Abstract
Over the past years, the poultry industry has been assigned to greater production performance but has become highly sensitive to environmental changes. The average world temperature has recently risen and is predicted to continue rising. In open-sided houses, poultry species confront high outside temperatures, which cause heat stress (HS) problems. Cellular responses are vital in poultry, as they may lead to identifying confirmed HS biomarkers. Heat shock proteins (HSP) are highly preserved protein families that play a significant role in cell function and cytoprotection against various stressors, including HS. The optimal response in which the cell survives the HS elevates HSP levels that prevent cellular proteins from damage caused by HS. The HSP have chaperonic action to ensure that stress-denatured proteins are folded, unfolded, and refolded. The HSP70 and HSP90 are the primary HSP in poultry with a defensive function during HS. HSP70 was the optimal biological marker for assessing HS among the HSP studied. The current review attempts to ascertain the value of HSP as a heat stress defense mechanism in poultry.
Key words: heat shock protein, hot season, performance, poultry
INTRODUCTION
Of all the agricultural industries worldwide, the poultry industry plays a major role (Farghly et al. 2017, 2018a, 2019, and 2021). Poultry demand for human consumption has grown with the world's population growth. The rise in poultry meat has averaged 5.7% annually since 1990, and considering the significance of this sector, the abnormal environmental factors that adversely impact it, such as HS, need to be tackled (Delgado 2005). Since Egypt is one of the countries identified as tropical or semi-tropical, raising poultry in such conditions makes them susceptible to extreme heat, especially in summer (Farghly et al. 2017, 2018b, and 2020). This results in developing the HS problem, which results from the imbalance between heat loss and production that disturbs birds’ performance rates, physiological status, immune status, and general health (Lara and Rostagno, 2013).
Heat stress affects poultry in 2 forms: acute (heat waves) or chronic, and both forms result in variable mortalities and lowered performance (Abd El-Hack et al., 2019). Economically, HS causes severe economic losses estimated at 240 million USA dollars per year in the poultry sector (Salem et al., 2022), representing, for example, about 7% of total HS-caused losses in the French livestock industry in 2003 (Abdelnour et al., 2020; Ashour et al., 2020). The European summer thermal waves caused roughly 4 million mortalities in broilers (Smith et al. 2014). Therefore, safe solutions must be found to solve the problems related to HS in birds (Saeed et al., 2019; Ezzat el al., 2024).
Previously, specific techniques have been employed to lessen the negative consequences of HS on chickens by implying some physiological measurements without discussing the perception of the underlying mechanism of the impact of these strategies. However, the cellular biological processes successfully reacted to any external stimuli such as HS, which in turn aided in offering a significant tool for a better understanding of HS impacts (Belhadj Slimen et al. 2016).
The production of HSP, which play a very influential role in defending against the impact of any stress on living organisms, particularly HS, are among these cellular responses (Gaughan et al. 2010; Ganaie et al. 2013). To evaluate the role of these proteins as one of the molecular chaperones in mitigating the adverse impacts of HS, it was essential for us to adopt modern science to investigate these abnormal environmental conditions, so the general aim of this review article is to clarify the role of HSP as a line of protection against HS which defend poultry from the HS adverse effects (Alagawany et al., 2016, 2017; Khafaga et al., 2019).
IMPACTS OF HEAT STRESS ON POULTRY
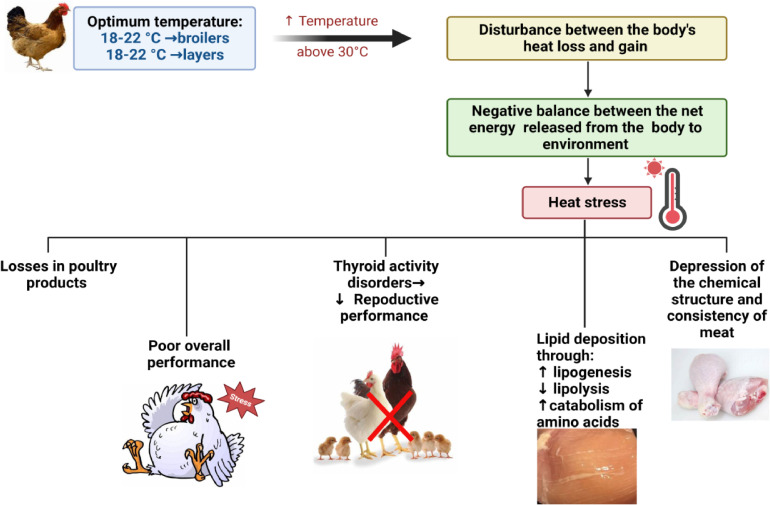
Stress is “an agent that exposes the organism to stress at any time,” meaning it is the body's nonspecific response to any stimuli. Stress is, therefore, the biological response of an organism to specific stimuli that disrupt its regular physiological functions or homeostasis (Chovatiya and Medzhitov 2014). This relates to heat stress, as it is one of the main types of stress confronting the tropical and semi-tropical poultry industry countries. It is known that the optimal performance temperature is between 18 to 22°C and 19 to 22°C for broilers and laying hens, respectively and when the thermos requirements of chickens are not met, HS can occur (Lin et al. 2006; Wasti et al. 2020). When the birds are raised under high-temperature conditions (an average of 30°C), a disturbance between the body's heat loss and gain is produced, resulting in a negative equilibrium between the bird's body's net energy release and its surroundings (Statistics Unit 2009). Such stress can be fatal for the birds if the internal heat produced exceeds the maximum heat loss, either instantly (acute HS) or over a long duration (chronic HS). Besides the environmental temperature and relative humidity, other factors cause thermal imbalance for the birds, such as housing systems and birds’ internal characteristics, like thermoregulatory mechanism and the metabolic rate (Lara and Rostagno 2013; Farghly et al. 2018a; Abd El-Hack et al. 2019; Farghly et al. 2020; Hassan et al. 2021).
Because of the enhanced production performance and feed conversion efficiency of todays’ poultry species, they become more vulnerable than ever to HS, which led to significant losses in poultry products due to the adverse impact of HS on birds’ physiological, immunological and behavioral responses (Lin et al. 2006; Rizk et al. 2019; Awad et al. 2021). These changes negatively affect birds’ feed intake, activate the hypothalamic-pituitary-adrenal (HPA) axis to cope with HS, alter the bird's neuroendocrine profile and eventually result in poor overall performance (Lara and Rostagno 2013; Attia et al. 2017).
In fact, body temperature and metabolic activity were found to be controlled by thyroid gland hormones, triiodothyronine (T3), thyroxine (T4), and thyroid activity disorders due to HS are anticipated to affect the chickens’ reproductive performance. Previous studies indicate that, under high-temperature conditions, T3 levels consistently decrease, while other studies indicate that T4 concentrations did not respond (Elnagar et al. 2010). Also, previous research shows a prominent impact of HS on meat quality. Dai et al. (2012) and Imik et al. (2012) found that HS in broilers decreased meat's chemical structure and consistency. Another work by Zhang et al. (2012) indicated that the percentage of breast muscle was diminished in broilers exposed to chronic HS; the same authors found more fats deposited and less protein (Figure 1).
Figure 1.
Impacts of heat stress (HS) on poultry performance.
