Abstract
Background
The prevalence of macrolide-resistant Mycoplasma pneumoniae has increased considerably. Treatment in children has become challenging. This study aimed to evaluate the efficacy of doxycycline therapy for macrolide-resistant Mycoplasma pneumoniae pneumonia in children at different periods.
Methods
We retrospectively analyzed the data of patients with macrolide-resistant Mycoplasma pneumoniae pneumonia hospitalized between May 2019 to August 2022. According to treatment, patients were divided into three groups: oral doxycycline treatment alone (DOX group), changed from intravenous azithromycin to oral doxycycline (ATD group), and intravenous azithromycin treatment alone (AZI group). ATD group cases were separated into two sub-groups: intravenous azithromycin treatment<3 days (ATD1 group) and ≥ 3 days (ATD2 group). Clinical symptoms were compared in each group and adjusted by Propensity score matching (PSM) analysis.
Results
A total of 106 were recruited in this study. 17 (16%) were in DOX group, 58 (55%) in ATD group, and 31(29%) in AZI group. Compared with ATD group and AZI group, the DOX group showed shorter hospitalization duration and fever duration after treatment, while higher rate of chest radiographic improvement. After using PSM analysis, shorter days to hospitalization duration (P = 0.037) and to fever duration after treatment (P = 0.027) in DOX + ATD1 group than in ATD2 group was observed. A higher number of patients in the DOX + ATD1 group achieved defervescence within 72 h (P = 0.031), and fewer children received glucocorticoid adjuvant therapy (P = 0.002). No adverse reactions associated with doxycycline was observed during treatment.
Conclusions
Children receiving early oral doxycycline had a shorter duration of fever and hospitalization in macrolide-resistant Mycoplasma pneumoniae patients.
Keywords: Macrolide-resistant, Mycoplasma pneumoniae, Doxycycline
Background
Mycoplasma pneumoniae (M. pneumoniae) is one of the most common causes of upper and lower respiratory tract infections, particularly in children and young adults. The majority of M. pneumoniae pneumonia are benign and self-limiting disease. However, some patients may develop severe M. pneumoniae pneumonia or refractory M. pneumoniae pneumonia, causing progressive pneumonia or various extrapulmonary complications [1]. These cases may be related to the occurrence of macrolide-resistant (MR) M. pneumoniae [2, 3]. This resistance is associated with point mutations in the V region of the 23S rRNA gene and leads to high-level resistance to macrolides [4]. Therefore, the efficacy of macrolide treatment was shown to be lower in patients infected with macrolide-resistant isolates than in patients infected with macrolide-sensitive isolates [5, 6].
In recent years, the global patterns in the proportion of MR M. pneumoniae infections showed an increasing trend, and the proportion of MR M. pneumoniae infections was highest in China [3, 7]. Treatment with the increase in MR M. pneumoniae has become challenging. Because this resistance may lead to more extrapulmonary complications and severe clinical features [2], alternative antibiotic treatment can be required, including tetracyclines or fluoroquinolones. To date, no tetracycline resistance has been reported in M. pneumoniae clinical isolates. In vitro antimicrobial susceptibility testing showed that M. pneumoniae in all cases was sensitive to tetracyclines, including doxycycline and minocycline [8].
MR M. pneumoniae pneumonia is characterized by an excessive immune response against the pathogen as well as direct injury caused by an increasing M. pneumoniae load [9]. Study indicates that children with higher M. pneumoniae abundance in the bronchoalveolar lavage fluid tend to have a longer hospital stay and higher fever peak [10]. This suggests that the loading of M. pneumoniae is associated with clinical severity [11]. Doxycycline, as an alternative drug for treating MR M. pneumoniae, can inhibit the replication of M. pneumoniae DNA and reduce the load of pulmonary pathogens [12]. However, the exact timing of doxycycline treatment has not been established at present. In this study, we aimed to evaluate the efficacy of doxycycline therapy for macrolide-resistant Mycoplasma pneumoniae pneumonia in children at different periods.
Materials and methods
Study subjects
We retrospectively reviewed the medical records of children without prior underlying diseases with Community acquired pneumonia hospitalized at the Ningbo Medical Center Lihuili Hospital between May 2019 and August 2022. All the evaluated patients had signs and symptoms indicative of pneumonia, such as fever, cough, and abnormal chest radiographic findings compatible with pneumonia [13]. The M. pneumoniae infection was determined by polymerase chain reaction test of nasopharyngeal aspirates obtained from the patients on admission. Samples positive for M. pneumoniae were subjected to direct DNA sequencing of the domain V of the 23S rRNA gene to identify A2063G or A2064G mutation site using real-time fluorescent PCR assay kit (Jiangsu Mole Bioscience Co., Ltd, China) in accordance with the manufacturer’s instructions. It took about 2 days to determine whether there is a resistance mutation.
