Abstract
Introduction
Physiology-guided cardiopulmonary resuscitation (CPR) offers the potential to optimize resuscitation and enable early prognosis.
Methods
Physiology-Guided CPR was one of six focus topic for the Wolf Creek XVII Conference held on June 14–17, 2023 in Ann Arbor, Michigan, USA. International thought leaders and scientists in the field of cardiac arrest resuscitation from academia and industry were invited. Participants submitted via online survey knowledge gaps, barriers to translation and research priorities for each focus topic. Expert panels used the survey results and their own perspectives and insights to create and present a preliminary unranked list for each category, which was then debated, revised and ranked by all attendees to identify the top 5 for each category.
Results
Top knowledge gaps include identifying optimal strategies for the evaluation of physiology-guided CPR and the optimal values for existing patients using patient outcomes. The main barriers to translation are the limited usability outside of critical care environments and the training and equipment required for monitoring. The top research priorities are the development of clinically feasible and reliable methods to continuously and non-invasively monitor physiology during CPR and prospective human studies proving targeting parameters during CPR improves outcomes.
Conclusion
Physiology-guided CPR has the potential to provide individualized resuscitation and move away from a one-size-fits-all approach. Current understanding is limited, and clinical trials are lacking. Future developments need to consider the clinical application and applicability of measurement to all healthcare settings. Therefore, clinical trials using physiology-guided CPR for individualisation of resuscitation efforts are needed.
Keywords: Heart arrest, Resuscitation, Physiology-guided CPR
Introduction
Cardiac arrest carries high mortality.1, 2 Cardiopulmonary resuscitation (CPR) is the cornerstone of initial management but follows a one-size-fits-all treatment paradigm. Physiology-guided CPR holds great potential to improve patient outcomes through optimising and individualising resuscitation efforts for each patient.
Physiology-guided CPR involves the continuous monitoring of various physiologic parameters, such as electrocardiogram (ECG) waveform, arterial blood pressure (BP), end-tidal carbon dioxide (PETCO2), regional cerebral oxygenation (rSO2) and electroencephalography (EEG). By leveraging real-time data, resuscitation strategies can be tailored and adjusted on a patient-specific basis in response to the individual’s unique physiological needs.3, 4 This approach moves away from rigid adherence to predefined cycles or fixed time intervals for resuscitation, allowing for a more dynamic and adaptive response to the patient’s evolving condition. Importantly, rSO2 and EEG may uniquely provide additional insights regarding real-time brain tissue perfusion. Continuous arterial BP monitoring will guide healthcare providers to tailor chest compressions to achieve the best blood pressure and, specifically, the highest coronary perfusion pressure. Furthermore, integrating physiological monitoring into CPR holds promise for early prognostication, enabling healthcare providers to assess the likelihood of a positive outcome more accurately.
Physiology-guided CPR represents a forward-looking approach that capitalises on continuous monitoring and data-driven decision-making to revolutionise the way we treat cardiac arrest, offering the potential for improved patient survival and long-term outcomes. This paper discusses the current state, potential future state, knowledge gaps, barriers to translation and research priorities for physiology-guided CPR.
Methods
Since its inception in 1975, the Wolf Creek Conference has a well-established tradition of providing a unique forum for robust intellectual exchange between thought leaders and scientists from academia and industry that focuses on advancing the science and practice of cardiac arrest resuscitation.5 The Wolf Creek XVII Conference was hosted by the Max Harry Weil Institute for Critical Care Research and Innovation in Ann Arbor, Michigan, USA on June 15–17 2023.6
Physiology-guided CPR was one of 6 focus topics for the Wolf Creek XVII Meeting. Meeting invitees included international academic and industry scientists as well as thought leaders in the field of cardiac arrest resuscitation. All participates were required to complete conflict of interest disclosures. Prior to the meeting, all participants were asked via an online survey to list up to three knowledge gaps, barriers to translation and research priorities for each topic. Participants were instructed that the topic of the physiology-guided CPR session would focus on physiologic monitoring techniques that can be used to guide and optimise therapy during CPR. Knowledge gaps were defined as areas where our understanding or knowledge is incomplete or limited. These gaps can arise due to various factors, such as lack of research, inadequate information, limited access to data or resources, or simply because the topic is new or complex. Barriers to translation were defined as obstacles that can prevent the successful transfer of knowledge or innovations from research or development settings to practical applications in the real world. Research priorities were defined as the areas of study that are considered most important or urgent by the scientific community or society as a whole. These priorities are often determined by a range of factors such as knowledge gaps, scientific breakthroughs, new challenges, societal needs or funding opportunities.
Expert panellists in each topic provided an overview of the field's current state and potential future state. This layed the groundwork for an informed debate. They also used the survey results and their own perspectives and insights to create an initial unranked list of up to ten items for each category. This was followed by the presentation and initial ranking of the knowledge gaps, barriers to translation, and research priorities by all attendees using electronic voting, discussion and revision by the panel and attendees, and then re-ranking (supplemental materials). The top five items in each category underwent final review on the last day of the conference. This manuscript presents and discusses an overview of the current and potential future state of the field and prioritized results for physiology-guided CPR.
Current state
Two mechanical effects are evident when blood flow occurs during cardiac activity or chest compressions. Firstly, the heart’s motion induces a recoil effect on the chest. Secondly, the motion of the blood generates a recoil effect throughout the entire body. Utilising physiological biosensors, including invasive arterial BP monitoring, ECG, PETCO2, pulse oximetry (SpO2), rSO2, ballistocardiography (BCG), point-of-care Ultrasound (POCUS) and echocardiography, enables the measurement of these effects (Table 1). These sophisticated tools offer a comprehensive approach to assessing the intricate dynamics of blood flow and cardiac activity during both natural heart function and resuscitation efforts, providing valuable insights into the physiological responses of the cardiovascular system.
Table 1.
