Abstract
Schwannomas are slow-growing benign tumors originating from the Schwann cells of the peripheral nerve sheaths. Herein, we report the first documented case of a schwannoma presenting as a painful nipple mass in a 32-year-old woman. This mass initially developed six years ago following a period of breastfeeding. Breast magnetic resonance imaging (MRI) scans revealed an iso-intense mass, with an approximate size of 2.2 cm, on a T1-weighted image with internal cystic changes. The mass exhibited heterogeneously delayed enhancement and restricted diffusion. Surgical excision was performed, and the diagnosis of cutaneous plexiform nipple schwannoma was confirmed histopathologically. A literature review revealed that the MRI findings of the nipple mass in our case were consistent with the common features of a schwannoma.
Keywords: Breast, Magnetic Resonance Imaging, Neurilemmoma, Nipples
INTRODUCTION
The nipple is an important part of the breast and is a complicated structure possessing various components such as the epithelium from the skin, columnar cells from ducts, sebaceous glands, Sappey’s plexus (subareolar lymphatics), and numerous sensory nerve endings. Nipple masses can originate from any of these tissues through diverse pathological processes. Nipple masses pose a diagnostic challenge due to their unique anatomy, nonspecific clinical and radiological findings, and inadequate imaging modalities.
Schwannomas, also known as neurilemmomas, are benign peripheral nerve sheath tumors that originate from Schwann cells. They can arise from any part of the body but are most commonly observed in the head and neck region [1]. They rarely occur in the breast and are associated with genetic conditions such as neurofibromatosis type 2. Intramammary schwannomas typically present as palpable breast lumps.
Herein, we report a case of schwannoma in the nipple based on magnetic resonance imaging (MRI) findings. To the best of our knowledge, Schwannomas of the nipple have not been reported in the literature. This rare form of schwannoma should be included in the differential diagnosis of nipple neoplasms.
CASE REPORT
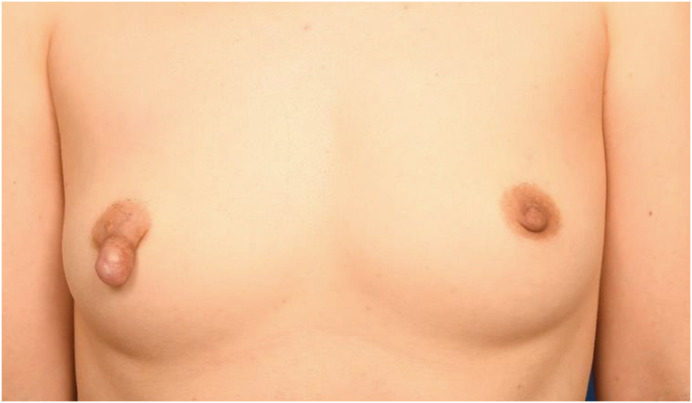
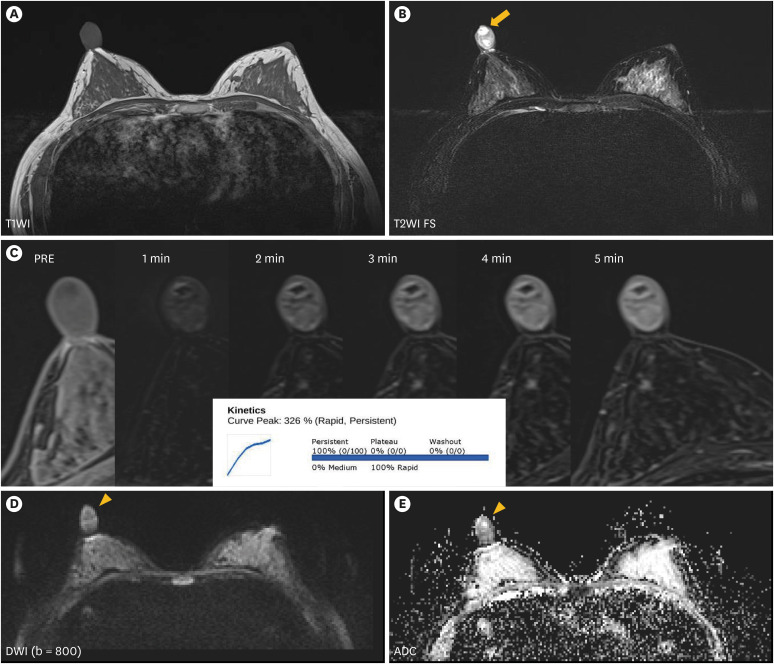
A 32-year-old woman presented with a painful mass in the right nipple that had persisted for six years. The lesion slowly grew in size after breastfeeding, and the patient was not under any medication. Physical examination revealed that the lesion was a hard mass with regular margins approximately 2 cm in size (Figure 1). No abnormal discharges, erythema, or adjacent skin changes were observed. Breast MRI (3T Magnetom Skyra; Siemens AG Healthcare, Erlangen, Germany), emphasizing the nipple, revealed a circumscribed, oval-shaped, heterogeneous enhancing mass measuring 2.2 × 1.8 × 1.7 cm in the right nipple. The mass exhibited iso-signal intensity on T1-weighted image (T1WI) and heterogeneous signal intensity with internal cystic changes on fat-saturated T2-weighted image (T2WI) (Figure 2A and B), with a type I kinetic curve and diffusion restriction (Figure 2C-E). The lesion was classified as BI-RADS category 3 (probably benign) based on an oval-shaped mass with a circumscribed margin and internal T2 high signal intensity. Nipple adenoma, papilloma, and leiomyoma were considered differential diagnoses. There were no other abnormal findings in the parenchyma of either the breast or the axillae on MRI.
