This randomized clinical trial investigates the effectiveness and safety of the neonatal fragment crystallizable receptor monoclonal antibody, batoclimab, in adults with generalized myasthenia gravis.
Key Points
Question
Is the neonatal fragment crystallizable receptor (FcRn) monoclonal antibody, batoclimab, effective and safe in adult patients with generalized myasthenia gravis (MG)?
Findings
In this multicenter, phase 3 randomized clinical trial including 131 patients, the rate of sustained improvement in the Myasthenia Gravis Activities of Daily Living score was significantly higher in the batoclimab group than in the placebo group. Batoclimab was well tolerated and no treatment-related serious adverse events were reported.
Meaning
The results of this trial established the efficacy and safety profile of batoclimab in Chinese patients with generalized MG, and batoclimab could be an important addition to the currently limited arsenal of FcRn inhibitors.
Abstract
Importance
Myasthenia gravis (MG) is caused by autoantibodies that disrupt the neuromuscular junction. The neonatal fragment crystallizable receptor (FcRn) antagonists, efgartigimod and rozanolixizumab, reduce immunoglobulin G (IgG) level in the circulation and alleviate symptoms in patients with generalized MG.
Objective
To examine the efficacy and safety profile of batoclimab, a monoclonal IgG1 antibody, in patients with generalized MG.
Design, Setting, and Participants
This was a multicenter randomized clinical trial conducted from September 15, 2021, to June 29, 2022, at 27 centers in China. Adult patients 18 years or older with generalized MG were screened, and those who were antibody positive were enrolled.
Intervention
Eligible patients received batoclimab or matching placebo in addition to standard of care. Each treatment cycle consisted of 6 weekly subcutaneous injections of batoclimab, 680 mg, or matching placebo followed by 4 weeks of observation. A second treatment cycle was conducted in patients who required continuing treatment.
Main Outcome and Measure
The primary outcome was sustained improvement, as defined by a 3-point or greater reduction in the Myasthenia Gravis Activities of Daily Living (MG-ADL) score from baseline for 4 or more consecutive weeks in the first cycle in individuals who were positive for acetylcholine receptor or muscle-specific kinase antibodies.
Results
A total of 178 adult patients with generalized MG were screened, 132 were randomly assigned, 131 tested positive for antibodies, and 1 tested negative for antibodies. A total of 132 patients (mean [SE] age, 43.8 [13.6] years; 88 women [67.2%]) were enrolled. The rate of sustained MG-ADL improvement in the first cycle in antibody-positive patients was 31.3% (20 of 64) in the placebo group vs 58.2% (39 of 67) in the batoclimab group (odds ratio, 3.45; 95% CI, 1.62-7.35; P = .001). The MG-ADL score diverged between the 2 groups as early as week 2. The mean (SE) maximum difference in MG-ADL score reduction occurred 1 week after the last dose (day 43, 1.7 [0.3] in the placebo group vs 3.6 [0.3] in the batoclimab group; group difference, −1.9; 95% CI, −2.8 to −1.0; nominal P < .001). The rates of treatment-related and severe treatment-emergent adverse events in patients were 36.9% (24 of 65) and 7.7% (5 of 65) in the placebo group vs 70.1% (47 of 67) and 3.0% (2 of 67) in the batoclimab group, respectively.
Conclusions and Relevance
Batoclimab increased the rate of sustained MG-ADL improvement and was well tolerated in adult patients with generalized MG. Clinical effects and the extent of IgG reduction were similar to those previously reported for efgartigimod and rozanolixizumab. Future studies of large sample size are needed to further understand the safety profile of batoclimab.
Trial Registration
ClinicalTrials.gov Identifier: NCT05039190
Introduction
Myasthenia gravis (MG) is a chronic disease characterized by fluctuating weakness of skeletal muscles. The estimated global prevalence in the general population is 15 to 25 per 100 000.1,2 The age- and sex-adjusted incidence is 6.8 per 100 000 person-years in China.3 It is caused by autoantibodies that disrupt the neuromuscular junction, most commonly against the nicotinic acetylcholine receptor (AChR) but also other proteins at the neuromuscular junction, eg, muscle-specific kinase (MuSK) and lipoprotein receptor–related peptide 4.4,5,6,7,8
Broad immunosuppressants, eg, glucocorticosteroids and nonsteroidal immunosuppressive therapies, are efficacious but are associated with long-term adverse effects.9 Furthermore, not all patients are responsive to the treatment. Treatments that selectively reduce immunoglobulin G (IgG) level in the circulation, including plasma exchange and high-dose intravenous immunoglobin and immunoadsorption, are effective for symptom relief but are associated with potential adverse effects, limited supply, and high cost.10,11
The neonatal fragment crystallizable receptor (FcRn) increases the half-life of IgG in the circulation by preventing its degradation by lysosomes.12,13 Antagonizing FcRn with efgartigimod and rozanolixizumab reduces IgG concentration and alleviates symptoms in patients with generalized MG.14,15 Based on the results of the efgartigimod and rozanolixizumab phase 3 trial ( Safety, Efficacy, and Tolerability of Efgartigimod in Patients With Generalized Myasthenia Gravis [ADAPT] and Safety and Efficacy of Rozanolixizumab in Patients With Generalized Myasthenia Gravis [MycarinG]), they were approved by the US Food and Drug Administration in December 2021 and June 2023, respectively, for use in adult patients with AChR antibody–positive generalized MG.16,17 Another FcRn inhibitor, nipocalimab, is currently under development.18 A recent network meta-analysis19 suggested a difference favoring anti-FcRn over anticomplement treatments for their ability to improve the Quantitative Myasthenia Gravis (QMG) score (−4.78 vs −2.6; net difference 2.18). Notably, efgartigimod and rozanolixizumab, showed similar effects.
Batoclimab is a fully humanized monoclonal IgG1 antibody that binds to FcRn. In healthy volunteers, a single subcutaneous injection of batoclimab, 680 mg, decreased serum IgG level by approximately 40% at day 11.20 Four weekly subcutaneous doses of 680 mg led to a 75% maximum reduction in total IgG concentration. A phase 2 trial has provided preliminary evidence to support the efficacy of batoclimab in Chinese patients with generalized MG.21 The phase 3 trial of batoclimab in patients with generalized MG has been completed, and the results are reported in this article.
Methods
This was a multicenter, double-blind, placebo-controlled, phase 3, randomized clinical trial. The trial was conducted at 27 centers in China (the trial protocol and statistical analysis plan are available in Supplement 1 and Supplement 2, respectively); patient screening started on September 15, 2021, and ended on June 29, 2022. The trial protocol adhered to the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) statement and was approved by the ethics committees of all participating centers. The trial is registered with ClinicalTrials.gov and was conducted in accordance with the Declaration of Helsinki. All patients provided written informed consent before participation in any trial-related procedures. The reporting of the study adhered to the Consolidated Standards of Reporting Trials (CONSORT) reporting guidelines.
