Abstract
This study aimed to compare the residue depletion of gamithromycin in yellow-feather and white-feather broilers, using Sanhuang and Arbor Acres chickens as typical examples, respectively. Each breed (54 chickens) received a single subcutaneous dose of gamithromycin at 7.5 mg/kg bodyweight (BW). Tissues, including muscle, skin + fat, liver, kidney, and injection site, were collected at 6 h, 3, 5, 7, 10, 14, 21, 28, and 35 d postdrug administration. Gamithromycin concentrations in these tissues were determined using ultra‐performance liquid chromatography–tandem mass spectrometry (UPLC‐MS/MS). The kinetics of gamithromycin were analyzed in different tissues using a noncompartmental method in the Phoenix software. Differences were observed in gamithromycin concentrations and kinetic characteristics in both breeds of chickens, with higher residue concentrations and longer residue times found in yellow-feathered broilers. In Sanhuang broilers, the elimination rates of gamithromycin followed this order: injection site > muscle > liver > kidney > skin + fat. The corresponding elimination half-lives (t1/2λzs) in these samples were 1.22, 1.30, 1.71, 2.04, and 2.52 d, respectively. In contrast, in Arbor Acres broilers, a different order was noted: muscle > injection site > kidney > liver > skin + fat, with corresponding t1/2λzs of 1, 1.23, 1.88, 1.93, and 2.21 d, respectively. These differences may be related to variations in pigments in various tissues of chickens of the 2 breeds. However, further investigations are warranted to discern the underlying reasons.
Key words: gamithromycin, residue depletion, yellow-feather broiler, white-feather broiler, comparison
INTRODUCTION
Poultry farming has achieved global prominence, with chicken meat surpassing beef and pork as the most widely consumed meat worldwide (Chowdhury et al., 2023). In China, chicken consumption is predominantly split between deep-processed white-feathered chickens and live sales of yellow-feathered broilers. Notably, these 2 segments contribute almost equally to the overall chicken consumption patterns in China (Yuan et al., 2022). Feather color variations are prevalent in numerous domesticated bird species, stemming from genetic mutations and rigorous selective breeding practices (Bartels, 2003). In essence, domestic chickens exhibiting different feather colors may exhibit significant differences at both molecular and visible levels.
Intensive breeding has become the predominant model for both yellow-feathered and white-feathered broilers (Rugani et al., 2023). However, this approach raises concerns, as chickens are at an increased risk of microbial infections due to suboptimal sanitary conditions during production and the high-density environment in which they are raised (Wang et al., 2022). These infections not only jeopardize the welfare of chickens but also result in substantial economic losses for farmers. Consequently, the use of effective antibacterial agents becomes imperative to mitigate these losses.
Macrolide antibiotics are commonly employed for treating bacterial infections in chickens. This antibiotic class includes structurally similar compounds classified as macrocyclic lactones, characterized by a lactone ring with 12 to 20 carbon atoms (Watteyn et al., 2013). Macrolides are primarily effective against gram-positive microorganisms and various intracellular bacteria. They exert their mode of action at the 23S ribosomal RNA in the 50S subunit of ribosomes (Arsic et al., 2018). By binding to different bases in the peptidyl transferase center, they impede protein elongation during the translocation process. In broiler production, macrolides like tylosin, tilmicosin, and erythromycin are used to treat the infections caused by Mycoplasma, Ornithobacterium rhinotracheale, P. multocida and sometimes Clostridium perfringens (Lhermie et al., 2020).
Gamithromycin, a semi-synthetic 15-membered macrolide, features a distinctive alkylated nitrogen at the 7a carbon of the lactone ring, a characteristic commonly associated with azalides (Watteyn et al., 2013). Approved for use in cattle, pigs, and sheep, it is specifically indicated for the treatment of respiratory disease and infectious pododermatitis (EMA, 2017). While not officially approved in poultry, studies have demonstrated the efficacy of subcutaneously injected gamithromycin against Ornithobacterium rhinotracheale in turkey (Watteyn et al., 2015; Watteyn et al., 2016). Furthermore, investigations into the pharmacokinetics of gamithromycin in broilers and turkeys revealed an excellent profile, including broad distribution and complete absorption following subcutaneous injection (Watteyn et al., 2013; Watteyn et al., 2015). These findings suggest the potential utility of gamithromycin for treating respiratory infections in poultry.
