Abstract
The promyelocytic leukemia zinc finger (PLZF) gene, which is disrupted in therapy-resistant, t(11;17)(q23;q21)-associated acute promyelocytic leukemia (APL), is expressed in immature hematopoietic cells and is down-regulated during differentiation. To determine the role of PLZF in myeloid development, we engineered expression of PLZF in murine 32Dcl3 cells. Expression of PLZF had a dramatic growth-suppressive effect accompanied by accumulation of cells in the G0/G1 compartment of the cell cycle and an increased incidence of apoptosis. PLZF-expressing pools also secreted a growth-inhibitory factor, which could explain the severe growth suppression of PLZF-expressing pools that occurred despite the fact that only half of the cells expressed high levels of PLZF. PLZF overexpression inhibited myeloid differentiation of 32Dcl3 cells in response to granulocyte and granulocyte-macrophage colony-stimulating factors. Furthermore, cells that expressed PLZF appeared immature as demonstrated by morphology, increased expression of Sca-1, and decreased expression of Gr-1. These findings suggest that PLZF is an important regulator of cell growth, death, and differentiation. Disruption of PLZF function associated with t(11;17) may be a critical event leading to APL.
Acute promyelocytic leukemia (APL) is defined as the accumulation of malignant myeloid cells blocked at the intermediate promyelocytic stage of myeloid differentiation. APL has now been associated with four chromosomal translocations, all resulting in the fusion of the retinoic acid receptor alpha (RARα) gene, located on chromosome 17, with genes encoding a number of nuclear proteins, including PML-t(15;17) (13, 17, 25), promyelocytic leukemia zinc finger (PLZF)-t(11;17)(q23;21) (9, 10, 36), nucleophosmin-t(5;17) (52), and nuclear matrix-associated antigen (NuMA)-t(11;17)(q13;q21) (67). Over 95% of patients with APL harbor chromosomal translocation t(15;17) (19, 65), and malignant promyelocytes from these patients express the PML-RARα fusion protein. These individuals can be successfully treated with all-trans retinoic acid (ATRA), resulting in the differentiation of immature promyelocytes into mature granulocyte forms and elimination of the malignant clone (11). Similarly, promyelocytes from a patient with t(5;17) and NPM-RARα expression can undergo terminal granulocytic differentiation in vitro (51). A patient with a NuMA-RARα fusion and t(11;17)(q13;q21) also clinically responded to ATRA therapy. In striking contrast, t(11;17)(q23;q21)-associated APL is not responsive to ATRA therapy (20, 36), and no patients with this disease have achieved lasting clinical remission with conventional chemotherapy. In all four forms of APL, an aberrant RAR is generated, perhaps leading to the promyelocytic phenotype due to a block in RA-mediated signaling. The singular failure of APL associated with fusion of PLZF and RARα to respond to ATRA may be due to disruption of PLZF function in hematopoiesis.
The PLZF protein, which is highly conserved among humans, mice, and chickens (12), is a transcription factor containing nine Krüppel-like C2H2 zinc fingers. PLZF binds DNA in a sequence-specific manner and represses transcription through its cognate binding site (35, 59). The amino terminus of PLZF contains a poxvirus and zinc finger (POZ) domain, which is the most highly conserved domain in the protein (6). POZ domains are also found in the mammalian zinc finger proteins Bcl-6 and ZF5, as well as in a number of Drosophila transcription factors and viral proteins (2, 46, 70). The POZ domain of PLZF mediates its abilities to homodimerize (14) and repress gene transcription (35), the latter likely by interaction with nuclear corepressors that affect deacetylation of histones (18, 21–23, 40).
PLZF, as expected for a transcriptional regulator, is localized to the nucleus, where, as seen in early hematopoietic progenitors and the more mature HL60 and MDS cell lines, it has a distinct speckled pattern of expression (30, 37, 53). PLZF colocalizes with the PML protein in large nuclear bodies of unknown function (8, 15, 29, 66). In t(15;17)-associated APL, these large nuclear-body structures are disrupted and both PML and PLZF are found in a diffuse microspeckled pattern throughout the cell due to the action of PML-RARα. Treatment of these leukemic cells with ATRA leads to the restoration of the wild-type staining pattern. PML was defined as a repressor of cell growth (1, 28, 44), and it was hypothesized that the nuclear body plays a role in cell growth control. This suggested that PLZF might also play a role in the control of cell proliferation. In the hematopoietic compartment, relatively high levels of PLZF are expressed in CD34+ cells of the bone marrow (10, 53) and in immature erythroid, lymphoid, and myeloid cell lines. When cells are induced to differentiate, PLZF levels generally decline, leading to the notion that down-regulation of PLZF may be required for the normal program of cell division accompanied by terminal cell differentiation.
To explore the role of the PLZF gene in myeloid development, we engineered the expression of PLZF in 32Dcl3G/GM (32DG/GM), a murine interleukin-3 (IL-3)-dependent myeloid cell line that differentiates into granulocytes after exposure to granulocyte colony-stimulating factor (G-CSF) and into macrophages and granulocytes after exposure to granulocyte-macrophage colony-stimulating factor (GM-CSF) (31). The 32Dcl3 cell line is an established model of hematopoietic development which has been used to study the effect of oncogenes (3, 41, 54), tumor suppressor genes (60), and growth factors (58). Our studies demonstrated that PLZF has pleiotropic effects when expressed in 32DG/GM cells: it inhibits transit through the cell cycle, blocking cells in G0/G1; it inhibits differentiation; and it yields cells with a more immature immunophenotypic profile. PLZF expression was also associated with the secretion of an autocrine growth-inhibitory factor. These data suggest that PLZF may help control the quiescent state of hematopoietic progenitor cells. Disruption of this function in t(11;17) APL may contribute to the ATRA-resistant phenotype of this disease.
MATERIALS AND METHODS
Generation of PLZF-expressing cell pools.
The murine IL-3-dependent 32DG/GM cell line, which was previously described (31), was grown in Iscove’s modified Dulbecco medium (Gibco BRL) supplemented with 10% heat-inactivated fetal bovine serum (FBS; HyClone), 50 U of penicillin per ml, 50 μg of streptomycin per ml, 2 mM glutamine (Gibco BRL), and 10 ng of recombinant murine IL-3 (Genzyme, Cambridge, Mass.) per ml. The Ψ2 retroviral packaging cell line was maintained in Dulbecco’s modified Eagle medium supplemented with 10% FBS, penicillin-streptomycin, and glutamine. Stable retroviral packaging lines were generated by using a retroviral vector, pBabepuro-PLZF, which was constructed by insertion of a 2.1-kb EcoRI fragment containing the PLZF cDNA (9) into pBabepuro (43). To create the packaging cell lines, Ψ2 cells were electroporated at 300 V and 250 μF with 10 μg of pBabepuro or pBabepuro-PLZF which had been linearized by digestion with NotI. The transfected cells were selected with 1.5 μg of puromycin per ml for 2 weeks, and individual clones were isolated. The supernatant from packaging clones was screened for its ability to confer puromycin resistance to 3T3 cells. The resulting positive clones were expanded, and lysates of these cells were screened by immunoblotting with an anti-PLZF antibody. To generate stable pools of 32DG/GM cells expressing PLZF, 5 × 106 cells were cocultivated with Ψ2 packaging cells overnight, removed, and allowed to grow without selection for 48 h. Stable pools were then selected in medium containing 1.5 μg of puromycin per ml for 2 to 3 weeks. PLZF-expressing 32Dcl3 cell pools 7 and 8 were generated by coculture with Ψ2 packaging cell line PLZF12, and pools 9 and 10 were generated from the Ψ2 line PLZF14. Pools 2 and 3 were created by infection of 32Dcl3 cells with a pool of PLZF-harboring retroviruses derived 48 h after transient transfection, utilizing calcium phosphate, of the BOSC packaging cell line with the pBABEpuro-PLZF vector (49).
Growth and morphological assessment.
Cells were plated at a density of 5 × 104/ml, and live, trypan blue-excluding cells were counted by using a Thomas’ hemocytometer every other day. 32DG/GM vector cells were plated at a density of 105/ml with or without 50% conditioned medium (CM). CM was generated by plating control or PLZF-expressing cells at a density of 104/ml and removing the medium after 3 days. The effect of IL-3 withdrawal on growth and survival was studied by washing cells twice with 5 ml of phosphate-buffered saline (PBS) and then incubating them in complete medium without IL-3. For morphological characterization, cells were collected, washed, and spun at 400 rpm in a cytocentrifuge (Shandon, Sewickly, Pa.) onto poly(l-lysine)-coated glass slides in PBS with 1% bovine serum albumin, after which they were treated with modified Giemsa stain (Sigma Diagnostics, St. Louis, Mo.). All slides were coded to eliminate bias and counted at least twice.
Immunoblotting.
