Abstract
A single‐center, phase I, partially double‐blind (double‐blind regarding doses of rimegepant and placebo, and open label with respect to moxifloxacin), randomized, 12‐sequence, four‐period crossover study of therapeutic (75 mg) and supratherapeutic (300 mg) doses of rimegepant with placebo and moxifloxacin (400 mg) controls was designed to evaluate drug effect on the Fridericia corrected QT (QTcF) interval in healthy fasted adults. A total of 38 participants were randomized and dosed in the study. Electrocardiogram (ECG) data were available from 37 participants in the rimegepant 75‐mg group, 38 participants in the rimegepant 300‐mg group, and 36 participants in the moxifloxacin and placebo groups. Both the 75‐ and 300‐mg doses of rimegepant had no clinically relevant effect on ECG parameters, including QTcF, heart rate, PR and QRS interval, T‐wave morphology, and U‐wave presence. All upper 90% confidence intervals for the QTcF effect with rimegepant were less than or equal to 4.69 ms, well below the 10‐ms threshold for potential clinical significance. Assay sensitivity was demonstrated by the QT effect of moxifloxacin. Using both by‐timepoint and concentration‐QTc analysis, a placebo‐corrected change‐from‐baseline QTcF greater than 10 ms could be excluded for rimegepant plasma concentrations up to ~10,000 ng/mL, representing concentrations at least 10.8‐fold the maximum observed concentration of the 75‐mg therapeutic dose of rimegepant.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
Rimegepant (Nurtec ODT; Pfizer) is an oral small molecule CGRP receptor antagonist and the first migraine treatment approved for acute and preventive treatment.
WHAT QUESTION DID THIS STUDY ADDRESS?
Identification of a drug's propensity to cause QT prolongation, a significant risk to patients, is an important part of drug development. Therefore, this study evaluated the effect of therapeutic (75 mg) and supratherapeutic (300 mg) rimegepant doses on Fridericia corrected QT (QTcF) interval in healthy adults, along with pharmacokinetics and safety of single‐dose rimegepant.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
A single dose up to 300‐mg rimegepant does not have a clinically relevant effect on electrocardiogram parameters. A QTcF effect (ΔΔQTcF) greater than 10 ms, the threshold of regulatory concern, can be excluded for rimegepant plasma concentrations up to ~10,000 ng/mL (18.71 μM), at least 10.8‐fold the maximum concentration of the 75‐mg therapeutic dose.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
This study supports cardiovascular safety of 75‐mg rimegepant which is approved for acute and preventive migraine treatment.
INTRODUCTION
Migraine is a chronic, debilitating primary headache disorder that affects one in six people in the United States. 1 Calcitonin gene‐related peptide (CGRP), an endogenous 37 amino acid peptide contained within pain signaling nociceptive afferents, plays a key role in migraine pathophysiology. 1 , 2 CGRP antagonist medications have demonstrated efficacy in the acute and preventive treatment of migraine. 3 Rimegepant is an orally administered small molecule CGRP receptor antagonist. Rimegepant is the first migraine treatment approved for use as an acute and preventive medication. The efficacy and safety of rimegepant have been demonstrated in several randomized, placebo‐controlled clinical trials. 4 , 5 , 6 , 7 , 8
Rimegepant pharmacokinetics (PK) at the therapeutic dose of 75 mg have been published. 7 , 9 , 10 , 11 , 12 , 13 , 14 The geometric mean maximum observed plasma concentration (C max) is ~850 ng/mL after a single 75 mg dose with a time to C max (T max) of ~1.5 h. 7 The area under the concentration‐time curve (AUC) from 0 extrapolated to infinity is ~4500 ng∙h/mL. 10 The half‐life of rimegepant is ~11 h. 12 The accumulation index is 1.5, with a more than dose proportional increase in exposure after multiple doses. 9 The geometric mean apparent total clearance is ~300 mL/min after single and multiple daily doses. 9 The steady‐state volume of distribution is 120 L. 13 Rimegepant is ~96% bound to human plasma proteins. 14 Food can delay T max and reduce rimegepant exposure but does not change the half‐life. 15 No clinically significant differences in PK are observed based on gender. 15 Rimegepant is metabolized by cytochrome P450 (CYP) 3A4 and to a lesser extent by CYP2C9 and is primarily eliminated unchanged. 15 Administration with strong CYP3A4 inhibitors or inducers should be avoided. Another dose of rimegepant within 48 h of a moderate CYP3A4 inhibitor should be avoided. 15
Drug‐induced QT prolongation can present a substantial risk to patients. Identification of a drug's propensity to cause QT prolongation is an important part of drug development. 16 , 17 Preclinical in vitro and in vivo studies with rimegepant indicated a low potential for rimegepant to cause clinically meaningful QT prolongation. 18 In addition, in the rimegepant single ascending dose study (25, 75, 150, 300, 600, 900, and 1500 mg) and multiple ascending dose study (75, 150, 300, 450, and 600 mg), no dose‐related trend in mean placebo‐corrected change‐from‐baseline Fridericia corrected QT (ΔΔQTcF) was observed in any dose group. 9 Therefore, the present study evaluated the effect of therapeutic (75 mg) and supratherapeutic (300 mg) doses of rimegepant on the QTcF interval in healthy adults, along with the PK, safety, and tolerability of single‐dose rimegepant. The study included moxifloxacin, widely used as a positive control in clinical studies of QTc prolongation, to establish assay sensitivity. 17
METHODS
Study design and participants
This was a single‐center, phase I, partially double‐blind (double‐blind regarding doses of rimegepant and placebo, and open label with respect to moxifloxacin), randomized, 12‐sequence, four‐period crossover study of therapeutic (75 mg) and supratherapeutic (300 mg) doses of rimegepant with placebo and moxifloxacin controls, all administered under fasted conditions.The first participant was dosed on August 5, 2018, with the last participant's visit on October 10, 2018. Moxifloxacin (400 mg) was selected as a positive control for this study. A single‐dose crossover design with a washout period of at least 7 days was determined to be adequate to avoid carry‐over effects from one period to the next. The supratherapeutic dose was determined in accordance with regulatory guidance and rimegepant exposure observed in a phase I single and multiple ascending dose study conducted in healthy participants with the original formulation of rimegepant at doses up to 1500 mg. A single 300‐mg rimegepant dose (4‐fold the 75‐mg therapeutic dose) produced an ~4.3‐ and 7.0‐fold increase in C max and AUC, respectively, of the 75‐mg rimegepant therapeutic dose. 19 Therefore, the 300‐mg rimegepant dose is a supratherapeutic dose that supports safety at C max exposures significantly above those identified from intrinsic or extrinsic factors.
