Abstract
Urinary tract infections (UTIs) are among the most common bacterial infections seen in clinical practice. The ascent of UTI-causing pathogens to the kidneys results in pyelonephritis, which can trigger kidney injury, scarring and ultimately impair kidney function. Despite sizable efforts to understand how infections develop or are cleared in the bladder, our appreciation of the mechanisms by which infections develop, progress or are eradicated in the kidney is limited. The identification of virulence factors that are produced by uropathogenic Escherichia coli to promote pyelonephritis have begun to fill this knowledge gap, as have insights into the mechanisms by which kidney tubular epithelial cells oppose uropathogenic E. coli infection to prevent or eradicate UTIs. Emerging data also illustrate how specific cellular immune responses eradicate infection whereas other immune cell populations promote kidney injury. Insights into the mechanisms by which uropathogenic E. coli circumvent host immune defences or antibiotic therapy to cause pyelonephritis is paramount to the development of new prevention and treatment strategies to mitigate pyelonephritis and its associated complications.
Introduction
Urinary tract infections (UTIs) are painful, costly and potentially harmful infections that are commonly caused by uropathogenic Escherichia coli (UPEC). Infections that are restricted to the bladder are termed cystitis and are associated with symptoms of dysuria, urinary frequency, haematuria or foul-smelling urine. By contrast, pyelonephritis is a severe and sometimes life-threatening infection that develops when bacteria ascend into the kidney from the bladder or invade the kidney from the bloodstream. Although pyelonephritis is commonly caused by UPEC, other Gram-negative and Gram-positive pathogens — including Klebsiella pneumoniae, Pseudomonas aeruginosa, Proteus mirabilis, Enterococcus faecalis and Staphylococcus spp. — can also cause pyelonephritis. Acute pyelonephritis can cause kidney injury, urosepsis or even death. Chronic or recurrent pyelonephritis can trigger kidney scarring and impair kidney function. A growing body of data suggests that the bladder and kidney engage adaptive or innate immune responses to prevent UTI and minimize tissue injury. However, despite considerable research into the mechanisms that underlie the pathogenesis of cystitis and the host immune responses through which the bladder protects itself against uropathogens1–5, efforts to better define the pathogenesis of pyelonephritis and the corresponding antibacterial defences of the kidney are relatively new. Recent discoveries have uncovered intrinsic immune defence mechanisms of kidney epithelial cells, the protective and damaging effects of infiltrating immune cells, and provided insights into the way by which UTI defences are orchestrated in different regions of the kidney to eradicate invading pathogens.
In this Review, we discuss the clinical relevance of pyelonephritis and the mechanisms employed by uropathogenic bacteria to establish and promote kidney infection. We also highlight host–pathogen interactions and the innate immune responses that are activated in the kidney to eradicate infection. Greater appreciation of the antibacterial defences of the kidney and the mechanisms employed by UPEC to circumvent host defences may facilitate the development of new personalized treatments for pyelonephritis.
Risk, outcomes and treatment of pyelonephritis
Cystitis and pyelonephritis have shared and distinct causes. Risk factors for cystitis, such as sexual activity, personal history of UTI or family history of UTI also predispose individuals to pyelonephritis. In children, bowel and bladder dysfunction, vesicoureteral reflux (VUR), and congenital anomalies of the kidney and urinary tract (CAKUT) also increase the risk of pyelonephritis6–8. Diabetes, pregnancy, use of bladder catheterization, urolithiasis and immunocompromised states also increase pyelonephritis risk across the lifespan7–13.
Unlike cystitis, pyelonephritis is generally associated with systemic signs of inflammation. Specifically, localized symptoms including dysuria, increased frequency of urination, foul-smelling urine and incontinence are associated with bladder involvement, whereas fever is a marker of renal parenchyma involvement. However, consensus on the diagnostic criteria for pyelonephritis is lacking. Hence, pyelonephritis is defined functionally, by the presence of bacteria in the kidney following their ascent from the bladder or spread from the bloodstream (referred to as haematogenous pyelonephritis). Unlike cystitis, in which uropathogens are restricted to the bladder, uropathogens in the context of pyelonephritis can enter the bloodstream from the kidney to cause bacteraemia and urosepsis. Among hospitalized adults, 42% of sepsis cases originate as UTIs and urosepsis remains a notable cause of mortality in the elderly14–16.
Prompt antibiotic treatment is the cornerstone of pyelonephritis treatment. However, even with antibiotic use, kidney scarring is a potential sequela that can lead to hypertension and chronic kidney disease (CKD)1. However, in the last 30 years, Gram-negative bacteria, including E. coli, have developed mechanisms to evade the bactericidal effects of antibiotics — including β-lactams and carbapenems, fluoroquinolones, polymyxins and aminoglycosides — that are routinely prescribed to treat UTIs. E. coli infections now account for half of the estimated global burden of antibiotic resistance, and up to 90% of E. coli strains are now resistant to at least one antibiotic2. Antibiotic misuse, overuse and suboptimal infection prevention practices have accelerated the prevalence of antibiotic resistance among UPEC. The mechanisms developed by UPEC to resist antibiotics are complex and varied, and include modifications in outer membrane proteins that decrease antibiotic accessibility, an enhanced capacity to enzymatically degrade antibiotics (for example, through the expression of β-lactamases or carbapenemases), modifications of antibiotic target sites, or the ability to eliminate antibiotics17,18 (Fig. 1). The prevalence of antibiotic-resistant E. coli highlights a need to interrogate the mechanisms by which UPEC establish pyelonephritis and the endogenous host responses that are activated to clear them from the kidney. Better understanding of these host–pathogen interactions may guide the development of new therapeutics that offer benefits over current antibiotics.
Fig. 1 |. Antibiotic targets and antibiotic resistance strategies deployed by uropathogenic Escherichia coli.
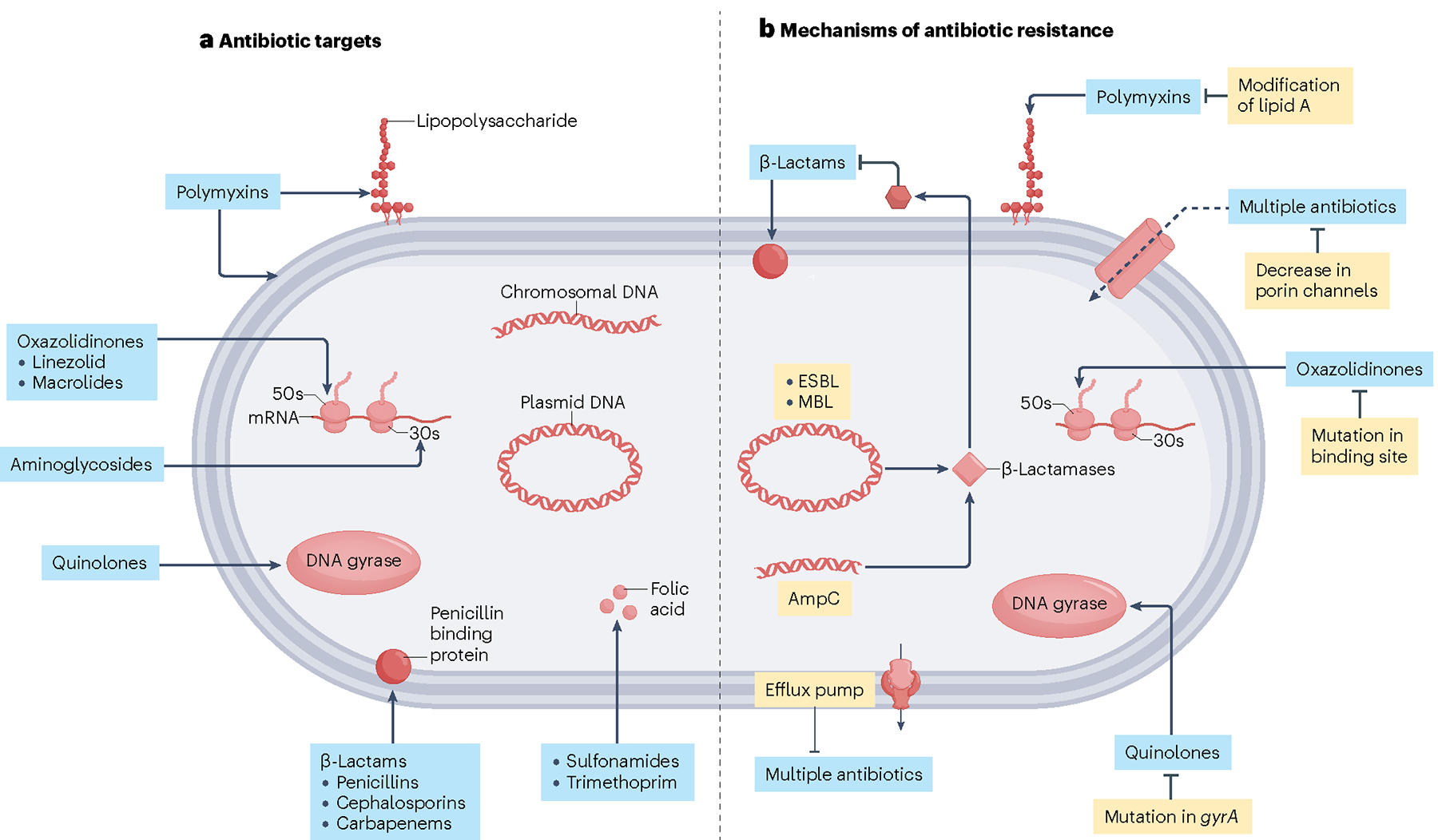
a, Targets of antibiotics used to treat uropathogenic Escherichia coli and Gram-negative uropathogens. Polymyxin antibiotics target outer membrane phospholipids and lipopolysaccharides of Gram-negative bacterial cell membranes. Oxazolidinones inhibit bacterial protein synthesis by blocking the large ribosomal subunit. Similarly, aminoglycosides inhibit bacterial protein synthesis by blocking the small ribosomal subunit. Quinolones target enzymes involved in bacterial DNA synthesis. β-lactam antibiotics prevent bacterial cell wall synthesis. Sulphonamides prevent folic acid synthesis in bacteria by targeting dihydropteroate synthase. b, Antibiotic resistance strategies adapted by uropathogenic E. coli and Gram-negative uropathogens. Bacteria can resist β-lactam antibiotics through the expression of β-lactamases, which disrupt the structure of the antibiotic and render it ineffective. They can also increase the expression of efflux pumps, which facilitate the removal of antibiotics from the bacterial cell interior. Conversely, bacteria can suppress expression of porin channels, reducing access to the bacterial cell interior to antibiotics. Bacteria can adapt to the selective pressure of antibiotics by modifying lipid A so that polymyxins no longer recognize their substrate. They can mutate their DNA or protein synthesis machinery so that oxazolidinones and quinolones can no longer bind their targets, respectively. ESBL, extended-spectrum β-lactamase; MBL, metallo-β-lactamase. Figure adapted from ref. 17, Springer Nature Ltd.
UPEC virulence factors
UPEC belong to a group of E. coli clones referred to as extraintestinal pathogenic E. coli (ExPEC) that have adapted to colonize and cause disease in extraintestinal host sites. Four UPEC phylogroups (A, B1, B2 and D) have been characterized based on their acquisition of pathogenicity-associated islands, which are large genetic elements (>10 kilobases) that encode virulence-associated genes that are absent in non-pathogenic E. coli species19,20. UPEC virulence factors can broadly be classified as cell surface structures or secreted toxins that promote colonization and disease progression in the kidney or bladder (Table 1). Cell surface virulence factors include adhesins, polysaccharides, flagella, and iron scavengers. Secreted toxins include α-haemolysin (hlyA), cytotoxic necrotizing factor 1 (cnf1), vacuolating autotransporter cytotoxin (vat), toll-interleukin-1 receptor domain-containing protein C (TcpC), and secreted autotransporter toxin (sat). The roles of these virulence factors in the pathogenesis of cystitis have been reviewed elsewhere20–25.
Tables 1 |.