Summary of the current state of physiological monitoring parameters (Adapted from Marquez3).
| Parameter | Advantages | Disadvantages |
|---|---|---|
| Coronary perfusion pressure |
|
|
| Diastolic blood pressure |
|
|
| ECG waveform |
|
|
| End-tidal carbon dioxide |
|
|
| Cerebral oximetry |
|
|
| Cardiac ultrasound |
|
|
Invasive arterial blood pressure
During CPR, invasive arterial BP can indicate CPR quality, measure systemic and organ perfusion pressures, and be a surrogate for the systemic blood flow generated by chest compressions. Restoration and maintenance of myocardial blood flow during CPR is critical to resuscitation success. Thus, longstanding clinical and laboratory data have demonstrated that higher intra-arrest coronary perfusion pressure values, the primary determinant of myocardial blood flow, are strongly associated with higher rates of return of spontaneous circulation (ROSC) and survival.7, 8 As the real-time determination of coronary perfusion pressure during CPR can be difficult, systemic diastolic BP has been endorsed as an alternative to coronary perfusion pressure with similar associations with survival outcomes.9, 10 Which part of the diastolic phase of the compression-decompression cycle the diastolic pressure should be measured is debated. The monitor’s algorithms for measuring and presenting the value on the screen are created for spontaneous beating hearts and not for chest compression-generated blood pressures. In laboratory studies, hemodynamic-directed CPR strategies, including real-time chest compression mechanics and vasopressors titration, improve intra-arrest physiology and superior survival outcomes.11, 12
Despite promising pre-clinical data and associations of invasively measured BP with CPR outcomes, the widespread use of BP for monitoring or guiding CPR has not occurred.13, 14 One significant barrier is the requirement for an invasive arterial catheter – as such, this is typically limited to in-hospital settings or out-of-hospital settings with physicians. Intra-arrest placement of an arterial catheter to assist with guiding CPR may divert attention from other aspects of high-quality resuscitation. Additionally, though coronary perfusion pressure or diastolic blood pressure thresholds are a valuable starting point, the actual strategies for achieving these goals have not been determined in humans and CPR guidelines do not instruct clinicians regarding how resuscitation may be optimized or tailored based on BP. Thus, the prospective study of BP-directed CPR is critically important to moving forward.
Cerebral oximetry
Cerebral oximetry measured by near-infrared spectroscopy (NIRS) is a noninvasive measure reflecting the balance of oxygen delivery and uptake in the cerebral circulation.15 It has been increasingly used during CPR.16, 17, 18, 19, 20, 21, 22 NIRS sensors placed on the forehead emit and detect near-infrared light that penetrates ∼3 cm into the brain's frontal region. Relying on the unique absorption spectra of oxy- and deoxyhemoglobin, NIRS devices calculate rSO2 using the Beer–Lambert Law.23, 24 As venous blood makes up ∼75% of blood in the sampled area, normal rSO2 values are 60–80 % and reflect the dynamic balance between oxygen delivery and uptake.24 Cerebral oximetry does not rely on pulsatile flow and has been used in diverse settings, including cardiac arrest, neurosurgery, and cardiothoracic surgery.25, 26, 27, 28, 29
Generally, rSO2 falls to critically low values with cardiac arrest and remains low,30, 31 but can increase with continued high-quality CPR.30, 32 Higher values of rSO2 are consistently associated with ROSC in multiple systematic reviews.17, 33, 34, 35, 36, 37 This suggests that rSO2 may indicate the quality of oxygen delivery to the brain but also other vital organs, including the heart, and potentially acts as a surrogate for coronary perfusion pressure.38
One meta-analysis reported better neurologic outcomes with higher rSO2 during resusciation.17 However, included studies were limited by the small number of survivors and the types of NIRS measurements during resuscitation influenced the association with ROSC.
ECG
Currently, most out-of-hospital resuscitations have rescuers use a snapshot every two minutes of the patient’s cardiac rhythm and vital status to help inform care (CPR, defibrillation, medications).39 Between these snapshots, rescuers are often blinded to the patient’s ECG rhythm and vital status as active CPR obscures the ECG and challenges the ready assessment of the patient’s vital status. The consequence is that CPR is interrupted every few minutes to update the patient’s underlying rhythm and vital status. And yet, the patient’s ECG and physiologic phenotype can be dynamic during these periods of CPR.40 Consequently, protocolised care may not align with a patient’s physiology. For example, the protocolised administration of a vasopressor during CPR despite an underlying (unrecognised) ROSC.
There is an advancing science that applies artificial intelligence to process bio-signals (ECG and impedance) to accurately provide real-time (continuous) rhythm identification during active CPR and gauge the rhythm’s vitality (i.e. whether the organised rhythm produces a spontaneous pulse, whether a shock for ventricular fibrillation (VF) produce an organised rhythm or spontaneous pulse).41, 42, 43, 44 These innovations have the potential to continuously inform the rescuer of the patient’s rhythm and physiologic status during CPR and, in turn, potentially improve care.
Moreover, these same types of advanced data interrogation methods can be used to predict early-on specific downstream clinical circumstances that may enable more directed care earlier in the course of resuscitation. For example, patients with refractory VF require 3 or more shocks often requiring additional therapies (i.e. antiarrhythmic medications, shock vector change, or double sequential defibrillation). Implementation of these treatments currently only occurs after the patient has demonstrated refractoriness. Innovative techniques of real-time ECG processing may predict at the outset of resuscitation which patients are most likely to manifest refractory VF, providing an opportunity for earlier interventions that may improve the course of resuscitation.45
Continuous waveform Capnography (end-tidal carbon dioxide)
A substantial body of pre-clinical evidence establishes that PETCO2 can serve as a surrogate marker of pulmonary blood flow and cardiac output during CPR.46, 47, 48, 49 Among clinical studies, there are four main themes that arise: 1) PETCO2 values are generally higher among patients who achieve ROSC50, 51, 52; 2) low PETCO2 values (<10 mmHg) are associated with a low chance of successful resuscitation without E-CPR support52, 53, 54; 3) a sudden rise in PETCO2 can be used to detect the onset of ROSC55; and 4) as CPR quality improves, so does PETCO2.56, 57, 58 Unfortunately, many factors can confound PETCO2 values during real-life use as a CPR quality monitor, including vasopressor administration, obstructed endotracheal tubes, PETCO2 measurement algorithm used, or clinical scenarios with extreme ventilation-perfusion (VQ) mismatch (e.g., pulmonary embolism).
Photoplethysmography (PPG) Waveforms: Pulse oximetry
A major barrier to the widespread adoption of physiologic-directed CPR is the indentification and validation of non-invasive monitors suitable for a diverse set of clinical environments. Extensive literature supports the use of photoplethysmography (PPG) waveforms to evaluate the cardiovascular system, particularly for assessing volume status or fluid responsiveness,59, 60, 61, 62, 63, 64, 65, 66, 67 and determining vascular distensibility, tone,68, 69, 70, 71 and BP.72, 73 Recent animal models indicate that PPG waveform characteristics (e.g., amplitude [Amp] and area under the curve [AUC]) can gauge CPR quality74 and detection of ROSC.75 A clinical observational study corroborated these PPG characteristics (Amp and AUC) as predictors of ROSC during in-hospital events.74 However, the current lack of clinical evidence supporting the prospective adjustment of resuscitation based on PPG values represents a notable knowledge gap.