Participants
Adult patients (≥18 years of age) with generalized MG were eligible to participate in the trial. All of the individuals included in our study were of Chinese Han ethnicity. The diagnosis of generalized MG was based on at least 1 of the following: (1) neuromuscular transmission abnormalities on repetitive electrical stimulations or a history thereof, (2) a positive neostigmine test or a history thereof, or (3) improvement of symptoms with oral acetylcholinesterase inhibitors. Patients were eligible irrespective of AChR/MuSK antibody status.
Additional inclusion criteria included the following: (1) Myasthenia Gravis Foundation of America (MGFA) clinical classification IIa to IVa; (2) Myasthenia Gravis Activities of Daily Living (MG-ADL) score of 5 or greater, with ocular score less than 50%; and (3) QMG score of 11 or greater at the time of screening and baseline irrespective of standard of care. Key exclusion criteria were as follows: (1) thymectomy within 3 months before screening or likely need for thymectomy during the trial as assessed by the investigator; (2) intravenous immunoglobin, plasma adsorption, or plasma exchange therapy within 4 weeks before screening; (3) use of an immunosuppressive monoclonal antibody (including, but not limited to, rituximab, belimumab, or eculizumab) within 6 months before screening or having a B-cell count below the lower limit of normal range in patients receiving rituximab or belimumab; and (4) hepatitis C or HIV infection. A full list of eligibility criteria are shown in Supplement 1.
Trial Design and Interventions
Eligible patients were randomized in a 1:1 ratio to receive either batoclimab or matching placebo. Each treatment cycle consisted of 6 weekly subcutaneous injections of batoclimab, 680 mg, or matching placebo, followed by 4 weeks of observation without treatment. Randomization and concealment were performed using an Interactive Web Response System (MediData). Randomization was stratified by AChR/MuSK antibody status (positive vs negative) and glucocorticosteroid use (yes vs no). Patients and investigators were blinded to group assignment.
A second treatment cycle would be initiated no later than week 18 if all the following criteria were met: (1) 4 weeks or more had passed since the final dose of initial treatment, (2) plasma albumin concentration was 3 g/dL or greater (to convert to grams per liter, multiply by 10), and (3) total MG-ADL score was 5 or greater or MG-ADL improvement from baseline was less than 3 points. All patients were followed up until week 24 or 5 weeks after the final dose, whichever was later. If retreatment criteria were met after week 18, the study participants proceeded to an ongoing open-label extension trial.22 Patients who completed the trial were provided the option to participate in the open-label extension trial at the discretion of the investigators.
All patients received standard of care in addition to the assigned treatment (batoclimab or placebo), but changes in dosage and/or dosing frequency were not permitted.
Outcomes
The patients were evaluated at baseline and then weekly for the MG-ADL, QMG, Myasthenia Gravis Composite (MGC), and 15-item revised version of the Myasthenia Gravis Quality of Life (MG-QOL15r) scores.23,24,25,26,27 Acetylcholinesterase inhibitors were suspended for at least 12 hours before evaluation at each visit.
Efficacy was primarily evaluated in antibody-positive (either AChR or MuSK) individuals. The primary outcome was sustained MG-ADL improvement (≥3-point reduction in MG-ADL score from baseline for at least 4 consecutive weeks) in the first cycle. Secondary outcomes included (1) sustained QMG improvement (≥3-point reduction for at least 4 consecutive weeks), (2) total duration of MG-ADL improvement of 3 points or greater relative to the 24 weeks treatment period in the antibody-positive patients, (3) minimal symptom expression (MSE; MG-ADL score of 0 or 1) in the first cycle, (4) early MG-ADL improvement (≥3-point reduction within the first 2 weeks of the first cycle), and (5) sustained MG-ADL improvement in the cycle 2.
Safety assessment included the frequency and severity of adverse events (AEs), vital signs, 12-lead electrocardiograms, and laboratory assessments that included albumin and low-density lipoprotein cholesterol (LDL-C) levels. AEs were coded using Medical Dictionary for Regulatory Activities (MedDRA [International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use]), version 25.1, and summarized by system organ class and preferred terms.
Pharmacokinetics, Pharmacodynamics, and Immunogenicity
Blood samples were collected on days 1, 22, 43, and 64 of each cycle and analyzed in a central laboratory to determine batoclimab concentration. Serum total IgG was measured weekly in each cycle and total hemolytic complement (CH50) and C3 were measured weekly within the active treatment period as well as at weeks 6 and 9 in each cycle. Serum AChR/MuSK antibodies were examined weekly in cycle 1 and at weeks 0 and 6 of cycle 2 using enzyme-linked immunosorbent assay kits (Tina-quant IgG-II [Roche Diagnostics]).
Blood samples obtained at day 1 and weeks 3 and 9 of each cycle were tested for antibatoclimab antibodies using a Meso Scale Discovery (Meso Scale Diagnostics) electrochemiluminescence method. Patients who were positive for antibatoclimab antibodies at week 9 were tested weekly thereafter until the antibodies became negative. Neutralizing antibodies were tested only in the patients who tested positive for antibatoclimab antibodies.
Statistical Analysis
Sample size was estimated based on the following assumptions: (1) the rate of the primary outcome of sustained MG-ADL improvement in the antibody-positive patients at 30% in the placebo control and 60% in the batoclimab group13 and (2) 90% power. The calculation yielded a need for 120 antibody-positive patients.
An interim analysis was preplanned when the primary efficacy outcome (sustained MG-ADL improvement in cycle 1) was available in approximately 80 antibody-positive patients. The interim analysis was conducted and reviewed by an independent data monitoring committee. The prespecified α-spending function was Hwang-Shih-DeCani (ρ = −4). Consequently, the 1-sided statistical significance level at the interim and final analyses were .006 and .023, respectively. The overall α was .025.
All efficacy outcomes were analyzed following the intention-to-treat principle. Effect sizes of dichotomous outcomes were estimated and compared using multivariable logistic regression that included the use of glucocorticoids at baseline (yes vs no) and baseline MG-ADL (sample median as cutoff) as independent variables. Relative treatment effect size is presented as odds ratio (OR) and 95% CI. Continuous variables that were repeatedly measured were analyzed using a mixed model for repeat measurements. Score change relative to baseline is shown as least squares (LS) mean (SE). Sensitivity analyses for the primary efficacy outcome included (1) excluding individuals with events that likely produced significant bias (ie, rescue treatment, poor compliance, missing MG-ADL score that made determination of sustained MG-ADL improvement impossible), (2) assuming a missing MG-ADL evaluation as no improvement, and (3) using the Cochran-Mantel-Haenszel stratified test (by concomitant use of glucocorticosteroid).