BIOLOGICAL CHANGES IN POULTRY DUE TO HEAT STRESS
Behavioral Changes
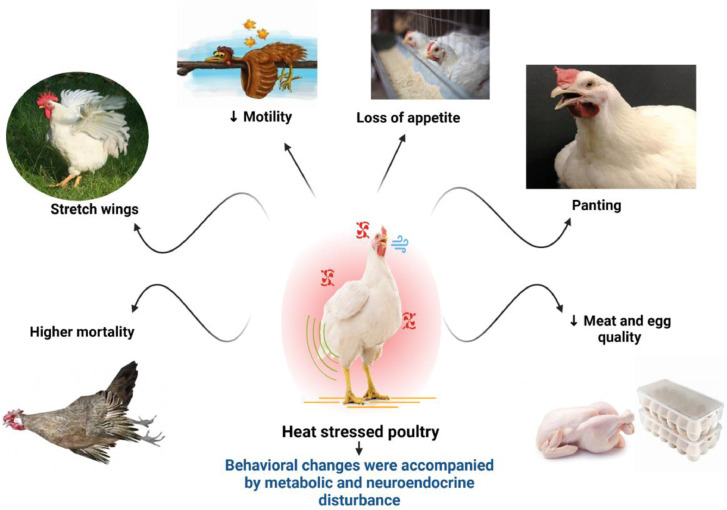
When poultry chickens are subjected to high atmospheric temperatures compared to the surrounding thermoneutral zone, they try to release the body's extra heat, which manifests as various behavioral changes. Heat-stressed chickens spend less time walking and standing, eat less feed and excess water, and stretch their wings. In addition, the typical signs of panting are often seen in chickens under HS conditions (Lara and Rostagno 2013). Such significant behavioral changes were accompanied by metabolic and neuroendocrine disturbances, which in turn contribute to more excellent feed conversion ratio, decreased feed intake, ultimate body weight, and higher mortality as well as higher-quality meat and eggs (Abd El-Hack et al. 2017a, 2017b,2019; Farghly et al. 2019). Thus, HS has been critically important, compelling researchers and farmers to use numerous strategies to deal with this issue (Lin et al., 2006; Farghly et al. 2020) (Figure 2).
Figure 2.
Behavioral changes in poultry due to heat stress.
Physiological Changes
Different physiological changes result from exposure to HS, which lead to various internal disturbances in chickens’ body:
Acid-Base Imbalance
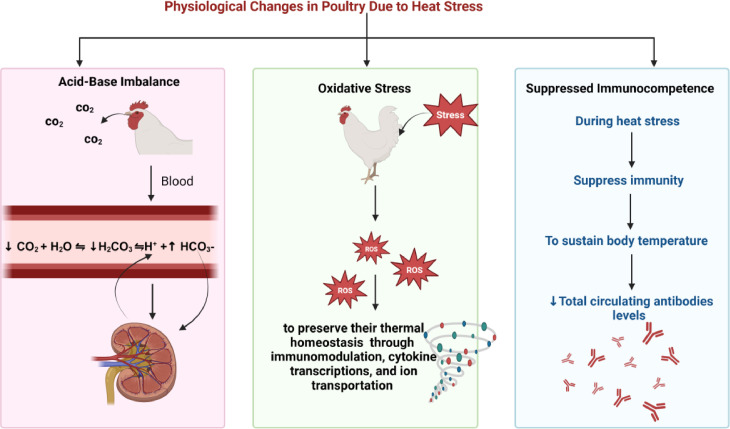
Poultry species do not have sweat glands and have feathers across their bodies, which hinder thermoregulation, and, as a result, at higher atmospheric temperatures, they must release heat through the active mechanism (i.e., panting). Panting is a behavior seen by birds in which they expand their beak to speed up respiration and evaporative cooling and eject a larger level of CO2 from the respiratory tract than cells produce (Wasti et al., 2020). These modifications result in a difference in bicarbonate's usual blood buffer system. Eventually, the abundance of hydrogen ions (H+) and carbonic acids (H2CO3) declines because of the CO2 decrease (Wasti et al. 2020).
On the other hand, the level of bicarbonate ions (HCO3-) increases; hence, blood pH rises, making the blood more alkaline. Birds continue to store H+ in the kidney and excrete more HCO3 to abide by this criterion and maintain the blood's normal pH. High H+ levels impact the acid-base balance, which can lead to metabolic acidosis and respiratory alkalosis. All these alterations adversely affect the overall productive performance of poultry (Borges et al. 2007; Farghly et al. 2018) (Figure 3).
Figure 3.
Physiological changes in poultry due to heat stress.
Oxidative Stress
Under stressful conditions, increased amounts of reactive oxygen species (ROS) are formed inside the cells as an attempt by the chickens’ bodies to preserve their thermal homeostasis and compete against such conditions through immunomodulation, cytokine transcriptions, and ion transportation (Surai et al. 2019) (Figure 3). The excess ROS are removed through physiological detoxifying methods present in the cells. Activation of various transcriptional factors induces additional production of a group of antioxidant compounds during the thermoneutral state, which deals with the increased ROS (Surai et al. 2019; Hassan et al. 2021). However, in stressful situations, an imbalance between both processes results from either excess reactive oxygen species or a decrease in the antioxidant defense mechanism's effectiveness, leading to oxidative stress (Betteridge, 2000; Mishra and Jha, 2019). The consequences of oxidative stress inside the cell vary from minor reversible modifications to the destruction of all cellular components, including lipids, proteins, and DNA, to apoptosis and cell death (Avery 2014).
In poultry species, previous research reported oxidative stress conditions to result in extreme health disorders, biological damage, lower growth rates, and severe economic losses, with an elevated risk of infection by microorganisms due to cell damage caused by hyperthermia (Dröge 2002; Oliver et al. 2012; Estévez, 2015; Hassan et al. 2016).
Suppressed Immunocompetence
Under normal conditions, the bird channels the energy from the feed to the immune system's development; however, in HS, the chickens’ body makes numerous physiological alterations to sustain body temperature, allowing immunity to be suppressed (Lara and Rostagno 2013; Farghly et al. 2017). As a result, the incidence of infectious diseases in poultry, like Newcastle disease and Gumboro disease, are comparatively higher during the summer period (Badruzzaman et al. 2015). Additionally, heat-stressed chickens exhibit changes in the sympathetic-adrenal medullar and hypothalamic-pituitary-adrenal axes, which result in a decrease in the relative weights of many organs, including the thymus and spleen (Bartlett and Smith 2003; Quinteiro-Filho et al. 2010); furthermore, a reduction in total circulating antibodies levels (i.e., IgM and IgG) was reported as a consequence of disturbed immune response in poultry species (Bartlett and Smith 2003) (Figure 3).
In the same context, previous findings of Mashaly et al. (2004) concluded a substantial reduction in the count of total leukocytes, with a higher heterophil to lymphocyte ratio (H/L) in birds raised under HS conditions. Also, Aengwanich (2010) stated decreased bursa relative weight, decreased cortex and bursa medulla lymphocyte numbers, and increased H/L in broilers exposed to HS (Wasef et al., 2020; Abd El-Hack et al., 2022).
The immunological response to HS in poultry chickens is still poorly understood regarding genetic and cellular pathways; nevertheless, research is progressing in identifying the cellular response concerning HS.
Reactions of Cells to Heat Stress
Studies on how HS affects cells were conducted as early as the 1970s and early 1980s, which revealed that HS can influence both the stability and cell membrane fluidity, block receptors, and stop trans-membrane protein activity (Hildebrandt et al. 2002). Since the cell membrane is a significant component of heat-induced apoptosis, this insight clarified the difference in membrane potential, the change in intracellular sodium and calcium concentrations, and the increase in potassium flux under conditions of HS (Zhao et al. 2006).
