ABSTRACT
Immunotherapy has shaped the treatment approach to diffuse large B-cell lymphoma (DLBCL), with rituximab leading to remarkable improvements in outcomes for both relapsed and treatment-naïve patients. Recently, groundbreaking immunotherapies like chimeric antigen receptor T-cells have entered the treatment arena for relapsed/refractory (R/R) DLBCL and gained regulatory approval in several countries. The concept of harnessing a patient’s own T-cells to combat cancer has been further explored through the development of bispecific antibodies (BsAbs), a class of engineered antibody products designed to simultaneously target two different antigens. These novel drugs have demonstrated impressive single-agent activity and manageable toxicity in patients with heavily pretreated B-cell non-Hodgkin lymphoma. In this review, we provide an up-to-date overview of recently completed or ongoing BsAbs trials in patients with R/R DLBCL, including single-agent results, emerging combination data, and novel constructs.
KEYWORDS: Bispecific antibodies, DLBCL, Epcoritamab, Glofitamab, Immunotherapy
1. Introduction
Relapsed or refractory (R/R) diffuse large B-cell lymphoma (DLBCL) represents a medical challenge, especially for patients affected by primary refractory or early relapsing disease after front-line anthracycline-based chemoimmunotherapy.1 Platinum-based combinations followed by high-dose therapy and autologous stem cell support (ASCS) have historically constituted the mainstay of second-line therapy, with an estimated 15%–20% cure rate in the rituximab era.2 Patients not eligible for, or relapsing after two or more treatment lines exhibit poor outcomes, with an overall survival (OS) estimated in months.1
While the advent of targeted agents such as polatuzumab vedotin,3 tafasitamab,4 and loncastuximab5 has resulted in incremental benefits for patients with R/R DLBCL, some of the greatest advances in this space have been made with the introduction of T‐cell-based immunotherapies, namely chimeric antigen receptor (CAR-) T cells, with mature data showing durable remissions in 30%–40% of patients.6–8 More recently, two randomized trials have shown the superiority of both axicabtagene ciloleucel and lisocabtagene maraleucel over standard second-line therapy in patients with high-risk R/R DLBCL.9,10
Despite significant clinical efficacy, several impediments stand in the way of effective CAR-T cell therapy delivery, including limited access outside large tertiary care centers, complex insurance approval processes, high costs, increased demand vis-à-vis limited manufacturing capability, and potentially long product turnaround, among others. Bispecific antibodies (BsAbs) are a novel class of off-the-shelf T-cell redirecting drugs with promising activity in B-cell non-Hodgkin lymphoma and the potential to play a major role in the treatment of R/R DLBCL.
Along the lines of the Trial Watch series,11–14 here we provide a state-of-the-art overview of BsAb trials for patients with R/R DLBCL, including single-agent and combination data, as well as a look ahead at the future of this field.
2. Structural properties of bispecific antibodies
BsAbs comprise a class of engineered antibody products designed to simultaneously target two different antigens. Various bioengineering technologies have been used for their production, each resulting in constructs with unique structural and pharmacologic properties.15 Early BsAbs were derived from fragments of monospecific Ab fused together. Such structure requires the administration of these drugs through a continuous intravenous (IV) infusion due to short half-life and rapid clearance from plasma. A major advance has been the introduction of immunoglobulin (Ig)Glike BsAbs, in which the preservation of an Fc region confers longer half-life and allows natural FcRn-mediated recycling processes.16 The first and most extensively studied method for producing IgG-like BsAbs has been the ‘knobs-into-holes’ technology, where complementary mutations are introduced within the CH3 domain of each antibody component, thus ensuring consistent pairing of heavy chains.17 Another approach utilizes the ‘knobs-into-holes’ or a similar technology to facilitate heterodimerization of heavy chains, while addressing light chain mispairing through domain crossover between the antibody’s variable and constant regions.18 Most BsAbs also feature silencing mutations in the Fc region, which abrogate untoward T-cell activation and fratricidal killing through antibody-dependent cellular cytotoxicity (ADCC) and complement fixation (CDC).18
BsAbs’ cytotoxicity is thought to be driven by intratumoral and peripheral endogenous immune cells recruited by simultaneously targeting tumor and immune effector cell antigens. The so formed immunological synapse triggers T-cell activation and cytotoxic killing in a major histocompatibility complex (MHC)-independent manner.15 The latter is a key mechanistic feature, as many DLBCL patients frequently exhibit genetic aberrations that abolish expression of MHC class I molecules.19
While various B‐cell target antigens have been tested (CD19, CD20, CD22, CD37, and CD79b)20–23 BsAbs against CD3 × CD20 have thus far undergone the most extensive clinical development. Structurally, these agents may possess one or more CD20-binding Fabs in different spatial configurations, conferring different target affinity and in vitro potency.16,24–26 Several CD20 × CD3 BsAbs, including glofitamab, epcoritamab, mosunetuzumab, and odronextamab, have been evaluated in clinical trials (Figure 1) and will be described below.
Figure 1.
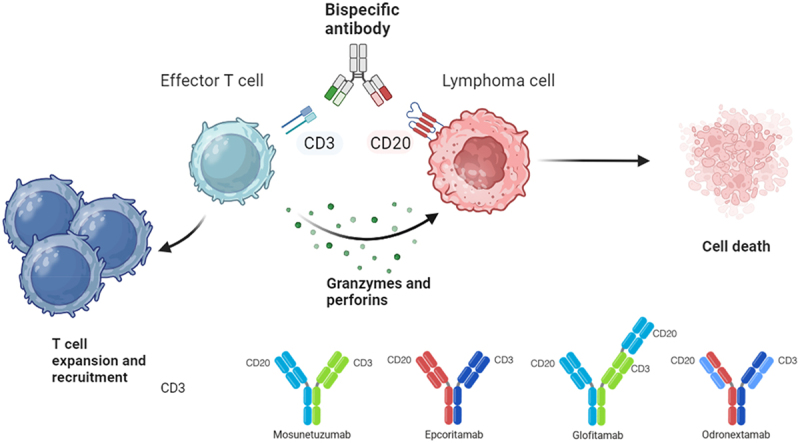
Mechanism of action of antiCD20 and antiCD3 bispecific antibodies. Mosunetuzumab, IgG1 ab with a rituximab-like antiCD20 domain; epcoritamab, IgG1 ab with an ofatumumab-like antiCD20 domain; glofitamab, IgG1 ab with a ratio 2:1 CD20:CD3 and an obinutuzumab-like antiCD20 domain; odronextamab, IgG4 ab with an ofatumumab-like antiCD20 domain.Illustration created with biorender-individual version.
3. Toxicity overview
The most important adverse events (AEs) associated with BsAb therapy in clinical trials were due to T-cell overactivation. Among these, cytokine release syndrome (CRS) was the most frequent, observed in 15%–80% of patients, depending on the specific agent, route of administration, and dosing regimen used.27–29 CRS onset occurred primarily during the initial treatment cycle, reflecting target-dependent T-cell activation. Clinically, this syndrome manifested with a range of symptoms, including chills, fever, hypotension, hypoxia, and confusion, usually emerging within the first 2 d post-administration and resolving within 3 d.
Although the term ‘immune effector cell – associated neurotoxicity syndrome (ICANS) – like’ was coined to characterize the neurological toxicity, prompted by its similarity to the syndrome observed following CAR-T administration (ICANS),30 its underlying pathogenesis remains less clear. Unlike CAR-T cells,31 IgG-like BsAbs are not expected to breach the blood–brain barrier and information on the presence of activated T cells or inflammatory cytokines in the cerebrospinal fluid (CSF) of affected patients is scarce. Neurological symptoms included delirium, dysphasia, tremor, lethargy, difficulty concentrating, agitation, aphasia, depressed level of consciousness, encephalopathy, seizures, and cerebral edema.
Various strategies were explored to mitigate T-cell-driven AEs, including step-up dosing during cycle 1,32,33 slower intravenous infusion,28,34 and prophylactic corticosteroid administration.35 Additionally, pretreatment with obinutuzumab was implemented in glofitamab trials based on preclinical evidence suggesting that depleting circulating B cells may attenuate T-cell activation.24
Cytopenias were frequently seen following treatment with BsAbs, especially neutropenia (15%–33%) and anemia (19%–38%), primarily of grades 1–2.36 Their pathogenesis, although poorly understood, is likely multifactorial in nature (e.g. related to disease involvement, prior therapies, and direct BsAb effect). Infectious complications were often linked to neutropenia and B-cell impairment and encompassed febrile neutropenia (affecting <5% of patients), urinary tract infections, and pneumonia.27,34,37
Overall, the safety profile of BsAbs proved to be consistent across trials, with the majority of AEs being manageable and leading to rare treatment interruptions or discontinuations. Consensus recommendations were recently published to aid clinicians in recognizing and managing BsAbrelated toxicities.38
4. Single-agent trials
The first T-cell redirecting antibody to show activity in B-cell non-Hodgkin lymphoma (NHL) was blinatumomab, a CD19 × CD3 fusion protein comprised of two single-chain Fragment variable (scFv) moieties. Despite encouraging clinical results,39 the requirement for multi-week continuous IV administration and the dose-limiting neurologic toxicity hindered further clinical development in lymphoma. In contrast, the more convenient dosing schedule and favorable safety profile seen with CD20 × CD3 IgG-like BsAbs have facilitated their development in this setting.