Exclusion criteria were as follow: (1) other pathogens were found before treatment, including bacteria, respiratory syncytial virus, influenza viruses, parainfluenza viruses, coronaviruses, human rhinoviruses, adenoviruses, human metapneumovirus and chlamydia pneumoniae; (2) patients who had been started on treatment for macrolide or doxycycline prior to admission; (3) children who have chronic disease (such as tuberculosis, asthma and immunodeficiency) states predisposing them to recurrent lung infections; (4) discharge within 48 h after enrollment and insufficient data; (5) children younger than 8 years were excluded because they could not be treated with doxycycline.
Methods
In our study, all patients were treated with intravenous azithromycin or oral doxycycline. The dosage of intravenous azithromycin was 10 mg/kg once daily, and oral doxycycline was administered once every 12 h at doses of 2.2 mg/kg, in accordance with the package insert accompanying each drug [13]. The primary antibiotic selection was made by the attending pediatrician. Oral doxycycline should be used if the patient had a history of exposure to MR M. pneumoniae. The preferred treatment for M. pneumoniae pneumonia is macrocyclic antibiotics [13]. Therefore, before the results of pathogen and M. pneumoniae mutation site testing are available, intravenous azithromycin was chosen as the primary antibiotic for patients with suspected M. pneumoniae pneumonia. After obtaining evidence of MR M. pneumoniae infection, some patients were changed from intravenous azithromycin to oral doxycycline, while others continued treatment with intravenous azithromycin due to inability to tolerate swallowing capsules. According to treatment, these patients were divided into three groups: oral doxycycline treatment alone (DOX group), changed from intravenous azithromycin to oral doxycycline (ATD group) and intravenous azithromycin treatment alone (AZI group). ATD group cases were separated into two sub-groups: intravenous azithromycin treatment<3 days (ATD1 group) and intravenous azithromycin treatment ≥ 3 days (ATD2 group).
Data collection
Clinical information was retrospectively collected from the medical records of the patients. The collected data included demographics, hospitalization period, duration of fever (febrile days before macrolide or doxycycline treatment, febrile days after treatment, time to defervescence), laboratory results upon admission, chest radiographic findings and adverse reactions during treatment. The duration of fever was defined as the number of days for which the patient had a body temperature of ≥ 38℃ with an interval of <24 h between each episode of fever. Defervescence of fever was defined as a decline in body temperature up to < 37.5℃ for > 48 h. All patients underwent chest radiographic examination before admission, and a second chest radiographic examination was performed 7 to 10 days after treatment. The chest radiographic findings were from the records read by two radiologists and classified according to the presence of consolidation lobar, patchy and effusion. If the results of the patient’s X-rays showed that reduction of more than 30% in consolidation and infiltration area compared to before treatment, we consider the patient to be a consolidation and/or infiltration absorption case.
Statistical analysis
SPSS 25.0 statistical software was applied for Propensity score matching (PSM) and analysis. The data were expressed as median (IQR) for continuous variables or as number of cases (percentage) of a specific group for categorical variables. The Kruskal-Wallis test was used for continuous variables. If the variables were statistically significant when compared among more than two groups, they were further analyzed by Mann-Whitney U test for comparing two groups. The Pearson’s Chi-squared or Fisher’s exact test were used for categorical variables. To reduce the effect of possible selective bias, patients in the DOX + ATD1 and ATD2 groups, were matched with those in non-biopsy group for a 1:1 PSM with a caliper value of 0.02. Matching factors included age, gender, fever duration prior to treatment, and chest radiographic before admission. Two-sided p-value < 0.05 was considered to be statistically significant.
Results
Demographic and clinical characteristics
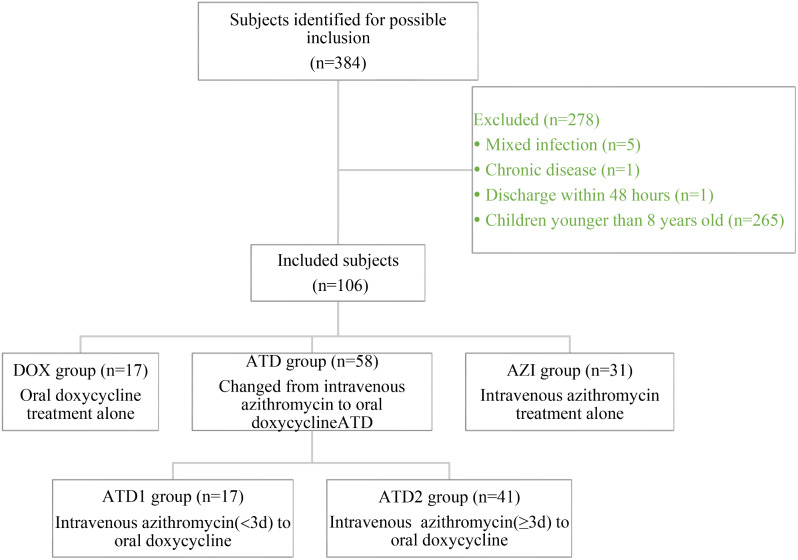
The total number of hospitalized patients was 5589, who were tested for M. pneumoniae PCR tests of the nasopharyngeal aspirates between May 2019 to August 2022 due to clinically suspected M. pneumoniae infection. Among the cases tested, 10% (533/5589) were M. pneumoniae PCR positive, and 72% (384/533) showed point mutations in domain V of 23S rRNA. The prevalence rate of patients tests positive for MR M. pneumoniae infection peaked in summer and autumn season of 2019 during the study period. Of the patients with MR M. pneumoniae pneumonia, we excluded 278 patients following the exclusion criteria. Consequently, a total of 106 were recruited in this study. 17 (16%) were in DOX group, 58 (55%) in ATD group, and 31(29%) in AZI group (Fig. 1).
