Abstract
Objective:
Diagnostic workup of Cushing disease (CD) involves imaging evaluation of the pituitary gland, but in many patients no tumour is visualised. The aim of this study is to describe the association of magnetic resonance imaging (MRI) findings with the postoperative course of paediatric and adolescent patients with CD.
Patients:
Patients with a diagnosis of CD at less than 21 years of age with MRI evaluation of the pituitary before first transsphenoidal surgery were included.
Measurements:
Clinical, imaging and biochemical data were analysed.
Results:
One hundred and eighty-six patients with paediatric or adolescent-onset CD were included in the study. Of all patients, 127 (68.3%) had MRI findings consistent with pituitary adenoma, while the remaining had negative or inconclusive MRI. Patients with negative MRI were younger in age and had lower morning cortisol and adrenocorticotropin levels. Of 181 patients with data on postoperative course, patients with negative MRI had higher odds of not achieving remission after the first surgery (odds ratio = 2.6, 95% confidence intervals [CIs] = 1.1–6.0) compared to those with positive MRI. In patients with remission after first transsphenoidal surgery, long-term recurrence risk was not associated with the detection of a pituitary adenoma in the preoperative MRI (hazard risk = 2.1, 95% CI = 0.7–5.8).
Conclusions:
Up to one-third of paediatric and adolescent patients with CD do not have a pituitary tumour visualised in MRI. A negative MRI is associated with higher odds of nonremission after surgery; however, if remission is achieved, long-term risk for recurrence is not associated with the preoperative MRI findings.
Keywords: Cushing, MRI, pituitary tumour
1 |. INTRODUCTION
Paediatric Cushing syndrome (CS) is a rare endocrine condition commonly caused by adrenocorticotropin (ACTH)-secreting pituitary adenomas, referred to as Cushing disease (CD).1 Prompt diagnosis of CS is imperative for the prevention or early treatment of CS-related complications such as obesity, growth stagnation, hypertension, metabolic abnormalities and others.2,3 The management of paediatric patients with suspected CD usually involves transsphenoidal surgery (TSS) for resection of the adenoma with high rates of remission in an experienced centre; however, a small percentage of patients may still experience recurrence.4
An important step in the diagnostic workup of patients with CS is the imaging evaluation of the pituitary, most commonly with magnetic resonance imaging (MRI).2 However, MRI is not confirmatory of a pituitary source in up to one-third of paediatric patients with later confirmed CD leading to the need for additional testing and delays in the final management.5,6 Newer MRI techniques and machines have provided more advanced imaging resolution with the hope of higher diagnostic yield; however, in a significant proportion of patients, MRI still does not identify a pituitary lesion.7–9 The prognostic value of a negative MRI with regard to the likelihood of successful management and risk for recurrence has been previously evaluated with inconsistent results in adults, and it is less clear in the paediatric population who often have smaller tumours at diagnosis.10–12
Here, we present a summary of MRI findings of paediatric patients with a confirmed diagnosis of CD. We describe their baseline characteristics and their postoperative course in association with the presence or absence of a distinct adenoma at the preoperative MRI.
2 |. METHODS
2.1 |. Subjects
Subjects enroled under the protocol 97-CH-0076 (ClinicalTrials.gov identifier NCT00001595) at the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), were screened for eligibility in this study. Paediatric and adolescent patients (<21 years old at the time of presentation) with the diagnosis of CD, for whom preoperative MRI was available for review before first TSS were included. CS diagnosis was based on criteria defined by the Endocrine Society guidelines and adjusted for the paediatric and adolescent population as previously described (abnormal measurements in at least two of the following criteria: Elevated 24 h urinary free cortisol [UFC], elevated midnight serum cortisol [>121 nmol/L in children] and/or failure to suppress cortisol to 1 mg [or weight-based equivalent dose] overnight dexamethasone suppression test [post-dexamethasone cortisol more than 50 nmol/L]).1,13 The diagnosis of ACTH-dependent CD was based on the results of plasma ACTH, corticotropin-releasing hormone stimulation test and high-dose dexamethasone suppression test as previously described.14 According to our protocol any patient with negative or inconclusive MRI and biochemical evidence of ACTH-dependent CS underwent bilateral inferior petrosal sinus sampling (BIPSS) for confirmation of pituitary source of CS. For patients with a previously identified lesion on the MRI, the neurosurgeon usually approached the side of the suspected adenoma first and performed resection of the tumour if identified. In patients with negative MRI who underwent TSS at the National Institute of Health (NIH; ClinicalTrials.gov identifier NCT00060541), sublabial TSS was performed as described elsewhere.15 Briefly, wide exposure of the dura overlying sella and medial cavernous sinuses was performed following the initial