results in slightly higher radiation exposure compared to other guiding techniques such as fluoroscopy, ENB, and R‐EBUS. We recommend that CBCT can be used as an auxiliary tool in pulmonary nodules that are difficult to be diagnosed and localized using conventional guiding techniques, including those that are invisible under fluoroscopy. The use of CBCT is also recommended in peripheral transbronchial needle aspiration biopsy, cryobiopsy, BTPNA, preoperative localization, or other special operations that require high precision. 21 , 36 , 37 In addition, the routine use of CBCT is recommended in transbronchial pulmonary nodule ablation to ensure that the ablation electrode position and ablation zone meet the therapeutic needs.
Using CBCT and other navigation techniques in combination
We recommend using CBCT in combination with other navigation techniques to ensure efficiency and accuracy.
One of the major challenges in transbronchial intervention is to guide the bronchoscope and tools to the target lesion through intricate bronchial passages in an efficient and precise way. Since CBCT is essentially a technique that helps confirm “tool‐in‐lesion”, it is recommended to be used in conjunction with a variety of other guiding tools. There are various guided bronchoscopy technologies such as VBN, ENB, robotic bronchoscopy, ultra‐thin bronchoscopy, and so on. The combined use of CBCT and these techniques can fully complement each other to achieve a better navigation and higher diagnostic yield. A prospective study used VBN to guide ultra‐thin bronchoscope to lesion and confirmed “tool‐in‐lesion” through CBCT, ultimately achieving a diagnostic yield of 90.0%. 64 Reisenauer et al. reported a combination use of robotic bronchoscopy and CBCT in a prospective study, achieving a “tool‐in‐lesion” rate of 96.7% and a diagnostic yield of 93.3%. 25 Another prospective study by Verhoeven et al. showed that when using CBCT alone and ENB alone to guide bronchoscopy for peripheral pulmonary nodules biopsy, the navigation success rates were only 76.3% and 52.2%, respectively. However, after the combined use of CBCT and ENB, the navigation success rates of the two groups were eventually increased to 89.9% and 87.5% respectively. 65
R‐EBUS has been widely used in transbronchial procedures due to its convenient and radiation‐free manner with capacity of lesion localization and adjacent tissue assessment in a cross‐sectional view. Most importantly, R‐EBUS can be used to determine the relationship between the lesion and surrounding blood vessels and bronchi, show the internal structure of lesion including blood vessels, calcification, necrosis and others to help initially evaluate the benign and malignant nature of the lesion. 66 However, using R‐EBUS alone for position confirmation has certain limitations: R‐EBUS cannot confirm the tool position in real time, and the final position of the interventional tools may deviate from the position of the probe and the nodule. 64 Moreover, R‐EBUS is susceptible to procedural complications, since complication like atelectasis may mimic a solid lesion with RP‐EBUS. In these circumstances, even fluoroscopy may have difficulty in identifying atelectasis, which is only detectable by CBCT, so that may easily mislead an inexperienced bronchoscopist. 52 The combined use of CBCT and R‐EBUS has the potential to increase the safety and efficiency of bronchoscopy. DiBardino et al. showed that the diagnostic yield was significantly higher when R‐EBUS was used in combination with CBCT (42.4% vs. 70.4%, respectively, p < 0.001) compared to using R‐EBUS alone. 67 On the other hand, using R‐EBUS for preliminary confirmation of arrival can help reduce repeated CBCT scans for frequent position adjustments and reduce radiation exposure dose. 68 Therefore, we recommend combining CBCT with other guiding techniques (thin/ultra‐thin bronchoscope, VBN, ENB, R‐EBUS, etc.) to improve operational efficiency and accuracy.
Value of CBCT in transthoracic interventions
We recommend that CBCT can be used to guide transthoracic interventions to improve operating efficiency and safety.
Although CBCT is inferior to conventional CT in terms of imaging range and density resolution, in most cases it is sufficient to meet the clinical needs of guiding puncture and confirming the positional relationship of lesions and needle tips. Previous studies have showed that CBCT‐guided PTNB has a noninferior diagnostic accuracy for malignancy compared to conventional CT (94.9%–96.0% vs. 89.9%–95.3%). 69 , 70 , 71 In comparison, conventional CT requires more room for the gantry frame and has a smaller inner space, which makes operations more challenging. Additionally, there is a higher chance of instrument displacement when patients are frequently moved in and out of the CT gantry for scanning and puncturing. In contrast, CBCT systems feature a relatively small and flexible C‐arm that can hover over the patient to enable real‐time CBCT scanning or to deliver fluoroscopic images at different angle as needed. With the help of augmented fluoroscopy and projecting laser mark towards the lesion, some fixed CBCT systems can also help identifying puncture entry point on the body surface and puncture direction based on the planned puncture path. For lesions that are small in size, deep in location, and have large path angles, this guidance allows for convenient and accurate punctures. Cazzato et al. retrospectively described their experience on 40 patients who underwent transthoracic ablation guided by CT and CBCT. The results showed that the CBCT‐guided group had less time to adjust the ablation needle position (2.5–4.5 min vs. 4.3–8 min) and fewer needle tip adjustment (27.8% vs. 45.4%) than the CT‐guided group. 72 The CBCT system's real‐time fluoroscopic images and capacity of puncture guidance can also assist in lowering the complication risk from repeated needle adjustment and the radiation exposure from repeated CT scan after corrections. Ren et al. analyzed 379 patients who underwent PTNB guided by CT or CBCT, and found that the CT‐guided group and the CBCT‐guided group had similar technical success rates (98.2% vs. 98.7%); however, for small lesions (diameter ≤2 cm), the CT‐guided group was associated with a higher incidence of pneumothorax (66.7% vs. 31%, p = 0.03) and higher radiation exposure (19.9 ± 8.4 mSv vs. 15.3 ± 5 mSv, p = 0.04) than the CBCT‐guided group. 69 Therefore, this consensus recommends that CBCT can be used to guide transthoracic interventions to improve operational efficiency and safety. When it is necessary to finely differentiate the internal components of the lesion or identify the relationship between the lesion and surrounding important tissue structures, conventional CT with better image quality may be preferred in this situation.
