Abstract
Background
There is evidence that patients with immune thrombocytopenia (ITP) are at increased risk of thrombosis. However, the association of clinical- and treatment-related factors with thrombosis remains controversial.
Objectives
To evaluate the incidence and impact of risk factors for arterial and venous thromboembolism (VTE) in patients with ITP and characterize the clinical features and management of patients.
Methods
We performed a retrospective cohort study (January 1, 2011, to October 30, 2022) of adult patients diagnosed with ITP from an Australian tertiary hospital. The incidence rates of thrombosis were calculated in terms of person-years of follow-up. Multiadjusted Cox regression was used to estimate associations.
Results
A total of 220 patients with 1365 person-years of follow-up since ITP diagnosis revealed 26 (11.8%) patients with a total of 37 thrombosis events, 29 (78%) VTE and 8 (22%) arterial thromboembolism (ATE). The incidence rate of thrombosis was 2.71 (95% CI, 1.97-3.72) (0.66 [95% CI, 0.33-1.26] for arterial thromboembolism and 2.05 [95% CI, 1.42-2.95] for VTE) per 100 person-years. Mean age and median time to first thrombosis diagnosis was 56 and 2.13 years, respectively. Age, secondary ITP, lines of therapy, thrombosis risk factors, and thrombopoietin receptor agonist therapy were independently associated with thrombosis. Almost all patients (25 of 26, [96%]) had good ITP disease control prior to thrombosis diagnosis, and antithrombotic therapy was deliverable and well tolerated.
Conclusion
Diagnosis of thrombosis in patients with ITP, while infrequent, is of clinical significance. We identified from a heterogeneous real-world cohort that older patients with multiply-treated secondary ITP receiving thrombopoietin receptor agonists are at the highest risk.
Keywords: hematologic agents, idiopathic, purpura, receptors, thrombocytopenia, thrombocytopenic, thrombopoietin, thrombosis
Essentials
-
•
Patients with immune thrombocytopenia (ITP) are at increased risk of thrombosis.
-
•
Age, secondary ITP, and thrombopoietin receptor agonists confer high thrombosis risk factors.
-
•
Antithrombotic therapy is deliverable and safe in patients with ITP.
-
•
Thrombosis risk factor assessment should be evaluated throughout the patient’s ITP history.
1. Introduction
Immune thrombocytopenia (ITP) is a rare autoimmune disorder characterized by immune-mediated platelet destruction and reduced platelet production, predisposing patients to an increased risk of bleeding [[1], [2], [3]]. Incongruously, patients with ITP may be at risk of thrombosis; large-scale population-based studies found increased rates of both venous thromboembolism (VTE) and arterial (ATE) thromboembolism in patients with ITP compared with age and gender-matched individuals without ITP [[4], [5], [6], [7]]. These observations led to systematic reviews and meta-analyses that provided supportive evidence that ITP may have a prothrombotic tendency influenced by therapy-specific factors, in particular thrombopoietin receptor agonists (TPO-RAs) [[8], [9], [10], [11]]. To date, delineating the risk factors for thrombosis in ITP remains incomplete but likely encompasses cumulative individual, ITP disease-specific, and ITP therapy-related risk factors [12].
While the estimated thrombosis incidence rate per 100 person-years in patients with ITP is only modest, 0.41 to 0.67 for VTE and 0.96 to 1.15 for ATE [9,11,13], it remains a clinically challenging problem. Not only do thrombotic events confer their own morbidity and mortality, but treatment with antithrombotic agents may further increase the bleeding risk. Currently, there are limited reports of the prevention, treatment, and adverse outcomes of thrombosis in ITP. Understanding the thrombotic risk and preventing thrombosis in patients with ITP is a significant clinical issue. Therefore, we evaluated the incidence and impact of risk factors for ATE and VTE from an Australian ITP cohort and characterized the clinical features and management of patients.
2. Methods
All patients newly diagnosed or with relapsed ITP who were added to the ITP repository at Royal Melbourne Hospital, Peter MacCallum Cancer Centre, and Bendigo Health during the study period (January 1, 2011 to October 30, 2022) were included in the study. Patients without detailed documentation of their diagnosis and each subsequent relapse, alongside data on their demographic features, comorbidities, medications, family history, and baseline organ function, were excluded.
This study was a retrospective cohort study of patients documented within the repository as of October 30, 2022. Due to the nature of data collection in the repository, it was not possible to determine the reason for the cessation of follow-up; therefore, loss to follow-up was not captured separately. Ethical approval was acquired from each site involved by their own reviewing ethical committees.
ATE included documentation of a confirmed diagnosis of cerebrovascular accident (CVA), transient ischemic attack, ST-elevated acute myocardial infarction (STEMI), non-STEMI, splenic infarction, bowel infarction, and peripheral limb infarction. VTE included documentation of a confirmed diagnosis of deep vein thrombosis (DVT), pulmonary embolus (PE), cavernous sinus thrombosis, Budd–Chiari syndrome, and splanchnic vein thrombosis. Details of thrombosis were characterized with attention to demographic features, time since diagnosis, ITP stage at diagnosis (new, persistent, chronic, or relapsed), previous ITP therapies, current ITP therapies, cardiac risk factors, thrombotic risk factors, platelet count, mean platelet volume and the subsequent management of the event. Cardiac risk factors were defined and evaluated according to the Framingham heart study: type 2 diabetes, smoking, hypertension, hypercholesterolemia, family history, and/or prior acute myocardial infarction/CVA [14]. Obesity as a risk factor was not analyzed, as this risk factor was not universally captured in the ITP repository.