Considering its prolonged elimination half-life (34.9 and 92.6 h in plasma and lung, respectively, after a single subcutaneous injection of 6 mg/kg BW in turkeys) (Watteyn et al., 2015), gamithromycin is anticipated to endure in poultry tissues for an extended period. Consequently, it becomes imperative to assess its residue depletion in poultry species. Currently, no data are available regarding gamithromycin residue in any poultry species. Hence, this study aims to compare the residue depletion of gamithromycin in both yellow-feathered and white-feathered broilers.
MATERIALS AND METHODS
Drugs and Reagents
The reference substance of gamithromycin (Lot No. HZD202201; purity: 99.1%) was donated by Luoyang Huizhong Animal Medicine Co., Ltd. (Luoyang, Henan). Gamithromycin injection (Lot No. 1304001; 50 mL: 7.5 g) was sourced from Qilu Animal Health Products Co., Ltd. (Jinan, Shandong). Methanol and acetonitrile of chromatographical grade were procured from Thermo Fisher Scientific (Shanghai, China), while other reagents, including n-hexane, acetic acid, ammonium acetate, and concentrated ammonia, were of analytical grade and obtained from Shanghai Aladdin Technology Co., Ltd. (Shanghai, China).
Experimental Animals
We obtained a total of 120 healthy 1-day-old broiler chickens from farms in the Luoyang area, representing 2 distinct breeds: Arbor Acres and Sanhuang Chicken, with 60 individuals of each breed. These breeds serve as typical examples of white-feather and yellow-feather broilers commonly raised in China. Each breed was equally housed in 4 wire cages, equipped with automatic water dispensers and troughs. Throughout the study, the broilers adhered to strict guidelines outlined in the Modern Broiler Production Manual. Their diet excluded antibiotics and coccidiostats, providing all broilers with unrestricted access to feed and water. The animal room maintained a temperature that gradually decreased from 34 to 24°C (d 1–21), subsequently remaining constant at 21°C from d 22 onwards. Humidity was consistently held at 60 ± 5%, with daily illumination lasting for 12 h. All procedures conducted in this study received ethical approval from the Institutional Animal Care and Use Committee (IACUC) of Henan University of Science and Technology, under the reference number 20220092.
Administration and Sampling
All broilers underwent a 6-d acclimatization period. On the 7th d of age, each breed was randomly and evenly divided into 10 groups. Nine of these groups received a single subcutaneous injection at a dosage of 7.5 mg/kg body weight (BW), a dosage commonly employed in clinical practice. The remaining groups served as controls, providing blank tissue. The injection site was beneath the skin of the neck, specifically targeting the subcutaneous muscle in that region. For each breed, one group was randomly euthanized via cervical dislocation at 6 h, 3, 5, 7, 10, 14, 21, 28, and 35 d postdrug administration. Samples, including liver, kidney, muscle, skin plus fat, and the muscle at injection site, were collected at each specified time point. The untreated group was euthanized at the last time point, and corresponding tissues were collected as blank samples.
Sample Preparation
Tissue sample processing followed a previously published method with slight modifications (Yan et al., 2021). Briefly, 1.0 g of tissue homogenate was accurately weighed and placed into a 10 mL centrifuge tube, followed by the addition of 3 mL of acetonitrile. After vortexing for 5 min and sonication for 10 min, the mixture underwent centrifugation for 10 min at 9,000 × g. The resulting supernatant was then transferred into a 15 mL polypropylene centrifuge tube, and 3 mL of acetonitrile was added to the tissue precipitate for repeated extraction. The collected supernatants were combined, and 3 mL of n-hexane saturated in acetonitrile was introduced. Following vortexing for 3 min and centrifugation at 5,000 × g for 10 min, the upper organic layer was discarded, and the lower layer was reserved for subsequent purification using an Oasis MCX solid-phase extraction (SPE) column (60 mg/3 mL, 30 µm; Waters Corporation, Shanghai, China).
The SPE column purification procedure commenced with the activation and equilibration of the column using 3 mL of methanol and 3 mL of 0.1 mol/L ammonium acetate solution (pH = 4.5). Subsequently, the reserved lower layer was passed through the SPE column. The loaded column was then washed with 6 mL of 0.1 mol/L ammonium acetate solution (pH = 4.5) and 6 mL of acetonitrile-methanol (70:30; v/v). Finally, elution was carried out with 3 mL of a mixed solution containing 5% ammonia in acetonitrile-methanol (70:30; v/v). The elution solvent was evaporated using a stream of nitrogen at 60°C and reconstituted in 2 mL of the mobile phase. After filtering through a 0.22-μm filter, a 2 μL aliquot was injected into the ultra‐performance liquid chromatography–tandem mass spectrometry (UPLC‐MS/MS) system for analysis.
