Abstract
Micro-abstract
The objective of this study was to examine outcomes of hormone receptor positive (HR+) HER2 non-amplified (HER2-) metastatic breast cancer (MBC), with prior exposure to everolimus, on palbociclib-based therapy. This retrospective study enrolled 23 eligible patients with HR+HER 2-MBC. Median progression free survival (PFS) was 2.9 months (95% CI 2.1–4.2), objective response rate (ORR) 0/23(0%) and clinical benefit ratio (CBR) of 4/23(17.4%). In PALOMA 3 trial, which studied the efficacy of palbociclib-based therapy in second line among everolimus naïve HR+ MBC, median PFS, ORR and CBR of palbociclib cohort were 9.5 months (95% CI 9.2–11.0), 19% and 66.5% respectively. Out study shows a limited clinical activity of palbociclib combinations after progression with everolimus combination.
Objectives
Outcome data on hormone receptor positive (HR+) HER 2 non-amplified (HER2-) metastatic breast cancer (MBC) treated with palbociclib after treatment with everolimus are lacking. The PALOMA-3 trial, showing benefit of palbociclib plus fulvestrant compared to fulvestrant alone in HR+HER2-MBC after progression on endocrine therapy excluded women previously treated with everolimus. The objective of this study was to examine outcomes of HR+HER2-MBC with prior exposure to everolimus, on palbociclib-based therapy.
Materials and Methods
This is a retrospective, single-institute review of HR+HER 2-MBC from Jan2014 to Nov2016 treated with palbociclib after prior treatment with everolimus. Progression free survival (PFS) was defined as the time from initiation of palbociclib to the date of progression as determined by treating physician based on radiological, biochemical and/or clinical criteria. Response rates were determined based on available radiological data. Objective response rate (ORR) was defined as the rate of any complete or partial responses (CR or PR); clinical benefit rate (CBR) was the rate of CR, PR or stable disease for at least 24 weeks.
Results
23 patients with mean age 68 years (42 to 81) were identified. Kaplan Meier estimate showed median PFS of 2.9 months (95% CI 2.1–4.2); ORR was 0/23(0%) and CBR was 4/23(17.4%). In PALOMA 3 trial, median PFS, ORR and CBR of palbociclib cohort were 9.5 months (95% CI 9.2–11.0), 19% and 66.5% respectively.
Conclusions
Our study shows a limited clinical activity of palbociclib combinations after progression with everolimus combination therapy. Further studies are necessary to confirm the findings.
Keywords: palbociclib, everolimus, CDK 4/6 inhibitor, mTOR inhibitor, endocrine therapy, hormone receptor positive metastatic breast cancer
Introduction
Endocrine therapy forms the backbone of anticancer therapy in hormone receptor positive (HR+), human epidermal growth factor receptor non-amplified (HER2-) metastatic breast cancer (MBC). Everolimus, an mTOR inhibitor, when combined with exemestane, a steroidal aromatase inhibitor (AI), improves progression free survival (PFS) compared to exemestane alone in HR+HER2-MBC who failed non-steroidal AI.1 Thus, everolimus in combination with exemestane has been approved for treatment of HR+HER2-MBC after failure of treatment with a non-steroidal AI. Cyclin Dependent Kinase (CDK) 4/6 inhibitors palbociclib, ribociclib, and abemaciclib each in combination with an AI have been shown to be efficacious against HR+HER2-MBC as first line of therapy and thus have been approved for treatment as an initial endocrine based therapy in such breast cancer.2–4 Similarly, the PALOMA-3 trial showed that palbociclib in combination with fulvestrant, a selective estrogen receptor degrader, was associated with significantly better progression free survival compared to fulvestrant alone in HR+HER2-MBC that has progressed on at least one line of endocrine therapy.5 This trial excluded patients who had previously received everolimus because multiple preclinical studies have suggested interactions between the PI3K/Akt/mTOR pathway and the Cyclin D/CDK4/6/Rb pathway.6–10 Currently, there is very limited clinical data on effectiveness of CDK 4/6 inhibitors in patients previously treated with mTOR inhibitors. With increasing number of therapeutic options for HR+HER2-MBC patients in both first and later lines, it is important to determine the best sequence of therapies. The objective of this study was to examine the clinical outcomes of HR+HER2-MBC patients with prior exposure to everolimus, on palbociclib-based therapy.
