Abstract
Objective
To evaluate the diagnostic accuracy and safety of using magnetically guided capsule endoscopy with a detachable string (ds-MCE) for detecting and grading oesophagogastric varices in adults with cirrhosis.
Design
Prospective multicentre diagnostic accuracy study.
Setting
14 medical centres in China.
Participants
607 adults (>18 years) with cirrhosis recruited between 7 January 2021 and 25 August 2022. Participants underwent ds-MCE (index test), followed by oesophagogastroduodenoscopy (OGD, reference test) within 48 hours. The participants were divided into development and validation cohorts in a ratio of 2:1.
Main outcome measures
The primary outcomes were the sensitivity and specificity of ds-MCE in detecting oesophagogastric varices compared with OGD. Secondary outcomes included the sensitivity and specificity of ds-MCE for detecting high risk oesophageal varices and the diagnostic accuracy of ds-MCE for detecting high risk oesophagogastric varices, oesophageal varices, and gastric varices.
Results
ds-MCE and OGD examinations were completed in 582 (95.9%) of the 607 participants. Using OGD as the reference standard, ds-MCE had a sensitivity of 97.5% (95% confidence interval 95.5% to 98.7%) and specificity of 97.8% (94.4% to 99.1%) for detecting oesophagogastric varices (both P<0.001 compared with a prespecified 85% threshold). When using the optimal 18% threshold for luminal circumference of the oesophagus derived from the development cohort (n=393), the sensitivity and specificity of ds-MCE for detecting high risk oesophageal varices in the validation cohort (n=189) were 95.8% (89.7% to 98.4%) and 94.7% (88.2% to 97.7%), respectively. The diagnostic accuracy of ds-MCE for detecting high risk oesophagogastric varices, oesophageal varices, and gastric varices was 96.3% (92.6% to 98.2%), 96.9% (95.2% to 98.0%), and 96.7% (95.0% to 97.9%), respectively. Two serious adverse events occurred with OGD but none with ds-MCE.
Conclusion
The findings of this study suggest that ds-MCE is a highly accurate and safe diagnostic tool for detecting and grading oesophagogastric varices and is a promising alternative to OGD for screening and surveillance of oesophagogastric varices in patients with cirrhosis.
Trial registration
ClinicalTrials.gov NCT03748563.
Introduction
Oesophagogastric varices occur in about half of patients with cirrhosis and are major causes of morbidity and mortality because of the risk of variceal bleeding.1 To identify people with high risk varices who need prophylactic treatment to prevent variceal bleeding, clinical guidelines recommend oesophagogastroduodenoscopy (OGD) as the standard diagnostic modality for screening and periodic surveillance of oesophagogastric varices.1 2 3 OGD is an unpleasant invasive procedure, however, which usually necessitates sedation, potentially leading to sedation related complications and low adherence to screening programmes in patients with cirrhosis.4 An alternative method to OGD that is minimally invasive and of comparable accuracy is thus needed for detecting and grading oesophagogastric varices in patients with cirrhosis.
Capsule endoscopy, involving the ingestion of a small battery powered device with a camera, has been proposed as a promising minimally invasive diagnostic modality for visualising oesophagogastric varices.5 6 7 8 The advantages of capsule endoscopy are that it is minimally invasive, requires no sedation, and is highly acceptable to patients.9 Studies have, however, reported that conventional small bowel capsule endoscopy and oesophageal capsule endoscopy are not accurate enough to replace OGD to detect or grade oesophageal varices.5 7 9 10 11 12 In a multicentre study of 330 participants with cirrhosis, the sensitivity of oesophageal capsule endoscopy to diagnose and correctly stage oesophageal varices was only 76% and 64%, respectively.7 Moreover, conventional capsule endoscopy has shown poor accuracy in detecting gastric varices and portal hypertensive gastropathy.5 6 13 During oesophageal examination, the image capture rate of conventional capsule endoscopy is insufficient compared to the rapid oesophageal transit time, and the passive movement of the capsule means the transit time cannot be controlled. During gastric examination, the passive movement also prevents complete visualisation of the capacious and non-uniform cavity of the stomach. These limitations preclude conventional capsule endoscopy from providing a complete view of areas with suspected oesophagogastric varices. To date, clinical guidelines do not recommend conventional capsule endoscopy for screening or surveillance of oesophagogastric varices.2
To achieve a complete examination of the oesophagus and stomach using capsule endoscopy, we developed magnetically guided capsule endoscopy with a detachable string (ds-MCE). The capsule can be actively controlled in the oesophagus via the string and in the stomach via magnetic force, and therefore offers a minimally invasive method to detect both oesophageal varices and gastric varices. In addition, the capsule has a long battery life, enabling further evaluation for portal hypertensive enteropathy in the small bowel. Our previous small sample studies showed the feasibility and safety of ds-MCE in healthy volunteers and in patients with compensated cirrhosis.14 15 In this paper, we conducted the CENTERS study to further assess the diagnostic accuracy of ds-MCE in detecting and grading oesophagogastric varices in patients with cirrhosis.
Methods
Study design
CENTERS is a prospective multicentre study reported according to the standards for reporting diagnostic accuracy studies.16 The study was performed at 14 hospitals in China between 7 January 2021 and 25 August 2022. Patients with cirrhosis were consecutively recruited to undergo ds-MCE (index test) first, then OGD (the reference standard) within 48 hours of the first procedure. Certified operators performed the ds-MCE and experienced endoscopists performed the OGD, each blinded to the results of the other test. All participants provided written informed consent. The study protocol and statistical analysis plan are included in the supplementary appendix.
Participants
Eligible patients were those aged at least 18 years with a diagnosis of cirrhosis. Exclusion criteria were dysphagia, Zenker’s diverticulum, gastrointestinal obstruction, pregnancy, gastrointestinal bleeding, a cardiac pacemaker or other implanted electromedical device, a life threatening condition, planned magnetic resonance imaging before excretion of the capsule endoscope, participation in another clinical study, and refusal to give informed consent or any condition that precluded compliance with the study. Supplementary method 2.1 lists the inclusion and exclusion criteria.
Study procedures
The ds-MCE examination (index test)
The ds-MCE system (Ankon Technologies, Wuhan, China) consists of a capsule endoscope with a camera for capturing images, a robotic arm with a magnet for guiding the capsule, a data recorder, a computer workstation with software for real time viewing, two joysticks for controlling the orientation of the capsule (see supplementary figure S1),17 18 19 20 and a detachable hollow latex string (see supplementary figure S2).14 The capsule endoscope measures 27×11.8 mm and has a battery life of more than 10 hours and a viewing field of 150 degrees. Images are captured at an adaptive rate of 0.5-6 frames per second, with a resolution of 480×480 pixels. The latex string is 120 cm in length and can be attached to the capsule endoscope at one end and a syringe at the other end.