Figure 1. Clinical appearance of the right nipple.
A hard mass with regular margins approximately 2 cm in size on the right nipple.
Figure 2. Breast magnetic resonance imaging evaluation of the nipple mass.
Breast MRI scans reveal an iso-intense mass of approximately 2.2 × 1.8 × 1.7 cm dimensions on the T1WI (A) with internal cystic change (arrow), (B) T2WI FS. The mass exhibits heterogeneous enhancement (C), and a restricted diffusion area (arrowhead) (D, E).
MRI = magnetic resonance imaging; T1WI = T1-weighted image; T2WI = T2-weighted image; FS = fat suppression; DWI = diffusion-weighted imaging; ADC = apparent diffusion coefficient.
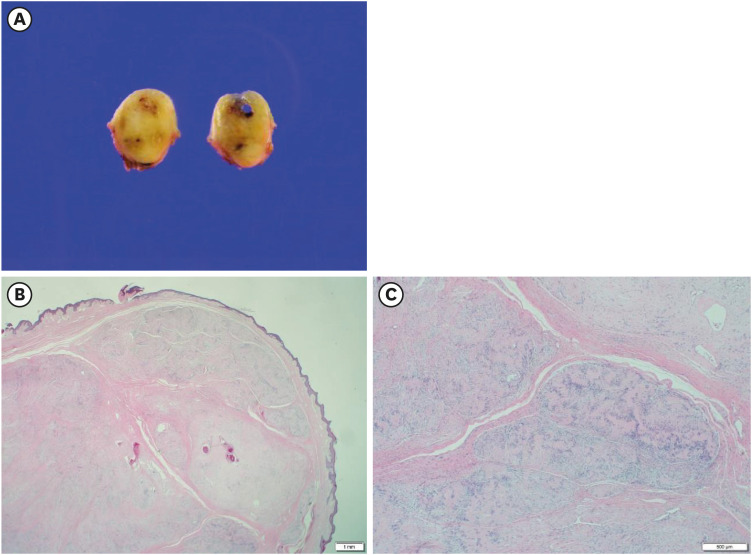
Surgical resection was then performed (Figure 3A). The histopathological examination revealed spindle cell proliferation with prominent Verocay bodies in the dermal layer (Figure 3B and C). No additional immunohistochemical staining was performed. The mass was confirmed to be a cutaneous plexiform schwannoma.
Figure 3. Gross and histopathologic characteristics of the nipple mass.
A gross specimen showing a well-circumscribed neoplasm with a partially cystic appearance (A). Histopathological findings revealed a spindle cell lesion with a plexiform appearance in the dermis layer (B, H&E stain, ×12.5). Parallel arrays of spindle cells with nuclear palisading, also known as Verocay bodies, are diffusely observed (C, H&E stain, ×40).
H&E = hematoxylin & eosin.
DISCUSSION
The nipple-areolar complex (NAC) has unique anatomical and histological characteristics. It is composed of multiple structures, including Montgomery glands, sebaceous glands, smooth muscles, lymphatics, and numerous sensory nerve endings. A wide range of neoplasms may arise from these structures. However, owing to its rarity and nonspecific clinical and radiological features, differential diagnosis remains challenging [2,3]. Moreover, evaluation of nipple masses is difficult because of their intricate anatomy, poor imaging resolution, and lack of specific imaging guidelines [4]. It is important to distinguish between benign and malignant nipple mass lesions. Although most nipple mass lesions are typically benign, it is crucial to consider the possibility of cases featuring suspicious imaging findings. These may encompass characteristics such as large tumor size, multifocality, or the presence of lymphovascular invasion accompanied by metastatic lymphadenopathy [5]. In MRI the presence of an irregular thickened enhancement often indicates a malignant process rather than a benign condition. Based on these radiological findings, we concluded that the nipple mass was benign.