UPLC-MS/MS Analysis
The analysis utilized an ACQUITY H-Class Series UPLC system (Waters Corporation; Shanghai, China) for injecting 2 μL aliquots of processed samples onto an ACQUITY UPLC BEH C18 Column (1.7 µm, 2.1 mm × 50 mm; Waters Corporation; Shanghai, China) maintained at 28°C. The mobile phase, comprising methanol and a 10 mmol/L ammonium acetate solution (pH = 3.6) in a 4:6 (v/v) ratio, was delivered at a flow rate of 0.2 mL/min.
For mass spectrometric analysis, the XEVO-TQ-S micro triple quadrupole mass spectrometer (Waters Corporation; Shanghai, China) was operated in positive electrospray ionization mode. The specific parameters were set as follows: capillary voltage at 1.5 kV, ion source temperature at 150°C, desolvation temperature at 500°C, desolvation flow at 1,000 L/h, and collision gas pressure at 7 psi. The mass spectrometer was employed in MS/MS mode using multiple reactions monitoring (MRM) mode. A summary of the adjusted MS conditions and parameters can be found in Table 1.
Table 1.
Liquid chromatography-tandem mass spectrometry: optimized tune parameters of gamithromycin.
| Qualitative ion pair (m/z) | Quantitative ion transitions (m/z) | Cone voltage (v) | Collision energy (eV) |
|---|---|---|---|
| 777.5>158.3 | 777.5>619.4 | 35.0 | 46.0 |
| 777.5>619.4 | 36.0 |
Validation of Determination Method
A standard stock solution of gamithromycin (1,000 µg/mL) was prepared using acetonitrile and stored at -20°C. Calibration standards for all collected tissue samples were established by spiking serial working solutions into blank samples. To assess the accuracy and precision of the methods, 5 replicate blank samples were spiked with 3 distinct concentrations of gamithromycin. In the kidney, spiked concentrations were set at 100, 200, and 1,000 ng/g. For muscle, skin + fat, and the injection site, spiked concentrations were 20, 100, and 2,000 ng/g, while in the liver, concentrations were 100, 1,000, and 20,000 ng/g. Each replicate sample underwent continuous measurement over 3 d to calculate the recovery rate, intra-day, and inter-day coefficients of variation. The limits of detection (LOD) and quantification (LOQ) were determined based on signal-to-noise (S/N) ratios, with thresholds set at ≥ 3 for LOD and ≥ 10 for LOQ.
Data Analysis
Concentrations in all collected tissues were presented as Mean ± SD. Statistical analyses were carried out using SPSS software (version 22.0; IBM, Armonk, NY), employing an independent samples t-test to assess gamithromycin tissue concentrations at each sampling point in both chicken breeds.
For pharmacokinetic analysis, a non-compartment model analysis (NCA) was employed in Phoenix software. This analysis determined the elimination equation, terminal half-life (t1/2λz), area under the concentration-time curve (AUC), and mean residence time (MRT) of gamithromycin in each collected tissue based on the average concentration-versus-time data. Additionally, the elimination curve was graphically represented using Origin software (version 2020; OriginLab Corporation, Northampton, MA).
RESULTS
Validation of Analytical Methods
No discernible differences were noted in the chromatographic behavior and mass spectrum response of gamithromycin within identical tissues from 2 broiler breeds. Consequently, for subsequent procedures, including the determination of calibration standards, recovery rates, coefficients of variation, LOD, and LOQ, blended blank tissues from both broiler breeds were utilized.
The extraction and detection method employed in this study effectively circumvents endogenous interference, showcasing high selectivity. Table 2 illustrates the robust linear range of gamithromycin across different samples at varying concentration levels, with correlation coefficients (R2) consistently exceeding 0.9949. The LOD ranged from 2 to 5 ng/g across all samples, while the LOQ fell within the range of 5 to 10 ng/g.
Table 2.