Tips for using the mobile CBCT system
We recommend the use of mobile CBCT system in consideration of some conditions such as facility conditions, size and density of the target lesion, anesthesia and intervention method and other factors.
Although the mobile CBCT system has disadvantages over the fixed CBCT system in terms of scanning speed, image quality and imaging range, it is adequate for most interventions for pulmonary nodules. Whether in biopsy, localization, or ablation, the mobile CBCT can conveniently provide real‐time 3D images to identify the relationship between tools and target lesion. 25 , 30 , 73 , 74 , 75 Some ground‐glass opacity nodules may be difficult to visualize due to the presence of instruments such as metal markers or needles in the surrounding area. 28 The image post‐processing function of reducing metal artifacts in the CBCT system can improve the image effect to some extent. Compared with the fixed CBCT system, the mobile CBCT system is more widely adopted because of its smaller space occupancy, its potential to be used in multiple operating rooms, and its affordability. It is important to use the mobile CBCT system with a radiographically penetrable interventional bed to avoid degrading the image quality. Currently, the mobile CBCT system lacks augmented fluoroscopy to label lesions, therefore, it is more dependent on other methods for guidance (e.g., ENB, R‐EBUS, robotic bronchoscopy). 25 , 29 , 75 In addition, the mobile CBCT system is unable to automatically project the laser to guide the puncture along a planned path during percutaneous transthoracic puncture operations and is therefore more commonly used in transbronchial interventions. The lack of the above functions limits the clinical application of the mobile CBCT system. Nonetheless, it is sufficient to meet the clinical demands such as fluoroscopic monitoring, position confirmation of lesions and tools, monitoring ablation zone, and checking for complications. Therefore, we recommend using the mobile CBCT system in consideration of some conditions such as facility conditions (e.g., navigation equipment, space in the operation room), size and density of the target lesion, anesthesia method, intervention method (transbronchial or transthoracic) and other factors.
Layout of the operating room
We recommend arranging the operation room properly to ensure the safety of patients and staff during CBCT procedures.
We recommend that each center establish and improve their own standard procedures of CBCT‐guided operations and the emergency plan for equipment failure according to their own conditions.
A CBCT scan requires the C‐arm to rotate 200° around the patient, as such ample space around the patient's cephalothorax is required. This means that CBCT requires a special layout of the equipment in the operating room. Most commonly, equipment such as anesthesia machines, bronchoscope towers, monitors, infusion pumps, and so on are placed on the side of the patient's head in a fan‐shaped arrangement. In the case of robotic CBCT, additional space around patient's head side is required for the C‐arm movements or activities. At the same time, the corresponding ventilator tubes, monitor wires, bronchoscope fiberoptic cables, and other tube bundles are sorted and fixed in a position where they will not be influenced by the C‐arm rotation. 21 Before CBCT scanning, a C‐arm rotation test without radiation exposure should be performed around the lesion. Also, the rotation test needs to be closely monitored to ensure the safety of the procedure. To minimize radiation exposure to the medical staffs, the operating room should be equipped with a control room or radiation shelter so that operators can be temporarily shielded during the scanning. During the transbronchial operation, the operator should ensure that the bronchoscope, guide sheath, ablation electrode, and other tools are properly fixed or secured before CBCT imaging acquisition. This avoids the position‐shifting of the bronchoscope and the intervention tool due to the C‐arm rotation or unstable fixation during the scanning process. In this case, the bronchoscope can be fixed with a specialized fixation bracket product or with a simple homemade stand. 29 We recommend arranging the operation room properly to ensure the safety of patients and staff during CBCT operation. Based on their own equipment and personnel, each medical center should establish and improve the standard procedures of CBCT‐guided interventions and emergency plan for equipment failure.
Radiation exposure related to CBCT
We recommend using CBCT in combination with other transbronchial or transthoracic guidance techniques to improve arrival rate and to reduce additional radiation exposure from repeated scans.