The following subgroup analyses were prespecified: age (<65 vs ≥65 years), sex, body weight (<60 vs ≥60 kg), body mass index (<25 vs ≥25; calculated as weight in kilograms divided by height in meters squared), MGFA clinical classification (IIa-IIb vs IIIa-IVa), thymectomy (yes vs no), baseline treatment (steroid plus nonsteroid immunosuppressant vs steroid but no other immunosuppressant vs nonsteroid immunosuppressant but no steroid vs others), and baseline MG-ADL score (less than vs greater than the sample median).
Multiplicity was controlled using a fixed sequence multiple comparison procedure. All statistical analyses were performed using SAS, version 9.4 (SAS Institute). Details of the statistical analyses are provided in Supplement 2.
Results
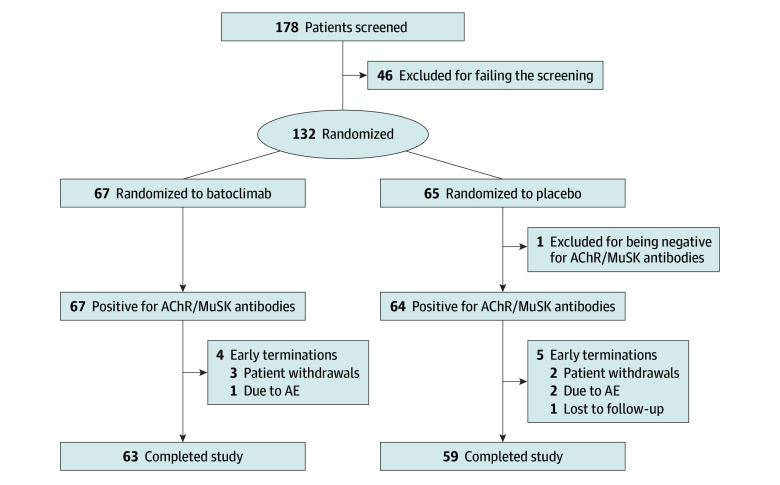
A total of 178 patients from 27 Chinese participating centers were screened between September 15, 2021, and June 29, 2022, of which 132 (mean [SE] age, 43.7 [13.6] years; 88 women [67.2%]; 43 men [32.8%]) were enrolled and randomly assigned to treatment groups. One individual was seronegative for both AChR and MuSK antibodies (randomized to the placebo group). Among the other 131 patients who were antibody positive, 64 (48.9%) were randomized to the placebo group and 67 (51.1%) to the batoclimab group (Figure 1). The mean (SE) MG-ADL score at baseline was 8.4 (2.5). Demographic and baseline characteristics are shown in Table 1.
Figure 1. Patient Flow Through the Trial.
AChR indicates acetylcholine receptor; AE, adverse event; MuSK, muscle-specific kinase.
Table 1. Demographic and Baseline Characteristics of the Acetylcholine Receptor (AChR)/Muscle-Specific Kinase (MuSK) Antibody-Positive Population.
| Characteristic | Batoclimab (n = 67) | Placebo (n = 64) | All (N = 131) |
|---|---|---|---|
| Age, y | |||
| Mean (SD) [range] | 43.8 (13.9) [20-75] | 43.7 (13.5) [18-71] | 43.8 (13.6) [18-75] |
| <65 | 59 (88.1) | 58 (90.6) | 117 (89.3) |
| Sex, No. (%) | |||
| Male | 27 (40.3) | 16 (25.0) | 43 (32.8) |
| Female | 40 (59.7) | 48 (75.0) | 88 (67.2) |
| Body weight, kg | |||
| Mean (SD) [range] | 65.13 (13.5) [38.5-97.0] | 63.8 (11.4) [43.3-95.0] | 64.5 (12.5) [38.5-97.0] |
| <60, No. (%) | 26 (38.8) | 23 (35.9) | 49 (37.4) |
| ≥60, No. (%) | 41 (61.2) | 41 (64.1) | 82 (62.6) |
| BMIa | |||
| Mean (SD) [range] | 23.8 (3.9) [15.2-32.4] | 24.0 (3.7) [16.8-32.9] | 23.9 (3.8) [15.2-32.9] |
| <25, No. (%) | 46 (68.7) | 34 (53.1) | 80 (61.1) |
| ≥25, No. (%) | 21 (31.3) | 30 (46.9) | 51 (38.9) |
| MGFA class at screening | |||
| IIa | 20 (29.9) | 16 (25.0) | 36 (27.5) |
| IIb | 16 (23.9) | 16 (25.0) | 32 (24.4) |
| IIIa | 12 (17.9) | 14 (21.9) | 26 (19.8) |
| IIIb | 13 (19.4) | 12 (18.8) | 25 (19.1) |
| IVa | 6 (9.0) | 6 (9.4) | 12 (9.2) |
| Duration of disease, y | |||
| ≤2, No. (%) | 17 (25.4) | 19 (29.7) | 36 (27.5) |
| >2, No. (%) | 50 (74.6) | 45 (70.3) | 95 (72.5) |
| Thymectomy, No. (%) | 23 (34.3) | 14 (21.9) | 37 (28.2) |
| Baseline score, mean (SD) | |||
| MG-ADL | 8.4 (2.8) | 8.5 (2.1) | 8.4 (2.5) |
| QMG | 17.9 (4.8) | 18.3 (4.9) | 18.1 (4.8) |
| MGC | 18.9 (5.3) | 19.7 (5.4) | 19.3 (5.3) |
| MG-QOL15r | 16.0 (6.0) | 16.5 (6.2) | 16.3 (6.1) |
| Total IgG, mean (SE), g/L | 11.3 (0.4) | 11.0 (0.4) | 11.2 (0.3) |
| Autoantibody status, No. (%) | |||
| AChR antibody-positive only | 65 (97.0) | 59 (92.2) | 124 (94.7) |
| MuSK antibody-positive only | 2 (3.0) | 3 (4.7) | 5 (3.8) |
| Both positive | 0 | 2 (3.1) | 2 (1.5) |
| MG crisis in the preceding year, No. (%) | 2 (3.0) | 4 (6.3) | 6 (4.6) |
| MG therapy at baseline, No. (%) | |||
| Steroid only | 16 (23.9) | 20 (31.3) | 36 (27.5) |
| NSIST only | 6 (9.0) | 6 (9.4) | 12 (9.2) |
| Steroid and NSIST | 31 (46.3) | 24 (37.5) | 55 (42.0) |
| Other | 14 (20.9) | 14 (21.9) | 28 (21.4) |
Abbreviations: BMI, body mass index; IgG, immunoglobulin G; MG-ADL, Myasthenia Gravis Activities of Daily Living; MGC, Myasthenia Gravis Composite; MGFA, Myasthenia Gravis Foundation of America; MG-QOL15r, Myasthenia Gravis Quality of Life 15–Revised; NSIST, nonsteroidal immunosuppressive therapy; QMG, Quantitative Myasthenia Gravis.
Calculated as weight in kilograms divided by height in meters squared.