Cellular exposure to HS triggers a variety of cell functioning abnormalities that modify biological molecules, interrupt functions of the cell, modulate metabolic responses, trigger oxidative cell destruction, and activate all the mechanisms of apoptosis and necrosis, eventually contributing to cell survival, acclimatization or apoptosis, based on the duration and success of these modifications (Pandey et al. 2012; Slimen et al. 2016). As a heat-shock response, unfolding for cellular proteins occurs with increased production of molecular chaperones and the activation of heat-shock transcription factors to protect the cell from the aggregation of foreign proteins; besides, the enhancement of Heat shock protein cytoprotective networks with maintenance of the master regulator of the body's oxygen homeostasis, hypoxia-inducible factor-Iα (Collier et al. 2012).
HEAT SHOCK PROTEINS
Heat shock proteins are widely distributed proteins found in the cells of every investigated species. Heat is one of the many types of stress that triggers the production of the HSP gene family. It was first discovered by the pioneering work of the Italian geneticist Ferruccio Ritossa in 1962 (Capocci et al. 2014) after raising the temperature in an incubator containing Drosophila cultures. Indeed, one of the key ways scientists worldwide assess the magnitude of HS is by HSP expression (Gaughan et al. 2010).
HSP over-expression protects against hyperthermia, cerebral ischemia, and circulatory shock during heatstroke, signifying the fundamental function of HSP in cytoprotection (Ganaie et al., 2013). According to reports, HS exposure increases the amounts of HSP in poultry chicks, which interact with several proteins inside the cell to retain them accurately together and keep them functional, thereby showing a significant involvement in cell integrity under stress conditions (Yu et al. 2008; Orhan et al. 2013). Barrett et al. (2004) suggested that HSP reactions are triggered by electron transport chain dysfunction and that oxidative stress mediates this response.
Many experiments on heat shock induction and its biological function have given rise to this initial biochemical result. HSP function as chaperones in the refolding of proteins that HS has weakened. In every species examined, ranging from bacteria to people, HSP have been identified, indicating that they evolved very early and have an essential role.
Molecular Chaperons: Heat Shock Proteins
Molecular chaperons are substantial protein classes’ at all cellular organization levels, ranging from bacteria to humans. Based on the cellular position and organism complexity, they have variable functions and organization. They are recognized as proteins that cling to and maintain the erratic conformation of other proteins to aid in the regular folding and compartmentalization process of newly formed proteins. They also participate in many other cellular functions which, in reality, ensure that molecular chaperons play a vital role in the newly shaped polypeptides to achieve a practical shape through folding and unfolding and restoring denatured proteins or facilitating their degradation after stress or injury due to this role they have been referred to as “molecular chaperones” (Blum et al. 2000).
Many chaperones are considered HSP for this effect, since the ability to accumulate increases when stress denatures proteins. In this situation, chaperones do not transmit any additional steric information required for folding proteins. Nevertheless, highly specialized “steric chaperones” convey special structural (steric) information into proteins, which cannot be randomly folded (Blum et al. 2000).
Heat Shock Transcription Factors
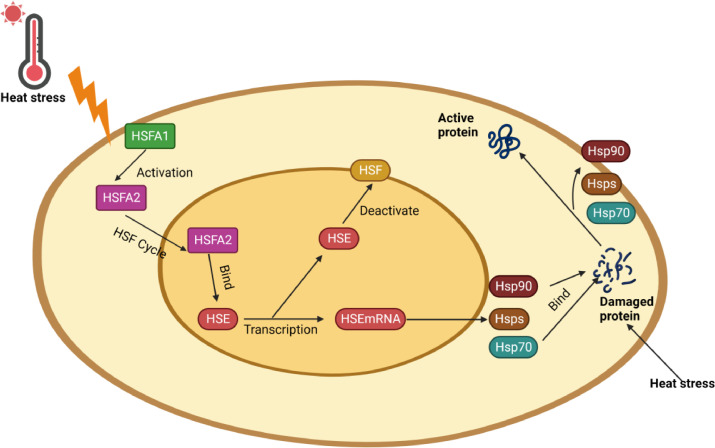
The heat shock response (HSR) is a crucial adaptive stress response in cells. Although HSR was first described as a reaction to thermal stress, other stressors such as cold, hypoxia, oxidative stress, pollutants, chemicals, pathogens, etc., can also activate HSR by interfering with protein folding and preventing the onset of inflammation and downstream events related to apoptosis. Four HSFs then play a crucial role in HSR activation at the transcriptional level by binding to the heat shock component (HSC), which triggers the expression of HSP to begin protecting cell proteins (Liu and Chang 2008; Kalmar and Greensmith 2009).
Indeed, species must conserve the integrity of their proteins to sustain essential life functions, and cooperative relationships between transcription factors and different homeostatic pathways lead to successful acclimatization to stressful environments that the organism is exposed to (Sakurai and Enoki 2010). The recruitment of HSR in vertebrates plays a main role in sustaining protein homeostasis by producing HSP (Velichko et al., 2013). At the transcriptional level, HSR is primarily mediated by 4 HSFs: HSF1, HSF2, HSF3, and HSF4. These HSFs bind with the heat shock element (HSE), which stimulates the expression of HSP (Fujimoto and Nakai 2010). HSF1 has gained considerable attention as the key force modulating stress-related transcription between HSFs to control the HSR (Richter et al. 2010). Activation of HSF1 is a multi-step mechanism that, within minutes of cellular stress, is negatively regulated by chaperones (i.e., HSP70 and HSP90) and prepares the ground for rapid gene expression activation (Stetler et al. 2010; de Thonel et al. 2012). HSF1 is a dormant monomeric molecule in the cytoplasm in an unstressed state. After heat shock, monomeric HSF1 gets hyperphosphorylated, transforming into a trimer that can bind DNA that assembles in the nucleus and binds to the heat shock component in the promoter region of the HSP gene. Moreover, significant posttranslational modifications such as acetylation and phosphorylation are suggested to adjust the HSF1 function (Meijering et al. 2015; Takii et al. 2015). The increased expression of HSP remains till the amount of HSP90 and HSP70 is adequate to restrict the HSFs activation domain, whereby increasing the quantities of HSP70 and HSP90 that are sufficient for the protective role, the processes of reverse transcription of the HSFs should cease since cells do not require more HSP70 and HSP90 (Kantidze et al. 2015) (Figure 4).
Figure 4.
Heat shock transcription factors.
In cells of avian species, HSFs (HSFs 1-3) express at least 3 different types of HSFs. Initially, 2 avian homologs of the mammalian HSF1 and 3 avian HSF genes corresponding to a new element, HSF3, were cloned. The amino acid sequence of HSF3 is associated with the sequence of HSF1 and 2 by approximately 40%, and its message is co-expressed during development, identical to HSF1 and HSF2. Avian cells express HSF1 and 3, which vary in their activation kinetics and initiation threshold temperature. For instance, in avian species, HSF3 is the primary regulator of the heat shock genes, whereas HSF1 slightly stimulates HSP70 production during heat shock (Inouye et al. 2003). Generally, it is impossible to overestimate the essential role of HSFs in the acclimatization of avian species to different stress conditions. However, recent genome-wide experiments have shown that HSF1 can reprogram transcription more widely than previously presumed; it is also active in many cell functions under stress and those not (Vihervaara and Sistonen 2014).
A core function of HSP is their capability to deliver cytoprotection, which, under stressful situations, produces proteins that highly conserve cell reaction mechanisms (Zilaee et al. 2014). The most common HSP that appear under heat stress conditions are HSP70 and HSP90, which effectively protect cells.
