Abstract
Background:
The usefulness of thiopurines has been poorly explored in pouchitis and other pouch disorders.
Objective:
To evaluate the effectiveness and safety of azathioprine as maintenance therapy in inflammatory pouch disorders.
Design:
This was a retrospective and multicentre study.
Methods:
We included patients diagnosed with inflammatory pouch disorders treated with azathioprine in monotherapy. Effectiveness was evaluated at 1 year and in the long term based on normalization of stool frequency, absence of pain, faecal urgency or fistula discharge (clinical remission), or any improvement in these symptoms (clinical response). Endoscopic response was evaluated using the Pouchitis Disease Activity Index (PDAI).
Results:
In all, 63 patients were included [54% males; median age, 49 (28–77) years]. The therapy was used to treat pouchitis (n = 37) or Crohn’s disease of the pouch (n = 26). The rate of clinical response, remission and non-response at 12 months were 52%, 30% and 18%, respectively. After a median follow-up of 23 months (interquartile range 11–55), 19 patients (30%) were in clinical remission, and 45 (66%) stopped therapy. Endoscopic changes were evaluated in 19 cases. PDAI score decreased from 3 (range 2–4) to 1 (range 0–3). In all, 21 patients (33%) presented adverse events and 16 (25%) needed to stop therapy.
Conclusion:
Azathioprine may be effective in the long term for the treatment of inflammatory pouch disorders and could be included as a therapeutic option.
Keywords: azathioprine, immunosuppressants, pouchitis, thiopurines
Introduction
Restorative proctocolectomy with ileal pouch–anal anastomosis remains the gold-standard procedure for the surgical treatment of ulcerative colitis (UC).1,2 Pouchitis is a non-specific inflammatory disorder that affects some pouch patients. While its aetiology remains unknown, it has been associated with risk factors such as the previous presence of inflammatory bowel disease (IBD).3–5 In fact, pouchitis is the most frequent non-mechanical complication in pouch patients with UC [prevalence, 29% (8–41%)].4–8
Clinical manifestations include increased stool frequency, faecal urgency, bloody diarrhoea and abdominal pain. Pouchitis can manifest as an acute, recurrent or chronic disease that interferes with quality of life. 7 Inflammatory pouch disorders other than pouchitis, such as cuffitis and Crohn’s disease (CD) of the pouch, may also appear.8–10 Cuffitis involves inflammation of the rectal cuff and resembles ulcerative proctitis. 11 CD of the pouch is a heterogeneous entity that includes inflammation of the pouch and/or the afferent ileal limb and may give rise to complications such as the stricturing and fistulizing phenotypes beyond 6–12 months after pouch surgery.12,13 CD of the pouch could be present in up to 25% of pouch patients.13–16
The therapeutic approach to inflammatory pouch disorders has been poorly explored, and many recommendations are based on non-controlled studies.17–20 The recommended treatment of pouchitis, cuffitis and CD of the pouch includes antibiotics,21–23 mesalamine, oral budesonide 24 and biological therapy.25–29 However, the absence of studies on pouchitis and other inflammatory pouch disorders treated with immunosuppressants such as azathioprine means that these drugs are not included in the therapeutic arsenal despite their known effectiveness and role as steroid-sparing agents in IBD. Given the unmet needs in the management of inflammatory pouch disorders, it would be interesting to know the role of azathioprine in this scenario.
Therefore, the main objective of this study was to assess the long-term effectiveness of azathioprine prescribed for pouch inflammatory disorder treatment. We also assessed endoscopic findings, duration of treatment, safety and long-term outcomes.
Methods
Study design and endpoints
This was an observational, retrospective and multicentre nationwide study undertaken by the Young Members Group of the Spanish Working Group on Crohn’s Disease and Ulcerative Colitis (GETECCU).
We selectively identified pouch patients aged 18 years or older who had undergone surgery between 1995 and 2020. All patients had been diagnosed with UC (resection specimen with compatible histology) and subsequently with inflammatory pouch disorders (pouchitis, CD of the pouch or cuffitis) following the diagnostic criteria of the European Crohn’s and Colitis Organization, GETECCU and the International Ileal Pouch Consortium (IIPC).9,10,15 The conditions were being treated with azathioprine in monotherapy based on clinical decisions. Pouchitis was classified according to the criteria of the IIPC (acute, chronic, recurrent or antibiotic-responsive). 15 We excluded patients who received these therapies for other clinical indications (e.g. extraintestinal manifestations or as part of a solid organ transplant immunosuppressant regimen), patients who were taking azathioprine in combination with biological therapy and patients who were lost to follow-up after initiation of therapy.
The demographic and clinical characteristics of the patients were recorded based on the Montreal and the IIPC classification. Perianal fistulas, strictures and penetrating complications were recorded if they appeared 6–12 months after stoma closure. We also recorded the presence of extraintestinal manifestations (articular, ocular, cutaneous, hepatic and other).15,30 Prior treatments and the indication for azathioprine therapy were also collected.
Participants were followed until withdrawal of therapy or their last clinical visit.
The reporting of this study conforms to the Strengthening the Reporting of Observational Studies in Epidemiology statement. 31
Definitions
Effectiveness was evaluated using clinical definitions. Clinical remission was defined as the normalization of stool frequency (this was the recovery of basal stool frequency) with the absence of abdominal pain, rectal bleeding, faecal urgency or cessation of fistula drainage. Clinical response was defined as any improvement in these parameters (over clinical worsening) without remission. Non-response was defined as no change in symptoms.25–29 This evaluation was used for pouchitis and CD of the pouch. We only considered that patients had experienced a clinical response and remission provided they were not receiving concomitant steroid therapy. Changes in endoscopic activity were evaluated (only for patients with baseline endoscopy assessment) using the endoscopic sub-score of the modified Pouchitis Disease Activity Index (PDAI). 32
Effectiveness was evaluated at 12 months and to the maximum follow-up. Treatment was discontinued in the case of non-response, loss of remission/response, adverse events, maintained remission and patient decision.
Finally, we also analysed the persistence of therapy defined as the time under active treatment.