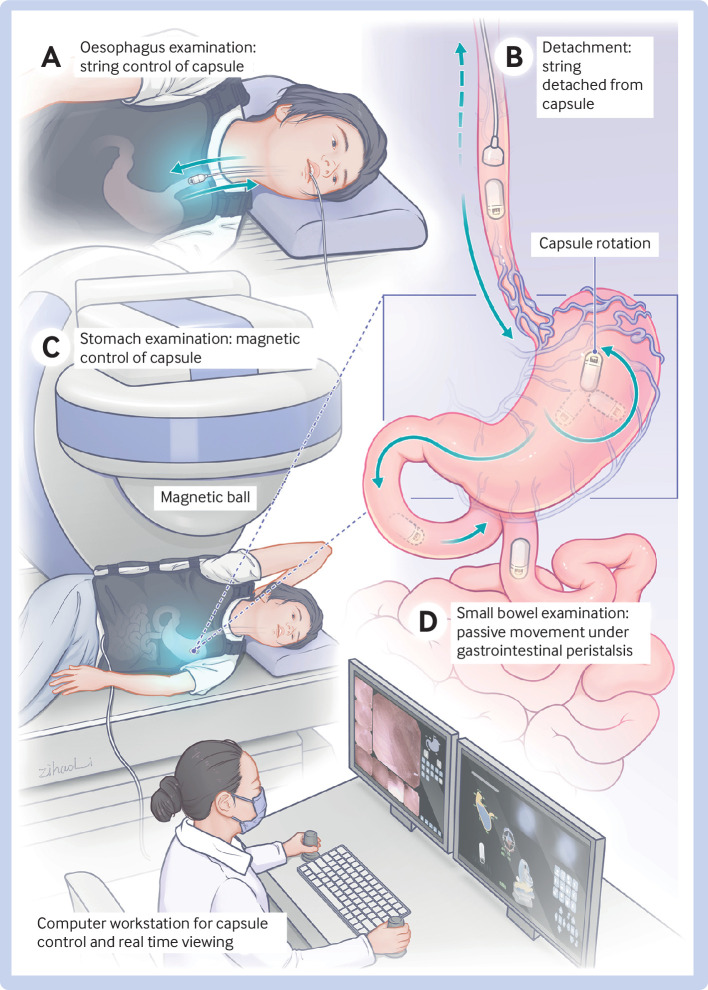
Figure 1 shows the ds-MCE procedure. Before the examination, participants were asked to fast overnight for 12 hours and to ingest 2.5 g of dimethicone as a defoaming agent 40 minutes before the procedure, followed by 500-1000 mL of water as tolerated before ingestion of the capsule to fill the stomach cavity and aid navigation of the capsule. Participants underwent a standardised gastrointestinal preparation regimen, swallowed the capsule with water, and were not sedated. During examination of the oesophagus, the capsule endoscope was controlled by the string and images were captured at a rate of six frames per second. Once the capsule had reached the gastric cardia by means of the swallowed water, the string was pulled up slowly so that the operator could inspect the oesophagus under real time viewing. This process was repeated at least three times, during which participants drank water to distend the distal oesophagus and to remove saliva and mucous bubbles for better observation. After completion of the examination, the syringe was used to administer 5 mL of air through the string to detach the capsule and the string was removed via the mouth. At this point the capsule endoscope entered the stomach and was controlled by an external magnetic field during the gastric examination. The procedure was performed twice according to standardised protocol, and the gastric cardia, fundus, body, angulus, antrum, and pylorus were fully examined.19 On completion of the gastric examination, the capsule endoscope was switched to the mode for examination of the small intestine and allowed to move passively under gastrointestinal peristalsis. In this mode, the frame rate of the capsule varies because the capsule automatically recognises the velocity at which it is moving and can adjust the camera to capture between 0.5-6 frames per second. At each centre, a dedicated certified operator performed the ds-MCE procedures. Staff at an independent core imaging laboratory reviewed the coded videos of the ds-MCE procedures (see supplementary methods 2.2 and 2.3).
Fig 1.
Magnetically guided capsule endoscopy with a detachable string (ds-MCE). During examination of the oesophagus, the capsule is controlled by a hollow latex string (A); after completion of the oesophageal examination, the string is separated from the capsule by injecting 5 mL of air through the syringe and then removed via the mouth, and the capsule enters the stomach (B); the capsule is then controlled by an external magnetic field during gastric examination (C); then is moved passively under gastrointestinal peristalsis during examination of the small bowel (D)
OGD examination (reference standard)
At each centre, experienced endoscopists performed conventional forward viewing OGD within 48 hours after ds-MCE. OGD was conducted with or without sedation, according to the centre’s standard procedure and preference of the patient. The endoscopist was blinded to results of the preceding ds-MCE. The oesophagus, stomach, and duodenum were examined routinely. The whole examination of each participant was recorded on video and digital images. An independent core imaging laboratory reviewed the coded videos and images of OGD from each centre (see supplementary methods 2.2 and 2.3).
After completion of both examinations, participants were administered a questionnaire on their satisfaction with the procedures. Two weeks after ds-MCE, participants were followed up to confirm excretion of the capsule endoscope. Any adverse events during the study were reported to the investigators and recorded.
Outcomes measures and definitions
The primary outcomes were the sensitivity and specificity of ds-MCE for detecting oesophagogastric varices in patients with cirrhosis, using OGD as the reference standard. The key secondary outcomes were the sensitivity and specificity of ds-MCE in detecting high risk oesophageal varices compared with OGD. Other secondary outcomes included the diagnostic accuracy of ds-MCE in detecting high risk oesophagogastric varices, oesophageal varices, large oesophageal varices, red colour signs of oesophageal varices, gastric varices, cardiofundal gastric varices, and portal hypertensive gastropathy compared with OGD; the findings of portal hypertensive enteropathy in small bowel under ds-MCE; the examination time of ds-MCE and OGD; assessment of patients’ satisfaction; and evaluation of safety.
Oesophagogastric varices occur in the oesophagus or stomach. Oesophageal varices detected during OGD were classified as large (diameter ≥5 mm) or small according to the Baveno III consensus.21 We used the de Franchis method5 to grade the size of oesophageal varices detected during ds-MCE according to the percentage of luminal circumference of oesophagus occupied by the largest oesophageal varices (see supplementary figure S3). Oesophageal varices detected during ds-MCE were defined as large when the varix occupied more than the optimal percentage threshold of the luminal circumference of the oesophagus. We defined high risk oesophageal varices as large varices or small varices occurring in the presence of red colour signs.22 Gastric varices in the stomach were classified as gastroesophageal varices and isolated gastric varices according to Sarin’s classification,23 and cardiofundal gastric varices included type 2 gastroesophageal varices and type 1 isolated gastric varices. We defined high risk oesophagogastric varices as high risk oesophageal varices or any gastric varices.24 25 Patient satisfaction assessments were based on satisfaction scores, with higher scores representing more comfort. Supplementary method 2.4 provides detailed definitions of outcomes.