HSP70 Family
HSP70, a 70 kDa chaperone protein, is among the most conserved and significant HSP studied extensively. Eight distinct HSP70 were established in eukaryote cells, which were spread in various subcellular compartments (i.e., nucleus, mitochondria, endoplasmic reticulum, and cytosol) (Mahalka et al. 2014). Some of the essential housekeeping roles assigned to HSP70 involve protein entry into cellular compartments; regulation of different regulatory proteins; folding of precursor proteins and misfolded protein trafficking into mitochondria; breakdown of defective proteins; dissolution of protein complexes; endoplasmic reticulum, mitochondrial and cytosol protein folding (Garrido et al. 2006). These kinds of molecular chaperones have a role in a broad range of cellular functions, like protein biosynthesis, proteome defense from negative stress effects, protein recovery from aggregates, protein translocation facilitation through membranes, and disintegration of certain protein complexes and signals from cells for cell growth, differentiation, and death (Clerico et al. 2015).
These ATP-dependent chaperones, core components of the cellular protein monitoring network, participate in various protein folding processes. Specifically, they effectively engage with almost all proteins in their unfolded, misfolded, or aggregated states; besides, a variety of eukaryotic proteins are regulated (i.e., kinases, transcription factors, and steroid hormone receptors) through transient interaction with HSP70 (Mayer 2013).
HSP70 expression during avian erythroid cell development has also been studied, revealing that conclusive red blood cells respond to heat shock via increasing HSP70 protein synthesis by 10- to 20-fold, with no difference in HSP70 mRNA expression levels. Such higher translation of HSP70 mRNA was therefore thought to be responsible for the production of HSP70 inside cells. Furthermore, preceding research reported that HSP70 expression in red blood cells is lineage-specific and that, although constitutively expressed, neither HSP70 mRNA levels nor HSP70 synthesis were heat-shock induced in primitive red blood cells. In avian species, despite the HSR being related to a temperature above 41°C, an increase in HSP70 level was determined during the first and the fifth hour of exposure to extreme heat (35°C), with the rise peaking at 3 h. (Gabriel et al. 2002).
In avian embryos, transcripts expressing HSP70 expressed spatially were observed under normal incubation conditions, and mild HS (41°C) didn't cause enhancement in HSP70 mRNA expression levels (Gabriel et al. 2002). Also, prolonged exposure for one hour to extreme HS (44°C) led to a 15-fold increase in HSP70 mRNA levels; however, it is important to notice that the reversion to normal incubation temperature of stressed embryos led to an increase in levels of HSP70 mRNA for 3 h, which stabilized after 6 h. Importantly, in chicken embryos, the elevated expression of HSP70 was affected by both heat and cold stress, depending on age and tissue (Leandro et al. 2004). Particularly, in the breast muscle, liver, heart, and lungs, HSP70 was found, with the brain containing 2 to 5 times more HSP70 than other embryonic tissues, which suggests that the rise in expression of HSP70 in different embryonic tissues is an adaptive mechanism for stress conditions (Leandro et al. 2004). Researchers found that younger embryos showed increased HSP70 synthesis than older ones, indicating that the embryonic defense mechanism depends on its maturation and growth stage (Gabriel et al. 2002).
Post hatch, HSP70 expression is both allele- and tissue-based. In the liver, the primary site of synthesis for many essential proteins, HSP70 gene expression was significantly higher (above 2-fold) than in the muscle tissue under normal growth conditions (Zhen et al. 2006). This finding highlights the roles of the HSP70 chaperone. Such tissue-based heat initiation expression of HSP70 was linked to DNA methylation in its promoter region (Gan et al. 2013). Increased HSP70 protein levels were seen in the kidney, liver, and heart after exposure to HS at 37°C. However, the HSP70 gene's expression in the brain during acute HS (44°C for 4 h) was the strongest, being slightly dissimilar from that in the muscle and liver, which indicates the important adaptive reaction for compensating for the comparatively low concentrations of antioxidants in chickens’ brain (Figueiredo et al. 2007).
Surai et al. (2019) stated HSP expression was an efficient antioxidant defense mechanism in chickens' gut. However, under HS conditions, there were no impacts of overexpression of the HSP70 on intestinal morphology; however, in hens, a noteworthy correlation was observed between the HSP70 expression and the activity of the digestive enzyme (Hao et al. 2012).
Other researchers showed that the induction of HSP70 was found to defend the intestinal mucosa from HS damage by enhancing broiler antioxidant ability by boosting the activity of antioxidant enzymes and preventing the peroxidation of lipids from progressing (Gu et al. 2012). Previous studies by Gu et al. (2012) demonstrated that under acute HS conditions, there was a substantial rise in serum levels of corticosterone and H/L, as well as a decrease in the antioxidant enzymatic reaction, indicating that quercetin-induced suppression of HSP70 expression in the intestinal mucosa of chickens caused an increased degree of stress. In laying hens, significantly elevated levels of HSP70 in mononuclear blood cells were associated with long-term, mild HS (30–32°C) (Maak et al. 2003). Moreover, the HSR of multiple genotypes was found to be nonconsistent concerning the birds’ age. Also, the birds’ gender affected HSR, as HSP70 gene expression was concluded to have a substantial increment in males compared to female chickens (Surai 2002).
To sum up, the findings of the above studies steadily show that enhanced HSP70 expression in various organs of chickens is one of the significant defensive responses to avoid or cope with adverse alterations in protein functions and structure because of different stresses. Nevertheless, more research is required to understand better the molecular mechanisms of HSP70 control in avian organisms.
HSP90 Family
HSP90, the cell's main soluble protein, has recently attracted considerable attention and its structure, functions, and regulation have been identified in many reviews. Under nonstress circumstances, HSP90 is thought to make up 1 to 2% of the overall protein content in the cell, and under stress, it is further boosted (Erlejman et al. 2014; Karagöz and Rüdiger 2015). HSP90 is categorized as a 90 kDa protein, and a homodimer (α/α or β/β) is its active molecule, and 3 domains are composed of each monomer. These are the middle domain (which binds to client proteins and the nuclear localization signal), the NH2-terminal nucleotide-binding domain (ATP/ADP binding site), and the C-terminal domain (where dimerization and co-chaperone binding occur) (Li et al. 2012). The 4 primary homologs that comprise the HSP90 family in mammalian cells are the constitutive (HSP90 β) and inducible (HSP90 α) cytoplasmic isoforms. That has an amino acid identity of about 86% and was expressed in all nucleated cells (Sreedhar et al. 2004; Revathi and Prashanth 2015). In highly skilled and ATP-dependent physiological and stress environments, HSP90 is a molecular chaperone that stabilizes and matures many proteins. It is an essential hub in the network of proteins that maintains homeostasis and cellular function. (Jackson 2012).
It has been documented that heat shock (37–42°C) induces HSP90 levels by as much as 2 times, which helps in correcting the folding, stabilization, and functioning of other proteins (Bagatell et al. 2000). HSP90 is found to be transcriptionally regulated through active connections with transcription factors such as HSF. Under normal physiological conditions, HSP90 is found in cells in a state of balance between low chaperoning activity in the “latent state” and enhanced chaperoning performance in the “activated state” under different kinds of stress (Hong et al. 2013).