Methods and Materials
This study is an IRB approved, single institution retrospective review of HR+HER2-MBC patients, who received treatment at the institute from Jan 2014 – Nov 2016. Women were included if they had been treated with palbociclib-based therapy after prior treatment with everolimus. Women who received everolimus for less than 1 month or palbociclib for less than 14 days were excluded. PFS was defined as the time from the initiation of palbociclib to the date of progression as determined by treating physician based on available radiological, biochemical and/or clinical information. Best overall response rates were determined based on available radiological data. Objective response rate (ORR) was defined as the best overall response of complete response (CR) or partial response (PR). Clinical benefit rate (CBR) was defined as a CR, PR or stable disease (SD) of at least 24 weeks. Kaplan Meier estimates were used for survival analysis. R software was used for statistical analysis and survival curve generation.
Results
Out of 28 patients obtained from initial computer based query in the Electronic Medical Record, only 23 patients were eligible for the final data collection and analysis. Out of 5 patients excluded, 3 received palbociclib for less than 14 days, 1 received everolimus for less than a month and 1 was found to have a HER2 amplified MBC on repeat biopsy thus were ineligible for the study. Median age of the patients was 68 years (42 to 81) and all were females. 95% of the women were postmenopausal, and 83% had visceral metastases i.e. lung, liver, brain, pleural, or peritoneal involvement. Median estrogen receptor Allred score from the most recent biopsy prior to start of palbociclib was 8 (5–8). 95% of the women had more than 2 lines of prior endocrine therapy including both adjuvant/neo-adjuvant therapies and treatment for advanced disease. 82% women showed prior sensitivity to endocrine therapy. This was defined as documented clinical benefit from at least one previous endocrine therapy (including everolimus based therapy) in the metastatic setting or treatment with at least 24 months of adjuvant therapy before disease recurrence. Overall, 82% of women received prior chemotherapy, of which 84% were in metastatic setting. Median duration of everolimus therapy was 6 months (1.7– 34.5 months). Everolimus therapy was terminated in 78% women due to progression while in remaining patients it was stopped due to intolerance or other reasons. Median number of chemotherapy agents or hormonal therapies between everolimus and palbociclib was 1 (0–6). Table 1 compares various parameter of study cohort with the historical palbociclib cohort of the PALOMA-3 trial.
Table 1:
Patients’ Characteristics of Study Cohort as Compared to Palbociclib Cohort of PALOMA-3 Trial.
| Variables | Study Cohort | Palbociclib Cohort of PALOMA-3 |
|---|---|---|
| Population | 23 | 347 |
| Median age, range | 68 (42–81) | 57 (30–88) |
| Race | ||
| White | 20 (88%) | 252 (73%) |
| Asian | 1 (4%) | 74 (21%) |
| Black and others | 2 (8%) | 21 (6%) |
| Menopausal status | ||
| Pre or peri-menopausal | 1 (5%) | 72 (21%) |
| Post-menopausal | 22 (95%) | 275 (79%) |
| Metastasis | ||
| Visceral with or without non-visceral | 19 (83%) | 206 (59%) |
| Non-visceral only | 4 (17%) | 141 (41%) |
| Number of previous line of endocrine therapy | ||
| 1 | 0 (0%) | 60 (46%) |
| 2 | 1 (5%) | 140 (40%) |
| 3 or more | 22 (95%) | 47 (14%) |
| Previous sensitivity to endocrine therapy | ||
| No | 4 (18%) | 73 (21%) |
| Yes | 19 (82%) | 274 (79%) |
| Previous chemotherapy | ||
| Yes | 19 (82%) | 242 (70%) |
| No | 4 (18%) | 105 (30%) |
| Reason for previous chemotherapy | ||
| Adjuvant/Neo-adjuvant | 3 (16%) | 139 (40%) |
| Metastatic disease with or without perioperative | 16 (84%) | 113 (33%) |
| Median duration of Everolimus therapy (months, range) | 6 (1.7–34.5) | NAP* |
| Reason for termination of Everolimus | ||
| Progression | 18 (78%) | NA** |
| Intolerance/others | 5 (22%) | NA** |
| Systemic therapy for metastatic disease prior to palbociclib | ||
| Median (range) number of systemic therapies | 3 (2–8) | NA** |
| Median (range) number of endocrine based therapy | 1 (0–5) | NA** |
| Median (range) number of chemotherapy | 3 (2–4) | NA** |
| Median number of chemo or hormonal therapies between Everolimus and Palbociclib, range | 1 (0–6) | NAP** |
Not applicable
Not available.