Mosunetuzumab, the first-in-class anti-CD3 × CD20 BsAb, was evaluated in B-NHL patients in a large phase 1/2 study (NCT02500407)29 with both fixed and step-up dosing schedules (the latter implemented to mitigate T-cell activation-related toxicities, see below). All patients received the drug IV every 3 weeks for up to eight cycles (for patients achieving a complete response (CR)) or 17 cycles (for those achieving partial response after eight cycles). The recommended phase 2 dose (RP2D) was 30 mg, after two initial 60 mg loading doses. Among 197 patients enrolled in the step-up dosing cohort, 129 with aggressive B-NHL (aNHL) achieved an overall response rate (ORR) and CR rate of 35% and 19%, respectively. Common AEs included CRS (27.4%), neutropenia (28.4%) and hypophosphatemia (23.4%). The most common neurologic AEs reported were headache (17.8%), insomnia (11.2%) and dizziness (10.2%).
An update of the dose expansion DLBCL cohort was recently published40 (Table 1). Eighty-eight patients received IV mosunetuzumab at the dose of 30 mg, achieving an ORR and CR rate of 40% and 23.9%, respectively. CR rates were consistent among patients with non-germinal center B (non-GCB) DLBCL (28%), transformed FL (tFL, 26%), disease refractory to previous anti-CD20 therapies (21%) and age ≥65 y (29%). Of note, patients with double-hit (DH)/triple-hit (TH) lymphoma (N = 17) and those with previous CAR-T therapy failure (N = 26) demonstrated lower CR rates of 6% and 12%, respectively. The median progression-free survival (mPFS) was 3.2 months. No new safety signals were recorded and the most common AEs were neutropenia (27.3%) and CRS (26.1%). The latter was mostly of grade 1–2, with only two patients experiencing a grade 3 event. Neurologic AEs occurred in two patients, and both were mild. While the development of mosunetuzumab as a stand-alone therapy for DLBCL has been limited, ongoing clinical trials are evaluating it in the R/R setting as consolidation therapy following ASCS (NCT05412290), CAR-T (NCT04889716), or in the first-line setting following immunochemotherapy (NCT03677154) (Table 3).
Table 1.
BsAb single-agent studies in r/r aNHL patients with published results.
| Clinical Trial | Phase | Drug(s) | Histology | Modifiers | N | ORR | CR | mPFS (months) | mDOR (months) | Grade 3–4 CRS |
Grade 3–4 ICANS |
Grade 3–4 NP |
mFU (months) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NCT02500407 | I/II | MOSUNETUZUMAB | DLBCL | Dose expansion cohort | 88 | 40% | 24% | 3.2 | 7 | 2.3% | None | 21.6% | 10.1 |
| NCT03625037 | I/II | EPCORITAMAB | DLBCL | Dose expansion cohort | 157 | 63% | 39% | 4.4 | 12 | 2.5% | 0.6% | 14.6% | 10.7 |
| NCT03075696 | II | GLOFITAMAB | DLBCL | Dose expansion cohort | 155 | 52% | 39% | 4.4 | 18.4 | 4% | 3% | 27% | 12.6 |
| NCT04657302 | I | GLOFITAMAB | DLBCL | Dose expansion cohort | 27 | 67% | 52% | 8.6 | 14.4 | 3.3% | 3.3% | 30% | 15 |
| NCT03888105 | II | ODRONEXTAMAB | DLBCL | Dose expansion cohort | 127 | 52% | 31% | N/A | 10.2 | 0.7% | None | N/A | 26.2 |
| NCT02924402 | I | PLAMOTAMAB | DLBCL | Dose escalation cohort | 19 | 47% | 26% | N/A | N/A | None | None | 16.7% | N/A |
| NCT04082936 | I | IgM-2323 | B-NHL | Dose escalation cohort | 23 | 35% | 22% | N/A | N/A | 3.4% | N/A | N/A | N/A |
| NCT04594642 | I | AZD0486 | DLBCL | Dose escalation cohort | 5 | 40% | 20% | N/A | N/A | None | 7% | 15% | 3.8 |
| NCT04923048 | I/II | GB261 | B-NHL | Dose escalation cohort | 22 | 73% | 45% | N/A | NR | None | None | 14.9% | 4.1 |
Reported abstract data refer to the time of their publication. DLBCL includes DLBCL, not otherwise specified, high-grade B-cell lymphoma, and transformed indolent NHL. Abbreviations: CRS, cytokine release syndrome; ICANS, immune effector cell-associated neurotoxicity syndrome; NP, neutropenia; ORR, overall response rate; CR, complete response; mFU, median follow-up; mPFS, median progression-free survival; mDOR, median duration of response; NR, not reached; N/A, not assessed.
Table 3.
BsAb studies in R/R aNHL patients without published results.
| Clinical Trial | Phase | Drug(s) | Histology | Comments | Primary Endpoints | Key Secondary endpoints | Estimated Enrollment |
|---|---|---|---|---|---|---|---|
| Planned ASCS Consolidation | |||||||
| NCT05464329 | I | MOSUNETUZUMAB + DHAX/ICE | B-NHL | Planned ASCS after cycle 3 | Frequencies and grades of TEAEs | CR rates; PFS and OS | 40 |
| NCT05852717 | II | EPCORITAMAB + GDP | DLBCL | Planned ASCS after cycle 3 | CR rates | ORR; PFS, DOR and OS; feasibility of ASCS or CAR-T cell consolidation | 32 |
| NCT05364424 | I | GLOFITAMAB + R-ICE | DLBCL | Planned ASCS after cycle 3 | ORR | CR rates; PFS, DOR and OS; TEAEs | 40 |
| Planned CAR-T Consolidation | |||||||
| NCT05260957 | II | MOSUNETUZUMAB + POLATUZUMAB | B-NHL | Mosunetuzumab + Anti-CD79b ADC; Planned CAR-T therapy after cycle 8 | CR rates | ORR; PFS, DOR and OS; CRS and ICANS rates following CAR-T | 40 |
| NCT05852717 | II | EPCORITAMAB + GDP | DLBCL | Planned CAR-T therapy after cycle 3 | CR rates | ORR; PFS, DOR and OS; feasibility of ASCS or CAR-T cell consolidation | 32 |
| No Consolidation Planned: Single Agent | |||||||
| NCT05412290 | I | MOSUNETUZUMAB | B-NHL | Administered following ASCS | Frequencies and grades of TEAEs | PFS and OS following ASCS | 15 |
| NCT04889716 | II | MOSUNETUZUMAB | B-NHL | Administered following CAR-T | CR rates; DLT | PFS, DOR and OS | 42 |
| NCT03677154 | I/II | MOSUNETUZUMAB | DLBCL | Administered either SC or IV following 1st line therapy | CR rates; ORR; frequencies and grades of TEAEs | PFS, DOR and OS; quality of life | 188 |
| NCT05451810 | II | EPCORITAMAB | B-NHL | Administered as Outpatient | Frequencies and grades of TEAEs | CR rates; best ORR; PFS, DOR and OS | 184 |
| NCT04628494 | III | EPCORITAMAB vs chemotherapy | DLBCL | Investigator’s choice as control arm, either BR or R-GemOx | OS | CR rates; ORR; PFS, DOR and TTNT; rate and duration of MRD negative status; TEAEs; quality of life | 552 |
| NCT04703686 | II | GLOFITAMAB | B-NHL | For patients with relapse after CAR-T | OS | CR rates; ORR; PFS, DOR; quality of life | 78 |
| NCT05619367 | ODRONEXTAMAB | B-NHL | Compassionate use program | N/A | N/A | N/A | |
| NCT05991388 | II | ODRONEXTAMAB | B-NHL | For pediatric and young adult patients | CR rates; ORR | PFS, DOR and OS; frequencies and grades of TEAEs | 210 |
| No Consolidation Planned: Combinations with Chemotherapeutic Agents | |||||||
| NCT04313608 | I | MOSUNETUZUMAB/GLOFITAMAB + GemOx | DLBCL | 8 cycles of combined therapy. Glofitamab monotherapy continued until C12 |
Frequencies and grades of TEAEs | CR rates; ORR; pharmacokinetics | 23 |
| NCT05533775 | I/II | GLOFITAMAB + R-ICE | B-NHL | Pediatric and young adult patients | CR rates; frequencies and grades of TEAEs | ORR; PFS, DOR and OS | 65 |
| NCT04408638 | III | GLOFITAMAB + GemOx vs R-GemOx |
DLBCL | 8 cycles of combined therapy. Glofitamab monotherapy continued until C12 |
OS | ORR; PF and DOR; frequencies and grades of TEAEs; quality of life | 270 |
| No Consolidation Planned: Combinations with Non-Chemotherapeutic Agents | |||||||
| NCT04970901 | I | MOSUNETUZUMAB/GLOFITAMAB + LONCASTUXIMAB | B-NHL | Mosunetuzumab/Glofitamab + anti-CD19 ADC | Frequencies and grades of TEAEs | CR rates; ORR; PFS, DOR; pharmacokinetics | 200 |
| NCT05672251 | II | MOSUNETUZUMAB + LONCASTUXIMAB | DLBCL | Mosunetuzumab + anti-CD19 ADC | ORR; Frequencies and grades of TEAEs | CR rates; ORR; PFS, DOR; TEAEs | 36 |