It isn't easy to overstate the importance of HSP90 chaperoning tasks connected to multiple nuclear proteins regulating DNA metabolism, repair, replication, as well as transcription and processing of RNA (Li et al. 2012). HSP90, on the other hand, is thought to be the primary component in maintaining cellular homeostasis and an effective stress response. It is engaged in various cellular processes, such as cell survival, cell cycle regulation, and other pathways for signaling transduction, frequently acting as hormone receptors. Interestingly, HSP90 was found to be involved in the activation, stabilization, and assembling of its client proteins by forming co-chaperone complexes such as HSP70 (Jackson 2012).
In broilers, HSP90 expression elevated in different tissues (i.e., liver, heart and kidney) for 2 h after high-temperature exposure (Lei et al. 2009). Seemingly, at an early stress state, HSP90 protein and HSP90 mRNA activation revealed that HS would directly induce and rapidly activate HSP90 protein translation and HSP90 mRNA transcription to protect cells. In embryos, in addition to defending cells from stress, the function of the HSP90 α gene during somitogenesis has been evolutionarily preserved (Lei et al. 2009).
CONCLUSIONS
Heat shock proteins contribute to a variety of physiological roles. Their roles extent ranges from the influence of spermatogenesis to infection and inflammation regulation, diabetes studies, and metastasis of cancer. As HSP, their name underestimates their various roles poorly. There is no denying that, in the years to come, HSP will be the focus of study. In the field of animal production and particularly poultry production, their functions in countering HS have been concentrated. The current literature, where HSP are frequently presented as a monolithic block, adds to the difficulty of understanding their functions. They belong to numerous families and subfamilies, each with distinctive and often complementary functions. It is intriguing to explore how each member will affect their positions in regulating physiological functions by themselves or coordinating with other family members. Dissecting the functions of each member of this complex family and their respective effect on various functions in poultry, immune response, HS, malignancy, and cancer will be a worthwhile undertaking for industrial poultry production and the entire field of animal production.
ACKNOWLEGMENTS
The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for supporting this work through large groups under grant number RGP2/304/44.
DISCLOSURES
The authors declare no conflict of interest.
Contributor Information
Mariusz Jaremko, Email: mariusz.jaremko@kaust.edu.sa.
Mohamed E. Abd El-Hack, Email: dr.mohamed.e.abdalhaq@gmail.com.
REFERENCES
- Abd El-Hack M.E., Alagawany M., Mahrose K.M., Arif M., Saeed M., Arain M.A., Siyal F.A., Fazlani S.A. Productive performance, egg quality, hematological parameters and serum chemistry of laying hens fed diets supplemented with certain fat-soluble vitamins, individually or combined, during summer season. Anim. Nutr. 2019;5:49–55. doi: 10.1016/j.aninu.2018.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Elbestawy A.R., Nahed A., Saad A.M., Salem H.M., El-Tarabily K.A. Necrotic enteritis in broiler chickens: disease characteristics and prevention using organic antibiotic alternatives–a comprehensive review. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2021.101590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd El-Hack M.E., Mahrose K.M., Arif M., Chaudhry M.T., Saadeldin I.M., Saeed M., Soomro R.N., Abbasi I.H., Rehman Z. Alleviating the environmental heat burden on laying hens by feeding on diets enriched with certain antioxidants (vitamin E and selenium) individually or combined. Environ. Sci. Pollut. Res. 2017;24:10708–10717. doi: 10.1007/s11356-017-8690-5. [DOI] [PubMed] [Google Scholar]
- Abd El-Hack M.E., Mahrose K.M., Askar A., Alagawany M., Arif M., Saeed M., Abbasi F., Soomro R.N., Siyal F.A., Chaudhry M.T. Single and combined impacts of vitamin A and selenium in diet on productive performance, egg quality, and some blood parameters of laying hens during hot season. Biol. Trace Elem. Res. 2017;177:169–179. doi: 10.1007/s12011-016-0862-5. [DOI] [PubMed] [Google Scholar]
- Abdelnour S.A., El-Saadony M.T., Saghir S.A.M., Abd El-Hack M.E., Al-Shargi O.Y.A., Al-Gabri N., Salama A. Mitigating negative impacts of heat stress in growing rabbits via dietary prodigiosin supplementation. Livest. Sci. 2020;240 [Google Scholar]
- Aengwanich W. Pathological changes and the effects of ascorbic acid on lesion scores of bursa of Fabricius in broilers under chronic heat stress. Res. J. Vet. Sci. 2010;3:74–78. [Google Scholar]
- Alagawany M., Abd El-Hack M.E., El-Kholy M.S. Productive performance, egg quality, blood constituents, immune functions, and antioxidant parameters in laying hens fed diets with different levels of Yucca schidigera extract. Environ. Sci. Poll. Res. 2016;23:6774–6782. doi: 10.1007/s11356-015-5919-z. [DOI] [PubMed] [Google Scholar]
- Alagawany M., Farag M.R., Abd El-Hack M.E., Patra A. Heat stress: effects on productive and reproductive performance of quail. World's Poult. Sci. J. 2017;73:747–756. [Google Scholar]
- Ashour E.A., Abd El-Hack M.E., Shafi, Alghamdi W.Y., Taha A.E., Swelum A.A., El-Saadony M.T. Impacts of green coffee powder supplementation on growth performance, carcass characteristics, blood indices, meat quality and gut microbial load in broilers. Agriculture. 2020;10:457. [Google Scholar]
- Attia Y.A., Al-Harthi M.A., El-Shafey A.S., Rehab Y.A., Kim W.K. Enhancing tolerance of broiler chickens to heat stress by supplementation with vitamin E, vitamin C and/or probiotics. Ann. Anim. Sci. 2017;17:1155–1169. [Google Scholar]
- Avery S. Oxidative stress and cell function. Syst. Biol. Free Radical Antioxidants. 2014;1(Part I):89–112. [Google Scholar]
- Awad A., Fahim H., El-shhat A., Mahrose K.M., Shazly S. Dietary Echinacea purpurea administration enhanced egg laying performance, serum lipid profile, antioxidant status and semen quality in duck breeders during summer season. J. Anim. Physiol. Anim. Nutr. 2021;105:757–765. doi: 10.1111/jpn.13488. [DOI] [PubMed] [Google Scholar]
- Badruzzaman A., Noor M., Mamun M., Husna A., Islam K., Alam K., Rahman M. Prevalence of diseases in commercial chickens at Sylhet Division of Bangladesh. Int. Clin. Pathol. J. 2015;1:104–108. [Google Scholar]