Whole-cell extracts from the stable cell pools were prepared by boiling an equal number of trypan blue-excluding cells in 1× sample buffer (6.25 mM Tris [pH 6.8], 2% sodium dodecyl sulfate, 10% glycerol, 5% 2-mercaptoethanol). The cellular proteins were separated through sodium dodecyl sulfate–7.5% or –10% polyacrylamide gels and transferred to Immobilon polyvinylidene difluoride membranes (Millipore, Bedford, Mass.) in a buffer (192 mM glycine, 25 mM Tris base) overnight at 25 V. The filters were blocked in 5% nonfat milk powder dissolved in TBST (10 mM Tris [pH 8.0], 150 mM NaCl, 0.05% Tween 20) for 1 h and then washed three times for 5 min each with 0.5% nonfat dry milk in TBST. The filters were incubated for 1 h with a 1:1,000 dilution of anti-PLZF polyclonal rabbit antiserum or affinity-purified anti-PLZF antibodies at a concentration of 0.5 μg/ml. The filters were washed three times for 5 min each with TBST and then incubated with a 1:7,500 dilution of goat anti-rabbit immunoglobulin G (IgG) or goat anti-mouse IgG coupled to horseradish peroxidase (Boehringer Mannheim, Indianapolis, Ind.) for 1 h; this was followed by five washes with TBST. Immunoreactive proteins were visualized by chemiluminescence and autoradiography (ECL; Amersham, Buckinghamshire, United Kingdom).
Immunostaining and flow cytometry.
Immunostaining was performed on 103 to 106 cells per sample, depending on the cell pool and cell availability. Cell surface staining was performed first, followed by the intranuclear antigen staining and, finally, by DNA staining when necessary. 32DG/GM cells were first blocked in PBS containing 2% FBS and 2% mouse serum for 30 min at room temperature; this was followed by addition of the primary antibody against the cell surface antigen and incubation for an additional 30 min. The cells were then washed three times with PBS–2% FBS. Secondary antibody was added only if cell surface staining was performed. When intranuclear staining was performed, cells were permeabilized by incubation in PBS–0.2% Tween for 15 min at 37°C followed by incubation with the antibody against the intranuclear antigen for 30 min. Next, the cells were washed three times in PBS, incubated with the appropriate fluorochrome-conjugated secondary antibody for 30 min, and washed three more times with PBS. All antibodies were diluted in the blocking solution, and all incubations were carried out in 100-μl volumes at room temperature. For DNA staining, cells were incubated with 10 mg of propidium iodide (PI) per ml in PBS and 2.5 μg of DNase-free RNase (Boehringer Mannheim) per ml for at least 30 min. Samples were kept at 4°C for up to 1 day and then subjected to flow cytometry and analyzed with CellFit or ModFit software (Nippon Becton Dickinson).
Antibodies.
Polyclonal rabbit anti-mouse IL-3R antibody (Santa Cruz Biotechnology) was used at a concentration of 0.3 μg/ml; polyclonal nonspecific rabbit IgG was purchased from R&D Systems; rat anti-GR-1-phycoerythrin (PE), PE-conjugated rat anti-Sca-1, and PE-conjugated rat IgG2b (PharMingen, San Diego, Calif.) were each used at a concentration of 0.2 μg/sample. Monoclonal mouse anti-PLZF antibody 2A9 was raised in the Hybridoma Core Facility of the Mount Sinai School of Medicine and used at a concentration of 1 μg/ml.
Flow cytometric analysis of apoptosis.
Apoptosis was assessed by a modified terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) assay (Apotag; Oncor, Gaithersburg, Md.). Cells were fixed in 1% paraformaldehyde followed by 70% ethanol. Free 3′ hydroxyl ends of DNA were extended with digoxigenin-conjugated nucleotides by using terminal transferase and labeled with fluorescein-conjugated antidigoxigenin antibody. Fluorescence emissions were recorded by using a FACscan flow cytometer and analyzed with LYSIS software (Nippon Becton Dickinson). Another approach for the assessment of apoptosis was based on the ability of annexin V to recognize phosphoserine-containing lipids on the surfaces of the dying cells (ApoAlert; Clontech, Palo Alto, Calif.). Live cells were stained with PI and fluorescein isothiocyanate (FITC)-conjugated annexin V, washed, and analyzed. PI-positive (necrotic) cells were excluded from analysis, and PI-negative cells were analyzed for FITC staining.
RESULTS
Generation of pools of 32DG/GM cells expressing PLZF.
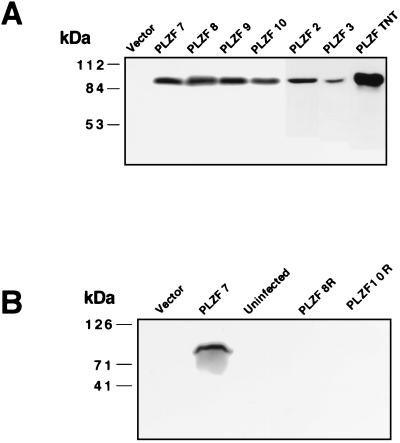
To address the biological role of PLZF in myeloid cell differentiation, we engineered the expression of PLZF in 32DG/GM cells. To avoid possible artifacts due to clonal selection, pools of PLZF-expressing cells were created by using three different sources of retrovirus. 32DG/GM cells were infected with a PLZF-expressing, replication-deficient retrovirus by coculture with two different Ψ2-PLZF packaging lines and by incubation with supernatant containing a pool of retroviruses derived after transient transfection of the BOSC packaging line with the PLZF vector. PLZF expression in the stable pools was demonstrated by immunoblotting (Fig. 1A). PLZF was detected as an 85- to 90-kDa protein in PLZF-infected pools but was not detected in control pools transduced with a pBabepuro vector lacking a cDNA insert. The six PLZF-expressing 32DG/GM cell pools analyzed all expressed similar levels of PLZF that were much higher than the amount of PLZF endogenously expressed in 32DG/GM cells, in which PLZF mRNA is detectable by reverse transcription-PCR (53) but the protein is not detected by immunoblot analysis (Fig. 1A).
FIG. 1.
Analysis of retrovirus-infected 32DG/GM cell pools and transfected NB4 cells for exogenous expression of PLZF. (A) In vitro-translated PLZF (TNT) and whole-cell lysates prepared from equal numbers of trypan blue-excluding cells from each of the pools infected with the pBabepuro or pBabepuro-PLZF retrovirus were separated by electrophoresis through a denaturing 10% polyacrylamide gel, transferred to a polyvinylidene difluoride membrane, and blotted with monoclonal mouse anti-PLZF antibody 2A9. (B) Immunoblot of extracts from a vector-infected pool, a growth-suppressed PLZF 7 pool, and two pools which had reverted from the growth-suppressed phenotype, PLZF 8R and PLZF 10R. The positions of molecular mass markers are shown to the left of the gels.
PLZF expression in 32DG/GM cells is associated with growth suppression and G1 arrest.
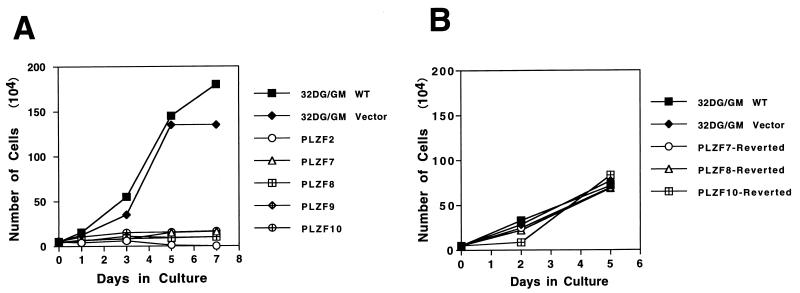
PLZF-expressing pools of 32DG/GM cells proliferated poorly in culture (Fig. 2A). Wild-type and vector-infected cells had very similar growth curves and reached plateaus of growth at about day 5 at densities exceeding 106/ml, while PLZF pools, despite being infected with viral vectors derived from three different packaging cell lines, all showed the same phenotype of severe growth suppression and never reached a density of greater than 105 cells/ml. The PLZF2 pool could be maintained in culture for a limited time period. The doubling time for wild-type and vector-control cell lines was less than 24 h. In contrast, it took PLZF pools on average more than 3 days to double their populations. Growth suppression by PLZF was not limited to the murine 32DG/GM cells, since human NB4 APL cells overexpressing PLZF were significantly growth retarded, as were lymphoid BaF/3 cells overexpressing PLZF (data not shown).
FIG. 2.
Effect of PLZF overexpression on cell proliferation. (A) Duplicate cultures derived from recently thawed PLZF-expressing or control 32DG/GM cells were plated at a density of 5 × 104/ml in complete medium. Live-cell numbers were determined at 2-day intervals, and the averages were plotted. (B) Growth curves of revertant 32DG/GM cell lines which lost PLZF expression and of control vector pools. The control and PLZF pools were maintained in continuous culture in parallel for at least 6 weeks prior to performance of the experiment at a time when the PLZF pools reverted to fast cell growth. WT, wild type.
The inhibiting effect of PLZF on cell growth was further confirmed by the finding that with prolonged incubation, PLZF-transduced cell lines would begin to grow rapidly. Expression of PLZF was monitored on a weekly basis. Even though the pools were constantly maintained under selective pressure by growth in the presence of puromycin, after 6 to 8 weeks all of the cell pools reverted to faster cell growth, correlating with the loss of PLZF expression as determined by immunoblot analysis. This may have been due to competition between higher- and lower-level expressors with an outgrowth of the faster-growing pools. However, the sudden reversion of the phenotype suggests a genetic event leading to the disruption of PLZF expression, perhaps methylation and inactivation of the retroviral vector (7). As an example, when PLZF pools 7 and 10 displayed rapid growth, matching that of cultures of 32D vector cells that were passaged in parallel, immunoblot analysis indicated a loss of PLZF expression (Fig. 1B and 2B), while the pools still maintained puromycin resistance. The vector cells passaged for several weeks grew less vigorously than newly thawed vector-containing cells (compare Fig. 2A and B), possibly due to senescence of the culture, but still grew substantially faster than PLZF-expressing cells and at the same rate as PLZF-revertant cell pools. The PLZF-revertant pools became responsive to CSFs, undergoing morphological differentiation after G-CSF and GM-CSF treatments (data not shown), and were no longer blocked in the cell cycle (see below).