The primary objective of the study was to evaluate the effect of therapeutic and supratherapeutic single rimegepant doses on the QTcF interval. Secondary objectives included assessment of PK, safety, and tolerability of single‐dose (75 and 300 mg) rimegepant, and the effects of single‐dose rimegepant on heart rate (HR), QRS and PR interval, T‐wave morphology and U‐wave presence.
This phase I study was conducted in Canada in compliance with Good Clinical Practice as referenced in the International Council for Harmonization (ICH) guidelines (E6), Good Laboratory Practice as referenced in the ICH guidelines, and all applicable regulations, including the Federal Food, Drug, and Cosmetic Act, US applicable Code of Federal Regulations Title 21, and any International Electrotechnical Commission requirements relative to clinical studies. The study was also conducted in compliance with the recommendations specified in the most recent version of the Declaration of Helsinki, with the exception of registration in a publicly accessible database as registration of phase I studies is not mandatory. The study protocol was reviewed and approved by Advarra, an independent institutional review board located in Columbia, MD. Informed consent was obtained from all study participants prior to start of the study.
Healthy adult, nonsmokers, between the ages of 18 and 55 years (inclusive), with a body mass index (BMI) greater than 18.5 and less than 30.0 kg/m2, body weight greater than or equal to 50.0 kg for men and greater than or equal to 45.0 kg for women, and a score of 0 on the Sheehan Suicidality Tracking Scale (S‐STS), 20 , 21 were eligible for the study.
Participants were randomized to receive study treatment under fasted conditions based on a four‐period, 12‐sequence, block randomization scheme using randomly assigned numbers corresponding to a previously generated randomization scheme generated by inVentiv, with a 7‐day washout between periods. Participants were administered treatment under fasted conditions in four treatment groups: group T received a therapeutic dose of 75‐mg rimegepant (1 × 75‐mg tablet and 3 matching placebo tablets); group ST received a supratherapeutic dose of 300‐mg rimegepant (4 × 75‐mg tablets), group P received placebo (4 rimegepant‐matching placebo tablets), and group M received moxifloxacin (400 mg).
The study included four patient populations (Figure 1). The safety population was defined as all participants who received at least one dose of rimegepant, moxifloxacin, or placebo. The QT/QTc population was defined as all participants in the safety population with QTcF measurements at baseline as well as on‐treatment, with at least one postdose timepoint with a valid change‐from‐baseline QTcF (ΔQTcF) value. The QT/QTc population was utilized for the by‐timepoint and categorical analyses of cardiodynamic electrocardiogram (ECG) parameters. The PK population was defined as all participants completing at least three periods, including at least rimegepant 75 mg, rimegepant 300 mg, and moxifloxacin 400 mg, and for whom the PK profile could be adequately characterized. The PK/QTc population included all participants who were in both the PK concentration population (those who received a dose of rimegepant or moxifloxacin and provided greater than or equal to 1 evaluable PK concentration for rimegepant or moxifloxacin) and the QT/QTc population with greater than or equal to one pair of matched postdose PK and QTcF data from the same timepoint.
FIGURE 1.
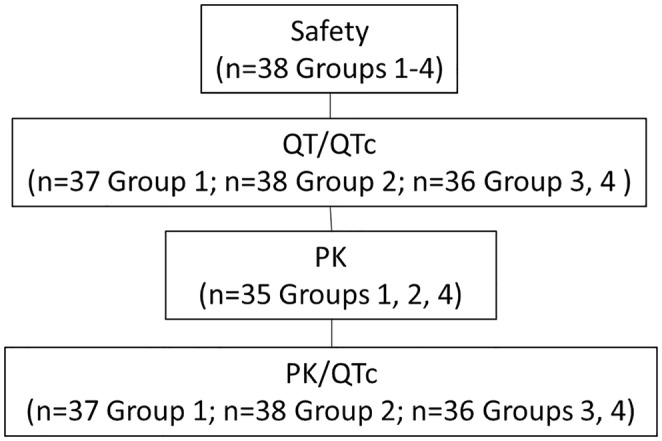
Analysis populations. M, moxifloxacin (400 mg); P, placebo (4 rimegepant‐matching placebo tablets); PK, pharmacokinetic; QTc, corrected QT interval; ST, rimegepant supratherapeutic dose (300 mg; 4 × 75 mg rimegepant tablets); T, rimegepant therapeutic dose (75 mg; 1 × 75 mg rimegepant tablet and 3 matching placebo tablets).
Electrocardiography
The cardiodynamic evaluation was performed with 12‐lead ECGs extracted from continuous recordings at prespecified timepoints (3 ECG timepoints prior to dosing [−45, −30, and −15 min] averaged as the baseline value) and 0.5, 1, 1.5, 2, 2.5, 3, 3.5, 4, 5, 6, 12, and 24 h postdose and paired with PK samples. At the central ECG laboratory (ERT), up to 10 replicate ECGs were extracted at each timepoint on Day 1 in each treatment period. The 12‐lead Holter and ECG equipment were supplied and supported by ERT. All ECG data were collected using Global Instrumentation's M12R ECG continuous 12‐lead digital recorder with data stored onto secure digital memory cards. ERT used TQT Plus, an advanced computer‐assisted, statistical process, to extract ECGs from continuous 24‐h recordings collected in thorough QT studies. Periods of stable HR on the continuous 12‐lead ECG tracing within the 5‐min extraction window were identified to decrease variability and noise. Participants were resting in a supine position for greater than or equal to 10 min prior to and 5 min after each timepoint for ECG extractions. Participants were required to avoid postural changes during ECG recordings.