Statistical analysis
The primary aims of our single arm diagnostic accuracy study were to test whether the sensitivity and specificity of ds-MCE for detecting oesophagogastric varices would be >85%. With an estimated sensitivity of 90%, specificity of 94%, two sided alpha of 5%, power of 80%, prevalence for oesophagogastric varices of 62%, and dropout rate of 3%, we determined that 591 participants would be needed.26
The diagnostic analyses are based on the results of participants with useable data after ds-MCE and OGD. Using OGD as the reference standard, we assessed the diagnostic accuracy of ds-MCE for detecting oesophagogastric varices, with sensitivity and specificity as the primary outcomes; with the positive predictive value, negative predictive value, and overall diagnostic accuracy of ds-MCE as other measures simultaneously, along with corresponding 95% confidence intervals using Wilson’s method, in per patient analysis. We compared the sensitivity and specificity of detecting oesophagogastric varices with the prespecified 85% threshold using the one sample exact test. The diagnostic performance of ds-MCE for detecting oesophageal varices, red colour signs of oesophageal varices, gastric varices, cardiofundal gastric varices, and portal hypertensive gastropathy was assessed using sensitivity, specificity, positive predictive value, negative predictive value, and overall diagnostic accuracy.
In addition, we divided the sample into development and validation cohorts in a ratio of 2:1 according to the temporal order of first-patient-in dates of each centre, such that centres with earlier first-patient-in dates were allocated to the development cohort (seven centres, n=393, supplementary table S1) and centres with later first-patient-in dates were allocated to the validation cohort (the remaining seven centres, n=189, supplementary table S1). From the development cohort we derived the optimal percentage threshold for luminal circumference of the oesophagus for detecting large oesophageal varices during ds-MCE. We calculated the Youden index, defined as [(sensitivity+specificity)−1], to determine the optimal percentage threshold for oesophageal luminal circumference, rounding down to the nearest whole percentage derived from the development cohort that resulted in the best combination of specificity and sensitivity for detecting large oesophageal varices during ds-MCE. And we internally validated the optimal threshold using the bootstrap method, with 1000 replications. The validation cohort was used to assess the diagnostic accuracy of ds-MCE for detecting high risk oesophageal varices, high risk oesophagogastric varices, and large oesophageal varices on the basis of the optimal threshold, using sensitivity, specificity, positive predictive value, negative predictive value, and diagnostic accuracy. P values <0.05 were considered to indicate statistical significance (see supplementary method 2.5).
Patient and public involvement
No patients or members of the public were directly involved in setting the research question or the outcome measures. At the protocol stage, more than 30 patients with cirrhosis were consulted about the ds-MCE procedure. Participants were aware of the purpose and content of this study during recruitment, although they were not involved in the initial design of the trial. Considering the confidentiality of clinical data, patients did not participate in the subsequent reporting of this research. The results were, however, communicated to patients who expressed an interest during clinic visits.
Results
Participants
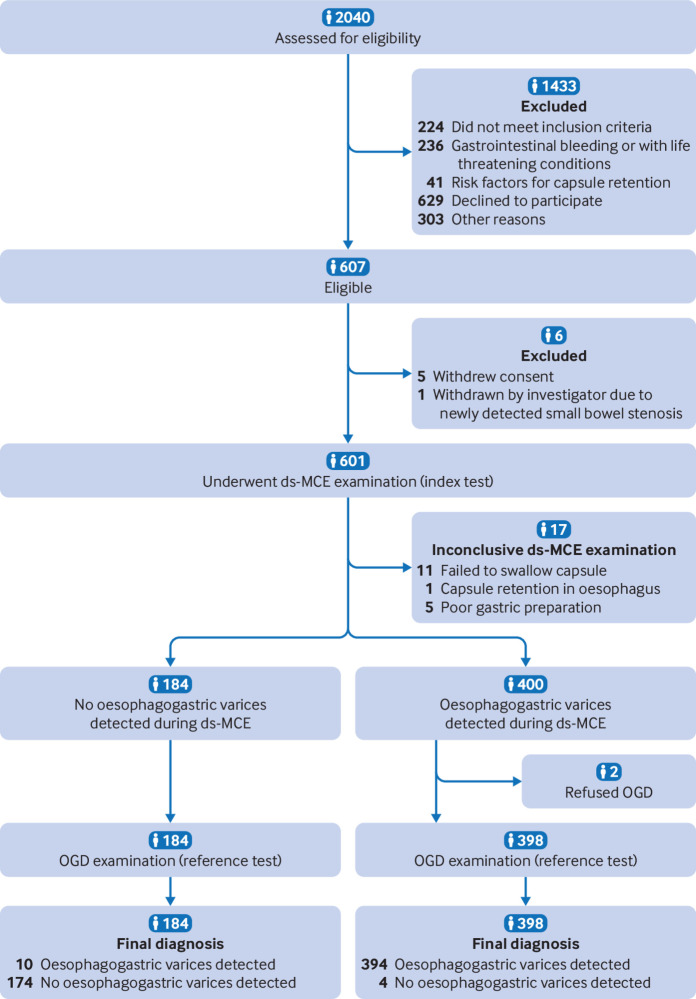
A total of 607 adults with cirrhosis from 14 centres in China were enrolled into the study between 7 January 2021 and 25 August 2022. Twenty five patients were excluded—in 17 patients it was not possible to complete the oesophageal and stomach examinations during ds-MCE. Both ds-MCE and OGD examinations were completed in 582 participants (fig 2). These participants were included in the accuracy analysis. Table 1 shows the baseline characteristics of the 582 participants.
Fig 2.
Flow of participants through study. ds-MCE=magnetically guided capsule endoscopy with a detachable string; OGD=oesophagogastroduodenoscopy
Table 1.
Baseline characteristics of adults with cirrhosis. Values are number (percentage) unless stated otherwise
| Characteristics | Participants (n=582) |
|---|---|
| Median (IQR) age (years) | 55.00 (48.00-64.00) |
| Sex: | |
| Male | 398 (68.4) |
| Female | 184 (31.6) |
| Median (IQR) time since cirrhosis diagnosis (years) | 2.5 (0.3-6.0) |
| Cause of cirrhosis: | |
| Hepatitis B virus infection | 340 (58.4) |
| Hepatitis C virus infection | 29 (5.0) |
| Alcoholic liver disease | 61 (10.5) |
| Autoimmune hepatitis | 44 (7.6) |
| Primary biliary cirrhosis | 16 (2.7) |
| Non-alcoholic steatohepatitis | 6 (1.0) |
| Cryptogenic | 60 (10.3) |
| Other* | 26 (4.5) |
| Median (IQR) Child-Pugh score† (points) | 6.0 (5.0-7.0) |
| Child-Pugh class‡: | |
| Class A | 402 (69.1) |
| Class B | 153 (26.3) |
| Class C | 27 (4.6) |
| Median (IQR) MELD score§ (points) | 9 (7-11) |
| Median (IQR) laboratory results: | |
| Platelet count (×109/L) | 94 (62-145) |
| Alanine transaminase (U/L) | 25.1 (18-40) |
| Aspartate aminotransferase (U/L) | 31 (24-46.75) |
| γ-glutaryltransferase (U/L) | 38 (22-77) |
| Total bilirubin (μmol/L) | 18.55 (13.4-28.55) |
| Albumin (g/L) | 38.75 (33-44.1) |
| Creatinine (μmol/L) | 66 (57-78) |
| Prothrombin time (s) | 13.7 (12.7-15.2) |
| International normalised ratio | 1.15 (1.035-1.28) |
| Decompensated cirrhosis | 356 (61.2) |
| Indication for endoscopy: | |
| Screening | 228 (39.2) |
| Surveillance | 354 (60.8) |
| Clinical events: | |
| Ascites | 264 (45.4) |
| History of splenectomy | 41 (7.0) |
| TIPS insertion | 39 (6.7) |
| History of endoscopic variceal treatment | 120 (20.6) |
| History of variceal oesophagogastric bleeding | 189 (32.5) |
IQR=interquartile range; MELD=model for end stage liver disease; TIPS=transjugular intrahepatic portosystemic shunt.