Incidence was calculated in terms of person-time (years) with 95% CI. Individual subject follow-up (person-years at risk) was calculated from the date of ITP diagnosis until the date of the first occurring event: VTE, ATE, death, loss to follow-up, and patients were censored after the event. Incidence was compared to reported literature of both VTE and ATE in the general population.
To assess for variable correlation, time-dependent multiple logistic regression analysis was performed with age, gender, splenectomy status, TPO-RA exposure, mean platelet volumes/immature platelet fraction, and past history of cardiovascular disease (CVD) as independent variables, and thrombosis as the dependent variable and patients were censored after the index thrombosis event.
3. Results
3.1. Cohort characteristics
We analyzed a total of 220 patients diagnosed with ITP (195 patients from Royal Melbourne Hospital/Peter MacCallum Cancer Centre and 25 patients from Bendigo Health) during the time period from January 1, 2011, to October 30, 2022. The mean (SD) age at ITP diagnosis was 45.3 (22.9) years, of which 55% were female. The baseline characteristics and main clinical features of the study cohort are shown in Table 1. Primary ITP comprised the majority of ITP diagnoses (in 65% of the cohort), while autoimmune disorders were the most common secondary ITP. Of the study cohort, the prevalence of CVD risk factors included 44 (20%) with ≥2 cardiovascular risk factors; of these, 27 (12%) had prior acute myocardial infarction/CVA. Seven (3.1%) patients had prior VTE. Median follow-up for all patients from ITP diagnosis was 3.4 years (IQR, 0.9-8.3) for a total observation of 1365 years from ITP diagnosis.
Table 1.
Baseline patient characteristics.
| Characteristic | Total cohort (N = 220) |
|---|---|
| Gender, n (%) | |
| Female | 122 (55) |
| Age at ITP diagnosis (y), mean (SD) | 45.3 (22.9) |
| Ethnicity, n (%) | |
| Caucasian | 162 (74) |
| Asian | 28 (13) |
| Arabic | 17 (8) |
| Other/unknown | 12 (5) |
| Primary ITP, n (%) | 141 (65) |
| Secondary ITP, n (%) | 78 (35) |
| Subtype, n (% of secondary ITP) | |
| Autoimmune disorder | 32 (41) |
| SLE | 11 (14) |
| APS | 4 (5) |
| Other | 17 (22) |
| Viral infection | 14 (18) |
| Hematologic malignancy | 6 (8) |
| Post vaccine | 9 (11) |
| Unknown | 3 (4) |
| Other | 14 (18) |
| Platelet nadir at ITP diagnosis (×109/L), median (IQR) | 13 (3-38) |
| No. of different previous ITP therapies, median (IQR) | 2 (1-3) |
| Previous splenectomy, n (%) | 39 (18) |
| Most common previous ITP therapies, n (%) | |
| Corticosteroids | 200 (91) |
| TPO-RAsa | 38 (17) |
| IVIG | 110 (50) |
| Rituximab | 29 (13) |
| Cardiovascular risk factors, n (%) | |
| ≥2 Risk factorsb | 44 (20) |
| Prior CVDc | 27 (12) |
| Prior VTE, n (%) | 7 (3) |
| Ongoing anticoagulation and/or antiplatelet therapy, n (%) | 21 (9) |
APS, antiphospholipid syndrome; CVD, cardiovascular disease; ITP, immune thrombocytopenia; IVIG, intravenous immune globulin; SLE, systemic lupus erythematosus; TPO-RAs, thrombopoietin receptor agonists; VTE, venous thromboembolism.
Included eltrombopag or romiplostim.
Smoking, type 2 diabetes mellitus, hypertension, hypercholesterolemia, family history, prior cardiovascular disease (including acute myocardial infarction, angina, and/or ischemic cerebrovascular accident).
Included acute myocardial infarction, angina, and/or ischemic cerebrovascular accident.
3.2. Incidence of thrombosis events
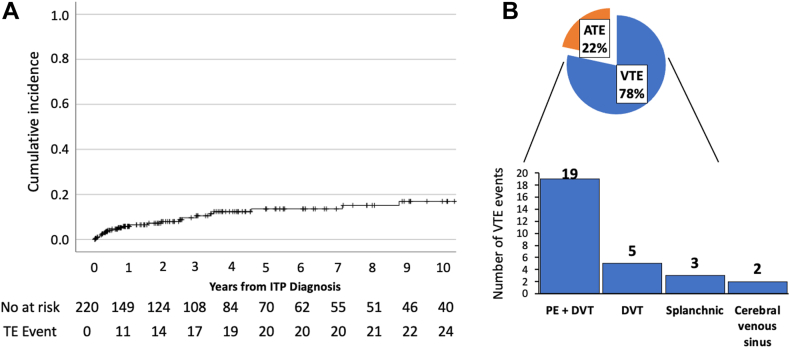
A total of 37 thrombotic events (29 venous and 8 arterial thromboses) occurred in 26 patients. The first thrombotic event mostly occurred within 5 years from ITP diagnosis (Figure 1A); the median time between ITP diagnosis and index thrombosis event was 2.13 years (IQR, 0.36-4.23). The index thrombosis incidence among the included patients was 2.71 per 100 person-years (95% CI, 1.97-3.72); the index VTE incidence was 2.05 per 100 person-years (95% CI, 1.42-2.95), and the index ATE incidence was 0.66 per 100 person-years (95% CI, 0.33-1.26). The most common VTE event was PE with or without DVT (19 events, 66%), followed by DVT alone (5 events, 17%), splanchnic vein thrombosis (3 events, 10%), and cerebral venous sinus thrombosis (2 events, 7%) (Figure 1B). Arterial thromboses included CVA (3 events), non-STEMI (3 events), STEMI (1 event), and transient ischemic attack (1 event).