Among all 132 enrolled patients, 119 (90.2%) completed all 6 doses in cycle 1 (eTable 1 in Supplement 3). The second treatment cycle was conducted in 115 patients (56 and 59 in the placebo and batoclimab groups, respectively), and 87.8% of patients (101 of 115) completed all 6 doses.
In the first treatment cycle, the rate of sustained MG-ADL improvement (≥3-point reduction) in the antibody-positive patients was 31.3% (20 of 64; 95% CI, 20.2%-44.1%) in the placebo group vs 58.2% (39 of 67; 95% CI, 45.5%-70.2%) in the batoclimab group (OR, 3.45; 95% CI, 1.62-7.35; P = .001) (Table 2 and eFigure 1 in Supplement 3). MG-ADL score diverged between the 2 groups as early as week 2 (Figure 2). The maximal between-group difference in score reduction was observed at week 6 (1 week after the last dose): the corresponding mean (SE) score change from baseline was −1.7 (0.3) in the placebo group vs −3.6 (0.3) in the batoclimab group, with a group difference of −1.9 (95% CI, −2.8 to −1.0; nominal P < .001). The percentage of patients achieving MSE was 4.7% (3 of 64) in the placebo group vs 25.4% (17 of 67) in the batoclimab group (P = .004) (Table 2). The rate of early MG-ADL improvement was 34.4% for patients (22 of 64) in the placebo group vs 32.8% for patients (22 of 67) in the batoclimab group (OR, 0.95; 95% CI, 0.44-2.05; P = .89) (Table 2). The higher rate of sustained MG-ADL improvement in the main analysis was supported by all sensitivity analyses (eTable 2 in Supplement 3). The results of all subgroup analyses were consistent with those in the overall cohort (eFigure 2 in Supplement 3). The rate of sustained QMG improvement in the first treatment cycle was 40.6% for patients (26 of 64) in the placebo group vs 64.2% for patients (43 of 67) in the batoclimab group (OR, 2.62; 95% CI, 1.29-5.33; P = .008) (Table 2 and eFigure 1 in Supplement 3). The 2 groups diverged as early as week 1. At the time of QMG reduction plateau (week 6), the mean (SE) change from baseline in QMG was −1.6 (0.5) in the placebo group vs −6.4 (0.5) in the batoclimab group, with a group difference of −4.7 (95% CI, −6.1 to −3.4; P < .001) (Figure 2). A clinically meaningful reduction from baseline in QMG score (by at least 3 points) was still observed at week 9 in the batoclimab group. The trajectories of MGC and MG-QOL15r scores were similar to those of QMG score, with improvement from week 2 through week 7 (MGC) or week 8 (MG-QOL15r) (Figure 2).
Table 2. Efficacy Outcomes in the First Treatment Cycle.
| Outcome | No. (%) | OR or LS mean difference (95% CI) | P value | |
|---|---|---|---|---|
| Batoclimab (n = 67) | Placebo (n = 64) | |||
| Primary outcome | ||||
| Sustained MG-ADL improvement | 39 (58.2) | 20 (31.3) | 3.45 (1.62-7.35) | .001 |
| Secondary outcomes | ||||
| Sustained QMG improvement | 43 (64.2) | 26 (40.6) | 2.62 (1.29-5.33) | .008 |
| Duration of ≥3-point MG-ADL improvement,a mean (SE), % | 51.5 (4.22) | 36.1 (4.48) | 15.4 (4.1-26.7) | .008 |
| Minimal symptom expression | 17 (25.4) | 3 (4.7) | 6.81 (1.85-25.11) | .004 |
| Early MG-ADL improvement | 22 (32.8) | 22 (34.4) | 0.95 (0.44-2.05) | .89 |
Abbreviations: LS mean, least squares mean; MG-ADL, Myasthenia Gravis Activities of Daily Living; OR, odds ratio; QMG, Quantitative Myasthenia Gravis.
Duration of 3-point or greater MG-ADL improvement was calculated relative to the 24-week treatment period (%), and presented as LS mean and corresponding SE. Outcomes are shown in the order of statistical test sequence. Sustained MG-ADL improvement was considered a 3-point or greater reduction in MG-ADL score from baseline for at least 4 consecutive weeks. Sustained QMG improvement was considered a 3-point or greater reduction for at least 4 consecutive weeks. Minimal symptom expression was considered an MG-ADL score of 0 or 1 at any visit. Early MG-ADL improvement was considered a 3-point or greater reduction within the first 2 weeks of the first cycle. All analyses were conducted in the antibody-positive patients.
Figure 2. Temporal Profile of Myasthenia Gravis Activities of Daily Living (MG-ADL), Quantitative Myasthenia Gravis (QMG), Myasthenia Gravis Composite (MGC), and MG Quality of Life 15–Revised (MG-QOL15r) Scores in Antibody-Positive Patients Through the First Cycle.
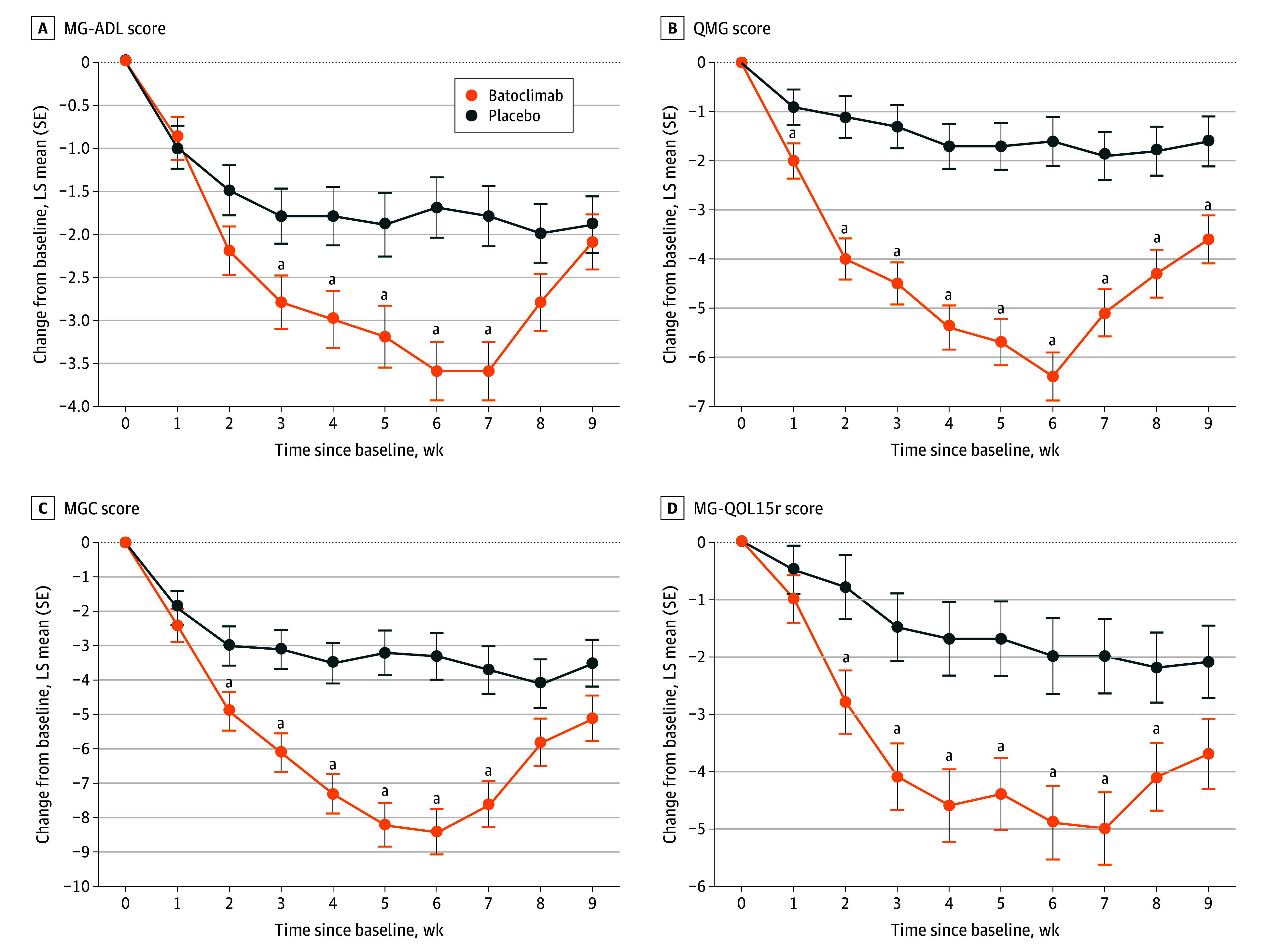
A, MG-ADL score. B, QMG score. C, MGC score. D, MG-QOL15r score. Weekly treatment was provided from 0 to 5 weeks.