The use of intraoperative CBCT to assist with pulmonary nodule biopsy, localization, and ablation has unique advantages, but the radiation exposure related to CBCT is also an important consideration. Using a body‐phantom experiment, Hohenforst‐Schmidt et al. showed that the average effective dose per CBCT scan was in the range of 0.98–1.15 mSv. 76 For different numbers of projected images, the effective CBCT doses were 0.98 mSv (248 images), 1.33 mSv (312 images), and 3.32 mSv (419 images). 76 , 77 , 78 Pritchett et al. combined CBCT with ENB for pulmonary nodules biopsy: an average of 1.5 CBCT scans were performed for the biopsy and the mean predicted effective doses for fluoroscopy‐related radiation and radiation exposure were 1.5 ± 0.7 and 3.0 ± 1.4 mSv, respectively. The predicted effective dose for a single CBCT scan was 2.0 mSv, 33 which is close to the effective dose of low‐dose CT (1.4–1.6 mSv) 79 and lower than the effective dose of conventional CT (7 mSv, 4–18 mSv). 80 The results obtained in the aforementioned studies are consistent with another randomized controlled trial, which reported that the predicted effective dose of preoperative CBCT scanning was 49% lower than that of conventional CT (p = 0.081). 81 A retrospective study by Ren et al. found that the mean radiation exposure dose for biopsies of larger pulmonary nodules (>2 cm in diameter) was similar using CBCT versus CT guidance (15.2 ± 4.0 mSv vs. 16.8 ± 6.1 mSv). However, when the lesions were smaller pulmonary nodules (≤2 cm in diameter), CBCT guidance generated a lower radiation dose (15.3 ± 5.0 mSv vs. 19.9 ± 8.4 mSv, p = 0.04), which may be attributed to more precise and convenient needle guidance. 69 In addition, a body‐phantom study attempted to focus the scanning range on the lesion area was performed (the scanning range was reduced from 18 to 6 cm in the head‐foot direction), and reported lower radiation dose (95.5 mGy vs. 147.7 mGy) while similar imaging quality to conventional methods. 82 However, this technique requires further clinical evidence to prove its value in patients with pulmonary nodules.
While radiation dose of a single CBCT scan is relatively low, the total dosage is multiplied by the number of scans performed and corresponding scanning parameters. Moreover, operator proficiency and experience in selecting acquisition procedures with shorter scanning times can also reduce radiation dose. 83 , 84 In general, we recommend using CBCT in combination with other transbronchial or transthoracic guidance techniques to improve arrival rate and to reduce additional radiation exposure from rescanning after position adjustments.
Measures to improve the imaging quality of CBCT
We recommend suspending patient's breathing during CBCT scan to ensure the image quality.
We recommend applying appropriate ventilation parameters to avoid atelectasis.
CBCT requires a longer scanning time and is susceptible to motion artifacts incurred from respiratory motion. Therefore, we recommend artificially pausing the patient's breathing during the scanning process to ensure imaging quality. For patients who undergo transbronchial interventions, general anesthesia with appropriate ventilator control can effectively pause respiratory movements to reduce motion artifacts. In order to keep the airway open and avoid atelectasis during scanning and operations, the airway pressure needs to be kept relatively high. Therefore, it is better to choose tracheal intubation rather than laryngeal mask to establish an artificial airway. 85 Volume‐controlled mode is used, and ventilation is performed with a tidal volume of 8–10 mL/kg (ideal bodyweight) and 8–15 cmH2O of PEEP. For obese patients or lesions located in the lower lungs, PEEP can be increased as hemodynamics allow. 86 To minimize the incidence of atelectasis, decreasing the fraction of inspired oxygen (while maintaining a saturation level above 94%), and keeping operation short are effective measures. During CBCT scanning, it is recommended to switch the ventilator to manual mode to suspend the ventilation, thus reducing imaging artifacts incurred from respiratory movements. Meanwhile, the APL valve is utilized to maintain a constant airway pressure (typically 10–20 cmH2O) during ventilation suspension to avoid diaphragm elevation or atelectasis. For lesions located in the lower lungs and dorsal side, a position that appropriately elevates the lesion side can be used.
Percutaneous transthoracic pulmonary nodule interventions are often performed under local anesthesia because of the relatively low difficulty and good patient tolerance. Therefore, it is necessary for patients to hold breath by themselves during CBCT scanning to avoid artifacts caused by respiratory movements. Since awake patients are always nervous during the puncture, drastic change in respiratory volume can happen and lead to inconsistent lung expansion. In order to minimize lesion location fluctuation incurred by respiratory motions between different sessions of CBCT imaging, we recommend that the patient hold their breath after a steady expiration. If the patient is unable to tolerate the breath hold after expiration or the lesion is not clearly visualized, CBCT scanning can be performed with breath holding after inspiration. Preoperative instructions and exercises can be given to the patient to ensure a satisfactory intraoperative cooperation.
CONCLUSIONS
The CBCT system can provide intraoperative fluoroscopy, augmented fluoroscopy, and real‐time 3D images to help operators identify the position of interventional tools and lesions, as well as monitor the operation area. These features facilitate accurate and efficient diagnosis, localization, and treatment of pulmonary nodules. At present, CBCT is widely used in clinical practice, and more and more clinical evidence confirms that it has good feasibility, safety and high clinical application value. With the rapid development of respiratory interventional technology, a standardized CBCT workflow is of great significance to improve the success rate of interventions and reduce adverse events. According to our practical experience and available clinical results, we formulated this expert consensus. This consensus statement will be continuously updated with the accumulation of more evidence in the use of the CBCT system, further development of the device and new functions.