UPEC virulence factors and their role in UTI pathogenesis
| Virulence factor | Positive isolates from cystitis cases (%)a | Positive isolates from pyelonephritis cases (%)a | Positive commensal isolates (%)a | Role in cystitis | Role in pyelonephritis |
|---|---|---|---|---|---|
| Secreted toxins | |||||
| Alpha haemoLysin (HLyA) | 39 | 54 | 17 | Activates caspase 1/caspase 4 and the NLRP3 infLammasome168 Promotes exfoliation of bladder urotheLiaL ceLLs116 | Activates caspase 3 apoptosis and permits paraceLLuLar UPEC translocation41–43 Induces haemolysis of tubular cells169 Activates the NLRP3 infLammasome117 Induces IL-6 and IL-8 production46 |
| Cytotoxic necrotizing factor (Cnf1) | 49 | 43 | 5.5 | Rearranges the actin cytoskeLeton, which promotes bacterial internaLization170 Induces apoptosis, which induces exfoLiation171 | Unknown |
| Secreted autotransporter toxin (Sat) | Not reported | 55 | 22 | Induces vacuoLation172 | Induces vacuoLation and ceLL detachment172 |
| ToLL/interLeukin-1 receptor domain-containing protein C (TcpC)111 | 21 | 40 | 8 | Unknown | Inhibits TLR4 by degrading MyD88 (ref. 113) Promotes inflammation by inducing MIP-2 in kidney ceLLs173 Inhibits NETosis114 |
| Adhesins | |||||
| Type I fimbriae | 64 | 47 | 53 | Bind uropLakins via FimH174 Activate TLR4175 |
Binds desmogLein-2 via FimH34 Activates TLR4 (ref. 32) Activates compLement via CD46 (refs. 39,40) |
| Type P fimbriae | 31 | 81 | 13 | Unknown | Binds gLycosphingoLipids via PapG34 |
| Type S/F1C fimbriae | 14 | 23 | 6.2 | Unknown | Binds gLycosphingoLipids176 |
| Afa/Dr adhesins | 19 | 16 | 8.2 | Unknown | Binds type IV coLLagen and decay-acceLerating factor (DAF)177 |
| Iron acquisition | |||||
| Aerobactin | 51 | 74 | 34 | Sequesters iron (siderophore)178 | |
| Immune evasion | |||||
| K1 capsule | Not reported | 28 | 22 | Involved in IBC formation179 | Resists phagocytosis and compLement180 Host immunomoduLation181 |
Despite the dedication of considerable effort to understanding the virulence factors used by UPEC to establish cystitis, our understanding of the UPEC virulence factors needed to establish pyelonephritis is incomplete. Available data show that some virulence factors are expressed at comparable levels in UPEC strains that cause asymptomatic bacteriuria, cystitis or pyelonephritis. However, other virulence factors are more highly expressed by UPEC strains that are more frequently identified with pyelonephritis. For example, type 1 pili, which promote attachment to kidney or bladder uroepithelial cells, are similarly expressed across UPEC strains; however, the majority of pyelonephritis-causing UPEC strains produce P fimbriae, which facilitates attachment to kidney epithelial cells. To a lesser degree, α-haemolysin, F1C/S fimbriae and aerobactin are more commonly expressed in pyelonephritis-causing UPEC strains20,23,25 (Table 1). As described below, the divergent expression patterns of these virulence factors provide insights into the mechanisms by which UPEC establish pyelonephritis.
Targeting the collecting duct
To establish pyelonephritis, UPEC must ascend the ureters and invade the collecting tubules26–28 — a component of the kidney tubules that are composed of principal cells (PCs) and intercalated cells (ICs). PCs are appreciated for their role in ion and water transport29. ICs regulate acid–base homeostasis and have historically been categorized into three subtypes – type-A (A-IC), type-B (B-IC), and non-A, non-B. A-ICs secrete hydrogen into the tubular lumen via an apical vacuolar H+-ATPase (V-ATPase) and regenerate bicarbonate using a basal chloride–bicarbonate transporter. B-ICs secrete bicarbonate via an apical chloride–bicarbonate transporter, pendrin, and express a basolateral H+-ATPase29,30 (Fig. 2a). However, data from single-cell RNA-sequencing (sc-RNAseq) analyses suggest that additional IC subtypes may exist31.
Fig. 2 |. Antibacterial responses of the kidney collecting duct to uropathogenic Escherichia coli.
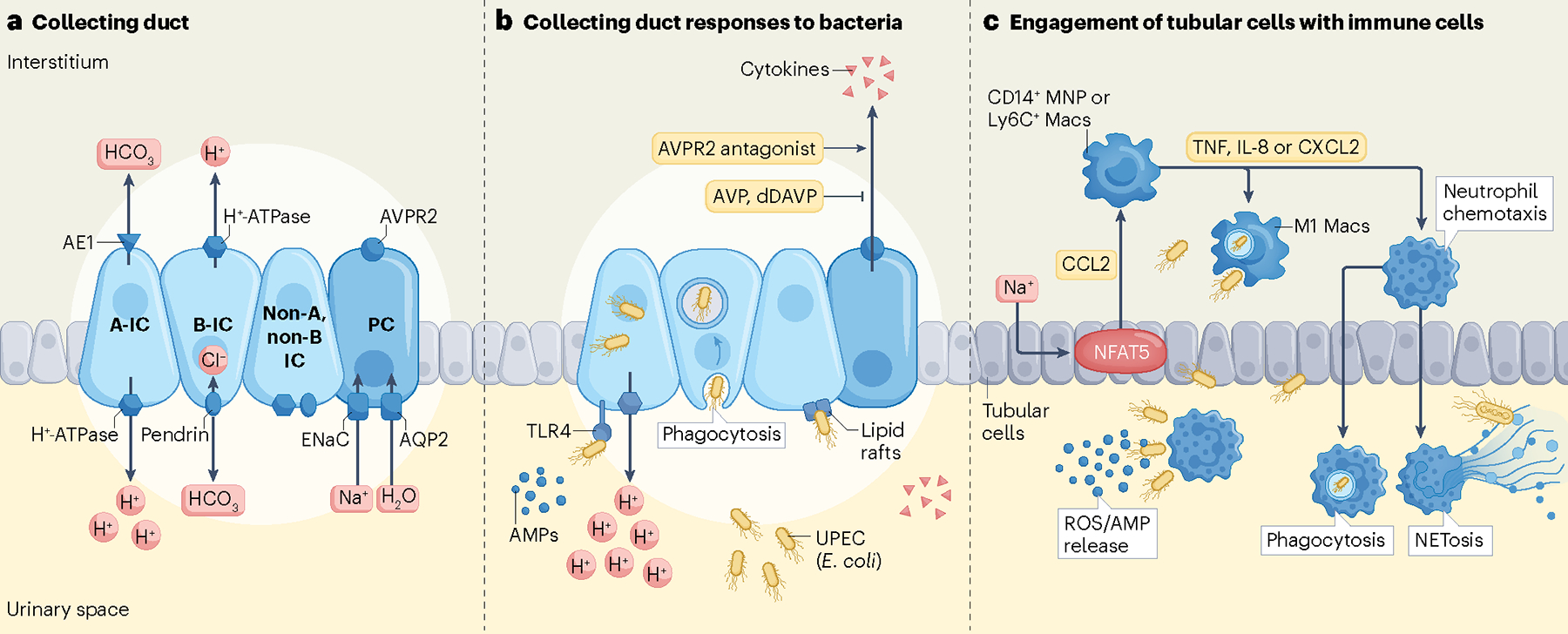
a, Collecting ducts are composed of principal cells (PCs) and three types of intercalated cells (A-IC; B-IC; and non-A, non-B IC) that express cell-specific transport proteins. A-IC express an apical H+-ATPase that interfaces with the urinary space and a Cl−/HCO3− exchanger AE1 along their basolateral surface. B-IC express pendrin, a Cl−/HCO3− exchanger, at their apical membrane and a basolateral H+-ATPase. In the connecting segment, non-A, non-B ICs express both H+-ATPase and pendrin at their apical surface. PCs express the epithelial sodium channel (ENaC), aquaporin 2 (AQP2) and an arginine vasopressin receptor (AVPR2). b, During pyelonephritis, uropathogenic Escherichia coli (UPEC) preferentially target the ICs in the collecting duct. UPEC are sensed by pattern recognition receptors, such as TLR4, on the surfaces of ICs. In addition, UPEC may access the interior of ICs via lipid rafts. ICs respond to uropathogens by releasing antimicrobial peptides (AMPs), cytokines and A-IC secrete protons into the urine. ICs also phagocytose UPEC. The role of PCs during pyelonephritis is less defined, but it is believed that activation of AVPR2 by vasopressin can induce secretion of cytokines by PCs and suppress endogenous dDAVP, ultimately reducing water reabsorption and increasing urine volume. c, (Top) In response to UPEC, the medullary sodium gradient orchestrates immune defences by stimulating NFAT5 in tubular epithelial cells to produce CCL2. CCL2 recruits human CD14+ mononuclear phagocytes (MNP) or mouse Ly6C+ macrophages (Macs) to the medulla where they differentiate into M1 macs and eliminate UPEC. Medullary epithelial cells and macs also secrete granulocyte-recruiting chemokines such as IL-8 (human), CXCL2 (mouse), or TNF to attract neutrophils to the source of infection. (Bottom) Neutrophil transepithelial migration into the urinary space combats UPEC by producing reactive oxygen species (ROS) and AMPs, phagocytosing UPEC and producing neutrophil extracellular traps (through NETosis).
Data from preclinical murine models show that UPEC establish pyelonephritis by attaching to the luminal surface of A-ICs32,33. Binding to these kidney epithelia is dependent on the ability of UPEC to produce type 1 or P-pili. P-pili bind glycosphingolipids in the kidney whereas the type 1 pilus adhesin, FimH, binds desmoglein-2 — a mannosylated junctional protein that is expressed by collecting duct cells34. Sc-RNAseq data show that desmoglein-2 is abundantly expressed in murine and human ICs35,36. UPEC mutants that lack type 1 pili or the P-pilus PapG adhesin have an attenuated capacity to colonize murine or human kidney cells37,38.
After binding to ICs, UPEC are internalized and invade the interstitium in a process that is facilitated by complement activation. Binding of type 1 fimbriae-expressing UPEC to kidney tubular cells engages CD46, a complement regulatory protein that promotes the production of the complement components C3 and C4 (ref. 39). C3 activation further enhances the internalization of UPEC. Mice that are deficient in C3 are resistant to pyelonephritis39,40. UPEC also translocate across tubular epithelial cells by two additional mechanisms. First, cytolytic UPEC strains express hlyA and sat (which encode the cytotoxins α-haemolysin and a secreted vacuolating cytotoxin, respectively) that activate caspase 3 and cause tubular cell apoptosis and kidney injury. Cellular injury compromises cellular junctions and facilitates paracellular bacterial translocation41–43. Second, non-cytolytic UPEC strains that lack hlyA and sat do not impair cellular junctions, but rather are internalized following remodelling of the actin cytoskeleton, after which they transcellularly translocate across kidney epithelial cells via lipid rafts and a clathrin-mediated process controlled by Toll-like receptor 4 (TLR4)41,44 (Fig. 2b).
Following their migration into the nephron, UPEC form microcolonies within the lumens of proximal tubules. Here, UPEC can trigger components of the innate immune response, including monocyte chemoattractant protein 1 (MCP-1; also known as CCL2) and RANTES (also known as CCL5) through TLR signalling45,46. In addition, cytotoxic UPEC strains secrete sub-lytic levels of α-haemolysin, which induce low-frequency oscillations in intracellular calcium concentrations. These intracellular calcium fluctuations result in the release of cytokines such as IL-6 and IL-8, which contribute to the pro-inflammatory environment46. Sustained calcium entry can also activate caspase-mediated pathways that cause tubular injury, apoptosis and paracellular bacterial dissemination47,48.
Host responses to UPEC infection
Innate immune responses
In response to UPEC infection, kidney epithelia engage their pattern recognition receptors, including TLRs, nucleotide oligomerization domain (NOD)-like receptors (NLRs), and mannose binding lectins. These pattern recognition receptors elicit inflammatory chemokines and cytokines that communicate with neutrophils and monocytes or macrophages to orchestrate the recruitment of immune cells for the purpose of eliminating UPEC (Fig. 2c).