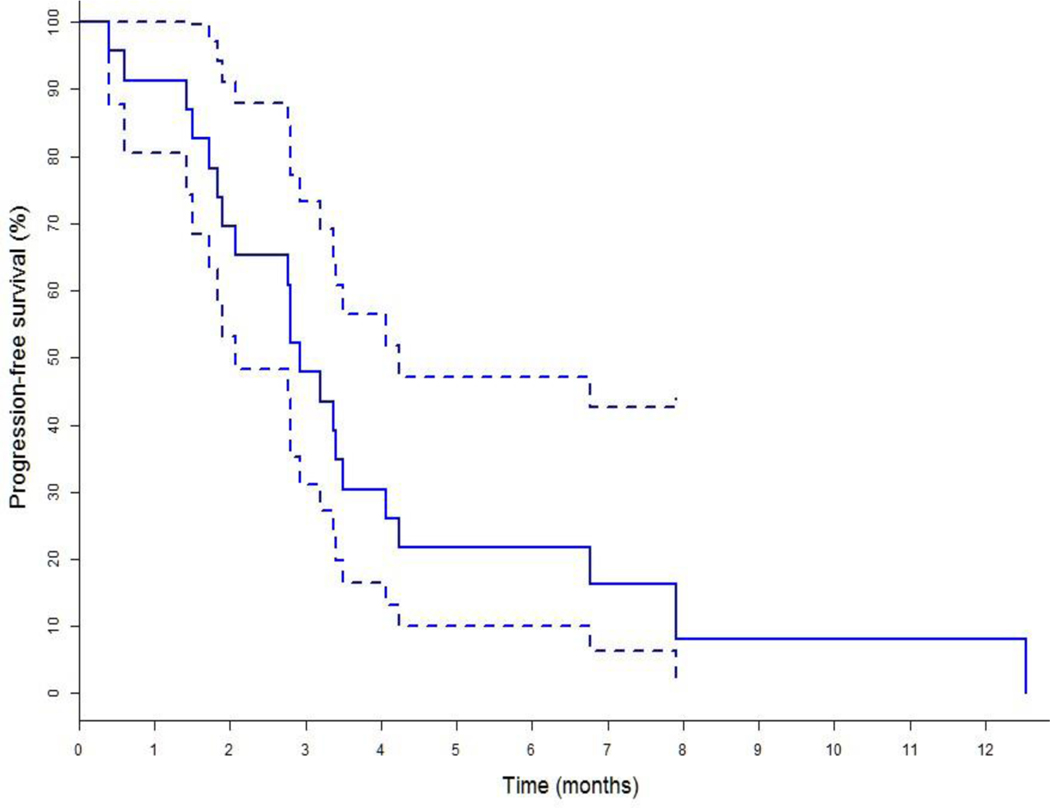
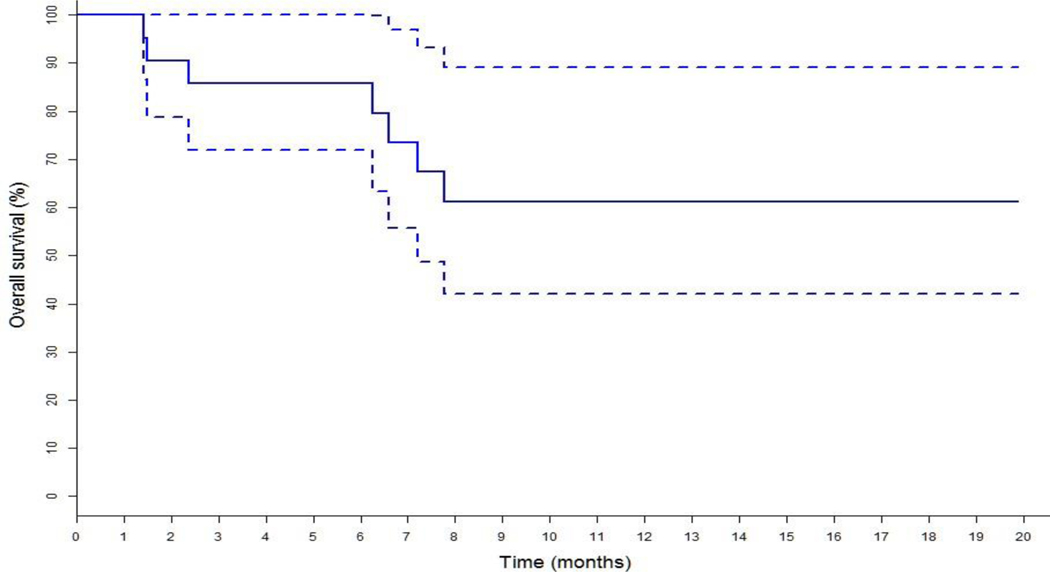
At the time of data extraction, 21 patients had progressed on palbociclib-based therapy and there were no treatment discontinuation due to toxicity. 17 patients were deemed progressed by treating physicians based on CT scan, bone scan or PET scan results while remaining 4 patients were deemed progressed based on relevant laboratory results (worsening hypercalcemia in 2 patients with extensive bone metastases, worsening liver function in 2 patients with extensive liver metastases, increasing tumor markers in all of them) and worsening clinical assessment. Kaplan Meier estimate of survival of the study cohort showed a median PFS of 2.9 months (95% CI 2.1–4.2) as shown in figure 1; ORR was 0/23(0%) and CBR 4/23(17.4%) (Table 2). Median PFS, ORR and CBR of the palbociclib cohort of the PALOMA-3 trial were 9.5 months (95% CI 9.2–11.0), 19% and 66.5% respectively.5 Median Overall Survival (OS) of the study cohort as shown in figure 2 had not been reached at the time of analysis and is > 19.8 months (95% CI 7.2–19.8).
Figure 1:
Kaplan Meier estimates for Progression Free Survival (PFS) with 95% CI of Study Cohort showing Median PFS 2.9 (95% CI 2.1–4.2) months.
Table 2:
Clinical Outcomes of Study Cohort as Compared to Palbociclib Cohort of PALOMA-3 Trial.
| Variables | Study Cohort | Palbociclib Cohort of PALOMA-3 |
|---|---|---|
| Population | 23 | 347 |
| Complete response | 0 (0%) | 0 (0%) |
| Partial Response | 0 (0%) | 66 (19%) |
| Stable disease | 5 (21.7%) | 213 (61%) |
| Progressive disease | 18 (78.2) | 58 (17%) |
| Objective tumor response | 0 (0%) | 66 (19%) |
| Clinical Benefit | 4 (17.4%) | 231 (67%) |
Figure 2:
Kaplan Meier estimate of Overall Survival (OS) with 95% Confidence Interval of Study Cohort. Median OS not reached at the time of analysis and is > 19.8 months (7.2–19.8)
Discussion
PALOMA-3 was a multi-center, double-blind, randomized phase III trial, which assessed the efficacy of palbociclib plus fulvestrant compared to fulvestrant alone in HR+HER2-MBC patients previously treated with endocrine therapy.5 Eligible patients were 18 years or older women with any menopausal status, ECOG performance status 0–1, with biopsy proven HR+HER2-MBC that had progressed on previous endocrine therapy, and had measurable or bone only metastatic diseases. Prior treatment with everolimus or a PI3K/mTOR pathway inhibitor was one of the exclusion criteria. Median progression-free survival was 9.5 months (95% CI 9.2–11.0) in the fulvestrant plus palbociclib