Includes schistosomiasis (n=12), drug induced cirrhosis (n=4), Budd-Chiari syndrome (n=3), co-infection with hepatitis C virus and hepatitis B virus (n=1), hepatitis B infection+alcoholic liver disease (n=5), alcoholic liver disease+schistosomiasis (n=1).
Child-Pugh score is a liver reserve function evaluation and scoring system, using five clinical and laboratory measures (total bilirubin, serum albumin, prothrombin time, ascites, and hepatic encephalopathy) to generate a total score between 5 and 15.
Class A represents a total Child-Pugh score of 5 to 6, class B a score of 7 to 9, and class C a score of 10 to 15.
MELD score measures the severity of liver disease, and is calculated based on serum creatinine and bilirubin levels, international normalised ratio of prothrombin time, and cause of liver disease.
Primary outcome
Overall, the findings for oesophagogastric varices were concordant between ds-MCE and OGD in 568 of the 582 participants (97.6%). Results were inconsistent between the two procedures in 14 participants, oesophagogastric varices detected by ds-MCE were not confirmed by OGD in four participants, and ds-MCE failed to detect oesophagogastric varices detected by OGD in 10 participants (see supplementary table S3). Using OGD as the reference standard, ds-MCE had a sensitivity of 97.5% (95% confidence interval 95.5% to 98.7%) and specificity of 97.8% (94.4% to 99.1%) for detecting oesophagogastric varices, and both sensitivity and specificity were significantly higher than the prespecified 85% threshold (both P<0.001) (table 2). The positive predictive value and negative predictive value was 99.0% (97.4% to 99.6%) and 94.6% (90.3% to 97.0%), respectively (table 2). Figure 3 shows representative oesophagogastric varices observed during ds-MCE and OGD.
Table 2.
Diagnostic performance of ds-MCE using oesophagogastroduodenoscopy as reference standard in main analysis for detection of oesophagogastric varices
| Outcome | Sensitivity | Specificity | Positive predictive value | Negative predictive value | Diagnostic accuracy | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % (95% CI) | No/total No | % (95% CI) | No/total No | % (95% CI) | No/total No | % (95% CI) | No/total No | % (95% CI) | No/total No | |||||
| Primary outcome | ||||||||||||||
| Oesophagogastric varices | 97.5 (95.5 to 98.7) | 394/404 | 97.8 (94.4 to 99.1) | 174/178 | 99.0 (97.4 to 99.6) | 394/398 | 94.6 (90.3 to 97.0) | 174/184 | 97.6 (96.0 to 98.6) | 568/582 | ||||
| Secondary outcomes | ||||||||||||||
| High risk oesophageal varices* | 95.8 (89.7 to 98.4) | 91/95 | 94.7 (88.2 to 97.7) | 89/94 | 94.8 (88.4 to 97.8) | 91/96 | 95.7 (89.5 to 98.3) | 89/93 | 95.2 (91.2 to 97.5) | 180/189 | ||||
| High risk oesophagogastric varices* | 96.6 (91.5 to 98.7) | 112/116 | 95.9 (88.6 to 98.6) | 70/73 | 97.4 (92.6 to 99.1) | 112/115 | 94.6 (86.9 to 97.9) | 70/74 | 96.3 (92.6 to 98.2) | 182/189 | ||||
| Large oesophageal varices* | 95.3 (88.5 to 98.2) | 81/85 | 93.3 (86.8 to 96.7) | 97/104 | 92.0 (84.5 to 96.1) | 81/88 | 96.0 (90.3 to 98.5) | 97/101 | 94.2 (89.9 to 96.7) | 178/189 | ||||
| Oesophageal varices | 96.4 (94.0 to 97.8) | 370/384 | 98.0 (94.9 to 99.2) | 194/198 | 98.9 (97.3 to 99.6) | 370/374 | 93.3 (89.0 to 96.0) | 194/208 | 96.9 (95.2 to 98.0) | 564/582 | ||||
| Oesophageal varices with red colour sign | 96.1 (92.5 to 98.0) | 196/204 | 97.6 (95.5 to 98.7) | 369/378 | 95.6 (91.9 to 97.7) | 196/205 | 97.9 (95.9 to 98.9) | 369/377 | 97.1 (95.4 to 98.2) | 565/582 | ||||
| Gastric varices | 96.2 (93.0 to 98.0) | 230/239 | 97.1 (94.7 to 98.4) | 333/343 | 95.8 (92.5 to 97.7) | 230/240 | 97.4 (95.1 to 98.6) | 333/342 | 96.7 (95.0 to 97.9) | 563/582 | ||||
| Cardiofundal gastric varices | 92.2 (85.8 to 95.8) | 106/115 | 99.6 (98.5 to 99.9) | 465/467 | 98.1 (93.5 to 99.5) | 106/108 | 98.1 (96.4 to 99.0) | 465/474 | 98.1 (96.7 to 98.9) | 571/582 | ||||
| Portal hypertensive gastropathy | 95.3 (90.9 to 97.6) | 161/169 | 98.3 (96.5 to 99.2) | 406/413 | 95.8 (91.7 to 98.0) | 161/168 | 98.1 (96.2 to 99.0) | 406/414 | 97.4 (95.8 to 98.4) | 567/582 | ||||
CI=confidence interval; ds-MCE=magnetically guided capsule endoscopy with detachable string.
Based on 18% optimal threshold for oesophageal luminal circumference: in the validation cohort (n=189), a threshold of 18% was applied for discriminating the size of oesophageal varices during ds-MCE. The diagnostic accuracy of ds-MCE for detecting high risk oesophageal varices, high risk oesophagogastric varices, and large oesophageal varices was assessed based on the optimal threshold of 18% in the validation cohort.
Fig 3.