Type 1 pili, P-pili and LPS on the surface of UPEC activates TLR4-independent immune responses and TLR4-driven responses involving NF-κB and downstream extracellular signal-regulated kinase (ERK) 1/2, mitogen-associated protein kinase (MAPK) and c-jun N-terminal kinase (JNK) that converge on specific transcription factors32,49,50. In the kidney, these pro-inflammatory cascades result in the production of cytokines from kidney epithelial cells, such as type I interferons, IL-6 and TNF, and chemokines such as IL-8, CCL3 (also known as MIP-1α), RANTES and MCP-1, which activate and recruit inflammatory cells for the eradication of UPEC. The importance of TLR4 to the host immune response is demonstrated by the inability of Tlr4-knockout mice to elicit immune responses in response to UPEC infection. Consequently, bacteria proliferate inside the lumens of kidney tubules and potentiate renal abscess formation51. Similarly, chimeric mice that express an inactivating Tlr4 mutation in their intrinsic kidney cells show impaired UPEC clearance and increased kidney abscess formation in response to UPEC infection. If UPEC are not contained within an abscess, mice succumb to disseminated infection52.
TLR4 closely regulates immune defences and imbalances in these responses impact pyelonephritis outcomes. For example, when P-pili-expressing UPEC strains bind kidney epithelial cells, TLR4 engages type I interferons to facilitate pathogen clearance through activation of the transcription factors interferon regulatory factor (IRF)-3 and IRF-7. Mice that are deficient in Irf3 hyperactivate Irf7-driven immune responses and develop pyelonephritis, renal abscesses and urosepsis following UPEC challenge53,54. By contrast, mice that are deficient in Irf7 do not show alterations in UPEC susceptibility, whereas combined Irf7 and Irf3 deletion reverses the pyelonephritis susceptibility of Irf3-deficient mice. Moreover, polymorphisms in the promoter region of IRF3 that confer reduced transcription factor activity have been identified in humans with pyelonephritis, whereas attenuating IRF7 promoter polymorphisms correlate negatively with infection risk53,54. These studies suggest that a balance in TLR4 and type I interferon responses influence susceptibility to UPEC infection.
Available data suggest that pattern recognition receptors other than TLR4 may also contribute to the host immune response to pyelonephritis. TLR2 acts synergistically with TLR4 to activate NF-κB55. TLR5 is expressed in the medullary collecting duct and is activated by flagellin. Tlr5 deletion promotes bacterial internalization, UPEC colonization, and suppresses the production of chemoattractant cytokines that recruit neutrophils to the site of infection56,57. A polymorphism in TLR5, which encodes a variant that negates flagellin-induced signalling, is associated with increased UTI risk58. Expression of TLR11 is restricted to mice and Tlr11 deficiency is associated with increased bacterial burden in the kidney following the induction of experimental UTI59. Although less frequently studied than TLRs, NLRs may also contribute to bacterial clearance in the context of pyelonephritis. Nod1-deficient mice are more susceptible to UPEC infection than wild type mice and demonstrate impaired neutrophil migration to the infected kidneys60. Nod2-deficient mice do not show impaired UPEC clearance but have a propensity to develop kidney abscesses following experimental UTI61. The involvement of different TLRs and NLRs in response to invading pathogens suggests that the interplay between pattern recognition receptors may be critical for the induction of protective immune responses following UPEC infection.
Antimicrobial peptides
In addition to activating innate immune signalling, host cells also eradicate invading pathogens by producing antimicrobial peptides and proteins. In the urinary tract, antimicrobial peptides are produced by the bladder urothelium, ureters, kidney epithelial cells and immune cells. In the kidney, ICs and PCs express antimicrobial peptides but ICs are viewed as the main source of production1,62,63. These antimicrobial peptides and proteins, including defensins, cathelicidin, metal binding proteins, ribonucleases and uromodulin, facilitate pathogen clearance by disrupting the integrity of the bacterial membrane, opsonizing bacteria, scavenging the nutrients needed for bacterial replication, preventing bacterial attachment, or modulating host responses. The utility of antimicrobial peptides in pyelonephritis antibacterial defences has been demonstrated in cultured human and mouse kidney cells, ex vivo in human and mouse urine samples, and in genetic mouse models1,33,64–79 (Table 2). These functional and proof-of-concept studies are complemented by findings from clinical studies, which show that individuals who experience recurrent cystitis have suppressed concentrations of urinary antimicrobial peptides76,80–82 (Table 2). In addition, genomic studies in children suggest that UTI susceptibility correlates with copy number variations or polymorphisms in antimicrobial peptide genes75,83,84. Thus, insufficient expression of antimicrobial peptides may augment UTI risk. Detailed prospective studies are needed to determine whether altered antimicrobial expression affects pyelonephritis severity or outcomes.
Table 2 |.
Antimicrobial peptides and proteins of relevance to UTIs
| Name | Source of production | Antimicrobial activity | In vivo preclinical evidence of UTI relevance | Clinical evidence of UTI relevance |
|---|---|---|---|---|
| β-defensins 1–3 (HNP1–3) | Collecting duct Immune cells | Bactericidal | Humanized DEFA1A3 mice are protected from UPEC65 | DEFA1A3 copy numbers correlate with UTI and pyelonephritis risk83 |
| β-defensin 1 | Nephron Collecting duct | Bactericidal |
Defb1 knockout mice develop Staphylococcal bacteriuria182 Defb1 knockout mice and controls have similar UTI risk67 |
Urine concentrations increase with pyelonephritis but not cystitis183 Urine concentrations increase in pregnancy183 |
| Cathelicidin (LL-37) | Nephron Collecting duct Immune cells | Bactericidal Chemoattractant Promotes NET formation |
Camp knockout mice on 129/SvJ background show increased UPEC burden (strain CFT073)184 Camp knockout mice on C57BI/6 background show decreased UPEC burden (strain UTI89)66 |
Urinary production increases with cystitis and pyelonephritis66 |
| Hepcidin | Nephron Collecting duct | Bacteriostatic Ferroportin inhibitor (iron export) | Hamp knockout mice have increased UPEC burden69,185 | Not tested |
| Lactoferrin | Collecting duct | Bacteriostatic Iron chelation | Oral lactoferrin treatment reduced UPEC bladder and kidney burden in mice186 | Urinary production increases with UTI187 |
| Pentraxin-related protein 3 | Kidney epithelia Immune cells | Promotes opsonization and phagocytosis | Ptx3 knockout mice have increased UPEC burden and renal abscess formation75 | Urinary production increases with cystitis and pyelonephritis75 |
| Lipocalin 2 (NGAL) | Nephron Intercalated cells Immune cells | Bacteriostatic Iron chelation | Lcn2 knockout mice have increased UPEC burden33,68 | Urinary production increases with UTI33,68 Girls with recurrent UTI have suppressed urinary concentrations Women with recurrent cystitis have elevated urinary concentrations68 |
| Ribonuclease 4 | Collecting duct Immune cells | Bactericidal | Not tested | Girls with recurrent UTI have suppressed urinary concentrations76 |
| Ribonuclease 7 | Intercalated cells | Bactericidal | Humanized RNASE7 mice are protected from UPEC73 | Girls with recurrent UTI have suppressed urinary concentrations73 RNASE7 polymorphism correlates with UTI risk84 |
| Uromodulin (Tamm-Horsfall protein) | Nephron | Prevents UPEC attachment | Lcn2 knockout mice have increased UPEC burden77 | UTI risk correlates with urine concentrations82 UMOD variants correlate with UTI risk188 |
UPEC, uropathogenic Escherichia coli; UTI, urinary tract infection.
Intercalated cells
Transcriptome analyses of human collecting duct cells suggest that UPEC exposure induces a shift in ICs towards an A-IC-like subtype31. Murine ICs also show increased V-ATPase mRNA expression following UPEC exposure31. These findings suggest that ICs may differentiate during infection — potentially to enhance urinary acidification, which inhibits bacterial replication. In support of this concept, one published dataset showed that mice acidify their urine during UTI; however, a later study did not replicate this finding33,85. Moreover, other studies have shown that the introduction of LPS into the lumen of rodent or rabbit collecting ducts or thick ascending limbs impairs urinary acidification through TLR4-mediated inhibition of bicarbonate absorption and/or hydrogen secretion86,87. The discordant results reported by different studies may stem from differences in the infecting UPEC strain, bacterial inoculum, or variations in experimental technique. However, urinary acidification or pH may not contribute notably to UTI defence as two studies suggest that urine pH neutralization does not affect UPEC clearance88,89.
In contrast to extracellular acidification, available evidence suggests that intracellular acidification can facilitate bacterial clearance. Exposure of ICs to UPEC induces the expression of genes that are associated with phagosome maturation31. In addition, single tubule microperfusion experiments and intravital microscopy studies show that murine ICs can phagocytose UPEC. Once internalized, phagosome acidification creates a microenvironment that kills UPEC31. These findings indicate that ICs may have an important role in UTI prevention. They also implicate the need to identify new approaches that facilitate A-IC differentiation, IC-mediated phagocytosis and acidification to treat pyelonephritis.
However, and as noted earlier, UPEC internalization by ICs is required to establish pyelonephritis. Thus, further insights are also needed to determine whether the process of UPEC internalization actually confers an advantage to UPEC and whether this process may potentiate pyelonephritis or UPEC survival within kidney tubules. In line with this suggestion, it is important to note that the induction of systemic metabolic acidosis through ammonium chloride supplementation exacerbates pyelonephritis in rodents89. Although in vitro and in vivo studies suggest that such acidosis enhances hypoxia-inducible factor (HIF)-1-mediated immune responses, it also predisposes rodents with VUR to pyelonephritis89–91. Acidosis may augment UTI by disrupting uroepithelial barriers, modulating cytokine production and immune cell recruitment, thereby impairing neutrophil bactericidal activity or suppressing antimicrobial peptide functions1,89.
The importance of ICs to the host response to UPEC is highlighted by the finding that reduced IC populations augment UPEC susceptibility. Mice that lack carbonic anhydrase 2 — an enzyme that is responsible for the generation of IC hydrogen gradients — have a 50% reduction in IC numbers and an impaired capacity to clear UPEC from the kidneys and bladder, independent of serum bicarbonate concentrations or urine pH88. Moreover, pyelonephritis risk increases when kidneys from carbonic anhydrase 2 knockout mice are transplanted into control syngeneic mice, suggesting that deficient kidney carbonic anhydrase 2 expression and reduced IC numbers are a risk factor for UTI92. In addition, ablation of IC populations by genetic deletion of the transcription factor Tfcp2l1 in murine collecting ducts, limits bacterial clearance following experimental UTI33. These results demonstrate that ICs are critical for UTI defence (Fig. 2b).
Dendritic cells and macrophages
In addition to epithelial cells, immune cell populations also have important roles in UPEC clearance during pyelonephritis. An intricate network of resident dendritic cells and macrophages survey the kidney interstitium, by probing glomerular and tubular self-antigens, danger signals and pathogen-associated molecular patterns5,93–96. The sentinel role of these dendritic cells and macrophages enables them to orchestrate TLR4-mediated antibacterial defence programmes. For example, macrophages respond by activating NF-κB signalling and murine CD11c+ dendritic cells respond by producing CXCL2 (MIP-2; the functional homologue of human IL-8) to recruit neutrophils to the site of infection. Ablation of CD11c+ dendritic cells decreases neutrophil recruitment and augments pyelonephritis in mouse kidneys52,97.
Two populations of macrophages are engaged during pyelonephritis — resident kidney macrophages, which self-renew in situ and originate mostly from the yolk sac, and bone marrow-derived macrophages, which originate from circulating monocytes and are recruited to the renal interstitium upon injury or infection. As described above, kidney-resident CX3CR1+/F480+ macrophages act as sentries that alert other immune phagocytes, such neutrophils, to the bacterial insult96,97. A similar role has been attributed to resident bladder macrophages, which produce chemokines to attract neutrophils and monocyte-derived Ly6C+ macrophages to the site of infection98,99.
The recruitment of circulating, monocyte-derived Ly6C+ macrophages to the kidney, particularly the renal medulla, is dependent on the C-C chemokine receptor type 2 (CCR2) and its chemokine C-C motif ligand 2 (CCL2). In mice with pyelonephritis, Ly6C+ macrophages have a pro-inflammatory (M1) profile, characterized by the expression of inducible nitric oxide synthase (iNOS) and inflammatory cytokines such as TNF and IL-1β100–102. The depletion of blood monocytes in these mice prevents the recruitment of Ly6C+ macrophages to the kidney and decreases inflammatory cytokine and chemokine expression. Notably, the bacterial burden in the kidney decreases after monocyte depletion, suggesting that monocyte-derived Ly6C+ macrophages may have dual roles as drivers of inflammation and of bacterial dissemination during pyelonephritis101. Notably, neutrophil depletion also increases the number of M1 macrophages, enhances bacterial burden and promotes tubulointerstitial nephritis and renal scarring in UPEC-infected mice101. Depletion of recruited Ly6C+ macrophages in neutropenic mice reduced these detrimental sequelae, indicating that dysregulated macrophage-dependent inflammation promotes kidney injury101. In support of this concept, monocyte-derived macrophages contribute to progressive kidney disease in the context of sterile inflammation103,104. The pro-inflammatory and profibrotic factors elicited by macrophages during pyelonephritis are unclear.