Statistical analysis of QT/QTc
A sample size of 32 evaluable participants provided greater than 95% power to exclude that rimegepant causes a greater than 10‐ms QTc effect at plasma levels that are clinically relevant, established by the upper bound of the two‐sided 90% confidence interval (CI) of the model‐predicted QTc effect (ΔΔQTcF) at the observed geometric mean C max of rimegepant. 22 , 23 A small underlying effect of rimegepant of 3 ms and a standard deviation (SD) of the ΔQTcF of 8 ms were assumed.
Moxifloxacin, a reversible blocker of the rapid component of the delayed rectifier potassium current of the cardiac inward‐rectifier potassium channel (IKr), is widely used as a positive control agent in studies evaluating the effects of investigational products on QT interval. 22 , 24 , 25 In this study, moxifloxacin (400 mg) was used as a positive drug control to establish assay sensitivity.
The primary ECG end point for the cardiodynamic ECG assessment was ΔΔQTcF. The ΔΔQTcF was generated using the individual ΔQTcF for placebo calculated at a specific timepoint, subtracted from ΔQTcF for the same participant on rimegepant at the same timepoint. Secondary end points included change‐from‐baseline HR, QTcF, and PR and QRS intervals (ΔHR, ΔQTcF, ΔPR, and ΔQRS), placebo‐corrected change‐from‐baseline HR and PR and QRS intervals (ΔΔHR, ΔΔPR, and ΔΔQRS), and frequency of changes in T‐wave morphology and U‐wave presence.
Pharmacokinetics
Blood samples for PK analysis were collected at baseline and 0.5, 1, 1.5, 2, 2.5, 3, 3.5, 4, 5, 6, 12, and 24 h postdose in each treatment period. PK parameters included AUC from time 0 to the last quantifiable concentration timepoint (AUC0–t ), AUC extrapolated from time 0 to infinity (AUC0–inf), C max, residual area, and T max. PK parameters were calculated by standard noncompartmental methods. PK analyses were performed using Phoenix WinNonlin version 8.0. The safety data tables and listings, as well as PK tables and listings, were created using SAS version 9.2. PK figures were created using R version 3.5.
Rimegepant bioanalytical assay
Rimegepant was measured in human ethylenediaminetetraacetic (EDTA) K2 plasma using rimegepant‐13C2‐d4 as the internal standard. Sample extraction was automated protein precipitation. The ultra‐performance liquid chromatography (UPLC) column was an Acquity UPLC BEH C18 (50 × 2.1 mm, 1.7 μM). Mobile phase A and B were Milli‐Q type water and acetonitrile with ammonium acetate and acetic acid (in different proportions). The within‐run accuracy was −0.64 to 5.00% (coefficient of variation [CV]: 2.09 to 4.30%). The between‐run accuracy was −2.49 to 3.39% (CV: 3.28 to 6.96%). The lower limit of quantification for rimegepant was 10 ng/mL using a 0.050‐mL aliquot of human plasma, and the upper limit of quantification was 5000 ng/mL.
Cardiodynamic ECG assessment
The primary analysis utilized concentration‐QTc modeling of the rimegepant and ΔΔQTcF relationship with the aim to exclude an effect greater than 10 ms at clinically relevant rimegepant plasma concentrations. 19 The modeling analysis included only timepoints with a matching placebo value.
Using the intersection union test, the effect of rimegepant on ΔΔQTcF was evaluated at each postdose timepoint (“by‐timepoint” analysis). An analysis of categorical outliers was performed for changes in HR, PR and QRS intervals, QTcF, T‐wave morphology, and U‐wave presence.
A model similar to the primary analysis was used to assess assay sensitivity evaluated by concentration‐QTc analysis of the moxifloxacin effect on ΔΔQTcF. Assay sensitivity requirements were met if the slope of the concentration‐QTc relationship was statistically significant at the 10% level in a two‐sided test and the predicted QT effect (i.e., the lower bound of the 2‐sided 90% CI) was above 5 ms at the observed geometric mean C max of 400‐mg moxifloxacin.
Linear mixed‐effects modeling was used to explore the relationship between rimegepant plasma concentration and ΔΔQTcF, with ΔΔQTcF as the dependent variable. Using QTcF, the 90% CIs and mean differences between the baseline‐adjusted QT interval durations of treatment and placebo were calculated and a concentration‐QT model analysis was performed on the rimegepant and moxifloxacin datasets.
Safety analysis
Rimegepant safety and tolerability were evaluated by assessing adverse events (AEs), clinical laboratory parameters (biochemistry, hematology, and urinalysis), 12‐lead safety ECG, S‐STS, physical examination findings, and vital signs.
RESULTS
Demographics
A total of 38 participants were randomized and dosed in the study. Because this was a crossover study, no notable differences in demographic characteristics (age, gender, ethnicity, race, height, weight, and BMI) were observed between the safety and PK populations. A summary of these characteristics for the overall population (safety population, N = 38) is presented in Table 1. The majority of participants were White (92.1%), not Hispanic or Latino (78.9%), and men (57.9%). The mean age was 42.2 years, and the mean BMI was 25.9 kg/m2. All the participants who received at least one dose of rimegepant, moxifloxacin, or placebo comprised the safety population (N = 38); 33 (86.8%) participants completed all the treatment periods.
TABLE 1.