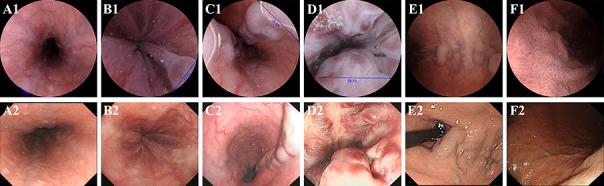
Representative oesophagogastric varices observed in patients A-F while standing for ds-MCE (panel 1) and OGD (panel 2). Small oesophageal varices (patient A), small oesophageal varices with red colour signs (patient B), large oesophageal varices (patient C), large oesophageal varices with red colour signs (patient D), gastric varices (patient E), and portal hypertensive gastropathy (patient F). ds-MCE=magnetically guided capsule endoscopy with detachable string; OGD=oesophagogastroduodenoscopy
Secondary outcomes
The development cohort included 393 participants from seven centres, and the validation cohort included 189 participants from the remaining seven centres (see supplementary table S1). Supplementary table S2 presents the clinical characteristics of participants in both cohorts. In the development cohort, the optimal percentage threshold circumference for the oesophageal lumen of ds-MCE in detecting large oesophageal varices was 18.45%, with a Youden’s index of 0.95 (see supplementary figure S4). Therefore, we chose a threshold of 18% for discriminating large oesophageal varices during ds-MCE. Using the 18% threshold in the development cohort, internal validation showed a sensitivity of 98.3% (94.9% to 100.0%) and a specificity of 97.6% (93.4% to 99.6%) for detecting large oesophageal varices (see supplementary table S4).
Using the 18% threshold in the validation cohort, the sensitivity and specificity of ds-MCE for detecting high risk oesophageal varices were 95.8% (89.7% to 98.4%) and 94.7% (88.2% to 97.7%), respectively (table 2). The diagnostic accuracy of ds-MCE for detecting high risk oesophagogastric varices was 96.3% (92.6% to 98.2%). The sensitivity and specificity of ds-MCE for detecting large oesophageal varices were 95.3% (88.5% to 98.2%) and 93.3% (86.8% to 96.7%), respectively (table 2). Supplementary table S5 presents subgroup analyses of the screening and surveillance populations, and supplementary table S6 presents subgroup analyses of the populations with compensated and decompensated cirrhosis.
Using OGD as the reference standard, the sensitivity and specificity of ds-MCE for detecting oesophageal varices were 96.4% (94.0% to 97.8%) and 98.0% (94.9% to 99.2%), respectively. The sensitivity and specificity of ds-MCE for oesophageal varices with red colour signs were 96.1% (92.5% to 98.0%) and 97.6% (95.5% to 98.7%), respectively (table 2). The sensitivity and specificity of ds-MCE in detecting gastric varices were, respectively, 96.2% (93.0% to 98.0%) and 97.1% (94.7% to 98.4%), cardiofundal gastric varices were 92.2% (85.8% to 95.8%) and 99.6% (98.5% to 99.9%), and portal hypertensive gastropathy were 95.3% (90.9% to 97.6%) and 98.3% (96.5% to 99.2%).
ds-MCE examination of the small bowel was completed in 510 participants. Supplementary table S7 summarises the detailed findings of portal hypertensive enteropathy. Portal hypertensive enteropathy was found in 333 (65.3%) participants, with spontaneous bleeding in three (0.6%) participants (see supplementary figure S5). Supplementary table S8 shows the durations of ds-MCE and OGD. During ds-MCE, the median examination time for the oesophagus and stomach was 4.74 minutes (interquartile range 3.12 to 7.15 minutes) and 15.78 (8.57 to 23.70) minutes, respectively. The examination time for oesophagus, stomach, and duodenum using OGD was 5.50 (4.50 to 7.00) minutes. The median overall satisfaction score for ds-MCE was higher than for OGD both without sedation (3 v 2), and with sedation (3 v 3) (see supplementary tables S9 and S10, respectively). A total of six (0.99%) adverse events were reported during the study. Two serious adverse events occurred in association with OGD (variceal oesophageal haemorrhage), with both participants requiring hospital admission and endoscopic band ligation. No serious adverse events were associated with ds-MCE, but four adverse events occurred during ds-MCE: capsule retention in the small bowel, with the capsule excreted spontaneously after 23 days (n=1 participant); capsule retention in the oesophagus owing to unexpected oesophageal stenosis, with the capsule pulled out using the string (n=1); syncope mainly due to glucopenia associated with gastrointestinal preparation (n=1); and rupture and bleeding of haemorrhoids related to small bowel preparation (n=1).
Discussion
In this prospective, multicentre study comparing ds-MCE with the standard reference of OGD among patients with cirrhosis, the results showed that the lower boundary of the 95% confidence interval for the sensitivity and specificity of ds-MCE in detecting oesophagogastric varices were both above the prespecified value of 0.85. This study suggested that ds-MCE has strong discriminative ability to detect oesophagogastric varices owing to technological advances in direct, real time visualisation of oesophagogastric varices under active control. Moreover, ds-MCE showed high accuracy in detecting high risk oesophageal varices in patients both with compensated cirrhosis and with decompensated cirrhosis, which outperformed the non-invasive diagnostic tools of Baveno VII criteria (liver stiffness measurement, spleen stiffness measurement, and platelet count)3 and conventional capsule endoscopy.5 6 7 8 9 27 28 In addition, ds-MCE enabled further exploration of the small bowel and thus provided a more comprehensive evaluation of gastrointestinal changes in patients with cirrhosis.
Current guidelines on the management of oesophagogastric varices stress the importance of detecting high risk oesophageal varices to prevent variceal bleeding.1 2 29 Three factors need to be identified for oesophageal varices to be classed as high risk: the presence of oesophageal varices, the detection of red colour signs, and the size grading of the oesophageal varices.2 ds-MCE showed high diagnostic accuracy in detecting oesophageal varices and red colour signs compared with OGD, which was higher than reported in previous studies on small bowel capsule endoscopy and oesophageal capsule endoscopy.5 7 8 9 The absence of a specific grading system for oesophageal varices under capsule endoscopy has been one obstacle to grading the size of oesophageal varices.9 The standard grading system for oesophageal varices used during OGD is not applicable to capsule endoscopy because air insufflation is required to fully distend the oesophagus2 21 30 31; a function that capsule endoscopy lacks. Such a difference may in theory impact the grading of oesophageal varices. Previous studies reported a specific oesophageal varices grading system for capsule endoscopy based on the percentage circumference of the oesophageal lumen occupied by the largest oesophageal varices, which showed good correlation with the standard OGD classification.5 7 8 28 However, the previously reported luminal circumference thresholds (25%, 1/6, 15%, or 12.5%) for grading oesophageal varices under capsule endoscopy were developed on the basis of small sample sizes and limited oesophageal images, as capsule endoscopy could not capture enough images under passive gastrointestinal movement.5 7 8 28 In this large prospective study, ds-MCE provided adequate oesophageal images for each participant to enable detection of the largest oesophageal varices and the luminal circumference percentage. We therefore developed a new threshold of 18%, and both internal and external validation verified that the new threshold presented high sensitivity and specificity in stratifying large oesophageal varices and high risk oesophageal varices compared with OGD, suggesting ds-MCE is an accurate diagnostic modality to determine prophylactic interventions for oesophageal varices.