aIndicates nominal P <.05.
Nine of 64 participants (14.1%) in the placebo group did not receive cycle 2 treatment (4 did not meet retreatment criteria, 3 received rescue therapy, and 1 lost to follow-up), and 8 of 67 participants (11.9%) in the batoclimab group did not receive cycle 2 treatment (7 did not meet retreatment criteria, and 1 received rescue therapy). The median (IQR) time to initiation of cycle 2 treatment was 9 (9-10) weeks and 10 (9-11) weeks after the first dose of treatment in cycle 1 in the placebo and batoclimab groups, respectively. In the second treatment cycle, the rate of sustained MG-ADL improvement was 36.4% for patients (20 of 55) in the placebo group vs 62.7% for patients (37 of 59) in the batoclimab group (nominal P = .002). The MG-ADL score in cycle 2 diverged as early as week 1; the maximal between-group difference in score reduction occurred at week 5 (eFigure 3 in Supplement 3). The percentage of the patients with sustained MG-ADL improvement in both cycles was 23.6% (13 of 55) in the placebo group vs 50.8% (30 of 59) in the batoclimab group. The percentage of the patients with no sustained MG-ADL improvement in both cycles was 58.2% (32 of 55) in the placebo group vs 32.2% (19 of 59) in the batoclimab group.
The mean (SE) duration of MG-ADL improvement of 3 points or greater relative to the 24 weeks treatment period was 36.1% (4.5%) in the placebo group vs 51.5% (4.2%) in the batoclimab group, with a group difference of 15.4% (95% CI, 4.1%-26.7%; P = .008) (Table 2).
The rate of treatment-emergent AEs (TEAEs) was 92.3% for patients (60 of 65) in the placebo group and 95.5% for patients (64 of 67) in the batoclimab group (Table 3). The rate of severe TEAEs was 7.7% for patients (5 of 65) in the placebo group vs 3.0% for patients (2 of 67) in the batoclimab group. The 3 most frequently reported TEAEs in study participants were upper respiratory tract infection in 14 of 65 (21.5%), urinary tract infection in 10 of 65 (15.4%), and MG worsening in 8 of 65 (12.3%) in the placebo group, and peripheral edema in 26 of 67 (38.8%), upper respiratory tract infection in 24 of 67 (35.8%), and urinary tract infection in 13 of 67 (19.4%) in the batoclimab group. Treatment was interrupted due to TEAEs in 2 patients (3.1%) and 3 patients (4.5%) in the placebo and batoclimab groups, respectively. Treatment was permanently discontinued due to TEAEs in 2 patients (3.1%) and 1 patient (1.5%) in the placebo (one each of MG worsening and MG crisis) and batoclimab (MG worsening) groups, respectively.
Table 3. Summary of Adverse Events.
| Event | No. (%) | |
|---|---|---|
| Batoclimab (n = 67) | Placebo (n = 65) | |
| TEAEs | 64 (95.5) | 60 (92.3) |
| Mild | 33 (49.3) | 32 (49.2) |
| Moderate | 29 (43.3) | 23 (35.4) |
| Severe | 2 (3.0) | 5 (7.7) |
| TEAEs causing treatment termination | 1 (1.5) | 2 (3.1) |
| TEAEs causing treatment interruptions | 3 (4.5) | 2 (3.1) |
| TEAEs (>5%) | ||
| Peripheral edema | 26 (38.8) | 3 (4.6) |
| Upper respiratory tract infection | 24 (35.8) | 14 (21.5) |
| Urinary tract infection | 13 (19.4) | 10 (15.4) |
| Diarrhea | 9 (13.4) | 7 (10.8) |
| Myasthenia gravis worsening | 8 (11.9) | 8 (12.3) |
| Hypercholesterolemia | 7 (10.4) | 1 (1.5) |
| Muscle spasms | 5 (7.5) | 3 (4.6) |
| Hyperlipidemia | 5 (7.5) | 2 (3.1) |
| Sleep disturbance | 5 (7.5) | 1 (1.5) |
| Cholesterol level increase | 5 (7.5) | 1 (1.5) |
| Hypertriglyceridemia | 5 (7.5) | 1 (1.5) |
| White blood cell level increase | 4 (6.0) | 6 (9.2) |
| CRP level increase | 4 (6.0) | 3 (4.6) |
| Headache | 4 (6.0) | 3 (4.6) |
| Nasopharyngitis | 4 (6.0) | 2 (3.1) |
| LDL-C level increase | 4 (6.0) | 1 (1.5) |
| Peripheral swelling | 4 (6.0) | 1 (1.5) |
| Hypocomplementemia | 4 (6.0) | 1 (1.5) |
| COVID-19 | 3 (4.5) | 4 (6.2) |
| Back pain | 3 (4.5) | 4 (6.2) |
Abbreviations: CRP, C-reactive protein; LDL-C, low-density lipoprotein cholesterol; TEAE, treatment-emergent adverse event.
