Abstract

3-Oxidopyraziniums are azomethine ylides derived from 2(1H)-pyrazinones that can undergo 1,3-dipolar cycloadditions with acrylate and acrylic acid derivatives. The cycloaddition of 1-(4-methoxybenzyl)-5,6-dimethyl-3-oxidopyrazinium with methyl and tert-butyl acrylate and with methyl crotonate afforded a 3,8-diazabicyclo[3.2.1]octane in 51–73% yield together with traces of the 2,5-diazabicyclo[2.2.2]octane. In contrast, cycloaddition of this 3-oxidopyrazinium with methyl 2-phenyl acrylate provided the [2.2.2] product in 40% yield. Herein, we show that the 2,5-diazabicyclo[2.2.2]octanes were formed from the [3.2.1] compounds via a Wagner–Meerwein rearrangement. Remarkably, when acrylic acid and 2-phenylacrylic acid were employed as dipolarophiles, novel tricyclic fused lactone-lactam systems were obtained in 71% and 50% yields, respectively. The formation of these tricyclic compounds can be rationalized via the mechanism described above followed by lactonization of the 2,5-diazabicyclo[2.2.2]octane.
Introduction
A 1989 review summarized Katritzky’s explorations of the reactivity of zwitterions 1, which were termed 3-oxidopyridiniums (Scheme 1).1 Thus, with R = Me, 1 is 1-methyl-3-oxidopyridinium (Chemical Abstracts uses the term “3-hydroxy-1-methylpyridinium, inner salt”). The interesting reactivity of these betaines has attracted the attention of researchers due to their application in the synthesis of natural products.2,3 These isolable species were accessed via quaternization of 3-hydroxypyridine (2) with an alkyl halide, giving 3-hydroxypyridinium salts 3, and then O-deprotonation under room-temperature conditions. The chief interest was in the reactivity of 3-oxidopyridiniums 1 as 1,3-dipoles in cycloaddition reactions with alkenes, across C-2 and C-6. Thus, for example, 1-methyl-3-oxidopyridinium reacted with ethyl acrylate producing ethyl 8-methyl-2-oxo-8-azabicyclo[3.2.1]oct-3-ene-6-carboxylate (4) (Scheme 1). Note that the regioselectivity of this reaction is determined by the polarization implied by resonance structure 1c.
Scheme 1. Main Resonance Contributors to 3-Oxidopyridiniums 1 and Reaction of 1-Methyl-3-oxidopyridinium with Ethyl Acrylate.
It occurred to us that comparable zwitterions and thence comparable cycloadditions might be possible in an analogous pyrazine series, though an alternative route to 3-oxidopyraziniums 5 would be required (Chemical Abstracts uses the term “3,4-dihydro-3-oxopyrazinium, inner salt”) (Scheme 2). This concept was put into practice by accessing 3-oxidopyraziniums 5 from 2(1H)-pyrazinones 6.4 Regioselective quaternization of the imine nitrogen of 2(1H)-pyrazinones 6 gives pyrazinium salts 7, and then N-deprotonation reveals 3-oxidopyraziniums 5. We showed that compounds 5 undergo ready dipolar cycloadditions with typical dipolarophiles like acrylates, acrylonitrile, vinyl sulfones, α,β-unsaturated ketones, and alkynyl esters.5,6 The cycloaddition occurred in the regiosense indicated by resonance contributor 5c and afforded 3,8-diazabicyclo[3.2.1]octanes 8–11 with an exo carboxylate group. The assumed intermediate products of the cycloadditions, 12 in Scheme 2, were never seen, the isolated material in each case being enamide tautomers 8–11.
Scheme 2. Synthesis of 3-Oxidopyraziniums 5 and Their Reaction with Acrylates.
Density functional theory (DFT) studies7−9 of the regio- and stereoselectivity of the 1,3-dipolar cycloaddition of 3-oxidopyraziniums 5 with methyl acrylate were consistent with the experimental results in terms of the regiochemistry of cycloaddition and the stereochemistry of the ester substituent in product 3,8-diazabicyclo[3.2.1]octanes. However, in an experiment designed to test the effect of a more bulky dipolarophile, 1,5,6-trimethyl-3-oxidopyrazinium (13) was reacted with methyl methacrylate but did not give the expected 3,8-diazabicyclo[3.2.1]octane 14 (Scheme 3, route a). Instead, tricyclic fused lactam-lactone product 15, derived from a [2.2.2] core,10 was obtained. Scheme 3 (route b) suggests a plausible mechanistic sequence that would lead to 15. The central difference from the previously assumed sequence leading to 3,8-diazabicyclo[3.2.1]octanes is that the initial interaction between the acrylate and the 3-oxidopyrazinium is proposed to occur across carbons C-2 and C-5; i.e., it is an aza-Diels–Alder type process: C-5–C-6–N-1–C-2 being the azadiene, yielding 2,5-diazabicyclo[2.2.2]octane 16. Afterward, an SN2 reaction, promoted by a halide anion, with concomitant nucleophilic attack of the created carboxylate anion on an iminium carbon, thus forms the lactone ring.
Scheme 3. Reaction of 1,5,6-Trimethyl-3-oxidopyrazinium 13 with Methyl Methacrylate Showing the Suggested Sequence of Bond Changes.

Alternatively, 2,5-diazabicyclo[2.2.2]octane 16 could be formed via a rearrangement of an initial 3,8-diazabicyclo[3.2.1]octane 14 obtained from a 1,3-dipolar type addition (Scheme 3, route a). In fact, this rearrangement might be reversible; i.e., 3,8-diazabicyclo[3.2.1]octane 14 might be formed from 2,5-diazabicyclo[2.2.2]octane 16. The feasibility of the mechanism of this rearrangement was assessed by the DFT method at the B3LYP/6-31G(d) level.11 The conclusion was that formation of lactone-lactam 15 is a domino process involving three consecutive reactions: first a 1,3-dipolar cycloaddition between 3-oxidopyrazinium 13 and methyl methacrylate that yields 3,8-diazabicyclo[3.2.1]octane 14, next a skeletal rearrangement, which converts this adduct into 2,5-diazabicyclo[2.2.2]octane 16, and finally lactone ring formation. This theoretical assessment therefore suggests that the 3,8-diazabicyclo[3.2.1]octane products (Scheme 3) are formed directly, and not via an aza-Diels–Alder intermediate.
In this work, we have explored the reactions of 1-(4-methoxybenzyl)-5,6-dimethyl-3-oxidopyrazinium (17) with acrylate and acrylic acid derivatives with the objective of gaining further insight into the mechanism of formation of 3,8-diazabicyclo[3.2.1]octanes (1,3-dipolar type addition) versus 2,5-diazabicyclo[2.2.2]octanes (aza-Diels–Alder type addition) and their possible interconversion (Scheme 4). The presence of the 4-methoxybenzyl (PMB) group at N-1 improves solubility, facilitates the interpretation of product 1H nuclear magnetic resonance (NMR) spectra, and was intended to be a late-stage removable substituent.
Scheme 4. Reactions of 3-Oxidopyrazinium 17 with an Acrylate Derivative and Possible Interconversions of the Corresponding Bicyclic Products.
Results and Discussion
Cycloaddition Reactions of 1-(4-Methoxybenzyl)-5,6-dimethyl-3-oxidopyrazinium 17 with Acrylate Derivatives
5,6-Dimethyl-2(1H)-pyrazinone (18) was prepared by Jones’ method12 through the condensation of butane-2,3-dione and glycinamide hydrochloride.13 N-1 alkylation with 4-methoxybenzyl bromide was carried out in acetonitrile under reflux for 6 h and then at room temperature overnight (Scheme 5). Purification of the resulting bromide salt by reversed-phase chromatography afforded 1-(4-methoxybenzyl)-5,6-dimethyl-3-oxo-3,4-dihydropyrazin-1-ium bromide (19) in 75% yield.
Scheme 5. Synthesis of Pyrazinium Bromide 19 and Cycloadditions of 3-Oxidopyrazinium 17 with Acrylates.
With pyrazinium bromide 19 in hand, we proceeded to explore the cycloaddition reactions of its corresponding 1-(4-methoxybenzyl)-5,6-dimethyl-3-oxidopyrazinium (17) with a range of acrylates (Table 1). Unlike our earlier studies, these cycloadditions were performed using a more convenient one-pot procedure, and without isolating the 3-oxidopyrazinium. Thus, a suspension of pyrazinium bromide 19 was treated with triethylamine at room temperature, leading to an orange solution of 3-oxidopyrazinium 17, to which was added the corresponding dipolarophile, while the mixture was kept at room temperature (Scheme 5). The formation of 3-oxidopyrazinium 17 was checked by electrospray ionization mass spectroscopy (ESI-MS) and 1H NMR.
