Abstract
The sphingomyelin derivative ceramide is a signaling molecule implicated in numerous physiological events. Recently published reports indicate that ceramide levels are elevated in insulin-responsive tissues of diabetic animals and that agents which trigger ceramide production inhibit insulin signaling. In the present series of studies, the short-chain ceramide analog C2-ceramide inhibited insulin-stimulated glucose transport by ∼50% in 3T3-L1 adipocytes, with similar reductions in hormone-stimulated translocation of the insulin-responsive glucose transporter (GLUT4) and insulin-responsive aminopeptidase. C2-ceramide also inhibited phosphorylation and activation of Akt, a molecule proposed to mediate multiple insulin-stimulated metabolic events. C2-ceramide, at concentrations which antagonized activation of both glucose uptake and Akt, had no effect on the tyrosine phosphorylation of insulin receptor substrate 1 (IRS-1) or the amounts of p85 protein and phosphatidylinositol kinase activity that immunoprecipitated with anti-IRS-1 or antiphosphotyrosine antibodies. Moreover, C2-ceramide also inhibited stimulation of Akt by platelet-derived growth factor, an event that is IRS-1 independent. C2-ceramide did not inhibit insulin-stimulated phosphorylation of mitogen-activated protein kinase or pp70 S6-kinase, and it actually stimulated phosphorylation of the latter in the absence of insulin. Various pharmacological agents, including the immunosuppressant rapamycin, the protein synthesis inhibitor cycloheximide, and several protein kinase C inhibitors, were without effect on ceramide’s inhibition of Akt. These studies demonstrate ceramide’s capacity to inhibit activation of Akt and imply that this is a mechanism of antagonism of insulin-dependent physiological events, such as the peripheral activation of glucose transport and the suppression of apoptosis.
Insulin stimulates glucose uptake into muscle and adipose tissues by effecting the redistribution of the insulin-responsive glucose transporter GLUT4 from intracellular stores to the plasma membrane. Subsequently, insulin activates numerous metabolic pathways which promote the storage of the incoming glucose as glycogen or fat. Insulin transmits its signals through a cell surface tyrosine kinase receptor which stimulates multiple intracellular signaling events (reviewed in reference 41). Activated insulin receptors phosphorylate adapter proteins, such as members of the insulin receptor substrate (IRS) family, which recruit and activate downstream effector molecules. One of these proteins, phosphatidylinositol 3-kinase (PI 3-kinase), is requisite for insulin’s acute regulation of glucose metabolism. Treatment with either of the PI 3-kinase inhibitors wortmannin or LY294002 blocks insulin’s effects on glucose metabolism (6, 7, 35, 49), while expression of constitutively active forms of PI 3-kinase stimulates them (14, 26, 33). In single-cell assays, microinjection of dominant negative forms of PI 3-kinase (19, 31) or inhibitory PI 3-kinase antibodies (20) blocks GLUT4 translocation.
Recent studies suggest a role for the serine/threonine kinase Akt/protein kinase B (PKB) as a mediator of PI 3-kinase’s metabolic effects. Akt/PKB was isolated independently by three laboratories in 1991. Two groups isolated the protein as a result of its homology with PKC and PKA; hence, one group named it PKB (8), and the other named it RAC-PK (related to A and C protein kinase) (23). Simultaneously, a third laboratory identified the protein as the transforming component of the AKT8 retrovirus found in a rodent T-cell lymphoma and named it Akt (3). Akt/PKB is activated by insulin and other growth factors in a variety of cell types, often in a manner dependent on PI 3-kinase (13). Expression of constitutively active forms of Akt in appropriate tissues stimulates glucose uptake, GLUT4 translocation, glycogen synthase, lipogenesis, and protein synthesis (9, 28, 41, 45, 47). Akt’s stimulation of glucose uptake and GLUT4 translocation is insensitive to inhibition by wortmannin (42), suggesting that Akt activates insulin signaling pathways downstream of PI 3-kinase. Furthermore, inducible expression of a constitutively active Akt is temporally associated with increases in glucose uptake, GLUT4 translocation, and glycogen synthesis (27).
Intramuscular ceramide concentrations are elevated in skeletal muscle obtained from insulin-resistant rats (46), and ceramide analogs inhibit insulin-stimulated glucose uptake in cultured adipocytes (48). Other studies report that ceramide antagonizes the earliest events in insulin signaling (25, 37), although these results are controversial (48). The experiments described herein tested the hypothesis that ceramide prevents activation of Akt. Specifically, studies of the effect of ceramide on insulin-dependent signaling and metabolic events in 3T3-L1 adipocytes were performed. Data presented below indicate that a short-chain ceramide analog, C2-ceramide, inhibits glucose uptake, GLUT4 translocation, and Akt phosphorylation and activation in 3T3-L1 adipocytes independently of any effect on IRS-1.
MATERIALS AND METHODS
Antibodies and reagents.
Polyclonal sheep anti-GLUT4 antibodies were raised against a glutathione S-transferase (GST) fusion protein containing the last 31 amino acids of the GLUT4 carboxyl terminus (GST-ISATFRRTPSLLEQEVKPSTELEYLGPDEND). Polyclonal rabbit antibodies were raised against the sequence CDQTHFPQFSYSASIRE found in Akt2 (anti-Akt2 antibodies). Polyclonal rabbit anti-phospho-S6 antibodies were raised against the major phosphorylation site in the ribosomal S6 subunit [CRRLS(P)S(P)LRAS(P)TSKS(P)EES(P)QK, where P is the phosphoryl associated with the amino acid which precedes it], Anti-p85 antibodies were raised by Rockland, Inc. (Gilbertsville, Pa.), against a protein in which GST is fused to the N-terminal SH2 domain of p85, kindly provided by Jonathan Backer (Albert Einstein College of Medicine). Anti-phospho-mitogen activated protein kinase (MAPK) antibodies were from Promega (Madison, Wis.), anti-phospho-Akt and anti-phospho-p70 antibodies were from New England Biolabs (Beverly, Mass.), and agarose-bound antiphosphotyrosine antibodies were from Upstate Biotechnology, Inc. (Lake Placid, N.Y.). Anti-insulin-responsive aminopeptidase (IRAP), anti-IRS-1, and anti-pp70 antibodies were generously donated by Metabolex (Hayward, Calif.), Miles Pharmaceuticals (West Haven, Conn.), and Margaret Chou (University of Pennsylvania), respectively.
C2-ceramide, C2-dihydroceramide, rapamycin, and the protein kinase inhibitors staurosporine, bisindolymaleimide, Gö 6976, Gö 6983, H-89 dihydrochloride, ML-7, and KN-93 were from Calbiochem (La Jolla, Calif.). Okadaic acid was from Gibco/BRL (Gaithersburg, Md.). Porcine insulin was a gift from Eli Lilly (Indianapolis, Ind.).