Benign tumors of the nipple include adenoma, papilloma, and leiomyoma in the order of incidence. Malignant conditions include Paget’s disease, breast cancer, and lymphoma. The reported clinical and radiological features of benign and malignant nipple neoplasms are listed in Table 1. Because the sonographic findings of nipple masses were similar, we skipped the sonographic evaluation of the nipple mass and immediately performed an MRI. If a malignant disease is suspected, a biopsy is required for pathological confirmation. The pathological findings, treatment, and prognosis of each disease are listed in Table 2.
Table 1. Clinical presentation and imaging features of reported nipple masses.
| Pathologies | Clinical presentation | Mammography | US | MRI |
|---|---|---|---|---|
| Adenoma | • Nipple enlargement | • Enlarged nipple with calcification | • Isoechoic, hypervascular mass | • T2 high SI homogeneous enhancement with plateau kinetics |
| Papilloma | • Unilateral | • Oval, well circumscribed mass | • Homogeneous hypoechoic mass | • Homogeneous enhancement with a plateau or wash-out kinetics |
| • Bloody nipple discharge | ||||
| Leiomyoma | • Nipple enlargement | • Well-circumscribed mass, slow-growing, rarely speculated | • Isoechoic mass | • Intermediate T1 and T2 SI, peripheral rim enhancement with persistent enhancement |
| • Skin thickening | ||||
| • Pain | ||||
| Invasive primary carcinoma | • Non-milky | • May have calcifications, nipple inversion | • Swollen nipple with a non-circumscribed, complex cystic-solid mass | • Heterogeneous enhancement with washout kinetics |
| • Unilateral single-duct discharge | • Vascular mass | |||
| • Skin change* | ||||
| Paget’s disease | • Eczematoid skin change† | • Skin thickening, nipple retraction, or a mass associated DCIS | • Nipple asymmetry | • Asymmetric nodular or irregular enhancement |
| • Non-circumscribed mass | ||||
| • Duct change | ||||
| Lymphoma | • Shiny reddish mass | • Dense mass | • Well/ill-defined homogeneous hypoechoic mass | • Heterogeneous enhancement with a plateau or wash-out kinetics |
| • Lymphadenopathy | • Lymphadenopathy |
US = ultrasonography; MRI = magnetic resonance imaging; SI = signal intensity; DCIS = ductal carcinoma in situ.
*Skin changes include skin retraction and thickening.
†Eczematoid skin changes include erythema, scaliness, and erosion.
Table 2. Pathologic features, treatment, and prognosis of reported nipple masses.
| Pathologies | Pathologic features | Treatment | Prognosis |
|---|---|---|---|
| Adenoma | • Epithelial proliferation arising in the large nipple ducts | • Surgical excision | • Recurs if incompletely excised, but rare after complete excision |
| Papilloma | • Arising in the walls of lactiferous sinuses | • Surgical excision | • Recurs if incompletely excised, but rare after complete excision |
| • Characteristic fibrovascular stalks | |||
| Leiomyoma | • Spindle cell proliferation | • Surgical excision | • Local recurrence after excision is rare |
| • Malignant transformation (−) | |||
| Invasive primary carcinoma | • Malignant epithelial cells present | • Mastectomy or breast-conserving surgery | • Depends on the histologic tumor grading and the stage |
| • Systemic chemotherapy | |||
| Paget’s disease | • Presence of malignant glandular epithelial cells (Paget cells) within the squamous epithelium of the nipple | • Mastectomy or breast-conserving surgery, followed by whole-breast radiation therapy | • Depends on the presence or absence of an underlying carcinoma and the stage |
| Lymphoma | • Resemble lymphomas of similar histologic types and stages presenting at other sites | • Local radiotherapy, often combined with systemic chemotherapy | • Depends on the histologic tumor grading and the stage |
Schwannomas are slow-growing benign tumors originating from the Schwann cells of the peripheral nerve sheaths. These tumors occur sporadically or in association with genetic conditions such as neurofibromatosis type 2 or von Recklinghousen’s disease. Few cases of intramammary schwannomas have been previously reported in the literature, and its incidence accounts for only 2.6% of all schwannomas [6,7]. To the best of our knowledge, this is the first reported case of a solitary schwannoma of the nipple described in the literature. Schwannomas of the nipple are presumed to develop from the sensory nerves that are normally present in the NAC.