Fig. 1.
Flow chart for the inclusion and classification of the study subjects
The demographics and clinical characteristics were shown in Table 1. The median age of patients in three groups was 10 (8.5, 11) years old, 9 (8.75, 10) years old, and 9 (9, 9) years old, respectively. The number of males in DOX, ATD, and AZI groups was 5 (29%), 32 (55%), and 18 (58%), and the number of febrile patients was 15 (88%), 55 (95%), and 31 (100%), respectively. Fever duration prior to treatment showed no differences compared among the three groups (P = 0.114). No statistically significant differences were found among the groups for the occurrence of pleural effusion (DOX 35%, ATD 26%, AZI 42%) (P = 0.288). There were no statistically significant differences among the three groups in terms of laboratory data, including white blood cell count, neutrophil percentage, platelets, C-reactive protein, procalcitonin and lactate dehydrogenase (P > 0.05).
Table 1.
Characteristics of patients with macrolide-resistant MP pneumonia in each treatment group
| DOX group | ATD group | AZI group | p-value* | |
|---|---|---|---|---|
| n = 17 | n = 58 | n = 31 | ||
| Age, years | 10 (8.5, 11) | 9 (8.75, 10) | 9 (9, 9) | 0.258 |
| Male gender | 5 (29%) | 32 (55%) | 18 (58%) | 0.125 |
| Fever (n, %) | 15 (88%) | 55 (95%) | 31 (100%) | 0.179 |
| Fever duration before treatment(d) | 3 (2, 6.5) | 3 (2, 4) | 4 (2, 5) | 0.114 |
| Chest radiograph findings (n, %) | 0.457 | |||
| Lobar consolidation | 13 (76%) | 37 (64%) | 23 (74%) | |
| Patchy | 4 (24%) | 21 (36%) | 8 (26%) | |
| Pleural effusion (n, %) | 6 (35%) | 15 (26%) | 13 (42%) | 0.288 |
| Laboratory findings | ||||
| White blood cell(×10^9/L) | 6.2 (5.15, 7.0) | 6.8 (5.5, 7.875) | 7.6 (5.6, 8.7) | 0.073 |
| Neutrophil (%) | 66.5 (56.9, 69.45) | 64.35 (58.725, 70.100) | 64.8 (53.9, 70.5) | 0.947 |
| Platelets (×10^9/L) | 229 (179, 262) | 212.5 (182.0, 244.5) | 203 (181, 256) | 0.766 |
| C-reactive protein(mg/L) | 17.7 (8.8, 35.9) | 13.1 (7.0, 29.75) | 17.1 (4.9, 24.5) | 0.683 |
| Procalcitonin(ng/mL) | 0.081 (0.0515, 0.1375) | 0.088 (0.0615, 0.1515) | 0.104 (0.710, 0.156) | 0.565 |
| Lactate dehydrogenase (U/L) | 269 (238.5, 308.5) | 274 (244.25, 330.5) | 297 (243.5, 334) | 0.699 |
*Comparison between three groups
Comparisons of clinical courses after therapy
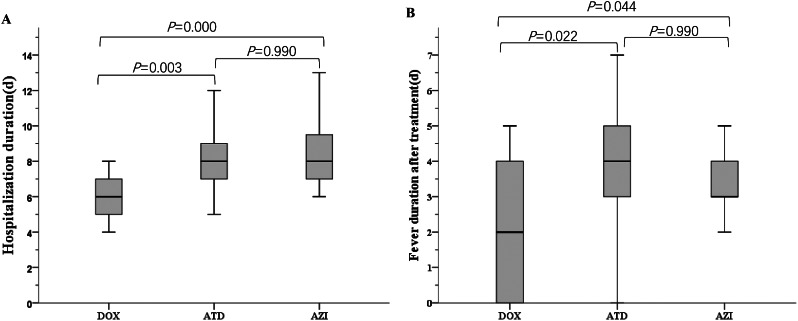
The efficacy of treatment in each group was compared (Table 2). Hospitalization duration in DOX group was shorter than that in ATD group (6 days vs. 8 days, P = 0.003) and AZI group (6 days vs. 8 days, P = 0.000) (Fig. 2). The median fever duration after treatment in DOX group was shorter than that in the other two groups (2 days vs. 4 days, P = 0.022 and 2 days vs. 3 days, P = 0.044, respectively) (Fig. 2). Hospitalization duration and fever duration after treatment were not significantly different between ATD group and AZI group (P = 0.867 and P = 0.990, respectively) (Fig. 2). The numbers of patients who achieved defervescence within 48 h and chest radiographic improvement after one week of treatment were higher in DOX group than that in in ATD group (P = 0.045 and P = 0.021, respectively) and AZI group (P = 0.000 and P = 0.003, respectively). The number of patients using glucocorticoid adjuvant therapy in DOX group was less than that in AZI group (P = 0.015). These indicators were no statistically significant differences compared between ATD group and AZI group (P > 0.05). Three patients in ATD group received nasal cannula oxygen supply, one patient in AZI group and none in DOX group. No patients were transferred to the intensive care unit or received mechanical ventilation during hospitalization. During intravenous azithromycin treatment, 10 patients had abdominal pain, 3 had vomiting, 3 had rash. All patients treated with doxycycline responded well. No adverse reactions associated with oral doxycycline were observed during treatment.