Akt constructs, retroviral infection, and cell culture.
3T3-L1 fibroblasts were differentiated into adipocytes 2 days postconfluence in Dulbecco’s modified Eagle’s-H21 medium supplemented with 10% fetal bovine serum, 1 μg of dexamethasone per ml, and 112 μg of isobutylmethylxanthine per ml. After 3 days, cells were maintained in Dulbecco’s modified Eagle’s-H21 medium supplemented with 10% fetal bovine serum. 3T3-L1 fibroblasts expressing a constitutively active form of Akt [myr-akt (Δ4–129)] and empty vector were generously provided by Richard Roth, Stanford University, Stanford, Calif. (29).
Glucose uptake and GLUT4 translocation assays.
Methods for measuring glucose uptake rates and plasma membrane GLUT4 levels (with the plasma membrane sheet assay) have been described (29).
IRAP translocation assay.
IRAP translocation was determined with an IRAP biotinylation assay similar to that described previously (38). 3T3-L1 adipocytes in 60-mm-diameter dishes were washed in phosphate-buffered saline and left in Leibovitz-15 medium (Sigma Chemical Co.) with 0.2% bovine serum albumin for 2 h at 37°C. The medium contained either ceramide, dihydroceramide, or carrier ethanol as described in the figure legends. Cells were stimulated with 20 nM insulin for 10 min in the same medium and moved to ice. All subsequent steps were performed at 4°C. Cells were washed twice in ice-cold KRPH (128 mM NaCl, 4.7 mM KCl, 1.25 mM CaCl2, 1.25 mM MgSO4, 5 mM NaPO4, 20 mM HEPES [pH 7.4]) and treated with 3 ml of 0.5-mg/ml sulfo-NHS-LC-LC-biotin (Pierce) for 30 min. Each plate was then bathed three times for 5 min each time in KRPH plus 20 mM glycine, bathed once in KRPH, and finally lysed in 500 μl of solubilization buffer (1% Triton, 150 mM NaCl, 20 mM Tris-Cl, 5 mM EDTA, 1 mM phenylmethanesulfonyl fluoride, 10 μg of aprotinin per ml, 10 μM leupeptin, 1 μM pepstatin A [pH 7.4]). The lysate was vortexed briefly, incubated for 10 min, and centrifuged at 23,000 × g for 20 min. The fat cake was removed, and 50 μl of the remaining lysate was diluted to 500 μl with solubilization buffer and immunoprecipitated with 3 μl of anti-IRAP serum (Metabolex) for 1 h, followed by overnight incubation in 30 μl of protein A-Sepharose (Gibco). Samples were eluted in sodium dodecyl sulfate (SDS), and the eluate was divided into two parts; 80% was allocated for a gel to quantitate the amount of biotin, and 20% was allocated for a gel to quantitate the amount of IRAP immunoprecipitated.
SDS gels intended for biotin quantitation were transferred to polyvinylidene difluoride membranes (Fisher), blocked in Tris-buffered saline containing 0.2% Tween with 6% bovine serum albumin, treated with 1 μg of streptavidin-horseradish peroxidase (HRP) (Pierce) per ml for 2 h, washed in Tris-buffered saline containing 0.2% Tween, and developed with an enhanced chemifluorescence kit (Amersham) on a STORM 860 scanner. SDS gels used for IRAP quantitation were treated identically to those for biotin quantitation except for the use of nonfat dry milk and application of anti-IRAP serum (1:2,000) and goat anti-rabbit HRP-conjugated antibody (1:5,000).
Protein immunoblotting, immunoprecipitation, and measurement of activity.
Western blots of total cell lysates were prepared and analyzed as described previously (43). Akt kinase assays were conducted as previously described (43), except that some of them were conducted with the phospho-Akt1-specific antibody from New England Biolabs instead of the anti-Akt2 antibody described above. Phospho-Akt1-specific antibodies were used at the concentrations indicated by the manufacturer. IRS-1 was immunoprecipitated by solubilizing cells as described for Akt kinase assays and incubating them with 5 μl of anti-IRS-1 antibodies. Following 1 to 3 h of incubation at 4°C, 20 μl of washed protein A-agarose (Gibco/BRL) was added and the incubation was extended for another 20 to 30 min. The protein A-agarose was then washed three times in ice-cold lysis buffer without protease inhibitors and solubilized in Laemmli solubilization buffer. PI 3-kinase assays were then performed by methods previously described (44).
RESULTS
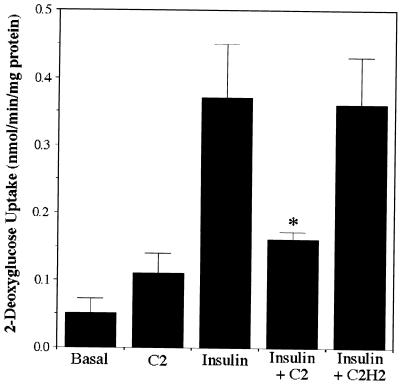
The effects of ceramide on insulin-mediated events were investigated by using the short-chain ceramide analog C2-ceramide. Although hydrophobic enough to cross the membrane bilayer, C2-ceramide disperses more easily in the incubation medium and is metabolized more slowly than ceramide, and it is thus a reagent commonly used for investigating ceramide’s effects. The closely related C2-dihydroceramide lacks biological activity (17) and is used as a negative control. Consistent with a previous study (48), C2-ceramide inhibited insulin-stimulated glucose uptake into 3T3-L1 adipocytes without affecting basal transport rates (Fig. 1).
FIG. 1.
C2-ceramide inhibits insulin-stimulated glucose uptake. 3T3-L1 adipocytes were incubated with C2-ceramide (C2) (100 μM) or C2-dihydroceramide (C2H2) (100 μM) for 2 h, with insulin present for the last 15 min. The uptake of radiolabeled 2-deoxyglucose was measured for 4 min. The asterisk denotes that the difference from the uptake in the presence of insulin alone was statistically significant at a P value of <0.05. Data presented are the averages ± standard errors of the means of three independent measurements.