| NCT05171647 | III | MOSUNETUZUMAB + POLATUZUMAB vs R-GemOx |
B-NHL | Mosunetuzumab + anti-CD79b ADC | PFS | CR rates; ORR; DOR and OS; TEAEs; quality of life | 222 |
| NCT05315713 | I/II | MOSUNETUZUMAB + TIRAGOLUMAB ± ATEZOLIZUMAB | B-NHL | Mosunetuzumab + anti-TIGIT Ab ± anti-PDL1 Ab | Frequencies and grades of TEAEs; ORR | CR rate; PFS, DOR and OS | 118 |
| NCT05615636 | II | MOSUNETUZUMAB + POLATUZUMAB + TAFASITAMAB + LENALIDOMIDE | DLBCL | Mosunetuzumab + anti-CD79b ADC + anti-CD19 Ab + immune modulator | Best ORR | N/A | 36 |
| NCT05169515 | I | MOSUNETUZUMAB + CC-220 MOSUNETUZUMAB + CC-99282 GLOFITAMAB + CC-99282 |
B-NHL | Mosunetuzumab/Glofitamab + cereblon E3 ligase modulator (CELMoD) | Best ORR; DLT; frequencies and grades of TEAEs | Best CR rate; PFS, DOR and OS up to 2 y; serum concentration of CC-220 and CC-99282 | 121 |
| NCT05283720a | I/II | EPCORITAMAB + immuno-modulating agents | B-NHL | Multi-arm study. | DLT | Best ORR; CR rates; PFS, DOR, OS and TTNT up to 5 y; rate and duration of MRD negative status | 394 |
| NCT04077723 | I/II | GLOFITITAMAB + RO7227166 | B-NHL | Glofitamab + CD19×CD137 BsAb | DLT; TEAEs; CR rates; ORR; PFS, DOR and OS | Pharmacokinetics; quality of life | 46 |
| NCT05219513 | I | GLOFITAMAB + RO7443904 | B-NHL | Glofitamab + CD19×CD28 BsAb | Frequencies and grades of TEAEs | Pharmacokinetics | 200 |
| NCT05896163 | I/II | GLOFITAMAB + MAPLIRPACEPT | DLBCL | Glofitamab + anti-CD47 Ab | DLT; ORR | CR rates; PFS and DOR; TEAEs | 70 |
| NCT02651662 | I | ODRONEXTAMAB + CEMIPLIMAB | B-NHL | Odronextamab + anti-PD1 Ab | Frequencies and grades of TEAEs | CR rates; ORR; DOR | 62 |
| NCT05685173 | I | ODRONEXTAMAB + REGN5837 | B-NHL | Odronextamab + CD22×CD28 BsAb | Frequencies and grades of TEAEs | CR rates; ORR; PFS, DOR and OS | 91 |
| NCT05328102 | II | PLAMOTAMAB + TAFASITAMAB + LENALIDOMIDE vs TAFASITAMAB + LENALIDOMIDE |
DLBCL | Study terminated early by the sponsor | frequencies and grades of TEAEs; PFS | N/A | 3 |
| Novel Constructs | |||||||
| NCT05210868 | I/II | CM355 | B-NHL | CD3×CD20 BsAb | frequencies and grades of TEAEs; ORR | CR rates; ORR; PFS, DOR and OS; pharmacokinetics endpoints | 184 |
| NCT05618327 | I | JS203 | B-NHL | CD3×CD20 BsAb | DLT | CR rates; ORR; PFS, DOR and OS; TEAEs; pharmacokinetics | 219 |
| NCT04056975 | I | A-319 | B-NHL | CD3×CD19 BsAb | Frequencies and grades of TEAEs | Pharmacokinetics endpoints | 54 |
| NCT04540796 | I | JNJ-75348780 | B-NHL | CD3×CD22 BsAb | Frequencies and grades of TEAEs | CR rates; ORR; DOR; pharmacokinetics | 148 |
| NCT05424822 | I | JNJ-80948543 | B-NHL | CD3×CD20×CD79b TsAb | Frequencies and grades of TEAEs | CR rates; ORR; DOR | 180 |
| NCT05348889 | I | CMG1A46 | B-NHL | CD3×CD19×CD20 TsAb | Frequencies and grades of TEAEs | CR rates; ORR; PFS and OS; pharmacokinetics | 165 |
| NCT05397496 | I | PIT565 | B-NHL | CD3×CD2×CD19 TsAb | Frequencies and grades of TEAEs | CR rates; ORR; PFS, DOR; pharmacokinetics | 140 |
| NCT05623982 | I/II | EMFIZATAMAB | B-NHL | CD3×CD137×PD-L1×CD19 TesAb | Frequencies and grades of TEAEs | CR rates; ORR; PFS, DOR and OS; pharmacokinetics | 40 |
| NCT06088654 | I/II | IPH6501 | B-NHL | NKp46×CD16a×CD122×CD20 TesAb | Frequencies and grades of TEAEs | ORR; DOR and PFS; pharmacokinetics | 184 |
Reported abstract data refer to the time of their publication. DLBCL includes DLBCL, not otherwise specified, high-grade B-cell lymphoma, and transformed indolent NHL.
aEpcoritamab investigated in combination with multiple agents across different histologies (DLBCL, FL, MZL). For r/r DLBCL patients, combination therapies encompass lenalidomide, ibrutinib, and CC-99282.
Abbreviations: TEAEs, treatment-emergent adverse events; ADAs, anti-drug antibodies; DLT, dose-limiting toxicity; SC, subcutaneous; IV, intravenous; PFS, progression-free survival; DOR, duration of response; TTNT, time to next treatment; OS, overall survival; ASCS, autologous stem cell support; CAR-T, chimeric antigen receptor T-cell; Cmax, maximum serum concentration; AUC, area under the curve; EORTC, European Organisation for Research and Treatment of Cancer; FACT-Lym, functional assessment of cancer therapy-lymphoma; DHAX, dexamethasone, cytarabine, oxaliplatin; R-ICE, rituximab, ifosfamide, carboplatin, etoposide; GDP, gemcitabine, dexamethasone, cisplatin; R-GemOx, rituximab, gemcitabine, oxaliplatin; BR, bendamustine, rituximab; Ab, antibody; ADC, antibody–drug conjugate; MRD, minimal residual disease; ORR, overall response rate; CR, complete remission; SD, stable disease; PR, partial response; N/A, not assessed; TsAb, trispecific antibody; TesAb, tetraspecific antibody.
Epcoritamab, a subcutaneously administered CD20 × CD3 BsAb produced via controlled Fab arm exchange,25 was initially studied in a phase 1/2 trial in patients with R/R B-NHL (NCT03625037). The drug was administered with a step-up dosing schedule in cycle 1, then weekly for two cycles, every other week for six cycles and every 28-d cycle thereafter, until progression or unacceptable toxicity.35 The RP2D was identified as 48 mg and at this dose level the ORR and CR rate of the eight DLBCL patients treated in the dose escalation cohort were 88% and 38%, respectively. Of note, five patients initially achieving a partial response (PR) subsequently converted to a CR. Epcoritamab demonstrated a favorable safety profile, with no treatment-related discontinuations or deaths.
Results from the dose expansion cohort of the trial was recently published.27 Among 157 patients with R/R DLBCL, ORR and CR rates were 63.1% and 38.9%, respectively, including nine patients who converted their PR into CR after week 36. Epcoritamab exhibited consistent efficacy across high-risk subgroups: CR rates were 30.2% in patients with primary refractory lymphoma (N = 96) and 34.4% in those previously exposed to CAR-T therapy (N = 61). Median PFS for the entire cohort was 4.4 months, though this was not reached in complete responders. The safety profile aligned with prior reports, with CRS (49.7%) and neutropenia (21.7%) being the most common AEs. Ten patients experienced neurologic toxicity, including one fatal event. Based on these results, in May 2023, the FDA granted accelerated approval to epcoritamab for treatment of adult patients with R/R DLBCL, not otherwise specified, including DLBCL arising from indolent lymphoma, and high-grade B-cell lymphoma after two or more lines of systemic therapy.41
A phase 3 trial of epcoritamab vs. physician’s choice in patients with R/R DLBCL ineligible for curative therapy is currently underway (NCT04628494). Finally, a study (NCT05451810) is currently evaluating the feasibility of outpatient subcutaneous administration.
Glofitamab is a peculiar CD20 × CD3 BsAb with two CD20-binding moieties and one CD3-binding site (also called ‘2:1 format’) created by domain crossover and head-to-tail fusion.28 It was initially studied in a phase 1 trial (NCT03075696) including 171 R/R B-NHL patients, 73 of whom had DLBCL.28 The CRS mitigating strategy included the step-up dosing and a pretreatment of 1000 mg dose of obinutuzumab. In the study, glofitamab was administered IV every 2 or 3 weeks. For all DLBCL patients, ORR was 41% and CR was 29%. Across the whole cohort, 50% of patients developed CRS, grade 1–2 in 47% and grade 3–4 in only 3%. ICANS-like symptoms were reported in nine patients, with no grade 3–4 events. Grade ≥3 neutropenia occurred in 25% of patients.