group and 4.6 months (3.5–5.6) in the fulvestrant plus placebo group (hazard ratio 0.46, 95% CI 0.36–0.59, p<0.0001). Our study has shown that palbociclib is associated with a short PFS of 2.9 months (95% CI 2.1–4.2) and no objective response among HR+HER2-MBC with prior treatment with everolimus. The main weakness is that we are comparing our small retrospective cohort with a cohort treated under the PALOMA-3 protocol, which is a randomized phase III trial. Assessment of response was less standardized and not using RECIST criteria. Compared to the PALOMA-3, present cohort is older; less fit, has more visceral metastases, and has received both more lines of endocrine and chemotherapy in metastatic setting. Despite having a short PFS of 2.9 months, the study cohort has a median OS of more than 19.8 months, which shows that their disease continued to be responsive to further therapies and that the patients were not in their terminal stage when starting palbociclib based therapy. Finally, not all patients in the cohort received fulvestrant as the endocrine therapy component. Some of the patients received AI in combination with palbociclib due to patients’ or physicians’ preference. Fulvestrant has a different mechanism of action than AI. Data suggest that it is potentially more effective than AI in patients with ESR1 mutation, a common mutation thought to cause resistance to endocrine therapy.11 This variance adds to the limitation of the study.
Despite limitations, outcome data of palbociclib after everolimus from our study are novel and the results are hypothesis generating. A prospective nonrandomized single arm study of efficacy of palbociclib plus fulvestrant after everolimus in France showed a median PFS after palbociclib combination was 5.8 months (95% CI 3.9–7.3).12 26.7% had partial response, while 45% had stable disease as best responses. No clinical benefit rate was reported. Both our results and published results from the French study suggest that tumor acquiring resistance to the PI3K/Akt/mTOR pathway inhibition may have cross-resistance to CDK 4/6 inhibition. Multiple preclinical studies have suggested interactions between the PI3K/Akt/mTOR pathway and the Cyclin D/CDK4/6/Rb pathway. Expression of Cyclin D is controlled post-transcriptionally via the PI3K/Akt/mTOR dependent pathway.10 mTOR inhibition in various cell lines has been associated with increased activity of p16INK4a leading to decreased activity of Cyclin D/CDK 4/6 complex ultimately causing cell cycle arrest.6–9 On the other hand, CDK 4/6 inhibition has been shown to have synergistic effect in producing tumor regression in combination with PI3K inhibitor in breast cancer cell lines with otherwise intrinsic or acquired resistance to PI3K inhibitors.13 Thus, there appears to be a complex interaction between aberrations in the PI3K/Akt/mTOR and the Cyclin D/CDK 4/6 pathways causing cell proliferation and decreased apoptosis.