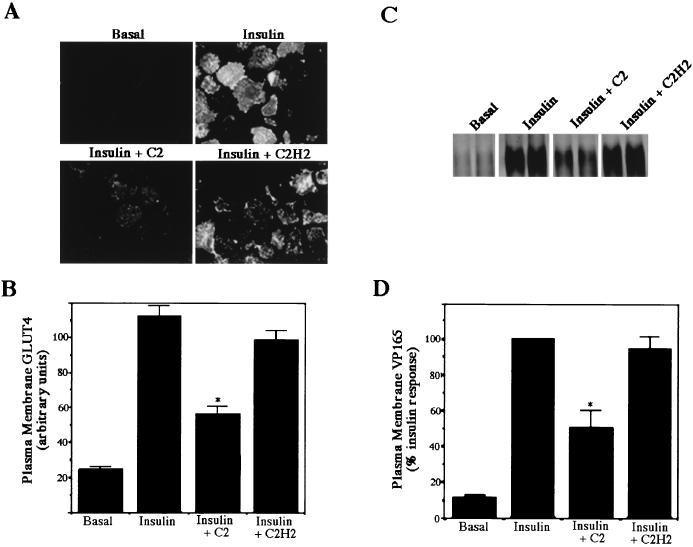
As described above, insulin accelerates glucose uptake by stimulating the translocation of GLUT4 from a poorly defined intracellular compartment to the plasma membrane. In addition, IRAP apparently resides in this compartment and translocates to the plasma membrane, like GLUT4 (24, 34). The effect of C2-ceramide on the insulin-dependent translocation of these proteins was determined by two independent methods. The GLUT4 sheet assay was used to measure plasma membrane GLUT4 levels (29). Briefly, 3T3-L1 adipocytes differentiated on coverslips were sonicated, liberating cellular structures from each coverslip but leaving an intact sheet containing the plasma membrane with its cytosolic face exposed. Probing the sheet with antibodies raised against the carboxyl terminus of GLUT4 reveals the total amount of GLUT4 on the membrane. Insulin caused a 10-fold increase in plasma membrane GLUT4 levels, which were reduced by ∼50% following a 2-h incubation with ceramide (Fig. 2A and B). This matched the inhibition in insulin-stimulated glucose transport observed under identical conditions. C2-ceramide similarly inhibited the translocation of IRAP. IRAP contains numerous lysine residues on its exofacial surface, allowing easy biotinylation of the protein (38). Cell surface biotinylation followed by specific immunoprecipitation of IRAP reveals the amount of cell surface IRAP. Insulin caused a 10-fold stimulation of IRAP biotinylation due to the translocation of IRAP to the plasma membrane. C2-ceramide inhibited IRAP translocation by 40 to 60%, again consistent with the inhibition of glucose transport and GLUT4 translocation. Basal plasma membrane GLUT4 and IRAP levels were not reduced by C2-ceramide. These data indicate that ceramide antagonizes insulin’s stimulation of glucose transport and its redistribution of these two proteins.
FIG. 2.
C2-ceramide inhibits insulin-stimulated GLUT4 and IRAP translocation. Plasma membrane GLUT4 (A and B) and IRAP (C and D) levels were measured as described in Materials and Methods. In both assays, C2-ceramide (C2) (100 μM) or C2-dihydroceramide (C2H2) (100 μM) was added 2 h prior to initiation of the assay. Insulin (20 nM) was present for the last 10 min. Immunofluorescence detection of GLUT4 on plasma membrane sheets was performed with polyclonal sheep anti-GLUT4 primary antibodies followed by rhodamine-conjugated anti-sheep secondary antibodies. Images were captured with a digital camera (A) and quantitated as described in Materials and Methods (B). Biotinylated IRAP was detected with streptavidin-HRP and visualized with enhanced chemifluorescence (C), and the results were quantitated on a phosphorimager (D). Each asterisk denotes that the difference from the value obtained in the presence of insulin alone was statistically significant at a P value of <0.05. Data presented are the averages ± standard errors of the means of three independent measurements.
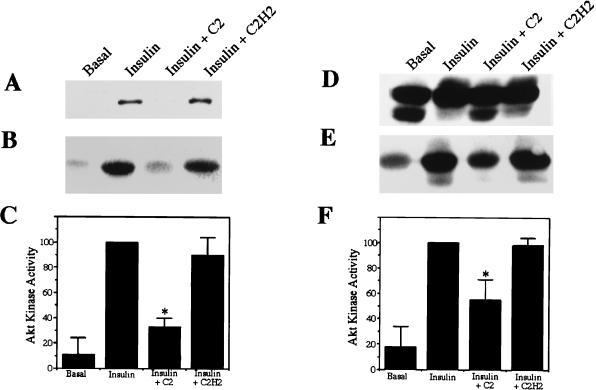
Since glucose uptake (29, 45) and GLUT4 (29, 45) and IRAP (16) translocation are known to be stimulated by constitutively active forms of Akt (29), the effect of C2-ceramide on Akt phosphorylation and activity was investigated. Commercially available antibodies raised against a regulatory phosphorylation site on Akt allow for a direct assessment of that protein’s phosphorylation state. Moreover, antibodies raised against the carboxyl terminus of Akt reveal a dramatic insulin-induced mobility shift when the protein is separated on low-percentage-polyacrylamide gels. Both types of antibodies precipitate insulin-dependent kinase activity as revealed by measuring 32P incorporation into histone. C2-ceramide markedly inhibited the phosphorylation, mobility shift, and precipitation of kinase activity by both types of antibodies (Fig. 3). C2-ceramide’s inhibition of Akt kinase activity was more pronounced when the anti-phospho-Akt antibody, which presumably recognizes only activated Akt and therefore precipitates less basal kinase activity, was used than when the anti-Akt2 antibody was used. In both cases, the degree of inhibition was consistent with that observed in glucose transport and GLUT4 and IRAP translocation assays.
FIG. 3.
C2-ceramide inhibits Akt. 3T3-L1 adipocytes were treated with C2-ceramide (C2) (100 μM) or C2-dihydroceramide (C2H2) (100 μM) for 2 h prior to solubilization. Insulin (20 nM) was present for the last 10 min. Western blots of total cell lysates were probed with anti-phospho-Akt1 antibodies (A) or anti-Akt2 antibodies (D). Kinase activities in immunocomplexes obtained with anti-phospho-Akt1 antibodies (B and C) or anti-Akt2 antibodies (E and F) were determined by measuring the incorporation of 32P into histone, which was either visualized by autoradiography (B and E) or quantitated on a phosphorimager (C and F). Each asterisk denotes that the difference from the activity in the presence of insulin alone was statistically significant at a P value of <0.05. Data are the averages ± standard errors of the means of three independent measurements.