AUTHOR CONTRIBUTIONS
Jiayuan Sun and Enguo Chen participated in the design of the expert consensus. Dongyang Xu, Fangfang Xie and Jisong Zhang conceived of the expert consensus, and participated in its design and other authors coordination and helped to draft the expert consensus. All authors read and approved the final manuscript.
CONFLICT OF INTEREST STATEMENT
This expert consensus was simultaneously submitted to <Chinese Journal of Cancer> in a Chinese version. The submission is agreed by all authors and both journals. The authors have no conflicts of interest to declare.
ACKNOWLEDGMENTS
This work was supported by the National Multi‐disciplinary Treatment Project for Major Diseases (2020NMDTP) and Shanghai Municipal Education Commission–Gaofeng Clinical Medicine Grant Support (20181815).
Xu D, Xie F, Zhang J, Chen H, Chen Z, Guan Z, et al. Chinese expert consensus on cone‐beam CT‐guided diagnosis, localization and treatment for pulmonary nodules. Thorac Cancer. 2024;15(7):582–597. 10.1111/1759-7714.15222
Dongyang Xu, Fangfang Xie and Jisong Zhang contributed equally to this work.
Contributor Information
Enguo Chen, Email: 3195024@edu.zju.cn.
Jiayuan Sun, Email: xkyyjysun@163.com.
REFERENCES
- 1. National Lung Screening Trial Research, Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, et al. Reduced lung‐cancer mortality with low‐dose computed tomographic screening. N Engl J Med. 2011;365(5):395–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chaddha U, Kurman JS, Mahajan A, Hogarth DK. Lung nodule management: an interventional pulmonology perspective. Semin Respir Crit Care Med. 2018;39(6):661–666. [DOI] [PubMed] [Google Scholar]
- 3. Nadig TR, Thomas N, Nietert PJ, Lozier J, Tanner NT, Wang Memoli JS, et al. Guided bronchoscopy for the evaluation of pulmonary lesions: an updated meta‐analysis. Chest. 2023;163(6):1589–1598. [DOI] [PubMed] [Google Scholar]
- 4. Ali MS, Trick W, Mba BI, Mohananey D, Sethi J, Musani AI. Radial endobronchial ultrasound for the diagnosis of peripheral pulmonary lesions: a systematic review and meta‐analysis. Respirology. 2017;22(3):443–453. [DOI] [PubMed] [Google Scholar]
- 5. Jiang S, Liu X, Chen J, Ma H, Xie F, Sun J. A pilot study of the ultrathin cryoprobe in the diagnosis of peripheral pulmonary ground‐glass opacity lesions. Transl Lung Cancer Res. 2020;9(5):1963–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zo S, Woo SY, Kim S, Lee JE, Jeong BH, Um SW, et al. Predicting the risk of malignancy of lung nodules diagnosed as indeterminate on radial endobronchial ultrasound‐guided biopsy. J Clin Med. 2020;9(11):3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Folch EE, Labarca G, Ospina‐Delgado D, Kheir F, Majid A, Khandhar SJ, et al. Sensitivity and safety of electromagnetic navigation bronchoscopy for lung cancer diagnosis: systematic review and meta‐analysis. Chest. 2020;158(4):1753–1769. [DOI] [PubMed] [Google Scholar]
- 8. Folch EE, Bowling MR, Pritchett MA, Murgu SD, Nead MA, Flandes J, et al. NAVIGATE 24‐month results: electromagnetic navigation bronchoscopy for pulmonary lesions at 37 centers in Europe and the United States. J Thorac Oncol. 2022;17(4):519–531. [DOI] [PubMed] [Google Scholar]
- 9. Zheng X, Cao L, Zhang Y, Xie F, Yang H, Liu J, et al. A novel electromagnetic navigation bronchoscopy system for the diagnosis of peripheral pulmonary nodules: a randomized clinical trial. Ann Am Thorac Soc. 2022;19(10):1730–1739. [DOI] [PubMed] [Google Scholar]
- 10. Kickuth R, Reichling C, Bley T, Hahn D, Ritter C. C‐arm cone‐beam CT combined with a new electromagnetic navigation system for guidance of percutaneous needle biopsies: initial clinical experience. Rofo. 2015;187(7):569–576. [DOI] [PubMed] [Google Scholar]
- 11. Meyer BC, Peter O, Nagel M, Hoheisel M, Frericks BB, Wolf KJ, et al. Electromagnetic field‐based navigation for percutaneous punctures on C‐arm CT: experimental evaluation and clinical application. Eur Radiol. 2008;18(12):2855–2864. [DOI] [PubMed] [Google Scholar]
- 12. Ikezawa Y, Shinagawa N, Sukoh N, Morimoto M, Kikuchi H, Watanabe M, et al. Usefulness of endobronchial ultrasonography with a guide sheath and virtual Bronchoscopic navigation for ground‐glass opacity lesions. Ann Thorac Surg. 2017;103(2):470–475. [DOI] [PubMed] [Google Scholar]
- 13. Zheng X, Zhang L, Zhong C, Xie F, Li SY, Wang G, et al. Virtual bronchoscopic navigation and endobronchial ultrasound with a guide sheath without fluoroscopy for diagnosing peripheral pulmonary lesions with a bronchus leading to or adjacent to the lesion: a randomized non‐inferiority trial. Respirology. 2023;28(4):389–398. [DOI] [PubMed] [Google Scholar]