For the detection of gastric varices and portal hypertensive gastropathy, the reported oesophageal capsule endoscopy and small bowel capsule endoscopy showed poor diagnostic performance owing to the inability to control the capsule endoscope during gastric examination.6 9 32 33 The reported non-invasive tests of the Baveno VI criteria variables had a limited role in predicting the presence of gastric varices or portal hypertensive gastropathy.29 In contrast with these non-invasive diagnostic modalities, ds-MCE is comparable to OGD in being able to detect gastric lesions.14 17 18 19 Notably, in the current study ds-MCE showed robust diagnostic performance for the detection of gastric varices and portal hypertensive gastropathy compared with OGD. Gastric varices were further classified based on Sarin’s classification.23 ds-MCE also showed high accuracy in detecting cardiofundal gastric varices, which represent the highest risk of bleeding.21 In the current study, the good performance of ds-MCE suggests that, besides detecting oesophageal varices, it is a promising minimally invasive alternative to OGD in detecting gastric varices and portal hypertensive gastropathy.
Portal hypertensive enteropathy is a potential source of gastrointestinal bleeding and may contribute to chronic anaemia.34 35 36 37 In the current study, ds-MCE enabled the small bowel to be examined, and portal hypertensive enteropathy was detected in 65% of participants with cirrhosis. Importantly, bleeding was observed in the small bowel of three participants. The overall prevalence of portal hypertensive enteropathy was similar to that reported in previous studies on capsule endoscopy.34 38 39 The results indicated that ds-MCE is able to recognise small bowel abnormalities and their underlying influence on patients with cirrhosis.
Mild or moderate adverse events specifically associated with ds-MCE occurred in four participants (0.7%). No serious adverse event occurred in association with ds-MCE. Two participants, however, experienced variceal bleeding during OGD examinations and required admission to hospital. In addition, patients’ satisfaction with ds-MCE was better than with OGD either with or without sedation, which may improve adherence to the screening and surveillance programme. Taken together, these results highlight that ds-MCE is safe and well tolerated in the detection of oesophagogastric varices without the need for conscious sedation.
Strengths and limitations of this study
We performed a prospective, multicentre study involving a large number of participants with cirrhosis to evaluate the diagnostic value of ds-MCE in detecting oesophagogastric varices. The sample size was sufficient to meet our objectives, with suitably narrow confidence intervals. Secondly, we proposed and validated a new capsule endoscopy grading standard for risk stratification of oesophageal varices. Besides, the diagnostic performance of ds-MCE in detecting oesophageal varices and red colour signs of oesophageal varices, gastric varices, and portal hypertensive gastropathy was also validated, providing a more comprehensive evaluation of ds-MCE.
Our study has limitations. Firstly, a small number of participants failed to swallow the capsule endoscope and detachable string. A smaller capsule could overcome this problem.40 In addition, ds-MCE takes a longer time to perform than OGD and the cost of the disposable ds-MCE is higher than OGD, whereas ds-MCE does not require preoperative anaesthesia and postoperative recovery and enables a more thorough evaluation of the upper gastrointestinal tract and small bowel. Thus, a cost efficacy analysis is required in future studies. Secondly, most of the participants had cirrhosis related to hepatitis B virus, resulting in unavoidable selection bias. The application value of ds-MCE in other populations requires validation. Thirdly, OGD was always carried out after ds-MCE in this study. This order effect may have influenced the evaluation of patient satisfaction. Fourthly, the size of oesophageal varices was clarified using the oesophageal varices diameter under insufflation of the oesophagus by OGD, whereas the size of oesophageal varices was graded using the luminal circumference percentage grading system during ds-MCE. The effectiveness of this specific grading system and the proposed threshold to detect large oesophageal varices under capsule endoscopy needs to be validated in further studies. Finally, other non-invasive tools, including Baveno VI and VII criteria, were not compared with ds-MCE, and further studies are warranted.
Implications for clinical practice
As the population with cirrhosis increases worldwide, the incidence of oesophagogastric varices will increase. It is therefore vital to improve the screening and surveillance of oesophagogastric varices and thus possibly to intervene at an early stage to prevent variceal haemorrhage. Our study has several important implications for clinical practice. Firstly, ds-MCE can be used as a minimally invasive screening tool for detecting oesophagogastric varices in patients with cirrhosis. As ds-MCE uses direct visualisation, it is able to provide clear images of oesophageal varices, gastric varices, and portal hypertensive gastropathy. Thus, as a screening tool, ds-MCE showed an advantage over other available non-invasive tests, including serum markers, doppler ultrasonography, contrast enhanced ultrasonography, and ultrasound elastography. Secondly, the accurate detection of high risk oesophageal varices during ds-MCE could provide evidence for clinical treatment decisions, which depends on risk stratification for the prevention of variceal bleeding. Therefore, ds-MCE also serves as a promising surveillance tool for stratification of variceal bleeding risk in patients with cirrhosis. Thirdly, apart from the oesophagus and stomach, ds-MCE can be further used to evaluate small bowel lesions in one process. Finally, ds-MCE is well tolerated, easy to perform, does not require sedation, and is potentially cost effective, and as such is suitable for use in primary, secondary, and tertiary healthcare settings, providing clear potential to optimise the screening and monitoring of oesophagogastric varices.
Conclusions
This study found that ds-MCE is a highly accurate, safe, and minimally invasive method for detecting and grading oesophagogastric varices in patients with cirrhosis. Moreover, ds-MCE is a reliable tool to evaluate portal hypertensive gastropathy and small bowel abnormalities during one examination. The better tolerance of patients to ds-MCE may also improve adherence to endoscopic follow-up. Therefore, ds-MCE provides a promising alternative to OGD for screening and surveillance of gastrointestinal lesions in patients with cirrhosis.
What is already known on this topic
Oesophagogastroduodenoscopy (OGD) is used to diagnose oesophagogastric varices in patients with cirrhosis, but it is invasive and involves sedation leading to patient discomfort, poor adherence, and potentially serious complications
Capsule endoscopy is a less invasive test than OGD as patients swallow a small device that produces images of the gastrointestinal tract
Conventional small bowel capsule endoscopy and oesophageal capsule endoscopy, however, are not accurate enough to replace OGD to detect oesophageal or gastric varices
What this study adds
In this diagnostic accuracy study of 607 patients with cirrhosis, magnetically controlled capsule endoscopy with a detachable string (ds-MCE) showed high sensitivity and specificity in diagnosing oesophagogastric varices, with OGD as the reference standard
The accuracy of ds-MCE for detecting high risk oesophageal varices was also comparable to OGD
The results indicate ds-MCE is a highly accurate and safe method for detecting and grading oesophagogastric varices, and it is a promising alternative to OGD for screening and surveillance of oesophagogastric varices in patients with cirrhosis
Acknowledgments
We thank the patients who participated in this study, their families, and the medical, nursing, and research staff at the study centres; Xiao-Hua Zhou and Yan Hou from the Department of Biostatistics, Peking University for their statistical support; and Ankon Technologies for providing software technical support.