In human kidney tissues, two resident mononuclear phagocyte populations have been identified. CD14+ cells are primarily located in the medulla where they might sense bacteria that ascend from the bladder into the kidney100. In support of this proposal, CD14+ cells demonstrate greater phagocytic activity and produce higher levels of IL-8 and TNF than CD14− cells when challenged with UPEC100. The functional contribution of these cells to pyelonephritis resistance remains to be elucidated.
Neutrophils
Neutrophils promote bacterial killing by releasing reactive oxygen species (ROS) and antimicrobial peptides, phagocytosing UPEC, and producing neutrophil extracellular traps through (NETosis) to capture and eliminate UPEC (Fig. 2c). The recruitment of neutrophils to infected kidneys is initiated when UPEC stimulate tubular epithelial cells, resident dendritic cells or macrophages to secrete granulocyte-recruiting chemokines such as IL-8 (or CXCL2 in mouse), which act via the CXC chemokine receptors CXCR1 and CXCR2 (ref. 105). Similar to neutrophil-depleted mice101,105, Cxcr2 knockout mice develop pyelonephritis, renal abscesses and kidney scarring when challenged with UPEC, eventually succumbing to sepsis owing to delayed neutrophil recruitment and inefficient bacterial killing106. Clinical studies also support a protective role for IL-8 and its receptor. Children who are prone to pyelonephritis show lower CXCR1 expression in neutrophils than age-matched controls107. Moreover, polymorphisms in IL8 and CXCR1 are associated with an increased risk of pyelonephritis108–110. This body of evidence suggests that variations in the genes that drive neutrophil chemotaxis influence pyelonephritis susceptibility and severity.
Impairment of immune cell responses
In addition to possessing virulence factors that help to establish infection, UPEC genomes also encode virulence factors that interfere with neutrophil and mononuclear phagocyte function and may render the host susceptible to pyelonephritis (Table 1). For example, the secretion of Toll/interleukin-1 receptor (TIR) domain containing-protein C (TcpC) by UPEC subverts host defences by binding to the adaptor protein, myeloid differentiation factor 88 (MyD88), and blocking TLR signalling, thereby preventing NF-κB activation in macrophages111. Mutation of TcpC attenuates UPEC virulence and renal abscess formation112,113. In addition, TcpC also blocks activation of the NACHT leucin-rich repeat PYD protein 3 (NLRP3) inflammasome, which serves a role in pathogen recognition in the cytoplasm. Consequently, TcpC blunts IL-1β secretion by UPEC-infected macrophages112.
Beyond its effects on macrophages, evidence supports a role for TcpC in suppressing the antimicrobial mechanisms of neutrophils. Specifically, TcpC inhibits NETosis by acting as an E3 ubiquitin ligase to enhance the ubiquitination and proteasomal degradation of the NETosis-related protein PAD4114 (Fig. 2c). In addition, TcpC inhibits the LPS-elicited production of ROS and pro-inflammatory cytokines114.
It has long been known that the secreted toxin, HlyA, is a potent leukocidal protein, as evidenced by the finding that low concentrations evoke membrane permeability defects in neutrophils, resulting in ATP release, granule exocytosis, loss of phagocytic killing and cell death115. Although these effects were initially presumed to result from its pore-forming ability, we now know that HlyA triggers the activation of serine proteases in macrophages, resulting in degradation of the cytoskeletal scaffolding protein, paxillin, along with components of the NF-κB signalling cascade116. In addition, HlyA triggers the NLRP3 inflammasome in a manner that is dependent on potassium efflux from the cell, resulting in increased IL-1β production, mitochondrial dysfunction and macrophage death through pyroptosis117.
Finally, changes in UPEC morphology suppress host phagocyte activity. UPEC that express type 1 fimbriae interact with neutrophils in a mannose- and LPS-dependent manner, which induces neutrophil apoptosis. This apoptosis is abolished by blocking FimH-mediated attachment118. In addition, the PapG tip adhesin of P fimbriae protects UPEC from neutrophil killing through an unknown mechanism119. Although not defined in the kidney, bacterial filamentation limits UPEC phagocytosis in the bladder and in vitro, supporting the notion that alterations in morphology help UPEC to elude host immune responses120,121.
Regulation of immunity
The immune defences of the kidney are temporally and spatially regulated. Single-cell transcriptomic studies show that immune cell populations differ between fetal and adult human kidneys, and are asymmetrically distributed across the kidney. In adult kidneys, for example, T cells and B cells are enriched in the cortex, whereas neutrophils and mononuclear phagocytes are predominant in the medulla100,122. These data also show that the expression of immune genes is much higher in the adult kidney pelvic epithelium and medulla than in the fetal kidney epithelium. Immune genes that are enriched in the adult pelvic epithelium and medulla are involved in innate immune responses, and encode pattern recognition receptors, chemokines and antimicrobial peptides122, suggesting that the defence capacity of the kidney is acquired in the postnatal period when the kidney is more likely to encounter pathogens. These findings also indicate that antibacterial defences in the kidney are strategically positioned to shield its most vulnerable regions from ascending pathogens122; however, and as described below, changes in the extracellular environment and hormone levels can affect kidney antibacterial defences.
The effect of sodium on kidney antibacterial defences
Available evidence suggests that the high interstitial sodium concentration in the medulla strengthens the antibacterial defences of the kidney. In the context of pyelonephritis, this hypersaline environment promotes nuclear factor of activated T cells 5 (NFAT5)-dependent cytokine production by kidney tubular cells, which stimulates the recruitment of neutrophils and CD14+ mononuclear phagocytes that phagocytose UPEC (Fig. 2c). Disruption of the renal sodium gradient in mice or humans triggers aberrant cytokine production, reduces the recruitment of mononuclear phagocytes to the medulla, and increases pyelonephritis risk100, suggesting that a critical relationship exists between the microenvironment within the medulla and UTI defences.
Despite this protective role, subsequent experiments have shown that a high salt intake increases pyelonephritis susceptibility via two mechanisms. First, the increased production of urea, which is needed to establish a medullary osmotic gradient in the context of increased sodium excretion, suppresses neutrophil antimicrobial functions. Second, a decrease in mineralocorticoid production, which is needed to enhance sodium excretion, promotes the accumulation of aldosterone precursors with glucocorticoid activity. The ensuing hyperglucocorticoidism impairs the ability of mouse neutrophils to kill UPEC. Concordantly, humans who consume a high salt diet develop suppressed plasma aldosterone, increased plasma corticosterone concentrations and decreased neutrophil antimicrobial capacity123. Together, these studies indicate that although the hypersaline environment of the medulla might prepare the kidney to sense and eliminate bacteria, high salt intake or hypernatraemia may in fact suppress UPEC defences and increase pyelonephritis risk.
Hormonal regulation of antibacterial defences
Hormones, including vasopressin, insulin and sex hormones, also affect the immune defences of the kidney.
Vasopressin.
Arginine vasopressin (AVP), which is released from the pituitary in response to hypernatraemia or hypovolaemia, binds to the vasopressin V2 receptor (AVPR2) expressed on the basolateral surface of PCs to increase intracellular cAMP production and induce aquaporin 2-mediated water reabsorption. However, AVP also promotes sodium chloride reabsorption via the epithelial sodium channel (ENaC) and activates the cAMP-sensitive cystic fibrosis transmembrane conductance regulator (CFTR) chloride channel. Preclinical data indicate that pharmacological antagonism of AVPR2 stimulates the production of cytokines by kidney epithelial cells to facilitate bacterial clearance in UPEC-infected mice. (Fig. 2b). Diamino-8-D-arginine vasopressin (dDAVP), a AVPR2 agonist, suppresses TLR4-mediated NF-κB activity, chemokine secretion, neutrophil recruitment and augmented UPEC susceptibility through a mechanism that requires functional CFTR channels124.
Insulin.
In the kidney, insulin regulates gluconeogenesis, blood flow, electrolyte and mineral balance, and vascular resistance125. Interestingly, the expression of some antimicrobial peptides may also be dependent on the bioavailability of insulin. For example, levels of Bd1, which encodes the antimicrobial peptide β-defensin 1, are lower in kidneys from rodents with type 1 and type 2 diabetes than in those from healthy control animals. Of note, kidney expression of Defb11 and urinary β-defensin 1 levels were restored in diabetic models following insulin treatment126,127. Similarly, insulin induces expression of the iron scavenging protein lipocalin 2 and ribonucleases in kidney epithelial cells via PI3K–AKT signalling81,85. Last, cohort studies show that individuals with diabetes have lower serum and urinary concentrations of the antimicrobial peptides ribonuclease 7, lipocalin 2, cathelicidin and β-defensin 1 than healthy controls81,85,128. In a mouse model of localized insulin resistance, deletion of the insulin receptor in the collecting duct and ICs promoted localized cellular insulin resistance and increased cystitis susceptibility by suppressing antimicrobial peptide production, but did not cause hyperglycaemia or glucosuria85. These data suggest that insulin and its receptor regulate host responses and impact UTI risk. In addition, they suggest that hyperglycaemia and/or glucosuria are not the only factors that promote UTI in individuals with diabetes. Given the clinical impact of diabetes on UTI susceptibility, further investigation into the mechanisms by which insulin signalling regulates UTI responses is warranted. Although not comprehensively studied in the kidney, an impact of deregulated glucose control on cystitis antibacterial defences has been demonstrated10,129.
Sex hormones.
Sex and sex hormones also shape immune responses5,130–132. Male mice develop pyelonephritis and form renal abscesses more frequently than female mice following a UTI133, suggesting that androgens may potentiate UTI. In support of this concept, UTI severity increases in male and female mice treated with testosterone. Elevated testosterone levels prime the kidney for fibrosis by increasing local TGFβ production and promoting macrophage polarization towards a pro-fibrotic M2 phenotype134. By contrast, castration of male mice reduces UTI severity and pharmacologic or genetic inhibition of androgen receptor signalling reduces UTI risk133–136. These studies identify potential roles for mediators within the TGFβ superfamily in renal fibrosis, but further studies are warranted to identify specific ligands, receptors and cellular sources to substantiate these roles.
The influence of oestrogens on pyelonephritis is less frequently studied. Although ovariectomy in female mice does not make a sizable difference in pyelonephritis susceptibility, treatment of mice with an oestrogen receptor agonist facilitated UPEC clearance from the kidney135,137. By contrast, ovariectomized mice are predisposed to cystitis. Oestrogen therapy may improve UTI outcomes by boosting the expression of antimicrobial peptides, strengthening the urothelial barrier, changing the vaginal microbiome, and suppressing inflammation130,138,139. As more is learned about the effects of sex on UTI, new interventions may be applied to modulate hormonal signalling to reduce infection susceptibility or kidney scarring.
Therapeutic approaches
Targeting UPEC for pyelonephritis therapy
Given the rising prevalence of antibiotic-resistant uropathogens and the potentially devastating effect of pyelonephritis, sizable efforts are underway to develop new UTI therapies. Our newfound appreciation of the mechanisms used by UPEC to develop and propagate UTI has led to the development of vaccine-based approaches to boosting immune responses (Fig. 3a). Currently, four vaccines — Uro-vaxom, SolcoUrovac, Uromune, and ExPEC4V — have shown promise in randomized control trials2,140–142. The immunogens on which these vaccines are based include whole-cell heat-killed bacteria, bacterial extracts and antigenic bacterial cell wall components. In addition, bacterial iron acquisition proteins have also been assessed as vaccine antigens. Murine studies show that these prophylactics protect against cystitis and pyelonephritis; moreover, clinical isolates from patients with acute pyelonephritis show that iron acquisition antigens elicit immune responses and represent viable vaccine targets143–146. Finally, FimH is being investigated as a vaccine immunogen with promising results in cystitis and pyelonephritis prevention in preclinical models147.
Fig. 3 |. Emerging and potential antibiotic-conserving therapeutics for pyelonephritis.