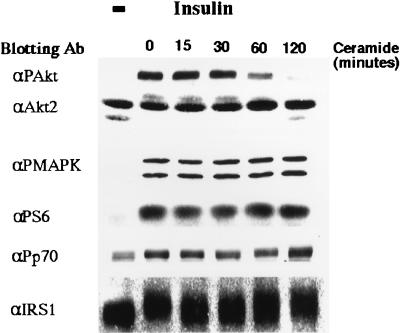
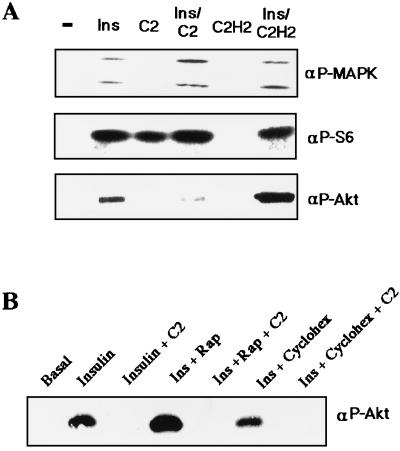
C2-ceramide’s effects on Akt were apparent within 1 h of treatment (Fig. 4). In contrast, C2-ceramide did not inhibit insulin-stimulated phosphorylation of MAPK, pp70 S6-kinase, or the ribosomal S6 subunit (Fig. 4). Moreover, C2-ceramide did not alter the insulin-induced mobility shift observed for IRS-1 (Fig. 4) or pp70 (data not shown). These results indicate that C2-ceramide specifically inhibits insulin’s stimulation of Akt1 and Akt2 without affecting other insulin-mediated pathways.
FIG. 4.
Time course of ceramide’s effects on Akt. Total cell lysates were prepared from 3T3-L1 adipocytes preincubated with C2-ceramide (100 μM) for the indicated periods of time, with insulin (20 nM) present for the last 10 min. Western blots were then probed with the indicated antibodies (Ab) and examined by enhanced chemiluminescence. Data are representative of five independent experiments. αPAkt, anti-phospho-Akt; αPMAPK, anti-phospho-MAPK; αP56, anti-phospho-S6; αPp70, anti-pp70; −, no ceramide present.
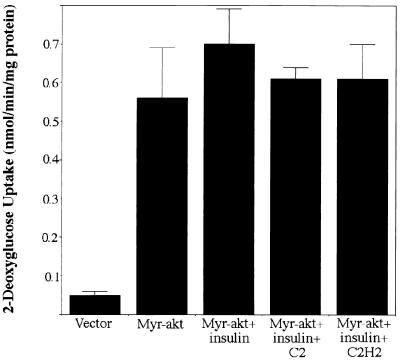
To investigate the effects of C2-ceramide on Akt-stimulated glucose transport, 3T3-L1 adipocytes stably expressing a constitutively active form of Akt (myr-akt) (described in reference 29) were evaluated. myr-akt expression caused a 10-fold glucose uptake stimulation which was insensitive to C2-ceramide (Fig. 5). These data indicate that the membrane targeting of Akt is sufficient to bypass the inhibitory actions of ceramide.
FIG. 5.
C2-ceramide does not inhibit myr-akt-stimulated glucose transport. 3T3-L1 adipocytes expressing empty vector or myr-akt were incubated with C2-ceramide (C2) (100 μM) or C2-dihydroceramide (C2H2) (100 μM) for 2 h, with insulin present for the last 15 min. The uptake of radiolabeled 2-deoxyglucose was measured for 4 min. Data presented are the averages ± standard errors of the means of three independent measurements.
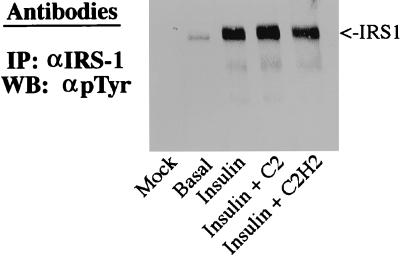
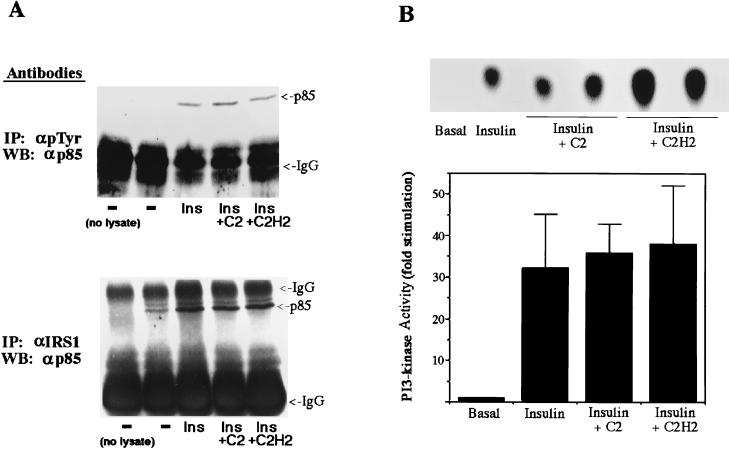
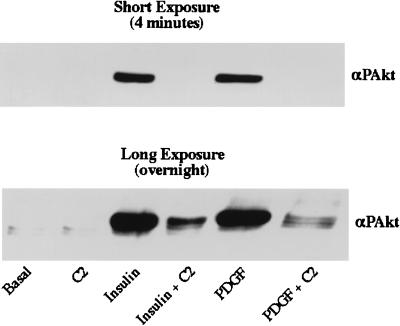
C2-ceramide concentrations which inhibited glucose transport and Akt activity did not affect very early insulin signaling events, including the IRS-1-mediated activation of PI 3-kinase. First, ceramide did not affect the tyrosine phosphorylation of IRS-1 as determined by immunoblotting of anti-IRS-1 precipitates with anti-phosphotyrosine antibodies (Fig. 6). Second, C2-ceramide did not inhibit the tyrosine phosphorylation of the insulin receptor examined by methods similar to those used to study the phosphorylation of IRS-1 (data not shown). Third, C2-ceramide did not affect the amount of p85 protein which immunoprecipitated with anti-IRS-1 or anti-phosphotyrosine antibodies (Fig. 7A). And fourth, C2-ceramide did not affect the amount of PI 3-kinase activity in anti-IRS-1 precipitates (Fig. 7B). To investigate whether ceramide also inhibited Akt phosphorylation or activation by agents which do not depend on IRS-1 for signaling, the effect of ceramide on platelet-derived growth factor (PDGF)-stimulated Akt phosphorylation was investigated. Since PDGF is a weak stimulator of Akt phosphorylation in 3T3-L1 adipocytes (45) but is a potent activator in fibroblasts (5), undifferentiated fibroblasts were used. Insulin stimulation and PDGF stimulation of Akt phosphorylation were equally sensitive to ceramide (Fig. 8). Taken together, these results indicate that altered phosphorylation of IRS-1 or activation of PI 3-kinase does not mediate ceramide’s inhibition of Akt activation.