Figure 1.
(A) Cumulative thrombosis incidence and (B) type of thrombosis. (A) Cumulative incidence graph of thrombosis events in all patients with immune thrombocytopenia (ITP) (n = 220) over a 10-year period. (B) Pie graph showing the type of thrombosis event as a percentage of all thrombosis events and a bar graph showing the frequency of venous thromboembolism (VTE) event type. ATE, arterial thromboembolism; DVT, deep vein thrombosis; PE, pulmonary embolism.
3.3. Characteristics of patients with thrombosis
Among the 26 patients who had thrombosis, the mean (SD) age at thrombosis diagnosis was 56 (21) years, with 13 (50%) patients aged 60 years or older and 15 (57.7%) patients being female. Thirteen (50%) patients had primary ITP, and 13 (50%) had secondary ITP, with autoimmune disorders the most common etiology (61.5%).
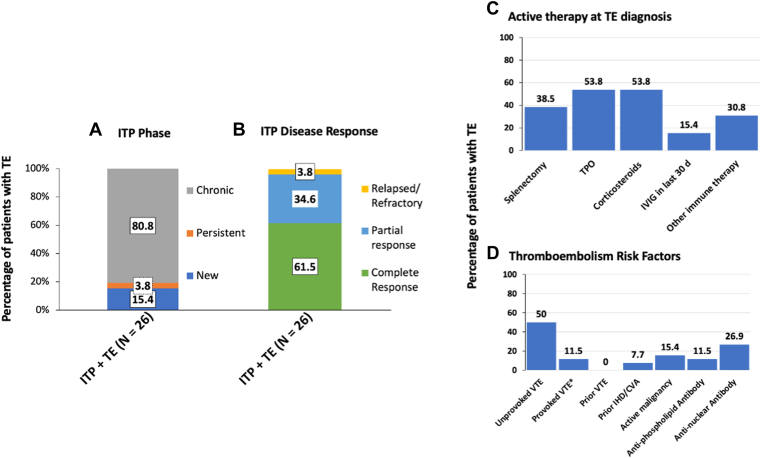
Characteristics of ITP at index thrombosis diagnosis, including ITP phase and ITP disease response, are shown in Figure 2; most had chronic ITP and were demonstrating ITP disease response (Figure 2A, B respectively). Median lines of ITP therapy at index thrombosis was 3.5 (IQR, 2-5), with 21 (81%) patients treated with 2 or more lines of ITP therapy. At the time of thrombosis diagnosis, most patients were receiving active ITP therapy, including TPO-RA therapy, corticosteroids, and other immune therapy, including mycophenolate, hydroxychloroquine, rituximab and azathioprine (Figure 2C). Most of the index thrombosis diagnoses were unprovoked VTE; however, some had identifiable thrombosis risk factors, which included postoperative, concurrent malignancy, and past ischemic heart disease (IHD)/CVA history (Figure 2D).
Figure 2.
Immune thrombocytopenia (ITP)–related clinical characteristics of patients with thrombosis. (A and B) Bar graph showing the percentage of patients with thrombosis (A) at the ITP phase of diagnosis and (B) demonstrable ITP disease response. ITP phase was defined as the duration of ITP: chronic: >12 months; persistent: 3-12 months; and new <3 months. ITP disease response was defined as relapsed/refractory: platelet count < 30 × 109/L and requiring therapy; partial response: platelet count > 30 × 109/L in response to therapy and in the absence of rescue therapy; complete response: platelet count > 100 × 109/L in response to therapy and in the absence of rescue therapy. (C) Data showing the percentage of patients on concomitant ITP therapy at thrombosis diagnosis. Corticosteroids included prednisolone or dexamethasone, and other immune therapy included mycophenolate, hydroxychloroquine, rituximab, and azathioprine. (D) Data showing the percentage of patients with thrombosis risk factors at thrombosis diagnosis. CVA, cerebral vascular accident; IHD, ischemic heart disease; IVIG, intravenous immunoglobulin; TPO, thrombopoietin; VTE, venous thromboembolism. ∗Provoked VTE (transient major risk factor) = major surgery >30 minutes, hospitalization or immobilization for >2 days.