According to previous reports, the radiologic features of intramammary schwannomas are usually described as those of benign tumors, while malignancies are not well-defined owing to their rare incidence. Mammographic features of the tumor were nonspecific and exhibited an oval mass with circumscribed margins. Occasionally, tumors are overlooked in mammography [7]. In ultrasonography images, schwannomas present as a solid hypoechoic, round, or oval-shaped, circumscribed mass with posterior acoustic enhancement [1]. The MRI characteristics of schwannoma typically reveal well-defined masses exhibiting iso-to-low signal intensity on T1WI and heterogeneous signal intensity on T2WI due to cystic or mucoid changes. Schwannomas typically exhibit intense enhancement [8,9]. In our case, the MRI features were consistent with the typical findings of schwannomas reported in previous studies.
The diagnosis of schwannoma is based on histopathological evaluations of needle or excisional biopsies, as in our case. Surgical excision was the treatment of choice. Malignant transformation is extremely rare; however, some cases have been reported [10,11].
In conclusion, we present a case of nipple schwannoma, which, to the best of our knowledge, has not been previously reported. Although extremely rare, radiologists should consider schwannoma as a differential diagnosis when encountering a benign-looking mass in the nipple.
Footnotes
Conflict of Interest: The authors declare that they have no competing interests.
Data Availability: Requests for data can be made by contacting the corresponding author.
- Conceptualization: Woo O.
- Data curation: Kang YJ.
- Methodology: Kang YJ, Woo O.
- Resources: Woo O.
- Supervision: Woo O.
- Writing - original draft: Kang YJ.
- Writing - review & editing: Kang YJ, Woo O, Kim A.
References
- 1.Cho KS, Choi HY, Lee SW, Sung SH. Sonographic findings in solitary schwannoma of the breast. J Clin Ultrasound. 2001;29:99–101. doi: 10.1002/1097-0096(200102)29:2<99::AID-JCU1005>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 2.Del Riego J, Pitarch M, Codina C, Nebot L, Andreu FJ, Aparicio O, et al. Multimodality approach to the nipple-areolar complex: a pictorial review and diagnostic algorithm. Insights Imaging. 2020;11:89. doi: 10.1186/s13244-020-00896-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nicholson BT, Harvey JA, Cohen MA. Nipple-areolar complex: normal anatomy and benign and malignant processes. Radiographics. 2009;29:509–523. doi: 10.1148/rg.292085128. [DOI] [PubMed] [Google Scholar]
- 4.Da Costa D, Taddese A, Cure ML, Gerson D, Poppiti R, Jr, Esserman LE. Common and unusual diseases of the nipple-areolar complex. Radiographics. 2007;27(Suppl 1):S65–S77. doi: 10.1148/rg.27si075512. [DOI] [PubMed] [Google Scholar]
- 5.Omofoye TS, Scoggins ME, Dogan BE. Imaging approach to nipple masses: what a radiologist should know. Contemporary Diagnostic Radiology. 2015;38:1–7. [Google Scholar]
- 6.Parikh Y, Sharma KJ, Parikh SJ, Hall D. Intramammary schwannoma: a palpable breast mass. Radiol Case Rep. 2016;11:129–133. doi: 10.1016/j.radcr.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bellezza G, Lombardi T, Panzarola P, Sidoni A, Cavaliere A, Giansanti M. Schwannoma of the breast: a case report and review of the literature. Tumori. 2007;93:308–311. [PubMed] [Google Scholar]
- 8.Whorms DS, Fishman MD, Slanetz PJ. Mesenchymal lesions of the breast: what radiologists need to know. AJR Am J Roentgenol. 2018;211:224–233. doi: 10.2214/AJR.17.19020. [DOI] [PubMed] [Google Scholar]
- 9.Lee EK, Kook SH, Kwag HJ, Park YL, Bae WG. Schwannoma of the breast showing massive exophytic growth: a case report. Breast. 2006;15:562–566. doi: 10.1016/j.breast.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 10.Yi JM, Moon EJ, Oh SJ, Lee A, Suh YJ, Baek JM, et al. Malignant peripheral nerve sheath tumor of the breast in a patient without neurofibromatosis: a case report. J Breast Cancer. 2009;12:223–226. [Google Scholar]
- 11.Malas S, Krawitz HE, Sur RK, Uijs RR, Nayler SJ, Levin CV. Von Recklinghausen’s disease associated with a primary malignant schwannoma of the breast. J Surg Oncol. 1995;59:273–275. doi: 10.1002/jso.2930590415. [DOI] [PubMed] [Google Scholar]