approach to the sella. Wide dural opening including medial cavernous sinuses was performed while preserving the underlying pituitary capsule. If an apparent ‘adenoma’ was evident on the surface, circumferential dissection along the pseudocapsular plane was performed to remove the lesion en-bloc. In other cases, serial vertical incisions were made through entire depth of the anterior pituitary gland to search for adenoma within the depths. If no adenoma was found subtotal hypophysectomy was performed. Early reoperation within 3 weeks of initial surgery was offered if biochemical evidence of remission from CD was not detected.16,17 Patients diagnosed and managed at outside Institutes followed the recommendations and management of their local physicians. We excluded patients with no available MRI in our electronic medical records (n = 39) and patients for whom MRI was only available at the time of recurrence (n = 4). Remission was defined as postoperative cortisol nadir level less than 138 nmol/L. After surgery, all patients were prescribed oral hydrocortisone at 8–12 mg/m2/day and weaning of the dose was performed according to previously described recommendations and guided by their biochemical results and symptoms.18
Informed consent was obtained from parents and assent from patients if developmentally appropriate. All study procedures were approved by the Eunice Kennedy Shriver NICHD Institutional Review Board.
2.2 |. Clinical and biochemical data
Clinical data and biochemical results were extracted from electronic medical records. Duration of disease was calculated as the time between the age at weight gain, in excess of the previous percentile, as noted on the patient’s growth chart, and the age at first biochemical diagnosis. Height, weight and body mass index (BMI) z-scores were calculated based on age- and sex-matched references from the 2000 CDC growth charts. Height deficit was calculated as the difference of the patient’s height z-score minus the mid-parental target height z-score. Serum cortisol and plasma ACTH levels were calculated as the average value of the corresponding levels performed at 23:30 and 00:00 h (reported as midnight values) and 07:30 and 08:00 h (reported as morning values). Twenty-four-hour UFC was calculated as the average of the first 2–3 samples reported in the electronic medical records. Given the possible differences in the assays and reported reference range for UFC, we calculated the increase of UFC based on the upper limit of normal according to the following formula: UFC fold change = UFC/upper limit of the reference range. Serum cortisol was measured with solid-phase, competitive chemiluminescent enzyme immunoassay on Siemens Immulite 2500 analyzer (Malvern). Plasma ACTH was measured with chemiluminescence immunoassay on Siemens Immulite 200 XPi analyzer (Malvern). UFC was measured with chemiluminescent enzyme immunoassay until 2011 and with high-performance liquid chromatography/tandem mass spectrometry since 2011 (LC-MS/MS).
2.3 |. Magnetic resonance imaging
MRI scans (MRIs) were performed based on standard clinical protocols of each institute. At the NIH, MRIs were performed as previously described.9 Briefly, most MRIs at NIH were performed before and after intravenous administration of gadolinium contrast material, with a gradient-echo sequence and thin slices (less or equal to 1.5 mm). MRIs were performed in either a 1.5 T (n = 107) or 3 T (n = 76) MR machine from various manufacturers over time, while for three studies the magnetic field of the machine used was not recorded in the available images. MRIs were reviewed by the radiology department and the principal investigator of the protocol. We classified MRIs as ‘positive’ when a lesion consistent with an adenoma (hypoenhancing after contrast administration) was identified by both the radiologist and the principal investigator. MRI studies with radiologic findings not clearly suggestive of a pituitary adenoma, such as stalk thickness, asymmetry of the pituitary gland or heterogenous enhancement after contrast administration, were considered inconclusive and were analysed under the category of ‘negative’ MRIs.
2.4 |. Statistical analysis
Categorical data are presented as counts and proportions and compared between subjects with positive versus negative MRIs using the χ2 test. Continuous data were checked for normality based on histogram distribution and the Shapiro–Wilk statistical test. Continuous data with normal distribution are described as mean (standard deviation) and were compared between groups using student’s t-test. Continuous data without normal distribution are presented as median (interquartile range, presented as the difference between the third and the first quartile) and were compared between groups using Wilcoxon rank-sum test. The frequency of remission versus nonremission in patients with positive versus negative MRIs was calculated with χ2 and presented as odds ratio (OR) and 95% confidence intervals (CIs). Logistic regression was used to adjust for the performing neurosurgeon. Multivariable Cox proportional hazard was used to compute the hazard risk (HR) and 95% CI for recurrence based on MRI findings, adjusting for the neurosurgeon. Results were considered significant if p value was less than .05. Statistical analyses were performed in R.