The recently published phase 2 expansion cohort (NCT03075696) enrolled 155 patients with R/R DLBCL treated at the target dose of 30 mg for up to 12 3-week cycles.37 The ORR and CR rates were 52% and 39%, respectively. Complete responses were similar among patients with a history of previous CAR T-cell therapy (35%) and those without (42%).37 Based on these results, in June 2023, the FDA granted accelerated approval to glofitamab for the treatment of adult patients with R/R DLBCL, not otherwise specified, or large B-cell lymphoma (LBCL) arising from follicular lymphoma, after two or more lines of systemic therapy.42
An updated analysis, after a median time on study of 25.8 months, revealed a median duration of CR (DoCR) of 26.9 months among all patients and 22.0 months in those previously treated with CAR-T cell therapy.43 For patients achieving a CR at the end of treatment (EOT), the 12-month PFS and OS rates were 80% and 90%, respectively. These findings suggest the potential for favorable long-term outcomes with the fixed-duration use of glofitamab in R/R LBCL.43
Study NCT04657302 is exploring glofitamab as a single agent in Chinese patients and showed an ORR of 67% and a CR rate of 52% among 27 patients; 8 out of 14 patients in CR had ongoing responses after a median follow-up of 15 months.44 Finally, a French-led phase 2 study is exploring glofitamab in patients who have relapsed after CAR-T therapy (NCT04703686, currently recruiting), a challenging clinical scenario.
Odronextamab is a fully humanized heterodimeric IgG4-based BsAb that was initially studied (NCT02290951) in a phase 1/2 trial in 145 patients with R/R B-NHL at doses ranging from 0.1 to 320 mg weekly for 9 weeks, then every other week until progression. In DLBCL patients without prior CAR-T therapy (N = 15), at 80 mg or higher, odronextamab achieved a 53% CR rate34 while, in the post-CAR-T setting (N = 44) at the RP2D of 160 mg, the CR rate was 30%.45 Common AEs included CRS (61%), anemia (38%), fatigue (33%), and neutropenia (25%). ICANS-like symptoms were reported in 12% of patients, with 3% exhibiting symptoms of grade ≥3.34
To further mitigate the CRS risk, a split step-up dosing approach was explored (NCT03888105). Among 127 evaluable patients, the ORR was 52%, with a CR rate of 31% with no significant differences in activity among selected high-risk subgroups. The toxicity profile was similar to that observed with other CD3 × CD20 BsAbs, including rates of CRS (55%), anemia (43%), and pyrexia (42%). Only one grade 3 CRS and no ICANS-like events were recorded with this optimized step-up regimen. Fourteen patients (10%) discontinued odronextamab due to treatment-related AE.46
Study NCT05991388 is exploring odronextamab in pediatric and young adult patients, while an open compassionate use program is available for enrollment (NCT05619367, Table 3).
Plamotamab (a fully humanized CD3 × CD20 BsAb) and imvotamab (IgM-like CD20 × CD3 BsAb) have been evaluated in the phase 1 NCT02924402 and NCT04082936 clinical trials, respectively, where they exhibited early clinical activity without safety signals.47,48 Their clinical development, however, is less clear at present.
5. Combination trials
Pre-clinical work suggested that the BsAb-mediated T-cell cytotoxicity may be retained with the co-administration of traditional T-cell cytotoxic agents like cyclophosphamide or dexamethasone.49,50 Furthermore, glofitamab and epcoritamab remained active in combination with anti‐CD20 monoclonal antibodies due to partially non-overlapping epitopes, lack of competition for the FcγR, and high activity even at low receptor occupancy rates.36 These observations sparked interest for BsAb-containing combination studies.
In transplant eligible patients with R/R DLBCL (Table 2), epcoritamab was combined with R-DHAX/C (dexamethasone, high-dose cytarabine, and oxaliplatin/carboplatin) as salvage prior to high-dose therapy (HDT)-ASCS in a phase 1/2 study (NCT04663347, arm 4).51 Patients who deferred ASCS were allowed to continue the BsAb until progression or unacceptable toxicity. Of 26 response-evaluable patients, 15 proceeded to consolidation, achieving an ORR of 100% and a CR rate of 80%. The remaining 11 who continued epcoritamab without HDT-ASCS had an ORR of 64% and a CR rate of 45%. CRS rate and severity were similar to those seen with single-agent epcoritamab; 21% of patients experienced grade 3 or 4 infections and one patient experienced grade 2 neurological toxicity.
Table 2.
BsAb combination studies in r/r aNHL patients with published results.
| Clinical Trial | Phase | Drug(s) | Modifiers | Histology | N | ORR | CR | mPFS (months) | mDOR (months) | G. 3–4 CRS | G. 3–4 ICANS | G. 3–4 NP | mFU (months) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Planned ASCS Consolidation | |||||||||||||
| NCT04663347 | I/II | EPCORITAMAB + R-DHAX/C |
Planned ASCS after cycle 3 | DLBCL | 15 | 100% | 80% | N/A | NR | None | None | N/A | 9.2 |
| No ASCS consolidation | |||||||||||||
| NCT04663347 | I/II | EPCORITAMAB + R-DHAX/C |
3 cycles of combined therapy. Epcoritamab monotherapy continued until PD or toxicity | DLBCL | 11 | 64% | 45% | N/A | NR | None | None | N/A | 9.2 |
| NCT04663347 | I/II | EPCORITAMAB + GemOx | 4 cycles of combined therapy. Epcoritamab monotherapy continued until PD or toxicity | DLBCL | 34 | 91% | 59% | N/A | N/A | 3% | None | N/A | 20.3 |
| NCT05283720 | I/II | EPCORITAMAB + LENALIDOMIDE | 12 cycles of combined therapy | DLBCL | 24 | 75% | 58% | N/A | N/A | 8% | 3.8% | 58% | N/A |
| NCT03671018 | II | MOSUNETUZUMAB + POLATUZUMAB | 6 cycles of combined therapy. If CR after C8, mosunetuzumab discontinued. If SD or PR after C8, mosunetuzumab continued up to C17 | DLBCL | 98 | 59% | 46% | 11.4 | 20.8 | 3.1% | 2% | 20.4% | 23.9 |
| NCT03533283 | I/II | GLOFITAMAB + POLATUZUMAB | 6 cycles of combined therapy. Glofitamab continued up to C12 | DLBCL | 125 | 80% | 59% | 10.4 | N/A | 0.8% | None | N/A | 21.6 |
| NCT05335018 | II | GLOFITAMAB + LENALIDOMIDE + POSELTINIB | 12 cycles of combined therapy | DLBCL | 6 | 100% | 50% | N/A | N/A | None | None | 33% | N/A |
Reported abstract data refer to the time of their publication. DLBCL includes DLBCL, not otherwise specified, high-grade B-cell lymphoma, and transformed indolent NHL. Abbreviations: G., grade; CRS, cytokine release syndrome; ICANS, immune effector cell- associated neurotoxicity syndrome; NP, neutropenia; ASCS, autologous stem cell support; R-DHAX/C, rituximab, dexamethasone, cytarabine, oxaliplatin/carboplatin; GemOx, gemcitabine, oxaliplatin; SUD, step-up dosing; Q3W, every 3 weeks; ORR, overall response rate; CR, complete response; SD, stable disease; PR, partial response; PD, progressive disease; mFU, median follow-up; mPFS, median progression-free survival; mDOR, median duration of response; NR, not reached; N/A, not assessed.
Table 3 outlines ongoing combination trials for epcoritamab (NCT05852717), mosunetuzumab (NCT05464329), and glofitamab (NCT05364424) including preplanned HDT-ASCS, with clinical data yet to be reported. CAR-T consolidation is also being explored in the NCT05260957 and NCT05852717 trial following mosunetuzumab and epcoritamab administration, respectively.
Early results of a study exploring epcoritamab in combination with GemOx (gemcitabine and oxaliplatin) in transplant-ineligible patients have been recently reported. In the NCT04663347 trial arm 5, patients received four cycles of GemOx along with concurrent epcoritamab, and subsequently continued epcoritamab until disease progression or unacceptable toxicity. Among 34 evaluable patients, 53% of whom had a primary refractory disease, the ORR was 91%, and the CR rate was 59%. The CR rates were of 57% and 59% among CAR-T exposed and CAR-T naïve patients, respectively. The most common AEs were thrombocytopenia (68%), diarrhea (59%), neutropenia (56%), and CRS (56%). Most CRS events were of low grade (53%) and only one ICANS event of grade 1 was reported.52
A phase 1/2 trial (NCT03671018) testing the safety and efficacy of combining mosunetuzumab with polatuzumab in patients with R/R DLBCL was recently reported.53 Ninety-eight patients received mosunetuzumab according to the single-agent protocol,40 plus polatuzumab administered on d 1 of cycles 1–6. Fifty-eight patients (59%) had a response, and 45 patients had (46%) a CR. At a median follow-up of 23.9 months, the mPFS was 11.4 months, and the median DoCR was not reached. Among patients who had previously undergone CAR-T therapy (N = 35), a CR was achieved in 40%, with a median PFS of 9.6 months. The incidence of CRS was generally low, with 18% of patients experiencing any grade CRS, and 3% experiencing grade ≥3. Additionally, 5% of patients developed neurotoxicity, with 2% experiencing symptoms of grade ≥3.