Participant demographic and baseline characteristics (safety population).
| Category | Statistic | Rimegepant 75 mg | Rimegepant 300 mg | Moxifloxacin 400 mg | Placebo | Overall |
|---|---|---|---|---|---|---|
| Age (years) | N | 37 | 38 | 36 | 36 | 38 |
| Mean (SD) | 42.6 (8.8) | 42.2 (9.1) | 42.2 (9.3) | 41.7 (9.1) | 42.2 (9.1) | |
| Age groups | ||||||
| <18 | n (%) | 0 | 0 | 0 | 0 | 0 |
| 18–40 | n (%) | 12 (32.4) | 13 (34.2) | 12 (33.3) | 13 (36.1) | 13 (34.2) |
| >40 | n (%) | 25 (67.6) | 25 (65.8) | 24 (66.7) | 23 (63.9) | 25 (65.8) |
| Gender | ||||||
| Female | n (%) | 16 (43.2) | 16 (42.1) | 15 (41.7) | 15 (41.7) | 16 (42.1) |
| Male | n (%) | 21 (56.8) | 22 (57.9) | 21 (58.3) | 21 (58.3) | 22 (57.9) |
| Ethnicity | ||||||
| Not Hispanic or Latino | n (%) | 29 (78.4) | 30 (78.9) | 28 (77.8) | 29 (80.6) | 30 (78.9) |
| Hispanic or Latino | n (%) | 8 (21.6) | 8 (21.1) | 8 (22.2) | 7 (19.4) | 8 (21.1) |
| Race | ||||||
| White | n (%) | 34 (91.9) | 35 (92.1) | 33 (91.7) | 33 (91.7) | 35 (92.1) |
| Black or African American | n (%) | 3 (8.1) | 3 (7.9) | 3 (8.3) | 3 (8.3) | 3 (7.9) |
| Height (cm) | N | 37 | 38 | 36 | 36 | 38 |
| Mean (SD) | 167.65 (8.14) | 167.68 (8.04) | 167.69 (8.19) | 167.69 (8.11) | 167.68 (8.04) | |
| Weight (kg) | N | 37 | 38 | 36 | 36 | 38 |
| Mean (SD) | 72.88 (9.07) | 72.87 (8.95) | 72.93 (9.09) | 72.53 (8.90) | 72.87 (8.95) | |
| BMI (kg/m2) | N | 37 | 38 | 36 | 36 | 38 |
| Mean (SD) | 25.889 (2.113) | 25.875 (2.086) | 25.893 (2.137) | 25.752 (2.073) | 25.875 (2.086) | |
Note: Last results (scheduled or unscheduled) obtained prior to the administration of study drug were used to generate this table.
Abbreviations: BMI, body mass index; N, number of participants dosed; n (%), number and percent of participants; SD, standard deviation.
Overall: Included counts from all treatment groups.
Electrocardiography
ECG data were available from 37 participants in the rimegepant 75‐mg group, 38 participants in the rimegepant 300‐mg group, and 36 participants each in the moxifloxacin 400‐mg and placebo groups. Baseline ECG values were within normal limits for a healthy population. Across treatment periods, mean HR was 54.1–55.2 beats per minute (bpm), mean PR interval was 139.8–142.7 ms, mean QRS interval was 105.0–105.5 ms, and mean QTcF interval was 405.7–407.5 ms. A single therapeutic (75 mg) and supratherapeutic (300 mg) dose of rimegepant had no clinically relevant effect on ECG parameters, including QTcF, HR, PR interval, and QRS duration. In addition, there were no treatment‐emergent T‐ or U‐wave morphology changes.
Mean ΔQTcF values for rimegepant were negative at all postdose timepoints, with a single exception of a ΔQTcF value of 0.2 ms at 5 h postdose among participants dosed with 300 mg. The mean ΔΔQTcF values for 75‐ and 300‐mg rimegepant and 400‐mg moxifloxacin are shown in Figure 2. The mean rimegepant plasma concentration for the 300‐mg supratherapeutic dose is presented in Figure 2 to illustrate the time course of rimegepant exposure. For rimegepant, mean ΔΔQTcF was small (−1.4 ms at 2 h postdose, to 2.0 ms at 3.5 h after dosing with rimegepant 75 mg). ΔΔQTcF was within ±1.6 ms after treatment with rimegepant 300 mg. All upper 90% CIs for the QTc effect with rimegepant were less than or equal to 4.69 ms, well below the 10‐ms threshold for potential clinical significance (Table 2). For moxifloxacin, there was a clear increase in mean ΔΔQTcF with a peak of 14.1 ms (90% CI: 12.10–16.20) at 3.5 h postdose (Figure 2; Table 2).
FIGURE 2.
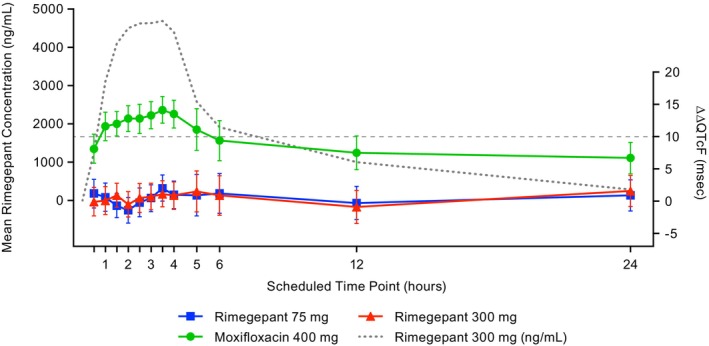
Mean plasma concentrations over 24 h for rimegepant 300 mg and ΔΔQTcF across time points (PK/QTc population). Error bars for ΔΔQTcF are 90% CI from statistical modeling. Mean concentration curve is overlaid to illustrate the time course of rimegepant exposure. CI, confidence interval; PK, pharmacokinetics; ΔΔQTcF, placebo‐corrected change‐from‐baseline in Fridericia corrected QT interval.