In recent years, everolimus, palbociclib, ribociclib and abemaciclib each in combination with an endocrine therapy have improved the outcomes of HR+HER2-MBC patients.1–5,14,15 Similarly, BOLERO 4, an open-label, phase-II study is currently investigating safety and efficacy of everolimus plus letrozole in postmenopausal women with HR+ HER2- metastatic or locally advanced breast cancer in the first line setting (NCT01698918). Other early stage clinical studies are underway, evaluating safety and efficacy of everolimus in combination with endocrine, targeted or cytotoxic chemotherapy (NCT00574366- phase I/II, erlotinib + everolimus in advanced MBC who have progressed on chemotherapy; NCT01783444- phase II, everolimus monotherapy vs. capecitabine monotherapy vs. everolimus/exemestane combination in HR+MBC after progression on AI; NCT02404051- phase III, fulvestrant followed, at progression, by exemestane + everolimus vs. exemestane + everolimus followed, at progression, by fulvestrant in HR+ HER2- locally advanced or MBC after progression on AI). As CDK 4/6 inhibitors and everolimus have emerged as the early therapeutic options for management of HR+HER2-MBC, it is important to understand how to use these drugs clinically to obtain best clinical benefit for patients. Understanding appropriate sequence of therapies in increasingly complex decision tree for treatment of these patients is necessary in order to delay emergence of resistance, enhance clinical benefit and decrease toxicities. In the future, the choices might become more difficult with results from the early stage clinical trials which are evaluating combination of mTOR inhibitor, CDK 4/6 inhibitor and an AI (NCT02871791- phase I, palbociclib + everolimus + exemestane; NCT02732119- phase I/II, ribociclib + everolimus + exemestane; NCT01857193- phase Ib, ribociclib + everolimus + exemestane). Currently there is no active effort to determine the optimal sequence of these agents leaving clinicians to make choices based on limited data and toxicity profiles. We designed our study to investigate the clinical outcome of CDK 4/6 inhibitor- palbociclib in HR+HER2-MBC after they have been treated with everolimus based therapy.
