Abstract
Parkinsonism-hyperpyrexia syndrome (PHS) is a rare neurological emergency that shares clinical features with neuroleptic malignant syndrome. It is usually due to sudden deprivation of dopaminergic treatment, although there are cases related to failure of the deep brain stimulation system.
Keywords: Deep brain stimulation, Malfunction, Parkinsonism-hyperpyrexia syndrome
Parkinsonism–hyperpyrexia syndrome (PHS) shares clinical features with neuroleptic malignant syndrome (NMS) and is characterized by high temperature, muscle stiffness, autonomic nervous system disorders, confusion, and increased creatine kinase (CK) [1]. Its incidence is estimated to be approximately 0.3%, with a mortality rate of 4%, being more frequent in patients with advanced Parkinson's disease (PD) and high doses of levodopa [2].
The main trigger for PHS is usually the sudden discontinuation of dopaminergic medications, especially levodopa, although cases have also been documented after discontinuation of catechol-O-methyltransferase (COMT) inhibitors. This abrupt interruption affects neurotransmission pathways in the hypothalamus, the nigrostriatal system, and the mesolimbic dopaminergic cortical system. However, it can also occur in the context of systemic conditions that affect drug absorption, infections, trauma, dehydration, and stress [1].
The treatment of PHS focuses on correcting the underlying cause and adjusting the dose of levodopa, gradually reestablishing the previous dosing regimen if necessary. Alternatively, if the enteral route is not feasible, the use of other dopamine agonists such as rotigotine or subcutaneous apomorphine pump may be considered [3]. Likewise, the pharmacological management of PHS includes other drugs such as dantrolene (which reduces stiffness by stabilizing the sarcoplasmic membrane of muscle fibers), bromocriptine and methylprednisolone, although scientific evidence of its use is limited [2], [3], [4].
Below, two cases of PHS secondary to failure of the deep brain stimulator in PD patients are reported.
1. Case 1
A 67-year-old man diagnosed with PD in an advanced stage underwent surgery in October 2016 for the placement of octopolar electrodes on subthalamic nucleus (STN) with the rechargeable Vercise® system (Boston Scientific). In April 2021, a technical failure that prevented the neurostimulator from connecting to the programmer control led to a second surgical procedure, replacing the neurostimulator with the Genus® system (Boston Scientific). The last DBS setting before admission was as follows:
-
•
Left: 3 (-) 5 (+); 4.5 mA; 60 mcs; 130 Hz.
-
•
Right: 10 (-) 12 (+); 4.5 mA; 60 mcs; 130 Hz.
Prior to his hospitalization, his usual medication included Sinemet 250 mg half a tablet every 12 h and amantadine 100 mg 1 tablet every 24 h.
At the end of November 2021, the patient came to the emergency department for a marked worsening of parkinsonian symptomatology of 24 h of evolution, suddenly presenting abruptly high temperature with increased stiffness and tremor. The neurostimulator was checked and it was confirmed that the battery was discharged. The person responsible for her care was charging the battery every 4 to 5 days, and the last recharge was 4 days before the emergency room visit.
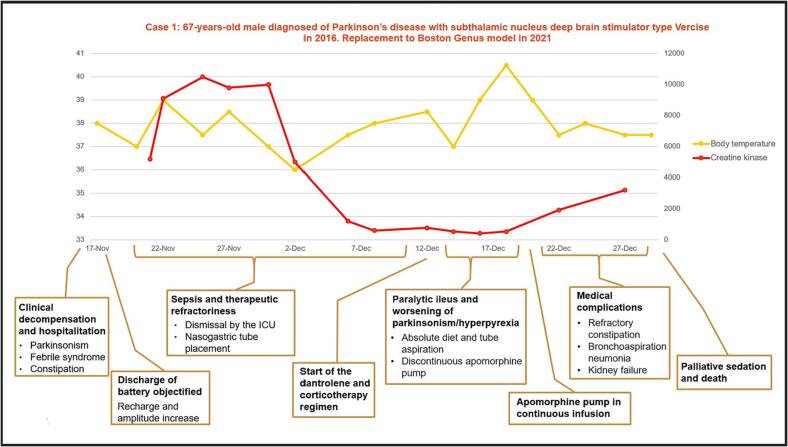
On admission, he did not present leukocytosis, no other analytical alterations suggesting the presence of infection at any level. The general physical examination was normal, and the neurological examination showed a marked worsening of parkinsonian symptoms with severe generalized rigidity, marked bradykinesia and inability to walk, without the presence of meningeal signs. Cranial CT scan was performed with no findings of acute pathology. On admission, empirical antibiotic treatment was started due to a temperature of 37.8 °C with no identified focus, and the neurostimulator was recharged establishing an amperage of 4.6 mA bilaterally and adding levodopa treatment of 325 mg every 8 h, without observing clinical improvement. CK levels were already very elevated, with figures of 5181U/L, with progressive increase in the following days, reaching 10476U/L. Lumbar puncture was normal, with negative film array and EEG showed no epileptic activity. During his stay, antiviral and antifungal treatment was added, despite which he remained with temperatures around 39 °C. Treatments with benzodiazepines, dantrolene and corticosteroids that were tried during admission were not effective, and apomorphin subcutaneous formulation in continuous infusion achieved a partial and transitory improvement of hyperthermia and CK levels. The associated complications, which included paralytic ileus, renal failure and metabolic alterations, prevented the patient's adequate recovery, leading to the fatal outcome (Fig. 1).