3 |. RESULTS
3.1 |. Characteristics of the cohort
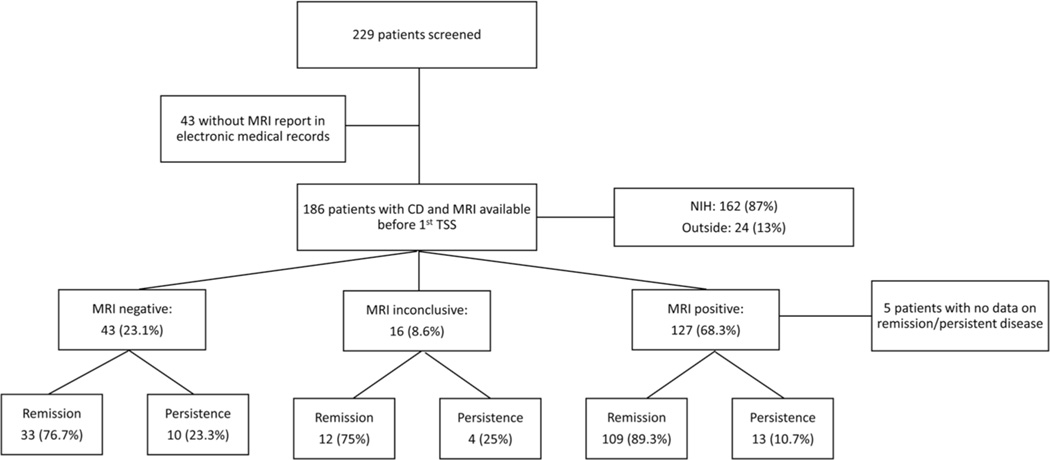
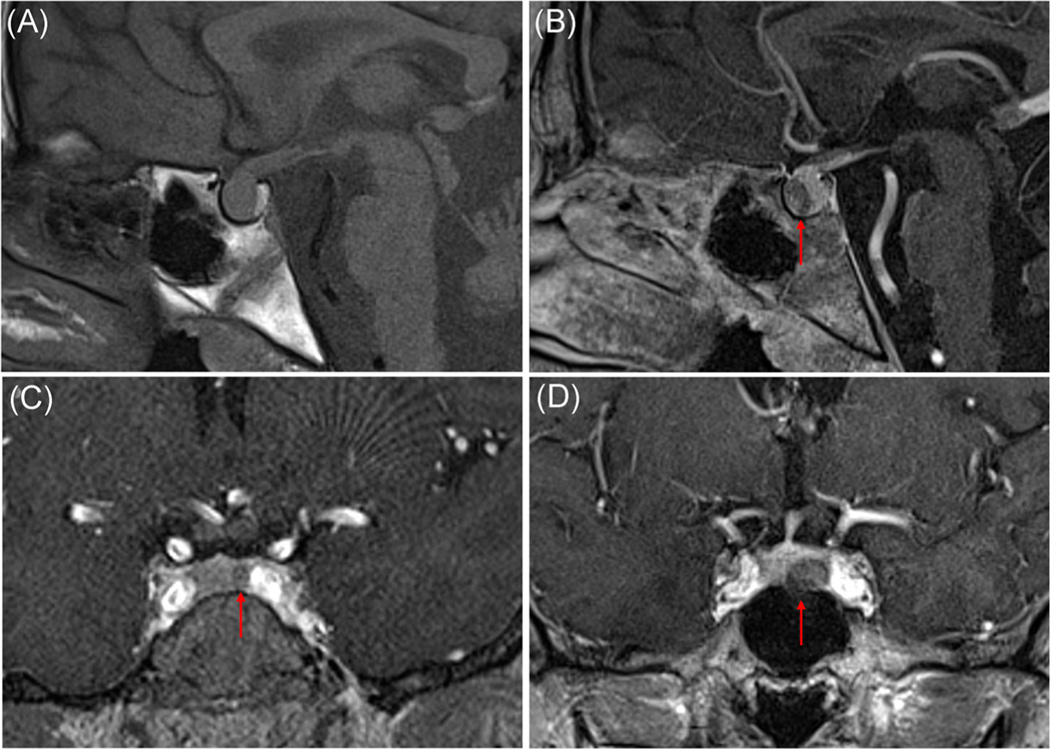
Of 229 patients with paediatric or adolescent onset of CD (<21 years of age) enroled in the protocol, 186 patients were included in this study (Figure 1). Most of the MRIs were performed at the NIH (n = 162, 87%), while the remaining (n = 24, 13%) were performed at outside facilities. Two-thirds of the patients (n = 127, 68.3%) had an MRIs suggestive of a pituitary lesion, most commonly a hypoenhancing lesion after contrast administration (representative MRIs shown in Figure 2a–d). Fifty-one adenomas were identified in the right side, 48 in the left, 2 patients had 2 or more possible lesions, while the remaining tumours were located in the middle aspect of the pituitary gland. The remaining patients (n = 59, 31.7%) had either an MRIs read as within the normal range (n = 43, 23.1%) or with subtle MRI findings without clear adenoma identification (n = 16, 8.6%). The frequency of positive MRIs was similar when we looked only at the cohort of patients who underwent MRIs at the NIH (66.7% of patients had positive MRIs vs. 33.3% with negative or inconclusive findings).