- Bagatell R., Paine-Murrieta G.D., Taylor C.W., Pulcini E.J., Akinaga S., Benjamin I.J., Whitesell L. Induction of a heat shock factor 1-dependent stress response alters the cytotoxic activity of HSP90-binding agents. Clin. Cancer. Res. 2000;6:3312–3318. [PubMed] [Google Scholar]
- Barrett M.J., Alones V., Wang K.X., Phan L., Swerdlow R.H. Mitochondria-derived oxidative stress induces a heat shock protein response. J. Neurosci. Res. 2004;78:420–429. doi: 10.1002/jnr.20249. [DOI] [PubMed] [Google Scholar]
- Bartlett J., Smith M. Effects of different levels of zinc on the performance and immunocompetence of broilers under heat stress. Poult Sci. 2003;82:1580–1588. doi: 10.1093/ps/82.10.1580. [DOI] [PubMed] [Google Scholar]
- Belhadj Slimen I., Najar T., Ghram A., Abdrrabba M. Heat stress effects on livestock: molecular, cellular and metabolic aspects, a review. J. Anim. Physiol. Anim. Nutr. 2016;100:401–412. doi: 10.1111/jpn.12379. [DOI] [PubMed] [Google Scholar]
- Betteridge D.J. What is oxidative stress? Metabolism. 2000;49:3–8. doi: 10.1016/s0026-0495(00)80077-3. [DOI] [PubMed] [Google Scholar]
- Blum J.H., Dove S.L., Hochschild A., Mekalanos J.J. Isolation of peptide aptamers that inhibit intracellular processes. Proc. Nat. Acad. Sci. 2000;97:2241–2246. doi: 10.1073/pnas.040573397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges S., Da Silva A.F., Maiorka A. Acid-base balance in broilers. World Poult. Sci. J. 2007;63:73–81. [Google Scholar]
- Capocci M., Santoro M.G., Hightower L.E. The life and times of Ferruccio Ritossa. Cell Stress Chaperones. 2014;19:599–604. doi: 10.1007/s12192-014-0525-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chovatiya R., Medzhitov R. Stress, inflammation, and defense of homeostasis. Molecul. Cell. 2014;54:281–288. doi: 10.1016/j.molcel.2014.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clerico E.M., Tilitsky J.M., Meng W., Gierasch L.M. How hsp70 molecular machines interact with their substrates to mediate diverse physiological functions. J. Mole. Biol. 2015;427:1575–1588. doi: 10.1016/j.jmb.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier R.J., Gebremedhin K., Macko A.R., Roy K.S. Pages 379-410 in Environmental Stress and Amelioration in Livestock Production. Springer; Berlin, Germany: 2012. Genes involved in the thermal tolerance of livestock. [Google Scholar]
- Dai S., Gao F., Xu X., Zhang W., Song S., Zhou G. Effects of dietary glutamine and gamma-aminobutyric acid on meat colour, pH, composition, and water-holding characteristic in broilers under cyclic heat stress. Br. Poult. Sci. 2012;53:471–481. doi: 10.1080/00071668.2012.719148. [DOI] [PubMed] [Google Scholar]
- de Thonel A., Mouël A.Le, Mezger V. Transcriptional regulation of small HSP—HSF1 and beyond. Inter. J. Biochem. Cell Biol. 2012;44:1593–1612. doi: 10.1016/j.biocel.2012.06.012. [DOI] [PubMed] [Google Scholar]
- Delgado C. Rising demand for meat and milk in developing countries: implications for grasslands-based livestock production. Grassland: a global resource. Pages 29-39 in Proceedings of the twentieth International Grassland Congress, Dublin, Ireland, 26-30 June 2005; Wageningen (The Netherlands); Wageningen Academic Publishers; 2005. [Google Scholar]
- Dröge W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- Elnagar S., Scheideler S., Beck M. Reproductive hormones, hepatic deiodinase messenger ribonucleic acid, and vasoactive intestinal polypeptide-immunoreactive cells in hypothalamus in the heat stress-induced or chemically induced hypothyroid laying hen. Poult. Sci. 2010;89:2001–2009. doi: 10.3382/ps.2010-00728. [DOI] [PubMed] [Google Scholar]
- Erlejman A.G., Lagadari M., Toneatto J., Piwien-Pilipuk G., Galigniana M.D. Regulatory role of the 90-kDa-heat-shock protein (HSP90) and associated factors on gene expression. Biochim. Biophysic. Acta (BBA)-Gene Regulatory Mech. 2014;1839:71–87. doi: 10.1016/j.bbagrm.2013.12.006. [DOI] [PubMed] [Google Scholar]
- Estévez M. Oxidative damage to poultry: from farm to fork. Poult. Sci. 2015;94:1368–1378. doi: 10.3382/ps/pev094. [DOI] [PubMed] [Google Scholar]
- Ezzat W., Mahrose K.M., Rizk A.M., Ouda M.M., Fathey I.A., Othman S.I., Abd El-Hack M.E. Impact of β-glucan dietary supplementation on productive, reproductive performance and physiological response of laying hens under heat stress conditions. Poult. Sci. 2024;103 doi: 10.1016/j.psj.2023.103183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farghly M.F., Mahrose K.M., Galal A.E., Ali R.M., Ahmad E.A.M., Rehman Z., Ullah Z., Ding C. Implementation of different feed withdrawal times and water temperatures in managing turkeys during heat stress. Poult. Sci. 2018;97:3076–3084. doi: 10.3382/ps/pey173. [DOI] [PubMed] [Google Scholar]
- Farghly M.F., Mahrose K.M., Cooper C., Metwally K.A., Abougabal M.Sh., El-Ratel I.T. Use of available crop by-products as alternative bedding materials to wheat straw for rearing broilers. Anim. 2021;15 doi: 10.1016/j.animal.2021.100260. [DOI] [PubMed] [Google Scholar]
- Farghly M.F., Mahrose K.M., Ahmad E.A.M., Rehman Z., Yu S. Implementation of different feeding regimes and flashing light in broiler chicks. Poult. Sci. 2019;98:2034–2042. doi: 10.3382/ps/pey577. [DOI] [PubMed] [Google Scholar]
- Farghly M.F., Mahrose K.M., Mahmoud G.B., Ali R.M., Daghash W., Metwally K.A., Abougabal M.S. Lighting programs as an appliance to improve growing New Zealand white rabbit's performance. Inter. J. Biometeorol. 2020;64:1295–1303. doi: 10.1007/s00484-020-01906-z. [DOI] [PubMed] [Google Scholar]
- Farghly M.F., Mahrose K.M., Cooper R.G., Ullah Z., Rehman Z., Ding C. Sustainable floor type for managing turkey production in a hot climate. Poult. Sci. 2018;97:3884–3890. doi: 10.3382/ps/pey280. [DOI] [PubMed] [Google Scholar]
- Farghly M.F., Mahrose K.M., Ullah Z., Rehman Z., Ding C. Influence of swimming time in alleviating the deleterious effects of hot summer on growing Muscovy duck performance. Poult. Sci. 2017;96:3912–3919. doi: 10.3382/ps/pex207. [DOI] [PubMed] [Google Scholar]
- Figueiredo D., Gertler A., Cabello G., Decuypere E., Buyse J., Dridi S. Leptin downregulates heat shock protein-70 (HSP-70) gene expression in chicken liver and hypothalamus. Cell Tissue Res. 2007;329:91–101. doi: 10.1007/s00441-007-0414-6. [DOI] [PubMed] [Google Scholar]
- Fujimoto M., Nakai A. The heat shock factor family and adaptation to proteotoxic stress. FEBS J. 2010;277:4112–4125. doi: 10.1111/j.1742-4658.2010.07827.x. [DOI] [PubMed] [Google Scholar]
- Gabriel J.E., da Mota A.F., Boleli I.C., Macari M., Coutinho L.L. Effect of moderate and severe heat stress on avian embryonic hsp70 gene expression. Growth Dev Aging: GDA. 2002;66:27–33. [PubMed] [Google Scholar]