Web extra.
Extra material supplied by authors
Supplementary information: Additional information, tables, and figures
Contributors: XJ, JP, QX, Y-HS, H-HS, CP, and X-LQ contributed equally to the study and are joint first authors. ZL and Z-SL conceived the study. ZL and JP designed the study. XJ, JP, QX, Y-HS, H-HS, CP, Y-YQ, W-BZ, YY, S-QJ, B-SD, SW, YC, D-HX, L-JH, J-ZC, J-FD, XL, TX, WZ, TC, C-HZ, WW, S-JL, Z-YY, FW, LZ, C-ZL, HX, J-XW, BW, YL, XD, L-HQ, Y-QS, HW, Y-FH, H-BB, SZ, LL, Y-HS, X-YW, D-WZ, X-JW, M-DX, HM, C-HH, ZL, X-LZ, S-XH, X-PX, JL, and C-QY conducted the study. XJ and JP drafted the manuscript. ZL, Z-SL, CS, and X-LQ critically revised the manuscript. All authors gave final approval of the manuscript. Z-SL and CS are the co-corresponding authors and can be reached at zhaoshenli@smmu.edu.cn and cristianospada@gmail.com. ZL is the guarantor. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: This study was supported by grants from the “Ten Thousand Plan”-National High-Level Talents Special Support Plan (to ZL) and Shanghai Municipal Hospital Emerging Frontier Technology Joint Project (to ZL, No SHDC12019105). The funders of the study had no role in considering the study design, data collection, data analysis, data interpretation, writing of the report, or decision to submit the article for publication.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare: support from the “Ten Thousand Plan”-National High-Level Talents Special Support Plan and Shanghai Municipal Hospital Emerging Frontier Technology Joint Project for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
The lead author (ZL) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Dissemination to participants and related patient and public communities: The results of this work will be disseminated to the public through the press, social media, and conferences. The lay summaries of the study results will be communicated to study participants and relevant patient groups.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Ethical approval
This study was approved by the medical ethics committee of Changhai Hospital (CHEC2020-121) as well as local approval from all participating hospitals. This study was conducted in accordance with Good Clinical Practice and the Declaration of Helsinki. All participants provided written informed consent. We attest that we have obtained appropriate permissions and paid any required fees for use of copyright protected materials.
Data availability statement
The steering committee of the CENTERS study will consider reasonable requests for the sharing of deidentified individual participant data. Requests should be made to the corresponding author.
References
- 1. Garcia-Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: Risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology 2017;65:310-35. 10.1002/hep.28906. [DOI] [PubMed] [Google Scholar]
- 2. Gralnek IM, Camus Duboc M, Garcia-Pagan JC, et al. Endoscopic diagnosis and management of esophagogastric variceal hemorrhage: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2022;54:1094-120. 10.1055/a-1939-4887. [DOI] [PubMed] [Google Scholar]
- 3. de Franchis R, Bosch J, Garcia-Tsao G, Reiberger T, Ripoll C, Baveno VII Faculty . Baveno VII - Renewing consensus in portal hypertension. J Hepatol 2022;76:959-74. 10.1016/j.jhep.2021.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yeo YH, Hwang J, Jeong D, et al. Surveillance of patients with cirrhosis remains suboptimal in the United States. J Hepatol 2021;75:856-64. 10.1016/j.jhep.2021.04.042. [DOI] [PubMed] [Google Scholar]
- 5. de Franchis R, Eisen GM, Laine L, et al. Esophageal capsule endoscopy for screening and surveillance of esophageal varices in patients with portal hypertension. Hepatology 2008;47:1595-603. 10.1002/hep.22227. [DOI] [PubMed] [Google Scholar]
- 6. Chavalitdhamrong D, Jensen DM, Singh B, et al. Capsule endoscopy is not as accurate as esophagogastroduodenoscopy in screening cirrhotic patients for varices. Clin Gastroenterol Hepatol 2012;10:254-8.e1. 10.1016/j.cgh.2011.11.027. [DOI] [PubMed] [Google Scholar]
- 7. Sacher-Huvelin S, Calès P, Bureau C, et al. Screening of esophageal varices by esophageal capsule endoscopy: results of a French multicenter prospective study. Endoscopy 2015;47:486-92. 10.1055/s-0034-1391393. [DOI] [PubMed] [Google Scholar]
- 8. Cardey J, Le Gall C, Michaud L, et al. Screening of esophageal varices in children using esophageal capsule endoscopy: a multicenter prospective study. Endoscopy 2019;51:10-7. 10.1055/a-0647-1709. [DOI] [PubMed] [Google Scholar]
- 9. Colli A, Gana JC, Turner D, et al. Capsule endoscopy for the diagnosis of oesophageal varices in people with chronic liver disease or portal vein thrombosis. Cochrane Database Syst Rev 2014;2014:CD008760. 10.1002/14651858.CD008760.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McCarty TR, Afinogenova Y, Njei B. Use of Wireless Capsule Endoscopy for the Diagnosis and Grading of Esophageal Varices in Patients With Portal Hypertension: A Systematic Review and Meta-Analysis. J Clin Gastroenterol 2017;51:174-82. 10.1097/MCG.0000000000000589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lapalus MG, Ben Soussan E, Gaudric M, et al. Esophageal capsule endoscopy vs. EGD for the evaluation of portal hypertension: a French prospective multicenter comparative study. Am J Gastroenterol 2009;104:1112-8. 10.1038/ajg.2009.66. [DOI] [PubMed] [Google Scholar]
- 12. Laurain A, de Leusse A, Gincul R, et al. Oesophageal capsule endoscopy versus oesophago-gastroduodenoscopy for the diagnosis of recurrent varices: a prospective multicentre study. Dig Liver Dis 2014;46:535-40. 10.1016/j.dld.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 13. Eisen GM, Eliakim R, Zaman A, et al. The accuracy of PillCam ESO capsule endoscopy versus conventional upper endoscopy for the diagnosis of esophageal varices: a prospective three-center pilot study. Endoscopy 2006;38:31-5. 10.1055/s-2005-921189. [DOI] [PubMed] [Google Scholar]
- 14. Chen YZ, Pan J, Luo YY, et al. Detachable string magnetically controlled capsule endoscopy for complete viewing of the esophagus and stomach. Endoscopy 2019;51:360-4. 10.1055/a-0856-6845. [DOI] [PubMed] [Google Scholar]