TABLE 2.
ΔΔQTcF in ms (QT/QTc population).
| Timepoint postdose (h) | Statistics | Rimegepant 75 mg | Rimegepant 300 mg | Moxifloxacin 400 mg |
|---|---|---|---|---|
| 0.5 | LS mean | 1.2 | −0.1 | 8.1 |
| 90% CI | (−1.05, 3.38) | (−2.29, 2.12) | (5.90, 10.37) | |
| 1.0 | LS mean | 0.6 | 0.1 | 11.6 |
| 90% CI | (−1.58, 2.80) | (−2.07, 2.27) | (9.37, 13.78) | |
| 1.5 | LS mean | −0.7 | 0.9 | 12.0 |
| 90% CI | (−2.54, 1.22) | (−0.94, 2.79) | (10.10, 13.89) | |
| 2 | LS mean | −1.4 | −0.5 | 12.8 |
| 90% CI | (−3.39, 0.55) | (−2.44, 1.47) | (10.80, 14.78) | |
| 2.5 | LS mean | −0.2 | 0.5 | 12.8 |
| 90% CI | (−2.42, 2.06) | (−1.72, 2.72) | (10.50, 15.00) | |
| 3 | LS mean | 0.5 | 0.7 | 13.3 |
| 90% CI | (−1.62, 2.54) | (−1.33, 2.79) | (11.23, 15.42) | |
| 3.5 | LS mean | 2.0 | 1.1 | 14.1 |
| 90% CI | (0.01, 4.06) | (−0.87, 3.16) | (12.10, 16.20) | |
| 4 | LS mean | 1.0 | 0.9 | 13.5 |
| 90% CI | (−1.16, 3.10) | (−1.26, 2.98) | (11.33, 15.64) | |
| 5 | LS mean | 0.9 | 1.5 | 11.1 |
| 90% CI | (−2.27, 4.15) | (−1.66, 4.69) | (7.88, 14.32) | |
| 6 | LS mean | 1.2 | 0.9 | 9.4 |
| 90% CI | (−1.86, 4.27) | (−2.17, 3.92) | (6.27, 12.47) | |
| 12 | LS mean | −0.3 | −0.9 | 7.5 |
| 90% CI | (−2.85, 2.30) | (−3.44, 1.68) | (4.89, 10.10) | |
| 24 | LS mean | 0.9 | 1.6 | 6.7 |
| 90% CI | (−1.51, 3.31) | (−0.80, 3.98) | (4.23, 9.10) |
Abbreviations: CI, confidence interval; LS, least squares; ΔΔQTcF, placebo‐corrected change‐from‐baseline in Fridericia corrected QT interval.
Concentration‐QTc analysis was based on ΔΔQTcF. Figure 3 shows the relationship between the individual observed rimegepant plasma concentrations and ΔΔQTcF.
FIGURE 3.
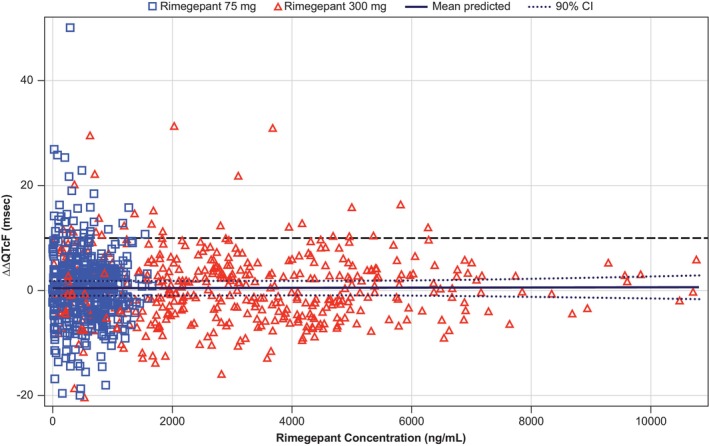
Scatter plot of observed rimegepant plasma concentrations and ΔΔQTcF by participant (PK/QTc population). The solid black line with dashed black lines denotes the model‐predicted mean ΔΔQTcF with 90% CI. The blue squares and red triangles denote the pairs of observed rimegepant plasma concentrations and observed ΔΔQTcF by participants for the rimegepant 75 mg and rimegepant 300 mg treatment periods, respectively. ΔΔQTcF, placebo‐corrected change‐from‐baseline in Fridericia corrected QT interval; CI, confidence interval; PK, pharmacokinetic; QTc, corrected QT interval.
Mean ΔΔHR was small and ranged from −0.2 bpm at 6 h postdose for 300‐mg rimegepant to 2.9 bpm at 1‐h postdose for 400‐mg moxifloxacin. Rimegepant at the study doses did not have an effect on cardiac conduction (i.e., PR and QRS intervals). Mean ΔΔPR values ranged from −1.7 ms (24 h postdose) to 2.0 ms (6 h postdose), both after dosing with 300‐mg rimegepant. Mean ΔQRS values were small and mean ΔΔQRS values were within ±0.6 ms across all postdose timepoints.
Pharmacokinetic and pharmacodynamic analysis
The C max and AUC0–t of rimegepant oral tablets administered as a single 300‐mg supratherapeutic dose produced an ~5.7 to 7.1‐fold increase in the C max and AUC0–t , respectively, over the 75‐mg therapeutic dose of rimegepant (Table 3). The mean (SD) C max of rimegepant was 924 (333) ng/mL and 5245 (1868) ng/mL at the 75‐ and 300‐mg dose, respectively. The mean (SD) AUC0–t was 5168 (1650) h*ng/mL and 36,907 (12,836) h*ng/mL at the 75‐ and 300‐mg dose, respectively. The mean (SD) AUC0–inf was 5354 (1735) h*ng/mL and 39,750 (15,291) h*ng/mL at the 75‐ and 300‐mg dose, respectively. Although the peak rimegepant concentration was observed ~29 min later for the 300‐mg dose with a median T max of 2.60 h postdose compared to a median T max of 2.11 h postdose for the 75‐mg dose, the range of the T max for the 300‐mg dose is within the range of the T max for the 75‐mg dose.