FIGURE 1.
Flowchart of patients screened and included in the study and their classification based on MRI findings and postoperative course. CD, Cushing disease; MRI, magnetic resonance imaging; NIH, National Institute of Health
FIGURE 2.
Representative pituitary MRI studies of patients with Cushing disease. T1-weighted precontrast (A) and postcontrast (B) images of the same patient. Precontrast study shows a large pituitary gland without distinct adenoma, which is visualised in the postcontrast images on the anterior aspect of the pituitary with diminished enhancement with respect to the normal pituitary tissue. (C) Hypoenhancing lesion seen on gradient-echo T1-weighted images consistent with microadenoma. (D) Hypoenhancing lesion seen on early postcontrast gradient-echo T1-weighted images consistent with macroadenoma. MRI, magnetic resonance imaging
3.2 |. Clinical and biochemical factors of patients with positive and negative MRI findings
When comparing the demographic and biochemical characteristics of patients with negative versus those with positive MRIs, patients with negative MRIs were younger at the time of diagnosis (median age: 11.1 years [4.5] vs. 14.4 years old [4.1]; p < .0001). They also had lower morning cortisol levels (median morning cortisol 429 nmol/L [211] vs. 510 nmol/L [298]; p = .001, reference range: 138–690] and ACTH levels (median morning ACTH: 7.24 pmol/L [5.1] vs. 10.4 pmol/L [7.7]; p < .0001, reference range: 1.1–10.1; Table 1). As expected, the tumour size calculated after surgical resection was smaller in patients with negative MRIs compared to those with positive MRIs (median tumour size: 4 [6]vs. 5 mm [5];p < .001). When excluding MRIs performed outside the NIH, results remained similar.
TABLE 1.
Clinical and biochemical characteristics of the patients overall and based on their MRI findings
| MRI negative or inconclusive (N = 59) | MRI positive (N = 127) | All patients (N = 186) | p Valuea | |
|---|---|---|---|---|
| Age at diagnosis (years)b | 11.1 [4.5] | 14.4 [4.1] | 13.3 [5.0] | <.0001 |
| Duration of disease (years)b | 2.50 [1.5] | 2.23 [2.5] | 2.45 [2.2] | .96 |
| N =57 | N = 122 | N = 179 | ||
| Gender | ||||
| Female, N (%) | 29 (49.2) | 76 (59.8) | 105 (56.5) | .17 |
| Male, N (%) | 30 (50.8) | 51 (40.2) | 81 (43.5) | |
| Height z-score | −1.26 (1.4) | −1.25 (1.4) | −1.26 (1.4) | .97 |
| N =56 | N = 112 | N = 168 | ||
| Height deficit z-score | −1.54 (1.5) | −1.08 (1.3) | −1.25 (1.4) | .07 |
| N =52 | N = 88 | N = 140 | ||
| Weight z-scoreb | 1.96 [1.4] | 1.46 [1.6] | 1.63 [1.7] | .12 |
| N = 56 | N = 112 | N = 168 | ||
| BMI z-scoreb | 2.32 [0.8] | 2.11 [1.1] | 2.15 [0.9] | .011 |
| N =56 | N = 112 | N = 168 | ||
| Morning cortisol (nmol/L)b (RR: 138–690) | 429 [211] | 510 [298] | 480 [268] | .001 |
| N =57 | N = 114 | N = 171 | ||
| Midnight cortisol (nmol/L)b (RR: < 50 nmol/L) | 366 [230] | 454 [309] | 424 [290] | .15 |
| N = 55 | N = 109 | N = 164 | ||
| UFC fold changeb | 4.24 [4.9] | 4.17 [5.0] | 4.24 [4.9] | .73 |
| N = 51 | N = 108 | N = 159 | ||
| Morning ACTH (pmol/L)b (RR: 1.1–10.1) | 7.24 [5.1] | 10.4 [7.7] | 9.45 [6.4] | <.0001 |
| N = 58 | N = 115 | N = 173 | ||
| Tumour size in histology (mm)b | 4.00 [6.0] | 5.00 [5.0] | 5.00 [4.0] | <.001 |
| N = 49 | N = 93 | 142 |
Abbreviations: ACTH, adrenocorticotropin; BMI, body mass index; MRI, magnetic resonance imaging; RR, reference range; UFC, urinary free cortisol.