Table 2.
Comparisons of clinical courses after therapy in macrolide-resistantMycoplasma pneumoniae pneumonia
| DOX group | ATD group | AZI group | p-value* | |
|---|---|---|---|---|
| n = 17 | n = 58 | n = 31 | ||
| Hospitalization duration(d) | 6 (5, 7) | 8 (7, 9) | 8 (7, 10) | 0.000 |
| Fever duration after treatment(d) | 2 (0, 4) | 4 (3, 5) | 3 (3, 4) | 0.020 |
| Defervescence within 48 h (n, %) | 8/15 (53%) | 10/55 (18%) | 1/31 (3%) | 0.000 |
| Chest X-ray improvement (n, %) | 15 (88%) | 30 (52%) | 12 (39%) | 0.004 |
| Glucocorticoid (n,%) | 1 (6%) | 16 (28%) | 14 (45%) | 0.015 |
*Comparison between three groups
Fig. 2.
The comparison between the DOX, ATD and AZI groups. (A) Hospitalization duration; (B) Fever duration after treatment
Comparison of doxycycline treatment in different periods
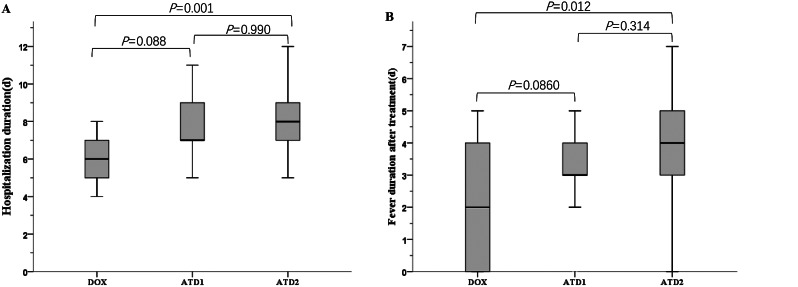
As shown in Table 3, the response to azithromycin and doxycycline among children with MR M. pneumoniae pneumonia at different time points was analyzed. The median of hospitalization duration and fever duration after treatment were shorter in DOX group than that in ATD2 group (P = 0.001 and P = 0.012, respectively) (Fig. 3). There was no difference in hospitalization duration and fever duration after treatment in ATD1 group compared with the DOX group (P = 0.088 and P = 0.860, respectively) and ATD2 group (P = 0.990 and P = 0.314, respectively). The numbers of patients who achieved defervescence within 72 h were higher in DOX group than that in ATD2 group (P = 0.039), and were not significantly different between ATD1 and ATD2 group (P = 0.741). The numbers of patients who achieved defervescence within 96 h were higher in DOX and ATD1 group than that in ATD2 group (P = 0.024 and P = 0.006, respectively), and were not significantly different between DOX and ATD1 group (P = 0.990). After one week of treatment, the number of patients who achieved chest radiographic improvement in DOX group and ATD1 group was higher than that in ATD2 group (P = 0.003 and P = 0.045, respectively). ATD2 group had the highest number of patients using glucocorticoid adjuvant therapy, followed by the other two groups (P = 0.007).
Table 3.