Of the patients who developed thrombosis, 3 were on aspirin as primary prophylaxis, while 1 patient with antiphospholipid syndrome (APLS) was on aspirin and warfarin as secondary prophylaxis for prior IHD/CVA. Antinuclear antibody (ANA) was positive in 7 patients (6 had systemic lupus erythematosus [SLE] diagnosis), negative in 13, and not available in 6. Similarly, an antiphospholipid antibody was positive in 3 patients (all had APLS), negative in 17, and not available in 6. Antithrombotic therapy was commenced at the VTE event in all patients except 1, where no treatment was given as the patient died immediately from massive PE. Choice of antithrombotic therapies used included direct oral anticoagulants as the most common 18 (62%), followed by warfarin 6 (21%), and low-molecular-weight heparin 4 (14%). The median duration of anticoagulation therapy for VTE treatment was 6 months (range, 5-14.3). Antiplatelet therapy was used to treat all ATE events; 1 patient was treated with dual antiplatelet and 1 in combination with direct oral anticoagulants (patient with recurrent thrombosis and APLS). Angiogram and percutaneous intervention were required in 3 patients with IHD.
3.4. ITP clinical features associated with thromboembolism
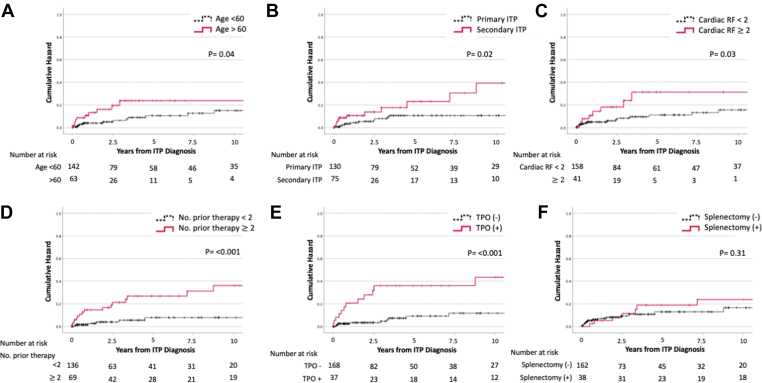
Univariate Cox proportional hazard analyses including age, gender, ethnicity, thrombosis risk factors (CVD risk factors), secondary ITP diagnosis, number of lines of therapy, splenectomy status, and TPO-RA therapy found a significant association between age >60 years (HR, 1.04; 95% CI, 1.02-1.06; P = .04), secondary ITP status (HR, 2.40; 95% CI, 1.10-5.21; P = .02), multiple prior ITP therapies (HR, 1.29; 95% CI, 1.12-1.48; P ≤ .001), presence of thrombosis risk factors (HR, 3.88; 95% CI, 1.55-9.71; P = .03), and treatment with TPO-RA (HR, 2.96; 95% CI, 1.36-6.44; P ≤ .001) to be associated with risk of developing thrombosis after ITP diagnosis (Figure 3A–F).
Figure 3.
Immune thrombocytopenia (ITP) clinical features and cumulative incidence of thrombosis. (A) Cumulative incidence graph of thrombosis events in patients greater than age 60 (n = 63) and less than age 60 (n = 142) (P = .04). (B) Cumulative incidence graph of thrombosis events in patients with primary ITP (n = 130) and secondary ITP (n = 75) (P = .02). (C) Cumulative incidence graph of thrombosis events in patients with <2 cardiac risk factors (RFs) (n = 158) and ≥2 cardiac RFs (n = 41) (P = .03). (D) Cumulative incidence graph of thrombosis events in patients with <2 number of prior therapies (n = 136) and ≥2 number of prior therapies (n = 69) (P ≤ .001). (E) Cumulative incidence graph of thrombosis events in patients with (n = 37) and without thrombopoietin (TPO) therapy (n = 168) (P ≤ .001). (F) Cumulative incidence graph of thrombosis events in patients with (n = 38) and without splenectomy (n = 163) (P = .31).
Age (HR, 1.04; 95% CI, 1.02-1.06; P ≤ .001), secondary ITP (HR, 2.63; 95% CI, 1.20-5.76; P = .02), ≥2 prior ITP therapies (HR, 1.21; 95% CI, 1.05-1.38; P ≤ .001), and TPO-RA (HR, 3.15; 95% CI, 1.45-6.86; P = .04) remained significant predictors for thrombosis in multivariable Cox regression (Table 2).
Table 2.
Predictors for thrombosis – multivariable Cox regression for risk factors of thrombosis.
| Predictor | HR | 95% CI | P value |
|---|---|---|---|
| Age | 1.04 | 1.02-1.06 | <.001 |
| Gender | 1.04 | 0.47-2.28 | .92 |
| Ethnicity | 1.14 | 0.45-2.85 | .79 |
| MPV/IPF | 1.18 | 0.43-3.28 | .75 |
| Prior splenectomy | 1.87 | 0.72-4.81 | .20 |
| ≥2 cardiac risk factorsa | 2.29 | 0.61-8.63 | .22 |
| ≥2 prior ITP therapies | 1.21 | 1.05-1.38 | <.001 |
| Secondary ITP | 2.63 | 1.20-5.76 | .02 |
| TPO-RA use | 3.15 | 1.45-6.86 | .04 |
Bold indicates significance.
HR, hazard ratio; IPF, immature platelet fraction, ITP, immune thrombocytopenia; MPV, mean platelet volume; TPO-RA, thrombopoietin receptor agonist.