The rate of treatment-related AEs (TRAEs) was 36.9% for patients (24 of 65) in the placebo group and 70.1% for patients (47 of 67) in the batoclimab group (eTable 3 in Supplement 3). The 3 most frequently reported TRAEs in patients in the placebo group were urinary tract infection in 6 of 65 (9.2%), upper respiratory tract infection in 5 of 65 (7.7%), and peripheral edema in 3 of 65 (4.6%). The 4 most frequently reported TRAEs in patients in the batoclimab group were peripheral edema in 26 of 67 (38.8%), hypercholesterolemia in 7 of 67 (10.4%), hyperlipidemia in 5 of 67 (7.5%), and upper respiratory tract infection in 5 of 67 (7.5%). Treatment was interrupted due to TRAE in 1 patient (1.5%) in each group (hematuria in the placebo group; anxiety in the batoclimab group). No permanent discontinuation of the test agent due to TRAE was reported, and no death was reported.
In the first treatment cycle, plasma albumin levels in the batoclimab group started to decline at week 1, reached nadir at week 6 (from a mean [SE] of 45.3 [0.3] g/L at baseline to 31.2 [0.5] g/L at week 6, corresponding to a 31% reduction), and returned to a level slightly lower than baseline at 3 weeks after the last dose (eFigure 4 in Supplement 3). Total cholesterol level in the batoclimab group increased at week 1, plateaued at week 6 (from a mean [SE] of 209 [5.4] mg/dL at baseline to 261 [6.8] mg/dL, corresponding to a 52.6 mg/dL increase; to convert to millimoles per liter, multiply by 0.0259), and returned to within 90% of the baseline 4 weeks after the last dose (eFigure 4 in Supplement 3). Plasma LDL-C level showed a similar trajectory (eFigure 4C in Supplement 3), with a maximal increase at week 6 (mean [SE] difference from baseline 39.1 [3.9] mg/dL; to convert to millimoles per liter, multiply by 0.0259).
During the 24-week study, the rate of hypercholesterolemia was 1.5% (1 of 65 patients) and 10.4% (7 of 67 patients) in the placebo and batoclimab groups, respectively. Hyperlipidemia occurred in 2 patients (3.1%) receiving placebo and 5 patients (7.5%) receiving batoclimab. LDL-C level was elevated in 1 patient (1.5%) and 4 patients (6.0%) in the placebo and batoclimab groups, respectively.
Batoclimab resulted in a rapid reduction in serum total IgG level (eFigure 5 in Supplement 3), reaching the maximal mean (SE) reduction of 70.8% (1.2%) at week 6 in the cycle 1 and returning to approximately 70% of baseline by week 9 (mean [SE], −30.4% [1.6%]). Batoclimab also led to a rapid and sustained reduction in AChR antibody, with a median (IQR) reduction of 81.1% (58.9%-86.3%) at week 6 (eFigure 5 in Supplement 3). Serum MuSK antibody was available in 1 patient in each group only and was not analyzed. Increase in C3 level was just observed in the batoclimab group, with a maximal increase from mean (SE) of 1.14 (0.02) g/L at baseline to 1.31 (0.3) g/L at week 6 (to convert to milligrams per deciliter, divide by 0.01). The 2 groups did not differ in CH50 throughout cycle 1 (eFigure 5 in Supplement 3).
Antibatoclimab antibodies were positive at the baseline in 1 patient (in the batoclimab group) and positive in 4 additional patients (6.0%) after batoclimab treatment in the batoclimab group.
Discussion
Consistent with a previous phase 2 trial, the results of this phase 3 trial confirmed the efficacy of batoclimab in patients with antibody-positive generalized MG.20 The rate of sustained MG-ADL improvement in the first treatment cycle was 31.3% in the placebo control group vs 58.2% in the batoclimab group. The efficacy of batoclimab in the main analysis was supported by all sensitivity analyses, indicating the robustness of study results. The rate of MSE in cycle 1 was also higher in the batoclimab group than in the placebo control group. Subgroup analyses confirmed consistent efficacy across various subpopulations. In addition to sustained MG-ADL improvement, batoclimab showed broad efficacy, including symptom relief and quality of life improvement as assessed by multiple instruments. The trajectory of total IgG and AChR antibodies mirrored that of improvements in the efficacy measures, providing additional evidence to support efficacy conclusion. The trial data also showed that batoclimab was safe and well tolerated, with no new safety concerns. Another important finding was sustained efficacy with batoclimab in the second treatment cycle.
The observed rate of sustained MG-ADL improvement in this trial (58%) is lower than that reported for efgartigimod in the ADAPT trial (68%).13 Such a discrepancy was primarily due to the more stringent definition of sustained MG-ADL improvement in this trial (a 3-point reduction vs a 2-point reduction from the baseline in the ADAPT trial). If a 2-point reduction in MG-ADL score was used as the threshold for sustained improvement, the rate of sustained improvement in this trial (65.7%) would have been similar to that reported for efgartigimod. Although the primary outcome of this study differs from that of the MycarinG study, the change in MG-ADL scores from baseline at day 43 in the batoclimab group (mean [SE], −3.6 [0.34]) was almost identical to that reported for rozanolixizumab at 10 mg/kg (mean [SE], −3.4 [0.49]).14
The MG-ADL score diverged between the 2 groups as early as week 2. Meaningful separation of QMG score occurred as early as week 1 in the first treatment cycle, suggesting rapid onset of action. This is similar to efgartigimod and rozanolixizumab, as both led to a reduction in QMG in week 1 of the cycle 1.13,14,15
The maximal reduction of serum total IgG level with batoclimab (70.8% at week 6) in this trial is similar to that reported for efgartigimod (57.6%).13 On discontinuation of batoclimab, serum total IgG gradually returned to a level comparable with the baseline after 4 weeks. Reversibility of the effect is important considering the risk of infection on prolonged immune suppression. Serum AChR antibody level exhibited a sustained reduction from week 1 through week 9 of cycle 1, with a median reduction of 81.1% at week 6.
A higher rates of upper respiratory tract infection (35.8% vs 21.5%) and urinary tract infection (19.4% vs 15.4%) were noted in the batoclimab group than in the placebo control group. These findings are generally consistent with those reported for rozanolixizumab (infection rate of 30% vs 19% with placebo) and for efgartigimod (upper respiratory tract infection 11% vs 5% with placebo; urinary tract infection 10% vs 5%).13,14 The rate of treatment-related upper respiratory tract infection was comparable between the 2 groups (batoclimab 7.5% vs placebo 7.7%), whereas treatment-related urinary tract infection was numerically lower with batoclimab (6.0% vs 9.2%). The majority of the infections were mild, supporting the overall safety of batoclimab.