Table 1. Reaction of 3-Oxidopyrazinium 17 with Acrylates.
| entry | R | R1 | R2 | T (°C) | reaction time (h) | [3.2.1] products [yield (%)] | [2.2.2] products [yield (%)] |
|---|---|---|---|---|---|---|---|
| 1 | CH3 | H | H | rt | 0.75 | 20a (73) | 21a (4) |
| 2 | t-Bu | H | H | rt | 1.5 | 20b (63) | 21b (7) |
| 3 | CH3 | H | CH3 | rt | 6 | 20c (51) | – |
| 4 | CH3 | H | Ph | rt | 72 | – | – |
| 5 | CH3 | H | Ph | 50 | 24 | – | – |
| 6 | CH3 | H | Ph | 80 | 4 | 20d (13)a | – |
| 7 | CH3 | H | 4-NO2-C6H4 | rt | 72 | – | – |
| 8 | CH3 | H | 4-NO2-C6H4 | 50 | 24 | 20e (4) | – |
| 9 | CH3 | H | 4-NO2-C6H4 | 80 | 4 | 20e (23)b | – |
| 10 | CH3 | H | 4-NH2-C6H4 | rt | 72 | – | – |
| 11 | CH3 | H | 4-NH2-C6H4 | 50 | 24 | – | – |
| 12 | CH3 | H | 4-NH2-C6H4 | 80 | 4 | – | – |
| 13 | CH3 | Ph | H | rt | 5 | – | 21f (40) |
Initially, we explored the reaction of 3-oxidopyrazinium 17 with methyl acrylate in acetonitrile under reflux, which afforded a mixture of adducts together with decomposition products after long reaction times. Next, this reaction was carried out at room temperature for 45 min (Table 1, entry 1), which led to a much cleaner result and the formation of cycloadduct 20a in 73% yield accompanied by a minor isomer, which was shown to have structure 21a, in 4% yield. This reaction was also attempted using tetrahydrofuran and CH2Cl2 as solvents, but they led to poorer results; thus, acetonitrile was the solvent of choice.
Diazabicyclo[3.2.1]octane 20a, resulting from the 1,3-dipolar cycloaddition, was fully characterized by NMR on the basis of a comparison with the data6 reported for comparable cycloadduct 9 containing a methyl instead of the 4-methoxybenzyl group. The most characteristic NMR data of 20a are the chemical shift of protons d and e and the coupling constants among protons a, b and c (Figure 1A). Protons d, corresponding to the exocyclic methylene, appeared as two doublets at 4.20 and 4.34 ppm, and benzylic protons e as two doublets at 3.29 and 3.75 ppm. Coupling constants 3JHa,Hb-exo and 3JHa,Hb-endo were 8.0 and 0.0 Hz, respectively, and 3JHc,Hb-exo and 3JHc,Hb-endo were 9.0 and 6.4 Hz, respectively. In addition, the 1H–1H NOESY spectrum of 20a showed cross-peaks between benzylic protons e and methyl f as well as between NH and one of the protons d of the exocyclic methylene (Figure 1C). In the 1H–13C HMBC spectrum, cross-peaks between proton a and the quaternary bridgehead carbon as well as between methyl f and proton c were observed. Accordingly, 20a contained the carboxylate group at carbon c with an exo configuration. Its structure was unambiguously confirmed by X-ray diffraction [CCDC 2297226 (Figure 1D)].
Figure 1.

Comparison of the 1H NMR spectra between 2.0 and 4.5 ppm showing (A) signals for protons of diazabicyclo[3.2.1]octane 20a, (B) signals for protons of diazabicyclo[2.2.2]octane 21a, (C) selected key 1H–13C HMBC (blue arrows) and 1H–1H NOESY correlations (red arrows), and (D) a capped-stick representation of the corresponding X-ray crystal structures (see the Supporting Information for the ORTEP representation showing thermal ellipsoids at the 50% probability level).
Minor product 21a obtained in this reaction had the same molecular composition as 20a but was more polar, as shown by thin layer chromatography (TLC) and high-performance liquid chromatography (HPLC). The structure of 21a was elucidated by NMR analysis, which mainly differed from the data for 20a in the chemical shift of protons d and e and the multiplicity pattern of protons a, b and c (Figure 1B). Protons d of 21a appeared as two doublets (3.66 and 3.69 ppm) at chemical shifts lower than those for 20a (4.20 and 4.34 ppm). Protons e, which were expected to be a methylene quartet, appeared as two doublets in 20a and were observed as an apparent singlet at 4.25 ppm in the spectrum of 21a. With regard to the vicinal coupling constants of protons a, b and c in 20a and 21a, those between protons a and b differed considerably whereas those between protons b and c were similar. In the case of compound 20a, the coupling constant 3JHa,Hb-endo of 0.0 Hz pointed out a perpendicular orientation between Ha and Hb-endo according to the Karplus equation.14 In contrast, in compound 21a the value of both coupling constants 3JHa,Hb-exo and 3JHa,Hb-endo was 2.0 Hz, which would correspond to a dihedral angle of ∼60° between Ha and both Hb-endo and Hb-exo. This result evidenced different bicyclic structures for 20a and 21a. Two-dimensional 1H–1H NOESY and 1H–13C HMBC experiments were crucial for the structural identification of compound 21a (Figure 1C). The 1H–1H NOESY cross-peaks between protons e and methyl f as well as between NH and one of the protons d were not observed for 21a. In this case, benzylic protons e correlated with both exocyclic protons d and NH correlated with methyl f. Unlike 20a, the 1H–13C HMBC spectrum of 21a showed a correlation between benzylic protons e and the quaternary carbon of the exocyclic methylene and no correlation was observed between proton a and the quaternary bridgehead carbon. Taking all of this data together, the structure of 21a was determined to be a 2,5-diazabicyclo[2.2.2]octane. Later, this structure was unambiguously confirmed by single-crystal X-ray diffraction analysis [CCDC 2297223 (Figure 1D)].
Next, the 1,3-dipolar reaction of 3-oxidopyrazinium 17 with tert-butyl acrylate was carried out (Table 1, entry 2). It required a longer reaction time (1.5 h) compared to that with methyl acrylate (Table 1, entry 1), and diazabicyclo[3.2.1]octane 20b was obtained in a yield (63%) that was slightly lower than that of 20a. This result could be attributed to the steric hindrance posed by the tert-butyl group. In addition, diazabicyclo[2.2.2]octane 21b was isolated in 7% yield. Both 20b and 21b were fully characterized by NMR comparisons to the data corresponding to their methyl ester analogues, 20a and 21a, respectively.
The influence of the substituents present in the dipolarophile on the cycloaddition reaction with 3-oxidopyrazinium 17 was then investigated. For this purpose, a methyl acrylate with a methyl, a phenyl, or a substituted phenyl at C-3 of the acrylate unit was utilized, i.e., MeCH=CHCO2Me, PhCH=CHCO2Me, 4-NO2-C6H4CH=CHCO2Me, and 4-NH2-C6H4CH=CHCO2Me (Table 1, entries 3–12). These dipolarophiles were subjected to reaction with 3-oxidopyrazinium 17. In general, in the presence of these substituents, the corresponding diazabicyclo[3.2.1]octanes 20 were formed in low yields and the formation of diazabicyclo[2.2.2]octanes 21 was not detected. The reaction with methyl crotonate afforded 20c in 51% yield after 6 h at room temperature (Table 1, entry 3). Compound 20c was characterized by NMR, showing a splitting pattern similar to that of 20a, and HMBC and NOESY experiments allowed establishment of the regio- and stereochemistry of the product. Thus, in the HMBC spectra, cross-peaks between proton b and carbons a and c were detected, while no NOEs were observed between the methyl group at carbon b and the aromatic ring. No reaction with methyl cinnamate took place either at room temperature for 72 h or at 50 °C for 24 h (Table 1, entries 4 and 5). At 80 °C for 4 h, an inseparable mixture of regioisomers 20da and 20db (3:4) was obtained in 13% yield (Table 1, entry 6; Figure 2). Longer reaction times led to decomposition products. Using methyl 4-nitrocinnamate, under the conditions developed for methyl cinnamate, the corresponding diazabicyclo[3.2.1]octane 20e was formed in 4% and 23% yields at 50 and 80 °C, respectively (Table 1, entries 8 and 9, respectively). Bicyclic compound 20e was obtained as a mixture of regioisomers 20ea and 20eb in a 3:2 ratio (Figure 2). Compounds 20d and 20e were characterized by NMR, which showed the characteristic signals and correlations of the diazabicyclo[3.2.1]octanes previously discussed. The structure of the corresponding regioisomers, 20da/20db and 20ea/20eb, was elucidated on the basis of the multiplicity of bridgehead proton a and the NOESY correlations observed for the phenyl protons of the dipolarophile. In the case of 20da and 20ea, proton a appeared as a doublet due to the coupling with vicinal proton b, while in 20db and 20eb, proton a appeared as a singlet with a dihedral angle close to 90° with proton b. In the NOESY spectra, the phenyl protons of the dipolarophile correlated with protons a, b and c in the case of 20da and 20ea and with protons b and c and methyl f for 20db and 20eb. The cycloaddition of 3-oxidopyrazinium 17 with methyl 4-aminocinnamate did not lead to a cycloadduct under any of the conditions assayed (Table 1, entries 10–12).
Figure 2.
Structures of diazabicyclo[3.2.1]octanes 20da/db and 20ea/eb.
The lower yields of the cycloadditions of 3-oxidopyrazinium 17 with an acrylate containing a substituent at C-3 compared to that with methyl acrylate suggested the importance of a positive charge density at C-3 of the dipolarophile (Table 1, entries 1–12). The presence of a methyl group at this position increases its electron density by hyperconjugation leading to a decrease in the reactivity of the dipolarophile and, therefore, to a decrease in the yield. This effect was more pronounced when using cinnamate derivatives as dipolarophiles. The aromatic ring further decreases the electrophilic character of C-3, and the cycloadditions required heating at 80 °C. In accordance with this reasoning, the presence of a nitro group in the phenyl favored the reaction, whereas the cycloaddition did not proceed upon incorporation of an amino group even at 80 °C.
Finally, 3-oxidopyrazinium 17 was treated with an acrylate bearing a phenyl group at C-2, in particular with methyl 2-phenyl acrylate, CH2=CPhCO2Me (Table 1, entry 13). To our surprise, a diazabicyclo[3.2.1]octane was not detected and only diazabicyclo[2.2.2]octane 21f was obtained in 40% yield after 5 h at room temperature. The NMR spectra of 21f exhibited a pattern analogous to that observed for 21a. Additionally, the NOESY spectra showed a correlation between methyl f and the phenyl protons of the dipolarophile unit, confirming that this aromatic ring is bound to carbon c in an endo configuration (Scheme 5).