A separate phase 1b/2 study (NCT03533283) explored glofitamab in association with polatuzumab.54 Among 125 R/R DLBCL patients treated at the glofitamab target dose of 30 mg, ORR was 80% and CR was 59%. After a median follow-up of 20.4 months, the mPFS and median DoCR were 10.4 and 28.6 months, respectively. CRS was mostly of grade 1–2 (45%), with one CRS-related death. ICANS-like symptoms occurred in four cases (3%), all of grade 1–2.55
Other trials are evaluating mosunetuzumab (NCT04313608) and glofitamab (NCT04313608, NCT05533775, and NCT04408638) in conjunction with either GemOx or R-ICE (rituximab, ifosfamide, carboplatin, and etoposide) for transplant-ineligible patients (Table 3).
6. Novel combinations
During the immune synapse formation, T-cell activation and proliferation are amplified by additional interactions with co-stimulatory receptors, such as CD28 and 4-1BB.56 This notion was leveraged to enhance T-cell-mediated killing and combat T-cell exhaustion during BsAb therapy.57–59 BsAbs simultaneously targeting B-cell antigens and T-cell co-stimulatory receptors have been developed and are currently in clinical trials, as outlined in Table 3.
RO7227166 is a novel BsAb that simultaneously targets CD19 on B-cells and 4-1BB on T-cells and is currently under investigation in the NCT04077723 phase 1 trial in combination with glofitamab. Preliminary findings comprising 56 efficacy-evaluable subjects indicated an ORR of 67% and a CR rate of 39% in R/R DLBCL, with no new safety signals reported.60
A similar antibody, RO7443904, which binds CD19 and CD28, is being studied in combination with glofitamab in another dose escalation trial (NCT05219513). In pre-clinical models, RO7443904 improved glofitamab’s anti-tumor effects by increasing intratumoral T-cells without displaying superagonistic activity or increasing cytokine release.61
REGN5837 is a human CD28 × CD22 IgG4-based bispecific antibody that provides a co-stimulatory signal. When combined with odronextamab, REGN5837 improved anti-tumor efficacy and survival in in vivo DLBCL tumor models via enhanced T-cell expansion.21 This combination is currently being studied in the phase 1 NCT05685173 trial.
Both glofitamab and epcoritamab have been combined with the immunomodulatory agent lenalidomide. A small experience of glofitamab plus lenalidomide and poseltinib, a novel irreversible BTK inhibitor, was recently reported (NCT05335018).62 In the clinical trial EPCORE NHL-5 (NCT05283720), epcoritamab was administered concurrently with other antineoplastic regimens. When combined with lenalidomide63 in patients with R/R DLBCL (N = 24), the ORR was 75%, with a CR rate of 58%. In this rituximab-free regimen, grade 3+ CRS and ICANS were observed in 8% and 4% of patients, respectively (Table 2), suggesting that the safety of epcoritamab and lenalidomide alone needs further exploration.
Ongoing studies are investigating BsAbs combined with different immune-modulatory agents, including the novel cereblon modulators CC-220 and CC-99282 (NCT05169515); the checkpoint inhibitors tiragolumab (NCT05315713), maplirpacept (NCT05896163), and cemiplimab (NCT02651662); tafasitamab (NCT05615636, NCT05328102); and the monoclonal antibody conjugates loncastuximab (NCT04970901, NCT05672251) and polatuzumab (NCT05171647), as outlined in Table 3.
7. Novel multi-specific antibodies
Given the vast combinatorial possibilities, the landscape of multispecific antibodies is rapidly expanding. Exploring alternative antigen targets beyond CD20, co-targeting two separate tumor-associated antigens, or two separate T-cell receptors, are all avenues under investigation to improve the activity of, and/or overcome resistance to, currently available BsAb.
AZD0486 is a novel anti-CD19 × CD3 BsAb designed to reduce CRS by binding to T-cells with low affinity. In a phase 1 dose escalation study (NCT04594642), among five evaluable R/R DLBCL patients, one achieved PR and one achieved CR.64 A recent update of the safety data following the introduction of a two-step-up dosing approach revealed a decrease in the incidence of grade 1–2 CRS from 62.5% to 22.2%, and grade 1–2 neurological AEs from 20% to 5.6%. No grade 3 events occurred in either category.65 GB261 is a CD20 × CD3 BsAb computationally designed to maintain Fc effector function. It also integrates de-tuned CD3 binding to reduce CRS incidence and improve safety features. Early activity was reported in 47 patients treated in the dose-escalation study (NCT04923048), 36 of whom had R/R DLBCL (Table 1).66 Novel CD3/CD20, CD3/CD19, and CD3/CD22 BsAb constructs are being evaluated in phase 1 studies (NCT05210868, NCT05618327, NCT04056975, and NCT04540796), as outlined in Table 3.
A number of other multispecific antibodies are currently under investigation in phase 1 trials. JNJ-80948543 (CD3 × CD20 × CD79b) and CMG1A46 (CD3 × CD20 × CD19) are trispecific Abs targeting two tumor-associated antigens, currently evaluated in the NCT05424822 and NCT05348889 trial, respectively.67,68 PIT565 is a CD3 × CD2 × CD19 trispecific Ab investigated in the NCT05397496 trial and designed to provide T-cell activation with both the initial signal (via CD3) and the co-stimulatory signal (via CD2).69 Emfizatamab is a CD3 × 4-1BB x CD19 × PDL-1 tetraspecific Ab combining a co-stimulatory domain and an immune checkpoint inhibitor target in a single agent, and is currently being explored in the NCT05623982 trial.
Finally, IPH6501 is a tetraspecific molecule designed to engage NK cells through two activating receptors (NKp46 and CD16a/FcγRIIIa) and the stimulatory β chain (CD122) of the interleukin-2 receptor, and simultaneously bind the CD20 antigen expressed on malignant B-cells.70 This drug is being studied in an ongoing dose-escalation trial in patients with various B-cell lymphoma subtypes (NCT06088654).
8. Conclusions
Since their introduction, CD3 × CD20 BsAbs have represented a breakthrough in the treatment of R/R aNHL, their development has been rapid, and their role in the therapeutic landscape is constantly being re-defined based on emerging data. Response rates are encouraging and the safety profile appears manageable. Clearly, longer follow-up is needed to ascertain durability of responses and curative potential, if any, of these drugs. Moreover, the regulatory approval of glofitamab and epcoritamab will lead to widespread adoption, and real-world efficacy and safety data are likely to inform clinical practice while potentially uncovering previously unidentified challenges.
The promising activity coupled with the unique toxicity profile of BsAbs highlights their combinability. Notably, the lymphoma-killing activity of CD20 × CD3 BsAbs appears unaffected when T-cell cytotoxic agents are co-administered, while concurrent use of immunomodulatory agents that restore the immune synapse may produce synergism with BsAbs. These combinatorial strategies hold promise for improving BsAb efficacy with an acceptable safety profile.
Finally, newer multi-specific Abs, designed to overcome resistance to CD20 × CD3 BsAbs, could improve our understanding of response and resistance, and further refine our approach to this promising therapeutic modality.
Funding Statement
G.C. was supported by Associazione italiana contro le leucemie-linfomi e mieloma Milano e Provincia ONLUS.
Disclosure statement
L.F. has served on advisory boards for ADC Therapeutics, Seagen, AstraZeneca, Ipsen, AbbVie, and Genentech, has consulted for and received Honoraria from Genmab, AbbVie, and Genetech, has consulted for and received research fundings from Genmab, Roche, AbbVie, Genetech, and Innate Pharma, has consulted for EvolveImmune, and has received travel reimbursement from Genmab and AbbVie.
G.C. and A.LdA have no conflict of interest to disclose.
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article.