Point-of-Care ultrasound (POCUS) and echocardiography
POCUS during cardiac arrest has traditionally been used to identify reversible causes of arrest (e.g., cardiac tamponade or tension pneumothorax) or to identify the underlying cardiac rhythm (PEA vs. asystole vs. fine VF), a finding that may have prognostic benefit.76 Despite promise in previous studies, images obtained via a transthoracic approach can be limited due to the inability of the proceduralist to obtain adequate cardiac windows. As an alternative, transesophageal echocardiography (TEE) has demonstrated clinical utility, including optimizing hand position during CPR, ensuring that the chest compressions are applied to the left ventricle, rather than the aortic outflow track.77, 78, 79 Of note, as chest compression fraction is highly associated with patient outcomes, and resuscitation teams should ensure that any use of POCUS or echocardiography does not result in increased interruptions in CPR.
Potential future state
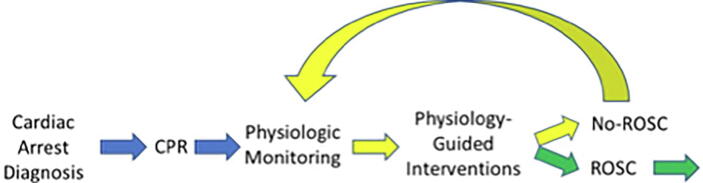
We increasingly understand that a spectrum of time-sensitive physiology can occur within a single or across the population of cardiac arrest patients. The understanding supports a more dynamic, precision approach whereby information from various biosensors could be smartly integrated to help guide a more patient-specific approach that aligns the choice, dose, and timing of treatments with the patient’s physiology (Fig. 1). This precision strategy of physiologic-guided treatment has inherent appeal as we understand treatment response is not uniform and instead may depend on patient characteristics.80, 81, 82
Fig. 1.
The patient-specific approach of physiological-guided CPR.
Although promising, substantial knowledge gaps must be addressed to meaningfully achieve this strategy.
Knowledge gaps
The top five knowledge gaps identified by the conference participants are listed in Fig. 2 and discussed below. Additional knowledge gaps can be found in the supplemental materials.
-
1.
Identifying the optimal strategies for the evaluation of physiologically-directed CPR
Fig. 2.
Physiological-guided cardiopulmonary resuscitation: Top 5 knowledge gaps as ranked by attendees at Wolf Creek XVII, June 15–17, 2023, Ann Arbor, MI, USA.
Physiologically-directed CPR relies on a nuanced understanding of physiological responses, which can be intricate and multifaceted. Translating these complex mechanisms into practical guidelines for healthcare providers requires clear and simplified frameworks without sacrificing essential details. While there is a growing body of research supporting the benefits of physiology-guided CPR, more robust high-quality evidence is needed.
-
2.
Identifying the optimal values for existing physiological parameters using patient outcomes
Many of the measures currently recommended are indirect measures of physiology (e.g. PETCO2 as an indirect measure of cardiac output). There is insufficient knowledge about how these modalities gauge acute physiology, distinguish physiological phenotypes, and predict short- and long-term outcomes in real-time. Ideally, continuous monitoring would provide ongoing resuscitation assessment and help measure the patient's acute physiology and predict prognosis.
Not all patients may respond the same way to physiological interventions, and individual responses may be unpredictable. This may make it challenging to develop standardized protocols for physiology-guided CPR.
-
3.
Accuracy of existing modalities in measuring perfusion and oxygenation in humans
Physiologically based measures often serve as surrogate indicators of the underlying physiological processes. Presently, our understanding of the efficacy of these modalities in accurately assessing acute physiology, such as tissue perfusion and oxygenation, remains incomplete. Additionally, there is a need to ascertain how reliably these measurements reflect the generation of blood flow and the perfusion of the lungs during chest compressions and, ultimately, the supply of oxygen to the brain.
-
4.
Understanding the impact of underlying etiology on physiological parameters
Distinguishing physiologic phenotypes holds the potential to tailor patient-specific care. For example, the decision between ongoing CPR and medication administration versus an immediate shock depends upon the patient's acute physiologic status. While it is useful to know if these measures can gauge CPR (flow and oxygenation), it is equally important to understand how these measures might gauge the patient’s acute physiology and how this information might guide differential treatment. The question remains: can these measures truly direct care, or do we still lack comprehensive knowledge about well-defined physiologic and prognostic phenotypes and their role in influencing the selection, timing, and dosage of various treatments, including CPR, medications, and defibrillation?
-
5.
Lack of human clinical trials of interventions in physiologically-directed CPR
Much of the evidence supporting these parameters is derived from animal studies,83 and there is a lack of human studies demonstrating that physiological-based CPR improves patient outcomes. Conducting rigorous research on CPR, especially in real-world clinical settings, is inherently complex. It can be challenging to control all variables, and ethical considerations may limit the extent to which experimental interventions can be applied to critically ill patients. The multifactorial nature of these interventions, which may include personalised adjustments based on continuous monitoring, makes designing and conducting clinical trials challenging. Establishing uniform protocols for a clinical trial while accommodating the personalised nature of these interventions poses a methodological challenge. However, establishing a clear evidence base through well-designed clinical trials is essential to validate the effectiveness of these approaches in real-world scenarios. Such trials will require patient-centred outcomes such as measures of quality of life,84 to provide evidence of effectiveness.
Dissenting opinions
There was a discussion supporting these knowledge gaps, the need for direct measures during CPR, and the lack of progress in this field.
Barriers to translation
The top five barriers to translation identified by the conference participants are listed in Fig. 3 and discussed below. Additional barriers to translation can be found in the supplemental materials.
-
1.
Modalities that can be used outside of critical care environments
Fig. 3.
Physiological-guided cardiopulmonary resuscitation: Top 5 barriers to translation as ranked by attendees at Wolf Creek XVII, June 15–17, 2023, Ann Arbor, MI, USA.
A significant barrier to the widespread adoption of physiological monitoring is its limited application beyond well-resourced critical care environments. Currently, the selection of a physiological monitoring modality is contingent upon the clinical context in which they are applied. Some measures require specialised medical expertise, involving invasive procedures and reliance on sophisticated and costly monitoring equipment.3 These factors restrict their use and applicability in prehospital and resource-limited settings. To address this challenge, future advancements in point-of-care modalities should consider the unique challenges of the prehospital and low-resource environments and aim to be universally applicable across all healthcare settings.
-
2.