FIG. 6.
C2-ceramide does not inhibit tyrosine phosphorylation of IRS-1. 3T3-L1 adipocytes were treated with C2-ceramide (C2) (100 μM) or C2-dihydroceramide (C2H2) (100 μM) for 2 h prior to solubilization, with insulin (20 nM) present for the last 10 min. IRS-1 was immunoprecipitated (IP), separated on SDS-polyacrylamide gel electrophoresis gels, and transferred to nitrocellulose. Western blots (WB), were probed with antiphosphotyrosine antibodies (αpTyr), and the blots shown are representative of two independent experiments.
FIG. 7.
C2-ceramide does not inhibit recruitment or activation of PI 3-kinase. 3T3-L1 adipocytes were treated with C2-ceramide (C2) (100 μM) or C2-dihydroceramide (C2H2) (100 μM) for 2 h prior to solubilization. Insulin (Ins) (20 nM) was present for the last 10 min. (A) Proteins were immunoprecipitated (IP) with antibodies to IRS-1 (αIRS1) or phosphotyrosine (αpTyr), separated on SDS-polyacrylamide gel electrophoresis gels, and transferred to nitrocellulose. Western blots (WB) were probed with anti-p85 antibodies and the blots shown are representative of two independent experiments. IgG, immunoglobulin G. (B) PI 3-kinase activity in anti-IRS-1 immunoprecipitates was measured as described in the text, and the radioactivity incorporated into phosphatidylinositol 3-phosphate was visualized by autoradiography (upper panel) or quantitated on a phosphorimager (lower panel).
FIG. 8.
C2-ceramide inhibits insulin- and PDGF-stimulated Akt phosphorylation in fibroblasts. Total cell lysates were prepared from 3T3-L1 fibroblasts preincubated with C2-ceramide (C2) (100 μM) for 2 h followed by a 10-min incubation with insulin (1 μM) or PDGF (50 ng/ml). Proteins were separated by SDS-polyacrylamide gel electrophoresis and transferred to nitrocellulose, and Western blots were then probed with anti-phospho-Akt antibodies (αPAkt).
In further studies we attempted to identify the ceramide target responsible for its inhibition of Akt phosphorylation. The addition of C2-ceramide in the absence of insulin stimulated the phosphorylation of the S6 ribosomal subunit, a marker of pp70 S6-kinase activity (Fig. 9A). To investigate whether activation of pp70 by C2-ceramide was responsible for the inhibited Akt, the immunosuppressant rapamycin was utilized. Under the conditions described, rapamycin completely inhibited S6 phosphorylation (data not shown) without affecting ceramide’s inhibition of Akt phosphorylation (Fig. 9B). Similarly, the protein synthesis inhibitor cycloheximide did not affect Akt phosphorylation.
FIG. 9.
C2-ceramide does not require protein synthesis to inhibit Akt. Total cell lysates were prepared from 3T3-L1 adipocytes incubated with C2-ceramide (C2) or C2 dihydroceramide (C2H2) (100 μM) for 2 h, with insulin (Ins) (20 nM) present of these lysates for the last 10 min. Western blots in the indicated samples were then probed with the indicated antibodies and examined by enhanced chemiluminescence. Data are representative of two independent experiments. For the blots shown in panel B, cells were treated as described for panel A, except that indicated samples additionally received rapamycin (Rap) (20 ng/ml) or cycloheximide (Cyclohex) (100 μM). αP-MAPK, anti-phospho-MAPK; αP-S6, anti-phospho-S6; αP-Akt, anti-phospho-Akt; −, no ceramide present.
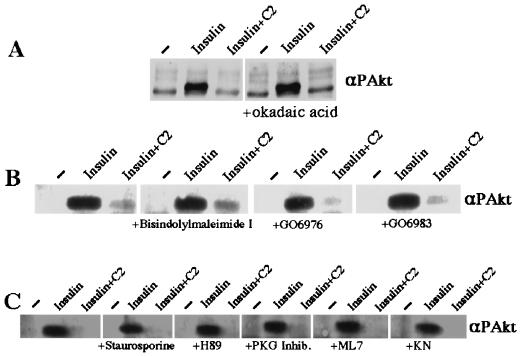
Akt is inactivated through dephosphorylation by protein phosphatase 2A (PP2A) (2), and a reported intracellular target of ceramide is the okadaic acid-sensitive PP2A-like phosphatase ceramide-activated protein phosphatase (11, 32). Treatment of cells with okadaic acid, which blocks PP2A activity, did not affect ceramide’s inhibition of Akt (Fig. 10A). Another target of ceramide is PKC isoform ζ (15), which also physically associates with Akt (30). However, Gö 6983 (40), an inhibitor of PKC isoforms α, β, δ, γ, and ζ did not affect ceramide’s inhibition of Akt phosphorylation (Fig. 10B). Similarly, a variety of other protein kinase inhibitors, including the general protein kinase inhibitor staurosporine, the PKC inhibitors bisindolymaleimide and Gö 6976 (40), the PKA inhibitor H-89 dihydrochloride, the myosin light-chain kinase (MLCK) inhibitor ML-7, and the Ca2+/calmodulin kinase II inhibitor KN-93, did not prevent ceramide’s inhibition of Akt phosphorylation (Fig. 10C).
FIG. 10.
Protein kinase inhibitors do not affect C2-ceramide’s inhibition of Akt. Total cell lysates were prepared from 3T3-L1 adipocytes incubated with C2-ceramide (C2) (100 μM) for 2 h. Insulin (20 nM) was present for the last 10 min. All of the protein kinase inhibitors were added to the incubation medium 2 h prior to addition of insulin. The final concentrations were 200 μM for the PKG inhibitor and 1 μM for all others. Western blots of these lysates were probed with anti-phospho-Akt and examined by enhanced chemiluminescence. Data are representative of two independent experiments. Inhib., inhibitor; KN, KN-93; αPAkt, anti-phospho-Akt antibodies.
DISCUSSION
These studies investigated the effects of ceramide on insulin signaling pathways as well as physiologically important metabolic responses. The experiments clearly demonstrate that C2-ceramide antagonizes insulin-stimulated glucose uptake and GLUT4 and IRAP translocation in 3T3-L1 adipocytes. The specific event modified apparently occurs between activation of PI 3-kinase and subsequent stimulation of Akt: (i) C2-ceramide inhibited insulin- and PDGF-stimulated Akt phosphorylation on a key regulatory residue (Fig. 3, 4, and 8), (ii) it reduced Akt kinase activities precipitated by two different antibodies (Fig. 3), (iii) it inhibited an insulin-induced mobility shift in Akt separated on polyacrylamide gels (Fig. 3 and 4), and (iv) its inhibitory actions on glucose transport were bypassed by overexpression of a constitutively active form of Akt. In contrast, C2-ceramide did not inhibit insulin’s activation of PI 3-kinase (Fig. 7) and other insulin-dependent signaling events (Fig. 4 and 6). This novel, antagonistic relationship between ceramide levels and the activation of Akt could have widespread implications, particularly given the numerous stimuli which modulate these signaling molecules.