The results of the LOD, LOQ, linear range, regression equation, and correlation coefficient (R2) of all samples.
| Samples | LOD (ng/g) | LOQ (ng/g) | Linear range (ng/g) | Regression Equation (R2) |
|---|---|---|---|---|
| Muscle | 2 | 5 | 5∼2000 | Y=170.679X+2488.11 (0.9984) |
| Skin and Fat | 2 | 5 | 5∼5000 | Y=354.716X-369.457 (0.9964) |
| Liver | 5 | 10 | 10∼20000 | Y=294.476X-1104.43 (0.9949) |
| Kidney | 5 | 10 | 10∼7000 | Y=375.858X -1637.48 (0.9991) |
| Injection site | 2 | 5 | 5∼10000 | Y= 359.538X+46.9288 (0.9985) |
In instances where gamithromycin concentrations surpassed the upper limit of quantification, a mobile phase-based dilution approach was applied to ensure the accuracy of sample quantification. Recovery rates for different gamithromycin concentrations in various samples ranged from 83.13 to 112.70% (Table 3). Both intra-day and inter-day coefficients of variation were below 9.06 and 7.12%, respectively, as detailed in Table 3.
Table 3.
Recovery rates, intra-day and inter-day coefficients of variation at different spiked concentrations of gamithromycin in blended tissues sourced from both broiler breeds.
| Samples | Concentrations (ng/g) | Recovery (%) | Intra-day CV (%) | Inter-day CV (%) |
|---|---|---|---|---|
| Muscle | 20 | 90.89 ± 2.41 | 1.19-4.26 | 2.65 |
| 100 | 93.91 ±1.80 | 1.73-2.38 | 1.91 | |
| 2,000 | 97.88 ± 2.94 | 2.53-4.62 | 3.00 | |
| Skin and Fat | 20 | 109.57 ± 5.29 | 2.63-6.90 | 4.82 |
| 100 | 106.37 ± 5.86 | 3.43-6.52 | 5.51 | |
| 2,000 | 108.34 ± 7.71 | 2.53-6.90 | 7.12 | |
| Liver | 100 | 83.13 ± 5.41 | 5.59-8.16 | 6.50 |
| 1,000 | 98.07 ± 5.38 | 2.31-5.99 | 5.48 | |
| 20,000 | 112.70 ± 6.13 | 1.22-5.06 | 5.44 | |
| Kidney | 100 | 92.43 ± 3.18 | 0.84-6.57 | 3.44 |
| 200 | 103.20 ± 6.79 | 2.06-9.06 | 6.57 | |
| 1,000 | 111.44 ± 6.14 | 4.40-7.54 | 5.51 | |
| Injection site | 20 | 111.72 ± 4.72 | 2.26-6.81 | 4.23 |
| 100 | 107.95 ± 4.83 | 4.72-5.37 | 4.47 | |
| 2,000 | 100.15 ± 6.39 | 5.17-7.46 | 6.38 |
Residue Depletion
Throughout the experiment, all broilers exhibited robust health, devoid of any discernible adverse reactions. Normal water and food intake were consistently recorded. During dissection, all tissues and organs were found to be in excellent condition, displaying no lesions. Importantly, none of the samples from the untreated chickens tested positive for gamithromycin.
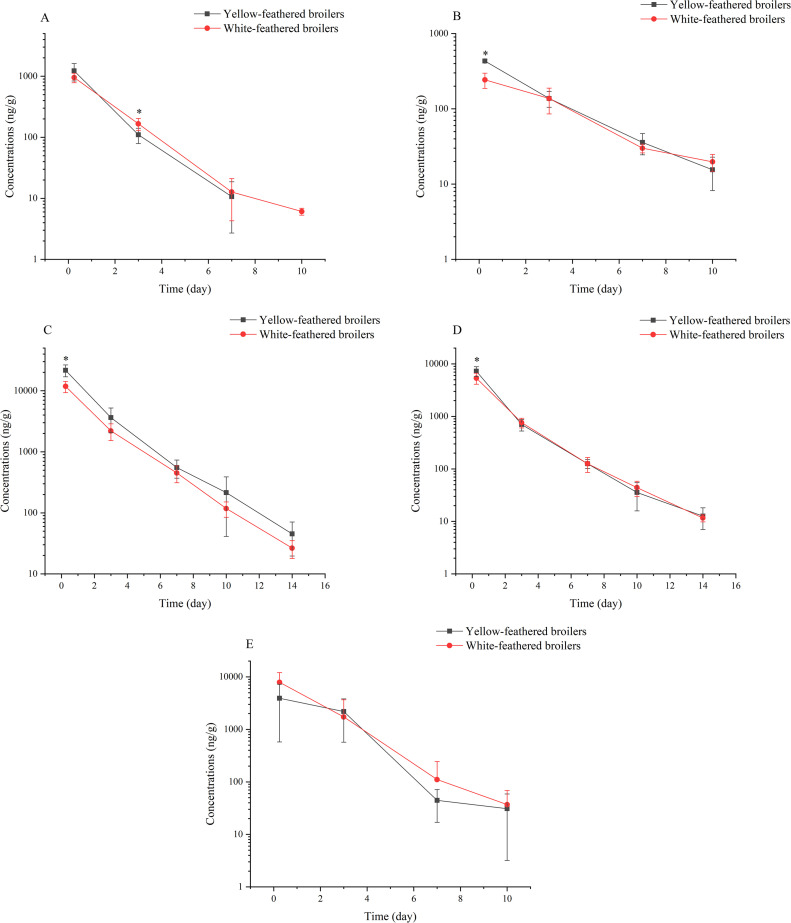