Skills, training and equipment required for monitoring strategies
Implementing physiology-guided CPR effectively requires healthcare providers to receive specialised training. For some measurements (e.g. ultrasound), simulation-based training may be required. Training includes understanding how to interpret physiological data, adjust interventions accordingly, and make real-time decisions based on this information. This can be resource-intensive and may not be feasible for all healthcare settings. Healthcare providers need to be well-trained and experienced in reading and responding to these data accurately. How innovative new methods for physiology monitoring can be integrated into understandable, easy-to-use systems for all professionals is not yet understood.
-
3.
Increasing complexity of monitoring and treatment during CPR could reduce focus on interventions proven to be effective
A significant concern is the additional monitoring and complication of advanced life support algorithms in physiological-based CPR, which has the potential to distract providers from tasks that are known to be effective. The real-time decision-making involved in physiology-guided CPR adds complexity to resuscitation efforts. Healthcare providers must balance the need for personalized care with the urgency of the situation, and this can be challenging in high-stress, time-sensitive situations. The continuous monitoring and adjustments required in physiology-guided CPR may consume more time compared to traditional CPR protocols. This could potentially delay other critical interventions or lead to longer resuscitation attempts. There are likely patient- and setting-specific scenarios in which physiology-directed CPR offers benefit and potentially alternative scenarios in which focusing on standard CPR algorithms is more ideal – understanding these relationships will be key to guiding the implementation of physiology-directed resuscitation strategies.
-
4.
Lack of coordination of basic science, experimental and human studies
A significant challenge in this field is translating scientific discovery into operational clinical actions. Most of the scientific evidence does not provide the best clinical thresholds for differential actions (e.g. when to change CPR, administer a drug, or immediately defibrillate), what are optimal clinical goals, whether physiological measurements can be combined or what outcomes are best to examine. In resuscitation, there is also the complexity of adding practices with the need to keep resuscitation algorithms as simple as possible to ensure maximal adherence to best practices. Bridging the gap between basic science, clinical and implementation science, and bedside practice is essential to ensure that the theoretical foundations of physiologically-directed CPR align seamlessly with practical application.
-
5.
Regulatory issues
Regulatory issues can effect both the implementation and study of physiology-directed CPR. Though the use of physiologic monitoring during CPR is endorsed by resuscitation guidelines, actual titration of resuscitation therapies to physiologic metrics may conversely require deviation from established CPR algorithms. The feasibility of obtaining informed consent must also be considered in designing prospective studies of physiology-directed CPR.
Dissenting opinions
There were no dissenting opinions for the barriers to translation.
Research priorities
The top five research priorities identified by the conference participants are listed in Fig. 4 and discussed below. Additional research priorities can be found in the supplemental materials. The Top 5 research priorities identified in the survey and supported in the polls were to assess the implementation of specific physiological-guided CPR measures in the clinical environment to determine optimal goals to achieve favourable patient outcomes.
-
1.
What interventions in physiological-guided CPR, compared to standard care, improve physiological parameters and patient outcomes?
Fig. 4.
Physiological-guided cardiopulmonary resuscitation: Top 5 research priorities as ranked by attendees at Wolf Creek XVII, June 15–17, 2023, Ann Arbor, MI, USA.
The top-ranked research priority highlights the distinct lack of clinical data testing of the use of interventions guided by physiological measures. A better understanding of the relationship between specific intra-arrest interventions and the achievement of physiologic goals is imperative to moving forward with physiologic-directed CPR.
-
2.
Development of clinically feasible and reliable methods to continuously and non-invasively monitor brain and heart perfusion, energy state and oxygenation during CPR in all care state.
The second-ranked research priority highlights the need for simple and reliable measures and measurement tools that can be easily and rapidly implemented. Many current physiologic measurement tools are limited in that they are surrogate measures of the physiology of interest (e.g., blood pressure as a surrogate of blood flow). Moreover, many established indicators of intra-arrest physiology require invasive monitoring and are thus limited in terms of the settings in which they can be readily deployed. Ideally, existing technology (e.g., pulse oximetry) will be studied and leveraged to monitor CPR across more diverse clinical scenarios and environments and new monitoring tools will be devised through the collaboration of basic scientists and clinicians and be usable in all healthcare settings.
-
3.
Does targeting a specific partial pressure of end-tidal carbon dioxide (PETCO2) goal, compared to standard care, during CPR improve outcomes?
The third-ranked research priority was to identify a target range of PETCO2. Capnography is widely available in a range of healthcare settings and is already familiar to healthcare professionals. Though studies in both adults and children have identified PETCO2 thresholds associated with superior outcomes, prospective studies are necessary to determine if these values can be targeted to improve outcomes. Identifying a target range of PETCO2 that improves patient outcomes holds great promise for a measurement that is already readily available and used during resuscitation. Another question is when during ventilation PETCO2 should be measured. Different monitors measure at different timepoints, and therefore may report different values for the same patient that will influence care.85, 86
-
4.
Does targeting a specific arterial relaxation pressure (i.e., diastolic BP) during CPR, compared to standard care, improve outcomes?
The fourth-ranked research priority was to identify a target range of arterial diastolic BP. Invasively measured arterial blood pressure measurements are readily available in some healthcare settings (e.g. critical care units), and given the importance of organ and coronary perfusion to resuscitation outcomes, it was prioritised by participants. The critical next steps are to 1) determine when during the diastolic phase to measure and 2) prospective studies titrating resuscitation therapies to diastolic BP to optimize physiology and improve patient outcomes.
-
5.
Does targeting a specific cerebral oximetry goal, compared to standard care, improve outcomes?
The fifth-ranked research priority, to identify a target range for cerebral oximetry, highlights the importance of cerebral perfusion, which is critical for minimising neurological damage. Larger prospective studies are needed to assess neurologic recovery and must consider the crucial impact of comprehensive post-resuscitation interventions.15 Effective delivery of critical care measures, such as targeted temperature management, treating blood gas abnormalities, and blood pressure control, are necessary to optimize neurologic outcomes, which NIRS findings may guide or complement.
Dissenting opinions
There was robust discussion about using “standard care” (i.e. standard CPR) as the control group in the proposed research questions. However, no better solution was proposed.
Conclusions
By leveraging real-time physiological data, physiology-guided CPR empowers healthcare professionals to adapt their approach dynamically, addressing each patient's unique needs. This precision approach represents a significant advancement in resuscitation strategies, as it prioritizes the individual patient's response over a one-size-fits-all approach, ultimately increasing the likelihood of a positive neurological outcome following a cardiac arrest event. However, there is limited evidence as to how these measures can be applied to guide resuscitation. Future research is needed to establish therapeutic targets and explore the impact on patient outcomes.
Funding
Janet Bray is funded by a National Heart Foundation of Australia Fellowship (#104751).