- 14. Ishida T, Asano F, Yamazaki K, Shinagawa N, Oizumi S, Moriya H, et al. Virtual bronchoscopic navigation combined with endobronchial ultrasound to diagnose small peripheral pulmonary lesions: a randomised trial. Thorax. 2011;66(12):1072–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fielding DIK, Bashirzadeh F, Son JH, Todman M, Chin A, Tan L, et al. First human use of a new robotic‐assisted fiber optic sensing navigation system for small peripheral pulmonary nodules. Respiration. 2019;98(2):142–150. [DOI] [PubMed] [Google Scholar]
- 16. Rojas‐Solano JR, Ugalde‐Gamboa L, Machuzak M. Robotic bronchoscopy for diagnosis of suspected lung cancer: a feasibility study. J Bronchol Interv Pulmonol. 2018;25(3):168–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Xie F, Wagh A, Wu R, Hogarth DK, Sun J. Robotic‐assisted bronchoscopy in the diagnosis of peripheral pulmonary lesions. Chin Med J Pulmon Crit Care Med. 2023;1(1):30–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Anzidei M, Argirò R, Porfiri A, Boni F, Anile M, Zaccagna F, et al. Preliminary clinical experience with a dedicated interventional robotic system for CT‐guided biopsies of lung lesions: a comparison with the conventional manual technique. Eur Radiol. 2015;25(5):1310–1316. [DOI] [PubMed] [Google Scholar]
- 19. Lanza C et al. Robotics in interventional radiology: review of current and future applications. Technol Cancer Res Treat. 2023;22:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hohenforst‐Schmidt W, Zarogoulidis P, Vogl T, Turner JF, Browning R, Linsmeier B, et al. Cone beam Computertomography (CBCT) in interventional chest medicine ‐ high feasibility for endobronchial Realtime navigation. J Cancer. 2014;5(3):231–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Setser R, Chintalapani G, Bhadra K, Casal RF. Cone beam CT imaging for bronchoscopy: a technical review. J Thorac Dis. 2020;12(12):7416–7428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lee SM, Park CM, Lee KH, Bahn YE, Kim JI, Goo JM. C‐arm cone‐beam CT‐guided percutaneous transthoracic needle biopsy of lung nodules: clinical experience in 1108 patients. Radiology. 2014;271(1):291–300. [DOI] [PubMed] [Google Scholar]
- 23. Cheng GZ, Liu L, Nobari M, Miller R, Wahidi M. Cone beam navigation bronchoscopy: the next frontier. J Thorac Dis. 2020;12(6):3272–3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jin KN, Park CM, Goo JM, Lee HJ, Lee Y, Kim JI, et al. Initial experience of percutaneous transthoracic needle biopsy of lung nodules using C‐arm cone‐beam CT systems. Eur Radiol. 2010;20(9):2108–2115. [DOI] [PubMed] [Google Scholar]
- 25. Reisenauer J, Duke JD, Kern R, Fernandez‐Bussy S, Edell E. Combining shape‐sensing robotic bronchoscopy with Mobile three‐dimensional imaging to Verify tool‐in‐lesion and overcome divergence: a pilot study. Mayo Clin Proc Innov Qual Outcomes. 2022;6(3):177–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sadoughi A, Virdi S. Mobile 3D intraprocedural fluoroscopy in combination with ultrathin bronchoscopy for biopsy of peripheral lung nodules. J Bronchol Interv Pulmonol. 2021;28(1):76–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chen J, Pan X, Gu C, Zheng X, Yuan H, Yang J, et al. The feasibility of navigation bronchoscopy‐guided pulmonary microcoil localization of small pulmonary nodules prior to thoracoscopic surgery. Transl Lung Cancer Res. 2020;9(6):2380–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chan JWY, Lau RWH, Chu CM, Ng CSH. Expanding the scope of electromagnetic navigation bronchoscopy‐guided transbronchial biopsy and ablation with mobile 3D C‐arm machine Cios spin((R))‐feasibility and challenges. Transl Lung Cancer Res. 2021;10(10):4043–4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chan JWY, Lau RWH, Ngai JCL, Tsoi C, Chu CM, Mok TSK, et al. Transbronchial microwave ablation of lung nodules with electromagnetic navigation bronchoscopy guidance‐a novel technique and initial experience with 30 cases. Transl Lung Cancer Res. 2021;10(4):1608–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chen J, Xie F, Zheng X, Li Y, Liu S, Ma KC, et al. Mobile 3‐dimensional (3D) C‐arm system‐assisted transbronchial biopsy and ablation for ground‐glass opacity pulmonary nodules: a case report. Transl Lung Cancer Res. 2021;10(7):3312–3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ghosn M, Elsakka AS, Ridouani F, Doustaly R, Mingione L, Royalty K, et al. Augmented fluoroscopy guided transbronchial pulmonary microwave ablation using a steerable sheath. Transl Lung Cancer Res. 2022;11(2):150–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hwang EJ, Kim H, Park CM, Yoon SH, Lim HJ, Goo JM. Cone beam computed tomography virtual navigation‐guided transthoracic biopsy of small (</= 1 cm) pulmonary nodules: impact of nodule visibility during real‐time fluoroscopy. Br J Radiol. 