- Gan J., Zhang D., He D., Zhang X., Chen Z., Luo Q. Promoter methylation negatively correlated with mRNA expression but not tissue differential expression after heat stress. Genet. Mol. Res. 2013;12:809–819. doi: 10.4238/2013.March.15.1. [DOI] [PubMed] [Google Scholar]
- Ganaie A., Ghasura R., Mir N., Bumla N., Sankar G., Wani S. Biochemical and physiological changes during thermal stress in bovines: a review. Iranian J. Appl. Anim. Sci. 2013;3:423–430. [Google Scholar]
- Garrido C., Brunet M., Didelot C., Zermati Y., Schmitt E., Kroemer G. Heat shock proteins 27 and 70: anti-apoptotic proteins with tumorigenic properties. Cell Cycle. 2006;5:2592–2601. doi: 10.4161/cc.5.22.3448. [DOI] [PubMed] [Google Scholar]
- Gaughan J., Mader T., Holt S., Sullivan M., Hahn G. Assessing the heat tolerance of 17 beef cattle genotypes. Int. J. Biometeorol. 2010;54:617–627. doi: 10.1007/s00484-009-0233-4. [DOI] [PubMed] [Google Scholar]
- Gu X., Hao Y., Wang X. Overexpression of heat shock protein 70 and its relationship to intestine under acute heat stress in broilers: 2. Intestinal oxidative stress. Poult. Sci. 2012;91:790–799. doi: 10.3382/ps.2011-01628. [DOI] [PubMed] [Google Scholar]
- Hao Y., Gu X., Wang X. Overexpression of heat shock protein 70 and its relationship to intestine under acute heat stress in broilers: 1. Intestinal structure and digestive function. Poult. Sci. 2012;91:781–789. doi: 10.3382/ps.2011-01627. [DOI] [PubMed] [Google Scholar]
- Hassan F., Mahrose Kh.M., Basyony M.M. Effects of grape seed extract as a natural antioxidant on growth performance, carcass characteristics and antioxidant status of rabbits during heat stress. Arch. Anim. Nutr. 2016;70:141–154. doi: 10.1080/1745039X.2016.1139609. [DOI] [PubMed] [Google Scholar]
- Hassan F., Mobarez S., Mohamed M., Attia Y., Mekawy A., Mahrose Kh. Zinc and/or selenium enriched Spirulina as antioxidants in growing rabbit diets to alleviate the deleterious impacts of heat stress during summer season. Animals. 2021;11:756. doi: 10.3390/ani11030756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt B., Wust P., Ahlers O., Dieing A., Sreenivasa G., Kerner T., Felix R., Riess H. The cellular and molecular basis of hyperthermia. Crit. Rev. Oncol. Hematol. 2002;43:33–56. doi: 10.1016/s1040-8428(01)00179-2. [DOI] [PubMed] [Google Scholar]
- Hong D.S., Banerji U., Tavana B., George G.C., Aaron J., Kurzrock R. Targeting the molecular chaperone heat shock protein 90 (HSP90): lessons learned and future directions. Cancer. Treat. Rev. 2013;39:375–387. doi: 10.1016/j.ctrv.2012.10.001. [DOI] [PubMed] [Google Scholar]
- Imik H., Atasever M.A., Urcar S., Ozlu H., Gumus R., Atasever M. Meat quality of heat stress exposed broilers and effect of protein and vitamin E. Br. Poult. Sci. 2012;53:689–698. doi: 10.1080/00071668.2012.736609. [DOI] [PubMed] [Google Scholar]
- Inouye S., Katsuki K., Izu H., Fujimoto M., Sugahara K., Yamada S., Shinkai Y., Oka Y., Katoh Y., Nakai A. Activation of heat shock genes is not necessary for protection by heat shock transcription factor 1 against cell death due to a single exposure to high temperatures. Molec. Cell. Biol. 2003;23:5882–5895. doi: 10.1128/MCB.23.16.5882-5895.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson S.E. Hsp90: structure and function. Top. Curr. Chem. 2012;328:155–240. doi: 10.1007/128_2012_356. [DOI] [PubMed] [Google Scholar]
- Kalmar B., Greensmith L. Induction of heat shock proteins for protection against oxidative stress. Adv. Drug. Deliver. Rev. 2009;61:310–318. doi: 10.1016/j.addr.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Kantidze O., Velichko A., Razin S. Heat stress-induced transcriptional repression. Biochem. 2015;80:990–993. doi: 10.1134/S0006297915080039. [DOI] [PubMed] [Google Scholar]
- Karagöz G.E., Rüdiger S.G. Hsp90 interaction with clients. Trend. Biochem. Sci. 2015;40:117–125. doi: 10.1016/j.tibs.2014.12.002. [DOI] [PubMed] [Google Scholar]
- Khafaga A.F., Abd El-Hack M.E., Taha A.E., Elnesr S.S., Alagawany M. The potential modulatory role of herbal additives against Cd toxicity in human, animal, and poultry: a review. Environ. Sci. Poll. Res. 2019;26:4588–4604. doi: 10.1007/s11356-018-4037-0. [DOI] [PubMed] [Google Scholar]
- Lara L.J., Rostagno M.H. Impact of heat stress on poultry production. Animals. 2013;3:356–369. doi: 10.3390/ani3020356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leandro N.S.M., Gonzales E., Ferro J.A., Ferro M.I.T., Givisiez P.E.N., Macari M. Expression of heat shock protein in broiler embryo tissues after acute cold or heat stress. Molec. Reprod. Dev. Incorporat. Gamete Res. 2004;67:172–177. doi: 10.1002/mrd.10397. [DOI] [PubMed] [Google Scholar]
- Lei L., Yu J., Bao E. Expression of heat shock protein 90 (Hsp90) and transcription of its corresponding mRNA in broilers exposed to high temperature. Br. Poult. Sci. 2009;50:504–511. doi: 10.1080/00071660903110851. [DOI] [PubMed] [Google Scholar]
- Li J., Soroka J., Buchner J. The Hsp90 chaperone machinery: conformational dynamics and regulation by co-chaperones. Biochim. Biophysic. Acta Molec. Cell Res. 2012;1823:624–635. doi: 10.1016/j.bbamcr.2011.09.003. [DOI] [PubMed] [Google Scholar]
- Lin H., Jiao H., Buyse J., Decuypere E. Strategies for preventing heat stress in poultry. World Poult. Sci. J. 2006;62:71–86. [Google Scholar]
- Liu Y., Chang A. Heat shock response relieves ER stress. EMBO J. 2008;27:1049–1059. doi: 10.1038/emboj.2008.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maak S., Melesse A., Schmidt R., Schneider F., Von Lengerken G. Effect of long-term heat exposure on peripheral concentrations of heat shock protein 70 (HSP70) and hormones in laying hens with different genotypes. Br. Poult. Sci. 2003;44:133–138. doi: 10.1080/0007166031000085319. [DOI] [PubMed] [Google Scholar]
- Mahalka A.K., Kirkegaard T., Jukola L.T., Jäättelä M., Kinnunen P.K. Human heat shock protein 70 (Hsp70) as a peripheral membrane protein. Biochimic. Biophysic. Acta Biomembrane. 2014;1838:1344–1361. doi: 10.1016/j.bbamem.2014.01.022. [DOI] [PubMed] [Google Scholar]
- Mashaly M., Hendricks G., Kalama M., Gehad A., Abbas A., Patterson P. Effect of heat stress on production parameters and immune responses of commercial laying hens. Poult. Sci. 2004;83:889–894. doi: 10.1093/ps/83.6.889. [DOI] [PubMed] [Google Scholar]
- Mayer M.P. HSP70 chaperone dynamics and molecular mechanism. Trend. Biochem. Sci. 2013;38:507–514. doi: 10.1016/j.tibs.2013.08.001. [DOI] [PubMed] [Google Scholar]