The mechanism by which ceramide inhibits Akt phosphorylation is unclear. Akt is phosphorylated predominantly at two regulatory sites (T308 and S473), both of which are required for complete activation (1). Phosphorylation at both sites is dependent upon PI 3-kinase activity (1), and kinases capable of phosphorylating the T308 residue have been identified (12). However, the kinase(s) responsible for S473 phosphorylation, a process inhibited by ceramide, has not been discovered. Whether ceramide (or one of its metabolites) acts directly on these kinases or acts through some other intermediate(s) is clearly of interest. Experiments investigating other known downstream effectors of ceramide failed to reveal a likely mediator of ceramide’s actions on Akt phosphorylation. Inhibitors of ceramide-activated protein phosphatase (11, 32), PKCζ (15), and pp70 S6-kinase did not block ceramide’s effects on Akt (Fig. 9 and 10). Moreover, protein synthesis inhibitors failed to affect ceramide’s antagonistic actions (Fig. 9), indicating that transcriptional or translational events are not required.
The results of the ceramide experiments presented above contradict some, but not all, reports regarding ceramide’s effects on early insulin signaling events. In agreement with the studies described above, Wang et al. reported that ceramide inhibited neither tyrosine phosphorylation of IRS-1 nor its recruitment of the regulatory p85 subunit of PI 3-kinase in 3T3-L1 adipocytes (48). In contrast, two other groups reported that ceramide inhibited insulin’s tyrosine phosphorylation of IRS-1 in multiple other cell lines (25, 37). These two groups did not investigate recruitment of p85, and none of the groups measured PI 3-kinase activity directly. The data described herein also failed to demonstrate an effect of ceramide on tyrosine phosphorylation of IRS-1 or its recruitment and activation of PI 3-kinase. However, regardless of the conditions under which ceramide is capable of inhibiting tyrosine phosphorylation of IRS-1, its blockade of Akt activation must also occur through an alternative mechanism. Perhaps the most convincing argument for this is that ceramide inhibits Akt phosphorylation in response to both insulin and PDGF (Fig. 8), despite the fact that the latter does not utilize IRS-1 as a signaling intermediate. Intriguingly, many events in which Akt is implicated, such as antiapoptosis, anabolic metabolism, and adipogenesis, are inhibited by agents which produce ceramide or the lipid itself (18, 21, 22, 36).
The finding that C2-ceramide suppresses GLUT4 translocation also contrasts with a prior study (48) in which ceramide inhibited insulin-stimulated glucose uptake into 3T3-L1 adipocytes but failed to affect the GLUT4 content of plasma membrane fractions from insulin-stimulated cells. The GLUT4 translocation data presented in the previous study are difficult to reconcile with both the transport data acquired in that study and the translocation data presented above. The studies described herein utilized the GLUT4 sheet and IRAP biotinylation assays (Fig. 2), which are more sensitive methods for detecting translocation than subcellular fractionation. The possibility of fraction impurity in the aforementioned studies is a plausible explanation for the inconsistencies between the two results.
Implications for insulin resistance.
Insulin resistance is an important contributor to the pathogenesis of type II diabetes mellitus. Since the majority of type II diabetics are obese, a search is under way for signaling molecules secreted from fat which could cause insulin resistance in the major site for glucose disposal, skeletal muscle. Tumor necrosis factor alpha (TNF) and free fatty acids (FFA) have been proposed as links between adiposity and the development of insulin resistance (4, 21), and ceramide has been invoked as a principal mediator of both agents (10, 39). TNF produces ceramide through the FAN-mediated activation of sphingomyelinase, while fatty acids contribute to de novo synthesis (reviewed in references 17 and 36). The studies described herein suggest a mechanism for either TNF- or FFA-induced ceramide production in the development of insulin resistance through indirect inhibition of Akt/PKB. Interestingly, a recent report (39) has shown that FFA-dependent increases in pancreatic β-cell ceramide levels account for the reduced insulin-secretory capacity in a rodent model of type II diabetes. The present report raises the intriguing idea that concerted effects of ceramide on muscle and the pancreas account for both the peripheral and insulin-secretory defects associated with type II diabetes mellitus.
ACKNOWLEDGMENTS
This work was supported by NIH grants DK39615 (to M.J.B.) and DK09375 (to S.A.S.) and a grant from the Cox Institute (to M.J.B.).
We express gratitude to several individuals and organizations for their generous contributions to this paper. Margaret Chou (University of Pennsylvania), Jonathan Backer (Albert Einstein College of Medicine), and Metabolex kindly donated anti-pp70 antibodies, p85-GST fusion proteins, and anti-IRAP antibodies, respectively. Aimee Kohn and Richard Roth (Stanford University) generated the 3T3-L1 adipocytes stably expressing constitutively active forms of Akt. Randy Pittman (University of Pennsylvania) shared valuable information from his own laboratory. Eileen Whiteman (University of Pennsylvania) provided useful technical assistance regarding Akt kinase assays, and Robyn Tuttle (University of Pennsylvania) donated a number of reagents, solutions, and cells. Finally, Cass Lutz (University of Pennsylvania) provided assistance in the typing and editing of the manuscript.