Smoking, type 2 diabetes mellitus, hypertension, hypercholesterolemia, family history, prior cardiovascular disease (including acute myocardial infarction, angina, and/or ischemic cerebrovascular accident).
3.5. Outcomes of thrombosis
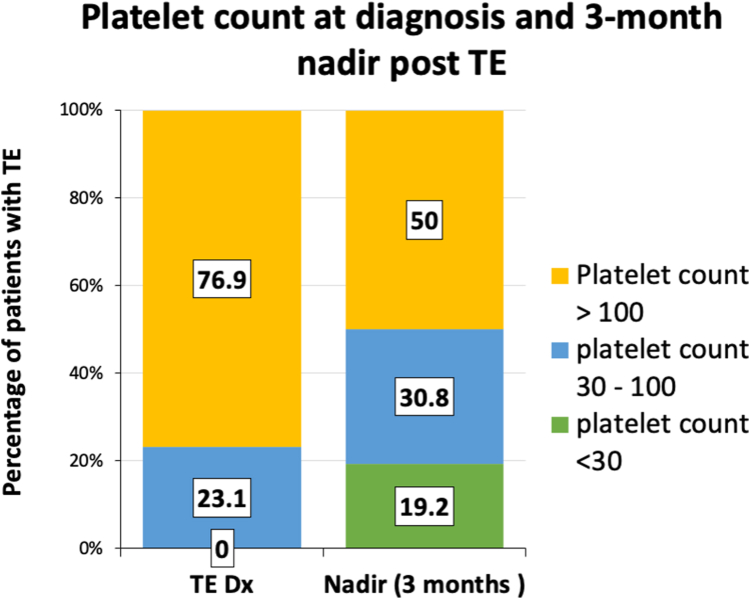
The median platelet count at index thrombosis diagnosis was 186 × 109/L (IQR, 87-244 × 109/L), and all patients had a platelet count of >30 × 109/L; however, within 3 months following thrombosis diagnosis, 9 (35%) patients developed thrombocytopenia, with 5 patients (19.2%) developing a platelet nadir of <30 × 109/L (Figure 4). The 2 patients with the most severe thrombocytopenia (platelet nadir < 10 × 109/L) had anticoagulation interruption and developed grade 1 bleeding symptoms.
Figure 4.
Platelet count at diagnosis and platelet nadir within 3 months of thrombosis. Bar graph representing the percentage of patients with thrombosis demonstrating platelet count <30, 30-100, or >100 × 109/L at thrombosis diagnosis and at nadir within 3 months from thrombosis diagnosis. TE Dx, thrombosis.
A total of 8 patients had grade 1-2 bleeding observed during antithrombotic therapy; sites of bleeding included ecchymoses, menstrual, and gastrointestinal bleeding, and none had ≥grade 3 or organ bleeding. At the time of the bleeding event, 3 patients had a platelet count of <30 × 109/L and required interruption of antithrombotic therapy and ITP rescue therapy with corticosteroids and intravenous immune globulin. Antithrombotic therapy was withheld for less than 1 week and until platelet count increased above 30 × 109/L with rescue therapy. No patient had recurrent thrombosis during the period antithrombotic therapy was withheld.
In 1 patient, TPO-RA was discontinued immediately following thrombosis diagnosis because of a platelet count >700 × 109/L. Otherwise, therapy continued unchanged in patients receiving ITP therapy at thrombosis diagnosis. Moreover, 13 of 14 patients receiving TPO-RA therapy continued without dose adjustment or interruption.
Seven of 26 patients experienced thrombosis recurrence during the follow-up period; the median time from first to second thrombosis recurrence was 25 months (range, 0.6-71). Notably, 5 patients were receiving anticoagulation at the time of thrombosis recurrence. Of the 7 patients with thrombosis recurrence, 5 received TPO-RA therapy at a standard dose of eltrombopag 50 mg or romiplostim 3 to 5 mcg/kg, and 2 had secondary ITP with APLS. Among the thrombosis cohort, 5 patients had died at the last follow-up, 1 from massive PE, while the other 4 were from non-thrombosis related causes, including catastrophic APLS, chronic lymphocytic leukaemia progression, pneumonia, and chronic myelomonocytic leukemia progression.
4. Discussion
In this retrospective cohort study of an Australian ITP population with demographic features comparable to the recent multicenter Australian ITP study [15], we provide additional evidence for the incidence of thrombosis in patients with ITP, which, while relatively uncommon, is of clinical significance. In patients with ITP, disease-related factors and treatment are important in determining the thrombosis risk; specifically, we identified that older patients with multiply-treated secondary ITP, thrombosis risk factors, and receiving TPO-RA are at the highest risk. We observed that thrombosis occurred in patients demonstrating a stable and favorable response to ITP treatment; however, within 3 months of thrombosis diagnosis, some patients developed transient thrombocytopenia, and in those patients who had severe thrombocytopenia, ITP rescue therapy and only a short pause in antithrombotic therapy was required. Moreover, antithrombotic therapy was deliverable and tolerated, with only minor bleeding events occurring in some patients during antithrombotic therapy.