Comparison of efficacy of doxycycline at different time
| DOX group | ATD1 group | ATD2 group | p-value* | |
|---|---|---|---|---|
| n = 17 | n = 17 | n = 41 | ||
| Age, years | 10 (8.5, 11) | 9 (8.5, 10.5) | 9 (8.5, 10) | 0.545 |
| Hospitalization duration(d) | 6 (5, 7) | 7 (7, 9) | 8 (7, 9) | 0.002 |
| Fever (n, %) | 15 (88.2%) | 15 (88.2%) | 40 (97.6%) | 0.234 |
| Fever duration before treatment(d) | 3 (2, 6.5) | 3 (1.5, 4) | 3 (2, 4) | 0.500 |
| Fever duration after treatment(d) | 2 (0, 4) | 3 (2.5, 4) | 4 (3, 5) | 0.011 |
| Defervescence within 72 h (n, %) | 10 /15(67%) | 7/15 (47%) | 12 /40(30%) | 0.044 |
| Defervescence within 96 h (n, %) | 12/15 (80%) | 13 /15(87%) | 16 /40(40%) | 0.001 |
| Chest X-ray improvement (n, %) | 15 (88%) | 13 (76%) | 17 (41%) | 0.001 |
| Glucocorticoid(n,%) | 1 (6%) | 1 (6%) | 15 (37%) | 0.007 |
*Comparison between three groups
Fig. 3.
The comparison between the DOX, ATD1 and ATD2 groups. (A) Hospitalization duration; (B) Fever duration after treatment
The efficacy of early oral doxycycline using PS matched analysis
The efficacy of early oral doxycycline was compared in the matched analysis between the DOX + ATD1group and ATD2 group (Table 4). There was no significant difference in baseline characteristics between the two groups. The hospitalization duration, fever duration after treatment were shorter in DOX + ATD1 group than that in ATD2 group (P = 0.037 and P = 0.027, respectively). The number of patients achieving defervescence within 72 h was higher in DOX + ATD1 group (P = 0.031). More children in ATD2 group were treated with glucocorticoid adjuvant therapy (P = 0.002).
Table 4.
Evaluate the efficacy of early oral doxycycline using PSM analysis
| DOX + ATD1 group | ATD2 group | p-value | |
|---|---|---|---|
| n = 28 | n = 28 | ||
| Age, years | 9 (9, 10) | 10 (9, 10) | 0.447 |
| Male gender | 13 (46%) | 16 (57%) | 0.422 |
| Fever duration before treatment(d) | 3 (2, 5) | 3 (2, 4) | 0.326 |
| Chest radiograph findings (n, %) | 0.783 | ||
| Lobar consolidation | 17 (61%) | 18 (64%) | |
| Patchy | 11 (39%) | 10 (36%) | |
| Hospitalization duration(d) | 7 (6, 8) | 8 (7, 9) | 0.037 |
| Fever duration after treatment(d) | 3 (2, 4) | 4 (3, 5) | 0.027 |
| Defervescence within 72 h (n, %) | 16 (57%) | 8 (29%) | 0.031 |
| Glucocorticoid(n,%) | 2 (7%) | 12 (43%) | 0.002 |
Discussion
The incidence of MR M. pneumoniae has recently increased and has been related to life-threatening or refractory M. pneumoniae pneumonia in children [14]. Since the emergence of macrolide resistance has been reported mainly in Asia [7, 15], prevalence of MR M. pneumoniae isolated in pediatric patients has increased annually in China [16]: 88.19% in 2016, 90.93% in 2017, 90.56% in 2018 and 92.90% in 2019. In this study, the total number of M. pneumoniae pneumonia was 533 cases between May 2019 to August 2022, and 72% (384/533) were MR M. pneumoniae pneumonia. The prevalence of MR M. pneumoniae was similar to the data reported, and peaked in 2019 during the study period. There was an uneven distribution among cases in the 2019–2022, which was related to the COVID-19 pandemic. Since January 2020, in response to various public health policies to control the spread of COVID-19 in the pandemic, there was a substantial decrease in respiratory infections in China and many resources were focused on diagnosis and management of COVID-19. During the COVID-19 pandemic, our study also showed an increase in MR M. pneumoniae infection rates in 2022. The increasing prevalence of MR M. pneumoniae has become a significant clinical issue in the pediatric patients. Treatment of MR M. pneumoniae pneumonia in children has become challenging.
M. pneumoniae lacks cell wall and consequently is resistant to beta-lactams and to all antimicrobials targeting the cell wall [17]. This mycoplasma is intrinsically susceptible to antibiotics that act on the bacterial ribosome and inhibit protein synthesis such as macrolides or tetracyclines or agents that inhibit DNA replication such as fluoroquinolones [18, 19]. MR M. pneumoniae is caused by mutations in domain V of the 23s rRNA gene that interfere with the binding of macrolides to rRNA [15]. A-to-G transition mutation at position 2063 in 23S rRNA genes is the most prevalent in MR M. pneumoniae isolates, and it is closely followed by the A2064G mutation [2, 3]. Both mutations can cause high-level resistance to erythromycin and azithromycin in M. pneumoniae [20]. This suggests that macrolide may have limited effects on MR M. pneumoniae infection. Therefore, in cases of MR M. pneumoniae strains, alternative antibiotic treatment can be required, including tetracyclines such as doxycycline and minocycline [21, 22]. To date, no tetracycline resistance has been reported in M. pneumoniae clinical isolates. Doxycycline has good activity against both macrolide-susceptible and macrolide-resistant strains [22, 23]. As expected, our study found that doxycycline regimens were shown to be more effective than macrolide regimens in patients infected by MR M. pneumoniae. The duration of fever and hospitalization were significantly longer in patients with macrolide regimens. Compared to intravenous azithromycin treatment, oral doxycycline is more acceptable to children. Therefore, oral doxycycline is likely to be a better treatment of MR M. pneumoniae infections than macrolide for children above the age of 8 years.