REFERENCES
- 1.Alessi D R, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, Hemmings B A. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 1996;15:6541–6551. [PMC free article] [PubMed] [Google Scholar]
- 2.Andjelkovic M, Jakubowicz T, Cron P, Ming X F, Han J W, Hemmings B A. Activation and phosphorylation of a pleckstrin homology domain containing protein kinase (RAC-PK/PKB) promoted by serum and protein phosphatase inhibitors. Proc Natl Acad Sci USA. 1996;93:5699–5704. doi: 10.1073/pnas.93.12.5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bellacosa A, Testa J R, Staal S P, Tsichlis P N. A retroviral oncogene, akt, encoding a serine-threonine kinase containing an SH2-like region. Science. 1991;254:274–277. doi: 10.1126/science.254.5029.274. [DOI] [PubMed] [Google Scholar]
- 4.Boden G. Role of fatty acids in the pathogenesis of insulin resistance and NIDDM. Diabetes. 1997;46:3–10. [PubMed] [Google Scholar]
- 5.Burgering M, Coffer P. Protein kinase B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction. Nature. 1995;376:599–602. doi: 10.1038/376599a0. [DOI] [PubMed] [Google Scholar]
- 6.Cheatham B, Vlahos C J, Cheatham L, Wang L, Blenis J, Kahn C R. Phosphatidylinositol 3-kinase activation is required for insulin stimulation of pp70 S6 kinase, DNA synthesis, and glucose transporter translocation. Mol Cell Biol. 1994;14:4902–4911. doi: 10.1128/mcb.14.7.4902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clarke J F, Young P W, Yonezawa K, Kasuga M, Holman G D. Inhibition of the translocation of GLUT1 and GLUT4 in 3T3-L1 cells by the phosphatidylinositol 3-kinase inhibitor, wortmannin. Biochem J. 1994;300:631–635. doi: 10.1042/bj3000631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coffer P J, Woodgett J R. Molecular cloning and characterization of a novel putative protein-serine kinase related to the cAMP-dependent and protein kinase C families. Eur J Biochem. 1991;201:475–481. doi: 10.1111/j.1432-1033.1991.tb16305.x. . (Erratum, 205:1217, 1992.) [DOI] [PubMed] [Google Scholar]
- 9.Cong L-N, Chen H, Li Y, Zhou L, McGibbon M, Taylor S, Quon M. Physiological role of Akt in insulin-stimulated translocation of GLUT4 in transfected rat adipose cells. Mol Endocrinol. 1997;11:1881–1890. doi: 10.1210/mend.11.13.0027. [DOI] [PubMed] [Google Scholar]
- 10.Darnay B G, Aggarwal B B. Early events in TNF signaling: a story of associations and dissociations. J Leukoc Biol. 1997;61:559–566. doi: 10.1002/jlb.61.5.559. [DOI] [PubMed] [Google Scholar]
- 11.Dobrowsky R T, Hannun Y A. Ceramide stimulates a cytosolic protein phosphatase. J Biol Chem. 1992;267:5048–5051. [PubMed] [Google Scholar]
- 12.Downward J. Lipid-regulated kinases: some common themes at last. Science. 1998;279:673–674. doi: 10.1126/science.279.5351.673. [DOI] [PubMed] [Google Scholar]
- 13.Franke T F, Kaplan D R, Cantley L C. PI3K: downstream AKTion blocks apoptosis. Cell. 1997;88:435–437. doi: 10.1016/s0092-8674(00)81883-8. [DOI] [PubMed] [Google Scholar]
- 14.Frevert E U, Kahn B B. Differential effects of constitutively active phosphatidylinositol 3-kinase on glucose transport, glycogen synthase activity, and DNA synthesis in 3T3-L1 adipocytes. Mol Cell Biol. 1997;17:190–198. doi: 10.1128/mcb.17.1.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galve-Roperh I, Haro A, Diaz-Laviada I. Ceramide-induced translocation of protein kinase C zeta in primary cultures of astrocytes. FEBS Lett. 1997;415:271–274. doi: 10.1016/s0014-5793(97)00985-x. [DOI] [PubMed] [Google Scholar]
- 16.Garza, L. A., and M. J. Birnbaum. Unpublished data.
- 17.Hannun Y. The sphingomyelin cycle and the second messenger function of ceramide. J Biol Chem. 1994;269:3125–3128. [PubMed] [Google Scholar]
- 18.Hannun Y A, Obeid L M. Ceramide: an intracellular signal for apoptosis. Trends Biochem Sci. 1995;20:73–77. doi: 10.1016/s0968-0004(00)88961-6. [DOI] [PubMed] [Google Scholar]
- 19.Haruta T, Morris A J, Rose D W, Nelson J G, Mueckler M, Olefsky J M. Insulin-stimulated GLUT4 translocation is mediated by a divergent intracellular signaling pathway. J Biol Chem. 1995;270:27991–27994. doi: 10.1074/jbc.270.47.27991. [DOI] [PubMed] [Google Scholar]
- 20.Hausdorff, S. E., and M. J. Birnbaum. Unpublished data.
- 21.Hotamisligil G S, Spiegelman B M. Tumor necrosis factor alpha: a key component of the obesity-diabetes link. Diabetes. 1994;43:1271–1278. doi: 10.2337/diab.43.11.1271. [DOI] [PubMed] [Google Scholar]
- 22.Jarvis W D, Grant S, Kolesnick R N. Ceramide and the induction of apoptosis. Clin Cancer Res. 1996;2:1–6. [PubMed] [Google Scholar]
- 23.Jones P F, Jakubowicz T, Pitossi F J, Maurer F, Hemmings B A. Molecular cloning and identification of a serine/threonine protein kinase of the second-messenger subfamily. Proc Natl Acad Sci USA. 1991;88:4171–4175. doi: 10.1073/pnas.88.10.4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kandror K V, Yu L, Pilch P F. The major protein of GLUT4-containing vesicles, gp160, has aminopeptidase activity. J Biol Chem. 1994;269:30777–30780. [PubMed] [Google Scholar]
- 25.Kanety H, Hemi R, Papa M Z, Karasik A. Sphingomyelinase and ceramide suppress insulin-induced tyrosine phosphorylation of the insulin receptor substrate-1. J Biol Chem. 1996;271:9895–9897. doi: 10.1074/jbc.271.17.9895. [DOI] [PubMed] [Google Scholar]
- 26.Katagiri H, Asano T, Ishihara H, Inukai K, Shibasaki Y, Kikuchi M, Yazaki Y, Oka Y. Overexpression of catalytic subunit p110 of phosphatidylinositol 3-kinase increases glucose transport activity with translocation of glucose transporters in 3T3-L1 adipocytes. J Biol Chem. 1996;271:16987–16990. doi: 10.1074/jbc.271.29.16987. [DOI] [PubMed] [Google Scholar]
- 27.Kohn A D, Boge A, Barthel A, Kovacina K, Wallach B, Summers S A, Birnbaum M J, Roth R A. Construction and characterization of a conditionally active version of the ser/thr kinase Akt. J Biol Chem. 1998;273:11937–11943. doi: 10.1074/jbc.273.19.11937. [DOI] [PubMed] [Google Scholar]