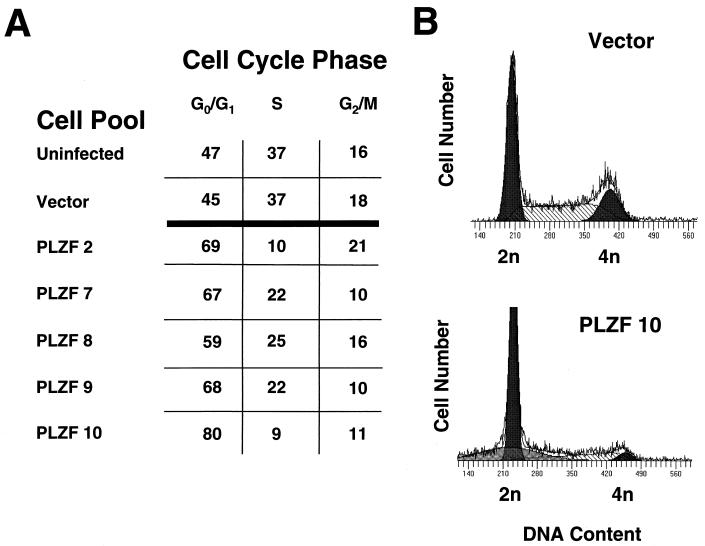
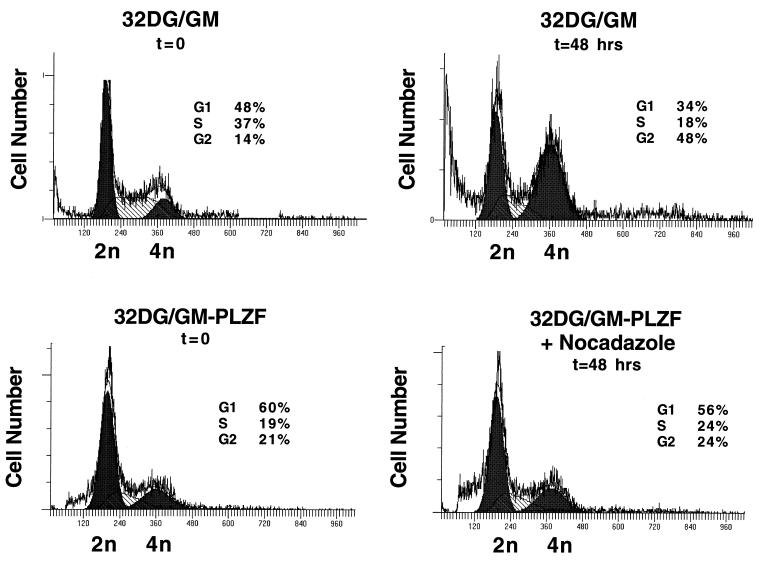
To understand the nature of the growth retardation associated with PLZF expression, we studied the cell cycle profiles of PLZF-expressing 32DG/GM pools. By PI staining and fluorescence-activated cell sorter (FACS) analysis for DNA content, there was no difference between the cell cycle profile of cells infected with insertless retrovirus and that of wild-type cells, with less than 50% of the cells of either pool being in the G0/G1 compartment and a substantial portion being in S phase (Fig. 3A). In contrast, the cell cycle profiles of PLZF-overexpressing pools were significantly altered, with up to 80% of cells accumulating in the G0/G1 phase of the cell cycle and a significantly smaller proportion being in S phase (Fig. 3A). This distortion of the cell cycle could reflect the prolonged G1 phase or a complete block of some cells in G1. This was investigated by treatment of control and PLZF-expressing cells with nocadazole to synchronize them in the G2/M phase of the cell cycle. At the start of the control experiment, nearly 50% of control cells were in G0/G1. After 48 h in the presence of nocadazole, there was a significant decrease in the number of cells in the G0/G1 and S phases, and nearly 50% of the cells accumulated in G2 (Fig. 4). In contrast, after 48 h of growth in the presence of nocadazole, most of the PLZF-expressing pool remained in G1, few of the cells trasversed S phase, and there was little accumulation of cells in G2 above the baseline level determined at the start of the experiment. Even after 72 h of growth in the presence of nocadazole, nearly 50% of PLZF-expressing cells remained in G0/G1 (data not shown). This indicates that most of the cells expressing PLZF are blocked in the G0/G1 phase of the cell cycle and are delayed in passage through S phase into G2. A minority of cells, perhaps those cells expressing little or no PLZF, may be cycling normally. However, less than 50% of the cells in the stable pools expressed high levels of PLZF (see Fig. 6). It is possible that expression of PLZF in a given cell fluctuates, since the pool does grow, escapes G0/G1 arrest, and at the same time maintains a steady level of PLZF expression. It is also possible that a subset of the pool exhibits no PLZF expression due to insertion of the retrovirus in a transcriptionally inactive portion of a chromosome. Nevertheless, the bulk of the population of PLZF retrovirus-infected 32DG/GM cells grew extremely slowly, which could imply that even low-level expression of PLZF, below the limits of detection of FACS analysis, was growth suppressive. Alternatively, the growth of cells expressing no or low levels of PLZF might have been slowed due to secretion of inhibitory factors by the cells expressing high levels of PLZF.
FIG. 3.
Effect of PLZF expression on cell cycle profile of 32DG/GM cells. (A) The cell cycle distributions of control and PLZF-expressing pools of 32DG/GM cells. Cells were plated at an initial density of 5 × 104/ml, grown for 2 days, and analyzed by PI staining and FACS analysis. Hypodiploid cells were excluded from this analysis. (B) Representative DNA content histograms of PLZF pool 10 and a vector-infected control pool. Curves modeling the G0/G1, S, and G2 compartments are shown.
FIG. 4.
Pools expressing PLZF are delayed in the G1 phase of the cell cycle. Vector control and PLZF-expressing 32DG/GM pool 7 cells were plated at an initial density of 5 × 104/ml and grown in the presence or absence of 1.5 μg of nocadazole/ml for 48 h. Cells were stained with PI and analyzed by FACS to determine their DNA content and cell cycle phase at the start of the experiment and after 48 h of growth in the presence of nocadazole. Curves modeling the G0/G1, S, and G2 compartments, derived by using the ModFit program, are shown. Cell number is plottted on the y axes, and DNA content is plotted on the x axes.
FIG. 6.
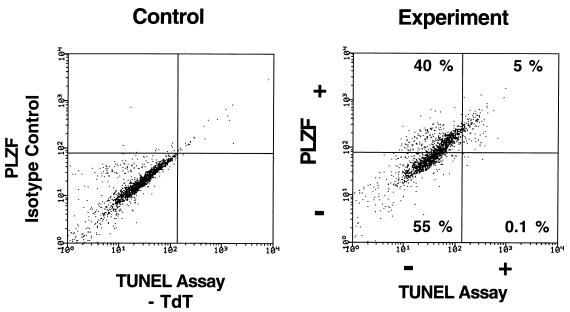
Expression of PLZF in 32DG/GM cells is closely linked to induction of apoptosis. 32DG/GM PLZF pool 2 cells were plated at a density of 5 × 104/ml and allowed to grow in complete medium containing IL-3 for 2 days. The cells were fixed in 1% formaldehyde and simultaneously stained for expression of PLZF with monoclonal mouse anti-PLZF antibody 2A9 (1 μg/ml) and for apoptosis by the TUNEL assay. After being stained, the cells were analyzed by two-channel FACS, with PLZF expression displayed on the ordinate and TUNEL-positive, apoptotic cells displayed on the abscissa. In parallel, the cells were stained with an isotype control for the PLZF monoclonal antibody and incubated with a Texas red-conjugated secondary antibody and a fluorescein-conjugated antidigoxigenin antibody in the absence of terminal transferase (TdT) to set the upper limits for nonspecific fluorescence.
PLZF overexpression leads to apoptosis.
Although PLZF repressed cell growth by inhibition of the cell cycle, we did studies to determine whether PLZF could also affect the process of programmed cell death. PI staining of PLZF-expressing cells generally showed a greatly expanded sub-G0/G1 shoulder, indicative of apoptosis (Fig. 3 and 5A). While less than 1% of the control pools contained hypodiploid cells, 7 to 20% of the PLZF-expressing pools contained such cells. To further demonstrate the occurrence of apoptosis, we used the TUNEL assay to detect free 3′ hydroxyl ends of DNA (Fig. 5B). As a positive control for apoptosis, 32DG/GM cells were withdrawn from IL-3 for 48 h; 25% of the cells subsequently stained positive by the TUNEL assay (data not shown). When 32DG/GM cells were infected with an insertless retroviral vector grown in the presence of IL-3, 0 to 3% of the cells stained dimly positive in the TUNEL assay, likely a reflection of a low-level physiological turnover of cells. For PLZF pool 2, in contrast, up to 5% of the cells stained brightly positive for apoptosis in the TUNEL assay (Fig. 5B). Apoptosis was also demonstrated by the binding of annexin V to phosphoserines on the outer membrane of the dying cell (63). PLZF-expressing 32DG/GM pools on average had two- to threefold more annexin V-positive, apoptotic cells (Fig. 5C) than did the vector pools. The results of the three assays for apoptosis are concordant with each other and demonstrate increased cell death in the PLZF pools. The correlation between PLZF expression and apoptosis was further demonstrated by dual-channel FACS analysis of a PLZF pool, assaying simultaneously for PLZF expression and the presence of 3′ hydroxyl ends by the TUNEL assay (Fig. 6). The experiment was internally controlled: the PLZF-positive or bright population was compared to the PLZF-negative or dim population. This experiment yielded two notable findings. First, virtually all of the TUNEL-positive apoptotic cells expressed high levels of PLZF. Second, less than half of the cell population expressed high levels of PLZF despite the marked growth suppression of the entire pool (Fig. 1).