- 15. Wang S, Huang Y, Hu W, et al. Detachable string magnetically controlled capsule endoscopy for detecting high-risk varices in compensated advanced chronic liver disease (CHESS1801): A prospective multicenter study. Lancet Reg Health West Pac 2020;6:100072. 10.1016/j.lanwpc.2020.100072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bossuyt PM, Reitsma JB, Bruns DE, et al. STARD Group . STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. BMJ 2015;351:h5527. 10.1136/bmj.h5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liao Z, Hou X, Lin-Hu EQ, et al. Accuracy of Magnetically Controlled Capsule Endoscopy, Compared With Conventional Gastroscopy, in Detection of Gastric Diseases. Clin Gastroenterol Hepatol 2016;14:1266-1273.e1. 10.1016/j.cgh.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 18. Jiang B, Qian YY, Pan J, et al. Second-generation magnetically controlled capsule gastroscopy with improved image resolution and frame rate: a randomized controlled clinical trial (with video). Gastrointest Endosc 2020;91:1379-87. 10.1016/j.gie.2020.01.027. [DOI] [PubMed] [Google Scholar]
- 19. Jiang X, Pan J, Li ZS, Liao Z. Standardized examination procedure of magnetically controlled capsule endoscopy. VideoGIE 2019;4:239-43. 10.1016/j.vgie.2019.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jiang B, Pan J, Qian YY, et al. Capsule Endoscopy Group of the Chinese Society of Digestive Endoscopy . Clinical guideline on magnetically controlled capsule gastroscopy (2021 edition). J Dig Dis 2023;24:70-84. 10.1111/1751-2980.13173. [DOI] [PubMed] [Google Scholar]
- 21. de Franchis R. Updating consensus in portal hypertension: report of the Baveno III Consensus Workshop on definitions, methodology and therapeutic strategies in portal hypertension. J Hepatol 2000;33:846-52. 10.1016/S0168-8278(00)80320-7. [DOI] [PubMed] [Google Scholar]
- 22. de Franchis R. Evolving consensus in portal hypertension. Report of the Baveno IV consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol 2005;43:167-76. 10.1016/j.jhep.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 23. Sarin SK, Lahoti D, Saxena SP, Murthy NS, Makwana UK. Prevalence, classification and natural history of gastric varices: a long-term follow-up study in 568 portal hypertension patients. Hepatology 1992;16:1343-9. 10.1002/hep.1840160607. [DOI] [PubMed] [Google Scholar]
- 24. Maurice JB, Brodkin E, Arnold F, et al. Validation of the Baveno VI criteria to identify low risk cirrhotic patients not requiring endoscopic surveillance for varices. J Hepatol 2016;65:899-905. 10.1016/j.jhep.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 25. Stafylidou M, Paschos P, Katsoula A, et al. Performance of Baveno VI and Expanded Baveno VI Criteria for Excluding High-Risk Varices in Patients With Chronic Liver Diseases: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol 2019;17:1744-1755.e11. 10.1016/j.cgh.2019.04.062. [DOI] [PubMed] [Google Scholar]
- 26. Korevaar DA, Gopalakrishna G, Cohen JF, Bossuyt PM. Targeted test evaluation: a framework for designing diagnostic accuracy studies with clear study hypotheses. Diagn Progn Res 2019;3:22. 10.1186/s41512-019-0069-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lapalus MG, Dumortier J, Fumex F, et al. Esophageal capsule endoscopy versus esophagogastroduodenoscopy for evaluating portal hypertension: a prospective comparative study of performance and tolerance. Endoscopy 2006;38:36-41. 10.1055/s-2006-924975. [DOI] [PubMed] [Google Scholar]
- 28. Schreibman I, Meitz K, Kunselman AR, Downey M, Le T, Riley T. Defining the threshold: new data on the ability of capsule endoscopy to discriminate the size of esophageal varices. Dig Dis Sci 2011;56:220-6. 10.1007/s10620-010-1272-8. [DOI] [PubMed] [Google Scholar]
- 29. de Franchis R, Baveno VI Faculty . Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol 2015;63:743-52. 10.1016/j.jhep.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 30. Jensen DM. Endoscopic screening for varices in cirrhosis: findings, implications, and outcomes. Gastroenterology 2002;122:1620-30. 10.1053/gast.2002.33419. [DOI] [PubMed] [Google Scholar]
- 31.Tajiri T, Yoshida H, Obara K, et al. General rules for recording endoscopic findings of esophagogastric varices (2nd edition). Digestive Endoscopy 2010;22:1-9. 10.1111/j.1443-1661.2009.00929.x [DOI] [PubMed]
- 32. Ramirez FC, Hakim S, Tharalson EM, Shaukat MS, Akins R. Feasibility and safety of string wireless capsule endoscopy in the diagnosis of esophageal varices. Am J Gastroenterol 2005;100:1065-71. 10.1111/j.1572-0241.2005.41037.x. [DOI] [PubMed] [Google Scholar]
- 33. Aoyama T, Oka S, Aikata H, et al. Is small-bowel capsule endoscopy effective for diagnosis of esophagogastric lesions related to portal hypertension? J Gastroenterol Hepatol 2014;29:511-6. 10.1111/jgh.12372. [DOI] [PubMed] [Google Scholar]
- 34. De Palma GD, Rega M, Masone S, et al. Mucosal abnormalities of the small bowel in patients with cirrhosis and portal hypertension: a capsule endoscopy study. Gastrointest Endosc 2005;62:529-34. 10.1016/S0016-5107(05)01588-9. [DOI] [PubMed] [Google Scholar]
- 35. Jeon SR, Kim JO. Capsule Endoscopy for Portal Hypertensive Enteropathy. Gastroenterol Res Pract 2016;2016:8501394. 10.1155/2016/8501394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Akyuz F, Pinarbasi B, Ermis F, et al. Is portal hypertensive enteropathy an important additional cause of blood loss in portal hypertensive patients? Scand J Gastroenterol 2010;45:1497-502. 10.3109/00365521.2010.510568. [DOI] [PubMed] [Google Scholar]
- 37. Kunihara S, Oka S, Tanaka S, et al. Predictive Factors of Portal Hypertensive Enteropathy Exacerbation in Patients with Liver Cirrhosis: A Capsule Endoscopy Study. Digestion 2018;98:33-40. 10.1159/000486666. [DOI] [PubMed] [Google Scholar]
- 38. Aoyama T, Oka S, Aikata H, et al. Major predictors of portal hypertensive enteropathy in patients with liver cirrhosis. J Gastroenterol Hepatol 2015;30:124-30. 10.1111/jgh.12658. [DOI] [PubMed] [Google Scholar]
- 39. Otani I, Oka S, Tanaka S, et al. Clinical significance of small-bowel villous edema in patients with liver cirrhosis: A capsule endoscopy study. J Gastroenterol Hepatol 2018;33:825-30. 10.1111/jgh.14016. [DOI] [PubMed] [Google Scholar]
- 40. Jiang X, Qiu XO, Li Z, et al. Small-sized versus standard magnetic capsule endoscopy in adults: a two-center, double-blinded randomized controlled trial. Endoscopy 2023;55:52-7. 10.1055/a-1881-4369. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information: Additional information, tables, and figures
Data Availability Statement
The steering committee of the CENTERS study will consider reasonable requests for the sharing of deidentified individual participant data. Requests should be made to the corresponding author.