Statistical analysis
In the descriptive analysis, categorical variables were expressed as absolute and relative frequencies. Quantitative variables were expressed as the mean and standard deviation (SD) or as the median and interquartile range (IQR) when they were not normally distributed. In the univariate analysis, categorical variables were compared using the chi-square test, and quantitative variables were compared using the appropriate test depending on the normality of the distribution. Factors that were found to be significantly associated with clinical response and remission were further explored in a multivariate logistic regression analysis with the odds ratio and 95% confidence interval. Persistence of therapy was assessed by the Kaplan–Meier method whereby patients in whom therapy was discontinued for any reason were right-censored at the time of discontinuation. Variables were included in the analysis if their p value was <0.1. Statistical significance was set at p < 0.05 for the rest of the statistical analysis. The analysis was performed using IBM SPSS Statistics for Windows, Version 24.
Results
Patient baseline characteristics
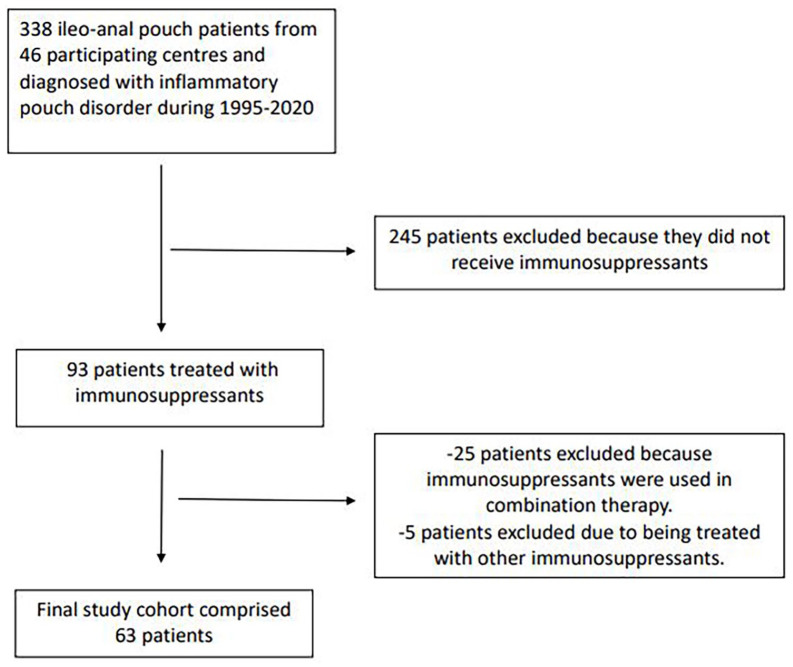
The cohort of the RESERVO study comprised 338 patients with inflammatory pouch disorder from 46 centres in Spain. A total of 93 patients from this cohort (27%) were treated with immunosuppressants. We excluded 25 patients who started immunosuppressants as combination therapy with biologics and 5 treated with other immunosuppressants (cyclosporine, methotrexate and tacrolimus). After applying the inclusion/exclusion criteria, the final study cohort consisted of 63 patients (Figure 1).
Figure 1.
Flowchart of population included.
The main demographic and disease-related characteristics of the study patients are provided in Table 1. Before therapy, 59 (94%) of participants had received antibiotics; of these, 65% were refractory or dependent. In all, 28 (46%) patients received probiotics (failure in 43%, recurrence in 13%), 37 (64%) rectal and/or oral mesalamine (38% without response) and 30 (50%) oral and/or topical steroids (29% were refractory, 42% developed dependence and 2 stopped due to adverse events). No patients received biological therapy before azathioprine for the treatment of their pouch disorders.
Table 1.
Demographic and disease-related characteristics of patients treated with azathioprine.
| Basal characteristics | Patients, N = 63 (%) |
|---|---|
| Male sex | 34 (54) |
| Age (median, range) | 49 (28–77) |
| Age at colectomy (median, range) | 31 (18–59) |
| Smoking (never/current/former) | 49/4/10 (78/6/16) |
| Indication for colectomy | Failure of medical treatment, 37 (59) |
| Acute severe flare, 22 (35) | |
| Dysplasia cancer, 3 (5) | |
| Lower gastrointestinal bleeding, 1 (2) | |
| J-type ileal pouch–anal anastomosis | 63 (100) |
| Inflammatory pouch disorder | Pouchitis, 37 (59) |
| Crohn’s disease pouch, 26 (41) | |
| Months between ileostomy closure and pouch disorder (median, range) | 7 (0–72) |
| Classification of pouchitis (n = 37)* | Acute, 4 (11) |
| Chronic, 33 (89) | |
| Recurrent, 36 (97) | |
| Antibiotic dependent, 10 (28) | |
| Antibiotic refractory, 16 (44) | |
| Location of Crohn’s disease (n = 26) | Pouch, 22 (85) |
| Ileal pre-pouch, 25 (96) | |
| Upper disease, 4 (15) | |
| Disease behaviour (Montreal B1/B2/B3) | 18/6/2 (69/23/8) |
| Perianal disease | 8 (31) |
| Extraintestinal manifestations | 19 (30) |
| Primary sclerosing cholangitis | 3 (5) |
| Optimal azathioprine dose (2.5 mg/kg) | 63 (100) |
Acute (<4 weeks of symptoms), chronic (⩾4 weeks of symptoms), recurrent and antibiotic dependent (⩾3–4 episodes per year). 15
B1, inflammatory; B2, stricturing; B3, penetrating.
Treatment characteristics
The indications for azathioprine therapy were a CD of the pouch (25, 40%), recurrent pouchitis (20, 32%) and antibiotic-refractory pouchitis (17, 27%). None of the patients who received this treatment presented cuffitis.
As previously mentioned, 50% of patients had previously been exposed to steroids, and they experienced refractoriness or dependence in 29% and 42%, respectively. Therapy was started at a median of 23 months (IQR 7–190 months) after the diagnosis of pouch disorder and this was longer for patients diagnosed with CD of the pouch (median, 89 months, IQR 25–205, difference 66 months, IQR 15–112 (p = 0.002).