Mean concentration values in each tissue at the same sampling time point were compared after a single subcutaneous injection of gamithromycin at 7.5 mg/kg BW in chickens with both feather colors. Except for specific time points in the liver, kidney, and skin plus fat (0.25 d after administration), as well as in muscle (3 d after administration), no significant differences were observed in the concentrations of gamithromycin in both breeds of chickens (Figure 1). The maximum average concentration was identified in the liver, reaching 21,664.2 ng/g. Furthermore, after a single subcutaneous injection, the concentration of gamithromycin in the 5 sampled tissues at the initial sampling point (6 h after administration) was the highest.
Figure 1.
Comparison of gamithromycin concentrations (ng/g; Mean ± SD) in various tissues of yellow-feathered and white-feathered broilers following a single subcutaneous injection at 7.5 mg/kg BW: (A) muscle, (B) skin + fat, (C) liver, (D) kidney, and (E) injection site.
Table 4 presents the essential pharmacokinetic parameters derived from a non-compartmental model using the average concentration versus time data. In yellow-feathered broilers, the elimination rates of gamithromycin followed this order: injection site > muscle > liver > kidney > skin + fat. The corresponding t1/2λzs in these samples are 1.22, 1.30, 1.71, 2.04, and 2.52 d, respectively. Contrastingly, in white-feathered broilers, a different order was noted: muscle > injection site > kidney > liver > skin + fat, with corresponding elimination half-lives of 1, 1.23, 1.88, 1.93, and 2.21 d, respectively.
Table 4.
Comparations of the elimination equation, elimination half-life (t1/2λz), the area under the concentration-time curve (AUC), and mean residence time (MRT) for gamithromycin in yellow-feathered and white-feathered broilers.
| Broiler breeds | Samples | Elimination equation | t1/2λz (day) | AUC (day·ng/g) | MRT (day) |
|---|---|---|---|---|---|
| Yellow-feathered broilers | Muscle | C = 1115.1e−0.53t (R2=0.9733) |
1.30 | 2,189.056 | 1.16 |
| Skin + fat | C =255.8e−0.27t (R2=0.973) |
2.52 | 1,056.695 | 3.19 | |
| Liver | C = 13734.6e−0.40t (R2=0.999) |
1.71 | 28,938 | 1.51 | |
| Kidney | C = 6402e−0.34t (R2=0.9998) |
2.04 | 12,067.41 | 1.26 | |
| Injection site | C = 9043.3e−0.57t (R2=0.9887) |
1.22 | 19,279.73 | 1.26 | |
| White-feathered broilers | Muscle | C = 1525.5e−0.69t (R2=0.9865) |
1.00 | 2,434.36 | 0.75 |
| Skin + fat | C = 478.5e−0.31t (R2=0.9973) |
2.21 | 1,367.38 | 2.41 | |
| Liver | C = 25482.4e−0.36t (R2=0.9964) |
1.93 | 50,845.74 | 1.36 | |
| Kidney | C = 8992.3e−0.37t (R2=0.9858) |
1.88 | 15,006.05 | 1.00 | |
| Injection site | C = 4138e−0.57t (R2=0.9172) |
1.23 | 14,044.94 | 1.83 |
C, concentration; t, time after the last dosing.