CRediT authorship contribution statement
Janet Bray: Conceptualization, Supervision, Writing – original draft, Writing – review & editing. Tom Rea: Conceptualization, Writing – original draft, Writing – review & editing. Sam Parnia: Conceptualization, Writing – original draft, Writing – review & editing. Ryan W. Morgan: Writing – original draft, Writing – review & editing. Lars Wik: Conceptualization, Writing – original draft, Writing – review & editing. Robert Sutton: Conceptualization, Writing – original draft, Writing – review & editing.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Janet Bray is an Associate Editor of Resuscitation Plus. Lars Wik is a medical advisor to Stryker Emergency Care and holds patents through his Hospital licenced to Stryker and Zoll.
Acknowledgment
The study team acknowledges the Weill Critical Care Institute at the University of Michigan for hosting and offering audiovisual support during the Wolff Creek Conference and providing data visualization support in the form of tables. We also wish to thank Robert Neumar for his insightful feedback.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.resplu.2024.100589.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Nishiyama C., Kiguchi T., Okubo M., et al. Three-year trends in out-of-hospital cardiac arrest across the world: Second report from the International Liaison Committee on Resuscitation (ILCOR) Resuscitation. 2023;186 doi: 10.1016/j.resuscitation.2023.109757. [DOI] [PubMed] [Google Scholar]
- 2.Schluep M., Gravesteijn B.Y., Stolker R.J., et al. One-year survival after in-hospital cardiac arrest: a systematic review and meta-analysis. Resuscitation. 2018;132:90–100. doi: 10.1016/j.resuscitation.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 3.Marquez A.M., Morgan R.W., Ross C.E., et al. Physiology-directed cardiopulmonary resuscitation: advances in precision monitoring during cardiac arrest. Curr Opin Crit Care. 2018;24:143–150. doi: 10.1097/MCC.0000000000000499. [DOI] [PubMed] [Google Scholar]
- 4.Kosmopoulos M, Kalra R, Bartos JA, et al. Contemporary approaches to cardiopulmonary resuscitation: physiology-guided approaches. J Emerg Crit Care Med; Vol 4 (April 2020): J Emerg Crit Care Med 2019.
- 5.Neumar R.W., Tang W. Wolf Creek XVII Part 2: the origin, evolution, and impact of the Wolf Creek Conference. Resuscitation Plus. 2023;16 doi: 10.1016/j.resplu.2023.100505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neumar R.W. Wolf Creek XVII Part 1: the future of cardiac arrest resuscitation. Resuscitation Plus. 2023;16 doi: 10.1016/j.resplu.2023.100504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kern K.B., Ewy G.A., Voorhees W.D., et al. Myocardial perfusion pressure: a predictor of 24-hour survival during prolonged cardiac arrest in dogs. Resuscitation. 1988;16:241–250. doi: 10.1016/0300-9572(88)90111-6. [DOI] [PubMed] [Google Scholar]
- 8.Brent C.M., Cheskes S., Castrén M., Brooks S.C. Wolf Creek XVII Part 5: Mobile AEDs. Resuscitation Plus. 2023;16 doi: 10.1016/j.resplu.2023.100500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meaney P.A., Bobrow B.J., Mancini M.E., et al. Cardiopulmonary resuscitation quality: [corrected] improving cardiac resuscitation outcomes both inside and outside the hospital: a consensus statement from the American Heart Association. Circulation. 2013;128:417–435. doi: 10.1161/CIR.0b013e31829d8654. [DOI] [PubMed] [Google Scholar]
- 10.Berg R.A., Morgan R.W., Reeder R.W., et al. Diastolic blood pressure threshold during pediatric cardiopulmonary resuscitation and survival outcomes: a multicenter validation study. Crit Care Med. 2023;51:91–102. doi: 10.1097/CCM.0000000000005715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friess S.H., Sutton R.M., Bhalala U., et al. Hemodynamic directed cardiopulmonary resuscitation improves short-term survival from ventricular fibrillation cardiac arrest. Crit Care Med. 2013;41:2698–2704. doi: 10.1097/CCM.0b013e318298ad6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lautz A.J., Morgan R.W., Karlsson M., et al. Hemodynamic-directed cardiopulmonary resuscitation improves neurologic outcomes and mitochondrial function in the heart and brain. Crit Care Med. 2019;47:e241–e249. doi: 10.1097/CCM.0000000000003620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kienzle M.F., Morgan R.W., Alvey J.S., et al. Clinician-reported physiologic monitoring of cardiopulmonary resuscitation quality during pediatric in-hospital cardiac arrest: a propensity-weighted cohort study. Resuscitation. 2023;188 doi: 10.1016/j.resuscitation.2023.109807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sutton R.M., French B., Meaney P.A., et al. Physiologic monitoring of CPR quality during adult cardiac arrest: a propensity-matched cohort study. Resuscitation. 2016;106:76–82. doi: 10.1016/j.resuscitation.2016.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abate S.M., Nega S., Basu B., et al. Global burden of out-of-hospital cardiac arrest in children: a systematic review, meta-analysis, and meta-regression. Pediatr Res. 2023;94:423–433. doi: 10.1038/s41390-022-02462-5. [DOI] [PubMed] [Google Scholar]
- 16.Ahn A., Nasir A., Malik H., et al. A pilot study examining the role of regional cerebral oxygen saturation monitoring as a marker of return of spontaneous circulation in shockable (VF/VT) and non-shockable (PEA/Asystole) causes of cardiac arrest. Resuscitation. 2013;84:1713–1716. doi: 10.1016/j.resuscitation.2013.07.026. [DOI] [PubMed] [Google Scholar]
- 17.Cournoyer A., Iseppon M., Chauny J.M., et al. Near-infrared spectroscopy monitoring during cardiac arrest: a systematic review and meta-analysis. Acad Emerg Med. 2016;23:851–862. doi: 10.1111/acem.12980. [DOI] [PubMed] [Google Scholar]