The occurrence of MR M. pneumoniae infections was likely to lead to treatment failure, which translates into a longer duration of therapy, persistent cough and increased time to resolution of fever compared with treatment-susceptible infection, both in children and in adults [6, 24]. For the treatment of MR M. pneumoniae pneumonia presenting clinical and radiological deterioration, adjunctive systemic corticosteroids are sometimes used [25]. However, too early use large doses corticosteroids could cause suppression of phagocytic function of alveolar macrophages and neutrophils, decrease mobilization of inflammatory cells into areas of infection, and cause changes in antigen presentation and lymphocyte mobilization [26]. In addition, corticosteroids did not significantly decrease the DNA load of M. pneumoniae in bronchoalveolar lavage fluid [27]. Therefore, untimely corticosteroid additional therapy may increase the risk of mixed infection and they may contribute to condition aggravation [26]. Tetracyclines can inhibit peptide chain lengthening of protein synthesis by acting on the 30 S subunit of M. pneumoniae ribosomes. Estimated M. pneumoniae amounts after 3 days clearly decreased from 106 copies/mL to 5 × 102 copies/mL in those receiving doxycycline [12]. That indicates doxycycline could decrease the DNA load of M. pneumoniae. As indicated by our results in, defervescence occurred within 72 h after initiation of doxycycline (66.7%), with poorer results using azithromycin (ATD1 46.7% and ATD2 30%, respectively). However, when doxycycline was administered within 3 days after azithromycin agents, almost 86.7% of patients showed defervescence within 96 h. Because the inflammatory indicators of our study subjects (such as white blood cells, CRP, LDH, etc.) are usually normal or slightly elevated in the early stages, only a very small number of patients have these inflammatory indicators rechecked after treatment. To avoid selection bias, these indicators were not used as evaluation indicators for efficacy. Due to its ability to inhibit virus replication and reduce viral load, patients receiving oral doxycycline treatment in the early stages can quickly improve clinical symptoms and promote the absorption of pulmonary inflammation. This resulted in a significantly lower number of patients using glucocorticoids as adjunctive therapy in the doxycycline group compared to the azithromycin group. Therefore, clinicians should be vigilant for macrolide treatment failure and consider using alternative drugs if symptoms persist or if there are signs of clinical deteriorations.
Tetracyclines are generally well tolerated, with common adverse reactions observed in patients receiving these agents including anorexia, nausea, vomiting, diarrhea, rash, photosensitivity, tooth discoloration [18, 28]. Most concerning side effect is permanent tooth discoloration. The affinity for mineralizing tissue leads to incorporation into calcifying tissues [29]. However, due to a low affinity for calcium of doxycycline [30], there is no or only negligible tooth staining, even in young children aged 2–8 years [31, 32]. Factors related to tooth discoloration are dosage, duration of treatment, stage of tooth mineralization, and activity of the mineralization process [33]. In our study, oral doxycycline treatment lengths usually range between 7 and 10 days. No adverse reactions associated with doxycycline was observed during treatment. Further studies were needed to evaluate the adverse effects of doxycycline.
This study has some limitations. The first limitation was its retrospective design, which had the potential to introduce memory bias and led to missing data, most notably for assessment of disease severity. Secondly, due to the limitations of the duration of this study, we collected all the qualified children rather than calculated the sample size in the study period. Therefore, it might lead to selective bias. Finally, all research subjects come from one center with limited sample size. Although the PSM method can deal with the issue of selection bias, the small sample size after matching may lead to less objective and complete display of data features. In the future, it is necessary to carry out prospective randomized studies or to conduct studies involving more subjects through multicenter studies.
Conclusions
Compared with intravenous azithromycin, cases with MR M. pneumoniae pneumonia showed significantly shorter duration of fever and hospitalization with oral doxycycline and more rapid improvement of radiologic findings. Most of MR M. pneumoniae pneumonia patients achieved rapid defervescence with oral doxycycline or treatment changes to oral doxycycline. Pediatricians should improve the early recognition of MR M. pneumoniae pneumonia, which is important for early conversion to doxycycline therapy. Furthermore, a large-scale prospective study is needed to guide appropriate treatment in children with MR M. pneumoniae.
Acknowledgements
We thank every patient who participated in this research.
Author contributions
HBS: study conception and design. YZ: data acquisition. QNT: analysis and data interpretation. YC: drafting of the manuscript. All authors read and approved the final manuscript.
Funding
The authors received no financial support for this study.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request. All the original data presented in the study are included in the article.