2018;91(1087): 20170805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pritchett MA, Schampaert S, de Groot JAH, Schirmer CC, van der Bom I. Cone‐beam CT with augmented fluoroscopy combined with electromagnetic navigation bronchoscopy for biopsy of pulmonary nodules. J Bronchol Interv Pulmonol. 2018;25(4):274–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yan GW, Bhetuwal A, Yan GW, Sun QQ, Niu XK, Zhou Y, et al. A systematic review and meta‐analysis of C‐arm cone‐beam CT‐guided percutaneous transthoracic needle biopsy of lung nodules. Pol J Radiol. 2017;82:152–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Professional Committee on Respiratory Equipment Technology of Chinese Medical Equipment, A. and B . Expert Group on Technical of Domestic Electromagnetic Navigation, [Expert Consensus on Technical Specifications of Domestic Electromagnetic Navigation Bronchoscopy System in Diagnosis, Localization and Treatment (2021 Edition)]. Zhongguo Fei Ai Za Zhi. 2021;24(8):529–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Piro R, Fontana M, Casalini E, Taddei S, Bertolini M, Iori M, et al. Cone beam CT augmented fluoroscopy allows safe and efficient diagnosis of a difficult lung nodule. BMC Pulm Med. 2021;21(1):327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Steinfort DP, D'Agostino RD, Vrjlic I, Einsiedel P, Prasad JD, Jennings BR, et al. CT‐fluoroscopic guidance for performance of targeted transbronchial Cryobiopsy: a preliminary report. Respiration. 2018;96(5):472–479. [DOI] [PubMed] [Google Scholar]
- 38. Ciriaco P, Negri G, Puglisi A, Nicoletti R, del Maschio A, Zannini P. Video‐assisted thoracoscopic surgery for pulmonary nodules: rationale for preoperative computed tomography‐guided hookwire localization. Eur J Cardiothorac Surg. 2004;25(3):429–433. [DOI] [PubMed] [Google Scholar]
- 39. Liu B, Gu C. Expert consensus workshop report: guidelines for preoperative assisted localization of small pulmonary nodules. J Cancer Res Ther. 2020;16(5):967–973. [DOI] [PubMed] [Google Scholar]
- 40. Liu BD, Ye X, Fan WJ, Li XG, Feng WJ, Lu Q, et al. Expert consensus on image‐guided radiofrequency ablation of pulmonary tumors: 2018 edition. Thorac Cancer. 2018;9(9):1194–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Interventional pulmonology group of the Chinese Thoracic Society . C.M.A., [Guideline for diagnostic flexible bronchoscopy in adults (2019)] . Zhonghua Jie He He Hu Xi Za Zhi. 2019;42(8):573–590. [DOI] [PubMed] [Google Scholar]
- 42. Nguyen ET, Bayanati H, Hurrell C, Aitken M, Cheung EM, Gupta A, et al. Canadian Association of Radiologists/Canadian Association for Interventional Radiology/Canadian Society of Thoracic Radiology Guidelines on thoracic interventions. Can Assoc Radiol J. 2023;74(2):272–287. [DOI] [PubMed] [Google Scholar]
- 43. Ye X, Fan W, Wang Z, Wang J, Wang H, Wang J, et al. Expert consensus on thermal ablation therapy of pulmonary subsolid nodules (2021 edition). J Cancer Res Ther. 2021;17(5):1141–1156. [DOI] [PubMed] [Google Scholar]
- 44. Orth RC, Wallace MJ, Kuo MD, R. Technology assessment Committee of the Society of interventional . C‐arm cone‐beam CT: general principles and technical considerations for use in interventional radiology. J Vasc Interv Radiol. 2009;20(7 Suppl):S538–S544. [DOI] [PubMed] [Google Scholar]
- 45. Avasarala SK, Machuzak MS, Gildea TR. Multidimensional precision: hybrid Mobile 2D/3D C‐arm assisted biopsy of peripheral lung nodules. J Bronchol Interv Pulmonol. 2020;27(2):153–155. [DOI] [PubMed] [Google Scholar]
- 46. Oki M, Saka H. Diagnostic value of ultrathin bronchoscopy in peripheral pulmonary lesions: a narrative review. J Thorac Dis. 2020;12(12):7675–7682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Xie F, Yang H, Huang R, Zheng X, Cao L, Liu J, et al. Chinese expert consensus on technical specifications of electromagnetic navigation bronchoscopy in diagnosing peripheral pulmonary lesions. J Thorac Dis. 2021;13(4):2087–2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ettinger DS, Wood DE, Aisner DL, Akerley W, Bauman JR, Bharat A, et al. Non‐small cell lung cancer, version 3.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2022;20(5):497–530. [DOI] [PubMed] [Google Scholar]
- 49. British Thoracic Society bronchoscopy guidelines committee, a.S.o.S.o.C.C.o.B.T.S . British Thoracic Society guidelines on diagnostic flexible bronchoscopy. Thorax. 2001;56(Suppl 1): i1‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mayo JR, Clifton JC, Powell TI, English JC, Evans KG, Yee J, et al. Lung nodules: CT‐guided placement of microcoils to direct video‐assisted thoracoscopic surgical resection. Radiology. 2009;250(2):576–585. [DOI] [PubMed] [Google Scholar]