TPO-RAs are pivotal in treating relapsed ITP; however, we showed that TPO-RA is an independent risk factor for developing thrombosis and remained significant with multivariate analysis together with age and secondary ITP diagnosis. Whether patients with ITP receiving TPO-RA are at higher risk of thrombosis remains controversial, as there have been no randomized studies powered to compare thrombosis rates in patients with ITP receiving TPO-RA vs placebo. The pooled analyses of the long-term open-label romiplostim extension studies observed higher thrombotic events in romiplostim-treated patients compared with control patients: 7.5 vs 5.5 thrombosis events per 100 patient-years [16]. Additionally, the safety data from the eltrombopag extend study reported a thrombosis incidence rate of 2.7 per 100 patient-years [17]. A recent systematic review and meta-analysis of the risk of thrombosis with TPO-RA showed a nonsignificant relative risk of 1.82 (95% CI, 0.78-4.24) of thrombosis in patients treated with TPO-RA compared with patients without TPO-RA treatment [18]. With respect to real-world evidence, a retrospective study of patients with ITP with higher thrombosis risk factors, which included elderly patients with cardiovascular risk factors, reported a thrombosis incidence of 1.1 per 100 patient-years, which increased to 3.6 per 100 patient-years following long-term TPO-RA therapy [19]. This study also demonstrated that older patients with thrombosis risk factors are not only at increased risk of developing thrombosis after commencing TPO-RA but are more likely to have thrombosis progression or recurrence [19]. Taken together, the risk of thrombosis associated with TPO-RA therapy in ITP remains debatable, and further studies are needed.
The thrombosis risk reported from the randomized clinical trials of patients with ITP treated with TPO-RA is likely underestimated as patients with high thrombosis risk factors were excluded. Thrombosis risk factors are frequent in patients with ITP. From our cohort of real-world unselected patients with ITP, 20% had 2 or more thrombosis risk factors, and this was an independent risk for developing thrombosis. Moulis et al. [20] reported data from the clinical CARMEN registry and found that CVD risk factors are frequent in patients at the time of ITP diagnosis, where 75.3% had at least 1 CVD risk factor. Furthermore, the frequency of thrombosis risk factors is likely to be higher in patients with chronic ITP; long-term safety data from the fostamatinib trials showed that 58% of patients had multiple risk factors for thrombosis [21]. Therefore, given the prevalence of thrombosis risk factors in patients with ITP, it is important that risk factor assessment and risk minimization are continuously evaluated and managed throughout the course of the patient’s ITP history.
We observed that patients with secondary ITP, in particular autoimmune disease, are at increased thrombosis risk after commencing TPO-RA. ITP is prevalent in other autoimmune disorders, specifically in SLE, where ITP develops in approximately 30% of patients [22]. In one retrospective study, patients with SLE and clinically significant ITP were observed to have higher thrombosis events [23]. Furthermore, a recent multicenter retrospective cohort study reported that patients with ITP associated with SLE and APS may be at increased risk of thrombosis after commencing TPO-RA [24]. Prior studies have shown a higher prevalence of other autoimmune markers, such as rheumatoid factor and ANA, in patients with primary ITP [25,26], and, specifically, ANA and antiphospholipid antibodies have been associated with increased thrombosis risk [27,28]. Our observations, together with previous studies, suggest that the dysregulation of immune tolerance may play a role in driving the pathogenesis of thrombosis in patients with ITP; further research is required to delineate this possible mechanism.
Splenectomy is a known risk factor for both VTE and ATE and in population-based studies of patients with ITP, higher rates of thrombosis were observed in patients who had been splenectomized compared with those who had not [29]. Interestingly, from our cohort, splenectomy was not an independent risk factor for thrombosis, even when combined with TPO-RA. The likely explanation was that most splenectomies were performed laparoscopically (74% of all splenectomies) and in younger patients (median age of patients having splenectomy 35 years old), both factors which are associated with lower postsplenectomy complications [30,31].
The strengths of this study include the large number of patients analyzed from an institutional registry with relatively long follow-up. This study also suffers from the potential limitation of being a retrospective case-finding series. While most patients in this study were enrolled in our institutional ITP registry, data collection from medical records would have been influenced by selection, recall, and information bias. Relevant information, such as bleeding events before and after thrombosis, may not have been accurately collected. Furthermore, patients with ITP who were managed in a shared-care model with external referring clinicians or General Practitioners would potentially have had limited and incomplete follow-up data, and importantly, it is possible that some thrombosis events may not have been reported during the follow-up period. The retrospective nature of the analysis limited the ability to precisely assess exposure to risk factors and was potentially underpowered to detect some risk factors.
Thrombosis is prevalent and a clinically challenging problem in patients with chronic ITP. Our observations from a heterogeneous real-world ITP population have identified that older patients with multiply-treated secondary ITP but in complete response to TPO-RA therapy are at the highest risk. Anticoagulation treatment is effective and safe, but monitoring of platelet count should be continued as thrombocytopenia and ITP rescue therapy are not infrequent following thrombosis. Studies providing more comprehensive details for improved patient selection for specific ITP therapy and thrombosis prevention are needed.
Acknowledgments
Funding
No funding or research grants were received in the course of the study, research, or assembly of the manuscript.
Ethics statement
Ethical approval was acquired from each site involved by their own reviewing ethical committees. A waiver for the requirement of patient consent was approved for this study.