Effectiveness of azathioprine
A total of 63 patients received azathioprine as monotherapy. The standard dose used was 2.5 mg/kg/day. At 12 months, 19 patients (30%) were in clinical remission and 33 (52%) had achieved a clinical response. In all, 11 patients (18%) achieved no response.
No differences in clinical response, clinical remission or non-response at 1 year were detected for various indications (recurrent pouchitis versus antibiotic-refractory pouchitis versus CD of the pouch). The only factor associated with clinical response and/or remission in the univariate analysis was steroid-dependent disease as the indication for azathioprine (85% versus 15%, p = 0.01) (Table 2). Therefore, the multivariable analysis was not performed.
Table 2.
Factors associated with clinical response and remission at 1 year: results of univariate analysis.
| Variables | Clinical remission/response (%) | OR (95% CI) | p Value | |
|---|---|---|---|---|
| Yes | No | |||
| Smoking (active smoker) | 5 (3.2) | 1 (0) | 3.6 (0.4–32) | 0.247 |
| Extraintestinal manifestations | 13 (19) | 6 (9.5) | 1.17 (0.5–2.9) | 0.578 |
| Pouch disorder diagnosed | ||||
| Pouchitis | 24 (65) | 13 (35) | 0.7 (0.3–1.7) | 0.306 |
| Crohn’s disease | 14 (54) | 12 (46) | ||
| Behaviour of Crohn’s | ||||
| B1 | 7 (39) | 9 (56) | 0.25 (0.1–1.7) | 0.184 |
| B2–3 | 5 (50) | 5 (50) | ||
| Perianal disease | 6 (67) | 3 (33) | 1.6 (0.4–6.6) | 0.391 |
| Pouch disorder with an indication for therapy: | ||||
| Recurrent pouchitis | 13 (62) | 8 (38) | 0.92 (0.3–2.7) | 0.792 |
| Antibiotic-refractory pouchitis | 11 (65) | 6 (35) | ||
| Previous steroid use | 18 (56) | 14 (44) | 0.8 (0.3–1.9) | 0.486 |
| Steroid dependency | 11 (85) | 2 (15) | 6.13 (1.4–26) | 0.01 |
In long term, after a median follow-up of 23 months (IQR 11–55) from the start of azathioprine, 19 patients (30%) were in clinical remission, 21 (33%) had achieved a clinical response and 12 (19%) who had initially experienced a clinical response/remission lost their response.
Finally, the persistence of azathioprine is represented in Figure 2. The percentage of patients who maintained treatment was 66%, 59% and 48% at 1, 2 and 3 years.
Figure 2.
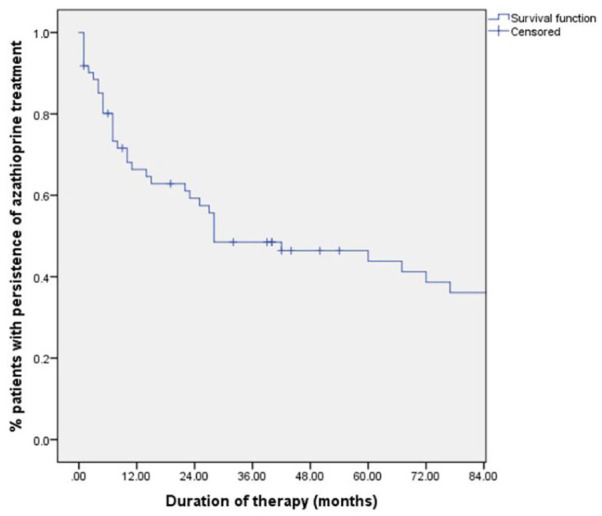
Persistence of azathioprine, survival analysis.
A total of 42 (66%) patients stopped therapy due to failure [non-response/remission or loss of response; 20 (47.6%)], adverse events (16, 38%) and sustained remission (6, 14%).
Re-evaluation of endoscopic activity
Endoscopic activity was re-evaluated in 19 patients (30%). After a median 9 months of endoscopic follow-up, the median PDAI endoscopic sub-score dropped from 3 (range 2–5) to 1 (range 0–5) (p = 0.020) (Figure 3). Histological data were not systematically obtained and could not be analysed.
Figure 3.
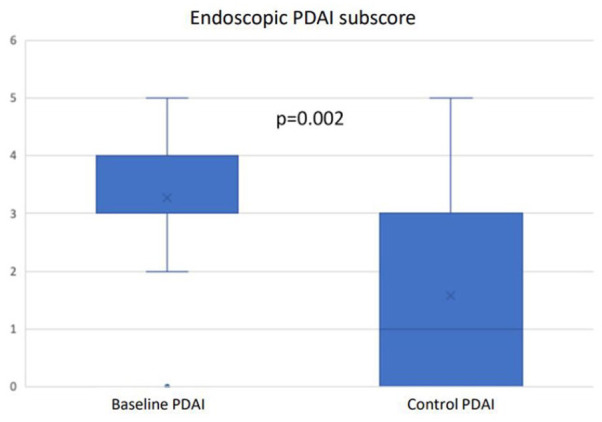
Endoscopic activity changes.
Safety
A total of 21 patients (33%) experienced adverse events secondary to azathioprine (Table 3). Finally, 16 (25%) patients were forced to stop therapy due to adverse effects.
Table 3.
Adverse events associated with azathioprine (N = 63).
| Adverse event | n (%) |
|---|---|
| Myelotoxicity | 8 (12.7) |
| Digestive intolerance | 4 (6.3) |
| Fever | 1 (1.6) |
| Neoplasm* | 2 (3) |
| Acute pancreatitis | 1 (1.6) |
| Liver toxicity | 2 (3) |
| Systemic CMV infection | 1 (1.6) |
| Other | 2 (3) |
Ovarian neoplasm, leukaemia.CMV, citomegalovirus.
Duration and outcomes
The median follow-up was 23 months (IQR 11–55). A total of 42 patients (66%) suspended therapy due to failure (20, 32%), adverse events (16, 25%) and sustained remission (6, 9%). After treatment withdrawal, 6 patients received another immunosuppressant, and 36 patients started biological therapy. Nevertheless, 22 patients needed pouch surgery owing to treatment refractoriness and, finally, 16 required a permanent ileostomy.