- 51. Jiao de C et al. Clinical applications of the C‐arm cone‐beam CT‐based 3D needle guidance system in performing percutaneous transthoracic needle biopsy of pulmonary lesions. Diagn Interv Radiol. 2014;20(6):470–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Casal RF, Sarkiss M, Jones AK, Stewart J, Tam A, Grosu HB, et al. Cone beam computed tomography‐guided thin/ultrathin bronchoscopy for diagnosis of peripheral lung nodules: a prospective pilot study. J Thorac Dis. 2018;10(12):6950–6959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hiraki T, Mimura H, Gobara H, Shibamoto K, Inoue D, Matsui Y, et al. Incidence of and risk factors for pneumothorax and chest tube placement after CT fluoroscopy‐guided percutaneous lung biopsy: retrospective analysis of the procedures conducted over a 9‐year period. AJR Am J Roentgenol. 2010;194(3):809–814. [DOI] [PubMed] [Google Scholar]
- 54. Manhire A, Charig M, Clelland C, Gleeson F, Miller R, Moss H, et al. Guidelines for radiologically guided lung biopsy. Thorax. 2003;58(11):920–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lordan JL, Gascoigne A, Corris PA. The pulmonary physician in critical care * illustrative case 7: assessment and management of massive haemoptysis. Thorax. 2003;58(9):814–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Grage RA, Naveed MA, Keogh S, Wang D. Efficacy of a dehydrated hydrogel plug to reduce complications associated with computed tomography‐guided percutaneous transthoracic needle biopsy. J Thorac Imaging. 2017;32(1):57–62. [DOI] [PubMed] [Google Scholar]
- 57. Lazguet Y, Maarouf R, Karrou M, Skiker I, Alloubi I. CT guided percutaneous needle biopsy of the chest: initial experience. Pan Afr Med J. 2016;23:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wang S, Tu J, Dong K. Nomogram to predict postoperative PR in patients undergoing CT‐guided transthoracic lung biopsy. J Thorac Dis. 2019;11(4):1705–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zhu JC, Yan TD, Morris DL. A systematic review of radiofrequency ablation for lung tumors. Ann Surg Oncol. 2008;15(6):1765–1774. [DOI] [PubMed] [Google Scholar]
- 60. Muth CM, Shank ES. Gas embolism. N Engl J Med. 2000;342(7):476–482. [DOI] [PubMed] [Google Scholar]
- 61. Ziser A, Adir Y, Lavon H, Shupak A. Hyperbaric oxygen therapy for massive arterial air embolism during cardiac operations. J Thorac Cardiovasc Surg. 1999;117(4):818–821. [DOI] [PubMed] [Google Scholar]
- 62. Zhou Q, Dong J, He J, Liu D, Tian DH, Gao S, et al. The Society for Translational Medicine: indications and methods of percutaneous transthoracic needle biopsy for diagnosis of lung cancer. J Thorac Dis. 2018;10(9):5538–5544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Park SC, Kim CJ, Han CH, Lee SM. Factors associated with the diagnostic yield of computed tomography‐guided transbronchial lung biopsy. Thorac Cancer. 2017;8(3):153–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ali EAA, Takizawa H, Kawakita N, Sawada T, Tsuboi M, Toba H, et al. Transbronchial biopsy using an ultrathin bronchoscope guided by cone‐beam computed tomography and virtual Bronchoscopic navigation in the diagnosis of pulmonary nodules. Respiration. 2019;98(4):321–328. [DOI] [PubMed] [Google Scholar]
- 65. Verhoeven RLJ, Futterer JJ, Hoefsloot W, van der Heijden E. Cone‐beam CT image guidance with and without electromagnetic navigation bronchoscopy for biopsy of peripheral pulmonary lesions. J Bronchol Interv Pulmonol. 2021;28(1):60–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zheng X, Wang L, Chen J, Xie F, Jiang Y, Sun J. Diagnostic value of radial endobronchial ultrasonographic features in predominant solid peripheral pulmonary lesions. J Thorac Dis. 2020;12(12):7656–7665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. DiBardino DM, Kim RY, Cao Y, Andronov M, Lanfranco AR, Haas A, et al. Diagnostic yield of cone‐beam‐derived augmented fluoroscopy and ultrathin bronchoscopy versus conventional navigational bronchoscopy techniques. J Bronchol Interv Pulmonol. 2023;30(4):335–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Verhoeven RLJ, Vos S, van der Heijden E. Multi‐modal tissue sampling in cone beam CT guided navigation bronchoscopy: comparative accuracy of different sampling tools and rapid on‐site evaluation of cytopathology. J Thorac Dis. 2021;13(7):4396–4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ren Q, Zhou Y, Yan M, Zheng C, Zhou G, Xia X. Imaging‐guided percutaneous transthoracic needle biopsy of nodules in the lung base: fluoroscopy CT versus cone‐beam CT. Clin Radiol. 2022;77(5):e394–e399. [DOI] [PubMed] [Google Scholar]