FIG. 5.
PLZF expression increases spontaneous apoptosis in 32DG/GM cells grown in the presence of IL-3. (A) The percentage of cells with a sub-G1/G0 content of DNA was derived by integration of the DNA content histograms from cells plated at an initial concentration of 5 × 104/ml and grown for 2 days in complete medium containing IL-3. (B) Apoptotic cells were detected by modified TUNEL assay. The percentage of TUNEL-positive cells and the mean fluorescence of the cells from two trials are indicated. (C) Apoptotic cells were stained with FITC-conjugated annexin V. Necrotic cells, which are nonspecifically stained by annexin V, were eliminated from the analysis by gating out cells which stained with PI. The data represent the averages (+ standard deviation) of from two to four independent determinations.
PLZF overexpression inhibits myeloid differentiation.
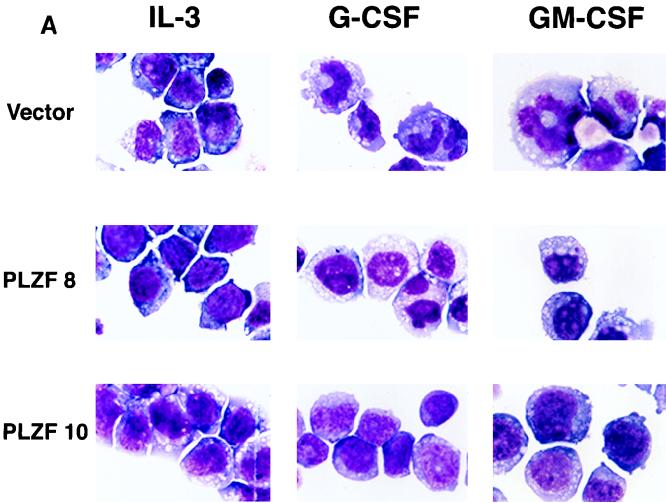
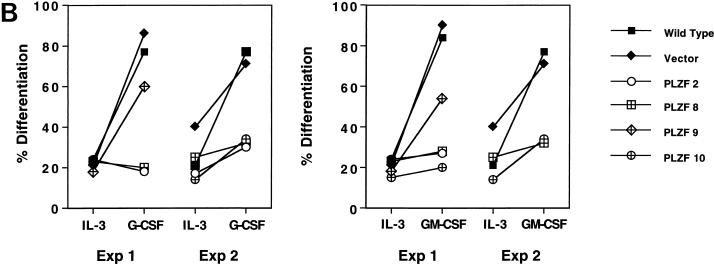
32DG/GM cells undergo myeloid differentiation in response to G-CSF and GM-CSF. The morphology of defined cell pools is demonstrated in Fig. 7A. When grown in medium containing IL-3, cells from all pools, including those expressing PLZF, demonstrated the immature morphology characteristic of myeloblasts, with round nuclei and a high nucleus/cytoplasm ratio. Wild-type and vector-infected pools, after exposure to G-CSF and GM-CSF, showed morphological evidence of maturation. The nuclei of the vector cells became lobulated, or bean shaped, and smaller in proportion to the cytoplasm, and in GM-CSF-treated cells the characteristic ring-nucleated murine neutrophils appeared (Fig. 7A, top row, extreme right panel). In contrast, PLZF-overexpressing pools showed fewer signs of differentiation, with their cell morphology being relatively unchanged after exposure to differentiating factors (Fig. 7A). Further examination of cells from each pool indicated that the block to differentiation was not complete. About 20% of the 32DG/GM cells spontaneously showed some evidence of differentiation when grown in the presence of IL-3, reflecting the partially differentiated phenotype of this subline of 32Dcl3 cells. The number of cells showing morphological differentiation at baseline was slightly depressed in the PLZF-expressing pools (Fig. 7A). Upon stimulation with G-CSF or GM-CSF, PLZF-expressing pools generally showed a minor increase in differentiated forms, such that generally 30 to 40% of the population of cells exhibited cytological differentiation. In contrast, G-CSF and GM-CSF induced morphological evidence of differentiation in about 90% of the control cells (Fig. 7B).
FIG. 7.
PLZF overexpression inhibits the ability of 32DG/GM cells to differentiate in response to G-CSF or GM-CSF. (A) 32DG/GM cells were grown in the presence of IL-3, G-CSF, or GM-CSF for 2 weeks and visualized by staining with modified Wright-Giemsa stains. Representative fields are shown. (B) The morphology of the cells grown in medium containing IL-3 or in the presence of G-CSF and GM-CSF was judged based on the nucleus/cytoplasm ratio and nuclear shape. At least 300 cells were counted on each slide, and the percentages of differentiated cells were plotted. Data from two independent experiments (Exp 1 and 2) are presented.
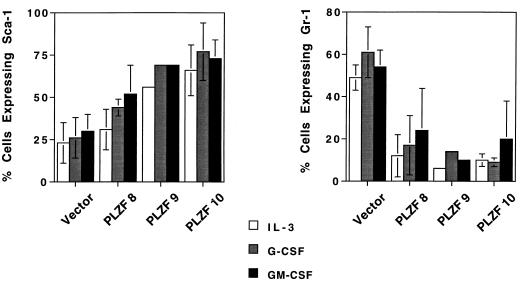
The state of differentiation of the 32D cells was further confirmed by examination of cell surface markers. Sca-1 (Ly-6A) was used as an early hematopoietic marker, while Gr-1 (Ly-6G) was chosen as a marker of myeloid differentiation (61). Less than 25% of cells from the vector-infected pool expressed Sca-1 (Fig. 8), indicative of the partially differentiated nature of the 32DG/GM line (31), while up to 75% of cells in the PLZF pools expressed Sca-1 when grown in medium containing IL-3. Furthermore, the intensity of Sca-1 staining was up to two times higher in PLZF pools (data not shown) than in the vector control cells. Paradoxically, after addition of G-CSF or GM-CSF differentiation factor, the PLZF-expressing pools tended to up-regulate Sca-1 expression (Fig. 8), while Sca-1 levels in the control pool did not consistently change with the addition of G-CSF or GM-CSF, perhaps due to the partial differentiation of the original cell line. The myeloid maturation marker Gr-1 (Fig. 8) showed the opposite pattern, with approximately 40 to 75% of vector-infected cells staining positive for this marker, confirming the partially differentiated state of the 32Dcl3G/GM subline. The percentage of cells expressing the Gr-1 marker in the PLZF-expressing pools was markedly decreased when the cells were grown in medium with IL-3. In addition, the Gr-1 intensity in PLZF pools was on average half that in vector-infected and uninfected cell pools (data not shown). Induction with G-CSF or GM-CSF in general mildly stimulated expression of the Gr-1 marker in control cells, increasing the proportion of positive cells to up to 75% (Fig. 8), with an up to twofold increase in the mean fluorescence of staining for this marker (data not shown). G-CSF and GM-CSF also modestly (by 5 to 10%) increased the percentage of cells expressing Gr-1, correlating with the limited morphological effects that these factors had on the PLZF-expressing pools. However, even after G-CSF or GM-CSF treatment, the number of Gr-1-positive cells in the PLZF pools was significantly lower than that in the control cells (Fig. 8).
FIG. 8.
Expression of myeloid cell surface differentiation markers in control and PLZF-expressing pools. Cells were maintained in medium containing IL-3, G-CSF, or GM-CSF for 2 weeks, harvested, stained with anti-Sca-1 or anti-Gr-1 antibody, and analyzed by FACS. The percentages (± the standard deviations for three to five trials) of cells positive for the expression of the indicated cell surface markers are plotted. Due to poor growth, pool 9 was assayed only once.
PLZF-expressing cells secrete a growth-suppressive factor.