Conclusion
This study has shown that palbociclib based therapy is associated with a short PFS and poor response rate in HR+HER2- heavily pretreated MBCs with prior treatment with everolimus The results from a recent French study also report short PFS with palbociclib combinations after progression on everolimus combination Both results show PFS shorter than reported in PALOMA-3. These results suggest that there might be limited clinical benefit with palbociclib combination after previous treatment with everolimus combination. With small cohort it is unclear if some patients continue to receive significant benefit from this therapy. Larger prospective studies comparing different sequence of therapies should be conducted to identify biomarkers and define optimal sequences of therapy in this patient population.
Clinical Practice Points
PALOMA 3 trial is a phase III multicenter study that showed significant clinical benefit of palbociclib-based therapy in second line among HR+ HER2- everolimus naïve MBC. As therapy options have increased for HR+ MBCs, it is important to find an optimal sequence of therapies. We performed a retrospective study to assess the clinical outcome of palbociclib-based therapy among HR+ MBCs with prior everolimus treatment. With palbociclib combinations, median PFS was short and ORR and CBR were poor in our cohort. This result suggests that palbociclib-based therapy is associated with limited clinical outcomes among heavily pretreated, everolimus exposed HR+ MBCs and supports the current practice of using palbociclib-based therapy prior to everolimus in the sequence. Further studies are required to confirm and build on this finding.
Acknowledgement
Clinical Data Network (CDN), Roswell Park Cancer Institute Shared Resource
Funding Information
This work was supported by National Cancer Institute (NCI) grant P30CA016056 involving the use of Roswell Park Cancer Institute’s Pathology Network, Genomic, and Clinical Data Network Shared Resources
Footnotes
Ethical Standard
This study was approved by Institutional Review Board, Roswell Park Cancer Institute. This research did not involve animals or human participants. This research was a retrospective chart review.
Declaration of Conflict of Interest
On behalf of all the authors of this manuscript, I, Ajay Dhakal, the corresponding author wish to draw the attention of the Editor to the following facts which may be considered as potential conflicts of interest and to significant financial contributions to this work:
Mateusz Opyrchal has received research fund from Pfizer/NCCN. All other authors declare that they have no conflict of interest.
I confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. I further confirm that the order of authors listed in the manuscript has been approved by all of authors.
I confirm that we have given due consideration to the protection of intellectual property associated with this work and that there are no impediments to publication, including the timing of publication, with respect to intellectual property. In so doing I confirm that we have followed the regulations of our institutions concerning intellectual property.
I further confirm that any aspect of the work covered in this manuscript that has involved either experimental animals or human patients has been conducted with the ethical approval of all relevant bodies and that such approvals are acknowledged within the manuscript.
Thank you.
Ajay Dhakal MBBS
Prior Publication
None. Abstract presented at ASCO annual meeting, 2017.
Conflict of Interest
Mateusz Opyrchal has received research fund from Pfizer/NCCN. All other authors declare that they have no conflict of interest.