TABLE 3.
PK parameters in rimegepant‐treated participants (PK population).
| Rimegepant 75 mg (N = 35) | Rimegepant 300 mg (N = 35) | |
|---|---|---|
| C max, ng/mL, mean (SD) | 924 (333) | 5245 (1868) |
| AUC0–t , h*ng/mL, mean (SD) | 5168 (1650) | 36,907 (12,836) |
| AUC0–inf, h*ng/mL, mean (SD) | 5354 (1735) | 39,750 (15,291) |
| T max, h, median (min, max) | 2.11 (0.61, 4.10) | 2.60 (1.10, 3.62) |
Abbreviations: AUC0–inf, area under the concentration‐time curve from time 0 to infinity (extrapolated); AUC0–t , area under the concentration‐time curve from time 0 to the last quantifiable concentration timepoint; C max, maximum observed plasma concentration; h, hour; max, maximum; min, minimum; N, number of observations; PK, pharmacokinetic; SD, standard deviation; T max, time of observed maximum plasma concentration.
A linear model with an intercept provided a reasonable data fit in the concentration‐QTc analysis (Figure 3, Figures S1–S3, Table S1). The concentration‐QTc relationship slope was shallow and not statistically significant (0.00002 ms per ng/mL [90% CI: −0.00022 to 0.00027]) with an intercept of 0.4 ms that was also not statistically significant. The geometric mean peak plasma level was 885 and 4963 ng/mL for 75‐ and 300‐mg, respectively (Figure 4). The model‐predicted ΔΔQTcF effect at the geometric mean peak plasma concentration was 0.45 ms (90% CI: −1.11 to 2.01) and 0.54 ms (90% CI: −0.96 to 2.05) for 75‐ and 300‐mg, respectively. Based on this concentration‐QTc analysis, a ΔΔQTcF greater than 10 ms can be excluded for rimegepant plasma concentrations up to ~10,000 ng/mL (18.71 μM).
FIGURE 4.
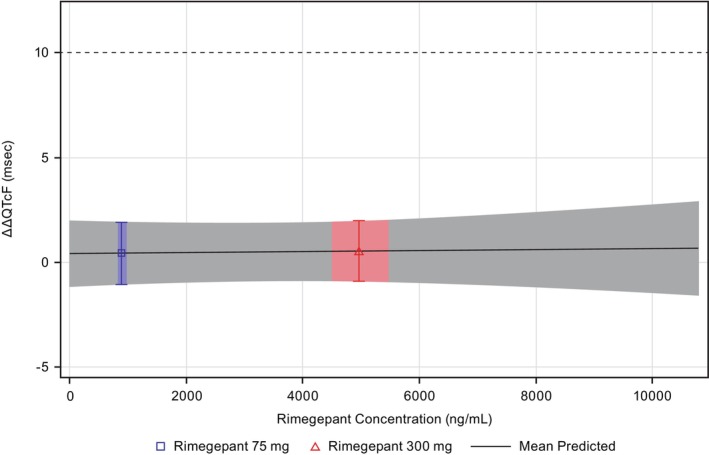
Predicted ΔΔQTcF interval at geometric mean peak rimegepant concentration (PK/QTc population). The blue and red areas denote the estimated mean (90% CI) ΔΔQTcF at the geometric mean (90% CI) C max of rimegepant for the 75‐ and 300‐mg dose levels, respectively. The solid black line with gray shaded area denotes the model‐predicted mean (90% CI) ΔΔQTcF. ΔΔQTcF, placebo‐corrected change‐from‐baseline in Fridericia corrected QT interval; C max, maximum plasma concentration; PK, pharmacokinetic; QTc, corrected QT interval.
Safety and tolerability
There were no deaths or other serious AEs reported in the study. Seventeen (44.7%) participants experienced at least one treatment‐emergent AE (TEAE). The majority of TEAEs (33/37, 89.2%) were mild; two placebo‐treated participants reported four moderate TEAEs. The majority of the TEAEs resolved spontaneously by the end of the study. Of the 26 TEAEs considered to be study‐drug related, 12 were considered to be possibly related to rimegepant. The most frequently reported TEAE considered possibly related to treatment was headache (15.8%), affecting one participant treated with 75‐mg rimegepant and four participants treated with 300‐mg rimegepant; no other TEAE was reported by greater than one participant. There were no reports of alanine aminotransferase, alkaline phosphatase, or aspartate aminotransferase values greater than three times the upper limit of normal (ULN), and no participants had total bilirubin levels greater than two times the ULN. No clinically meaningful changes in laboratory parameters, vital signs, physical measurements, or safety ECGs were identified. The S‐STS total score was 0 for all participants at screening and at study exit.
DISCUSSION
This randomized, partially double‐blind, placebo‐controlled, four‐period crossover study demonstrated that a single dose of up to 300‐mg rimegepant (supratherapeutic dose) does not have a clinically relevant effect on ECG parameters including HR, PR, QRS, QTcF, T‐wave morphology, or U‐wave presence. Using both by‐timepoint and concentration‐QTc analysis, a QTcF effect (ΔΔQTcF) greater than 10 ms, the threshold of regulatory concern, 19 could be excluded for rimegepant plasma concentrations up to ~10,000 ng/mL (18.71 μM); all QTcF effects were less than or equal to 2 ms. The safety profiles of orally administered single therapeutic and supratherapeutic doses of rimegepant were comparable to that of placebo in healthy adult participants.