Comparison of patients with negative or inconclusive MRI versus patients with positive MRI.
Data not normally distributed.
3.3 |. MRI findings over time
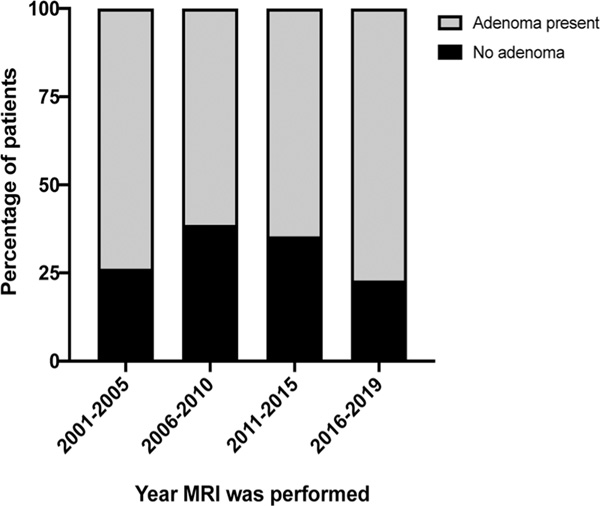
To look into the diagnostic yield of MRIs over time, we grouped MRI studies in approximately 5-year periods and compared the frequency of positive vs negative results. Overall, there was no significant change overtime (overall p = .36; Figure 3). Results were similar when we included only MRIs performed at the NIH (overall p = .37).
FIGURE 3.
Percentage of patients with positive (grey) and negative (black) MRI over time grouped in approximate 5-year time periods. MRI, magnetic resonance imaging
3.4 |. Postoperative course
We were able to retrieve data on the extent of surgery performed from 154 patients who were operated at the NIH. Of these, 145 (94.2%) underwent single lesion resection (‘adenectomy’) while 9 patients (5.8%) underwent subtotal hypophysectomy, including up to 75% of the pituitary gland. There was no association between the presence of an adenoma on MRI and the frequency of subtotal hypophysectomy at first operation (p = .17).
Data on the immediate postoperative course were available for 181 patients; of these, 154 patients had undergone surgery at the NIH and the remaining at outside hospitals. Among patients with data on the postoperative course, 27 (14.9%, 14 from the NIH cohort) did not achieve immediate remission after the first surgery. Of these patients, 13 (12 at the NIH) underwent second surgery within 3 weeks after first TSS as previously described,17 and 11 of those achieved remission. In all, 140/154 (91%) patients at the NIH achieved remission during initial hospitalisation.