Fig. 1.
Timeline of patient 1.
2. Case 2
A 73-year-old man was diagnosed with advanced-stage PD with a NST implant using the Kinetra® system (Medtronic) in November 2005. The last configuration of the system was as follows:
-
•
Left side: 2 (-); 3.3 V; 60 mcs; 180 Hz.
-
•
Right side: 6 (-); 3.3 V; 90 mcs; 180 Hz.
Before his admission, his usual treatment consisted of a combination of levodopa 100 mg with carbidopa and entacapone administered in 4–5 daily doses. He was admitted due to subacute worsening of body stiffness, marked bradykinesia, inability to swallow levodopa tablets, a high temperature of up to 38.9 °C, and profuse respiratory secretions. Due to lack of response to antibiotic treatment, normal brain imaging tests, normal cerebrospinal fluid analysis, and observation of a discharge on the neurostimulator battery, PHS syndrome was considered.
Despite increases in the dose of dopaminergic therapy, the patient experienced respiratory deterioration, dysautonomic symptoms and renal failure due to an increase in CK (from 530U/L to 7500 U/L over 7 days), requiring transfer to the ICU. After stabilization, the neurostimulator was replaced with the Libra XP® model (Abbott) without initial complications.
During his stay, he received respiratory and motor rehabilitation, which allowed his discharge at home with an adjustment in the dopaminergic treatment using Madopar® 200/500 mg and the following settings for the neurostimulator:
-
•
Left side: 2 (-); 3.8 V; 65 mcs; 180 Hz.
-
•
Right side: 6 (-); 3.7 V; 90 mcs; 180 Hz.
Although the sudden withdrawal of antiparkinson drugs, particularly levodopa [5], is the most common trigger of PHS, the scientific literature describes cases in which a malfunction of the DBS device is the cause [1].
From a surgical perspective, as the literature points out, in situations where the implantable pulse generator (IPG) is depleted, replacing it appears to be more effective than medical treatment alone [5]. In addition, increasing the amperage and pulse width has been shown to provide clinical benefits [4]. However, the rapid onset of PHS can lead to serious complications, such as aspiration pneumonia, renal failure due to rhabdomyolysis, disseminated intravascular coagulation, and venous thromboembolism. Regarding the indications for the surgical replacement procedure, there are no specific recommendations on when it should be performed [1], [2], [5], however, some patients evolve unfavorably if the neurostimulator replacement is delayed, being unable to recover their original baseline state. From our experience in clinical practice, we tend to replace the neurostimulator battery when it is close to running out without waiting for it to run out completely.
The rarity of PHS caused by the depletion of the DBS battery has resulted in the absence of specific guidelines for the management of these patients. However, considering the negative repercussions of this situation, it is crucial to adopt a proactive approach to establish guidelines that prevent this condition in patients with PD.
Ethical statement: This study has been conducted in accordance with the Declaration of Helsinki. All patients or their families (in cases of patient death) have provided informed consent prior to being included in the study.
CRediT authorship contribution statement
Manuel Díaz Castela: Writing – review & editing, Writing – original draft. Patricia Prendes Fernández: Writing – review & editing. Sonia Heres Bruck: Resources. Esther Suárez San Martín: Resources. Ciara García Fernández: Resources. Javier Sol Álvarez: Resources. Beatriz Lozano Aragoneses: Resources. Antonio Sáiz Ayala: Resources. Elena Santamarta Liébana: Resources. Juan Álvarez Carriles: Resources. Lorena González Álvarez: Resources. Marta Blázquez Estrada: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Methodology, Data curation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Raquel Martínez Campo, medical writer, for her collaboration.
References
- 1.Azar J, Elinav H, Safadi R, Soliman M. Malignant deep brain stimulator withdrawal syndrome. BMJ Case Rep CP. 1 de mayo de 2019;12(5):e229122. [DOI] [PMC free article] [PubMed]
- 2.Simonet C., Tolosa E., Camara A., Valldeoriola F. Emergencies and critical issues in parkinson’s disease. Pract Neurol. Febrero De. 2020;20(1):15. doi: 10.1136/practneurol-2018-002075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Newman E.J., Grosset D.G., Kennedy P.G. The parkinsonism-hyperpyrexia syndrome. Neurocrit Care. 2009;10(1):136–140. doi: 10.1007/s12028-008-9125-4. [DOI] [PubMed] [Google Scholar]
- 4.Akçakaya M.O., Akçakaya N.H., Kasımcan M.Ö., Kırış T. Life-threatening parkinsonism-hyperpyrexia syndrome following bilateral deep brain stimulation of the subthalamic nucleus. Neurol Neurochir Pol. Marzo De. 2018;52(2):289–292. doi: 10.1016/j.pjnns.2017.11.012. [DOI] [PubMed] [Google Scholar]
- 5.Reuter S., Deuschl G., Falk D., Mehdorn M., Witt K. Uncoupling of dopaminergic and subthalamic stimulation: life-threatening DBS withdrawal syndrome: life-threatening DBS withdrawal syndrome. Mov Disord. Septiembre De. 2015;30(10):1407–1413. doi: 10.1002/mds.26324. [DOI] [PubMed] [Google Scholar]