Discussion
To our knowledge, this nationwide study comprises the largest series to date of pouch patients with IBD and subsequent inflammatory pouch disorders (mainly chronic pouchitis) treated with azathioprine. One in four patients from the RESERVO study used this drug as a therapeutic option, even before biological therapy, resulting in an effective long-term strategy in around two-thirds of patients. However, half of them interrupted therapy for several reasons.
Various pharmacologic options are available for the management of inflammatory pouch disorders. However, published data are often scarce, and the strongest recommendations are based on antibiotic and/or biological therapy.9,18,19 Although immunosuppressants such as thiopurines and methotrexate have proven effective in the management of CD and UC, these drugs have been poorly explored in the case of pouch disorders, although they may be more widely used than previously thought. 33 To date, only two studies have evaluated the effectiveness of thiopurines in CD of the pouch (two case reports and a short clinical series of eight patients).34,35 Based on this evidence, GETECCU 18 recommended against the use of immunosuppressants in the case of chronic refractory pouchitis. However, clinical experience with thiopurines has been reported, since many studies that have evaluated the effectiveness of biological therapy in pouch disorders included patients previously exposed to thiopurines.25,26,28,29,36 Consensus guidelines from the IIPC are also based on limited evidence in favour of immunosuppressants for chronic pouchitis and CD of the pouch; nevertheless, the authors suggest the potential use of immunosuppressants (thiopurines) as monotherapy with a low grade of evidence, supported mainly by expert opinion. 19 This is supported as thiopurines are used in IBD, an entity that shares many characteristics with pouch inflammatory disorders. 37
Our results show that azathioprine seems an interesting additional option in patients with inflammatory pouch disorders even in the biological era (in this series, more than 60% of patients received this treatment after 2010, data not shown). We found this therapy was associated with clinical response and remission during the first year in more than 80% of cases and with clinical remission in the long term in 30% of cases. The profile of patients in this study includes those whose previous treatment (antibiotics, mesalamine, probiotics and steroids) had failed. It is important to highlight that azathioprine was chosen as a therapeutic option first than biological therapy. This was a clinical decision and it could be due to several reasons such as clinical experience with thiopurines or the scarce and limited evidence of biological therapy in previous decades among others.
Azathioprine was especially useful for previously steroid-dependent patients, with 84% long-term steroid-free clinical remission and response. However, two out of three patients needed to stop therapy in the long term owing to non-response (32%) or adverse events (25%), and more than half switched to biological therapy.
Our findings lead us to ask how to include and position thiopurines in the treatment algorithm of inflammatory pouch disorders. However, thiopurines are not an appropriate choice for inducing remission. Biological therapy is a good therapeutic option in patients with chronic refractory pouchitis and CD of the pouch.25–29,33,36,38 One meta-analysis reported that clinical remission after induction of anti-TNF agents was more common in CD than in chronic pouchitis (64% versus 10%). 27 Nonetheless, our data show that they would be a useful alternative long-term option, especially for patients with previous exposure to steroids who develop dependence (85% versus 15%, p = 0.01). Considering effectiveness and safety data, the different maintenance therapeutic options should be discussed and balanced with the patient.
The patients in our cohort achieved similar clinical benefits with azathioprine, irrespective of the type of inflammatory pouch disorder [chronic recurrent (62%), refractory (64%) and CD of the pouch (60%)]. In their single-centre study of 21 patients with CD of the pouch, Haveran et al. reported infliximab and/or azathioprine to be effective. 34 Eight patients received thiopurines in monotherapy, and the success of treatment was defined as complete resolution of or significant improvement in symptoms without requiring ileostomy. Treatment proved successful for all patients after a median of 38 months. This excellent result may be because physicians selected patients who received thiopurines in monotherapy instead of biological therapy or, even, combination therapy.
Finally, safety is one of the most relevant factors in therapy with azathioprine. In our study, 33% of patients presented adverse events, mainly affecting the haematological and digestive systems. More than three out of four were forced to stop therapy because of the event. These results resembled those reported in the literature and could limit the use of this therapy in many cases. 39 However, there are several strategies to optimize the management of azathioprine (some of them to improve tolerance) that have not been analysed in this study and could related to the high rate of discontinuation. We did not evaluate whether patients were exposed to azathioprine before colectomy or whether they had tolerated treatment previously.
This study is subject to a series of limitations. First, the design was retrospective and the number of patients included was limited to obtain firm conclusions. Nevertheless, pouchitis and other pouch inflammatory disorders are uncommon diseases within IBD. Second, baseline clinical activity and effectiveness were not evaluated using scores such as the PDAI or modified PDAI: the retrospective nature of this study meant that we used a less standardised definition, as analysed in many previously published studies.25–29 Moreover, none of these activity indices have previously been validated. A recent expert consensus group used a RAND/UCLA process to help clinicians and investigators assess the activity of pouchitis based on clinical factors such as stool frequency, faecal urgency and endoscopic and histological activity. 40 In our cohort, 19 patients (30%) were also evaluated using endoscopy, which revealed a decrease in the PDAI sub-score. On the other hand, ours is the first publication to date to report the effectiveness of azathioprine in inflammatory pouch disorders and to include a relevant number of patients belonging to a large multicentre and nationwide cohort affected by a rare condition within IBD. Furthermore, it enabled us to explore future options for this complex scenario.
In conclusion, the therapeutic arsenal for inflammatory pouch disorders remains open to various pharmacologic options. Azathioprine would also be a feasible maintenance therapeutic option for these disorders, especially in the case of steroid dependence. Further studies may confirm our findings and position these old but effective drugs appropriately.
Acknowledgments
The authors thank the Spanish Working Group on Crohn’s Disease and Ulcerative Colitis (GETECCU) for sponsoring this study.