DISCUSSION
In this study, we conducted a comparative analysis of gamithromycin residue depletion in broiler chickens with distinct feather colors. Following a single subcutaneous injection of 7.5 mg/kg BW of gamithromycin to yellow-feathered chickens, we observed varied peak concentrations across different tissues, ranked as followed: liver (21,664.2 ng/g), kidney (7,268.6 ng/g), injection site (3,924.8 ng/g), muscle (1,225.2 ng/g), and skin + fat (431.3 ng/g) (Table 4). In white-feathered broilers, the order of kidneys and injection sites was reversed, while the order of other tissues remained consistent with that of yellow-feathered broilers, showing peak concentrations of 11,791, 7,878.7, 5,361.8, 951.5, and 242.9 ng/g, respectively (Table 4). The comparison indicates that, excluding the injection site, the peak concentrations of gamithromycin in all other tissues of yellow-feathered broilers surpass those in the corresponding tissues of white-feathered broilers.
An additional noteworthy discovery in broilers with 2 distinct feather colors is that, at the initial sampling time point (6 h postadministration), concentrations in all 5 collected tissues reached peak levels. In a prior plasma pharmacokinetic study in Ross broiler chickens, the absorption half-life of gamithromycin was determined to be 0.021 h after a single subcutaneous injection at 6 mg/kg BW. And the plasma peak concentration of 889.46 ng/mL was observed around 0.13 h after administration (Watteyn et al., 2013). It is essential to note that the present study design, focused on comparing gamithromycin residue depletion in yellow-feathered and white-feathered broilers, did not include time points specifically aimed at characterizing gamithromycin absorption.
Beyond variations in tissue concentrations of gamithromycin, distinctions in the gamithromycin kinetics were evident between the 2 breeds of broiler chickens. In both breeds, the slowest elimination rate for gamithromycin was consistently observed in the skin plus fat. However, in yellow-feathered broilers, the subsequent order of slowest elimination was kidney, liver, muscle, and injection site, with elimination half-lives (t1/2λzs) at 2.04, 1.71, 1.30, and 1.22 d, respectively. Conversely, in white-feathered broilers, the sequence of slowest elimination included skin plus fat (2.21 d), followed by liver (1.93 d), kidney (1.88 d), injection site (1.23 d), and muscle (1 d) (Table 4). These differences in elimination speed underscore the varied dynamics of gamithromycin removal across different tissues and feather colors in broiler chickens.
The AUC is a crucial parameter reflecting internal exposure. After one single subcutaneous injection of gamithromycin at 7.5 mg/kg BW, the AUC values (day·ng/g) in yellow-feathered broilers were ranked as followed: liver (28,938), injection site (19,279.73), kidney (12,067.41), muscle (2,189.06), and skin + fat (1,056.70). In white-feathered broilers, the AUC values (day·ng/g) ranked from highest to lowest were liver (50,845.74), kidney (15,006.05), injection site (14,044.94), muscle (2,434.36), and skin + fat (1,367.38) (Table 4). Except for the injection site, the AUC values in all other tissues of yellow-feathered broilers were smaller than those in white-feathered broilers, with corresponding AUC ratios ranging from 0.57 to 0.90. These differences suggest a higher internal exposure of gamithromycin in white-feathered broilers after a single subcutaneous injection. Our current understanding of these differences is not precise. However, several general possibilities could contribute to these variations in the internal exposure. One potential factor is distribution variability due to differences in pigment distribution in the feathers of the 2 types of broilers. Additionally, genetic factors may play a role in influencing the pharmacokinetics of gamithromycin. Specific genetic variations could impact the expression of enzymes involved in drug metabolism or other relevant pathways. Further research is necessary to uncover the actual cause.
While the actual reasons for the observed differences in gamithromycin concentration and kinetic characteristics influenced by feather color are not conclusively known, it is plausible that these variations could be related to pigments. Birds exhibit a diverse range of colors across their bodies, including bills, legs, and feather. The hues of brown, grey, and black always result from the deposition of eumelanin pigments, while reddish-brown colorations are attributed to pheomelanin. Yellow to red colorations typically arise from carotenoid pigments (Roulin and Ducrest, 2013). Differences in pigment expression and deposition contribute to the diverse array of colors observed in chicken feathers (Mundy, 2005). Research on the impact of feather color on drug disposition is currently limited. However, findings indicate that the elimination time of some drugs in silky chickens is significantly longer compared to other chicken breeds (Liu et al., 2021; Chen et al., 2023; Hu et al., 2023; Yuan et al., 2023). The prevailing consensus suggests that silkie tissue exhibits a heightened affinity for drugs, primarily attributed to elevated melanin levels (Chen et al., 2023).