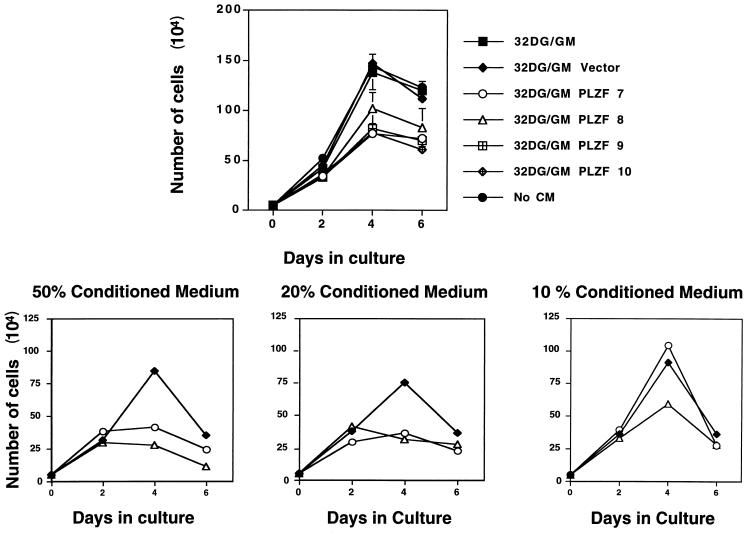
Growth suppression by PLZF was profound in 32DG/GM cells (Fig. 1), although FACS analysis (Fig. 6) indicated that high levels of PLZF were found in less than 50% of the cells in a pool. Therefore, we hypothesized that PLZF pools of 32DG/GM cells could secrete an inhibitory factor into the growth medium. To detect such a factor, control 32DG/GM cells stably infected with the parental pBabepuro vector were cultured in medium conditioned by PLZF-expressing cells. As a result, the growth of these cells was inhibited, reaching a 50% lower peak density and exhibiting an increase in estimated doubling time from 24 to 48 h (Fig. 9). Contamination with PLZF-expressing cells was eliminated by filtering the CM. Furthermore, the medium from PLZF-expressing 32DG/GM cells did not contain packaged retroviruses because the CM failed to confer puromycin resistance to NIH 3T3 cells (data not shown). When CM was taken from denser cultures (∼105 to 106 cells/ml) of 32DG/GM-PLZF cells, the effect of CM on the growth of control cells was more pronounced. The growth-suppressive effect was strongest when cells were cultivated with 50% CM, with cells reaching a threefold-lower cell density; it diminished with 20% CM and, in one case, was lost with 10% CM (Fig. 9). CM derived from the pBabepuro-infected pool did not suppress the growth of a fresh culture of 32DG/GM cells despite the fact that the vector-infected cells grew noticeably faster than the PLZF-expressing pools, leading to increased acidity of the CM and, presumably, depletion of some nutrients.
FIG. 9.
PLZF-expressing pools secrete a growth-inhibitory factor. 32DG/GM cells infected with the pBabepuro retroviral vector lacking an insert were plated in triplicate at a density of 105/ml in the presence or absence of medium conditioned by growth in the presence of PLZF-expressing 32DG/GM cell pools. Live cells were counted by hemocytometer and plotted (± standard deviations). The upper panel represents the effect on cell growth produced by 50% CM derived from an initial culture of 104 PLZF-expressing cells/ml. The lower panel displays the effect of different dilutions of CM derived from higher-density cultures (approximately 2 × 105 cells/ml) of PLZF-expressing cells on the growth of 32DG/GM vector cells.
PLZF expression increases survival of 32DG/GM cells after IL-3 withdrawal.
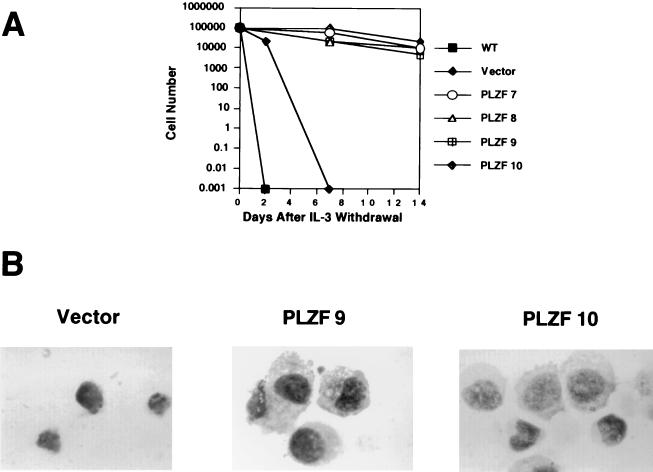
Since PLZF is expressed in quiescent and apoptosis-resistant CD34+ progenitor cells (53), we studied the effect of PLZF overexpression on cell death induced by withdrawal of IL-3. Within 24 h after IL-3 withdrawal, wild-type and vector-infected cells underwent apoptosis, as determined by TUNEL assay and the appearance of cells with a hypodiploid DNA content (data not shown). Since PLZF cells have a higher incidence of apoptosis when cultured in the presence of IL-3, we expected to see a much faster induction of the death program in PLZF-expressing pools after IL-3 withdrawal. Surprisingly, PLZF-expressing 32DG/GM cells survived longer in the absence of the growth factor than did the control cells (Fig. 10A). Whereas all control cells were dead within 1 week of IL-3 withdrawal, more than 10% of the cells derived from PLZF-expressing pools of 32DG/GM survived 2 weeks without the growth factor (Fig. 10A) and exhibited the typical appearance of undifferentiated 32DG/GM cells (Fig. 10B). FACS analysis indicated that these surviving cells were blocked in the G1 phase of the cell cycle (data not shown).
FIG. 10.
PLZF prolongs survival of 32DG/GM cells after IL-3 withdrawal. (A) Cells were washed free from IL-3 and plated in culture medium lacking IL-3; live cells were counted at the indicated time points. (B) Morphology of cells maintained for 2 weeks without IL-3. Cells were centrifuged onto slides, air dried, and visualized with a modified Wright-Giemsa stain.
DISCUSSION
APL associated with t(11;17)(q23;q21) is unique in its resistance to differentiation therapy with ATRA and to conventional chemotherapy (20, 36). Since the RARα gene is disrupted in a similar manner in all translocations associated with APL (19, 52, 62, 67, 68), we surmised that the properties of the PLZF gene disrupted in t(11;17) might explain the unique properties of the disease. PLZF encodes a DNA-binding transcriptional regulator (35, 59) which is expressed in the early hematopoietic precursor lineage (53). When such cells were placed into culture and allowed to differentiate, PLZF levels transiently increased and then declined (32). Engineered stable overexpression of PLZF in 32DG/GM cells yielded a striking suppression of cell growth, accumulation of cells in the G0/G1 phase of the cell cycle, and inhibition of differentiation in response to G-CSF and GM-CSF. Expression of PLZF was also associated with a more immature immunophenotype characterized by up-regulation of Sca-1, down-regulation of Gr-1, and a twofold decline in surface expression of the IL-3 receptor (data not shown). Hence, PLZF-expressing myeloid cells share many characteristics with hematopoietic progenitor/stem cells, including cell quiescence, expression of early hematopoietic markers, and reduced expression of growth factor receptors (47, 61). Together these data indicate that PLZF expression may be important for the maintenance or survival of hematopoietic stem cells.
Growth suppression and cell cycle delay induced by PLZF.
Growth suppression associated with expression of PLZF was linked to inhibition of the cell cycle. When acutely expressed in 32Dcl3 cells by retroviral infection, PLZF can cause arrest in S phase, and when expressed in NIH 3T3 cells, it can lengthen the G1/S transition (73). After selection of stable pools of 32Dcl3 cells expressing PLZF, the resulting population of cells showed an increased fraction in G0/G1. When treated with nocadazole, the PLZF cells failed to significantly accumulate in G2, slowly passing through the G1 and S phases of the cell cycle. The ability of PLZF to inhibit transit through the G1 and S phases may be due to the ability of PLZF to repress transcription of the cyclin A2 gene (73). However, other cyclin or cyclin-dependent complexes involved in the G1/S transition may be affected in 32Dcl3 cells, either by the direct action of PLZF or indirectly through secretion of growth-inhibitory substances.
PLZF expression appears to affect general components of the cell growth machinery rather than components exclusive to 32DG/GM or myeloid cells, since overexpression of PLZF represses growth of human promyelocytic NB4 cells, which express low levels of PLZF, and of lymphoid Ba/F3 cells and NIH 3T3 fibroblasts, which do not express PLZF (data not shown). Given that PLZF expression was compatible with the creation of stable pools, cell cycle blockade by PLZF in 32DG/GM cells was not complete. This could be due to the fact that only about 50% of the cells in the PLZF-expressing 32DG/GM pools expressed high levels of PLZF at any given time, and that only PLZF-negative cells could proliferate, or that the PLZF-expressing cells could proliferate, albeit slowly. We favor the latter explanation, given the fact that PLZF expression could be stably detected in the pools for up to 2 months. After prolonged culture, reversion to wild-type growth was observed, accompanied by the loss of PLZF expression (Fig. 2). This may have been due to selection of cells that never expressed PLZF, to progressive elimination of PLZF-expressing cells by apoptosis, or to inactivation of the retroviral vector, possibly by methylation.
Growth suppression by PLZF also occurred in a non-cell-autonomous manner, presumably due to the secretion of a factor which repressed cell growth in a concentration-dependent manner. This mechanism may be unique to hematopoietic cells. While CM from 32DG/GM cells expressing PLZF could inhibit the growth of 32DG/GM cells not harboring PLZF, the same CM could not inhibit the growth of NIH 3T3 cells (Fig. 9 and data not shown). Growth suppression by the inhibitory factor was more likely to occur by a cell cycle regulatory mechanism rather than by induction of apoptosis, since two-channel FACS staining indicated that apoptotic cells were exclusively brightly PLZF positive (Fig. 6). Likely candidates for the antiproliferative cytokine(s) elicited by PLZF-expressing 32DG/GM cells include IL-6, transforming growth factor β, and interferons (reviewed in reference 5), which are known to produce growth arrest in G0/G1 by acting on cyclins and cyclin-dependent kinases, leading to the accumulation of hypophosphorylated Rb (26, 38). Hematopoietic progenitors secrete transforming growth factor β in an autocrine manner (42), and it is tempting to speculate that PLZF plays a role in the expression of this cytokine.