Footnotes
ORCID iDs: Francisco Mesonero  https://orcid.org/0000-0002-7864-8187
https://orcid.org/0000-0002-7864-8187
María José García  https://orcid.org/0000-0002-6517-7005
https://orcid.org/0000-0002-6517-7005
Margalida Calafat  https://orcid.org/0000-0003-2335-3792
https://orcid.org/0000-0003-2335-3792
Laura Ramos  https://orcid.org/0000-0001-7015-1742
https://orcid.org/0000-0001-7015-1742
Contributor Information
Francisco Mesonero, Inflammatory Bowel Disease Unit, Department of Gastroenterology and Hepatology, Hospital Universitario Ramón y Cajal, Cra. Colmenar km 9.1, Madrid 28034, Spain.
Yamile Zabana, Hospital Universitario Mútua Terrassa, Terrassa, Spain; Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas (CIBERehd), Madrid, Spain.
Agnès Fernández-Clotet, Hospital Clínic Barcelona, Institut d’Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS), Barcelona, Spain; Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas (CIBERehd), Madrid, Spain.
Eduardo Leo-Carnerero, Hospital Universitario Virgen del Rocío, Sevilla, Spain.
Berta Caballol, Hospital Clínic Barcelona, Institut d’Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS), Barcelona, Spain; Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas (CIBERehd), Madrid, Spain.
Andrea Núñez-Ortiz, Hospital Universitario Virgen del Rocío, Sevilla, Spain.
María José García, Hospital Universitario Marqués de Valdecilla, IDIVAL, Santander, Spain.
Federico Bertoletti, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain.
Alejandro Mínguez, Hospital Universitario y Politécnico La Fe, Valencia, Spain.
Gerard Suris, Hospital Universitario de Bellvitge, Barcelona, Spain.
Begoña Casis, Hospital Universitario 12 de Octubre, Madrid, Spain.
Rocío Ferreiro-Iglesias, Hospital Clínico Universitario Santiago, Santiago de Compostela, Spain.
Margalida Calafat, Hospital Universitario Germans Trias i Pujol, Badalona, Spain; Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas (CIBERehd), Madrid, Spain.
Itxaso Jiménez, Hospital Universitario de Galdakao, Biocruces Bizkaia Health Research Institute, Galdakao, Spain.
José Miranda-Bautista, Hospital Universitario Gregorio Marañón, Madrid, Spain.
Luis Javier Lamuela, Hospital Universitario Miguel Servet, Zaragoza, Spain.
Ingrid Fajardo, Hospital Universitario Mútua Terrassa, Terrassa, Spain.
Leyanira Torrealba, Hospital Universitario Doctor Josep Trueta, Girona, Spain.
Rodrigo Nájera, Hospital Universitario Río Hortega, Valladolid, Spain.
Rosa María Sáiz-Chumillas, Hospital Universitario Burgos, Burgos, Spain.
Irene González, Hospital Universitario Puerta de Hierro, Madrid, Spain.
Miren Vicuña, Complejo Hospitalario Navarra, Pamplona, Spain.
Natalia García-Morales, Hospital Álvaro Cunqueiro, Vigo, Spain.
Ana Gutiérrez, Hospital General Universitario Alicante Doctor Balmis (Alicante), Instituto de Investigación Sanitaria y Biomédica de Alicante (ISABIAL), Alicante, Spain; Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas (CIBERehd), Madrid, Spain.
Alicia López-García, Hospital del Mar i Institut Mar d’Investigacions Mediques (IMIM), Barcelona, Spain.
José Manuel Benítez, Hospital Universitario Reina Sofía, Córdoba, Spain.
Cristina Rubín de Célix, Hospital Universitario de La Princesa, Instituto de Investigación Sanitaria Princesa (IIS-Princesa), Universidad Autónoma de Madrid (UAM), Madrid, Spain; Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas (CIBERehd), Madrid, Spain.
Coral Tejido, Complejo Hospitalario Universitario Ourense, Ourense, Spain.
Eduard Brunet, Hospital Universitari Parc Taulí, Sabadell, Spain Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas (CIBERehd), Madrid, Spain.
Alejandro Hernández-Camba, Hospital Universitario Nuestra Señora de Candelaria, Santa Cruz, Spain.
Cristina Suárez, Hospital Universitario La Paz, Instituto de Investigación Sanitaria del Hospital Universitario La Paz (IdiPAZ), Madrid, Spain.
Iago Rodríguez-Lago, Hospital Universitario de Galdakao, Biocruces Bizkaia Health Research Institute, Galdakao, Spain.
Marta Piqueras, Consorcio Sanitario Terrassa, Terrassa, Spain.
Andrés Castaño, Hospital Universitario Central Asturias, Oviedo, Spain.
Laura Ramos, Hospital Universitario de Canarias, Santa Cruz, Spain.
Ana Sobrino, Hospital General Universitario Ciudad Real, Ciudad Real, Spain.
María Carmen Rodríguez-Grau, Hospital Universitario del Henares, Coslada, Spain.
Alfonso Elosua, Hospital García Orcoyen, Estella, Spain.
Miguel Montoro, Hospital General Universitario San Jorge, Huesca, Spain.
Ruth Baltar, Hospital Universitario Álava, Vitoria, Spain.
José María Huguet, Hospital General Universitario Valencia, Valencia, Spain.
Benito Hermida, Hospital Universitario Cabueñes, Gijón, Spain.
Antonio Caballero-Mateos, Hospital Santa Ana Motril, Motril, Spain.
Luis Sánchez-Guillén, Hospital General Universitario Elche, Elche, Spain.
Abdel Bouhmidi, Hospital Santa Bárbara, Puertollano, Spain.
Ramón Pajares, Hospital Universitario Infanta Sofía, San Sebastián de los Reyes, Spain.
Iria Baston-Rey, Hospital Clínico Universitario Santiago, Santiago de Compostela, Spain.
Antonio López-Sanromán, Hospital Universitario Ramón y Cajal, Madrid, Spain.
Agustin Albillos, Hospital Universitario Ramón y Cajal, Madrid, Spain.
Manuel Barreiro-de Acosta, Hospital Clínico Universitario Santiago, Santiago de Compostela, Spain.
Declarations
Ethics approval and consent to participate: The study design and its procedures were approved by the Clinical Research Ethics Committee of the steering centre, Hospital Universitario Ramón y Cajal (Madrid, Spain) with approval number 156/20 and the research committee of GETECCU. Written informed consent to participate in the study was obtained from all patients.