Consistent with the previously demonstrated favorable safety profile of rimegepant, administration in this trial of single doses of rimegepant, 75 and 300 mg, was safe and generally well‐tolerated. 8 , 26 A single 300‐mg rimegepant dose (4‐fold the 75‐mg therapeutic dose) produced an ~4.3‐ and 7.0‐fold increase in the C max and AUC, respectively, of the 75‐mg rimegepant therapeutic dose. 19 A 300‐mg rimegepant dose is therefore a supratherapeutic dose that supports cardiovascular safety at C max exposures significantly above those associated with labeled therapeutic use.
The results of this clinical thorough QT study demonstrating that even a supratherapeutic dose of rimegepant does not have an effect on QTcF interval in healthy adults, corroborate the results from preclinical in vitro and in vivo studies with rimegepant which indicated a low potential for rimegepant to cause clinically meaningful QT prolongation. 18 In in vitro studies, 30 μM rimegepant (>20× the repeat‐dose 75 mg human C max) was a weak human ether‐a‐go‐go related gene inhibitor and had no effect on action potentials in rabbit Purkinje fibers. In ex vivo studies, 3 to 10 μM rimegepant showed no vasoconstriction of human coronary or cerebral arteries. In in vivo studies in cynomolgus monkeys, single doses and 9‐month daily doses had no effect on hemodynamic/electrocardiographic parameters. In addition, pooled results from the three clinical trials showed no cardiovascular AEs. 18
A potential limitation of the study is the enrollment of a healthy, largely White, adult population that may not share the same comorbidities or cardiovascular conditions as a migraine population.
In conclusion, in healthy adults, a single therapeutic, as well as a supratherapeutic, dose of rimegepant had no clinically meaningful effect on ECG parameters, including QTcF. The single therapeutic and supratherapeutic doses of rimegepant were safe and well‐tolerated with safety profiles comparable to placebo.
AUTHOR CONTRIBUTIONS
B.A.M., M.S.H., and R.S.C. designed the research. R.Bh., and R.S.C. performed the research. M.S.H., R.Be., R.S.C., and J.L. analyzed the data. All authors wrote the manuscript.
FUNDING INFORMATION
This study was sponsored by Biohaven, which was acquired by Pfizer in October 2022.
CONFLICT OF INTEREST STATEMENT
M.S.H., B.A.M., and R.Bertz are employees of Biohaven and own Biohaven stock and/or stock options. R.S.C. was an employee of Biohaven Pharmaceuticals, owns stock in Biohaven Ltd, was an employee of Pfizer, has received research payments from Pfizer, and provides services to Collima LLC which has had consulting agreements with Pfizer, Aptose Biosciences Inc., Manistee Therapeutics, and Vida Ventures Management Co., LLC. R.Bhardwaj is an employee of Certara Strategic Consulting and was a paid consultant to Biohaven acquired by Pfizer in October 2022, for this study. J.L. and K.T.M. are employed by and hold stock/options in Pfizer.
Supporting information
Data S1
ACKNOWLEDGMENTS
Medical writing support was provided by Amy C. Porter, PhD, ISMPP, CMPP, and Scott C. Thompson, ELS, of Certara Synchrogenix, funded by Biohaven Pharmaceuticals Inc. which was acquired by Pfizer Inc. in October 2022.
Bhardwaj R, Hanna MS, Morris BA, et al. No clinically relevant electrocardiogram effects in a randomized TQT study of single therapeutic/supratherapeutic rimegepant doses in healthy adults. Clin Transl Sci. 2024;17:e13727. doi: 10.1111/cts.13727
Data from this study were presented at the 19th International Headache Congress, September 5–8, 2019, Dublin, Ireland (Poster No. IHC‐PO‐131).
DATA AVAILABILITY STATEMENT
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions and exceptions, Pfizer may also provide access to the related individual de‐identified participant data. See https://www.pfizer.com/science/clinical‐trials/trial‐data‐and‐results for more information.
REFERENCES
- 1. Peters GL. Migraine overview and summary of current and emerging treatment options. Am J Manag Care. 2019;25:S23‐S34. [PubMed] [Google Scholar]
- 2. International Headache Society . Headache Classification Committee of the International Headache Society (IHS) the International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38:1‐211. doi: 10.1177/0333102417738202 [DOI] [PubMed] [Google Scholar]
- 3. Ailani J, Burch RC, Robbins MS. The American headache society consensus statement: update on integrating new migraine treatments into clinical practice. Headache. 2021;61:1021‐1039. doi: 10.1111/head.14153 [DOI] [PubMed] [Google Scholar]
- 4. Croop R, Goadsby PJ, Stock DA, et al. Efficacy, safety, and tolerability of rimegepant orally disintegrating tablet for the acute treatment of migraine: a randomised, phase 3, double‐blind, placebo‐controlled trial. Lancet. 2019;394:737‐745. doi: 10.1016/s0140-6736(19)31606-x [DOI] [PubMed] [Google Scholar]
- 5. Lipton RB, Croop R, Stock EG, et al. Rimegepant, an oral calcitonin gene‐related peptide receptor antagonist, for migraine. N Engl J Med. 2019;381:142‐149. doi: 10.1056/NEJMoa1811090 [DOI] [PubMed] [Google Scholar]
- 6. Marcus R, Goadsby PJ, Dodick D, Stock D, Manos G, Fischer TZ. Bms‐927711 for the acute treatment of migraine: a double‐blind, randomized, placebo controlled, dose‐ranging trial. Cephalalgia. 2014;34:114‐125. doi: 10.1177/0333102413500727 [DOI] [PubMed] [Google Scholar]