- 18.Ito N., Nanto S., Nagao K., et al. Regional cerebral oxygen saturation predicts poor neurological outcome in patients with out-of-hospital cardiac arrest. Resuscitation. 2010;81:1736–1737. doi: 10.1016/j.resuscitation.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 19.Newman D.H., Callaway C.W., Greenwald I.B., Freed J. Cerebral oximetry in out-of-hospital cardiac arrest: standard CPR rarely provides detectable hemoglobin-oxygen saturation to the frontal cortex. Resuscitation. 2004;63:189–194. doi: 10.1016/j.resuscitation.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 20.Parnia S., Nasir A., Shah C., et al. A feasibility study evaluating the role of cerebral oximetry in predicting return of spontaneous circulation in cardiac arrest. Resuscitation. 2012;83:982–985. doi: 10.1016/j.resuscitation.2012.01.039. [DOI] [PubMed] [Google Scholar]
- 21.Parnia S., Yang J., Nguyen R., et al. Cerebral oximetry during cardiac arrest: a multicenter study of neurologic outcomes and survival. Crit Care Med. 2016;44:1663–1674. doi: 10.1097/CCM.0000000000001723. [DOI] [PubMed] [Google Scholar]
- 22.Genbrugge C., De Deyne C., Eertmans W., et al. Cerebral saturation in cardiac arrest patients measured with near-infrared technology during pre-hospital advanced life support. Results from Copernicus I cohort study. Resuscitation. 2018;129:107–113. doi: 10.1016/j.resuscitation.2018.03.031. [DOI] [PubMed] [Google Scholar]
- 23.Pollard V., Prough D.S., DeMelo A.E., et al. Validation in volunteers of a near-infrared spectroscope for monitoring brain oxygenation in vivo. Anesth Analg. 1996;82:269–277. doi: 10.1097/00000539-199602000-00010. [DOI] [PubMed] [Google Scholar]
- 24.Pollard V.P. McGraw-Hill; 1998. Principles and Practice of Intensive Care Monitoring. [Google Scholar]
- 25.Genbrugge C., Eertmans W., Jans F., et al. Regional cerebral saturation monitoring during withdrawal of life support until death. Resuscitation. 2017;121:147–150. doi: 10.1016/j.resuscitation.2017.07.023. [DOI] [PubMed] [Google Scholar]
- 26.Vernieri F., Tibuzzi F., Pasqualetti P., et al. Transcranial Doppler and near-infrared spectroscopy can evaluate the hemodynamic effect of carotid artery occlusion. Stroke. 2004;35:64–70. doi: 10.1161/01.STR.0000106486.26626.E2. [DOI] [PubMed] [Google Scholar]
- 27.Taillefer M.C., Denault A.Y. Cerebral near-infrared spectroscopy in adult heart surgery: systematic review of its clinical efficacy. Can JAnaesth. 2005;52:79–87. doi: 10.1007/BF03018586. [DOI] [PubMed] [Google Scholar]
- 28.Shojima M, Watanabe E, Mayanagi Y. Cerebral blood oxygenation after cerebrospinal fluid removal in hydrocephalus measured by near infrared spectroscopy. Surg Neurol 2004;62(4):312-8; discussion 8. [DOI] [PubMed]
- 29.Gracias VH, Guillamondegui OD, Stiefel MF, et al. Cerebral cortical oxygenation: a pilot study. J Trauma 2004;56(3):469–72; discussion 72–4. [DOI] [PubMed]
- 30.Prosen G., Strnad M., Doniger S.J., et al. Cerebral tissue oximetry levels during prehospital management of cardiac arrest - A prospective observational study. Resuscitation. 2018;129:141–145. doi: 10.1016/j.resuscitation.2018.05.014. [DOI] [PubMed] [Google Scholar]
- 31.Nemoto E.M., Yonas H., Kassam A. Clinical experience with cerebral oximetry in stroke and cardiac arrest. Crit Care Med. 2000;28:1052–1054. doi: 10.1097/00003246-200004000-00023. [DOI] [PubMed] [Google Scholar]
- 32.Berve P.O., Hardig B.M., Skalhegg T., et al. Mechanical active compression-decompression versus standard mechanical cardiopulmonary resuscitation: a randomised haemodynamic out-of-hospital cardiac arrest study. Resuscitation. 2022;170:1–10. doi: 10.1016/j.resuscitation.2021.10.026. [DOI] [PubMed] [Google Scholar]
- 33.Skhirtladze-Dworschak K., Dworschak M. Cerebral oximetry and cardiac arrest. Semin Cardiothorac Vasc Anesth. 2013;17:269–275. doi: 10.1177/1089253213492861. [DOI] [PubMed] [Google Scholar]
- 34.Sanfilippo F., Murabito P., Messina A., et al. Cerebral regional oxygen saturation during cardiopulmonary resuscitation and return of spontaneous circulation: a systematic review and meta-analysis. Resuscitation. 2021;159:19–27. doi: 10.1016/j.resuscitation.2020.12.002. [DOI] [PubMed] [Google Scholar]
- 35.Liu Y., Jing K., Liu H., et al. Association between cerebral oximetry and return of spontaneous circulation following cardiac arrest: a systematic review and meta-analysis. PLoS One. 2020;15 doi: 10.1371/journal.pone.0234979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schnaubelt S., Sulzgruber P., Menger J., et al. Regional cerebral oxygen saturation during cardiopulmonary resuscitation as a predictor of return of spontaneous circulation and favourable neurological outcome - A review of the current literature. Resuscitation. 2018;125:39–47. doi: 10.1016/j.resuscitation.2018.01.028. [DOI] [PubMed] [Google Scholar]
- 37.Sanfilippo F., Serena G., Corredor C., et al. Cerebral oximetry and return of spontaneous circulation after cardiac arrest: a systematic review and meta-analysis. Resuscitation. 2015;94:67–72. doi: 10.1016/j.resuscitation.2015.06.023. [DOI] [PubMed] [Google Scholar]
- 38.Paradis N.A., Martin G.B., Rivers E.P., et al. Coronary perfusion pressure and the return of spontaneous circulation in human cardiopulmonary resuscitation. J Am Med Assoc. 1990;263:1106–1113. [PubMed] [Google Scholar]
- 39.Panchal A.R., Bartos J.A., Cabanas J.G., et al. Part 3: adult basic and advanced life support: 2020 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2020;142:S366–S468. doi: 10.1161/CIR.0000000000000916. [DOI] [PubMed] [Google Scholar]
- 40.Bhandari S., Doan J., Blackwood J., et al. Rhythm profiles and survival after out-of-hospital ventricular fibrillation cardiac arrest. Resuscitation. 2018;125:22–27. doi: 10.1016/j.resuscitation.2018.01.037. [DOI] [PubMed] [Google Scholar]