Cycloaddition Reactions of 3-Oxidopyrazinium 17 with Acrylic Acids
Having studied the reaction of 3-oxidopyrazinium 17 with various acrylic esters as dipolarophiles, we proposed to explore its reactivity with acrylic acids to yield products that would allow further functionalization. This is the first time that acrylic acids have been directly employed as dipolarophiles in these cycloadditions.
After reaction of 3-oxidopyrazinium 17 with acrylic acid for 45 min at room temperature, a 1:1 mixture of 22 and 23 was obtained (Scheme 6), which during purification evolved into a 1:3 mixture. The structure of 22 corresponds to the expected diazabicyclo[3.2.1]octane from a 1,3-dipolar cycloaddition, whereas 23 is a tricyclic fused lactone-lactam system derived from a [2.2.2] core. Unexpectedly, compound 22 gradually converted into 23 over time. After 10 days, conversion to 23 was total, and this compound was obtained in 71% yield.
Scheme 6. Reaction of 3-Oxidopyrazinium 17 with Acrylic and 2-Phenylacrylic Acid.

To gain further insight into this cycloaddition, we performed the reaction with a bulkier acid, in particular with 2-phenylacrylic acid. In this case, after reaction for 1.5 h at room temperature, only compound 24 was isolated in 50% yield.
Characterization of compounds 23 and 24 was achieved by two-dimensional 1H–13C HMBC and 1H–1H NOESY experiments (Figure 3). Their HMBC spectra showed cross-peaks between NH and both C-3 and the methyl group bound to C-7a. In addition, the benzylic protons correlated with C-7 and H-4exo correlated with the two carbonyl carbons. In contrast, the methyl bound to C-7 did not show any HMBC correlations. The most relevant NOESY correlations were those observed for the methyl bound to C-7a that correlated with NH, with the methyl bound to C-7, and with the protons of the phenyl (24) or with H-4a (23). Moreover, the methyl bound to C-7 showed cross-peaks with protons H-3′ of the 4-methoxybenzyl unit. The structures of compounds 23 and 24 were unambiguously established by single-crystal X-ray diffraction [CCDC 2297225 and 2297224 (Figure 3)].
Figure 3.
Selected key 1H–13C HMBC (blue arrows), 1H–1H NOESY correlations (red arrows), and capped-stick representations of the X-ray crystal structures of 23 and 24 (see the Supporting Information for the ORTEP representation showing thermal ellipsoids at the 50% probability level).
Mechanism of the Formation of the Diazabicyclo[2.2.2]octanes from the Reaction of 3-Oxidopyrazinium 17 with Acrylates and Acrylic Acids
The formation of diazabicyclo[2.2.2]octanes from the reaction of 3-oxidopyrazinium 17 with acrylate derivatives together with the observed progressive conversion of diazabicyclo[3.2.1]octane 22 into tricyclic fused lactone-lactam system 23 prompted us to explore whether the diazabicyclo[2.2.2]octanes are formed through an aza-Diels–Alder type reaction or derived from a diazabicyclo[3.2.1]octane via a rearrangement.
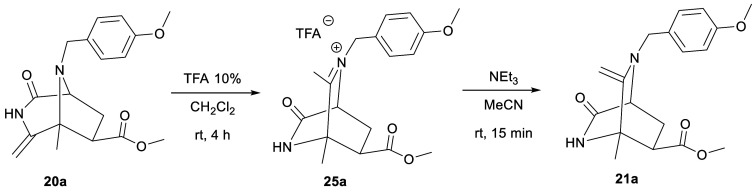
To shed light on this question, we treated diazabicyclo[3.2.1]octane 20a with 10% TFA in CH2Cl2 (Scheme 7). After 4 h, we observed full conversion of 20a into a very polar compound 25a, and its structure was elucidated by ESI-MS and NMR as the iminium salt derivative of diazabicyclo[2.2.2]octane 21a. The NMR spectra did not show the signals corresponding to the exocyclic methylene protons. In contrast, in the 1H NMR spectrum appeared a significantly deshielded methyl group at 2.86 ppm corresponding to the CH3 attached to the iminium group and the 13C{1H} NMR spectrum showed a low field signal at 190.1 ppm that can be assigned to the carbon of this iminium. The presence of this cationic group also caused an important upfield shift of the bridgehead proton (4.75 ppm) and of the benzyl protons (5.07 and 5.41 ppm). In addition, the 1H–13C HMBC spectrum did not show cross-peaks between the bridgehead atoms. All of these data pointed toward the formation of diazabicyclo[2.2.2]octane 25 resulting from the rearrangement of diazabicyclo[3.2.1]octane 20a. Treatment of iminium salt 25 with triethylamine yielded diazabicyclo[2.2.2]octane 21a, providing further evidence of the structure of 25.
Scheme 7. Formation of Diazabicyclo[2.2.2]octane 21a from Diazabicyclo[3.2.1]octane 20a.
The result presented above made clear that the diazabicyclo[2.2.2]octanes obtained in the reactions of 3-oxidopyrazinium 17 with acrylate derivatives are formed from the corresponding diazabicyclo[3.2.1]octanes. A plausible mechanism for this transformation is depicted in Scheme 8 for 20a. The first step would involve the C protonation of the enamide producing cation 26. Next, a Wagner–Meerwein rearrangement that implies the 1,2-migration of the C-5–C-6 bond to the C-4 carbocation followed by deprotonation would provide bicyclic compound 21a. This result confirms the theoretical assessment previously obtained by the DFT method.11
Scheme 8. Acid-Catalyzed Mechanism for the Conversion of 20a into 21a.

The formation of tricyclic fused lactone-lactam systems 23 and 24 in the cycloadditions of 17 with an acrylic acid can be rationalized via a mechanism analogous to that described in Scheme 8 followed by a lactonization step. Thus, the initial cycloaddition would provide diazabicyclo[3.2.1]octanes 22 and 27, which would be readily transformed into iminium salts 28 and 29, respectively, via a 1,2-migration (Scheme 9). Finally, the nucleophilic attack of the carboxylate on the carbocation would yield tricyclic compounds 23 and 24.
Scheme 9. Mechanism for the Conversion of Diazabicyclo[3.2.1]octanes 22 and 27 into Tricyclic Fused Lactone-Lactam Systems 23 and 24.
Conclusions
3-Oxidopyraziniums generated by simple deprotonation of pyrazin-2-one N-alkyl salts react with acrylates forming 3,8-diazabicyclo[3.2.1]octanes. In some cases, isomeric 2,5-diazabicyclo[2.2.2]octanes are also formed. Evidence is presented that suggests that the [2.2.2] products are formed from the [3.2.1] products through a mechanism that involves a Wagner–Meerwein rearrangement. Further evidence of this [3.2.1] to [2.2.2] product conversion is provided from the fused lactone-lactam system obtained from the reaction of 3-oxidopyraziniums with acrylic acids. In this case, after the Wagner–Meerwein rearrangement, a subsequent lactonization occurs. To the best of our knowledge, these are the first examples of the cycloaddition of 3-oxidopyraziniums with acrylic acids. We note that, the tricyclic products from these reactions are highly functionalized and can be obtained from commercially available starting materials in three steps.
Experimental Section
General Methods
Anhydrous CH3CN and CH2Cl2 were obtained from an MBraun SPS-800 (Garching, Germany) solvent purification system. TLC analyses were performed on precoated TLC plates with silica gel 60 F254 (Merck), and detection was done with ultraviolet light (254 nm). Flash chromatography purifications were performed on silica gel 60 (0.040–0.063 mm, Merck). Analysis by HPLC was carried out with a model 1260 Infinity II instrument (Agilent Technologies) consisting of a 1260 vial sampler, a Pump VL quaternary pump, and a diode array HS detector and controlled by OpenLab CDS ChemStation software. The analysis was performed in reverse phase using a Kromasil 100 C18 column (3 μm, 4.6 mm × 40 mm) and a mobile phase consisting of H2O with 0.1% TFA (solvent A) and CH3CN with 0.1% TFA (solvent B) and a flow rate of 1 mL/min. For the elution, a linear gradient from 2% to 100% was applied over 12 min. NMR experiments were performed in the Serveis Tècnics de Recerca de la Universitat de Girona (STR-UdG) with an Ultrashield Avance III 400 (9.4 T) spectrometer from Bruker (1H, 400 MHz; 13C, 100 MHz), equipped with an RT BBI probe and a temperature control unit (BCU Xtreme) or with an Ultrashield ASCEND Nanobay 400 instrument (9.4 T) from Bruker (1H, 400 MHz; 13C, 100 MHz). Structural assignments were made with additional information from gCOSY, gHSQC, and gHMBC experiments. NMR spectra were processed and analyzed using TopSpin 3.6.2. Chemical shifts were reported as δ (parts per million) directly calibrated with the solvent signal. IR spectra were recorded with a Cary 630 FT-IR spectrophotometer (Agilent Technologies) equipped with a Golden Gate Single Reflection, ATR MK-II system. For the acquisition, the instrument was controlled with MicroLabPC software, and spectra were analyzed with ResolutionsPro version 5.2.0. ESI-MS analyses were performed with an Esquire 6000 ESI Bruker ion trap LC/MS instrument equipped with an electrospray ion source (STR-UdG) operating in both positive and negative ion modes. Samples (5 μL) were introduced into the mass spectrometer ion source directly through a 1200 series Agilent HPLC autosampler. The mobile phase, CH3CN/H2O (80:20), was delivered by an Agilent 1200 series HPLC pump at a flow rate of 100 μL/min. Nitrogen was employed as the drying and nebulizing gas. HRMS spectra were recorded under ESI conditions with a Bruker MicroTOF-Q II instrument using a hybrid quadrupole time-of-flight mass spectrometer (STR-UdG). Samples were introduced into the mass spectrometer ion source by direct infusion through a syringe pump and externally calibrated using sodium formate. Single-crystal X-ray diffraction (XRD) data were acquired on a Bruker D8 Quest Eco diffractometer (STR-UdG) equipped with graphite-monochromated molybdenum Kα radiation (λ = 0.71073 Å) and a Photon II area detector. The melting point of the compounds was determined with a Melting Point SMP10 (Stuart) instrument, and their values are expressed in degrees Celsius.