Declarations
Ethics approval and consent to participate
This study was approved by the institutional research ethics committee of the Ningbo Medical Center Lihuili Hospital (Date-11/18/2022/NO-KY2022SL379-01), and was conducted in compliance with ethical principles originating in the Declaration of Helsinki. The ethics committee listed above waived the requirement for informed consent, because this study used currently existing medical records collected during the course of routine medical care and did not pose any additional risks to the patients.
Consent to publish
Not applicable.
Conflict of interest
All authors had no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Izumikawa K, Izumikawa K, Takazono T, et al. Clinical features, risk factors and treatment of fulminant Mycoplasma pneumoniae pneumonia: a review of the Japanese literature. J Infect Chemother. 2014;20(3):181–5. doi: 10.1016/j.jiac.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 2.Zhou Y, Zhang Y, Sheng Y, Zhang L, Shen Z, Chen Z. More complications occur in macrolide-resistant than in macrolide-sensitive Mycoplasma pneumoniae pneumonia. Antimicrob Agents Chemother. 2014;58(2):1034–8. doi: 10.1128/AAC.01806-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu Y, Ye X, Zhang H, et al. Characterization of macrolide resistance in Mycoplasma pneumoniae isolated from children in Shanghai, China. Diagn Microbiol Infect Dis. 2010;67(4):355–8. doi: 10.1016/j.diagmicrobio.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 4.Principi N, Esposito S. Macrolide-resistant Mycoplasma pneumoniae: its role in respiratory infection. J Antimicrob Chemother. 2013;68(3):506–11. doi: 10.1093/jac/dks457. [DOI] [PubMed] [Google Scholar]
- 5.Pereyre S, Goret J, Bébéar C. Mycoplasma pneumoniae: current knowledge on Macrolide Resistance and Treatment. Front Microbiol. 2016;7:974. doi: 10.3389/fmicb.2016.00974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsubara K, Morozumi M, Okada T, et al. A comparative clinical study of macrolide-sensitive and macrolide-resistant Mycoplasma pneumoniae infections in pediatric patients. J Infect Chemother. 2009;15(6):380–3. doi: 10.1007/s10156-009-0715-7. [DOI] [PubMed] [Google Scholar]
- 7.Kim K, Jung S, Kim M, Park S, Yang HJ, Lee E. Global trends in the proportion of Macrolide-Resistant Mycoplasma pneumoniae infections: a systematic review and Meta-analysis. JAMA Netw Open. 2022;5(7):e2220949. doi: 10.1001/jamanetworkopen.2022.20949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao F, Liu G, Wu J, et al. Surveillance of macrolide-resistant Mycoplasma pneumoniae in Beijing, China, from 2008 to 2012. Antimicrob Agents Chemother. 2013;57(3):1521–3. doi: 10.1128/AAC.02060-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang HJ, Song DJ, Shim JY. Mechanism of resistance acquisition and treatment of macrolide-resistant Mycoplasma pneumoniae pneumonia in children. Korean J Pediatr. 2017;60:167–74. doi: 10.3345/kjp.2017.60.6.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dai W, Wang H, Zhou Q, et al. The concordance between upper and lower respiratory microbiota in children with Mycoplasma pneumoniae pneumonia. Emerg Microbes Infect. 2018;7:92. doi: 10.1038/s41426-018-0097-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang C, Zhang Q, Du JL, et al. Correlation between the clinical severity, bacterial load, and inflammatory reaction in children with Mycoplasma Pneumoniae Pneumonia. Curr Med Sci. 2020;40:822–8. doi: 10.1007/s11596-020-2261-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okada T, Morozumi M, Tajima T, et al. Rapid effectiveness of minocycline or doxycycline against macrolide-resistant Mycoplasma pneumoniae infection in a 2011 outbreak among Japanese children. Clin Infect Dis. 2012;55(12):1642–9. doi: 10.1093/cid/cis784. [DOI] [PubMed] [Google Scholar]
- 13.National Health Commission of the People’s Republic of China SAoTCM Guideline for diagnosis and treatment of community acquired pneumoniain children(2019 version) Chin J Clin Infect Dis. 2019;12(1):1674–2397. [Google Scholar]
- 14.Hsieh YC, Tsao KC, Huang CG, et al. Life-threatening pneumonia caused by macrolide-resistant Mycoplasma pneumoniae. Pediatr Infect Dis J. 2012;31(2):208–9. doi: 10.1097/INF.0b013e318234597c. [DOI] [PubMed] [Google Scholar]
- 15.Okazaki N, Narita M, Yamada S, et al. Characteristics of macrolide-resistant Mycoplasma pneumoniae strains isolated from patients and induced with erythromycin in vitro. Microbiol Immunol. 2001;45(8):617–20. doi: 10.1111/j.1348-0421.2001.tb01293.x. [DOI] [PubMed] [Google Scholar]