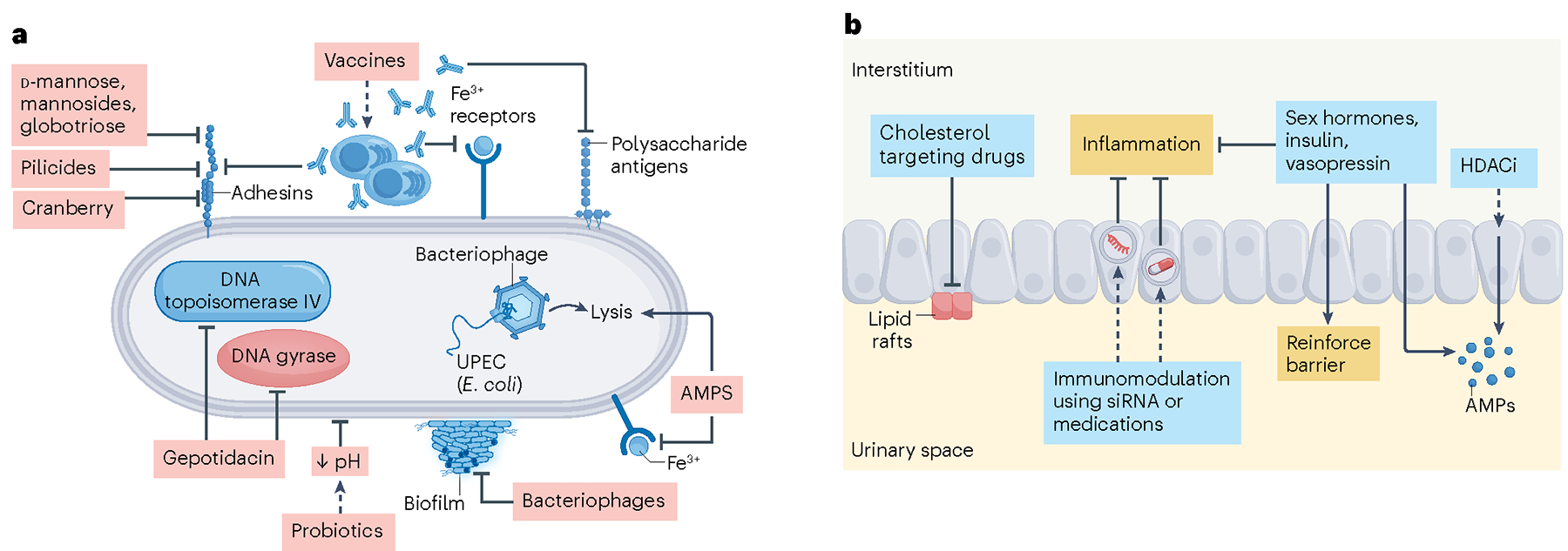
a, Vaccines induce immunity against uropathogenic Escherichia coli (UPEC) bacterial antigens including adhesins and polysaccharide antigens, such as FimH and lipopolysaccharide (LPS), respectively. Vaccines can also induce immunity against iron receptors. Exogenous mannose or its derivatives, pilicides and cranberry extracts can reduce UPEC binding to host cells. Gepotidacin is a novel antibiotic with a dual-targeting mechanism of action, disrupting both DNA gyrase and topoisomerase IV in bacteria. Probiotics can prevent UPEC attachment to host cells by altering the microenvironment, including pH. Bacteriophages can inhibit biofilm formation and induce lysis of cells that they infect. Antimicrobial peptides (AMPs) are small, cationic peptides that can directly kill bacteria by disrupting their cell membranes or by sequestering metal ions that are required for bacterial enzyme activity. b, Host defences against urinary tract infections (UTIs) can be boosted in a number of ways. Cholesterol targeting drugs can prevent UPEC from utilizing lipid rafts to gain entry into host cells. Immunomodulation of pro-inflammatory factors including interferon regulatory factor 7 (Irf7) has been shown to reduce pyelonephritis in rodent models (dashed line). Additionally, hormones including oestrogen, insulin and vasopressin suppress the inflammatory responses that contribute to pyelonephritis and strengthen the epithelial barrier. These hormones may also boost expression of AMPs. Finally, histone deacetylase inhibitors (HDACi) increase AMP production and reduce UTIs in rodent and cell-culture models. siRNA, small interfering RNA.
Beyond vaccines, approaches to minimizing UPEC attachment represents another promising strategy for preventing cystitis and pyelonephritis (Fig. 3a). Cell culture and murine studies have demonstrated the ability of D-mannose and globotriose to prevent binding of type 1 or P-pili to urothelial and kidney tubule cells, respectively41,148. Clinically, prophylactic administration of D-mannose reduces recurrent UTIs and prospective studies to test its benefits are ongoing148–150. This preliminary success with D-mannose has led to the development of synthetic mannosides — high-affinity receptor analogues for FimH on type I pili. In mice, mannosides prevent the binding of type 1 fimbriae to mannosylated desmoglein-2 on kidney epithelial cells34. Small molecules called pilicides represent another strategy for minimizing UPEC attachment by inhibiting the assembly of pili, which as noted above, have a role in the pathogenesis of cystitis and pyelonephritis151,152. Cranberry extracts have also been tested to prevent UPEC attachment in vitro and in vivo, but results from these experiments are inconclusive and their impact on improving pyelonephritis outcomes is unclear153.
Preventing bacterial colonization and internalization are other approaches to limiting infection. Probiotics such as lactobacillus can limit the growth and survival of pathogenic and antibiotic-resistant bacteria by releasing lactic acid, hydrogen peroxide, and bacteriocin. The utility of probiotics as anti-UPEC therapies has been reviewed elsewhere but is understudied in pyelonephritis153,154. Cholesterol-lowering drugs such as statins prevent UPEC invasion by altering the composition of lipid rafts41. In addition to preventing UPEC internalization, statins can modulate inflammation, which affects UTI-associated morbidity41,155–157.
New bactericidal approaches also show promise in UTI. Bacteriophages are highly effective at introducing their genetic material into host cells and can promote bacterial lysis. Prospective clinical studies have evaluated the use of bacteriophage instillation following kidney or prostate surgery to reduce UPEC titres. In addition to no adverse effects, these studies have reported a significant reduction in urinary UPEC concentrations158,159. Bacteriophages are also effective against biofilms and multidrug-resistant pathogens, and can restore antibiotic sensitivity160–162. Alternatively, antimicrobial peptides, which directly lyse UPEC and disrupt bacterial biofilms, show potential as UTI therapies. Further studies are needed to determine if synthetic antimicrobial peptides can be safely administered in a cost-effective manner to treat or prevent UTI1. Although in the early phases of development, bacteriophage therapies and antimicrobial peptides represent promising antibiotic alternatives or adjuvants for cystitis and pyelonephritis and warrant further exploration.
For the first time in almost two decades, a new antibiotic has been developed and shows promise for UTI treatment. Gepotidacin targets DNA gyrase and topoisomerase IV and has bactericidal activity against E. coli, including UPEC strains that are resistant to fluoroquinolones. Studies in rat models of pyelonephritis show that it is effective in reducing UPEC bladder and kidney burden163. Phase III clinical trials ended enrolment early owing to overwhelming evidence of efficacy in treating uncomplicated UTI164,165.
Engaging kidney immune responses
Boosting kidney immune mechanisms to control infection may represent an alternative therapeutic approach to preventing or treating pyelonephritis (Fig. 3b). Given the importance of the collecting duct in shaping UTI responses, strategies that enhance bacterial phagocytosis or UPEC acidification, TLR4-mediated inflammatory defences, or antimicrobial peptide secretion may represent new therapeutic avenues. For example, targeting developmental programmes that regulate IC and PC fate may alter collecting duct phenotypes to enable UPEC clearance166. Alternatively, silencing of the transcription factor, IRF-7, represents an opportunity to transiently reduce toxic inflammation and minimize kidney injury53. Lessons learned from advances in therapeutic mRNA delivery contend that similar strategies could be utilized to deliver IRF7 small interfering RNA or other target small interfering RNAs to dampen pro-inflammatory signalling following an infection. Moreover, our understanding of the response of the collecting duct to UPEC infection insinuates that medications such as the AVPR2 antagonist tolvaptan, insulin-sensitizing agents, or sex hormone therapy might lead to antibiotic-conserving therapies81,124,130. Last, repurposing clinically available drugs such as histone deacetylase inhibitors may reduce cystitis or pyelonephritis sequelae by augmenting antimicrobial peptide expression167.
Conclusions
The clinical ramifications of pyelonephritis are vast and represent a global challenge given the increasing prevalence of antibiotic-resistant UPEC and limited availability of approaches with which to treat or prevent kidney infections. This unmet need underscores a need for greater insights into mechanisms used by UPEC to establish pyelonephritis and the responses activated in the kidney to combat infection. Greater understanding of the mechanisms by which UPEC impacts the kidney and the epithelial or immune cells that impart immediate and sustained protection may provide a foundation on which to develop new immunotherapies for pyelonephritis.
Key points.
Uropathogenic Escherichia coli (UPEC) is the most common bacterial cause of pyelonephritis; virulence factors expressed by UPEC promote survival in the kidney by expediating cellular invasion and neutralizing host defences.
Within the collecting duct, intercalated cells are targeted by UPEC; intercalated cells respond by activating acid–base machinery, phagocytosing bacteria, producing cytokines and chemokines and releasing antimicrobial peptides.
An intricate network of macrophages and dendritic cells, in close proximity to collecting tubules, survey the renal interstitium and respond to UPEC by producing neutrophil and monocyte chemoattractants to combat bacteria during pyelonephritis.
Extracellular environmental factors and endogenous hormones influence or control important antibacterial defences in the kidney.
The increasing prevalence of antibiotic-resistant uropathogens highlights an urgent need for new therapeutic approaches that conserve antibiotic use and mitigate the morbidity associated with pyelonephritis; better understanding of host–pathogen interactions in the kidney may aid these efforts.
Acknowledgements
We apologize to the authors whose important work could not be included in this article owing to space limitations. J.D.S. discloses support for publication of this work from the National Institutes of Health (NIDDK) R01 DK115737, DK114035, and DK128088 (J.D.S.). J.D.R.R. discloses support by the National Institutes of Health (NIDDK) K01 DK128379.
Footnotes
Competing interests
The authors declare no competing interests.