5,6-Dimethyl-2(1H)-pyrazinone (18)
In a 250 mL round-bottom flask, glycinamide hydrochloride (2.5 g, 22.6 mmol, 1 equiv) was dissolved in MeOH (40 mL) and the solution cooled to −30 to −40 °C. Aqueous NaOH (12.5 M, 56.5 mmol, 2.5 equiv) was added, and the mixture stirred for 10 min. Then, a solution of butane-2,3-dione (2.4 mL, 27.2 mmol, 1.2 equiv) in MeOH (10 mL), previously cooled, was added dropwise, and the mixture was maintained at −30 °C for 30 min and then at room temperature for 3 h. The formation of the 2(1H)-pyrazinone was monitored by TLC, and once it was complete, concentrated HCl was added to the crude reaction mixture followed by neutralization with a saturated aqueous solution of NaHCO3. MeOH was evaporated under reduced pressure, and the remaining aqueous solution extracted with CHCl3 (3 × 50 mL). The organic extract was dried over anhydrous MgSO4, filtered, and evaporated to give 5,6-dimethyl-2(1H)-pyrazinone. Recrystallization of the crude product from acetone afforded 5,6-dimethyl-2(1H)-pyrazinone as a pale pink solid (1.21 g, 43% yield):13 MW (C6H8N2O) 124.1 g/mol; TLC (8:6:0.5 CHCl3/MeOH/AcOH) Rf = 0.63; mp 194–196 °C; FT-IR (ATR) v (cm–1) 3271 (N–H), 2855 (C–H), 1683 (C=O), 1607 (N–C=O); 1H NMR (400 MHz, CDCl3) δ 13.90 (br, H-1), 8.01 (s, 1H, H-3), 2.32 (s, 3H, 5-CH3), 2.29 (s, 3H, 6-CH3); 13C{1H} NMR (100 MHz, CDCl3) δ 159.2 (C-2), 144.4 (CH, C-3), 134.5 (C-6), 132.4 (C-5), 19.3 (6-CH3), 17.1 (5-CH3); ESI-MS (m/z) 125.1 [M + H]+, 147.0 [M + Na]+; ESI-HRMS (m/z) calcd for C6H8N2ONa [M + Na]+ 147.0529, found 147.0537; ESI-HRMS (m/z) calcd for (C6H8N2O)2Na [2M + Na]+ 271.1165, found 271.1169. Spectral data in accordance with literature values.14
1-(4-Methoxybenzyl)-5,6-dimethyl-3-oxo-3,4-dihydropyrazin-1-ium Bromide (19)
In a round-bottom flask, 5,6-dimethyl-1H-pyrazin-2-one (1.18 g, 9.52 mmol, 1 equiv) was dissolved in anhydrous CH3CN under N2 and, subsequently, p-methoxybenzyl bromide (3.83 g, 19.04 mmol, 2 equiv) was added. The mixture was heated at reflux using an oil bath for 6 h and then stirred at room temperature for 18 h, until the reaction had reached completion as evidenced by TLC. Then, the solvent was evaporated under reduced pressure and the residue was purified by reversed-phase chromatography. Elution with H2O/CH3CN (85:15) afforded 1-(4-methoxybenzyl)-5,6-dimethyl-3-oxo-3,4-dihydropyrazin-1-ium bromide (2.33 g, 75% yield) as a pale brown solid: MW (C14H17N2O2Br) 325.2 g/mol; TLC (9:1 CHCl3/MeOH) Rf = 0.14; mp 108–112 °C; FT-IR (ATR) v (cm–1) 3338 (N–H), 2917 (C–H), 1673 (C=O), 1606 (Ar), 1510 (N–C=O), 1247 and 1176 (C–O); 1H NMR (400 MHz, CDCl3) δ 8.23 (s, 1H, H-2), 7.30 (d, J = 8.4 Hz, 2H, H-3′), 6.89 (d, J = 8.4 Hz, 2H, H-4’), 5.72 (s, 2H, H-1′), 3.75 (s, 3H, 5′-OCH3), 2.59 (s, 3H, 6-CH3), 2.58 (s, 3H, 5-CH3); 13C{1H} NMR (100 MHz, CDCl3) δ 160.8 (C-5′), 159.4 (C-3), 155.6 (C-5), 133.6 (C-6), 130.8 (2CH, C-3′), 128.9 (CH, C-2), 121.7 (C-2′), 115.2 (2CH, C-4′), 62.9 (CH2, C-1′), 55.4 (5′-OCH3), 21.6 (5-CH3), 15.2 (6-CH3); ESI-MS (m/z) 121.0 [CH3O – C6H4 – CH2]+, 245.1 [M]+, 267.1 [M – H + Na]+; ESI-HRMS (m/z) calcd for C14H16N2O2Na [M – H + Na]+ 267.1104, found 267.1116; ESI-HRMS (m/z) calcd for (C14H16N2O2)2Na [2M – 2H + Na]+ 511.2316, found 511.2327.
General Procedure for Cycloadditions
To a suspension of 1-(4-methoxybenzyl)-5,6-dimethyl-3-oxo-3,4-dihydropyrazin-1-ium bromide (1 equiv) in anhydrous CH3CN (2 mL) was added dropwise triethylamine (1.5 equiv), and the mixture was stirred at room temperature under nitrogen. After 10 min, the formation of an orange transparent solution evidenced the formation of the 3-oxidopyrazinium. Then, the corresponding dipolarophile (1.5 equiv) was added dropwise to the ylide and the resulting mixture was stirred under nitrogen. The progress of the reaction was monitored by TLC. Once the reaction had reached completion, the solvent was removed under reduced pressure and the resulting residue was purified by flash chromatography using CH2Cl2/MeOH mixtures of increasing polarity.
Methyl 8-(4-Methoxybenzyl)-5-methyl-4-methylene-2-oxo-3,8-diazabicyclo[3.2.1]octane-6-carboxylate (20a)
This compound was prepared following the general procedure described above starting from 1-(4-methoxybenzyl)-5,6-dimethyl-3-oxo-3,4-dihydropyrazin-1-ium bromide (88 mg, 0.27 mmol, 1 equiv) and methyl acrylate (37 μL, 0.41 mmol, 1.5 equiv) as the dipolarophile. The reaction was performed at room temperature for 45 min. Final purification by flash chromatography eluting with CH2Cl2/MeOH (99:1) afforded 20a (66 mg, 73% yield) as a pale yellow solid. Suitable crystals for X-ray diffraction were obtained by slow diffusion of hexane into a CH2Cl2 solution of the compound: MW (C18H22N2O4) 330.4 g/mol; TLC (9:1 CHCl3/MeOH) Rf = 0.58; HPLC (λ = 220 nm) tR = 6.33 min (94% purity); mp 143–145 °C; FT-IR (ATR) v (cm–1) 2925 (C–H), 1773 and 1663 (C=O), 1507 (Ar), 1180 (C–O); 1H NMR (400 MHz, CDCl3) δ 8.53 (s, 1H, H-3), 7.22 (d, J = 8.8 Hz, 2H, H-3′), 6.83 (d, J = 8.8 Hz, 2H, H-4′), 4.34 (d, J = 1.6 Hz, 1H, H-9), 4.20 (d, J = 1.6 Hz, 1H, H-9), 3.78 (s, 3H, CO2CH3), 3.75 (d, J = 13.2 Hz, 1H, H-1′), 3.73 (s, 3H, 5′-OCH3), 3.50 (d, J = 8.0 Hz, 1H, H-1), 3.29 (d, J = 13.2 Hz, 1H, H-1′), 3.07 (dd, J = 9.0 Hz, J′ = 6.4 Hz, 1H, H-6), 2.56 (ddd, J = 12.8 Hz, J′ = 8.0 Hz, J″ = 6.4 Hz, 1H, H-7exo), 2.10 (dd, J = 12.8 Hz, J′ = 9.0 Hz, 1H, H-7endo), 1.36 (s, 3H, 5-CH3); 13C{1H} NMR (100 MHz, CDCl3) δ 173.3 (CO2CH3), 172.2 (C-2), 158.7 (C-5′), 146.2 (C-4), 130.2 (C-2′), 129.6 (2CH, C-3′), 113.7 (2CH, C-4′), 90.3 (CH2, C-9), 63.5 (CH, C-1), 62.7 (C-5), 55.2 (CO2CH3), 52.5 (CH, C-6), 51.9 (5′-OCH3), 49.0 (CH2, C-1′), 31.8 (CH2, C-7), 17.8 (5-CH3); ESI-MS (m/z) 331.2 [M + H]+; ESI-HRMS (m/z) calcd for C18H22N2O4Na [M + Na]+ 353.1472, found 353.1478.