Authorship contributions
Contributed to the design of the study: I.G., C.L., B.G., S.-R.P., J.S., K.M. Participated in patient data collection and assembly of data and performed the research: I.G., C.L., B.G., S.-R.P., J.S., K.M., N.L., R.D. Analyzed data: I.G., C.L., B.G. Participated in manuscript writing: I.G. wrote the initial manuscript draft. All authors provided their reviews and feedback during the development of the manuscript and provided final approval for the manuscript prior to submission.
Relationship Disclosure
There are no competing interests to disclose.
Footnotes
Handling Editor: Dr Kristen Sanfilippo
References
- 1.Cines D.B., Blanchette V.S. Immune thrombocytopenic purpura. N Engl J Med. 2002;346:995–1008. doi: 10.1056/NEJMra010501. [DOI] [PubMed] [Google Scholar]
- 2.Cooper N., Ghanima W. Immune thrombocytopenia. N Engl J Med. 2019;381:945–955. doi: 10.1056/NEJMcp1810479. [DOI] [PubMed] [Google Scholar]
- 3.Bussel J., Cooper N., Boccia R., Zaja F., Newland A. Immune thrombocytopenia. Expert Rev Hematol. 2021;14:1013–1025. doi: 10.1080/17474086.2021.1995347. [DOI] [PubMed] [Google Scholar]
- 4.Ekstrand C., Linder M., Baricault B., Lafaurie M., Sailler L., Lapeyre-Mestre M., et al. Impact of risk factors on the occurrence of arterial thrombosis and venous thromboembolism in adults with primary immune thrombocytopenia - Results from two nationwide cohorts. Thromb Res. 2019;178:124–131. doi: 10.1016/j.thromres.2019.04.016. [DOI] [PubMed] [Google Scholar]
- 5.Machin N., Ragni M.V., Comer D.M., Yabes J.G. Prevalence and correlates of thrombosis in adults with immune thrombocytopenia: an NIS study. Thromb Res. 2018;172:80–85. doi: 10.1016/j.thromres.2018.10.017. [DOI] [PubMed] [Google Scholar]
- 6.Nørgaard M., Severinsen M.T., Lund Maegbaek M., Jensen A.Ø., Cha S., Sørensen H.T. Risk of arterial thrombosis in patients with primary chronic immune thrombocytopenia: a Danish population-based cohort study. Br J Haematol. 2012;159:109–111. doi: 10.1111/j.1365-2141.2012.09231.x. [DOI] [PubMed] [Google Scholar]
- 7.Sarpatwari A., Bennett D., Logie J.W., Shukla A., Beach K.J., Newland A.C., et al. Thromboembolic events among adult patients with primary immune thrombocytopenia in the United Kingdom General Practice Research Database. Haematologica. 2010;95:1167–1175. doi: 10.3324/haematol.2009.018390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doobaree I.U., Nandigam R., Bennett D., Newland A., Provan D. Thromboembolism in adults with primary immune thrombocytopenia: a systematic literature review and meta-analysis. Eur J Haematol. 2016;97:321–330. doi: 10.1111/ejh.12777. [DOI] [PubMed] [Google Scholar]
- 9.Rodeghiero F. Is ITP a thrombophilic disorder? Am J Hematol. 2016;91:39–45. doi: 10.1002/ajh.24234. [DOI] [PubMed] [Google Scholar]
- 10.Langeberg W.J., Schoonen W.M., Eisen M., Gamelin L., Stryker S. Thromboembolism in patients with immune thrombocytopenia (ITP): a meta-analysis of observational studies. Int J Hematol. 2016;103:655–664. doi: 10.1007/s12185-016-1974-6. [DOI] [PubMed] [Google Scholar]
- 11.Swan D., Newland A., Rodeghiero F., Thachil J. Thrombosis in immune thrombocytopenia - current status and future perspectives. Br J Haematol. 2021;194:822–834. doi: 10.1111/bjh.17390. [DOI] [PubMed] [Google Scholar]
- 12.Abdelrahim M., Mamlouk O., Lin H., Lin J., Page V., Abdel-Wahab N., et al. Incidence, predictors, and survival impact of acute kidney injury in patients with melanoma treated with immune checkpoint inhibitors: a 10-year single-institution analysis. Oncoimmunology. 2021;10 doi: 10.1080/2162402X.2021.1927313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruggeri M., Tosetto A., Palandri F., Polverelli N., Mazzucconi M.G., Santoro C., et al. Thrombotic risk in patients with primary immune thrombocytopenia is only mildly increased and explained by personal and treatment-related risk factors. J Thromb Haemost. 2014;12:1266–1273. doi: 10.1111/jth.12636. [DOI] [PubMed] [Google Scholar]
- 14.Tsao C.W., Vasan R.S. Cohort profile: the Framingham Heart Study (FHS): overview of milestones in cardiovascular epidemiology. Int J Epidemiol. 2015;44:1800–1813. doi: 10.1093/ije/dyv337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenberg A., Cashion C., Ali F., Haran H., Biswas R.K., Chen V., et al. Treatment of immune thrombocytopenia in Australian adults: a multicenter retrospective observational study. Res Pract Thromb Haemost. 2022;6 doi: 10.1002/rth2.12792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodeghiero F., Stasi R., Giagounidis A., Viallard J.F., Godeau B., Pabinger I., et al. Long-term safety and tolerability of romiplostim in patients with primary immune thrombocytopenia: a pooled analysis of 13 clinical trials. Eur J Haematol. 2013;91:423–436. doi: 10.1111/ejh.12181. [DOI] [PubMed] [Google Scholar]