References
- 1.Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366(6):520–529. doi: 10.1056/NEJMoa1109653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Finn RS, Crown JP, Lang I, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 2015;16(1):25–35. doi: 10.1016/S1470-2045(14)71159-3 [DOI] [PubMed] [Google Scholar]
- 3.Hortobagyi GN, Stemmer SM, Burris HA, et al. Ribociclib as First-Line Therapy for HR-Positive, Advanced Breast Cancer. N Engl J Med. 2016;375(18):1738–1748. doi: 10.1056/NEJMoa1609709 [DOI] [PubMed] [Google Scholar]
- 4.Goetz MP, Toi M, Campone M, et al. MONARCH 3: Abemaciclib As Initial Therapy for Advanced Breast Cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2017;35(32):3638–3646. doi: 10.1200/JCO.2017.75.6155 [DOI] [PubMed] [Google Scholar]
- 5.Cristofanilli M, Turner NC, Bondarenko I, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016;17(4):425–439. doi: 10.1016/S1470-2045(15)00613-0 [DOI] [PubMed] [Google Scholar]
- 6.Albers MW, Williams RT, Brown EJ, Tanaka A, Hall FL, Schreiber SL. FKBP-rapamycin inhibits a cyclin-dependent kinase activity and a cyclin D1-Cdk association in early G1 of an osteosarcoma cell line. J Biol Chem. 1993;268(30):22825–22829. [PubMed] [Google Scholar]
- 7.Gao N, Flynn DC, Zhang Z, et al. G1 cell cycle progression and the expression of G1 cyclins are regulated by PI3K/AKT/mTOR/p70S6K1 signaling in human ovarian cancer cells. Am J Physiol Cell Physiol. 2004;287(2):C281–291. doi: 10.1152/ajpcell.00422.2003 [DOI] [PubMed] [Google Scholar]
- 8.Grewe M, Gansauge F, Schmid RM, Adler G, Seufferlein T. Regulation of cell growth and cyclin D1 expression by the constitutively active FRAP-p70s6K pathway in human pancreatic cancer cells. Cancer Res. 1999;59(15):3581–3587. [PubMed] [Google Scholar]
- 9.Sabatini DM. mTOR and cancer: insights into a complex relationship. Nat Rev Cancer. 2006;6(9):729–734. doi: 10.1038/nrc1974 [DOI] [PubMed] [Google Scholar]
- 10.Muise-Helmericks RC, Grimes HL, Bellacosa A, Malstrom SE, Tsichlis PN, Rosen N. Cyclin D expression is controlled post-transcriptionally via a phosphatidylinositol 3-kinase/Akt-dependent pathway. J Biol Chem. 1998;273(45):29864–29872. [DOI] [PubMed] [Google Scholar]
- 11.Fribbens C, O’Leary B, Kilburn L, et al. Plasma ESR1 Mutations and the Treatment of Estrogen Receptor-Positive Advanced Breast Cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2016;34(25):2961–2968. doi: 10.1200/JCO.2016.67.3061 [DOI] [PubMed] [Google Scholar]
- 12.du Rusquec P, Palpacuer C, Campion L, et al. Efficacy of palbociclib plus fulvestrant after everolimus in hormone receptor-positive metastatic breast cancer. Breast Cancer Res Treat. December 2017. doi: 10.1007/s10549-017-4623-8 [DOI] [PubMed] [Google Scholar]
- 13.Vora SR, Juric D, Kim N, et al. CDK 4/6 inhibitors sensitize PIK3CA mutant breast cancer to PI3K inhibitors. Cancer Cell. 2014;26(1):136–149. doi: 10.1016/j.ccr.2014.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fasching PA, Jerusalem GHM, Pivot X, et al. Phase III study of ribociclib (LEE011) plus fulvestrant for the treatment of postmenopausal patients with hormone receptor-positive (HR+), human epidermal growth factor receptor 2-negative (HER2–) advanced breast cancer (aBC) who have received no or only one line of prior endocrine treatment (ET): MONALEESA-3. J Clin Oncol. 2016;34(15_suppl):TPS624–TPS624. doi: 10.1200/JCO.2016.34.15_suppl.TPS624 [DOI] [Google Scholar]
- 15.Sledge GW, Toi M, Neven P, et al. MONARCH 2: Abemaciclib in Combination With Fulvestrant in Women With HR+/HER2- Advanced Breast Cancer Who Had Progressed While Receiving Endocrine Therapy. J Clin Oncol Off J Am Soc Clin Oncol. June 2017:JCO2017737585. doi: 10.1200/JCO.2017.73.7585 [DOI] [PubMed] [Google Scholar]