tert-Butyl 8-(4-Methoxybenzyl)-5-methyl-4-methylene-2-oxo-3,8-diazabicyclo[3.2.1]octane-6-carboxylate (20b) and tert-Butyl 5-(4-Methoxybenzyl)-1-methyl-6-methylene-3-oxo-2,5-diazabicyclo[2.2.2]octane-7-carboxylate (21b)
These compounds were prepared following the general procedure described above starting from 1-(4-methoxybenzyl)-5,6-dimethyl-3-oxo-3,4-dihydropyrazin-1-ium bromide (104 mg, 0.32 mmol, 1 equiv) and tert-butyl acrylate (71 μL, 0.48 mmol, 1.5 equiv) as the dipolarophile. The reaction was performed at room temperature for 1.5 h. Final purification by flash chromatography eluting with CH2Cl2/MeOH (99:1) afforded 20b (76 mg, 63% yield) as a white solid. Also, 21b (8 mg, 7% yield) was isolated as a byproduct as a white solid.
tert-Butyl 8-(4-Methoxybenzyl)-5-methyl-4-methylene-2-oxo-3,8-diazabicyclo[3.2.1]octane-6-carboxylate (20b)
MW (C21H28N2O4) 372.5 g/mol; TLC (9:1 CHCl3/MeOH) Rf = 0.67; HPLC (λ = 220 nm) tR = 8.04 min (97% purity); mp 142–145 °C; FT-IR (ATR) v (cm–1) 2974 (C–H), 1722 and 1669 (C=O), 1509 (Ar), 1147 (C–O); 1H NMR (400 MHz, CDCl3) δ 8.72 (s, 1H, H-3), 7.24 (d, J = 8.4 Hz, 2H, H-3′), 6.83 (d, J = 8.4 Hz, 2H, H-4′), 4.35 (d, J = 1.4 Hz, 1H, H-9), 4.20 (d, J = 1.4 Hz, 1H, H-9), 3.78 (s, 3H, 5′-OCH3), 3.76 (d, J = 13.2 Hz, 1H, H-1′), 3.49 (d, J = 7.6 Hz, 1H, H-1), 3.30 (d, J = 13.2 Hz, 1H, H-1′), 2.96 (dd, J = 9.2 Hz, J′ = 6.8 Hz, 1H, H-6), 2.52 (ddd, J = 12.8 Hz, J′ = 7.6 Hz, J″ = 6.8 Hz, 1H, H-7exo), 2.08 (dd, J = 12.8 Hz, J′ = 9.2 Hz, 1H, H-7endo), 1.49 [s, 9H, C(CH3)3], 1.44 (s, 3H, 5-CH3); 13C{1H} NMR (100 MHz, CDCl3) δ 172.6 (C-2), 172.0 (CO2t-Bu), 158.7 (C-5′), 146.5 (C-4), 130.5 (C-2′), 129.5 (2CH, C-3′), 113.7 (2CH, C-4′), 90.3 (CH2, C-9), 81.2 [C(CH3)3], 63.5 (CH, C-1), 62.7 (C-5), 55.2 (5′-OCH3), 53.2 (CH, C-6), 49.0 (CH2, C-1′), 32.0 (CH2, C-7), 28.1 [3CH3, C(CH3)3], 17.9 (5-CH3); ESI-MS (m/z) 373.2 [M + H]+; ESI-HRMS (m/z) calcd for C21H28N2O4Na [M + Na]+ 395.1941, found 395.1941; ESI-HRMS (m/z) calcd for (C21H28N2O4)2Na [2M + Na]+ 767.3990, found 767.3982.
tert-Butyl 5-(4-Methoxybenzyl)-1-methyl-6-methylene-3-oxo-2,5-diazabicyclo[2.2.2]octane-7-carboxylate (21b)
MW (C21H28N2O4) 372.5 g/mol; TLC (9:1 CHCl3/MeOH) Rf = 0.41; 1H NMR (400 MHz, CDCl3) δ 7.23 (d, J = 8.5 Hz, 2H, H-3′), 6.86 (d, J = 8.5 Hz, 2H, H-4′), 6.32 (br, 1H, H-2), 4.23 (s, 2H, H-1′), 3.79 (s, 3H, 5′-OCH3), 3.70–3.67 (m, 2H, H-9), 3.59 (m, 1H, H-4), 2.79 (dd, J = 11.7 Hz, J′ = 4.6 Hz, 1H, H-7), 2.52 (dt, J = 11.7 Hz, J′ = 4.6 Hz, 1H, H-8exo), 2.00 (t, J = 11.7 Hz, 1H, H-8endo), 1.58 (s, 3H, 1-CH3), 1.46 [s, 9H, C(CH3)3]; ESI-MS (m/z) 373.2 [M + H]+.
Methyl 8-(4-Methoxybenzyl)-5,7-dimethyl-4-methylene-2-oxo-3,8-diazabicyclo[3.2.1]octane-6-carboxylate (20c)
This compound was prepared following the general procedure described above starting from 1-(4-methoxybenzyl)-5,6-dimethyl-3-oxo-3,4-dihydropyrazin-1-ium bromide (107 mg, 0.33 mmol, 1 equiv) and methyl crotonate (54 μL, 0.50 mmol, 1.5 equiv) as the dipolarophile. The reaction was performed at room temperature for 6 h. Final purification by flash chromatography eluting with CH2Cl2/MeOH (99:1) afforded 20c (58 mg, 51% yield) as a white solid: MW (C19H24N2O4) 344.4 g/mol; TLC (9:1 CHCl3/MeOH) Rf = 0.70; HPLC (λ = 220 nm) tR = 6.49 min (98% purity); mp 179–182 °C; FT-IR (ATR) v (cm–1) 2951 (C–H), 1733 and 1680 (C=O), 1509 (Ar), 1165 (C–O); 1H NMR (400 MHz, CDCl3) δ 8.04 (s, 1H, H-3), 7.22 (d, J = 8.6 Hz, 2H, H-3′), 6.83 (d, J = 8.6 Hz, 2H, H-4′), 4.31 (d, J = 1.6 Hz, 1H, H-9), 4.19 (d, J = 1.6 Hz, 1H, H-9), 3.79 (s, 3H, 5′-OCH3), 3.76 (s, 3H, CO2CH3), 3.72 (d, J = 13.6 Hz, 1H, H-1′), 3.38 (dd, J = 7.2 Hz, J′ = 1.2 Hz, 1H, H-1), 3.26 (d, J = 13.6 Hz, 1H, H-1′), 2.95 (sext, J = 7.2 Hz, 1H, H-7), 2.60 (d, J = 7.2 Hz, 1H, H-6), 1.36 (s, 3H, 5-CH3), 1.04 (d, J = 7.2 Hz, 3H, 7-CH3); 13C{1H} NMR (100 MHz, CDCl3) δ 173.2 (CO2CH3), 170.3 (C-2), 158.9 (C-5′), 146.8 (C-4), 130.3 (C-2′), 129.8 (2CH, C-3′), 113.9 (2CH, C-4′), 90.0 (CH2, C-9), 67.7 (CH, C-1), 64.0 (C-5), 60.9 (CH, C-6), 55.4 (5′-OCH3), 52.1 (CO2CH3), 49.4 (CH2, C-1′), 38.7 (CH, C-7), 17.7 (5-CH3), 15.0 (7-CH3); ESI-MS (m/z) 345.1 [M + H]+; ESI-HRMS (m/z) calcd for C19H24N2O4Na [M + Na]+ 367.1628, found 367.1625; ESI-HRMS (m/z) calcd for (C19H24N2O4)2Na [2M + Na]+ 711.3364, found 711.3327.
Methyl 8-(4-Methoxybenzyl)-5-methyl-4-methylene-2-oxo-7-phenyl-3,8-diazabicyclo[3.2.1]octane-6-carboxylate (20da) and Methyl 8-(4-Methoxybenzyl)-1-methyl-2-methylene-4-oxo-7-phenyl-3,8-diazabicyclo[3.2.1]octane-6-carboxylate (20db)
These compounds were prepared following the general procedure described above starting from 1-(4-methoxybenzyl)-5,6-dimethyl-3-oxo-3,4-dihydropyrazin-1-ium bromide (218 mg, 0.67 mmol, 1 equiv) and methyl cinnamate (163 mg, 1.00 mmol, 1.5 equiv) as the dipolarophile. The reaction was performed by heating with an oil bath at 80 °C for 4 h. Final purification by flash chromatography eluting with CH2Cl2/MeOH (99:1) afforded an inseparable mixture of regioisomers 20da and 20db (3:4) (36 mg, 13% yield): MW (C24H26N2O4) 406.5 g/mol; TLC (9:1 CHCl3/MeOH) Rf = 0.43; 1H NMR (400 MHz, CDCl3) δ 8.28 (s, 1H, H-3a), 8.06 (s, 1H, H-3b), 7.29–7.20 (m, 14H, H-3′a, H-3′b, H-11a, H-11b, H-12a, H-12b, H-13a, H-13b), 6.87–6.85 (m, 4H, H-4′a, H-4′b), 4.33 (d, J = 1.6 Hz, 1H, H-9a), 4.26 (d, J = 1.6 Hz, 1H, H-9a), 4.24 (m, 1H, H-7a), 4.16 (d, J = 1.6 Hz, 1H, H-9b), 3.89 (d, 1H, J = 13.6 Hz, H-1′a), 3.86 (s, 1H, H-5b), 3.80 (d, J = 13.2 Hz, 1H, H-1′b), 3.80 (s, 3H, 5′-OCH3-a), 3.79 (s, 3H, 5′-OCH3-b), 3.73 (m, 1H, H-7b), 3.73 (s, 3H, CO2CH3-a), 3.68 (d, J = 6.4 Hz, 1H, H-1a), 3.65 (d, J = 1.6 Hz, 1H, H-9b), 3.62 (s, 3H, CO2CH3-b), 3.40 (d, J = 8.4 Hz, 1H, H-6a), 3.38 (d, J = 13.6 Hz, 1H, H-1′a), 3.31 (d, J = 13.6 Hz, 1H, H-1′b), 3.21 (d, J = 6.4 Hz, 1H, H-6b), 1.46 (s, 3H, 5-CH3-a), 1.39 (s, 3H, 1-CH3-b); 13C{1H} NMR (100 MHz, CDCl3) δ 172.9 (CO2CH3-b), 172.8 (CO2CH3-a), 170.5 (C-4b), 169.5 (C-2a), 159.0 (2C, C-5′a and C-5′b), 146.7 (C-4a), 141.5 (C-2b), 137.0 (C-10b), 136.2 (C-10a), 130.1, 130.0, 129.9, 129.6, 128.9, 128.7, 128.6, 128.4, 128.0 (C-2′, C-3′, C-11, C-12, C-13a and -b), 114.0 (2CH, C-4′a), 113.9 (2CH, C-4′b), 95.0 (CH2, C-9b), 90.3 (CH2, C-9a), 68.7 (CH, C-1a), 67.3 (C-1b), 65.4 (CH, C-5b), 64.3 (C-5a), 58.7 (CH, C-7b), 58.0 (CH, C-6a), 55.4 (2CH3, 5′-OCH3-a and 5′-OCH3-b), 52.5 (CO2CH3-b), 52.2 (CO2CH3-a), 51.2 (CH, C-6b), 50.1 (CH, C-7a), 49.2 (2CH2, C-1′a and C-1′b), 20.7 (1-CH3-b), 17.8 (5-CH3-a); ESI-MS (m/z) 407.2 [M + H]+; ESI-HRMS (m/z) calcd for C24H27N2O4 [M + H]+ 407.1965, found 407.1978.