References
- 1.Crump M, Neelapu SS, Farooq U, Van Den Neste E, Kuruvilla J, Westin J, Link BK, Hay A, Cerhan JR, Zhu L, et al. Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood. 2017;130(16):1800–12. doi: 10.1182/BLOOD-2017-03-769620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hagberg H, Gisselbrecht C.. Randomised phase III study of R-ICE versus R-DHAP in relapsed patients with CD20 diffuse large B-cell lymphoma (DLBCL) followed by high-dose therapy and a second randomisation to maintenance treatment with rituximab or not: an update of the CORAL study. Ann Oncol. 2006;17(SUPPL. 4):iv31–iv32. doi: 10.1093/ANNONC/MDJ996. [DOI] [PubMed] [Google Scholar]
- 3.Sehn LH, Herrera AF, Flowers CR, Kamdar MK, McMillan A, Hertzberg M, Assouline S, Kim TM, Kim WS, Ozcan M, et al. Polatuzumab vedotin in relapsed or refractory diffuse large B-Cell lymphoma. J Clin Oncol. 2020;38(2):155–165. doi: 10.1200/JCO.19.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salles G, Duell J, González Barca E, Tournilhac O, Jurczak W, Liberati AM, Nagy Z, Obr A, Gaidano G, André M, et al. Tafasitamab plus lenalidomide in relapsed or refractory diffuse large B-cell lymphoma (L-MIND): a multicentre, prospective, single-arm, phase 2 study. Lancet Oncol. 2020;21(7):978–988. doi: 10.1016/S1470-2045(20)30225-4. [DOI] [PubMed] [Google Scholar]
- 5.Caimi PF, Ai W, Alderuccio JP, Ardeshna KM, Hamadani M, Hess B, Kahl BS, Radford J, Solh M, Stathis A, et al. Loncastuximab tesirine in relapsed or refractory diffuse large B-cell lymphoma (LOTIS-2): a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol. 2021;22(6):790–800. doi: 10.1016/S1470-2045(21)00139-X. [DOI] [PubMed] [Google Scholar]
- 6.Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, Braunschweig I, Oluwole OO, Siddiqi T, Lin Y, et al. Axicabtagene ciloleucel CAR T-Cell therapy in refractory large B-Cell lymphoma. N Engl J Med. 2017;377(26):2531–2544. doi: 10.1056/nejmoa1707447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, Jäger U, Jaglowski S, Andreadis C, Westin JR, et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N Engl J Med. 2019;380(1):45–56. doi: 10.1056/nejmoa1804980. [DOI] [PubMed] [Google Scholar]
- 8.Abramson JS, Palomba ML, Gordon LI, Lunning MA, Wang M, Arnason J, Mehta A, Purev E, Maloney DG, Andreadis C, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396(10254):839–852. doi: 10.1016/S0140-6736(20)31366-0. [DOI] [PubMed] [Google Scholar]
- 9.Locke FL, Miklos DB, Jacobson CA, Perales M-A, Kersten M-J, Oluwole OO, Ghobadi A, Rapoport AP, McGuirk J, Pagel JM, et al. Axicabtagene ciloleucel as second-line therapy for large B-Cell Lymphoma. N Engl J Med. 2022;386(7):640–654. doi: 10.1056/NEJMoa2116133. [DOI] [PubMed] [Google Scholar]
- 10.Abramson JS, Solomon SR, Arnason J, Johnston PB, Glass B, Bachanova V, Ibrahimi S, Mielke S, Mutsaers P, Hernandez-Ilizaliturri F, et al. Lisocabtagene maraleucel as second-line therapy for large B-cell lymphoma: primary analysis of the phase 3 transform study. Blood. 2023;141(14):1675–1684. doi: 10.1182/blood.2022018730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petrazzuolo A, Maiuri MC, Zitvogel L, Kroemer G, Kepp O. Trial watch: combination of tyrosine kinase inhibitors (TKIs) and immunotherapy. Oncoimmunology. 2022;11(1). doi: 10.1080/2162402X.2022.2077898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Le Naour J, Kroemer G. Trial watch: toll-like receptor ligands in cancer therapy. Oncoimmunology. 2023;12(1). doi: 10.1080/2162402X.2023.2180237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laureano RS, Sprooten J, Vanmeerbeerk I, Borras DM, Govaerts J, Naulaerts S, Berneman ZN, Beuselinck B, Bol KF, Borst J, et al. Trial watch: dendritic cell (DC)-based immunotherapy for cancer. Oncoimmunology. 2022;11(1). doi: 10.1080/2162402X.2022.2096363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sprooten J, Laureano RS, Vanmeerbeek I, Govaerts J, Naulaerts S, Borras DM, Kinget L, Fucíková J, Špíšek R, Jelínková LP, et al. Trial watch: chemotherapy-induced immunogenic cell death in oncology. Oncoimmunology. 2023;12(1). doi: 10.1080/2162402X.2023.2219591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kontermann RE, Brinkmann U. Bispecific antibodies. Drug Discov Today. 2015;20(7):838–847. doi: 10.1016/J.DRUDIS.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 16.Sun LL, Ellerman D, Mathieu M, Hristopoulos M, Chen X, Li Y, Yan X, Clark R, Reyes A, Stefanich E. et al, Anti-CD20/CD3 T cell-dependent bispecific antibody for the treatment of B cell malignancies. Sci Transl Med. 2015;7(287). doi: 10.1126/scitranslmed.aaa4802. [DOI] [PubMed] [Google Scholar]
- 17.Atwell S, Ridgway JBB, Wells JA, Carter P. Stable heterodimers from remodeling the domain interface of a homodimer using a phage display library. J Mol Biol. 1997;270(1):26–35. doi: 10.1006/JMBI.1997.1116. [DOI] [PubMed] [Google Scholar]
- 18.Klein C, Schaefer W, Regula JT. The use of CrossMAb technology for the generation of bi- and multispecific antibodies. MAbs. 2016;8(6):1010–1020. doi: 10.1080/19420862.2016.1197457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Challa-Malladi M, Lieu YK, Califano O, Holmes AB, Bhagat G, Murty VV, Dominguez-Sola D, Pasqualucci L, Dalla-Favera R. Combined genetic inactivation of β2-microglobulin and CD58 reveals frequent escape from immune recognition in diffuse large B cell lymphoma. Cancer Cell. 2011;20(6):728–740. doi: 10.1016/J.CCR.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rothe A, Sasse S, Topp MS, Eichenauer DA, Hummel H, Reiners KS, Dietlein M, Kuhnert G, Kessler J, Buerkle C, et al. A phase 1 study of the bispecific anti-CD30/CD16A antibody construct AFM13 in patients with relapsed or refractory Hodgkin lymphoma. Blood. 2015;125(26):4024–4031. doi: 10.1182/BLOOD-2014-12-614636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wei J, Montalvo-Ortiz W, Yu L, Krasco A, Olson K, Rizvi S, Fiaschi N, Coetzee S, Wang F, Ullman E, et al. CD22-targeted CD28 bispecific antibody enhances antitumor efficacy of odronextamab in refractory diffuse large B cell lymphoma models. Sci Transl Med. 2022;14(670). doi: 10.1126/scitranslmed.abn1082. [DOI] [PubMed] [Google Scholar]
- 22.Wang J, Li C, He K, Kuang Z, Lu J, Yao Y, He F, Li N, Li L, Fu F, et al. Characterization of anti-CD79b/CD3 bispecific antibody, a potential therapy for B cell malignancies. Cancer Immunol Immunother. 2023;72(2):493–507. doi: 10.1007/s00262-022-03267-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buatois V, Johnson Z, Salgado-Pires S, Papaioannou A, Hatterer E, Chauchet X, Richard F, Barba L, Daubeuf B, Cons L, et al. Preclinical development of a bispecific antibody that safely and effectively targets CD19 and CD47 for the treatment of B-cell lymphoma and leukemia. Mol Cancer Ther. 2018;17(8):1739–1751. doi: 10.1158/1535-7163.MCT-17-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bacac M, Colombetti S, Herter S, Sam J, Perro M, Chen S, Bianchi R, Richard M, Schoenle A, Nicolini V, et al. CD20-TCB with obinutuzumab pretreatment as next-generation treatment of hematologic malignancies. Clin Cancer Res. 2018;24(19):4785–4797. doi: 10.1158/1078-0432.CCR-18-0455. [DOI] [PubMed] [Google Scholar]
- 25.Engelberts PJ, Hiemstra IH, de Jong B, Schuurhuis DH, Meesters J, Beltran Hernandez I, Oostindie SC, Neijssen J, van den Brink EN, Horbach GJ, et al. DuoBody-CD3xCD20 induces potent T-cell-mediated killing of malignant B cells in preclinical models and provides opportunities for subcutaneous dosing. EBioMedicine. 2020;52:102625. doi: 10.1016/j.ebiom.2019.102625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith EJ, Olson K, Haber LJ, Varghese B, Duramad P, Tustian AD, Oyejide A, Kirshner JR, Canova L, Menon J, et al. A novel, native-format bispecific antibody triggering T-cell killing of B-cells is robustly active in mouse tumor models and cynomolgus monkeys. Sci Rep. 2015;5(1):1–12. doi: 10.1038/srep17943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thieblemont C, Phillips T, Ghesquieres H, Cheah CY, Clausen MR, Cunningham D, Do YR, Feldman T, Gasiorowski R, Jurczak W, et al. Epcoritamab, a novel, subcutaneous CD3xCD20 bispecific T-Cell-engaging antibody, in relapsed or refractory large B-Cell lymphoma: dose expansion in a phase I/II trial. J Clin Oncol. 2023;41(12):2238–2247. doi: 10.1200/JCO.22.01725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hutchings M, Morschhauser F, Iacoboni G, Carlo-Stella C, Offner FC, Sureda A, Salles G, Martinez-Lopez J, Crump M, Thomas DN, et al. Glofitamab, a novel, bivalent CD20-targeting T-Cell-engaging bispecific antibody, induces durable complete remissions in relapsed or refractory B-Cell lymphoma: a phase I trial. J Clin Oncol. 2021;39(18):1959–1970. doi: 10.1200/JCO.20.03175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Budde LE, Assouline S, Sehn LH, Schuster SJ, Yoon S-S, Hyun Yoon D, Matasar MJ, Bosch F, Seog Kim W, Nastoupil LJ, et al. Single-agent mosunetuzumab shows durable complete responses in patients with relapsed or refractory B-Cell Lymphomas: phase I dose-escalation study. J Clin Oncol. 