PLZF expression alters myeloid differentiation.
Enforced expression of PLZF inhibited myeloid differentiation induced by G-CSF or GM-CSF. It was suggested that differentiation of myeloid cells usually occurs in two steps: a precommitment stage, in which inducers of differentiation alter the genetic program of the cell, and terminal differentiation, which is accompanied by G1 arrest, generally occurring after a few rounds of cell division (71, 72). Since 60 to 80% of 32DG/GM-PLZF cells were already arrested in G1 before the addition of G-CSF or GM-CSF differentiation factor, the failure of the PLZF-expressing pools to undergo differentiation cannot be explained by their inability to cease cell division. Instead, the problem may reside in the inability of the PLZF-expressing cells to undergo the rounds of division which accompany terminal differentiation. It is also possible that PLZF prevents expression of genes determining differentiation during the precommitment phase, maintaining the cells in an undifferentiated state. Sca-1 and Gr-1 themselves could be targeted by PLZF. This is supported by the fact that even when grown in the absence of differentiation factors, PLZF-expressing cells expressed considerably less Gr-1 and more Sca-1. PLZF expression led to either the induction of a more undifferentiated phenotype or preferential selection of 32DG/GM cells with this phenotype. This is consistent with the fact that PLZF is expressed in CD34+ cells and further supports the hypothesis that PLZF is necessary for the maintenance of dormant immature hematopoietic progenitors. PLZF-expressing 32DG/GM cells, in an aberrant manner, up-regulated Sca-1 after G-CSF or GM-CSF treatment. This altered phenotype also indicates that the growth suppression induced by PLZF is unlikely to be a nonspecific toxic effect but rather is due to a blockade of normal pathways of myeloid development. As a result, signals received by the cells through the actions of cytokines may be diverted to an aberrant differentiation program.
PLZF and altered cell death.
Constitutive PLZF expression protected 10% of 32DG/GM cells from apoptotic death after 2 weeks of IL-3 withdrawal, with the surviving cells arrested in the G0/G1 phase of the cell cycle. This novel phenotype further indicates that PLZF has specific effects on cell growth and survival. The antiapoptotic effect of PLZF may reflect an important biological function, namely, to enhance the survival of quiescent stem cells. It is possible that decreased sensitivity to IL-3 withdrawal is the reflection of the decreased dependence on IL-3 to provide the proliferative signal, due to the diminished numbers of IL-3 receptors on the surfaces of the PLZF pool cells (73). It will be important to determine if other apoptotic stimuli, such as genotoxic damage and treatment with tumor necrosis factor, are also blunted in the PLZF-expressing pools. This would determine whether PLZF expression causes a more global change in the balance between cell life and death, possibly by altering the expression of bcl2 or related proteins. The effect of PLZF is very similar to that of bcl2, which, when overexpressed in 32Dcl3 cells, retards apoptosis after IL-3 deprivation (4, 64). Strikingly, bcl2 overexpression also inhibits differentiation of 32Dcl3 cells (39) even though it does not interfere with differentiation in other cell lines (45, 48, 69). By itself, protection from apoptosis is not sufficient to inhibit differentiation, since overexpression of bcl2 family member A1 protects 32Dcl3 from cell death but has no effect on differentiation (39).
In an apparent paradox, PLZF expression was associated with an increase in the rate of apoptosis in cells maintained in the presence of IL-3. PLZF leads to a G1/S arrest or delay by cell autonomous and probable autocrine mechanisms likely leading to the inhibition of cyclin–cyclin-dependent kinase activity and the accumulation of hypophosphorylated Rb. At the same time, the IL-3 cytokine yields a proliferative signal mediated by STAT activation, tyrosine phosphorylation of Shc and Grb2, and stimulation of mitogen-activated protein kinase (24, 27). As a result, the cytokine increases transcription of cyclins, driving the cell cycle forward by the phosphorylation of Rb (reviewed in reference 50). The observed phenotype of increased apoptosis may occur due to a clash of cellular growth signals. In an analogous manner, serum deprivation, which is a growth-inhibitory signal, clashes with constitutive c-myc or E2F expression to induce apoptosis (16, 57). PLZF function itself may be changed by IL-3 treatment, since PLZF is phosphorylated on serine and threonine residues and can be phosphorylated in vitro by mitogen-activated protein kinase (56). Elucidation of the mechanism of increased apoptosis by PLZF will require the identification of genes directly or indirectly regulated by the factor, which may in turn reset the cell life/death balance.
The role of PLZF in t(11;17) APL.
Our data have implications for understanding the pathogenesis of APL associated with t(11;17)(q23;q21). The blockade of the normal RA signal plays a critical role in the pathogenesis of APL, likely in selecting the promyelocyte phenotype. Both PML-RARα and PLZF-RARα inhibit the transcription of key myeloid differentiation target genes at low (10−9 M) concentrations of ATRA. While PML-RARα can activate these genes after treatment with pharmacological doses of ATRA (10−6 M), PLZF-RARα fails to activate such genes (55) due to the ability of the PLZF moiety of the fusion protein to interact with SMRT and N-Cor corepressor proteins even in the presence of ATRA (18, 21–23, 40). Thus, part of the resistant phenotype of t(11;17)-associated APL may be due to a reduced capacity of the PLZF-RARα chimeric protein to transduce ATRA-mediated differentiation signals. However, a transgenic model for t(11;17) APL yielded a myeloid leukemia that did respond to high-dose ATRA with an increase in differentiated granulocyte forms, suggesting that in the organism, PLZF-RARα, though leukemogenic, does not completely block differentiation (22).
We suggest that the aberrant regulation of targets of the PLZF protein may have a significant role in refractoriness of the t(11;17) APL syndrome. It can be argued that PLZF is in fact a tumor suppressor. Patients with t(11;17) have one disrupted PLZF allele, and the products resulting from the reciprocal PLZF-RARα fusion genes generated by the translocation, RARα-PLZF and PLZF-RARα, may both have deleterious, dominant-negative effects on the remaining cellular PLZF. The PLZF-RARα protein contains the POZ (2, 6) domain of PLZF, which mediates self-association of PLZF (14) as well as transcriptional repression (35). Consequently, PLZF-RARα might sequester the remaining PLZF in the cell in a multiprotein complex or bind to cofactors utilized by PLZF to repress its gene targets. Furthermore, the RARα-PLZF protein can exert a second effect on PLZF function by binding to the same sequences as wild-type PLZF protein and blocking its normal transcriptional effects (35). Hence, APL cells from t(11;17) patients might be functionally null for the PLZF protein, and the growth-regulatory checks normally afforded by PLZF would be abrogated. The situation is in reality more complex, since RARα-PLZF is actually a PLZF gain-of-function mutant. In cotransfection experiments, RARα-PLZF was found to activate, rather than repress, a model PLZF target gene (35). It would therefore be predicted that RARα-PLZF would be a growth activator or oncogene, and in fact RARα-PLZF stimulates growth of NIH 3T3 cells (73) and can transform murine marrow progenitor cells (33). ATRA therapy may begin to stimulate myeloid differentiation, but these effects could be blocked by the stimulation of a growth program mediated by PLZF target genes. Since the second promoter of the RARα gene is ATRA responsive (34), ATRA treatment might stimulate the production of the RARα-PLZF fusion protein, further deregulating genes normally repressed by PLZF and, hence, possibly worsening the clinical status of a t(11;17) APL patient. Proof of this hypothesis awaits the further identification of PLZF target genes and the construction and characterization of animal and cell culture models engineered to express both PLZF-RARα and RARα-PLZF.
In summary, we defined PLZF as a potent suppressor of cell growth with prominent effects on the cell cycle, myeloid differentiation, and programmed cell death. PLZF may play a key role in maintaining the quiescent, undifferentiated state of hematopoietic progenitors. Disruption of these functions could play a principal role in the pathogenesis of APL.
ACKNOWLEDGMENTS
We thank Alan Rosmarin, David Sassoon, and Heidi Stuhlmann for helpful discussions and Steven Arkin and Christine Arkin for assistance with FACS analysis.
This work was supported by NIH grants CA59936 (J.D.L., S.W., and A.Z.), CA73762 (A.M.), American Cancer Society grant DHP-160 (J.D.L.), and the Leukemia Research Fund of Great Britain (A.Z.). J.D.L. is a Scholar of the Leukemia Society of America. R.S.S. was supported by Medical Scientist Training Program grant GM0707280-17 from the NIH. P.L.Y. was supported by the Lauri Strauss Leukemia Fund.
Footnotes
Publication no. 249 from the Brookdale Center for Developmental and Molecular Biology.