Methyl 8-(4-Methoxybenzyl)-5-methyl-4-methylene-7-(4-nitrophenyl)-2-oxo-3,8-diazabicyclo[3.2.1]octane-6-carboxylate (20ea) and Methyl 8-(4-Methoxybenzyl)-1-methyl-2-methylene-7-(4-nitrophenyl)-4-oxo-3,8-diazabicyclo[3.2.1]octane-6-carboxylate (20eb)
Compound 20ea was prepared following the general procedure described above starting from 1-(4-methoxybenzyl)-5,6-dimethyl-3-oxo-3,4-dihydropyrazin-1-ium bromide (182 mg, 0.56 mmol, 1 equiv) and methyl p-nitrocinnamate15 (173 mg, 0.84 mmol, 1.5 equiv) as the dipolarophile. The reaction was performed by heating with an oil bath at 50 °C for 24 h. Final purification by flash chromatography eluting with CH2Cl2/MeOH (99.5:0.5) afforded only regioisomer 20ea (11 mg, 4% yield) as a white solid. When the reaction was performed under reflux at 80 °C, an inseparable mixture of regioisomers 20ea and 20eb (3:2) was obtained in 23% yield.
Methyl 8-(4-Methoxybenzyl)-5-methyl-4-methylene-7-(4-nitrophenyl)-2-oxo-3,8-diazabicyclo[3.2.1]octane-6-carboxylate (20ea)
MW (C24H25N3O6) 451.5 g/mol; TLC (9:1 CHCl3/MeOH) Rf = 0.53; HPLC (λ = 220 nm) tR = 8.41 min (>99% purity); mp 188–192 °C; FT-IR (ATR) v (cm–1) 2849 (C–H), 1735 and 1511 (C=O), 1684 (Ar), 1346 (NO2), 1147 (C–O); 1H NMR (400 MHz, CDCl3) δ 8.11 (d, J = 8.8 Hz, 2H, H-12), 8.04 (s, 1H, H-3), 7.36 (d, J = 8.8 Hz, 2H, H-11), 7.23 (d, J = 8.6 Hz, 2H, H-3′), 6.86 (d, J = 8.6 Hz, 2H, H-4′), 4.32 (t, J = 7.2 Hz, 1H, H-7), 4.26 (s, 2H, H-9), 3.81 (d, J = 13.2 Hz, 1H, H-1′), 3.81 (s, 3H, 5′-OCH3), 3.78 (s, 3H, CO2CH3), 3.72 (dd, J = 7.2 Hz, J′ = 1.2 Hz, 1H, H-1), 3.34 (d, J = 7.2 Hz, 1H, H-6), 3.26 (d, J = 13.2 Hz, 1H, H-1′), 1.47 (s, 3H, 5-CH3); 13C{1H} NMR (100 MHz, CDCl3) δ 172.1 (CO2CH3), 168.6 (C-2), 159.0 (C-5′), 147.2 (C-13), 145.9 (C-4), 143.8 (C-10), 129.8 (2CH, C-3′), 129.4 (C-2′), 128.8 (2CH, C-11), 123.7 (2CH, C-12), 113.9 (2CH, C-4′), 90.7 (CH2, C-9), 67.9 (CH, C-1), 64.2 (C-5), 57.6 (CH, C-6), 55.3 (5′-OCH3), 52.4 (CO2CH3), 49.6 (CH, C-7), 49.1 (CH2, C-1′), 17.5 (5-CH3); ESI-MS (m/z) 452.2 [M + H]+; ESI-HRMS (m/z) calcd for C24H26N3O6 [M + H]+ 452.1816, found 452.1811.
Methyl 8-(4-Methoxybenzyl)-1-methyl-2-methylene-7-(4-nitrophenyl)-4-oxo-3,8-diazabicyclo[3.2.1]octane-6-carboxylate (20eb)
NMR data were obtained from a 3:2 mixture with 20ea: 1H NMR (400 MHz, CDCl3) δ 8.14 (d, J = 8.8 Hz, 2H, H-12), 7.36 (d, J = 8.8 Hz, 2H, H-11), 7.23 (d, J = 8.6 Hz, 2H, H-3′), 6.85 (d, J = 8.6 Hz, 2H, H-4’), 4.15 (d, J = 1.6 Hz, 1H, H-9), 3.92 (d, J = 13.2 Hz, 1H, H-1′), 3.91 (s, 1H, H-5), 3.87 (d, J = 6.4 Hz, 1H, H-7), 3.81 (s, 3H, 5′-OCH3), 3.64 (d, J = 1.6 Hz, 1H, H-9), 3.62 (s, 3H, CO2CH3), 3.38 (d, J = 13.2 Hz, 1H, H-1′), 3.19 (d, J = 6.4 Hz, 1H, H-6), 1.42 (s, 3H, 1-CH3); 13C{1H} NMR (100 MHz, CDCl3) δ 171.9 (CO2CH3), 169.8 (C-4), 159.0 (C-5′), 147.4 (C-13), 144.5 (C-10), 140.7 (C-2), 129.6 (4CH, C-11, C-3′), 128.6 (C-2′), 123.5 (2CH, C-12), 113.9 (2CH, C-4′), 95.3 (CH2, C-9), 67.4 (C-1), 65.0 (CH, C-5), 58.1 (CH, C-7), 55.3 (5′-OCH3), 52.6 (CO2CH3), 51.0 (CH, C-6), 49.1 (CH2, C-1′), 20.5 (1-CH3).
Methyl 5-(4-Methoxybenzyl)-1-methyl-6-methylene-3-oxo-7-phenyl-2,5-diazabicyclo[2.2.2]octane-7-carboxylate (21f)
This compound was prepared following the general procedure described above starting from 1-(4-methoxybenzyl)-5,6-dimethyl-3-oxo-3,4-dihydropyrazin-1-ium bromide (198 mg, 0.61 mmol, 1 equiv) and methyl 2-phenyl acrylate16 (149 mg, 0.92 mmol, 1.5 equiv) as the dipolarophile. The reaction was performed at room temperature for 5 h. Final purification by flash chromatography eluting with CH2Cl2/MeOH (99:1) afforded 21f (96 mg, 40% yield) as a white solid: MW (C24H26N2O4) 406.2 g/mol; TLC (9:1 CHCl3/MeOH) Rf = 0.38; mp 88–90 °C; FT-IR (ATR) v (cm–1) 2946 (C–H), 1686 (C=O), 1509 (Ar), 1240 (C–O); 1H NMR (400 MHz, CDCl3) δ 7.27–7.19 (m, 3H, H-12, H-13), 7.13–7.10 (m, 4H, H-3′, H-11), 6.79 (d, J = 8.4 Hz, 2H, H-4′), 6.16 (s, 1H, H-2), 4.22 (d, J = 15.4 Hz, 1H, H-1′), 4.06 (d, J = 15.4 Hz, 1H, H-1′), 3.89 (s, 1H, H-9), 3.74 (s, 1H, H-9), 3.72 (s, 3H, 5′-OCH3), 3.69 (s, 3H, CO2CH3), 3.62–3.60 (m, 1H, H-4), 3.19 (dd, J = 14.8 Hz, J′ = 4.4 Hz, 1H, H-8exo), 2.33 (dd, J = 14.8 Hz, J′ = 1.6 Hz, 1H, H-8endo), 1.31 (s, 3H, 1-CH3); 13C{1H} NMR (100 MHz, CDCl3) δ 173.1 (CO2CH3), 172.9 (C-3), 159.0 (C-5′), 151.7 (C-6), 139.6 (C-10), 130.0 (C-2′), 128.7 (2CH, C-3′), 128.5 (2CH, C-12), 127.8 (2CH, C-11), 127.7 (CH, C-13), 114.1 (2CH, C-4′), 78.3 (CH2, C-9), 62.1 (C-1), 59.8 (CH, C-4), 58.5 (CH, C-7), 55.4 (5′-OCH3), 54.8 (CH2, C-1′), 52.5 (CH3, CO2CH3), 39.7 (CH2, C-8), 18.7 (1-CH3); ESI-MS (m/z) 407.1 [M + H]+; ESI-HRMS (m/z) calcd for C24H27N2O4 [M + H]+ 407.1965, found 407.1964.