- 16.Wang Yacui1 WX Liu Fang3 YQ, Jieqiong1 L. Epidemiological characteristics and macrolide-resistance of children hospitalized with Mycoplasma pneumoniae infection in Beijing from 2016 to 2019. Chin J Appl Clin Pediatr. 2022;37(14):1082–5. [Google Scholar]
- 17.Bébéar CM, Pereyre S. Mechanisms of drug resistance in Mycoplasma pneumoniae. Curr Drug Targets Infect Disord. 2005;5(3):263–71. doi: 10.2174/1568005054880109. [DOI] [PubMed] [Google Scholar]
- 18.Lee H, Yun KW, Lee HJ, Choi EH. Antimicrobial therapy of macrolide-resistant Mycoplasma pneumoniae pneumonia in children. Expert Rev Anti Infect Ther. 2018;16(1):23–34. doi: 10.1080/14787210.2018.1414599. [DOI] [PubMed] [Google Scholar]
- 19.Waites KB, Xiao L, Liu Y, Balish MF, Atkinson TP. Mycoplasma pneumoniae from the respiratory tract and Beyond. Clin Microbiol Rev. 2017;30(3):747–809. doi: 10.1128/CMR.00114-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Y, Ye X, Zhang H, et al. Antimicrobial susceptibility of Mycoplasma pneumoniae isolates and molecular analysis of macrolide-resistant strains from Shanghai, China. Antimicrob Agents Chemother. 2009;53(5):2160–2. doi: 10.1128/AAC.01684-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin M, Shi L, Huang A, Liang D, Ge L, Jin Y. Efficacy of levofloxacin on macrolide-unresponsive and corticosteroid-resistant refractory Mycoplasma pneumoniae pneumonia in children. Ann Palliat Med. 2019;8(5):632–9. doi: 10.21037/apm.2019.10.05. [DOI] [PubMed] [Google Scholar]
- 22.Lung DC, Yip EK, Lam DS, Que TL. Rapid defervescence after doxycycline treatment of macrolide-resistant Mycoplasma pneumoniae-associated community-acquired pneumonia in children. Pediatr Infect Dis J. 2013;32(12):1396–9. doi: 10.1097/INF.0b013e3182a25c71. [DOI] [PubMed] [Google Scholar]
- 23.Lee H, Choi YY, Sohn YJ et al. Clinical efficacy of doxycycline for treatment of Macrolide-Resistant Mycoplasma pneumoniae Pneumonia in Children. Antibiot (Basel). 2021;10(2). [DOI] [PMC free article] [PubMed]
- 24.Suzuki S, Yamazaki T, Narita M, et al. Clinical evaluation of macrolide-resistant Mycoplasma pneumoniae. Antimicrob Agents Chemother. 2006;50(2):709–12. doi: 10.1128/AAC.50.2.709-712.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang EA, Kang HM, Rhim JW, Kang JH, Lee KY. Early corticosteroid therapy for Mycoplasma pneumoniae pneumonia irrespective of used antibiotics in children. J Clin Med. 2019;8(5). [DOI] [PMC free article] [PubMed]
- 26.Gavrilovic S, Andrijevic A, Mujakovic A, Odeyemi Y, Paralija B, Gajic O. Adjunct corticosteroid treatment in patients with pneumonia: a precision medicine approach. Bosn J Basic Med Sci. 2019;19(4):315–20. doi: 10.17305/bjbms.2019.3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song HM. Rational use of glucocorticoids. Zhonghua Er Ke Za Zhi. 2018;56(3):161–2. doi: 10.3760/cma.j.issn.0578-1310.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 28.Carris NW, Pardo J, Montero J, Shaeer KM. Minocycline as a substitute for doxycycline in targeted scenarios: a systematic review. Open Forum Infect Dis. 2015;2(4):ofv178. doi: 10.1093/ofid/ofv178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith K, Leyden JJ. Safety of doxycycline and minocycline: a systematic review. Clin Ther. 2005;27(9):1329–42. doi: 10.1016/j.clinthera.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 30.Forti G, Benincori C. Doxycycline and the teeth. Lancet. 1969;1(7598):782. doi: 10.1016/S0140-6736(69)91787-5. [DOI] [PubMed] [Google Scholar]
- 31.Todd SR, Dahlgren FS, Traeger MS, et al. No visible dental staining in children treated with doxycycline for suspected Rocky Mountain spotted fever. J Pediatr. 2015;166(5):1246–51. doi: 10.1016/j.jpeds.2015.02.015. [DOI] [PubMed] [Google Scholar]
- 32.Volovitz B, Shkap R, Amir J, Calderon S, Varsano I, Nussinovitch M. Absence of tooth staining with doxycycline treatment in young children. Clin Pediatr (Phila) 2007;46(2):121–6. doi: 10.1177/0009922806290026. [DOI] [PubMed] [Google Scholar]
- 33.Sánchez AR, Rogers RS, 3rd, Sheridan PJ. Tetracycline and other tetracycline-derivative staining of the teeth and oral cavity. Int J Dermatol. 2004;43(10):709–15. doi: 10.1111/j.1365-4632.2004.02108.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request. All the original data presented in the study are included in the article.