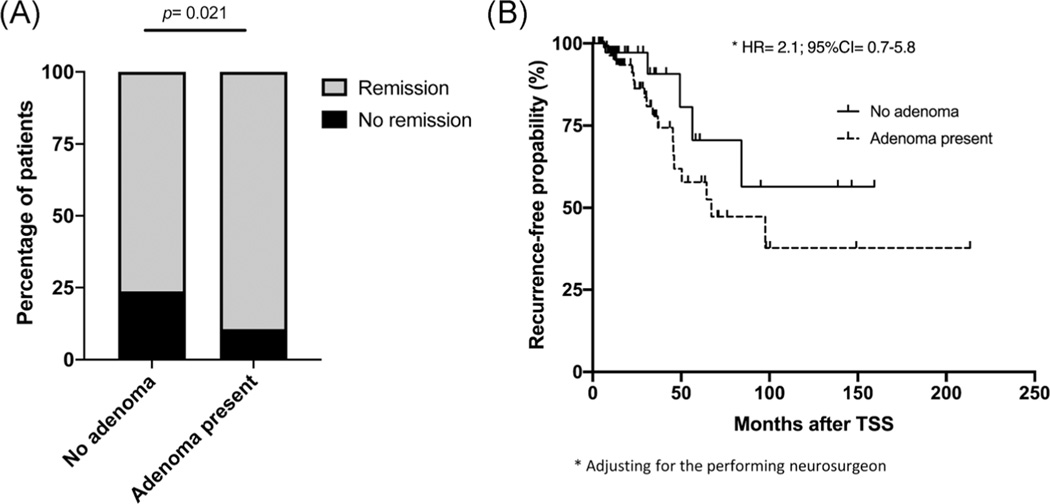
A ‘positive’ preoperative MRI was associated with higher odds of achieving immediate postoperative remission after the first surgery (OR = 2.6, 95% CI = 1.1–6.0; Figure 4a). This finding remained significant after adjusting for the performing neurosurgeon (p = .003, all surgeries performed outside the NIH were grouped in the same category as ‘other’) and when the analysis was restricted to patients who had MRIs at the NIH (OR = 6.3, 95% CI = 1.9–21.0) or only those who were operated at the NIH (OR = 6.6, 95% CI = 2.0–21.8).
FIGURE 4.
(A) Percentage of patients with remission and nonremission based on the MRI findings. (B) Kaplan–Meir plot of risk for recurrence based on the preoperative MRI findings in patients who achieved initial remission. CI, confidence interval; HR, hazard risk; MRI, magnetic resonance imaging; TSS, transsphenoidal surgery
Looking into long-term remission and risk for recurrence of patients who have initially achieved remission after the first surgery (n = 154), at a median follow-up time of 15.8 [25.6] months, there was no difference in the risk for recurrence when comparing patients with positive vs negative MRIs, adjusting for the neurosurgeon who performed the surgery (HR = 2.1, 95% CI = 0.7–5.8; Figure 4b). Similarly, no difference was identified in the risk for recurrence based on preoperative MRI for patients who had an MRI at the NIH (HR = 1.8, 95% CI = 0.5–6.7), at a median follow-up time of 14.5 months [26.5], or only those who were operated at the NIH (HR = 1.8, 95% CI = 0.5–6.9), at a median follow-up time of 14.6 months [25.2], adjusting for the performing neurosurgeon.
For 126 patients with available data on the pituitary function after first surgery, the odds of developing any pituitary hormone deficiency (including isolated posterior pituitary dysfunction/diabetes insipidus) were not different between patients with positive or negative preoperative MRI (p = .8).
4 |. DISCUSSION
The imaging evaluation of children with suspected CD is an imperative step of their diagnostic workup, but unfortunately, results sometimes do not confirm the diagnosis. Consistent with previous studies, we present here that MRI results may be negative in up to one-third of patients with a final diagnosis of CD. This finding seems to be more common in younger children with lower morning cortisol and ACTH levels. A negative MRI suggests a higher risk for non-remission after surgery; however, for patients who achieve remission, the long-term risk for recurrence is not associated with the preoperative imaging findings.
The frequency of negative MRI findings in patients with CD has been previously described. In the paediatric population as confirmed also in our study, the rate of MRI negative patients varies from 30% to 50%; this is not unexpected since most pituitary adenomas are small (in our cohort median size of 5 mm).4,6 We also describe that patients with negative MRI tend to be younger in age and have lower biochemical markers of hypercortisolemia (as shown by cortisol and ACTH levels). This may imply that these patients are identified earlier in the course of their disease, although this hypothesis is not confirmed based on the disease duration as extrapolated by medical records.
The possibility of a negative MRI should constitute an important part of the discussion with the patient and the family during the workup. In our experience, a negative MRI often increases the family’s anxiety which may delay further testing often required, such as BIPSS. To prepare families and patients, we often discuss in advance that a negative MRI is not uncommon, and we have additional strategies in confirming the diagnosis in these patients.