The current findings align with those for silkie and other chickens, indicating that the t1/2λz values of gamithromycin in muscle, skin + fat, and kidney of yellow-feathered broilers were consistently longer than the corresponding values in white-feathered broilers (Chen et al., 2023; Hu et al., 2023; Liu et al., 2021; Yuan et al., 2023). Nevertheless, further confirmation is required to determine whether differences exist in melanin content in various tissues of white-feathered and yellow-feathered broilers. Additionally, investigation is needed to ascertain whether gamithromycin also exhibits specific binding to melanin.
This study has 2 limitations. First, concerning the statistical analysis of the kinetic parameters, utilizing average concentration-time data resulted in obtaining only one value for each parameter. Consequently, a comparative statistical analysis of these parameters between white-feathered and yellow-feathered broilers was not feasible. The second limitation is the absence of confirmed MRL data for gamithromycin in various tissues of broiler chickens, impeding the precise determination of the withdrawal period.
Another study characterized the depletion behavior of tylosin, another macrolide, in broiler feather samples. Following an oral dose of 32 mg/kg BW of tylosin for 5 d, consistently high concentrations of tylosin were observed in feather samples for up to 15 d after the last dose. The withdrawal period for feather samples was determined as 27 d, markedly longer than the corresponding values in muscle and liver (Cornejo et al., 2018). The residue concentration and elimination time of gamithromycin in chicken feathers warrant further investigation. We intend to undertake similar experiments in the future to ascertain whether gamithromycin exhibits characteristics akin to tylosin.
CONCLUSIONS
The present study is the first to compare the residue depletion of gamithromycin in yellow-feather and white-feather broilers. Notably, differences were observed in gamithromycin concentrations and kinetic characteristics in both breeds of chickens, with higher residue concentrations and longer residue times found in yellow-feathered broilers. However, further investigations are warranted to discern the underlying reasons for these differences.
ACKNOWLEDGMENTS
This work was supported by funding from the Foundation for the University Young Key Teacher Program of Henan Province (no. 2021GGJS044) and the Doctoral Scientific Research Program of Henan University of Science and Technology (no. 13480091).
Ethical Approval: All applicable international, national and institutional guidelines for the care and use of animals were followed. And the animal experimentation protocol adhered to the guidelines and approvals set forth by the Experimental Animal Management and Use Committee of Henan University of Science and Technology (approved # 20220092).
DISCLOSURES
All authors declare that they have no conflict of interest.
REFERENCES
- Arsic B., Barber J., Cikos A., Mladenovic M., Stankovic N., Novak P. 16-membered macrolide antibiotics: a review. Int. J. Antimicrob. Agents. 2018;51:283–298. doi: 10.1016/j.ijantimicag.2017.05.020. [DOI] [PubMed] [Google Scholar]
- Bartels T. Variations in the morphology, distribution, and arrangement of feathers in domesticated birds. J. Exp. Zool. Part B. 2003;298:91–108. doi: 10.1002/jez.b.28. [DOI] [PubMed] [Google Scholar]
- Chen Z., Liu W., Wu Q., Li Z., Tan L., Ding H., Liu W., Shen X. Withdrawal time of danofloxacin and difloxacin and in vitro binding phenomenon to melanin in black-boned silky fowl. J. Food Sci. 2023;88:4773–4783. doi: 10.1111/1750-3841.16753. [DOI] [PubMed] [Google Scholar]
- Chowdhury M.A.H., Ashrafudoulla M., Mevo S.I.U., Mizan M.F.R., Park S.H., Ha S.D. Current and future interventions for improving poultry health and poultry food safety and security: a comprehensive review. Compr. Rev. Food. Sci. Food Saf. 2023;22:1555–1596. doi: 10.1111/1541-4337.13121. [DOI] [PubMed] [Google Scholar]
- Cornejo J., Pokrant E., Carvallo C., Maddaleno A., San Martin B. Depletion of tylosin residues in feathers, muscle and liver from broiler chickens after completion of antimicrobial therapy. Food Addit. Contam. Part A-Chem. 2018;35:448–457. doi: 10.1080/19440049.2017.1401740. [DOI] [PubMed] [Google Scholar]