- 70. Rotolo N, Floridi C, Imperatori A, Fontana F, Ierardi AM, Mangini M, et al. Comparison of cone‐beam CT‐guided and CT fluoroscopy‐guided transthoracic needle biopsy of lung nodules. Eur Radiol. 2016;26(2):381–389. [DOI] [PubMed] [Google Scholar]
- 71. Akkakrisee S, Hongsakul K. Percutaneous transthoracic needle biopsy for pulmonary nodules: a retrospective study of a comparison between C‐arm cone‐beam computed tomography and conventional computed tomography guidance. Pol J Radiol. 2020;85:e309–e315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Cazzato RL, Battistuzzi JB, Catena V, Grasso RF, Zobel BB, Schena E, et al. Cone‐beam computed tomography (CBCT) versus CT in lung ablation procedure: which is faster? Cardiovasc Intervent Radiol. 2015;38(5):1231–1236. [DOI] [PubMed] [Google Scholar]
- 73. Hsieh MJ, Chou PL, Fang HY, Wen CT, Chao YK. Single‐step localization and excision of small pulmonary nodules using a mobile 3D C‐arm. Interact Cardiovasc Thorac Surg. 2021;33(6):885–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kalchiem‐Dekel O, Fuentes P, Bott MJ, Beattie JA, Lee RP, Chawla M, et al. Multiplanar 3D fluoroscopy redefines tool‐lesion relationship during robotic‐assisted bronchoscopy. Respirology. 2021;26(1):120–123. [DOI] [PubMed] [Google Scholar]
- 75. Duke JD, Fernandez‐Bussy S, Reisenauer J. Combined portable cone beam computed tomography and robotic‐assisted bronchoscopy impacting diagnosis of a solitary pulmonary nodule: a case report. AME Case Rep. 2022;6:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Hohenforst‐Schmidt W, Banckwitz R, Zarogoulidis P, Vogl T, Darwiche K, Goldberg E, et al. Radiation exposure of patients by cone beam CT during endobronchial navigation ‐ a phantom study. J Cancer. 2014;5(3):192–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Choi JW, Park CM, Goo JM, Park YK, Sung W, Lee HJ, et al. C‐arm cone‐beam CT‐guided percutaneous transthoracic needle biopsy of small (</= 20 mm) lung nodules: diagnostic accuracy and complications in 161 patients. AJR Am J Roentgenol. 2012;199(3):W322–W330. [DOI] [PubMed] [Google Scholar]
- 78. Choo JY, Park CM, Lee NK, Lee SM, Lee HJ, Goo JM. Percutaneous transthoracic needle biopsy of small (</= 1 cm) lung nodules under C‐arm cone‐beam CT virtual navigation guidance. Eur Radiol. 2013;23(3):712–719. [DOI] [PubMed] [Google Scholar]
- 79. Larke FJ, Kruger RL, Cagnon CH, Flynn MJ, McNitt‐Gray MM, Wu X, et al. Estimated radiation dose associated with low‐dose chest CT of average‐size participants in the National Lung Screening Trial. AJR Am J Roentgenol. 2011;197(5):1165–1169. [DOI] [PubMed] [Google Scholar]
- 80. Mettler FA Jr, Huda W, Yoshizumi TT, Mahesh M. Effective doses in radiology and diagnostic nuclear medicine: a catalog. Radiology. 2008;248(1):254–263. [DOI] [PubMed] [Google Scholar]
- 81. Abi‐Jaoudeh N, Fisher T, Jacobus J, Skopec M, Radaelli A, van der Bom IM, et al. Prospective randomized trial for image‐guided biopsy using cone‐beam CT navigation compared with conventional CT. J Vasc Interv Radiol. 2016;27(9):1342–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Werncke T, von Falck C, Luepke M, Stamm G, Wacker FK, Meyer BC. Collimation and image quality of C‐arm computed tomography: potential of radiation dose reduction while maintaining equal image quality. Invest Radiol. 2015;50(8):514–521. [DOI] [PubMed] [Google Scholar]
- 83. Yang SM, Yu KL, Lin KH, Liu YL, Sun SE, Meng LH, et al. Localization of small pulmonary nodules using augmented fluoroscopic bronchoscopy: experience from 100 consecutive cases. World J Surg. 2020;44(7):2418–2425. [DOI] [PubMed] [Google Scholar]
- 84. Yang SM, Yu KL, Lin JH, Lin KH, Liu YL, Sun SE, et al. Cumulative experience of preoperative real‐time augmented fluoroscopy‐guided endobronchial dye marking for small pulmonary nodules: an analysis of 30 initial patients. J Formos Med Assoc. 2019;118(8):1232–1238. [DOI] [PubMed] [Google Scholar]
- 85. Pritchett MA, Bhadra K, Calcutt M, Folch E. Virtual or reality: divergence between preprocedural computed tomography scans and lung anatomy during guided bronchoscopy. J Thorac Dis. 2020;12(4):1595–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Katsis J, Roller L, Aboudara M, Pannu J, Chen H, Johnson J, et al. Diagnostic yield of digital tomosynthesis‐assisted navigational bronchoscopy for indeterminate lung nodules. J Bronchol Interv Pulmonol. 2021;28(4):255–261. [DOI] [PubMed] [Google Scholar]