8-(4-Methoxybenzyl)-5-methyl-4-methylene-2-oxo-3,8-diazabicyclo[3.2.1]octane-6-carboxylic Acid (22) and 8-(4-Methoxybenzyl)-7,7a-dimethyl-1,3,4,4a-tetrahydro-3,7-epiminofuro[3,4-b]pyridine-2,5-dione (23)
Compound 23 was prepared following the general procedure described above starting from 1-(4-methoxybenzyl)-5,6-dimethyl-3-oxo-3,4-dihydropyrazin-1-ium bromide (275 mg, 0.85 mmol, 1 equiv) and acrylic acid (87 μL, 1.28 mmol, 1.5 equiv) as the dipolarophile. The reaction was performed at room temperature for 45 min, and the mixture purified by flash chromatography eluting with CH2Cl2/MeOH (99:1). After purification, an initial mixture of 22 and 23 (1:3) was obtained, which gradually was converted into 23, isolated as a white solid (189 mg, 71% yield). Crystals suitable for X-ray diffraction of 23 were obtained by slow evaporation of a saturated CH2Cl2 solution of the compound.
8-(4-Methoxybenzyl)-5-methyl-4-methylene-2-oxo-3,8-diazabicyclo[3.2.1]octane-6-carboxylic Acid (22)
NMR data were obtained from a 1:3 mixture with compound 23: MW (C17H20N2O4) 316.4 g/mol; TLC (9:1 CHCl3/MeOH) Rf = 0.47; 1H NMR (400 MHz, CDCl3) δ 8.58 (br, 1H, H-3), 7.18 (d, J = 8.8 Hz, 2H, H-3′), 6.85 (d, J = 8.8 Hz, 2H, H-4′), 4.41 (d, J = 1.8 Hz, 1H, H-9), 4.27 (d, J = 1.8 Hz, 1H, H-9), 3.81 (d, J = 13.2 Hz, 1H, H-1′), 3.78 (s, 3H, 5′-OCH3), 3.51–3.49 (m, 1H, H-1), 3.31 (d, J = 13.2 Hz, 1H, H-1′), 3.03 (dd, J = 9.2 Hz, J′ = 6.0 Hz, 1H, H-6), 2.48 (ddd, J = 13.4 Hz, J′ = 9.2 Hz, J″ = 6.0 Hz, 1H, H-7exo), 2.14 (dd, J = 13.4 Hz, J′ = 9.2 Hz, 1H, H-7endo), 1.53 (s, 3H, 5-CH3); 13C{1H} NMR (100 MHz, CDCl3, assigned from HSQC and HMBC) δ 175.0 (COOH), 171.4 (C-2), 158.7 (C-5′), 146.2 (C-4), 130.2 (C-2′), 129.6 (2CH, C-3′), 113.7 (2CH, C-4′), 91.9 (CH2, C-9), 63.4 (C-5), 61.5 (CH, C-1), 55.4 (5′-OCH3), 53.3 (CH, C-6), 49.1 (CH2, C-1′), 31.8 (CH2, C-7), 18.1 (5-CH3); (−)-ESI-MS (m/z) 314.9 [M – H]−.
8-(4-Methoxybenzyl)-7,7a-dimethyl-1,3,4,4a-tetrahydro-3,7-epiminofuro[3,4-b]pyridine-2,5-dione (23)
MW (C17H20N2O4) 316.4 g/mol; TLC (9:1 CHCl3/MeOH) Rf = 0.47; mp 182–186 °C; FT-IR (ATR) v (cm–1) 1785 and 1694 (C=O), 1510 (Ar), 1238 (C–O); 1H NMR (400 MHz, CDCl3) δ 7.18 (d, J = 8.8 Hz, 2H, H-3′), 6.86 (d, J = 8.8 Hz, 2H, H-4′), 4.13 (d, J = 15.2 Hz, 1H, H-1′), 3.78 (s, 3H, 5′-OCH3), 3.69 (d, J = 15.2 Hz, 1H, H-1′), 3.36–3.34 (m, 1H, H-3), 2.58 (dd, J = 10.4 Hz, J′ = 1.2 Hz, 1H, H-4a), 2.25 (ddd, J = 14.4 Hz, J′ = 4.4 Hz, J″ = 1.2 Hz, 1H, H-4endo), 2.01 (ddd, J = 14.4 Hz, J′ = 10.4 Hz, J″ = 0.8 Hz, 1H, H-4exo), 1.54 (s, 3H, 7-CH3), 1.48 (s, 3H, 7a-CH3); 13C{1H} NMR (100 MHz, CDCl3) δ 176.0 (C-5), 174.1 (C-2), 159.1 (C-5′), 129.7 (C-2′), 129.1 (2CH, C-3′), 114.2 (2CH, C-4′), 101.7 (C-7), 61.8 (C-7a), 55.3 (5′-OCH3), 54.8 (CH, C-3), 48.7 (CH2, C-1′), 45.6 (CH, C-4a), 24.6 (CH2, C-4), 18.2 (7a-CH3), 18.0 (7-CH3); ESI-MS (m/z) 317.1 [M + H]+; ESI-HRMS (m/z) calcd for C17H21N2O4 [M + H]+ 317.1496, found 317.1500.
8-(4-Methoxybenzyl)-7,7a-dimethyl-4a-phenyl-3,4-dihydro-3,7-epiminofuro[3,4-b]pyridine-2,5(1H)-dione (24)
This compound was prepared following the general procedure described above starting from 1-(4-methoxybenzyl)-5,6-dimethyl-3-oxo-3,4-dihydropyrazin-1-ium bromide (111 mg, 0.34 mmol, 1 equiv) and 2-phenylacrylic acid (76 mg, 0.51 mmol, 1.5 equiv) as the dipolarophile. The reaction was performed at room temperature for 5 h. Final purification by flash chromatography eluting with CH2Cl2/MeOH (99:1) afforded 24 (67 mg, 50% yield) as a white solid. Crystals suitable for X-ray diffraction were obtained by slow evaporation of a saturated CH2Cl2 solution of the compound: MW (C23H24N2O4) 392.5 g/mol; TLC (9:1 CHCl3/MeOH) Rf = 0.78; HPLC (λ = 220 nm) tR = 7.73 min (>99% purity); mp 208–210 °C; FT-IR (ATR) v (cm–1) 1755 and 1703 (C=O), 1510 (Ar), 1246 (C–O); 1H NMR (400 MHz, CDCl3) δ 7.45–7.43 (m, 2H, H-10), 7.41–7.34 (m, 3H, H-11, H-12), 7.22 (d, J = 8.8 Hz, 2H, H-3′), 6.87 (d, J = 8.8 Hz, 2H, H-4′), 6.22 (br, 1H, H-1), 4.24 (d, J = 15.2 Hz, 1H, H-1′), 3.80 (s, 3H, 5′-OCH3), 3.75 (d, J = 15.2 Hz, 1H, H-1′), 3.49–3.48 (m, 1H, H-3), 2.68 (dd, J = 15.2 Hz, J′ = 4.0 Hz, 1H, H-4exo), 2.36 (dd, J = 15.2 Hz, J′ = 1.2 Hz, 1H, H-4endo), 1.61 (s, 3H, 7-CH3), 0.94 (s, 3H, 7a-CH3); 13C{1H} NMR (100 MHz, CDCl3) δ 175.5 (C-5), 172.9 (C-2), 159.1 (C-5′), 135.1 (C-9), 129.6 (C-2′), 129.0 (2CH, C-3′), 128.6 (2CH, C-11), 128.1 (CH, C-12), 127.7 (2CH, C-10), 114.2 (2CH, C-4′), 98.7 (C-7), 66.0 (C-7a), 55.6 (CH, C-3), 55.3 (5′-OCH3), 53.7 (C-4a), 48.6 (CH2, C-1′), 33.5 (CH2, C-4), 18.6 (7-CH3), 17.3 (7a-CH3); ESI-MS (m/z) 393.1 [M + H]+; ESI-HRMS (m/z) calcd for C23H24N2O4Na [M + Na]+ 415.1628, found 415.1631; ESI-HRMS (m/z) calcd for (C23H24N2O4)2Na [2M + Na]+ 807.3364, found 807.3358.
2-(4-Methoxybenzyl)-8-(methoxycarbonyl)-3,4-dimethyl-6-oxo-2,5-diazabicyclo[2.2.2]oct-2-en-2-ium Trifluoroacetate (25a)
Cycloadduct 20a was treated with 10% TFA in anhydrous CH2Cl2, and the mixture stirred at room temperature for 4 h. Subsequently, the solvents were removed under reduced pressure, affording quantitatively 25a as a yellowish oil: MW ([C18H23N2O4]+[CF3COO]−) 444.4 g/mol; TLC (9:1 CHCl3/MeOH) Rf = 0.02; HPLC (λ = 220 nm) tR = 4.21 min (94% purity); 1H NMR (400 MHz, CDCl3) δ 7.32 (d, J = 8.4 Hz, 2H, H-3′), 6.92 (d, J = 8.4 Hz, 2H, H-4′), 5.41 (d, J = 14.4 Hz, 1H, H-1′), 5.07 (d, J = 14.4 Hz, 1H, H-1′), 4.75 (m, 1H, H-1), 3.81 (s, 3H, 5′-OCH3), 3.71 (s, 3H, CO2CH3), 3.31 (dd, J = 11.8 Hz, J′ = 5.2 Hz, 1H, H-8), 2.86 (s, 3H, 3-CH3), 2.44 (t, J = 11.8 Hz, 1H, H-7endo), 1.77 (s, 3H, 4-CH3), 1.42–1.39 (m, 1H, H-7exo); 13C{1H} NMR (100 MHz, CDCl3) δ 190.1 (C-3), 172.7 (CO2CH3), 167.0 (C-6), 161.1 (C-5′), 160.4 (q, J = 38 Hz, CF3COO–), 131.1 (2CH, C-3′), 120.5 (C-2′), 115.6 (q, J = 289 Hz, CF3COO–), 115.0 (2CH, C-4′), 64.2 (CH, C-1), 61.0 (C-4), 58.7 (CH2, C-1′), 55.4 (5′-OCH3), 53.3 (CO2CH3), 51.1 (CH, C-8), 29.5 (CH2, C-7), 19.3 (3-CH3), 17.0 (4-CH3); 19F NMR (377 MHz, CDCl3) δ −76.8 (s, 3F, CF3COO–); ESI-MS (m/z) 331.2 [C18H23N2O4]+; ESI-HRMS (m/z) calcd for C18H23N2O4 [M]+ 331.1652, found 331.1656.