Peer review information Nature Reviews Nephrology thanks Michael Zasloff and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
References
- 1.Becknell B, Schwaderer A, Hains DS & Spencer JD Amplifying renal immunity: the role of antimicrobial peptides in pyelonephritis. Nat. Rev. Nephrol. 11, 642–655 (2015). [DOI] [PubMed] [Google Scholar]
- 2.Butler D et al. Immunomodulation therapy offers new molecular strategies to treat UTI. Nat. Rev. Urol. 19, 419–437 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mulvey MA, Schilling JD, Martinez JJ & Hultgren SJ Bad bugs and beleaguered bladders: interplay between uropathogenic Escherichia coli and innate host defenses. Proc. Natl Acad. Sci. USA 97, 8829–8835 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ambite I et al. Molecular determinants of disease severity in urinary tract infection. Nat. Rev. Urol. 18, 468–486 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lacerda Mariano L & Ingersoll MA The immune response to infection in the bladder. Nat. Rev. Urol. 17, 439–458 (2020). [DOI] [PubMed] [Google Scholar]
- 6.Morello W, La Scola C, Alberici I & Montini G Acute pyelonephritis in children. Pediatr. Nephrol. 31, 1253–1265 (2016). [DOI] [PubMed] [Google Scholar]
- 7.Keren R et al. Risk factors for recurrent urinary tract infection and renal scarring. Pediatrics 136, e13–e21 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoberman A et al. Antimicrobial prophylaxis for children with vesicoureteral reflux. N. Engl. J. Med. 370, 2367–2376 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson JR & Russo TA Acute pyelonephritis in adults. N. Engl. J. Med. 378, 48–59 (2018). [DOI] [PubMed] [Google Scholar]
- 10.Geerlings SE Urinary tract infections in patients with diabetes mellitus: epidemiology, pathogenesis and treatment. Int. J. Antimicrob. Agents 31, S54–S57 (2008). [DOI] [PubMed] [Google Scholar]
- 11.Habak PJ & Griggs JRP Urinary Tract Infection in Pregnancy. StatPearls https://www.ncbi.nlm.nih.gov/books/NBK537047/ (2023). [PubMed]
- 12.Wu SY et al. Long-term surveillance and management of urological complications in chronic spinal cord-injured patients. J. Clin. Med. 10.3390/jcm11247307 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morris BJ & Wiswell TE Circumcision and lifetime risk of urinary tract infection: a systematic review and meta-analysis. J. Urol. 189, 2118–2124 (2013). [DOI] [PubMed] [Google Scholar]
- 14.Nordenstam GR, Brandberg CA, Oden AS, Svanborg Eden CM & Svanborg A Bacteriuria and mortality in an elderly population. N. Engl. J. Med. 314, 1152–1156 (1986). [DOI] [PubMed] [Google Scholar]
- 15.Hatfield KM et al. Assessing variability in hospital-level mortality among U.S. Medicare beneficiaries with hospitalizations for severe sepsis and septic shock. Crit. Care Med. 46, 1753–1760 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gharbi M et al. Antibiotic management of urinary tract infection in elderly patients in primary care and its association with bloodstream infections and all cause mortality: population based cohort study. Br. Med. J. 364, l525 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang TZ, Kodiyanplakkal RPL & Calfee DP Antimicrobial resistance in nephrology. Nat. Rev. Nephrol. 15, 463–481 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zowawi HM et al. The emerging threat of multidrug-resistant Gram-negative bacteria in urology. Nat. Rev. Urol. 12, 570–584 (2015). [DOI] [PubMed] [Google Scholar]
- 19.Desvaux M et al. Pathogenicity factors of genomic islands in intestinal and extraintestinal Escherichia coli. Front. Microbiol. 11, 2065 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mobley HL, Donnenberg MS & Hagan EC Uropathogenic Escherichia coli. EcoSal 10.1128/ecosalplus.8.6.1.3 (2009). [DOI] [PubMed] [Google Scholar]
- 21.Terlizzi ME, Gribaudo G & Maffei ME UroPathogenic Escherichia coli (UPEC) infections: virulence factors, bladder responses, antibiotic, and non-antibiotic antimicrobial strategies. Front. Microbiol. 8, 1566 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wiles TJ, Kulesus RR & Mulvey MA Origins and virulence mechanisms of uropathogenic Escherichia coli. Exp. Mol. Pathol. 85, 11–19 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nielubowicz GR & Mobley HL Host-pathogen interactions in urinary tract infection. Nat. Rev. Urol. 7, 430–441 (2010). This review comprehensively outlines the UPEC virulence factors needed to establish pyelonephritis.
- 24.Flores-Mireles AL, Walker JN, Caparon M & Hultgren SJ Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol. 13, 269–284 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaper JB, Nataro JP & Mobley HL Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2, 123–140 (2004). [DOI] [PubMed] [Google Scholar]
- 26.Li B et al. Inflammation drives renal scarring in experimental pyelonephritis. Am. J. Physiol. Renal Physiol. 312, F43–F53 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deguchi T et al. Electron microscopic study of acute retrograde pyelonephritis in mice. Urology 35, 423–427 (1990). [DOI] [PubMed] [Google Scholar]
- 28.Sanford JP, Hunter BW & Donaldson P Localization and fate of Escherichia coli in hematogenous pyelonephritis. J. Exp. Med. 116, 285–294 (1962). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roy A, Al-bataineh MM & Pastor-Soler NM Collecting duct intercalated cell function and regulation. Clin. J. Am. Soc. Nephrol. 10, 305–324 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kriz W, Kaissling B, Alpern R, Caplan M & Moe O Seldin and Giebisch’s the kidney: physiology and pathophysiology. 5th edn. (eds Alpern RJ, Moe OW & Caplan M) (Elsevier, 2013). [Google Scholar]
- 31. Saxena V et al. Kidney intercalated cells are phagocytic and acidify internalized uropathogenic Escherichia coli. Nat. Commun. 12, 2405 (2021). This study uses intravital microscopy and single kidney tubule perfusion to show that murine intercalated cells phagocytose UPEC to prevent pyelonephritis.
- 32. Chassin C et al. Renal collecting duct epithelial cells react to pyelonephritis-associated Escherichia coli by activating distinct TLR4-dependent and -independent inflammatory pathways. J. Immunol. 177, 4773–4784 (2006). This landmark study identifies TLR4-dependent and -independent epithelial mechanisms that are activated in the kidney when challenged with UPEC.
- 33. Paragas N et al. α-Intercalated cells defend the urinary system from bacterial infection. J. Clin. Invest. 124, 2963–2976 (2014). This study shows that intercalated cell deletion increases pyelonephritis susceptibility.
- 34.McLellan LK et al. A host receptor enables type 1 pilus-mediated pathogenesis of Escherichia coli pyelonephritis. PLoS Pathog. 17, e1009314 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu H, Kirita Y, Donnelly EL & Humphreys BD Advantages of single-nucleus over single-cell RNA sequencing of adult kidney: rare cell types and novel cell states revealed in fibrosis. J. Am. Soc. Nephrol. 30, 23–32 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu H et al. Comparative analysis and refinement of human PSC-derived kidney organoid differentiation with single-cell transcriptomics. Cell Stem Cell 23, 869–881 e868 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Korhonen TK, Virkola R & Holthofer H Localization of binding sites for purified Escherichia coli P fimbriae in the human kidney. Infect. Immun. 54, 328–332 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roberts JA et al. The Gal(alpha 1–4)Gal-specific tip adhesin of Escherichia coli P-fimbriae is needed for pyelonephritis to occur in the normal urinary tract. Proc. Natl Acad. Sci. USA 91, 11889–11893 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li K, Zhou W, Hong Y, Sacks SH & Sheerin NS Synergy between type 1 fimbriae expression and C3 opsonisation increases internalisation of E. coli by human tubular epithelial cells. BMC Microbiol. 9, 64 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Springall T et al. Epithelial secretion of C3 promotes colonization of the upper urinary tract by Escherichia coli. Nat. Med. 7, 801–806 (2001). [DOI] [PubMed] [Google Scholar]
- 41.Chassin C et al. TLR4 facilitates translocation of bacteria across renal collecting duct cells. J. Am. Soc. Nephrol. 19, 2364–2374 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang C et al. Alpha-hemolysin of uropathogenic Escherichia coli induces GM-CSF-mediated acute kidney injury. Mucosal Immunol. 13, 22–33 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu JH, Billings BJ & Balkovetz DF Hepatocyte growth factor alters renal epithelial cell susceptibility to uropathogenic Escherichia coli. J. Am. Soc. Nephrol. 12, 2543–2553 (2001). [DOI] [PubMed] [Google Scholar]
- 44.Trifillis AL et al. Binding to and killing of human renal epithelial cells by hemolytic P-fimbriated E. coli. Kidney Int. 46, 1083–1091 (1994). [DOI] [PubMed] [Google Scholar]
- 45.Tsuboi N et al. Roles of toll-like receptors in C-C chemokine production by renal tubular epithelial cells. J. Immunol. 169, 2026–2033 (2002). [DOI] [PubMed] [Google Scholar]
- 46.Uhlen P et al. Alpha-haemolysin of uropathogenic E. coli induces Ca2+ oscillations in renal epithelial cells. Nature 405, 694–697 (2000). [DOI] [PubMed] [Google Scholar]
- 47.Chakrabarti G & McClane BA The importance of calcium influx, calpain and calmodulin for the activation of CaCo-2 cell death pathways by Clostridium perfringens enterotoxin. Cell Microbiol. 7, 129–146 (2005). [DOI] [PubMed] [Google Scholar]
- 48.Melican K et al. Bacterial infection-mediated mucosal signalling induces local renal ischaemia as a defence against sepsis. Cell Microbiol. 10, 1987–1998 (2008). [DOI] [PubMed] [Google Scholar]
- 49.Kuper C, Beck FX & Neuhofer W Toll-like receptor 4 activates NF-κB and MAP kinase pathways to regulate expression of proinflammatory COX-2 in renal medullary collecting duct cells. Am. J. Physiol. Renal Physiol. 302, F38–F46 (2012). [DOI] [PubMed] [Google Scholar]
- 50.Saxena V, Arregui S, Kamocka MM, Hains DS & Schwaderer A MAP3K7 is an innate immune regulatory gene with increased expression in human and murine kidney intercalated cells following uropathogenic Escherichia coli exposure. J. Cell Biochem. 123, 1817–1826 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hagberg L et al. Difference in susceptibility to Gram-negative urinary tract infection between C3H/HeJ and C3H/HeN mice. Infect. Immun. 46, 839–844 (1984). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Patole PS et al. Toll-like receptor-4: renal cells and bone marrow cells signal for neutrophil recruitment during pyelonephritis. Kidney Int. 68, 2582–2587 (2005). [DOI] [PubMed] [Google Scholar]
- 53. Puthia M et al. IRF7 inhibition prevents destructive innate immunity — a target for nonantibiotic therapy of bacterial infections. Sci. Transl. Med. 8, 336ra359 (2016). This study shows the fine balance of the type I interferon response during pyelonephritis and the damaging effects of IRF-7 hyperactivation.
- 54.Fischer H et al. Pathogen specific, IRF3-dependent signaling and innate resistance to human kidney infection. PLoS Pathog. 6, e1001109 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chowdhury P, Sacks SH & Sheerin NS Toll-like receptors TLR2 and TLR4 initiate the innate immune response of the renal tubular epithelium to bacterial products. Clin. Exp. Immunol. 145, 346–356 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bens M et al. Flagellin/TLR5 signalling activates renal collecting duct cells and facilitates invasion and cellular translocation of uropathogenic Escherichia coli. Cell Microbiol. 16, 1503–1517 (2014). [DOI] [PubMed] [Google Scholar]
- 57.Andersen-Nissen E et al. Cutting edge: Tlr5−/− mice are more susceptible to Escherichia coli urinary tract infection. J. Immunol. 178, 4717–4720 (2007). [DOI] [PubMed] [Google Scholar]
- 58.Hawn TR et al. Toll-like receptor polymorphisms and susceptibility to urinary tract infections in adult women. PLoS One 4, e5990 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang D et al. A toll-like receptor that prevents infection by uropathogenic bacteria. Science 303, 1522–1526 (2004). [DOI] [PubMed] [Google Scholar]
- 60.Tourneur E et al. Cyclosporine A impairs nucleotide binding oligomerization domain (Nod1)-mediated innate antibacterial renal defenses in mice and human transplant recipients. PLoS Pathog. 9, e1003152 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang C et al. NOD2 is dispensable for ATG16L1 deficiency-mediated resistance to urinary tract infection. Autophagy 10, 331–338 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Saxena V et al. Cell specific qRT-PCR of renal epithelial cells reveals a novel innate immune signature in murine collecting duct. Am. J. Physiol. Renal Physiol. 315, F812–F823 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Saxena V et al. Whole transcriptome analysis of renal intercalated cells predicts lipopolysaccharide mediated inhibition of retinoid X receptor α function. Sci. Rep. 9, 545 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zasloff M Antimicrobial peptides, innate immunity, and the normally sterile urinary tract. J. Am. Soc. Nephrol. 18, 2810–2816 (2007). [DOI] [PubMed] [Google Scholar]
- 65.Canas JJ et al. Human neutrophil peptides 1–3 protect the murine urinary tract from uropathogenic Escherichia coli challenge. Proc. Natl Acad. Sci. USA 119, e2206515119 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chromek M et al. The antimicrobial peptide cathelicidin protects the urinary tract against invasive bacterial infection. Nat. Med. 12, 636–641 (2006). [DOI] [PubMed] [Google Scholar]
- 67.Becknell B et al. Expression and antimicrobial function of β-defensin 1 in the lower urinary tract. PLoS One 8, e77714 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Steigedal M et al. Lipocalin 2 imparts selective pressure on bacterial growth in the bladder and is elevated in women with urinary tract infection. J. Immunol. 193, 6081–6089 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Houamel D et al. Hepcidin as a major component of renal antibacterial defenses against uropathogenic Escherichia coli. J. Am. Soc. Nephrol. 27, 835–846 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Spencer JD et al. Ribonuclease 7, an antimicrobial peptide upregulated during infection, contributes to microbial defense of the human urinary tract. Kidney Int. 83, 615–625 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Spencer JD et al. Ribonuclease 7 is a potent antimicrobial peptide within the human urinary tract. Kidney Int. 80, 174–180 (2011). [DOI] [PubMed] [Google Scholar]
- 72.Hains DS et al. Deleted in malignant brain tumor 1 genetic variation confers urinary tract infection risk in children and mice. Clin. Transl. Med. 11, e477 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Eichler T et al. Ribonuclease 7 shields the kidney and bladder from invasive uropathogenic Escherichia coli infection. J. Am. Soc. Nephrol. 30, 1385–1397 (2019). This original work uses in vitro human models and a humanized transgenic mouse to show that the antimicrobial peptide RNase 7 has a role in UTI prevention.