2021;40(5):481–491. doi: 10.1200/JCO.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee DW, Santomasso BD, Locke FL, Ghobadi A, Turtle CJ, Brudno JN, Maus MV, Park JH, Mead E, Pavletic S, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant. 2019;25(4):625–638. doi: 10.1016/J.BBMT.2018.12.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gust J, Finney OC, Li D, Brakke HM, Hicks RM, Futrell RB, Gamble DN, Rawlings-Rhea SD, Khalatbari HK, Ishak GE, et al. Glial injury in neurotoxicity after pediatric CD19-directed chimeric antigen receptor T cell therapy. Ann Neurol. 2019;86(1):42–54. doi: 10.1002/ANA.25502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hosseini I, Gadkar K, Stefanich E, Li CC, Sun LL, Chu YW, Ramanujan S. Mitigating the risk of cytokine release syndrome in a phase I trial of CD20/CD3 bispecific antibody mosunetuzumab in NHL: impact of translational system modeling. NPJ Syst Biol Appl. 2020;6(1):1–11. doi: 10.1038/s41540-020-00145-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bartlett NL, Giri P, Budde LE, Schuster SJ, Assouline S, Matasar MJ, Yoon S-S, Canales M, Gutierrez NC, Fay K, et al. Subcutaneous (SC) administration of mosunetuzumab with cycle 1 step-up dosing is tolerable and active in patients with relapsed/refractory B-Cell Non-Hodgkin Lymphomas (R/R B-NHL): initial results from a phase I/II study. Blood. 2021;138(Supplement 1):3573–3573. doi: 10.1182/BLOOD-2021-147937. [DOI] [Google Scholar]
- 34.Bannerji R, Arnason JE, Advani RH, Brown JR, Allan JN, Ansell SM, Barnes JA, O’Brien SM, Chávez JC, Duell J, et al. Odronextamab, a human CD20×CD3 bispecific antibody in patients with CD20-positive B-cell malignancies (ELM-1): results from the relapsed or refractory non-Hodgkin lymphoma cohort in a single-arm, multicentre, phase 1 trial. Lancet Haematol. 2022;9(5):e327–e339. doi: 10.1016/S2352-3026(22)00072-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hutchings M, Mous R, Clausen MR, Johnson P, Linton KM, Chamuleau MED, Lewis DJ, Sureda Balari A, Cunningham D, Oliveri RS, et al. Dose escalation of subcutaneous epcoritamab in patients with relapsed or refractory B-cell non-Hodgkin lymphoma: an open-label, phase 1/2 study. Lancet. 2021;398(10306):1157–1169. doi: 10.1016/S0140-6736(21)00889-8. [DOI] [PubMed] [Google Scholar]
- 36.Falchi L, Vardhana SA, Salles GA. Bispecific antibodies for the treatment of B-cell lymphoma: promises, unknowns, and opportunities. Blood. 2023;141(5):467–480. doi: 10.1182/blood.2021011994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dickinson MJ, Carlo-Stella C, Morschhauser F, Bachy E, Corradini P, Iacoboni G, Khan C, Wróbel T, Offner F, Trněný M, et al. Glofitamab for relapsed or refractory diffuse large B-Cell lymphoma. N Engl J Med. 2022;387(24):2220–2231. doi: 10.1056/NEJMoa2206913. [DOI] [PubMed] [Google Scholar]
- 38.Crombie JL, Graff T, Falchi L, Karimi YH, Bannerji R, Nastoupil LJ, Thieblemont C, Ursu R, Bartlett NL, Nachar VR, et al. Consensus recommendations on the management of toxicity associated with CD3xCD20 bispecific antibody therapy. Blood J. 2024 Jan 22. doi: 10.1182/BLOOD.2023022432. [DOI] [PubMed] [Google Scholar]
- 39.Viardot A, Goebeler M-E, Hess G, Neumann S, Pfreundschuh M, Adrian N, Zettl F, Libicher M, Sayehli C, Stieglmaier J, et al. Phase 2 study of the bispecific T-cell engager (BiTE) antibody blinatumomab in relapsed/refractory diffuse large B-cell lymphoma. Blood. 2016;127(11):1410–1416. doi: 10.1182/blood-2015-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bartlett NL, Assouline S, Giri P, Schuster SJ, Cheah CYY, Matasar MJ, Gregory GP, Yoon D-H, Shadman M, Fay K, et al. Mosunetuzumab monotherapy is active and tolerable in patients with relapsed/refractory diffuse large B-cell lymphoma. Blood Adv. 2023;7(17):4926–4935. doi: 10.1182/BLOODADVANCES.2022009260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.EPKINLYTM for 3L+ large B-cell lymphoma - official HCP site. [accessed 2023 Dec 25]. https://www.epkinlyhcp.com/.
- 42.Genentech: ColumviTM (glofitamab-gxbm) - information for patients. [accessed 2023 Dec 26]. https://www.gene.com/patients/medicines/columvi.
- 43.Hutchings M, Carlo-Stella C, Morschhauser F, Falchi L, Bachy E, Cartron G, Khan C, Tani M, Martinez-Lopez J, Bartlett NL, et al. Glofitamab monotherapy in relapsed or refractory large B-Cell lymphoma: extended follow-up from a pivotal phase II study and subgroup analyses in patients with prior chimeric antigen receptor T-Cell therapy and by baseline total metabolic tumor volume. Blood. 2023;142(Supplement 1):433–433. doi: 10.1182/BLOOD-2023-173951. [DOI] [Google Scholar]
- 44.Song Y-Q, Zhang H-L, Huang H-Q, Zhang Q-Y, Jing H-M, Wang C, Wu C, Li D-H, Dai Y, Humphrey K, et al. Glofitamab monotherapy induces high complete response rates and manageable safety in Chinese patients with heavily pretreated relapsed or refractory diffuse large B-cell lymphoma. Haematologica. 2023 Oct 19. doi: 10.3324/HAEMATOL.2023.283802. [DOI] [PubMed] [Google Scholar]
- 45.Crombie JL, Matasar M, Topp MS, Allan JN, Barnes JA, Arnason JE, Michot J-M, Goldschmidt N, O’Brien SM, Abadi U, et al. Odronextamab demonstrates durable complete responses in patients with diffuse large B-Cell lymphoma (DLBCL) progressing after CAR-T therapy: outcomes from the ELM-1 study. Blood. 2023;142(Supplement 1):4461–4461. doi: 10.1182/BLOOD-2023-181602. [DOI] [Google Scholar]
- 46.Ayyappan S, Kim WS, Kim TM, Walewski J, Cho S-G, Jarque I, Iskierka-Jazdzewska E, Poon M, Oh SY, Inng Lim FLW, et al. Final analysis of the phase 2 ELM-2 study: odronextamab in patients with relapsed/refractory (R/R) diffuse large B-Cell lymphoma (DLBCL). Blood. 2023;142(Supplement 1):436–436. doi: 10.1182/BLOOD-2023-179818. [DOI] [Google Scholar]
- 47.Patel K, Riedell PA, Tilly H, Ahmed S, Michot J-M, Ghesquieres H, Schiano de Collela JM, Chanan-Khan A, Bouabdallah K, Tessoulin B, et al. A phase 1 study of plamotamab, an anti-CD20 x anti-CD3 bispecific antibody, in patients with Relapsed/Refractory Non-Hodgkin’s lymphoma: recommended dose Safety/Efficacy update and escalation exposure-response analysis. Blood. 2022;140(Supplement 1):9470–9472. doi: 10.1182/BLOOD-2022-159586. [DOI] [Google Scholar]
- 48.Budde E, Gopal AK, Kim WS, Flinn IW, Cheah CYY, Nastoupil L, Matasar MJ, Diefenbach CS, Gregory GP, Qazi I, et al. A phase 1 dose escalation study of igm-2323, a novel anti-CD20 x anti-CD3 IgM T cell engager (TCE) in patients with advanced B-Cell malignancies. Blood. 2021;138(Supplement 1):132–132. doi: 10.1182/BLOOD-2021-153355. [DOI] [Google Scholar]
- 49.Li C-C, Bender B, Yin S, Li Z, Zhang C, Hernandez G, Kwan A, Sun L, Adamkewicz JI, Wang H, et al. Exposure-response analyses indicate a promising benefit/risk profile of mosunetuzumab in relapsed and refractory Non-Hodgkin Lymphoma. Blood. 2019;134(Supplement_1):1285. doi: 10.1182/BLOOD-2019-123961. [DOI] [Google Scholar]
- 50.Chiu CW, Hiemstra IH, ten HW, Snijdewint-Nkairi R, de JB, Castro PG, Kweekel C, Oliveri RS, Elliot B, Wielgos-Bonvallet M, et al. Preclinical evaluation of epcoritamab combined with standard of care agents for the treatment of B-cell lymphomas. Hematol Oncol. 2021;39(S2). doi: 10.1002/HON.78_2881. [DOI] [Google Scholar]
- 51.Abrisqueta P, Falchi L, Phillips TJ, De Vos S, Nijland M, Offner F, Bykhovski I, Wu J, Wang L, Rana A, et al. Subcutaneous epcoritamab + R-DHAX/C in patients (pts) with relapsed or refractory (R/R) diffuse large B-cell lymphoma (DLBCL) eligible for autologous stem cell transplant (ASCT): preliminary phase 1/2 results. J Clin Oncol. 2022;40(16_suppl):7528–7528. doi: 10.1200/JCO.2022.40.16_SUPPL.7528. [DOI] [Google Scholar]
- 52.Brody J, Joergensen JM, Belada D, Costello RT, Trněný M, Vitolo U, Lewis D, Karimi YH, Sureda Balari AM, André M, et al. Epcoritamab SC + GemOx leads to high complete metabolic response rates in patients with Relapsed/Refractory diffuse large B-Cell lymphoma ineligible for autologous stem cell transplant: updated results from Epcore NHL-2. Blood. 2023;142(Supplement 1):3092–3092. doi: 10.1182/BLOOD-2023-180246. [DOI] [Google Scholar]
- 53.Budde LE, Olszewski AJ, Assouline S, Lossos IS, Diefenbach C, Kamdar M, Ghosh N, Modi D, Sabry W, Naik S, et al. Mosunetuzumab with polatuzumab vedotin in relapsed or refractory aggressive large B cell lymphoma: a phase 1b/2 trial. Nat Med. 2023. Dec;10(1):229–239. doi: 10.1038/s41591-023-02726-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hutchings M, Avigdor A, Sureda Balari AM, Terol MJ, Bosch F, Corradini P, Larsen TS, Rueda Dominguez A, Skarbnik A, Joergensen JM, et al. Glofitamab plus polatuzumab vedotin continues to demonstrate frequent and durable responses and has a manageable safety profile in patients with ≥2L relapsed/refractory DLBCL, including HGBCL, and in patients with prior CAR T-Cell therapy: updated results from a phase Ib/II study. Blood. 2023;142(Supplement 1):4460–4460. doi: 10.1182/BLOOD-2023-174213. [DOI] [Google Scholar]
- 55.https://www.postersessiononline.eu173580348_eucongresosASH2023aula-MON_4460_ASH2023.pdf.