Methyl 5-(4-Methoxybenzyl)-1-methyl-6-methylene-3-oxo-2,5-diazabicyclo[2.2.2]octane-7-carboxylate (21a)
This compound was prepared by deprotonation of iminium salt 25a with triethylamine in anhydrous acetonitrile. Final purification by flash chromatography (elution with 98:2 CH2Cl2/MeOH) afforded quantitatively the title heterocycle as a white solid. Crystals suitable for X-ray diffraction were obtained by recrystallization from EtOAc: MW (C18H22N2O4) 330.4 g/mol; TLC (9:1 CHCl3/MeOH) Rf = 0.35; HPLC (λ = 220 nm) tR = 4.29 min (94% purity); mp 132–134 °C; FT-IR (ATR) v (cm–1) 3072 (C–H), 1687 (C=O), 1510 (Ar), 1139 (C–O); 1H NMR (400 MHz, CDCl3) δ 7.25 (d, J = 8.4 Hz, 2H, H-3′), 6.87 (d, J = 8.4 Hz, 2H, H-4′), 4.25 (s, 2H, H-1′), 3.79 (s, 3H, 5′-OCH3), 3.71 (s, 3H, CO2CH3), 3.69 (d, J = 1.8 Hz, 1H, H-9), 3.66 (d, J = 1.8 Hz, 1H, H-9), 3.61 (t, J = 2.0 Hz, 1H, H-4), 2.93 (dd, J = 10.4 Hz, J′ = 4.8 Hz, 1H, H-7), 2.55 (ddd, J = 13.6 Hz, J′ = 4.8 Hz, J″ = 2.0 Hz, 1H, H-8exo), 2.05 (ddd, J = 13.6 Hz, J′ = 10.4 Hz, J″ = 2.0 Hz, 1H, H-8endo), 1.57 (s, 3H, 1-CH3); 13C{1H} NMR (100 MHz, CDCl3) δ 173.8 (C-3), 172.4 (CO2CH3), 159.5 (C-5′), 151.0 (C-6), 130.7 (C-2′), 129.1 (2CH, C-3′), 114.7 (2CH, C-4′), 77.0 (CH2, C-9), 59.7 (CH, C-4), 59.3 (C-1), 55.9 (5′-OCH3), 55.1 (CH2, C-1′), 52.8 (CO2CH3), 50.2 (CH, C-7), 29.7 (CH2, C-8), 20.7 (1-CH3); ESI-MS (m/z) 331.2 [M + H]+; ESI-HRMS (m/z) calcd for C18H23N2O4 [M + H]+ 331.1652, found 331.1661; ESI-HRMS (m/z) calcd for C18H22N2O4Na [M + Na]+ 353.1472, found 353.1480.
Acknowledgments
G.R.-L. is the recipient of a predoctoral fellowship from the University of Girona (IFUdG2020). The authors acknowledge the Serveis Tècnics de Recerca of the University of Girona for the NMR, ESI-MS, HRMS, and XRD experiments. Open Access funding provided thanks to the CRUE-CSIC agreement with ACS.
Data Availability Statement
The data underlying this study are available in the published article and its Supporting Information.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.joc.3c02273.
Characterization data (NMR, FT-IR, HPLC, ESI-MS, and HRMS) for all compounds (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Katritzky A. R.; Dennis N. Cycloaddition Reactions of Heteroaromatic Six-Membered Rings. Chem. Rev. 1989, 89, 827–861. 10.1021/cr00094a006. [DOI] [Google Scholar]
- Lamhauge J. N.; McLeod D. A.; Barløse C. L.; Oliver G. A.; Viborg L.; Warburg T.; Anker Jørgensen K. Enantioselective Synthesis of Tropane Scaffolds by an Organocatalyzed 1,3-Dipolar Cycloaddition of 3-Oxidopyridinium Betaines and Dienamines. Chem. - Eur. J. 2023, 29, e202301830 10.1002/chem.202301830. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y.; Shizume Y.; Tazawa S.; Yasui T. Oxidopyridinium Cycloadditions Revisited: A Combined Computational and Experimental Study on the Reactivity of 1-(2-Pyrimidyl)-3-Oxidopyridinium Betaine. J. Org. Chem. 2023, 88, 3193–3207. 10.1021/acs.joc.2c02971. [DOI] [PubMed] [Google Scholar]
- Riesco-Llach G.; Planas M.; Feliu L.; Joule J. A. 2(1H)-Pyrazinones from acyclic building blocks: methods of synthesis and further derivatizations. RSC Adv. 2023, 13, 1162–1184. 10.1039/D2RA07227K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates N. D.; Peters D. A.; Allway P. A.; Beddoes R. L.; Scopes D. I. C.; Joule J. A. 1,3-Dipolar cycloadditions to oxidopyraziniums. Heterocycles 1995, 40, 331–347. 10.3987/COM-94-S37. [DOI] [Google Scholar]
- Helliwell M.; You Y.; Joule J. A. The 1,3-dipolar cycloaddition of methyl acrylate to hindered 3-oxidopyraziniums. Heterocycles 2006, 70, 87–91. 10.3987/COM-06-S(W)22. [DOI] [Google Scholar]
- Rhyman L.; Abdallah H. H.; Jhaumeer-Laulloo S.; Domingo L. R.; Joule J. A.; Ramasami P. Regio- and Stereoselectivity of the 1,3-Dipolar Cycloaddition of Pyridinium-3-olates and Pyrazinium-3-olates with Methyl Methacrylate: A Density Functional Theory Exploration. Curr. Org. Chem. 2012, 16, 1711–1722. 10.2174/138527212800840883. [DOI] [Google Scholar]
- Rhyman L.; Abdallah H. H.; Jhaumeer-Laulloo S.; Domingo L. R.; Joule J. A.; Ramasami P. The 1,3-dipolar cycloaddition of 1H-pyridinium-3-olate and 1-methylpyridinium-3-olate with methyl acrylate: a density functional theory study. Tetrahedron 2010, 66, 9187–9193. 10.1016/j.tet.2010.09.071. [DOI] [Google Scholar]
- Rhyman L.; Abdallah H. H.; Jhaumeer-Laulloo S.; Domingo L. R.; Joule J. A.; Ramasami P. 1,3-Dipolar cycloaddition of 1H-pyrazinium-3-olate and N1- and C-methyl substituted pyrazinium-3-olates with methyl acrylate: A density functional theory study. Tetrahedron 2011, 67, 8383–8391. 10.1016/j.tet.2011.08.021. [DOI] [Google Scholar]
- Joomun Z.; Raftery J.; Delawarally K.; Laulloo S. J.; Joule J. A. 3-Oxidopyraziniums – [4 + 2] versus [3 + 2] cycloadditions. Arkivoc 2008, 2007, 51–57. 10.3998/ark.5550190.0008.g05. [DOI] [Google Scholar]
- Domingo L. R.; Sáez J. A.; Joule J. A.; Rhyman L.; Ramasami P. A DFT study of the [3 + 2] versus [4 + 2] cycloaddition reactions of 1,5,6-trimethylpyrazinium-3-olate with methyl methacrylate. J. Org. Chem. 2013, 78, 1621–1629. 10.1021/jo302730q. [DOI] [PubMed] [Google Scholar]
- Jones R. G. Pyrazines and Related Compounds. I. A New Synthesis of Hydroxypyrazines. J. Am. Chem. Soc. 1949, 71, 78–81. 10.1021/ja01169a023. [DOI] [PubMed] [Google Scholar]
- Pitchaiah A.; Hwang I.; Hwang J. S.; Kim H.; Lee K. I. Regioselective synthesis of trialkylpyrazines via nickel-catalyzed Negishi cross-coupling of pyrazine triflate. Synthesis (Stuttg). 2012, 44, 1631–1636. 10.1055/s-0031-1290972. [DOI] [Google Scholar]
- Karplus M. Vicinal Proton Coupling in Nuclear Magnetic Resonance. J. Am. Chem. Soc. 1963, 85, 2870–2871. 10.1021/ja00901a059. [DOI] [Google Scholar]
- Faridoon; Edkins A. L.; Isaacs M.; Mnkandhla D.; Hoppe H. C.; Kaye P. T. Synthesis and evaluation of substituted 4-(N-benzylamino)cinnamate esters as potential anti-cancer agents and HIV-1 integrase inhibitors. Bioorg. Med. Chem. Lett. 2016, 26, 3810–3812. 10.1016/j.bmcl.2016.05.023. [DOI] [PubMed] [Google Scholar]
- Clarke M. L.; Roff G. J. Highly regioselective rhodium-catalysed hydroformylation of unsaturated Esters: The first practical method for quaternary selective carbonylation. Chem. - A Eur. J. 2006, 12, 7978–7986. 10.1002/chem.200600914. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this study are available in the published article and its Supporting Information.