An important finding of our study is the association of a negative preoperative MRI with the patient’s postoperative course regarding the higher risk for nonremission and the possible need for repeat surgery. Previous studies have not consistently revealed a similar risk, but most of them included adult patients.10,11,19 A study in paediatric patients with CD, suggested no association of preoperative MRI with the outcome of the TSS.4 Preparing the family for the postoperative course is always a component of our team’s discussion. Encouragingly, the lack of preoperative imaging identification of a tumour did not predict long-term recurrence in our cohort when remission is achieved after surgery. This suggests that if the operation is successful and the neurosurgeon resects the tumour, the risk for long-term recurrence is similar as in patients with a visualised tumour. Of note, the incidence of recurrence and non-remission reported in this study is biased since it is mainly driven by patients referred to our centre after recurrent or difficult to treat adenomas initially operated at outside institutes, and represent that portion of patients that required further management.
Over the last two decades, advances in MRI techniques have improved their diagnostic yield and newer sequences have been tested in pituitary imaging. For example, Patronas et al.9 and Batista et al.5 reported increased sensitivity of spoiled gradient recall acquisition compared to conventional spin-echo images. Recently, Cristante et al.19 suggested a higher proportion of MRIs identifying a pituitary lesion over time. The progress of the diagnostic yield of MRI over years described in our study is interesting. We were not able to confirm a higher yield of positive results overtime, although a trend is clearly obvious in our findings, with more recent studies having only 20% rate of negative MRIs. However, it should be noted that the current study was not designed to compare MRI sequences and although the imaging evaluation of the patients was not the same in all patients, most studies, particularly those performed at the NIH, were performed with similar protocols. Our data were rather meant to describe the value of a positive or negative MRI as performed in general clinical endocrine practice and its utility in counselling patients.
There are several strengths and limitations for this study. A strength of the study is the large number of paediatric patients with CD studied, the largest cohort to our knowledge. We also have long-term follow-up evaluations for most of our patients providing sufficient data to analyse long-term recurrence risk. However, as mentioned above, MRIs were not performed under the same sequence, which constitutes a limitation. Additionally, patients were operated by different neurosurgeons over time, which may affect the outcome of TSS. For that reason, we adjusted the results for the operating neurosurgeon.
As a conclusion, we present a summary of imaging studies of patients with CD over years and the predictive utility of a positive or negative MRI in the prognosis of the patients. A negative MRI appears to signal an overall higher risk for nonremission; however, if TSS is successful in cure early on, then the long-term risk for recurrence is not dependent on the preoperative imaging findings. These results provide additional data for informative counselling of patients and their families before surgery.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
ACKNOWLEDGEMENTS
The work was supported by the Intramural Research Programs of the Eunice Kennedy Shriver National Institute of Child Health & Human Development and the National Institute of Neurological Diseases and Stroke, National Institutes of Health, Bethesda, MD, USA.
Funding information
Eunice Kennedy Shriver National Institute of Child Health and Human Development, Grant/Award Number: Intramural Research Program
CONFLICT OF INTERESTS
Dr. Stratakis holds patents on technologies involving PRKAR1A and related genes; his laboratory has received research funding support by Pfizer Inc., on growth hormone producing pituitary tumours and for work unrelated to this project.