- 56.Esensten JH, Helou YA, Chopra G, Weiss A, Bluestone JA. CD28 costimulation: from mechanism to therapy. Immunity. 2016;44(5):973–988. doi: 10.1016/j.immuni.2016.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Herter S, Sam J, Ferrara Koller C, Diggelmann S, Bommer E, Schönle A, Claus C, Bacac M, Klein C, Umana P. RG6076 (CD19-4-1BBL): CD19-targeted 4-1BB ligand combination with glofitamab as an off-the-shelf, enhanced T-Cell redirection therapy for B-Cell malignancies. Blood. 2020;136(Supplement 1):40–40. doi: 10.1182/BLOOD-2020-134782. [DOI] [Google Scholar]
- 58.Sam J, Hofer T, Kuettel C, Claus C, Herter S, Georges G, Thom JT, Kunz L, Gebhardt S, Limani F, et al. RG6333 (CD19-CD28), a CD19-targeted affinity-optimized CD28 bispecific antibody, enhances and prolongs the anti-tumor activity of glofitamab (CD20-TCB) in preclinical models. Blood. 2022;140(Supplement 1):3142–3143. doi: 10.1182/BLOOD-2022-159941. [DOI] [Google Scholar]
- 59.Claus C, Ferrara C, Xu W, Sam J, Lang S, Uhlenbrock F, Albrecht R, Herter S, Schlenker R, Hösser T, et al. Tumor-targeted 4-1BB agonists for combination with T cell bispecific antibodies as off-the-shelf therapy. Sci Transl Med. 2019;11(496). doi: 10.1126/SCITRANSLMED.AAV5989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hutchings M, Carlo-Stella C, Gritti G, Bosch F, Morschhauser F, Townsend W, Offner F, Walter HS, Ghesquieres H, Houot R, et al. CD19 4-1BBL (RO7227166) a novel costimulatory bispecific antibody can be safely combined with the T-Cell-engaging bispecific antibody glofitamab in relapsed or refractory B-Cell Non-Hodgkin Lymphoma. Blood. 2022;140(Supplement 1):9461–9463. doi: 10.1182/BLOOD-2022-157011. [DOI] [Google Scholar]
- 61.Dickinson M, Gritti G, Carlo-Stella C, Walter HS, Carlile D, Getzmann N, Curdt S, Harrop E, Keelara A, Korfi K, et al. Phase 1 study of CD19 targeted CD28 costimulatory agonist in combination with glofitamab to enhance T cell effector function in relapsed/refractory B cell lymphoma. Blood. 2022;140(Supplement 1):3818–3820. doi: 10.1182/BLOOD-2022-156808. [DOI] [Google Scholar]
- 62.Byun JM, Yoon S-S, Hong J, Shin D-Y, Koh Y. P1157 Phase ii study of glofitamab, poseltinib and lenalidomide in patients with relapsed/refractory diffuse b cell lymphomas. HemaSphere. 2023;7(Supplement 3): e36320c6. doi: 10.1097/01.HS9.0000971524.36320.c6. [DOI] [Google Scholar]
- 63.Avivi Mazza I, Kim WS, Ko P-S, Grande C, Lavie D, Chism D, Seliem M, Jeng EE, Joshi N, Siddani S, et al. Subcutaneous epcoritamab plus lenalidomide in patients with Relapsed/Refractory diffuse large B-Cell lymphoma from EPCORE NHL-5. Blood. 2023;142(Supplement 1):438–438. doi: 10.1182/BLOOD-2023-180089. [DOI] [Google Scholar]
- 64.Hou J-Z, Jacobs R, Cho S-G, Devata S, Gaballa S, Yoon DH, Stevens DA, Kim JS, Buelow B, Nair R. Interim results of the phase 1 study of tnb-486, a Novel CD19xCD3 T-Cell engager, in patients with Relapsed/Refractory (R/R) B-NHL. Blood. 2022;140(Supplement 1):1474–1475. doi: 10.1182/blood-2022-166385. [DOI] [Google Scholar]
- 65.Gaballa S, Nair R, Jacobs RW, Devata S, Cho S-G, Stevens DA, Yoon DH, Shah NN, Brennan D, Law J, et al. Double Step-up Dosing (2SUD) regimen mitigates severe icans and CRS while maintaining high efficacy in subjects with Relapsed/Refractory (R/R) B-Cell Non-Hodgkin Lymphoma (NHL) treated with AZD0486, a novel CD19xCD3 T-Cell engager (TCE): updated safety and efficacy data from the ongoing first-in-human (FIH) phase 1 trial. Blood. 2023;142(Supplement 1):1662–1662. doi: 10.1182/BLOOD-2023-174668. [DOI] [Google Scholar]
- 66.Song Y, Li Z, Li L, Qian Z, Zhou K, Fan L, Tan P, Giri P, Li Z, Kenealy M, et al. GB261, an Fc-Function enabled and CD3 affinity De-tuned CD20/CD3 bispecific antibody, demonstrated a highly advantageous Safety/Efficacy balance in an ongoing first-in-human dose-escalation study in patients with Relapsed/Refractory Non-Hodgkin Lymphoma. Blood. 2023;142(Supplement 1):1719–1719. doi: 10.1182/BLOOD-2023-188444. [DOI] [Google Scholar]
- 67.Zhang J, Zhou Z. Preclinical study of a novel tri-specific anti-CD3/CD19/CD20 T cell-engaging antibody as a potentially better treatment for NHL. Blood. 2020;136(Supplement 1):22. doi: 10.1182/BLOOD-2020-140154. [DOI] [Google Scholar]
- 68.Kuchnio A, Yang D, Vloemans N, Lowenstein C, Cornelissen I, Amorim R, Han C, Sukumaran S, Janssen L, Suls T, et al. Characterization of JNJ-80948543, a novel CD79bxCD20xCD3 trispecific T-Cell redirecting antibody for the treatment of B-Cell Non-Hodgkin Lymphoma. Blood. 2022;140(Supplement 1):3105–3106. doi: 10.1182/BLOOD-2022-168739. [DOI] [Google Scholar]
- 69.Lu H, Oka A, Coulson M, Polli JR, Aardalen K, Ramones M, Walker DB, Carrion A, Alexander D, Klopfenstein M, et al. PIT565, a first-in-class anti-CD19, anti-CD3, anti-CD2 trispecific antibody for the treatment of B cell malignancies. Blood. 2022;140(Supplement 1):3148–3148. doi: 10.1182/BLOOD-2022-168904. [DOI] [Google Scholar]
- 70.Demaria O, Gauthier L, Vetizou M, Blanchard Alvarez A, Vagne C, Habif G, Batista L, Baron W, Belaïd N, Girard-Madoux M, et al. Antitumor immunity induced by antibody-based natural killer cell engager therapeutics armed with not-alpha IL-2 variant. Cell Rep Med. 2022;3(10):100783. doi: 10.1016/j.xcrm.2022.100783. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article.