- 17.Wong R.S.M., Saleh M.N., Khelif A., Salama A., Portella M.S.O., Burgess P., et al. Safety and efficacy of long-term treatment of chronic/persistent ITP with eltrombopag: final results of the EXTEND study. Blood. 2017;130:2527–2536. doi: 10.1182/blood-2017-04-748707. [DOI] [PubMed] [Google Scholar]
- 18.Tjepkema M., Amini S., Schipperus M. Risk of thrombosis with thrombopoietin receptor agonists for ITP patients: a systematic review and meta-analysis. Crit Rev Oncol Hematol. 2022;171 doi: 10.1016/j.critrevonc.2022.103581. [DOI] [PubMed] [Google Scholar]
- 19.Palandri F., Rossi E., Bartoletti D., Ferretti A., Ruggeri M., Lucchini E., et al. Real-world use of thrombopoietin receptor agonists in older patients with primary immune thrombocytopenia. Blood. 2021;138:571–583. doi: 10.1182/blood.2021010735. [DOI] [PubMed] [Google Scholar]
- 20.Moulis G., Germain J., Comont T., Arrouy A., Lapeyre-Mestre M., Adoue D., et al. Cardiovascular risk factors in immune thrombocytopenia adults: results from the CARMEN registry. Am J Hematol. 2018;93:E181–E184. doi: 10.1002/ajh.25127. [DOI] [PubMed] [Google Scholar]
- 21.Cooper N., Altomare I., Thomas M.R., Nicolson P.L.R., Watson S.P., Markovtsov V., et al. Assessment of thrombotic risk during long-term treatment of immune thrombocytopenia with fostamatinib. Ther Adv Hematol. 2021;12 doi: 10.1177/20406207211010875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cines D.B., Liebman H., Stasi R. Pathobiology of secondary immune thrombocytopenia. Semin Hematol. 2009;46:S2–S14. doi: 10.1053/j.seminhematol.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roussotte M., Gerfaud-Valentin M., Hot A., Audia S., Bonnotte B., Thibault T., et al. Immune thrombocytopenia with clinical significance in systemic lupus erythematosus: a retrospective cohort study of 90 patients. Rheumatology (Oxford) 2022;61:3627–3639. doi: 10.1093/rheumatology/keab925. [DOI] [PubMed] [Google Scholar]
- 24.Guitton Z., Terriou L., Lega J.C., Nove-Josserand R., Hie M., Amoura Z., et al. Risk of thrombosis with anti-phospholipid syndrome in systemic lupus erythematosus treated with thrombopoietin-receptor agonists. Rheumatology (Oxford) 2018;57:1432–1438. doi: 10.1093/rheumatology/key119. [DOI] [PubMed] [Google Scholar]
- 25.Pratt E.L., Tarantino M.D., Wagner D., Hirsch Pescovitz O., Bowyer S., Shapiro A.D. Prevalence of elevated antithyroid antibodies and antinuclear antibodies in children with immune thrombocytopenic purpura. Am J Hematol. 2005;79:175–179. doi: 10.1002/ajh.20299. [DOI] [PubMed] [Google Scholar]
- 26.Demir C., Esen R., Atmaca M., Efe S. Prevalence of autoantibodies related to some autoimmune disorders in patients with chronic idiopathic thrombocytopenic purpura. Clin Appl Thromb Hemost. 2011;17:E114–E118. doi: 10.1177/1076029610387588. [DOI] [PubMed] [Google Scholar]
- 27.Hollenhorst M.A., Al-Samkari H., Kuter D.J. Markers of autoimmunity in immune thrombocytopenia: prevalence and prognostic significance. Blood Adv. 2019;3:3515–3521. doi: 10.1182/bloodadvances.2019000400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pierrot-Deseilligny Despujol C., Michel M., Khellaf M., Gouault M., Intrator L., Bierling P., et al. Antiphospholipid antibodies in adults with immune thrombocytopenic purpura. Br J Haematol. 2008;142:638–643. doi: 10.1111/j.1365-2141.2008.07228.x. [DOI] [PubMed] [Google Scholar]
- 29.Thomsen R.W., Schoonen W.M., Farkas D.K., Riis A., Fryzek J.P., Sørensen H.T. Risk of venous thromboembolism in splenectomized patients compared with the general population and appendectomized patients: a 10-year nationwide cohort study. J Thromb Haemost. 2010;8:1413–1416. doi: 10.1111/j.1538-7836.2010.03849.x. [DOI] [PubMed] [Google Scholar]
- 30.Chaturvedi S., Arnold D.M., McCrae K.R. Splenectomy for immune thrombocytopenia: down but not out. Blood. 2018;131:1172–1182. doi: 10.1182/blood-2017-09-742353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kojouri K., Vesely S.K., Terrell D.R., George J.N. Splenectomy for adult patients with idiopathic thrombocytopenic purpura: a systematic review to assess long-term platelet count responses, prediction of response, and surgical complications. Blood. 2004;104:2623–2634. doi: 10.1182/blood-2004-03-1168. [DOI] [PubMed] [Google Scholar]