- 74.Becknell B et al. Ribonucleases 6 and 7 have antimicrobial function in the human and murine urinary tract. Kidney Int. 87, 151–161 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jaillon S et al. The humoral pattern recognition molecule PTX3 is a key component of innate immunity against urinary tract infection. Immunity 40, 621–632 (2014). [DOI] [PubMed] [Google Scholar]
- 76.Bender K et al. Expression and function of human ribonuclease 4 in the kidney and urinary tract. Am. J. Physiol. Renal Physiol. 320, F972–F983 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bates JM et al. Tamm-Horsfall protein knockout mice are more prone to urinary tract infection: rapid communication. Kidney Int. 65, 791–797 (2004). [DOI] [PubMed] [Google Scholar]
- 78.Pak J, Pu Y, Zhang ZT, Hasty DL & Wu XR Tamm-Horsfall protein binds to type 1 fimbriated Escherichia coli and prevents E. coli from binding to uroplakin Ia and Ib receptors. J. Biol. Chem. 276, 9924–9930 (2001). [DOI] [PubMed] [Google Scholar]
- 79.Weiss GL et al. Architecture and function of human uromodulin filaments in urinary tract infections. Science 369, 1005–1010 (2020). [DOI] [PubMed] [Google Scholar]
- 80.Forster CS et al. Urinary NGAL deficiency in recurrent urinary tract infections. Pediatr. Nephrol. 32, 1077–1080 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Eichler TE et al. Insulin and the phosphatidylinositol 3-kinase signaling pathway regulate ribonuclease 7 expression in the human urinary tract. Kidney Int. 90, 568–579 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Garimella PS et al. Urinary uromodulin and risk of urinary tract infections: the Cardiovascular Health Study. Am. J. Kidney Dis. 69, 744–751 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schwaderer AL et al. Polymorphisms in alpha-defensin-encoding DEFA1A3 associate with urinary tract infection risk in children with vesicoureteral reflux. J. Am. Soc. Nephrol. 27, 3175–3186 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pierce KR et al. Ribonuclease 7 polymorphism rs1263872 reduces antimicrobial activity and associates with pediatric urinary tract infections. J. Clin. Investig. 10.1172/JCI149807 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Murtha MJ et al. Insulin receptor signaling regulates renal collecting duct and intercalated cell antibacterial defenses. J. Clin. Invest. 128, 5634–5646 (2018). This study shows that deletion of insulin receptor in the collecting duct or intercalated cells increases UTI susceptibility by suppressing antimicrobial peptide expression.
- 86.Watts BA 3rd, George T & Good DW Lumen LPS inhibits HCO3− absorption in the medullary thick ascending limb through TLR4-PI3K-Akt-mTOR-dependent inhibition of basolateral Na+/H+ exchange. Am. J. Physiol. Renal Physiol. 305, F451–F462 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tsuruoka S, Purkerson JM & Schwartz GJ Lipopolysaccharide directly inhibits bicarbonate absorption by the renal outer medullary collecting duct. Sci. Rep. 10, 20548 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hains DS et al. Carbonic anhydrase 2 deficiency leads to increased pyelonephritis susceptibility. Am. J. Physiol. Renal Physiol. 307, F869–F880 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Purkerson JM, Corley JL & Schwartz GJ Metabolic acidosis exacerbates pyelonephritis in mice prone to vesicoureteral reflux. Physiol. Rep. 8, e14525 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Peng H, Purkerson JM, Freeman RS, Schwaderer AL & Schwartz GJ Acidosis induces antimicrobial peptide expression and resistance to uropathogenic E. coli infection in kidney collecting duct cells via HIF-1α. Am. J. Physiol. Renal Physiol. 318, F468–F474 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Peng H, Purkerson JM, Schwaderer AL & Schwartz GJ Metabolic acidosis stimulates the production of the antimicrobial peptide cathelicidin in rabbit urine. Am. J. Physiol. Renal Physiol. 313, F1061–F1067 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ketz J et al. Developmental loss, but not pharmacological suppression, of renal carbonic anhydrase 2 results in pyelonephritis susceptibility. Am. J. Physiol. Renal Physiol. 318, F1441–F1453 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hayes BW & Abraham SN Innate immune responses to bladder infection. Microbiol. Spectr. 10.1128/microbiolspec.UTI-0024-2016 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Soos TJ et al. CX3CR1+ interstitial dendritic cells form a contiguous network throughout the entire kidney. Kidney Int. 70, 591–596 (2006). [DOI] [PubMed] [Google Scholar]
- 95. Weisheit CK, Engel DR & Kurts C Dendritic cells and macrophages: sentinels in the kidney. Clin. J. Am. Soc. Nephrol. 10, 1841–1851 (2015). This review summarizes the classification of dendritic cells and macrophages in the kidney and their roles in pyelonephritis, acute kidney disease, chronic kidney disease and renal transplantation.
- 96.Sedin J et al. High resolution intravital imaging of the renal immune response to injury and infection in mice. Front. Immunol. 10, 2744 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tittel AP et al. Kidney dendritic cells induce innate immunity against bacterial pyelonephritis. J. Am. Soc. Nephrol. 22, 1435–1441 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schiwon M et al. Crosstalk between sentinel and helper macrophages permits neutrophil migration into infected uroepithelium. Cell 156, 456–468 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mora-Bau G et al. Macrophages subvert adaptive immunity to urinary tract infection. PLoS Pathog. 11, e1005044 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Berry MR et al. Renal sodium gradient orchestrates a dynamic antibacterial defense zone. Cell 170, 860–874 e819 (2017). This work demonstrates that the renal sodium stimulates NFAT5-mediated epithelial CCL2 production, which recruits mononuclear phagocytes to renal medulla and forms a medullary defence zone against uropathogens.
- 101. Ruiz-Rosado JD et al. Neutrophil-macrophage imbalance drives the development of renal scarring during experimental pyelonephritis. J. Am. Soc. Nephrol. 32, 69–85 (2021). The data demonstrate that a balance between antimicrobial and inflammatory responses orchestrated by neutrophils and monocyte-derived macrophages, respectively, is required to effectively control acute pyelonephritis and prevent deteriorating kidney function.
- 102.Tsou CL et al. Critical roles for CCR2 and MCP-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. J. Clin. Investig. 117, 902–909 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Han HI, Skvarca LB, Espiritu EB, Davidson AJ & Hukriede NA The role of macrophages during acute kidney injury: destruction and repair. Pediatr. Nephrol. 34, 561–569 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wen Y, Yan HR, Wang B & Liu BC Macrophage heterogeneity in kidney injury and fibrosis. Front. Immunol. 12, 681748 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Haraoka M et al. Neutrophil recruitment and resistance to urinary tract infection. J. Infect. Dis. 180, 1220–1229 (1999). [DOI] [PubMed] [Google Scholar]
- 106.Svensson M et al. Acute pyelonephritis and renal scarring are caused by dysfunctional innate immunity in mCxcr2 heterozygous mice. Kidney Int. 80, 1064–1072 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Godaly G, Proudfoot AE, Offord RE, Svanborg C & Agace WW Role of epithelial interleukin-8 (IL-8) and neutrophil IL-8 receptor A in Escherichia coli-induced transuroepithelial neutrophil migration. Infect. Immun. 65, 3451–3456 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Javor J et al. Genetic variations of interleukin-8, CXCR1 and CXCR2 genes and risk of acute pyelonephritis in children. Int. J. Immunogenet. 39, 338–345 (2012). [DOI] [PubMed] [Google Scholar]
- 109.Artifoni L et al. Interleukin-8 and CXCR1 receptor functional polymorphisms and susceptibility to acute pyelonephritis. J. Urol. 177, 1102–1106 (2007). [DOI] [PubMed] [Google Scholar]
- 110.Han SS, Lu Y, Chen M, Xu YQ & Wang Y Association between interleukin 8-receptor gene (CXCR1 and CXCR2) polymorphisms and urinary tract infection: evidence from 4097 subjects. Nephrology 24, 464–471 (2019). [DOI] [PubMed] [Google Scholar]
- 111. Cirl C et al. Subversion of Toll-like receptor signaling by a unique family of bacterial Toll/interleukin-1 receptor domain-containing proteins. Nat. Med. 14, 399–406 (2008). This study identifies TcpC as a UPEC-derived virulence factor that blunts TLR signalling and NF-κB activation in macrophages.
- 112. Waldhuber A et al. Uropathogenic Escherichia coli strain CFT073 disrupts NLRP3 inflammasome activation. J. Clin. Investig. 126, 2425–2436 (2016). This study demonstrates that the TcpC protein blocks activation of the NLRP3 inflammasome, which serves a key role in intracellular recognition of UPEC.
- 113. Fang JQ et al. TcpC inhibits Toll-like receptor signaling pathway by serving as an E3 ubiquitin ligase that promotes degradation of myeloid differentiation factor 88. PLoS Pathog. 17, e1009481 (2021). This study demonstrates that TcpC serves as an E3 ubiquitin ligase to direct the proteosomal degradation of MyD88.
- 114.Ou Q et al. TcpC inhibits neutrophil extracellular trap formation by enhancing ubiquitination mediated degradation of peptidylarginine deiminase 4. Nat. Commun. 12, 3481 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bhakdi S et al. Potent leukocidal action of Escherichia coli hemolysin mediated by permeabilization of target cell membranes. J. Exp. Med. 169, 737–754 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dhakal BK & Mulvey MA The UPEC pore-forming toxin α-hemolysin triggers proteolysis of host proteins to disrupt cell adhesion, inflammatory, and survival pathways. Cell Host Microbe 11, 58–69 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Verma V et al. α-Hemolysin of uropathogenic E. coli regulates NLRP3 inflammasome activation and mitochondrial dysfunction in THP-1 macrophages. Sci. Rep. 10, 12653 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Blomgran R, Zheng L & Stendahl O Uropathogenic Escherichia coli triggers oxygen-dependent apoptosis in human neutrophils through the cooperative effect of type 1 fimbriae and lipopolysaccharide. Infect. Immun. 72, 4570–4578 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tewari R et al. The PapG tip adhesin of P fimbriae protects Escherichia coli from neutrophil bactericidal activity. Infect. Immun. 62, 5296–5304 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Horvath DJ Jr. et al. Morphological plasticity promotes resistance to phagocyte killing of uropathogenic Escherichia coli. Microbes Infect. 13, 426–437 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Justice SS, Hunstad DA, Seed PC & Hultgren SJ Filamentation by Escherichia coli subverts innate defenses during urinary tract infection. Proc. Natl Acad. Sci. USA 103, 19884–19889 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Stewart BJ et al. Spatiotemporal immune zonation of the human kidney. Science 365, 1461–1466 (2019). This elegant study provides evidence of the spatial arrangement of immune cells in the human kidney and how it changes over developmental time and anatomical space. The results from this study suggest that antimicrobial immunity is spatially zonated but this feature is only evident postnatally.
- 123. Jobin K et al. A high-salt diet compromises antibacterial neutrophil responses through hormonal perturbation. Sci. Transl. Med. 10.1126/scitranslmed.aay3850 (2020). This original work demonstrates that experimental pyelonephritis is aggravated in mice on a high salt diet.
- 124. Chassin C et al. Hormonal control of the renal immune response and antibacterial host defense by arginine vasopressin. J. Exp. Med. 204, 2837–2852 (2007). This study shows that arginine vasopressin modulates antibacterial defences in the kidney.