REFERENCES
- 1.Ahn M J, Nason-Burchenal K, Moasser M M, Dmitrovsky E. Growth suppression of acute promyelocytic leukemia cells having increased expression of the non-rearranged alleles: RAR alpha or PML. Oncogene. 1995;10:2307–2314. [PubMed] [Google Scholar]
- 2.Albagli O, Dhordain P, Deweindt C, Lecocq G, Leprince D. The BTB/POZ domain: a new protein-protein interaction motif common to DNA- and actin-binding proteins. Cell Growth Differ. 1995;6:1193–1198. [PubMed] [Google Scholar]
- 3.Anderson S A, Carrol P M, Lee F. Abrogation of IL-3 dependent growth requires a functional v-src gene product: evidence for an autocrine growth cycle. Oncogene. 1990;5:317–325. [PubMed] [Google Scholar]
- 4.Baffy G, Miyashita T, Williamson J R, Reed J C. Apoptosis induced by withdrawal of interleukin-3 (IL-3) from an IL-3-dependent hematopoietic cell line is associated with repartitioning of intracellular calcium and is blocked by enforced Bcl-2 oncoprotein production. J Biol Chem. 1993;268:6511–6519. [PubMed] [Google Scholar]
- 5.Bagby G J. Hematopoiesis. In: Stamatoyannopoulos G, Nienhaus A W, Majerus P W, Varmus H, editors. The molecular basis of blood diseases. W. B. Philadelphia, Pa: Saunders; 1994. pp. 71–103. [Google Scholar]
- 6.Bardwell V J, Treisman R. The POZ domain: a conserved protein-protein interaction motif. Genes Dev. 1994;8:1664–1677. doi: 10.1101/gad.8.14.1664. [DOI] [PubMed] [Google Scholar]
- 7.Challita P M, Kohn D B. Lack of expression from a retroviral vector after transduction of murine hematopoietic stem cells is associated with methylation in vivo. Proc Natl Acad Sci USA. 1994;91:2567–2571. doi: 10.1073/pnas.91.7.2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang K S, Fan Y H, Andreeff M, Liu J, Mu Z M. The PML gene encodes a phosphoprotein associated with the nuclear matrix. Blood. 1995;85:3646–3653. [PubMed] [Google Scholar]
- 9.Chen S J, Zelent A, Tong J H, Yu H Q, Wang Z Y, Derre J, Berger R, Waxman S, Chen Z. Rearrangements of the retinoic acid receptor alpha and promyelocytic leukemia zinc finger genes resulting from t(11;17)(q23;q21) in a patient with acute promyelocytic leukemia. J Clin Invest. 1993;91:2260–2267. doi: 10.1172/JCI116453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Z, Brand N J, Chen A, Chen S J, Tong J H, Wang Z Y, Waxman S, Zelent A. Fusion between a novel Kruppel-like zinc finger gene and the retinoic acid receptor-alpha locus due to a variant t(11;17) translocation associated with acute promyelocytic leukaemia. EMBO J. 1993;12:1161–1167. doi: 10.1002/j.1460-2075.1993.tb05757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chomienne C, Fenaux P, Degos L. Retinoid differentiation therapy in promyelocytic leukemia. FASEB J. 1996;10:1025–1030. doi: 10.1096/fasebj.10.9.8801163. [DOI] [PubMed] [Google Scholar]
- 12.Cook M, Gould A, Brand N, Davies J, Strutt P, Shaknovich R, Licht J, Waxman S, Chen Z, Gluecksohn-Waelsch S, Krumlauf R, Zelent A. Expression of the zinc-finger gene PLZF at rhombomere boundaries in the vertebrate hindbrain. Proc Natl Acad Sci USA. 1995;92:2249–2253. doi: 10.1073/pnas.92.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Thé H, Chomienne C, Lanotte M, Degos L, Dejean A. The t(15;17) translocation of acute promyelocytic leukaemia fuses the retinoic acid receptor alpha gene to a novel transcribed locus. Nature. 1990;347:558–561. doi: 10.1038/347558a0. [DOI] [PubMed] [Google Scholar]
- 14.Dong S, Zhu J, Reid A, Strutt P, Guidez F, Zhong H J, Wang Z Y, Licht J, Waxman S, Chomienne C, Chen Z, Zelent A, Chen S J. Amino-terminal protein-protein interaction motif (POZ-domain) is responsible for activities of the promyelocytic leukemia zinc finger-retinoic acid receptor-alpha fusion protein. Proc Natl Acad Sci USA. 1996;93:3624–3629. doi: 10.1073/pnas.93.8.3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dyck J A, Maul G G, Miller W H, Jr, Chen J D, Kakizuka A, Evans R M. A novel macromolecular structure is a target of the promyelocytic-retinoic acid receptor oncoprotein. Cell. 1994;76:333–343. doi: 10.1016/0092-8674(94)90340-9. [DOI] [PubMed] [Google Scholar]
- 16.Evan G I, Wyllie A H, Gilbert C S, Littlewood T D, Land H, Brooks M, Waters C M, Penn L Z, Hancock D C. Induction of apoptosis in fibroblasts by c-myc protein. Cell. 1992;69:119–128. doi: 10.1016/0092-8674(92)90123-t. [DOI] [PubMed] [Google Scholar]
- 17.Goddard A, Borrow J, Freemont P, Solomon E. Characterization of a zinc finger gene disrupted by the t(15;17) in acute promyelocytic leukemia. Science. 1991;254:1371–1374. doi: 10.1126/science.1720570. [DOI] [PubMed] [Google Scholar]
- 18.Grignani F, De Metteis S, Nervi C, Tomassoni L, Gelmetti V, Cioce M, Fanelli M, Ruthardt M, Ferrara F F, Zamir I, Seiser C, Grignani F, Lazar M A, Minucci S, Pelicci P G. Fusion proteins of the retinoic acid receptor-α recruit histone deacetylase in promyelocytic leukemia. Nature. 1998;391:815–818. doi: 10.1038/35901. [DOI] [PubMed] [Google Scholar]
- 19.Grignani F, Fagioli M, Alcalay M, Longo L, Pandolfi P P, Donti E, Biondi A, Lo Coco F, Grignani F, Pelicci P G. Acute promyelocytic leukemia: from genetics to treatment. Blood. 1994;83:10–25. [PubMed] [Google Scholar]
- 20.Guidez F, Huang W, Tong J H, Dubois C, Balitrand N, Waxman S, Michaux J L, Martiat P, Degos L, Chen Z, Chomienne C. Poor response to all-trans retinoic acid therapy in a t(11;17) PLZF/RAR alpha patient. Leukemia. 1994;8:312–317. [PubMed] [Google Scholar]
- 21.Guidez F, Ivins S, Zhu J, Söderström M, Waxman S, Zelent A. Reduced retinoic acid sensitivities of nuclear receptor co-repressor binding to PML- and PLZF-RARα underline molecular pathogenesis and treatment of acute promyelocytic leukemia. Blood. 1998;91:2634–2642. [PubMed] [Google Scholar]
- 22.He L-Z, Guidez F, Triboli C, Peruzzi D, Ruthardt M, Zelent A, Pandolfi P P. Distinct interactions of PML-RARα and PLZF-RARα with co-repressors determine differential responses to RA in APL. Nat Genet. 1998;18:126–135. doi: 10.1038/ng0298-126. [DOI] [PubMed] [Google Scholar]
- 23.Hong S H, David G, Wong C W, Dejean A, Privalsky M L. SMRT corepressor interacts with PLZF and with the PML-retinoic acid receptor alpha (RARα) and PLZF-RARα oncoproteins associated with acute promyelocytic leukemia. Proc Natl Acad Sci USA. 1997;94:9028–9033. doi: 10.1073/pnas.94.17.9028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ihle J N, Witthuhn B A, Quelle F W, Yamamoto K, Thierfelder W E, Kreider B, Silvennoinen O. Signaling by the cytokine receptor superfamily: JAKs and STATs. Trends Biochem Sci. 1994;4:222–227. doi: 10.1016/0968-0004(94)90026-4. [DOI] [PubMed] [Google Scholar]
- 25.Kakizuka A, Miller W H, Jr, Umesono K, Warrell R P, Jr, Frankel S R, Murty V V, Dmitrovsky E, Evans R M. Chromosomal translocation t(15;17) in human acute promyelocytic leukemia fuses RAR alpha with a novel putative transcription factor, PML. Cell. 1991;66:663–674. doi: 10.1016/0092-8674(91)90112-c. [DOI] [PubMed] [Google Scholar]
- 26.Kimchi A. Cytokine triggered molecular pathways that control cell cycle arrest. J Cell Biochem. 1992;50:1–9. doi: 10.1002/jcb.240500102. [DOI] [PubMed] [Google Scholar]
- 27.Kishimoto T, Taga T, Akira S. Cytokine signal transduction. Cell. 1994;76:253–262. doi: 10.1016/0092-8674(94)90333-6. [DOI] [PubMed] [Google Scholar]
- 28.Koken M H, Linares-Cruz G, Quignon F, Viron A, Chelbi-Alix M K, Sobczak-Thepot J, Juhlin L, Degos L, Calvo F, de Thé H. The PML growth-suppressor has an altered expression in human oncogenesis. Oncogene. 1995;10:1315–1324. [PubMed] [Google Scholar]
- 29.Koken M H M, Puvion-Dutilleul F, Guillemin M C, Viron A, Linares-Cruz G, Stuurman N, de Jong L, Szostecki C, Calvo F, Chomienne C, Degos L, Puvion E, de Thé H. The t(15;17) translocation alters a nuclear body in a retinoic acid-reversible fashion. EMBO J. 1994;13:1073–1083. doi: 10.1002/j.1460-2075.1994.tb06356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koken M H M, Reid A, Quignon F, Chelbi-Alix M K, Davies J M, Kabarowski J H S, Zhu J, Dong S, Chen S, Chen Z, Tan C C, Licht J, Waxman S, de Thé H, Zelent A. Leukemia-associated retinoic acid receptor alpha fusion partners, PML and PLZF, heterodimerize and colocalize to nuclear bodies. Proc Natl Acad Sci USA. 1997;94:10255–10260. doi: 10.1073/pnas.94.19.10255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kreider B L, Phillips P D, Prystowsky M B, Shirsat N, Pierce J H, Tushinski R, Rovera G. Induction of the granulocyte-macrophage colony-stimulating factor (CSF) receptor by granulocyte CSF increases the differentiative options of a murine hematopoietic progenitor cell. Mol Cell Biol. 1990;10:4846–4853. doi: 10.1128/mcb.10.9.4846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Labbaye, C., and J. D. Licht. Unpublished data.