Consent for publication: All participants reviewed and approved the manuscript and gave their consent for publication.
Author contributions: Francisco Mesonero: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Resources; Software; Writing – original draft; Writing – review & editing.
Yamile Zabana: Formal analysis; Methodology; Project administration; Supervision; Validation; Writing – review & editing.
Agnès Fernández-Clotet: Data curation; Writing – review & editing.
Eduardo Leo-Carnerero: Data curation; Writing – review & editing.
Berta Caballol: Data curation.
Andrea Núñez-Ortiz: Data curation.
María José García: Data curation; Visualization; Writing – review & editing.
Federico Bertoletti: Data curation; Visualization; Writing – review & editing.
Alejandro Mínguez: Data curation; Visualization.
Gerard Suris: Data curation.
Begoña Casis: Data curation; Visualization; Writing – review & editing.
Rocío Ferreiro-Iglesias: Conceptualization; Methodology; Visualization; Writing – review & editing.
Margalida Calafat: Data curation; Visualization.
Itxaso Jiménez: Data curation.
José Miranda-Bautista: Data curation; Visualization; Writing – review & editing.
Luis Javier Lamuela: Data curation.
Ingrid Fajardo: Data curation.
Leyanira Torrealba: Data curation.
Rodrigo Nájera: Data curation.
Rosa María Sáiz-Chumillas: Data curation.
Irene González: Data curation.
Miren Vicuña: Data curation.
Natalia García-Morales: Data curation.
Ana Gutiérrez: Data curation; Visualization.
Alicia López-García: Conceptualization; Data curation; Methodology; Validation; Visualization; Writing – review & editing.
José Manuel Benítez: Data curation; Visualization; Writing – review & editing.
Cristina Rubín de Célix: Data curation; Visualization; Writing – review & editing.
Coral Tejido: Data curation.
Eduard Brunet: Data curation; Visualization; Writing – review & editing.
Alejandro Hernández-Camba: Data curation; Visualization.
Cristina Suárez: Data curation; Visualization; Writing – review & editing.
Iago Rodríguez-Lago: Conceptualization; Methodology; Visualization; Writing – review & editing.
Marta Piqueras: Data curation; Visualization.
Andrés Castaño: Data curation.
Laura Ramos: Data curation.
Ana Sobrino: Data curation.
María Carmen Rodríguez-Grau: Data curation; Visualization.
Alfonso Elosua: Data curation; Visualization.
Miguel Montoro: Data curation; Visualization.
Ruth Baltar: Data curation.
José María Huguet: Data curation.
Benito Hermida: Data curation.
Antonio Caballero-Mateos: Data curation.
Luis Sánchez-Guillén: Data curation.
Abdel Bouhmidi: Data curation.
Ramón Pajares: Data curation.
Iria Baston-Rey: Data curation; Visualization.
Antonio López-Sanromán: Conceptualization; Supervision; Validation; Visualization; Writing – review & editing.
Agustin Albillos: Validation.
Manuel Barreiro-de Acosta: Conceptualization; Formal analysis; Methodology; Project administration; Supervision; Validation; Writing – review & editing.
Funding: The authors received no financial support for the research, authorship and/or publication of this article.
Francisco Mesonero has served as a speaker for and received consulting fees from MSD, AbbVie, Takeda, Janssen, Pfizer, Ferring, Kern-Pharma, Dr. Falk Pharma, Galapagos, Chiesi and Faes Farma. Yamile Zabana has received support for conference attendance, speaker fees, research support and consulting fees from AbbVie, Adacyte, Almirall, Amgen, Dr. Falk Pharma, FAES Pharma, Ferring, Janssen, MSD, Otsuka, Pfizer, Shire, Takeda, Galapagos, Boehringer Ingelheim and Tillots. Agnés Fernández-Clotet has served as a speaker for and received educational funding from Dr. Falk Pharma, Janssen, Takeda, Chiesi and Pfizer. Eduardo Leo-Carnerero has served as a speaker and consultant for and received educational funding from AbbVie, Janssen, Takeda, Ferring, Gilead, Pfizer and Dr. Falk Pharma. Andrea Núñez-Ortiz has received educational funding from Janssen, Takeda, Ferring and Pfizer. Berta Caballol has served as a speaker for and received consulting fees from MSD, AbbVie, Janssen, Takeda, Ferring, Shire Pharmaceuticals and Faes Pharma. Rocío Ferreiro-Iglesias has received support for conference attendance, speaker fees, research support and consulting fees from AbbVie, Adacyte, Dr. Falk Pharma, FAES Pharma, Ferring, Janssen, MSD, Kern, Chiesi, Gebro Pharma, Pfizer, Shire, Takeda and Tillots. José Miranda-Bautista has received support for conference attendance, speaker fees, and consulting and advisory fees from AbbVie, Adacyte, Dr. Falk Pharma, FAES Pharma, Ferring, Janssen, Pfizer, Takeda and Tillots. Leyanira Torrealba has served as a speaker for and received educational funding from AbbVie, Janssen, Takeda, Ferring, Gilead, Pfizer, Dr. Falk Pharma and Tillotts Pharma. Rosa María Sáiz-Chumillas has served as a speaker for and received consulting fees from Janssen, Ferring and Faes Farma. Alicia López-García has received support for conference attendance, educational funding and speaker fees from Chiesi, AbbVie, Janssen, Ferring, Takeda, Pfizer and Tillots. Cristina Rubín de Célix has received educational funding from Ferring, Tillotts Pharma, AbbVie, Norgine, MSD, Pfizer, Takeda and Janssen and is supported by a grant from the Ministerio de Economía y Competitividad (Instituto de Salud Carlos III, Rio Hortega CM21/00025) and co-financed by the European Social Fund Plus (ESF+) ‘Co-financed by the European Union’. Eduard Brunet has served as a speaker and consultant for and received educational funding from Janssen, Takeda, Ferring, Kern-Pharma, AbbVie and Chiesi. Alejandro Hernández-Camba has served as a speaker for and received educational funding from AbbVie, Takeda, Kern Pharma, Pfizer, Janssen, Adacyte Therapeutics, Chiesi, Galapagus and Ferring. Iago Rodríguez-Lago has received financial support for travelling and educational activities from or has served as an advisory board member for AbbVie, Adacyte, Celltrion, Chiesi, Danone, Dr. Falk Pharma, Ferring, Faes Farma, Janssen, Galapagos, MSD, Otsuka Pharmaceutical, Pfizer, Roche, Takeda and Tillotts Pharma. Iago Rodríguez-Lago has also received financial support for research from Tillotts Pharma and is supported by a research grant from Gobierno Vasco – Eusko Jaurlaritza [Grant No. 2020222004]. Alfonso Elosua has served as a speaker for and has received educational funding from AbbVie, Adacyte, Takeda, FAES Farma, Ferring, Janssen and Tillots Pharma. José María Huguet has received fees for educational activities, research projects, scientific meetings and advisory boards sponsored by MSD, Ferring, AbbVie, Janssen, Biogen, Sandoz, Kern Pharma, Faes Farma and Takeda. Manuel Barreiro-de Acosta has served as a speaker, consultant and advisory member for and has received research funding from MSD, AbbVie, Janssen, Kern Pharma, Celltrion, Takeda, Gilead, Celgene, Pfizer, Sandoz, Biogen, Fresenius, Ferring, Faes Farma, Dr. Falk Pharma, Chiesi, Gebro Pharma, Adacyte and Vifor Pharma.