- 125.Hale LJ & Coward RJ Insulin signalling to the kidney in health and disease. Clin. Sci. 124, 351–370 (2013). [DOI] [PubMed] [Google Scholar]
- 126.Froy O, Hananel A, Chapnik N & Madar Z Differential effect of insulin treatment on decreased levels of β-defensins and Toll-like receptors in diabetic rats. Mol. Immunol. 44, 796–802 (2007). [DOI] [PubMed] [Google Scholar]
- 127.Hiratsuka T et al. Structural analysis of human β-defensin-1 and its significance in urinary tract infection. Nephron 85, 34–40 (2000). [DOI] [PubMed] [Google Scholar]
- 128.Brauner H et al. Markers of innate immune activity in patients with type 1 and type 2 diabetes mellitus and the effect of the anti-oxidant coenzyme Q10 on inflammatory activity. Clin. Exp. Immunol. 177, 478–482 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Mohanty S et al. Diabetes downregulates the antimicrobial peptide psoriasin and increases E. coli burden in the urinary bladder. Nat. Commun. 13, 4983 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Albracht CD, Hreha TN & Hunstad DA Sex effects in pyelonephritis. Pediatr. Nephrol. 36, 507–515 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ingersoll MA Sex differences shape the response to infectious diseases. PLoS Pathog. 13, e1006688 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zychlinsky Scharff A et al. Sex differences in IL-17 contribute to chronicity in male versus female urinary tract infection. JCI Insight 10.1172/jci.insight.122998 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Olson PD, Hruska KA & Hunstad DA Androgens enhance male urinary tract infection severity in a new model. J. Am. Soc. Nephrol. 27, 1625–1634 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hreha TN et al. Androgen-influenced polarization of activin A-producing macrophages accompanies post-pyelonephritic renal scarring. Front. Immunol. 11, 1641 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Olson PD et al. Androgen exposure potentiates formation of intratubular communities and renal abscesses by Escherichia coli. Kidney Int. 94, 502–513 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hreha TN et al. TGFβ1 orchestrates renal fibrosis following Escherichia coli pyelonephritis. Physiol. Rep. 8, e14401 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sen A, Iyer J, Boddu S, Kaul A & Kaul R Estrogen receptor α differentially modulates host immunity in the bladder and kidney in response to urinary tract infection. Am. J. Clin. Exp. Urol. 7, 110–122 (2019). [PMC free article] [PubMed] [Google Scholar]
- 138.Luthje P et al. Estrogen supports urothelial defense mechanisms. Sci. Transl. Med. 5, 190ra180 (2013). [DOI] [PubMed] [Google Scholar]
- 139.Wang C, Symington JW, Ma E, Cao B & Mysorekar IU Estrogenic modulation of uropathogenic Escherichia coli infection pathogenesis in a murine menopause model. Infect. Immun. 81, 733–739 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Mobley HL & Alteri CJ Development of a vaccine against Escherichia coli urinary tract infections. Pathogens 10.3390/pathogens5010001 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lorenzo-Gomez MF et al. Comparison of sublingual therapeutic vaccine with antibiotics for the prophylaxis of recurrent urinary tract infections. Front. Cell Infect. Microbiol. 5, 50 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Prattley S, Geraghty R, Moore M & Somani BK Role of vaccines for recurrent urinary tract infections: a systematic review. Eur. Urol. Focus. 6, 593–604 (2020). [DOI] [PubMed] [Google Scholar]
- 143.Alteri CJ, Hagan EC, Sivick KE, Smith SN & Mobley HL Mucosal immunization with iron receptor antigens protects against urinary tract infection. PLoS Pathog. 5, e1000586 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Forsyth VS et al. Optimization of an experimental vaccine to prevent Escherichia coli urinary tract infection. mBio 10.1128/mBio.00555-20 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Spurbeck RR et al. Escherichia coli isolates that carry vat, fyuA, chuA, and yfcV efficiently colonize the urinary tract. Infect. Immun. 80, 4115–4122 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lloyd AL, Rasko DA & Mobley HL Defining genomic islands and uropathogen-specific genes in uropathogenic Escherichia coli. J. Bacteriol. 189, 3532–3546 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Langermann S et al. Vaccination with FimH adhesin protects cynomolgus monkeys from colonization and infection by uropathogenic Escherichia coli. J. Infect. Dis. 181, 774–778 (2000). [DOI] [PubMed] [Google Scholar]
- 148.Kranjcec B, Papes D & Altarac S D-mannose powder for prophylaxis of recurrent urinary tract infections in women: a randomized clinical trial. World J. Urol. 32, 79–84 (2014). [DOI] [PubMed] [Google Scholar]
- 149.De Nunzio C, Bartoletti R, Tubaro A, Simonato A & Ficarra V Role of D-Mannose in the prevention of recurrent uncomplicated cystitis: state of the art and future perspectives. Antibiotics 10.3390/antibiotics10040373 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Franssen M et al. D-Mannose to prevent recurrent urinary tract infections (MERIT): protocol for a randomised controlled trial. BMJ Open 11, e037128 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Greene SE et al. Pilicide ec240 disrupts virulence circuits in uropathogenic Escherichia coli. mBio 5, e02038 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Piatek R et al. Pilicides inhibit the FGL chaperone/usher assisted biogenesis of the Dr fimbrial polyadhesin from uropathogenic Escherichia coli. BMC Microbiol. 13, 131 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Loubet P et al. Alternative therapeutic options to antibiotics for the treatment of urinary tract infections. Front. Microbiol. 11, 1509 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sihra N, Goodman A, Zakri R, Sahai A & Malde S Nonantibiotic prevention and management of recurrent urinary tract infection. Nat. Rev. Urol. 15, 750–776 (2018). [DOI] [PubMed] [Google Scholar]
- 155.Pouwels KB, Visser ST, Bos HJ & Hak E Angiotensin-converting enzyme inhibitor treatment and the development of urinary tract infections: a prescription sequence symmetry analysis. Drug Saf. 36, 1079–1086 (2013). [DOI] [PubMed] [Google Scholar]
- 156.Hall SA et al. Commonly used antihypertensives and lower urinary tract symptoms: results from the Boston area community health (BACH) survey. BJU Int. 109, 1676–1684 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Blanco-Colio LM, Tunon J, Martin-Ventura JL & Egido J Anti-inflammatory and immunomodulatory effects of statins. Kidney Int. 63, 12–23 (2003). [DOI] [PubMed] [Google Scholar]
- 158.Leitner L et al. Bacteriophages for treating urinary tract infections in patients undergoing transurethral resection of the prostate: a randomized, placebo-controlled, double-blind clinical trial. BMC Urol. 17, 90 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ujmajuridze A et al. Adapted bacteriophages for treating urinary tract infections. Front. Microbiol. 9, 1832 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zulk JJ et al. Phage resistance accompanies reduced fitness of uropathogenic Escherichia coli in the urinary environment. mSphere 7, e0034522 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Gu Y et al. Identification of novel bacteriophage vB_EcoP-EG1 with lytic activity against planktonic and biofilm forms of uropathogenic Escherichia coli. Appl. Microbiol. Biotechnol. 103, 315–326 (2019). [DOI] [PubMed] [Google Scholar]
- 162.Pires DP, Melo L, Vilas Boas D, Sillankorva S & Azeredo J Phage therapy as an alternative or complementary strategy to prevent and control biofilm-related infections. Curr. Opin. Microbiol. 39, 48–56 (2017). [DOI] [PubMed] [Google Scholar]
- 163.Hoover JL, Singley CM, Elefante P & Rittenhouse S Efficacy of human exposures of gepotidacin (GSK2140944) against Escherichia coli in a rat pyelonephritis model. Antimicrob. Agents Chemother. 10.1128/AAC.00086-19 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Scangarella-Oman NE et al. Dose selection for phase III clinical evaluation of gepotidacin (GSK2140944) in the treatment of uncomplicated urinary tract infections. Antimicrob. Agents Chemother. 66, e0149221 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Perry C et al. Design of two phase III, randomized, multicenter studies comparing gepotidacin with nitrofurantoin for the treatment of uncomplicated urinary tract infection in female participants. Infect. Dis. Ther. 11, 2297–2310 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Park J et al. Single-cell transcriptomics of the mouse kidney reveals potential cellular targets of kidney disease. Science 360, 758–763 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Schwartz L et al. Repurposing HDAC inhibitors to enhance ribonuclease 4 and 7 expression and reduce urinary tract infection. Proc. Natl Acad. Sci. USA 120, e2213363120 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Nagamatsu K et al. Dysregulation of Escherichia coli α-hemolysin expression alters the course of acute and persistent urinary tract infection. Proc. Natl Acad. Sci. USA 112, E871–E880 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Skals M, Jorgensen NR, Leipziger J & Praetorius HA Alpha-hemolysin from Escherichia coli uses endogenous amplification through P2X receptor activation to induce hemolysis. Proc. Natl Acad. Sci. USA 106, 4030–4035 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Garcia TA, Ventura CL, Smith MA, Merrell DS & O’Brien AD Cytotoxic necrotizing factor 1 and hemolysin from uropathogenic Escherichia coli elicit different host responses in the murine bladder. Infect. Immun. 81, 99–109 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Mills M, Meysick KC & O’Brien AD Cytotoxic necrotizing factor type 1 of uropathogenic Escherichia coli kills cultured human uroepithelial 5637 cells by an apoptotic mechanism. Infect. Immun. 68, 5869–5880 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Guyer DM, Radulovic S, Jones FE & Mobley HL Sat, the secreted autotransporter toxin of uropathogenic Escherichia coli, is a vacuolating cytotoxin for bladder and kidney epithelial cells. Infect. Immun. 70, 4539–4546 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.He Y et al. TcpC secreting uropathogenic E. coli promoted kidney cells to secrete MIP-2 via p38 MAPK pathway. Mol. Med. Rep. 16, 3528–3534 (2017). [DOI] [PubMed] [Google Scholar]
- 174.Wu XR, Sun TT & Medina JJ In vitro binding of type 1-fimbriated Escherichia coli to uroplakins Ia and Ib: relation to urinary tract infections. Proc. Natl Acad. Sci. USA. 93, 9630–9635 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Mulvey MA Adhesion and entry of uropathogenic Escherichia coli. Cell Microbiol. 4, 257–271 (2002). [DOI] [PubMed] [Google Scholar]
- 176.Backhed F et al. Identification of target tissue glycosphingolipid receptors for uropathogenic, F1C-fimbriated Escherichia coli and its role in mucosal inflammation. J. Biol. Chem. 277, 18198–18205 (2002). [DOI] [PubMed] [Google Scholar]
- 177.Nowicki B, Hart A, Coyne KE, Lublin DM & Nowicki S Short consensus repeat-3 domain of recombinant decay-accelerating factor is recognized by Escherichia coli recombinant Dr adhesin in a model of a cell-cell interaction. J. Exp. Med. 178, 2115–2121 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Torres AG, Redford P, Welch RA & Payne SM TonB-dependent systems of uropathogenic Escherichia coli: aerobactin and heme transport and TonB are required for virulence in the mouse. Infect. Immun. 69, 6179–6185 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Anderson GG, Goller CC, Justice S, Hultgren SJ & Seed PC Polysaccharide capsule and sialic acid-mediated regulation promote biofilm-like intracellular bacterial communities during cystitis. Infect. Immun. 78, 963–975 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Goh KGK et al. Genome-wide discovery of genes required for capsule production by uropathogenic Escherichia coli. mBio 10.1128/mBio.01558-17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Corbett D & Roberts IS The role of microbial polysaccharides in host-pathogen interaction. F1000 Biol. Rep. 1, 30 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Morrison G, Kilanowski F, Davidson D & Dorin J Characterization of the mouse beta defensin 1, Defb1, mutant mouse model. Infect. Immun. 70, 3053–3060 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Valore EV et al. Human β-defensin-1: an antimicrobial peptide of urogenital tissues. J. Clin. Investig. 101, 1633–1642 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Danka ES & Hunstad DA Cathelicidin augments epithelial receptivity and pathogenesis in experimental Escherichia coli cystitis. J. Infect. Dis. 10.1093/infdis/jiu577 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Bauckman KA et al. Dietary restriction of iron availability attenuates UPEC pathogenesis in a mouse model of urinary tract infection. Am. J. Physiol. Renal Physiol. 316, F814–F822 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Haversen LA et al. Human lactoferrin and peptides derived from a surface-exposed helical region reduce experimental Escherichia coli urinary tract infection in mice. Infect. Immun. 68, 5816–5823 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Arao S et al. Measurement of urinary lactoferrin as a marker of urinary tract infection. J. Clin. Microbiol. 37, 553–557 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Ghirotto S et al. The uromodulin gene locus shows evidence of pathogen adaptation through human evolution. J. Am. Soc. Nephrol. 27, 2983–2996 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]