- 41.Brown G., Conway S., Ahmad M., et al. Role of artificial intelligence in defibrillators: a narrative review. Open Heart. 2022;9 doi: 10.1136/openhrt-2022-001976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kwok H., Coult J., Blackwood J., et al. Electrocardiogram-based pulse prediction during cardiopulmonary resuscitation. Resuscitation. 2020;147:104–111. doi: 10.1016/j.resuscitation.2019.11.021. [DOI] [PubMed] [Google Scholar]
- 43.Kwok H., Coult J., Blackwood J., et al. A method for continuous rhythm classification and early detection of ventricular fibrillation during CPR. Resuscitation. 2022;176:90–97. doi: 10.1016/j.resuscitation.2022.05.019. [DOI] [PubMed] [Google Scholar]
- 44.Coult J., Kwok H., Eftestol T., et al. Continuous assessment of ventricular fibrillation prognostic status during CPR: Implications for resuscitation. Resuscitation. 2022;179:152–162. doi: 10.1016/j.resuscitation.2022.08.015. [DOI] [PubMed] [Google Scholar]
- 45.Coult J., Yang B.Y., Kwok H., et al. Prediction of shock-refractory ventricular fibrillation during resuscitation of out-of-hospital cardiac arrest. Circulation. 2023;148:327–335. doi: 10.1161/CIRCULATIONAHA.122.063651. [DOI] [PubMed] [Google Scholar]
- 46.Idris A.H., Staples E.D., O'Brien D.J., et al. End-tidal carbon dioxide during extremely low cardiac output. Ann Emerg Med. 1994;23:568–572. doi: 10.1016/s0196-0644(94)70080-x. [DOI] [PubMed] [Google Scholar]
- 47.Ornato J.P., Garnett A.R., Glauser F.L. Relationship between cardiac output and the end-tidal carbon dioxide tension. Ann Emerg Med. 1990;19:1104–1106. doi: 10.1016/s0196-0644(05)81512-4. [DOI] [PubMed] [Google Scholar]
- 48.Gudipati C.V., Weil M.H., Bisera J., et al. Expired carbon dioxide: a noninvasive monitor of cardiopulmonary resuscitation. Circulation. 1988;77:234–239. doi: 10.1161/01.cir.77.1.234. [DOI] [PubMed] [Google Scholar]
- 49.Weil M.H., Bisera J., Trevino R.P., Rackow E.C. Cardiac output and end-tidal carbon dioxide. Crit Care Med. 1985;13:907–909. doi: 10.1097/00003246-198511000-00011. [DOI] [PubMed] [Google Scholar]
- 50.Sorcher J.L., Hunt E.A., Shaffner D.H., et al. Association of end-tidal carbon dioxide levels during cardiopulmonary resuscitation with survival in a large paediatric cohort. Resuscitation. 2022;170:316–323. doi: 10.1016/j.resuscitation.2021.10.029. [DOI] [PubMed] [Google Scholar]
- 51.Callaham M., Barton C. Prediction of outcome of cardiopulmonary resuscitation from end-tidal carbon dioxide concentration. Crit Care Med. 1990;18:358–362. doi: 10.1097/00003246-199004000-00002. [DOI] [PubMed] [Google Scholar]
- 52.Levine R.L., Wayne M.A., Miller C.C. End-tidal carbon dioxide and outcome of out-of-hospital cardiac arrest. N Engl J Med. 1997;337:301–306. doi: 10.1056/NEJM199707313370503. [DOI] [PubMed] [Google Scholar]
- 53.Wayne M.A., Levine R.L., Miller C.C. Use of end-tidal carbon dioxide to predict outcome in prehospital cardiac arrest. Ann Emerg Med. 1995;25:762–767. doi: 10.1016/s0196-0644(95)70204-0. [DOI] [PubMed] [Google Scholar]
- 54.Sanders A.B., Kern K.B., Otto C.W., et al. End-tidal carbon dioxide monitoring during cardiopulmonary resuscitation. A prognostic indicator for survival. J Am Med Assoc. 1989;262:1347–1351. [PubMed] [Google Scholar]
- 55.Pokorna M., Necas E., Kratochvil J., et al. A sudden increase in partial pressure end-tidal carbon dioxide (P(ET)CO(2)) at the moment of return of spontaneous circulation. J Emerg Med. 2010;38:614–621. doi: 10.1016/j.jemermed.2009.04.064. [DOI] [PubMed] [Google Scholar]
- 56.Hamrick J.T., Hamrick J.L., Bhalala U., et al. End-Tidal CO2-guided chest compression delivery improves survival in a neonatal asphyxial cardiac arrest model. Pediatr Crit Care Med. 2017;18:e575–e584. doi: 10.1097/PCC.0000000000001299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hamrick J.L., Hamrick J.T., Lee J.K., et al. Efficacy of chest compressions directed by end-tidal CO2 feedback in a pediatric resuscitation model of basic life support. J Am Heart Assoc. 2014;3 doi: 10.1161/JAHA.113.000450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sheak K.R., Wiebe D.J., Leary M., et al. Quantitative relationship between end-tidal carbon dioxide and CPR quality during both in-hospital and out-of-hospital cardiac arrest. Resuscitation. 2015;89:149–154. doi: 10.1016/j.resuscitation.2015.01.026. [DOI] [PubMed] [Google Scholar]
- 59.Golparvar M., Naddafnia H., Saghaei M. Evaluating the relationship between arterial blood pressure changes and indices of pulse oximetric plethysmography. Anesth Analg. 2002;95:1686–1690. doi: 10.1097/00000539-200212000-00040. table of contents. [DOI] [PubMed] [Google Scholar]
- 60.Shaltis P., Reisner A., Asada H. Calibration of the photoplethysmogram to arterial blood pressure: capabilities and limitations for continuous pressure monitoring. Conf Proc IEEE Eng Med Biol Soc. 2005;2005:3970–3973. doi: 10.1109/IEMBS.2005.1615331. [DOI] [PubMed] [Google Scholar]
- 61.Shelley K.H. Photoplethysmography: beyond the calculation of arterial oxygen saturation and heart rate. Anesth Analg. 2007;105:S31–S36. doi: 10.1213/01.ane.0000269512.82836.c9. [DOI] [PubMed] [Google Scholar]
- 62.Cannesson M., Attof Y., Rosamel P., et al. Respiratory variations in pulse oximetry plethysmographic waveform amplitude to predict fluid responsiveness in the operating room. Anesthesiology. 2007;106:1105–1111. doi: 10.1097/01.anes.0000267593.72744.20. [DOI] [PubMed] [Google Scholar]
- 63.Reisner A., Shaltis P.A., McCombie D., Asada H.H. Utility of the photoplethysmogram in circulatory monitoring. Anesthesiology. 2008;108:950–958. doi: 10.1097/ALN.0b013e31816c89e1. [DOI] [PubMed] [Google Scholar]
- 64.Pizov R., Eden A., Bystritski D., et al. Arterial and plethysmographic waveform analysis in anesthetized patients with hypovolemia. Anesthesiology. 2010;113:83–91. doi: 10.1097/ALN.0b013e3181da839f. [DOI] [PubMed] [Google Scholar]