Availability of data and materials: The data underlying this article will be shared on reasonable request to the corresponding author.
Guarantors of the article: Francisco Mesonero, Yamile Zabana and Manuel Barreiro-de Acosta are the guarantors of the article.
References
- 1. Scoglio D, Ahmend Ali U, Fichera A. Surgical treatment of ulcerative colitis: ileorectal vs ileal pouch-anal anastomosis. World J Gastroenterol 2014; 20: 13211–13218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Parks AG, Nicholls RJ. Proctocolectomy without ileostomy for ulcerative colitis. Br Med J 1978; 2: 85–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shen B. Acute and chronic pouchitis-pathogenesis, diagnosis and treatment. Nat Rev Gastroenterol Hepatol 2012; 9: 323–333. [DOI] [PubMed] [Google Scholar]
- 4. Fazio VW, Kiran RP, Remzi FH, et al. Ileal pouch anal anastomosis: analysis of outcome and quality of life in 3707 patients. Ann Surg 2013; 257: 679–685. [DOI] [PubMed] [Google Scholar]
- 5. Dozois RR, Kelly KA, Welling DR, et al. Ileal pouch-anal anastomosis: comparison of results in familial adenomatous polyposis and chronic ulcerative colitis. Ann Surg 1989; 210: 268–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hueting WE, Buskens E, van der Tweel I, et al. Results and complications after ileal pouch anal anastomosis: a meta-analysis of 43 observational studies comprising 9371 patients. Dig Surg 2005; 22: 69–79. [DOI] [PubMed] [Google Scholar]
- 7. Barnes EL, Herfarth HH, Sandler RS, et al. Pouch-related symptoms and quality of life in patients with ileal pouch-anal anastomosis. Inflamm Bowel Dis 2017; 23: 1218–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Peyrin-Biroulet L, Germain A, Patel AS, et al. Systematic review: outcomes and post-operative complications following colectomy for ulcerative colitis. Aliment Pharmacol Ther 2016; 44: 807–816. [DOI] [PubMed] [Google Scholar]
- 9. Magro F, Gionchetti P, Eliakim R, et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 1: Definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer surveillance, surgery and ileo-anal pouch disorders. J Crohns Colitis 2017; 11: 649–670. [DOI] [PubMed] [Google Scholar]
- 10. Barreiro de-Acosta M, Gutierrez A, Rodríguez-Lago I, et al. Recommendations of the Spanish Working Group on Crohn’s Disease and Ulcerative Colitis (GETECCU) on pouchitis in ulcerative colitis. Part 1: Epidemiology, diagnosis and prognosis. Gastroenterol Hepatol 2019; 42: 568–578. [DOI] [PubMed] [Google Scholar]
- 11. Wu B, Lian L, Li Y, et al. Clinical course of cuffitis in ulcerative colitis patients with restorative proctocolectomy and ileal pouch-anal anastomoses. Inflamm Bowel Dis 2013; 19: 404–410. [DOI] [PubMed] [Google Scholar]
- 12. Shen B. Crohn’s disease of the ileal pouch: reality, diagnosis and management. Inflamm Bowel Dis 2009; 15: 284–294. [DOI] [PubMed] [Google Scholar]
- 13. Lightner AL, Pemberton JH, Loftus EJ, et al. Crohn’s disease of the ileoanal pouch. Inflamm Bowel Dis 2016; 22: 1502–1508. [DOI] [PubMed] [Google Scholar]
- 14. Barnes EL, Kochar B, Jessup HR, et al. The incidence and definition of Crohn’s disease of the pouch: a systematic review and meta-analysis. Inflamm Bowel Dis 2019; 25: 1474–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shen B, Kochhar GS, Kariv R, et al. Diagnosis and classification of ileal pouch disorders: consensus guidelines from the International Ileal Pouch Consortium. Lancet Gastroenterol Hepatol 2021; 6: 826–849. [DOI] [PubMed] [Google Scholar]
- 16. Shamah S, Schneider J, Korelitz BI. High incidence of recurrent Crohn’s disease following colectomy for ulcerative colitis revealed with long follow-up. Dig Dis Sci 2018; 63: 446–451. [DOI] [PubMed] [Google Scholar]
- 17. Dalal RL, Shen B, Schwartz DA. Management of pouchitis and other common complications of the pouch. Inflamm Bowel Dis 2018; 24: 989–996. [DOI] [PubMed] [Google Scholar]
- 18. Barreiro-de Acosta M, Marín-Jimenez I, Rodríguez-Lago I, et al. Recommendations of the Spanish Working Group on Crohn’s Disease and Ulcerative Colitis (GETECCU) on pouchitis in ulcerative colitis. Part 2: Treatment. Gastroenterol Hepatol 2020; 43:649–658. [DOI] [PubMed] [Google Scholar]
- 19. Shen B, Kochhar GS, Rubin DT, et al. Treatment of pouchitis, Crohn’s disease, cuffitis, and other inflammatory disorders of the pouch: consensus guidelines from the International Ileal Pouch Consortium. Lancet Gastroenterol Hepatol 2022; 7: 69–95. [DOI] [PubMed] [Google Scholar]