- EMA (European Medicines Agency) Gamithromycin (All Ruminants Except Bovine Species): European Public Maximum-Residue-Limit Assessment Report. European Medicines Agency; London, UK: 2017. https://www.ema.europa.eu/en/documents/mrl-report/gamithromycin-all-ruminants-except-bovine-species-european-public-maximum-residue-limit-assessment-report-epmar-cvmp_en.pdf Accessed Jan. 2024. [Google Scholar]
- Hu H., Qiu J., Li R., Li D., Wang Q., Wang Q., Ma Y., Yang W., Xu R., Liu L., Su Y., Song H., Yang B. Comparative study of the plasma pharmacokinetics and tissue residues of trimethoprim in silky fowls and 817 broilers after single oral administration. Poult. Sci. 2023;102 doi: 10.1016/j.psj.2023.103060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lhermie G., La Ragione R.M., Weese J.S., Olsen J.E., Christensen J.P., Guardabassi L. Indications for the use of highest priority critically important antimicrobials in the veterinary sector. J. Antimicrob. Chemother. 2020;75:1671–1680. doi: 10.1093/jac/dkaa104. [DOI] [PubMed] [Google Scholar]
- Liu B.T., Sun S.K., Chen L., Yu J.J. Residue depletion of sarafloxacin in black-bone silky fowl tissues after oral administration. Turk. J. Vet. Anim. Sci. 2021;45:470–477. [Google Scholar]
- Mundy N.I. A window on the genetics of evolution: MC1R and plumage colouration in birds. Proc. R. Soc. B-Biol. Sci. 2005;272:1633–1640. doi: 10.1098/rspb.2005.3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roulin A., Ducrest A.L. Genetics of colouration in birds. Semin. Cell Dev. Biol. 2013;24:594–608. doi: 10.1016/j.semcdb.2013.05.005. [DOI] [PubMed] [Google Scholar]
- Rugani R., Zhang Y., Scarsi B., Regolin L. Hybro chicks outperform Ross308 in a numerical-ordinal task. Cognitive and behavioral comparisons between 2 broiler strains of newborn domestic chicks (Gallus gallus) Poult. Sci. 2023;102 doi: 10.1016/j.psj.2023.103148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Yang F., Song Z.W., Shao H.T., Bai D.Y., Ma Y.B., Kong T., Yang F. The influence of immune stress induced by Escherichia coli lipopolysaccharide on the pharmacokinetics of danofloxacin in broilers. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2021.101629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watteyn A., Devreese M., De Baere S., Wyns H., Plessers E., Boyen F., Haesebrouck F., De Backer P., Croubels S. Pharmacokinetic and pharmacodynamic properties of gamithromycin in turkey poults with respect to Ornithobacterium rhinotracheale. Poult. Sci. 2015;94:2066–2074. doi: 10.3382/ps/pev217. [DOI] [PubMed] [Google Scholar]
- Watteyn A., Devreese M., Plessers E., Wyns H., Garmyn A., Reddy V.R., Pasmans F., Martel A., Haesebrouck F., De Backer P., Croubels S. Efficacy of gamithromycin against Ornithobacterium rhinotracheale in turkey poults pre-infected with avian metapneumovirus. Avian Pathol. 2016;45:545–551. doi: 10.1080/03079457.2016.1183764. [DOI] [PubMed] [Google Scholar]
- Watteyn A., Plessers E., Wyns H., De Baere S., De Backer P., Croubels S. Pharmacokinetics of gamithromycin after intravenous and subcutaneous administration in broiler chickens. Poult. Sci. 2013;92:1516–1522. doi: 10.3382/ps.2012-02932. [DOI] [PubMed] [Google Scholar]
- Yan Y., Zhang H., Ai L., Kang W., Lian K., Wang J. Determination of gamithromycin residues in eggs, milk and edible tissue of food-producing animals by solid phase extraction combined with ultrahigh-performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. B. 2021;1171 doi: 10.1016/j.jchromb.2021.122637. [DOI] [PubMed] [Google Scholar]
- Yuan C., Jiang Y., Wang Z., Chen G., Bai H., Chang G. Indigenous, yellow-feathered chickens body measurements, carcass traits, and meat quality depending on marketable age. Animals. 2022;12:2422. doi: 10.3390/ani12182422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L., Wu H., Wang J., Zhou M., Zhang L., Xiang J., Liao Q., Luo L., Qian M., Zhang D. Pharmacokinetics, withdrawal time, and dietary risk assessment of enrofloxacin and its metabolite ciprofloxacin, and sulfachloropyridazine-trimethoprim in Taihe black-boned silky fowls. J. Food Sci. 2023;88:1743–1752. doi: 10.1111/1750-3841.16501. [DOI] [PubMed] [Google Scholar]