REFERENCES
- 1.Stratakis CA. An update on Cushing syndrome in pediatrics. Ann Endocrinol. 2018;79(3):125-131. 10.1016/j.ando.2018.03.010 [DOI] [PubMed] [Google Scholar]
- 2.Lodish MB, Keil MF, Stratakis CA. Cushing’s syndrome in pediatrics: an update. Endocrinol Metab Clin North Am. 2018;47(2):451-462. 10.1016/j.ecl.2018.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keil MF, Graf J, Gokarn N, Stratakis CA. Anthropometric measures and fasting insulin levels in children before and after cure of Cushing syndrome. Clin Nutr. 2012;31(3):359-363. 10.1016/j.clnu.2011.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lonser RR, Wind JJ, Nieman LK, Weil RJ, DeVroom HL, Oldfield EH. Outcome of surgical treatment of 200 children with Cushing’s disease. J Clin Endocrinol Metab. 2013;98(3):892-901. 10.1210/jc.2012-3604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Batista D, Courkoutsakis NA, Oldfield EH, et al. Detection of adrenocorticotropin-secreting pituitary adenomas by magnetic resonance imaging in children and adolescents with Cushing disease. J Clin Endocrinol Metab. 2005;90(9):5134-5140. 10.1210/jc.2004-1778 [DOI] [PubMed] [Google Scholar]
- 6.Batista DL, Riar J, Keil M, Stratakis CA. Diagnostic tests for children who are referred for the investigation of Cushing syndrome. Pediatrics. 2007;120(3):e575-e586. 10.1542/peds.2006-2402 [DOI] [PubMed] [Google Scholar]
- 7.Chatain GP, Patronas N, Smirniotopoulos JG, et al. Potential utility of FLAIR in MRI-negative Cushing’s disease. J Neurosurg. 2018;129(3):620-628. 10.3171/2017.4.JNS17234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fukuhara N, Inoshita N, Yamaguchi-Okada M, et al. Outcomes of three-Tesla magnetic resonance imaging for the identification of pituitary adenoma in patients with Cushing’s disease. Endocr J. 2019; 66(3):259-264. 10.1507/endocrj.EJ18-0458 [DOI] [PubMed] [Google Scholar]
- 9.Patronas N, Bulakbasi N, Stratakis CA, et al. Spoiled gradient recalled acquisition in the steady state technique is superior to conventional postcontrast spin echo technique for magnetic resonance imaging detection of adrenocorticotropin-secreting pituitary tumors. J Clin Endocrinol Metab. 2003;88(4):1565-1569. 10.1210/jc.2002-021438 [DOI] [PubMed] [Google Scholar]
- 10.Sun Y, Sun Q, Fan C, et al. Diagnosis and therapy for Cushing’s disease with negative dynamic MRI finding: a single-centre experience. Clin Endocrinol. 2012;76(6):868-876. 10.1111/j.1365-2265.2011.04279.x [DOI] [PubMed] [Google Scholar]
- 11.Cebula H, Baussart B, Villa C, et al. Efficacy of endoscopic endonasal transsphenoidal surgery for Cushing’s disease in 230 patients with positive and negative MRI. Acta Neurochir. 2017;159(7):1227-1236. 10.1007/s00701-017-3140-1 [DOI] [PubMed] [Google Scholar]
- 12.Yamada S, Fukuhara N, Nishioka H, et al. Surgical management and outcomes in patients with Cushing disease with negative pituitary magnetic resonance imaging. World Neurosurg. 2012;77(3-4):525-532. 10.1016/j.wneu.2011.06.033 [DOI] [PubMed] [Google Scholar]
- 13.Nieman LK, Biller BM, Findling JW, et al. The diagnosis of Cushing’s syndrome: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2008;93(5):1526-1540. 10.1210/jc.2008-0125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tatsi C, Stratakis C. Cushing Disease: Diagnosis and Treatment. In: Kohn B, ed. Pituitary Disorders of Childhood Diagnosis and Clinical Management. Cham, Switzerland: Springer Nature Switzerland AG; 2019:89-114. [Google Scholar]
- 15.Oldfield EH, Vortmeyer AO. Development of a histological pseudocapsule and its use as a surgical capsule in the excision of pituitary tumors. J Neurosurg. 2006;104(1):7-19. 10.3171/jns.2006.104.1.7 [DOI] [PubMed] [Google Scholar]
- 16.Locatelli M, Vance ML, Laws ER. Clinical review: the strategy of immediate reoperation for transsphenoidal surgery for Cushing’s disease. J Clin Endocrinol Metab. 2005;90(9):5478-5482. 10.1210/jc.2004-2436 [DOI] [PubMed] [Google Scholar]
- 17.Ram Z, Nieman LK, Cutler GB Jr., Chrousos GP, Doppman JL, Oldfield EH. Early repeat surgery for persistent Cushing’s disease. J Neurosurg. 1994;80(1):37-45. 10.3171/jns.1994.80.1.0037 [DOI] [PubMed] [Google Scholar]
- 18.Lodish M, Dunn SV, Sinaii N, Keil MF, Stratakis CA. Recovery of the hypothalamic-pituitary-adrenal axis in children and adolescents after surgical cure of Cushing’s disease. J Clin Endocrinol Metab. 2012;97(5):1483-1491. 10.1210/jc.2011-2325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cristante J, Lefournier V, Sturm N, et al. Why we should still treat by neurosurgery patients with Cushing’s disease and a normal or inconclusive pituitary MRI. J Clin Endocrinol Metab. 2019;104(9):4101-4113. 10.1210/jc.2019-00333 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.