- Meijering R.A., Henning R.H., Brundel B.J. Reviving the protein quality control system: therapeutic target for cardiac disease in the elderly. Trend. Cardiovasc. Med. 2015;25:243–247. doi: 10.1016/j.tcm.2014.10.013. [DOI] [PubMed] [Google Scholar]
- Mishra B., Jha R. Oxidative stress in the poultry gut: potential challenges and interventions. Front. Vet. Sci. 2019;6:60. doi: 10.3389/fvets.2019.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver S.R., Phillips N.A., Novosad V.L., Bakos M.P., Talbert E.E., Clanton T.L. Hyperthermia induces injury to the intestinal mucosa in the mouse: evidence for an oxidative stress mechanism. Am. J. Physiol. Reg. Integr. Comp. Physiol. 2012;302:R845–R853. doi: 10.1152/ajpregu.00595.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orhan C., Tuzcu M., Gencoglu H., Sahin N., Hayirli A., Sahin K. Epigallocatechin-3-gallate exerts protective effects against heat stress through modulating stress-responsive transcription factors in poultry. Br. Poult. Sci. 2013;54:447–453. doi: 10.1080/00071668.2013.806787. [DOI] [PubMed] [Google Scholar]
- Pandey N., Kataria N., Kataria A.K., Joshi A., Sankhala L.N., Asopa S., Pachaury R. Extreme ambiences vis-à-vis endogenous antioxidants of Marwari goat from arid tracts in India. Extreme Life Biospeol. Astrobiol. 2012;4:29–33. [Google Scholar]
- Quinteiro-Filho W., Ribeiro A., Ferraz-de-Paula V., Pinheiro M., Sakai M., Sá L., Ferreira A., Palermo-Neto J. Heat stress impairs performance parameters, induces intestinal injury, and decreases macrophage activity in broiler chickens. Poult. Sci. 2010;89:1905–1914. doi: 10.3382/ps.2010-00812. [DOI] [PubMed] [Google Scholar]
- Revathi B., Prashanth K. Potential Hsp90 Inhibitors: a novel target for cancer therapy. Chemother. 2015;4:146. [Google Scholar]
- Richter K., Haslbeck M., Buchner J. The heat shock response: life on the verge of death. Molec. Cell. 2010;40:253–266. doi: 10.1016/j.molcel.2010.10.006. [DOI] [PubMed] [Google Scholar]
- Rizk Y.S., Fahim H.N., Beshara M.M., Mahrose Kh., Awad A.L. Response of duck breeders to dietary L-Carnitine supplementation during summer season. An. Acad. Bras. Ciên. 2019;91 doi: 10.1590/0001-3765201920180907. [DOI] [PubMed] [Google Scholar]
- Saeed M., Babazadeh D., Naveed M., Alagawany M., Abd El-Hack M.E., Arain M.A., Chao S. In ovo delivery of various biological supplements, vaccines and drugs in poultry: current knowledge. J. Sci. Food Agric. 2019;99:3727–3739. doi: 10.1002/jsfa.9593. [DOI] [PubMed] [Google Scholar]
- Sakurai H., Enoki Y. Novel aspects of heat shock factors: DNA recognition, chromatin modulation and gene expression. FEBS. J. 2010;277:4140–4149. doi: 10.1111/j.1742-4658.2010.07829.x. [DOI] [PubMed] [Google Scholar]
- Salem H.M., Alqhtani A.H., Swelum A.A., Babalghith A.O., Melebary S.J., Soliman S.M., Abd El-Hack M.E. Heat stress in poultry with particular reference to the role of probiotics in its amelioration: an updated review. J. Thermal Biol. 2022;103302 doi: 10.1016/j.jtherbio.2022.103302. [DOI] [PubMed] [Google Scholar]
- Smith J.B., McCarl B.A., Kirshen P., Jones R., Deck L., Abdrabo M.A., Borhan M., El-Ganzori A., El‑Shamy M., Hassan M. Egypt's economic vulnerability to climate change. Climate Res. 2014;62:59–70. [Google Scholar]
- Sreedhar A.S., Kalmár É., Csermely P., Shen Y.F. HSP90 isoforms: functions, expression and clinical importance. FEBS. Let. 2004;562:11–15. doi: 10.1016/s0014-5793(04)00229-7. [DOI] [PubMed] [Google Scholar]
- Statistics Unit F. 2009. Defra (Department for Environment Food and RuralAffairs, UK). United Kingdom Sea Fisheries Statistics.
- Stetler R.A., Gan Y., Zhang W., Liou A.K., Gao Y., Cao G., Chen J. Heat shock proteins: cellular and molecular mechanisms in the central nervous system. Pro.g Neurobiol. 2010;92:184–211. doi: 10.1016/j.pneurobio.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surai P.F. Nottingham University Press; Nottingham: 2002. Natural Antioxidants in Avian Nutrition and Reproduction. [Google Scholar]
- Surai P.F., Kochish I.I., Fisinin V.I., Kidd M.T. Antioxidant defence systems and oxidative stress in poultry biology: an update. Antioxidant. 2019;8:235. doi: 10.3390/antiox8070235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takii R., Fujimoto M., Tan K., Takaki E., Hayashida N., Nakato R., Shirahige K., Nakai A. ATF1 modulates the heat shock response by regulating the stress-inducible heat shock factor 1 transcription complex. Molec. Cell Biol. 2015;35:11–25. doi: 10.1128/MCB.00754-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velichko A.K., Markova E.N., Petrova N.V., Razin S.V., Kantidze O.L. Mechanisms of heat shock response in mammals. Cell Molec. Life. Sci. 2013;70:4229–4241. doi: 10.1007/s00018-013-1348-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vihervaara A., Sistonen L. HSF1 at a glance. J. Cell Sci. 2014;127:261–266. doi: 10.1242/jcs.132605. [DOI] [PubMed] [Google Scholar]
- Wasef L.G., Shaheen H.M., El-Sayed Y.S., Shalaby T.I., Samak D.H., Abd El-Hack M.E., Swelum A.A. Effects of silver nanoparticles on burn wound healing in a mouse model. Biol. Trace. Elem. Res. 2020;193:456–465. doi: 10.1007/s12011-019-01729-z. [DOI] [PubMed] [Google Scholar]
- Wasti S., Sah N., Mishra B. Impact of heat stress on poultry health and performances, and potential mitigation strategies. Animals. 2020;10:1266. doi: 10.3390/ani10081266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J., Bao E., Yan J., Lei L. Expression and localization of Hsps in the heart and blood vessel of heat-stressed broilers. Cell Stress Chaperon. 2008;13:327–335. doi: 10.1007/s12192-008-0031-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Jia G., Zuo J., Zhang Y., Lei J., Ren L., Feng D. Effects of constant and cyclic heat stress on muscle metabolism and meat quality of broiler breast fillet and thigh meat. Poult. Sci. 2012;91:2931–2937. doi: 10.3382/ps.2012-02255. [DOI] [PubMed] [Google Scholar]
- Zhao Q.L., Fujiwara Y., Kondo T. Mechanism of cell death induction by nitroxide and hyperthermia. Free Rad. Biol. Med. 2006;40:1131–1143. doi: 10.1016/j.freeradbiomed.2005.10.064. [DOI] [PubMed] [Google Scholar]
- Zhen F.S., Du H.L., Xu H.P., Luo Q.B., Zhang X.Q. Tissue and allelic-specific expression of hsp70 gene in chickens: basal and heat-stress-induced mRNA level quantified with real-time reverse transcriptase polymerase chain reaction. Br. Poult. Sci. 2006;47:449–455. doi: 10.1080/00071660600827690. [DOI] [PubMed] [Google Scholar]
- Zilaee M., Ferns G.A., Ghayour-Mobarhan M. Heat shock proteins and cardiovascular disease. Adv. Clin. Chem. 2014;64:73–115. doi: 10.1016/b978-0-12-800263-6.00002-1. [DOI] [PubMed] [Google Scholar]