- 7. Li Y, Wang X, Ballesteros‐Perez A, Bertz R, Lu Z. Pharmacokinetics and safety of single and multiple daily dosing of 75‐mg rimegepant orally disintegrating tablets in healthy Chinese adults: a randomized placebo‐controlled trial. Clin Pharmacol Drug Dev. 2023;12:594‐601. doi: 10.1002/cpdd.1230 [DOI] [PubMed] [Google Scholar]
- 8. Croop R, Lipton RB, Kudrow D, et al. Oral rimegepant for preventive treatment of migraine: a phase 2/3, randomised, double‐blind, placebo‐controlled trial. Lancet. 2021;397:51‐60. doi: 10.1016/s0140-6736(20)32544-7 [DOI] [PubMed] [Google Scholar]
- 9. Bertz R, Bhardwaj R, Morris BA, Ashbrenner E, Coric V, Croop R. A placebo‐controlled, randomized, single and multiple dose study to evaluate the safety, tolerability, and pharmacokinetics of rimegepant in healthy participants. Cephalalgia. 2023;43:3331024231179131. doi: 10.1177/03331024231179131 [DOI] [PubMed] [Google Scholar]
- 10. Bhardwaj R, Collins JL, Stringfellow J, et al. P‐glycoprotein and breast cancer resistance protein transporter inhibition by cyclosporine and quinidine on the pharmacokinetics of oral rimegepant in healthy subjects. Clin Pharmacol Drug Dev. 2022;11:889‐897. doi: 10.1002/cpdd.1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Croop R, Ivans A, Stock DA, et al. A phase 1 study to evaluate the bioequivalence of oral table and orally dissolving tablet formulations of rimegepant, a small molecule CGRP receptor antagonist. Cephalalgia. 2018;38:142‐143. [Google Scholar]
- 12. Croop R, Ivans A, Anderson MS, et al. A phase 1 randomized study of hemodynamic effects and pharmacokinetic interactions during concomitant use of rimegepant and sumatriptan in healthy adults. Cephalalgia Rep. 2021;4:1‐10. doi: 10.1177/25158163211007922 [DOI] [Google Scholar]
- 13. Szkutnik‐Fiedler D. Pharmacokinetics, pharmacodynamics and drug‐drug interactions of new anti‐migraine drugs‐lasmiditan, gepants, and calcitonin‐gene‐related peptide (CGRP) receptor monoclonal antibodies. Pharmaceutics. 2020;12:1‐22. doi: 10.3390/pharmaceutics12121180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bhardwaj R, Ivans A, Stringfellow J, et al. Rimegepant 75 mg in subjects with hepatic impairment: results of a phase 1, open‐label, single‐dose, parallel‐group study. Clin Pharmacol Drug Dev. 2023;12:790‐800. doi: 10.1002/cpdd.1244 [DOI] [PubMed] [Google Scholar]
- 15. FDA . Rimegepant prescribing information. Accessed on 27 July 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/212728s006lbl.pdf
- 16. Valentin JP, Hoffmann P, Ortemann‐Renon C, et al. The challenges of predicting drug‐induced QTc prolongation in humans. Toxicol Sci. 2022;187:3‐24. doi: 10.1093/toxsci/kfac013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Grenier J, Paglialunga S, Morimoto BH, Lester RM. Evaluating cardiac risk: exposure response analysis in early clinical drug development. Drug Healthc Patient Saf. 2018;10:27‐36. doi: 10.2147/dhps.S133286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Conway CM, Croop R, Dubowchik GM, Coric V, Lipton RB. Cardiovascular safety of rimegepant 75 mg in 3 randomized clinical trials and systematic evaluations from in vitro, ex vivo, and in vivo nonclinical assays (2141). Neurology. 2020;94:200. [Google Scholar]
- 19. U.S. Food and Drug Adminstration . E14 clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for non‐antiarrhythmic drugs. Accessed June 27, 2023. https://www.fda.gov/media/71372/download [PubMed]
- 20. Coric V, Stock EG, Pultz J, Marcus R, Sheehan DV. Sheehan suicidality tracking scale (Sheehan‐STS): preliminary results from a multicenter clinical trial in generalized anxiety disorder. Psychiatry (Edgmont). 2009;6:26‐31. [PMC free article] [PubMed] [Google Scholar]
- 21. Sheehan DV, Giddens JM, Sheehan IS. Status update on the sheehan‐suicidality tracking scale (S‐STS) 2014. Innov Clin Neurosci. 2014;11:93‐140. [PMC free article] [PubMed] [Google Scholar]
- 22. Darpo B, Benson C, Dota C, et al. Results from the IQ‐csrc prospective study support replacement of the thorough QT study by QT assessment in the early clinical phase. Clin Pharmacol Ther. 2015;97:326‐335. doi: 10.1002/cpt.60 [DOI] [PubMed] [Google Scholar]
- 23. Ferber G, Zhou M, Darpo B. Detection of QTc effects in small studies—implications for replacing the thorough QT study. Ann Noninvasive Electrocardiol. 2015;20:368‐377. doi: 10.1111/anec.12227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Darpo B. The thorough QT/QTc study 4 years after the implementation of the ich E14 guidance. Br J Pharmacol. 2010;159:49‐57. doi: 10.1111/j.1476-5381.2009.00487.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Florian JA, Tornøe CW, Brundage R, Parekh A, Garnett CE. Population pharmacokinetic and concentration—QTc models for moxifloxacin: Pooled analysis of 20 thorough QT studies. J Clin Pharmacol. 2011;51:1152‐1162. doi: 10.1177/0091270010381498 [DOI] [PubMed] [Google Scholar]
- 26. Conway CM, Dubowchik GM, Croop R, Coric V. Phase 1 safety, tolerability and pharmacokinetics of single and multiple dose rimegepant as compared to the predicted clinically efficacious dose range. Cephalalgia. 2019;39:201. doi: 10.1177/0333102419859835 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Data Availability Statement
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions and exceptions, Pfizer may also provide access to the related individual de‐identified participant data. See https://www.pfizer.com/science/clinical‐trials/trial‐data‐and‐results for more information.