- 28.Kohn A D, Kovacina K S, Roth R A. Insulin stimulates the kinase activity of RAC-PK, a pleckstrin homology domain containing ser/thr kinase. EMBO J. 1995;14:4288–4295. doi: 10.1002/j.1460-2075.1995.tb00103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kohn A D, Summers S A, Birnbaum M J, Roth R A. Expression of a constitutively active Akt Ser/Thr kinase in 3T3-L1 adipocytes stimulates glucose uptake and GLUT4 translocation. J Biol Chem. 1996;271:31372–31378. doi: 10.1074/jbc.271.49.31372. [DOI] [PubMed] [Google Scholar]
- 30.Konishi H, Shinomura T, Kuroda S, Ono Y, Kikkawa U. Molecular cloning of rat RAC protein kinase alpha and beta and their association with protein kinase C zeta. Biochem Biophys Res Commun. 1994;205:817–825. doi: 10.1006/bbrc.1994.2738. [DOI] [PubMed] [Google Scholar]
- 31.Kotani K, Carozzi A J, Sakaue H, Hara K, Robinson L J, Clark S F, Yonezawa K, James D E, Kasuga M. Requirement for phosphoinositide 3-kinase in insulin-stimulated GLUT4 translocation in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 1995;209:343–348. doi: 10.1006/bbrc.1995.1509. [DOI] [PubMed] [Google Scholar]
- 32.Kowluru A, Metz S A. Ceramide-activated protein phosphatase-2A activity in insulin-secreting cells. FEBS Lett. 1997;418:179–182. doi: 10.1016/s0014-5793(97)01379-3. [DOI] [PubMed] [Google Scholar]
- 33.Martin S, Haruta T, Morris A, Klippel A, Williams L, Olefsky J. Activated phosphatidylinositol 3-kinase is sufficient to mediate actin rearrangement and GLUT4 translocation in 3T3-L1 adipocytes. J Biol Chem. 1996;271:17605–17608. doi: 10.1074/jbc.271.30.17605. [DOI] [PubMed] [Google Scholar]
- 34.Mastick C C, Aebersold R, Lienhard G E. Characterization of a major protein in GLUT4 vesicles. Concentration in the vesicles and insulin-stimulated translocation to the plasma membrane. J Biol Chem. 1994;269:6089–6092. [PubMed] [Google Scholar]
- 35.Okada T, Kawano Y, Sabakibara T, Hazeki O, Ui M. Essential role of phosphatidylinositol 3-kinase in insulin-induced glucose transport and antilipolysis in rat adipocytes. Studies with a selective inhibitor, wortmannin. J Biol Chem. 1994;269:3568–3573. [PubMed] [Google Scholar]
- 36.Pena L A, Fuks Z, Kolesnick R. Stress-induced apoptosis and the sphingomyelin pathway. Biochem Pharmacol. 1997;53:615–621. doi: 10.1016/s0006-2952(96)00834-9. [DOI] [PubMed] [Google Scholar]
- 37.Peraldi P, Hotamisligil G S, Buurman W A, White M F, Spiegelman B M. Tumor necrosis factor (TNF)-α inhibits insulin signaling through stimulation of the p55 TNF receptor and activation of sphingomyelinase. J Biol Chem. 1996;271:13018–13022. doi: 10.1074/jbc.271.22.13018. [DOI] [PubMed] [Google Scholar]
- 38.Ross S A, Scott H M, Morris N J, Liung W-Y, Mao F, Lienhard G E, Keller S R. Characterization of the insulin-regulated membrane aminopeptidase in 3T3-L1 adipocytes. J Biol Chem. 1996;271:3328–3332. doi: 10.1074/jbc.271.6.3328. [DOI] [PubMed] [Google Scholar]
- 39.Shimabukuro M, Zhou Y-T, Levi M, Unger R H. Fatty acid-induced β cell apoptosis: a link between obesity and diabetes. Proc Natl Acad Sci USA. 1998;95:2498–2502. doi: 10.1073/pnas.95.5.2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stempka L, Girod A, Muller H-J, Rincke G, Marks F, Gschwendt M, Bossemeyer D. Phosphorylation of protein kinase Cδ (PKCδ) at threonine 505 is not a prerequisite for enzymatic activity. Expression of rat PKCδ and alanine 505 mutant in bacteria in a functional form. J Biol Chem. 1997;272:6805–6811. doi: 10.1074/jbc.272.10.6805. [DOI] [PubMed] [Google Scholar]
- 41.Summers S A, Birnbaum M J. A role for the serine/threonine kinase, Akt, in insulin-stimulated glucose uptake. Biochem Soc Trans. 1997;25:981–988. doi: 10.1042/bst0250981. [DOI] [PubMed] [Google Scholar]
- 42.Summers, S. A., and M. J. Birnbaum. Unpublished data.
- 43.Summers S A, Lipfert L, Birnbaum M J. Polyoma middle T antigen activates the serine/threonine kinase Akt in a PI3-kinase dependent manner. Biochem Biophys Res Commun. 1998;246:76–81. doi: 10.1006/bbrc.1998.8575. [DOI] [PubMed] [Google Scholar]
- 44.Sun X J, Rothenberg P, Kahn C R, Backer J M, Araki E, Wilden P A, Cahill D A, Goldstein B J, White M F. Structure of the insulin receptor substrate IRS-1 defines a unique signal transduction protein. Nature. 1991;352:73–77. doi: 10.1038/352073a0. [DOI] [PubMed] [Google Scholar]
- 45.Tanti J F, Grillo S, Gremeaux T, Coffer P J, Van Obberghen E, Le Marchand-Brustel Y. Potential role of protein kinase B in glucose transporter 4 translocation in adipocytes. Endocrinology. 1997;138:2005–2010. doi: 10.1210/endo.138.5.5136. [DOI] [PubMed] [Google Scholar]
- 46.Turinsky J, O’Sullivan D M, Bayly B P. 1,2-Diacylglycerol and ceramide levels in insulin-resistant tissues of the rat in vivo. J Biol Chem. 1990;265:16880–16885. [PubMed] [Google Scholar]
- 47.Ueki K, Yamamoto-Honda R, Kaburagi Y, Yamauchi T, Tobe K, Burgering B M T, Coffer P J, Komuro I, Akanuma Y, Yazaki Y, Kadowaki T. Potential role of protein kinase B in insulin-induced glucose transport, glycogen synthesis, and protein synthesis. J Biol Chem. 1998;273:5315–5322. doi: 10.1074/jbc.273.9.5315. [DOI] [PubMed] [Google Scholar]
- 48.Wang C-N, O’Brien L, Brindley D N. Effects of cell-permeable ceramides and tumor necrosis factor-α on insulin signaling and glucose uptake in 3T3-L1 adipocytes. Diabetes. 1998;47:24–31. doi: 10.2337/diab.47.1.24. [DOI] [PubMed] [Google Scholar]
- 49.Yeh J I, Gulve E A, Rameh L, Birnbaum M J. The effects of wortmannin on rat skeletal muscle. Dissociation of signaling pathways for insulin- and contraction-activated hexose transport. J Biol Chem. 1995;270:2107–2111. doi: 10.1074/jbc.270.5.2107. [DOI] [PubMed] [Google Scholar]