- 20. Singh S, Stroud AM, Holubar SD, et al. Treatment and prevention of pouchitis after ileal pouch-anal anastomosis for chronic ulcerative colitis. Cochrane Database Syst Rev 2015; 23: CD001176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shen B, Achkar JP, Lashner BA, et al. A randomized clinical trial of ciprofloxacin and metronidazole to treat acute pouchitis. Inflamm Bowel Dis 2001; 7: 301–305. [DOI] [PubMed] [Google Scholar]
- 22. Gionchetti P, Rizzello F, Venturi A, et al. Antibiotic combination therapy in patients with chronic, treatment-resistant pouchitis. Aliment Pharmacol Ther 1999; 13: 713–718. [DOI] [PubMed] [Google Scholar]
- 23. Madden MV, McIntyre AS, Nicholls RJ. Double-blind crossover trial of metronidazole versus placebo in chronic unremitting pouchitis. Dig Dis Sci 1994; 39: 1193–1196. [DOI] [PubMed] [Google Scholar]
- 24. Gionchetti P, Rizzello F, Poggioli G, et al. Oral budesonide in the treatment of chronic refractory pouchitis. Aliment Pharmacol Ther 2007; 25: 1231–1236. [DOI] [PubMed] [Google Scholar]
- 25. Barreiro-de Acosta M, García-Bosch O, Souto R, et al. Efficacy of infliximab rescue therapy in patients with chronic refractory pouchitis: a multicenter study. Inflamm Bowel Dis 2012; 18: 812–817. [DOI] [PubMed] [Google Scholar]
- 26. Barreiro-de Acosta M, García-Bosch O, Gordillo J, et al. Efficacy of adalimumab rescue therapy in patients with refractory pouchitis previously treated with infliximab: a case series. Eur J Gastroenterol Hepatol 2012; 24: 756–758. [DOI] [PubMed] [Google Scholar]
- 27. Huguet M, Pereira B, Goutte M, et al. Systematic review with meta-analysis: anti-TNF therapy in refractory pouchitis and Crohn’s disease-like complications of the pouch after ileal pouch-anal anastomosis following colectomy for ulcerative colitis. Inflamm Bowel Dis 2018; 24: 261–268. [DOI] [PubMed] [Google Scholar]
- 28. Colombel JF, Ricart E, Loftus EV, Jr, et al. Management of Crohn’s disease of the ileoanal pouch with infliximab. Am J Gastroenterol 2003; 98:2239–2244. [DOI] [PubMed] [Google Scholar]
- 29. Ferrante M, D’Haens G, Dewit O, et al. Efficacy of Infliximab in refractory pouchitis and Crohn’s disease-related complications of the pouch: a Belgian case series. Inflamm Bowel Dis 2010; 16: 243–249. [DOI] [PubMed] [Google Scholar]
- 30. Silverberg MS, Satsangi J, Ahmad T, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol 2005; 19(Suppl. A): 5A–36A. [DOI] [PubMed] [Google Scholar]
- 31. von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med 2007; 147: 573–577. [DOI] [PubMed] [Google Scholar]
- 32. Shen B, Achkar JP, Connor JT, et al. Modified pouchitis disease activity index: a simplified approach to the diagnosis of pouchitis. Dis Colon Rectum 2003; 46: 748–753. [DOI] [PubMed] [Google Scholar]
- 33. Núñez L, Mesonero F, Rodríguez de Santiago E, et al. High incidence of surgery and initiation of medical therapies after colectomy for ulcerative colitis or inflammatory bowel disease unclassified. Gastroenterol Hepatol 2023; 46: 369–375. [DOI] [PubMed] [Google Scholar]
- 34. Haveran LA, Sehgal R, Poritz LS, et al. Infliximab and/or azathioprine in the treatment of Crohn’s disease-like complications after IPAA. Dis Colon Rectum 2011; 54: 15–20. [DOI] [PubMed] [Google Scholar]
- 35. Berrebi W, Chaussade S, Bruhl AL, et al. Treatment of Crohn’s disease recurrence after ileoanal anastomosis by azathioprine. Dig Dis Sci 1993; 38: 1558–1560. [DOI] [PubMed] [Google Scholar]
- 36. Bermejo F, Aguas M, Chaparro M, et al. Recommendations of the Spanish Working Group on Crohn’s Disease and Ulcerative Colitis (GETECCU) on the use of thiopurines in inflammatory bowel disease. Gastroenterol Hepatol 2018; 41: 205–221. [DOI] [PubMed] [Google Scholar]
- 37. Yadav A, Foromera J, Falchuk KR, et al. Biologics and immunomodulators for treating Crohn’s disease developing after surgery for an initial diagnosis of ulcerative colitis: a review of current literature. Scand J Gastroenterol 2018; 53: 813–817. [DOI] [PubMed] [Google Scholar]
- 38. Chandan S, Mohan BP, Kumar A, et al. Safety and efficacy of biological therapy in chronic antibiotic refractory pouchitis: a systematic review with meta-analysis. J Clin Gastroenterol 2021; 55: 481–491. [DOI] [PubMed] [Google Scholar]
- 39. Chaparro M, Ordás I, Cabré E, et al. Safety of thiopurine therapy in inflammatory bowel disease: long-term follow-up study of 3931 patients. Inflamm Bowel Dis 2013; 19: 1404–1410. [DOI] [PubMed] [Google Scholar]
- 40. Sedano R, Ma C, Pai RK, et al. An expert consensus to standardise clinical, endoscopic and histologic items and inclusion and outcome criteria for evaluation of pouchitis disease activity in clinical trials. Aliment Pharmacol Ther 2021; 53: 1108–1117. [DOI] [PubMed] [Google Scholar]