- 65.Chen L., Reisner A.T., Gribok A., Reifman J. Is respiration-induced variation in the photoplethysmogram associated with major hypovolemia in patients with acute traumatic injuries? Shock. 2010;34:455–460. doi: 10.1097/SHK.0b013e3181dc07da. [DOI] [PubMed] [Google Scholar]
- 66.Convertino V.A., Howard J.T., Hinojosa-Laborde C., et al. Individual-specific, beat-to-beat trending of significant human blood loss: the compensatory reserve. Shock. 2015;44:27–32. doi: 10.1097/SHK.0000000000000323. [DOI] [PubMed] [Google Scholar]
- 67.Stewart C.L., Nawn C.D., Mulligan J., et al. Compensatory reserve for early and accurate prediction of hemodynamic compromise: case studies for clinical utility in acute care and physical performance. J Spec Oper Med. 2016;16:6–13. [PubMed] [Google Scholar]
- 68.Tanaka G., Sawada Y. Examination of normalized pulse volume-blood volume relationship: toward a more valid estimation of the finger sympathetic tone. Int J Psychophysiol. 2003;48:293–306. doi: 10.1016/s0167-8760(03)00056-4. [DOI] [PubMed] [Google Scholar]
- 69.Luginbuhl M., Reichlin F., Sigurdsson G.H., et al. Prediction of the haemodynamic response to tracheal intubation: comparison of laser-Doppler skin vasomotor reflex and pulse wave reflex. Br J Anaesth. 2002;89:389–397. doi: 10.1093/bja/89.3.389. [DOI] [PubMed] [Google Scholar]
- 70.Ezri T., Steinmetz A., Geva D., Szmuk P. Skin vasomotor reflex as a measure of depth of anesthesia. Anesthesiology. 1998;89:1281–1282. doi: 10.1097/00000542-199811000-00041. [DOI] [PubMed] [Google Scholar]
- 71.Dorlas J.C., Nijboer J.A. Photo-electric plethysmography as a monitoring device in anaesthesia. Application and interpretation. Br J Anaesth. 1985;57:524–530. doi: 10.1093/bja/57.5.524. [DOI] [PubMed] [Google Scholar]
- 72.Talke P., Nichols R.J., Jr., Traber D.L. Does measurement of systolic blood pressure with a pulse oximeter correlate with conventional methods? J Clin Monit. 1990;6:5–9. doi: 10.1007/BF02832176. [DOI] [PubMed] [Google Scholar]
- 73.Wallace C.T., Baker J.D., 3rd, Alpert C.C., et al. Comparison of blood pressure measurement by Doppler and by pulse oximetry techniques. Anesth Analg. 1987;66:1018–1019. [PubMed] [Google Scholar]
- 74.Xu J., Li C., Zheng L., et al. Pulse oximetry: a non-invasive, novel marker for the quality of chest compressions in porcine models of cardiac arrest. PLoS One. 2015;10 doi: 10.1371/journal.pone.0139707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wijshoff R.W., van der Sar T., Peeters W.H., et al. Detection of a spontaneous pulse in photoplethysmograms during automated cardiopulmonary resuscitation in a porcine model. Resuscitation. 2013;84:1625–1632. doi: 10.1016/j.resuscitation.2013.07.019. [DOI] [PubMed] [Google Scholar]
- 76.Gaspari R., Weekes A., Adhikari S., et al. Emergency department point-of-care ultrasound in out-of-hospital and in-ED cardiac arrest. Resuscitation. 2016;109:33–39. doi: 10.1016/j.resuscitation.2016.09.018. [DOI] [PubMed] [Google Scholar]
- 77.Hwang S.O., Zhao P.G., Choi H.J., et al. Compression of the left ventricular outflow tract during cardiopulmonary resuscitation. Acad Emerg Med. 2009;16:928–933. doi: 10.1111/j.1553-2712.2009.00497.x. [DOI] [PubMed] [Google Scholar]
- 78.Shin J., Rhee J.E., Kim K. Is the inter-nipple line the correct hand position for effective chest compression in adult cardiopulmonary resuscitation? Resuscitation. 2007;75:305–310. doi: 10.1016/j.resuscitation.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 79.Nestaas S., Stensaeth K.H., Rosseland V., Kramer-Johansen J. Radiological assessment of chest compression point and achievable compression depth in cardiac patients. Scand J Trauma Resusc Emerg Med. 2016;24:54. doi: 10.1186/s13049-016-0245-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rea T.D., Cook A.J., Stiell I.G., et al. Predicting survival after out-of-hospital cardiac arrest: role of the Utstein data elements. Ann Emerg Med. 2010;55:249–257. doi: 10.1016/j.annemergmed.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 81.Daya M.R., Leroux B.G., Dorian P., et al. Survival after intravenous versus intraosseous amiodarone, lidocaine, or placebo in out-of-hospital shock-refractory cardiac arrest. Circulation. 2020;141:188–198. doi: 10.1161/CIRCULATIONAHA.119.042240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Naim M.Y., Griffis H.M., Berg R.A., et al. Compression-only versus rescue-breathing cardiopulmonary resuscitation after pediatric out-of-hospital cardiac arrest. J Am Coll Cardiol. 2021;78:1042–1052. doi: 10.1016/j.jacc.2021.06.042. [DOI] [PubMed] [Google Scholar]
- 83.Chopra A.S., Wong N., Ziegler C.P., Morrison L.J. Systematic review and meta-analysis of hemodynamic-directed feedback during cardiopulmonary resuscitation in cardiac arrest. Resuscitation. 2016;101:102–107. doi: 10.1016/j.resuscitation.2016.01.025. [DOI] [PubMed] [Google Scholar]
- 84.Haywood K., Whitehead L., Nadkarni V.M., et al. COSCA (Core Outcome Set for Cardiac Arrest) in adults: an advisory statement from the International Liaison Committee on Resuscitation. Resuscitation. 2018;127:147–163. doi: 10.1016/j.resuscitation.2018.03.022. [DOI] [PubMed] [Google Scholar]
- 85.Leturiondo M., Ruiz de Gauna S., Ruiz J.M., et al. Influence of chest compression artefact on capnogram-based ventilation detection during out-of-hospital cardiopulmonary resuscitation. Resuscitation. 2018;124:63–68. doi: 10.1016/j.resuscitation.2017.12.013. [DOI] [PubMed] [Google Scholar]
- 86.Leturiondo M., Ruiz de Gauna S., Gutierrez J.J., et al. Chest compressions induce errors in end-tidal carbon dioxide measurement. Resuscitation. 2020;153:195–201. doi: 10.1016/j.resuscitation.2020.05.029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.