In addition to immunoglobins, FcRn prevents albumin from lysosomal degradation.28 Similar to reduced albumin reported for rozanolixizumab, albumin level in the batoclimab group decreased significantly throughout the treatment cycle.14 Consistent with reduced albumin, the rate of peripheral edema was significantly higher in the batoclimab group (38.8% vs 4.6% with placebo). However, all cases were mild (26.9%) or moderate (11.9%) and deemed not clinically significant. In addition, albumin level increased rapidly toward baseline on treatment discontinuation. Ascites was not reported in either group, and pleural effusion occurred in 1 patient each in both groups. Because albumin is the key contributor in maintaining plasma osmotic pressure, the risk of ascites and pleural effusion remains a concern in patients with existing conditions (eg, hepatic cirrhosis). In such patients, efgartigimod may be more appropriate due to the lack of significant impact on albumin level.13
Consistent with the previous phase 2 trial, individuals in the current trial who received batoclimab had a higher rate of hypercholesterolemia (10.4% vs 1.5% in the placebo control).14 LDL-C level was elevated in 6.0% of the patients in the batoclimab group vs 1.5% in the placebo control. LDL-C and cholesterol level elevation was transient and reversible on drug discontinuation. No elevation in triglyceride level was observed in the batoclimab group, nor were any cardiovascular events reported in either this trial or in the previous phase 2 trial.20 Increased LDL-C level with concomitant albumin decrease in the batoclimab group seems to support the putative negative correlation between cholesterol and albumin metabolism.29
Headache is a primary TEAE reported for rozanolixizumab (38%) and efgartigimod (29%).13,14 In this trial, the rate of headache did not differ between the batoclimab and placebo groups (both at 6.0%). This finding may seem minor but could potentially translate into improved adherence in daily practice settings. The rate of newly emerged antibatoclimab antibodies after batoclimab treatment (6.0%) in this trial was lower than that reported for antidrug antibody with efgartigimod (20%).13 The clinical relevance of this finding requires further investigation.
The findings of this trial are clinically important for a variety of reasons. First, the efficacy of FcRn inhibitors in the Asian population has only been examined in subgroup analyses with a limited number of participants in previous studies.13,14 Most notably, the rate of sustained MG-ADL improvement in the Asian subpopulation of the ADAPT trial was similar between the treatment and placebo groups.13 Second, batoclimab is an important addition to the FcRn antagonist family from a global perspective, especially as a valuable alternative source of FcRn antagonist for China and the surrounding regions. This is particularly important considering the high mortality rate in hospitalized generalized MG patients in China.2,30
Strengths and Limitations
A major strength of this trial was the large number of participating centers (n = 27) across wide geographical areas in China, thereby broadly representing the overall generalized MG patient population. However, this trial also has several limitations. First, the trial just included 2 treatment cycles. Efficacy and long-term safety require further investigation. Second, the trial was not designed for investigation of long-term safety, particularly infections and cardiovascular events. An open-label extension trial is ongoing to examine the long-term safety and efficacy of batoclimab. Third, only 1 patient with negative AChR/MuSK antibodies was enrolled, preventing us from assessing the efficacy of batoclimab in this subpopulation.
Conclusions
In this randomized clinical trial, batoclimab resulted in a higher rate of sustained MG-ADL improvement in adult patients with generalized MG compared with placebo. An open-label extension trial is currently ongoing to monitor long-term safety.
Trial Protocol.
Statistical Analysis Plan.
eTable 1. Exposure of Study Agent
eTable 2. Sensitivity Analyses of Sustained Improvement in MG-ADL From Baseline to Week 9
eTable 3. Summary of Treatment-Related Adverse Events (TRAEs)
eFigure 1. Rate of Sustained Reduction of MG-ADL (A) of MG-QMG (B) for at Least 4 Weeks in AChR/MuSK Antibody-Positive Patients in the First Cycle
eFigure 2. Subgroup Analysis of Sustained Reduction of MG-ADL Stratified by Baseline Characteristics
eFigure 3. Mean Changes From Baseline in MG-ADL Score in the Antibody-Positive Patients Throughout the Second Cycle
eFigure 4. Mean (SE) Serum Albumin (A), Total Cholesterol (B), and LDL-C (C) in the Safety Set Through the First Cycle
eFigure 5. Change From Baseline in Total IgG (A), AChR Antibodies (B), C3 (C) and CH50 (D) in the Antibody-Positive Patients Through the First Cycle
Nonauthor Collaborators. Batoclimab Study Team.
Data Sharing Statement.
References
- 1.Carr AS, Cardwell CR, McCarron PO, McConville J. A systematic review of population based epidemiological studies in myasthenia gravis. BMC Neurol. 2010;10:46. doi: 10.1186/1471-2377-10-46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salari N, Fatahi B, Bartina Y, et al. Global prevalence of myasthenia gravis and the effectiveness of common drugs in its treatment: a systematic review and meta-analysis. J Transl Med. 2021;19(1):516. doi: 10.1186/s12967-021-03185-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen J, Tian DC, Zhang C, et al. Incidence, mortality, and economic burden of myasthenia gravis in China: a nationwide population-based study. Lancet Reg Health West Pac. 2020;5:100063. doi: 10.1016/j.lanwpc.2020.100063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patrick J, Lindstrom J. Autoimmune response to acetylcholine receptor. Science. 1973;180(4088):871-872. doi: 10.1126/science.180.4088.871 [DOI] [PubMed] [Google Scholar]
- 5.Fambrough DM, Drachman DB, Satyamurti S. Neuromuscular junction in myasthenia gravis: decreased acetylcholine receptors. Science. 1973;182(4109):293-295. doi: 10.1126/science.182.4109.293 [DOI] [PubMed] [Google Scholar]
- 6.Gilhus NE, Nacu A, Andersen JB, Owe JF. Myasthenia gravis and risks for comorbidity. Eur J Neurol. 2015;22(1):17-23. doi: 10.1111/ene.12599 [DOI] [PubMed] [Google Scholar]
- 7.Hoch W, McConville J, Helms S, Newsom-Davis J, Melms A, Vincent A. Autoantibodies to the receptor tyrosine kinase MuSK in patients with myasthenia gravis without acetylcholine receptor antibodies. Nat Med. 2001;7(3):365-368. doi: 10.1038/85520 [DOI] [PubMed] [Google Scholar]
- 8.Gilhus NE. Myasthenia gravis. N Engl J Med. 2016;375(26):2570-2581. doi: 10.1056/NEJMra1602678 [DOI] [PubMed] [Google Scholar]
- 9.Vanoli F, Mantegazza R. Current drug treatment of myasthenia gravis. Curr Opin Neurol. 2023;36(5):410-415. doi: 10.1097/WCO.0000000000001196 [DOI] [PubMed] [Google Scholar]
- 10.Dalakas MC. Immunotherapy in myasthenia gravis in the era of biologics. Nat Rev Neurol. 2019;15(2):113-124. doi: 10.1038/s41582-018-0110-z [DOI] [PubMed] [Google Scholar]
- 11.Alcantara M, Barnett C, Katzberg H, Bril V. An update on the use of immunoglobulins as treatment for myasthenia gravis. Expert Rev Clin Immunol. 2022;18(7):703-715. doi: 10.1080/1744666X.2022.2084074 [DOI] [PubMed] [Google Scholar]
- 12.Pyzik M, Kozicky LK, Gandhi AK, Blumberg RS. The therapeutic age of the neonatal Fc receptor. Nat Rev Immunol. 2023;23(7):415-432. doi: 10.1038/s41577-022-00821-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gable KL, Guptill JT. Antagonism of the neonatal Fc receptor as an emerging treatment for myasthenia gravis. Front Immunol. 2020;10:3052. doi: 10.3389/fimmu.2019.03052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Howard JF Jr, Bril V, Vu T, et al. ; ADAPT Investigator Study Group . Safety, efficacy, and tolerability of efgartigimod in patients with generalised myasthenia gravis (ADAPT): a multicentre, randomised, placebo-controlled, phase 3 trial. Lancet Neurol. 2021;20(7):526-536. doi: 10.1016/S1474-4422(21)00159-9 [DOI] [PubMed] [Google Scholar]
- 15.Bril V, Drużdż A, Grosskreutz J, et al. ; MG0003 study team . Safety and efficacy of rozanolixizumab in patients with generalised myasthenia gravis (MycarinG): a randomised, double-blind, placebo-controlled, adaptive phase 3 study. Lancet Neurol. 2023;22(5):383-394. doi: 10.1016/S1474-4422(23)00077-7 [DOI] [PubMed] [Google Scholar]
- 16.Heo YA. Efgartigimod alfa in generalised myasthenia gravis: a profile of its use. CNS Drugs. 2023;37(5):467-473. doi: 10.1007/s40263-023-01000-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoy SM. Rozanolixizumab: first approval. Drugs. 2023;83(14):1341-1347. doi: 10.1007/s40265-023-01933-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guptill J, Antozzi C, Bril V, et al. Vivacity-MG: a phase 2, multicenter, randomized, double-blind, placebo-controlled study to evaluate the safety, tolerability, efficacy, pharmacokinetics, pharmacodynamics, and immunogenicity of nipocalimab administered to adults with generalized myasthenia gravis. Abstract presented at: American Academy of Neurology Annual Meeting; April 21, 2021; Auckland, New Zealand. Abstract S29.002. [Google Scholar]
- 19.Saccà F, Pane C, Espinosa PE, Sormani MP, Signori A. Efficacy of innovative therapies in myasthenia gravis: a systematic review, meta-analysis and network meta-analysis. Eur J Neurol. 2023;30(12):3854-3867; Epub ahead of print. doi: 10.1111/ene.15872 [DOI] [PubMed] [Google Scholar]
- 20.Yap DYH, Hai J, Lee PCH, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of HBM9161, a novel FcRn inhibitor, in a phase 1 study for healthy Chinese volunteers. Clin Transl Sci. 2021;14(5):1769-1779. doi: 10.1111/cts.13019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yan C, Duan RS, Yang H, et al. Therapeutic effects of batoclimab in Chinese patients with generalized myasthenia gravis: a double-blinded, randomized, placebo-controlled phase 2 study. Neurol Ther. 2022;11(2):815-834. doi: 10.1007/s40120-022-00345-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Evaluate the Safety of HBM9161 (HL161) Subcutaneous Injection in Patients With Generalized Myasthenia Gravis. ClinicalTrials.gov identifier: NCT05332210. Updated March 8, 2023. Accessed March 8, 2023. https://clinicaltrials.gov/study/NCT05332210
- 23.Wolfe GI, Herbelin L, Nations SP, Foster B, Bryan WW, Barohn RJ. Myasthenia gravis activities of daily living profile. Neurology. 1999;52(7):1487-1489. doi: 10.1212/WNL.52.7.1487 [DOI] [PubMed] [Google Scholar]
- 24.Bedlack RS, Simel DL, Bosworth H, Samsa G, Tucker-Lipscomb B, Sanders DB. Quantitative myasthenia gravis score: assessment of responsiveness and longitudinal validity. Neurology. 2005;64(11):1968-1970. doi: 10.1212/01.WNL.0000163988.28892.79 [DOI] [PubMed] [Google Scholar]
- 25.Burns TM, Conaway M, Sanders DB; MG Composite and MG-QOL15 Study Group . The MG Composite: a valid and reliable outcome measure for myasthenia gravis. Neurology. 2010;74(18):1434-1440. doi: 10.1212/WNL.0b013e3181dc1b1e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burns TM, Grouse CK, Conaway MR, Sanders DB; mg composite and mg-qol15 study group . Construct and concurrent validation of the MG-QOL15 in the practice setting. Muscle Nerve. 2010;41(2):219-226. doi: 10.1002/mus.21609 [DOI] [PubMed] [Google Scholar]
- 27.Burns TM, Grouse CK, Wolfe GI, Conaway MR, Sanders DB; MG Composite and MG-OL15 Study Group . The MG-QOL15 for following the health-related quality of life of patients with myasthenia gravis. Muscle Nerve. 2011;43(1):14-18. doi: 10.1002/mus.21883 [DOI] [PubMed] [Google Scholar]
- 28.Ward ES, Gelinas D, Dreesen E, et al. Clinical significance of serum albumin and implications of FcRn inhibitor treatment in IgG-Mediated autoimmune disorders. Front Immunol. 2022;13:892534. doi: 10.3389/fimmu.2022.892534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hossain MA, Deb KP, Mannan KA, et al. Correlation between serum cholesterol and serum albumin level in childhood nephrotic syndrome. Urol Nephrol Open Access J. 2016;3:00086. doi: 10.15406/unoaj.2016.03.00086 [DOI] [Google Scholar]
- 30.Zhang C, Wang F, Long Z, et al. Mortality of myasthenia gravis: a national population-based study in China. Ann Clin Transl Neurol. 2023;10(7):1095-1105. doi: 10.1002/acn3.51792 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol.
Statistical Analysis Plan.
eTable 1. Exposure of Study Agent
eTable 2. Sensitivity Analyses of Sustained Improvement in MG-ADL From Baseline to Week 9
eTable 3. Summary of Treatment-Related Adverse Events (TRAEs)
eFigure 1. Rate of Sustained Reduction of MG-ADL (A) of MG-QMG (B) for at Least 4 Weeks in AChR/MuSK Antibody-Positive Patients in the First Cycle
eFigure 2. Subgroup Analysis of Sustained Reduction of MG-ADL Stratified by Baseline Characteristics
eFigure 3. Mean Changes From Baseline in MG-ADL Score in the Antibody-Positive Patients Throughout the Second Cycle
eFigure 4. Mean (SE) Serum Albumin (A), Total Cholesterol (B), and LDL-C (C) in the Safety Set Through the First Cycle
eFigure 5. Change From Baseline in Total IgG (A), AChR Antibodies (B), C3 (C) and CH50 (D) in the Antibody-Positive Patients Through the First Cycle
Nonauthor Collaborators. Batoclimab Study Team.
Data Sharing Statement.