- 33.Lavau, C., and J. D. Licht. Unpublished data.
- 34.Leroy P, Krust A, Zelent A, Mendelsohn C, Garnier J-M, Kastner P, Dierich A, Chambon P. Multiple isoforms of the mouse retinoic acid receptor α are generated by alternative splicing and differential induction by retinoic acid. EMBO J. 1991;10:59–69. doi: 10.1002/j.1460-2075.1991.tb07921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li J-Y, English M A, Ball H J, Yeyati P L, Waxman S, Licht J D. Sequence-specific DNA binding and transcriptional regulation by the promyelocytic leukemia zinc finger protein. J Biol Chem. 1997;272:22447–22455. doi: 10.1074/jbc.272.36.22447. [DOI] [PubMed] [Google Scholar]
- 36.Licht J D, Chomienne C, Goy A, Chen A, Scott A A, Head D R, Michaux J L, Wu Y, DeBlasio A, Miller W H, Jr, Zelenetz A D, Wilman C L, Chen Z, Chen S-J, Zelent A, Macintyre E, Veil A, Cortes J, Kantarjian H, Waxman S. Clinical and molecular characterization of a rare syndrome of acute promyelocytic leukemia associated with translocation (11;17) Blood. 1995;85:1083–1094. [PubMed] [Google Scholar]
- 37.Licht J D, Shaknovich R, English M A, Melnick A, Li J Y, Reddy J C, Dong S, Chen S J, Zelent A, Waxman S. Reduced and altered DNA-binding and transcriptional properties of the PLZF-retinoic acid receptor-alpha chimera generated in t(11;17)-associated acute promyelocytic leukemia. Oncogene. 1996;12:323–336. [PubMed] [Google Scholar]
- 38.Liebermann D A, Hoffman B, Steinman R A. Molecular controls of growth arrest and apoptosis: p53-dependent and independent pathways. Oncogene. 1995;11:199–210. [PubMed] [Google Scholar]
- 39.Lin E, Orlofsky A, Wang H-G, Reed J C, Prystowsky M B. A1, a Bcl-2 family member, prolongs survival and permits myeloid differentiation. Blood. 1996;87:983–992. [PubMed] [Google Scholar]
- 40.Lin R J, Nagy L, Inoue S, Shao W, Miller W H, Jr, Evans R M. Role of the histone deacetylase complex in acute promyelocytic leukemia. Nature. 1998;391:811–814. doi: 10.1038/35895. [DOI] [PubMed] [Google Scholar]
- 41.Mavilio F, Kreider B L, Valtieri M N, Naso G, Shirsat N, Venturelli D, Reddy E P, Rovera G. Alteration of growth and differentiation factors response by Kirsten and Harvey sarcoma viruses in the IL-3-dependent murine hematopoietic cell line 32DCL3(G) Oncogene. 1989;4:301–308. [PubMed] [Google Scholar]
- 42.Moore M. Clinical implications of positive and negative hematopoietic stem cell regulators. Blood. 1991;78:1–19. [PubMed] [Google Scholar]
- 43.Morgenstern P J, Land H. Advanced mammalian gene transfer: high titer retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res. 1990;18:3587–3596. doi: 10.1093/nar/18.12.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mu Z-M, Chin K-V, Liu J-H, Lozano G, Chang K-S. PML, a growth suppressor disrupted in acute promyelocytic leukemia. Mol Cell Biol. 1994;14:6858–6867. doi: 10.1128/mcb.14.10.6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Naumovski L, Cleary M L. Bcl2 inhibits apoptosis associated with terminal differentiation of HL-60 myeloid leukemia cells. Blood. 1994;83:2261–2267. [PubMed] [Google Scholar]
- 46.Numoto M, Niwa O, Kaplan J, Wong K K, Merrell K, Kamiya K, Yanagihara K, Calame K. Transcriptional repressor ZF5 identifies a new conserved domain in zinc finger proteins. Nucleic Acids Res. 1993;21:3767–3775. doi: 10.1093/nar/21.16.3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ogawa M. Differentiation and proliferation of hematopoietic stem cells. Blood. 1993;81:2844–2853. [PubMed] [Google Scholar]
- 48.Park J R, Robertson K, Hickstein D D, Tsai S, Hockenbery D M, Collins S J. Dysregulated bcl-2 expression inhibits apoptosis but not differentiation of retinoic acid-induced HL-60 granulocytes. Blood. 1994;84:440–445. [PubMed] [Google Scholar]
- 49.Pear W S, Nolan G P, Scott M L, Baltimore D. Production of high-titer helper-free retroviruses by transient transfection. Proc Natl Acad Sci USA. 1993;90:8392–8396. doi: 10.1073/pnas.90.18.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pines J, Hunter T. Cyclin-dependent kinases: an embarassment of riches? In: Hutchison C, Glover D, editors. Cell cycle control. Oxford, United Kingdom: IRL Press; 1995. pp. 144–176. [Google Scholar]
- 51.Redner R L, Corey S J, Rush E A. Differentiation of t(5;17) variant acute promyelocytic leukemic blasts by all-trans retinoic acid. Leukemia. 1997;11:1014–1016. doi: 10.1038/sj.leu.2400661. [DOI] [PubMed] [Google Scholar]
- 52.Redner R L, Rush E A, Faas S, Rudert W A, Corey S J. The t(5;17) variant of acute promyelocytic leukemia expresses a nucleophosmin-retinoic acid receptor fusion. Blood. 1996;87:882–886. [PubMed] [Google Scholar]
- 53.Reid A, Gould A, Brand N, Cook M, Strutt P, Li J, Licht J, Waxman S, Krumlauf R, Zelent A. Leukemia translocation gene, PLZF, is expressed with a speckled nuclear pattern in early hematopoietic progenitors. Blood. 1995;86:4544–4552. [PubMed] [Google Scholar]
- 54.Rovera G, Valtieri M, Mavilio F, Reddy E P. Effect of Abelson murine leukemia virus on granulocytic differentiation and interleukin-3 dependence of a murine progenitor cell line. Oncogene. 1987;1:29–35. [PubMed] [Google Scholar]
- 55.Ruthardt M, Testa U, Nervi C, Ferrucci P F, Grignani F, Puccetti E, Grignani F, Peschle C, Pelicci P G. Opposite effects of the acute promyelocytic leukemia PML-retinoic acid receptor alpha (RARα) and PLZF-RARα fusion proteins on retinoic acid signalling. Mol Cell Biol. 1997;17:4859–4869. doi: 10.1128/mcb.17.8.4859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shaknovich, R., S. Ivers, A. Zelent, and J. D. Licht. Unpublished data.
- 57.Shan B, Lee W-H. Deregulated expression of E2F-1 induces S-phase entry and leads to apoptosis. Mol Cell Biol. 1994;14:8166–8173. doi: 10.1128/mcb.14.12.8166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shimada Y, Migliaccio G, Shaw H, Migliaccio A R. Erythropoietin-specific cell cycle progression in erythroid subclones of the interleukin-3-dependent cell line 32d. Blood. 1993;81:935–941. [PubMed] [Google Scholar]
- 59.Sitterlin D, Tiollais P, Transy C. The RARα-PLZF chimera associated with acute promyelocytic leukemia has retained a sequence-specific DNA binding domain. Oncogene. 1997;14:1067–1074. doi: 10.1038/sj.onc.1200916. [DOI] [PubMed] [Google Scholar]
- 60.Soddu S, Blandino G, Scardigli R, Martinelli R, Rizzo M G, Crescenzi M, Sacchi A. Wild-type p53 induces diverse effects in 32D cells expressing different oncogenes. Mol Cell Biol. 1996;16:487–495. doi: 10.1128/mcb.16.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Spangrude G J, Heimfeld S, Weissman I L. Purification and characterization of mouse hematopoietic stem cells. Science. 1988;241:58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- 62.Tenen D G, Hromas R, Licht J D, Zhang D E. Transcription factors, normal myeloid development, and leukemia. Blood. 1997;90:489–519. [PubMed] [Google Scholar]
- 63.van Engeland M, Ramaekers F C, Schutte B, Reutelingsperger C P. A novel assay to measure loss of plasma membrane asymmetry during apoptosis of adherent cells in culture. Cytometry. 1996;24:131–139. doi: 10.1002/(SICI)1097-0320(19960601)24:2<131::AID-CYTO5>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 64.Vanhaesebroeck B, Reed J C, De Valck D, Grooten J, Miyashita T, Tanaka S, Beyaert R, Van Roy F, Fiers W. Effect of bcl-2 proto-oncogene expression on cellular sensitivity to tumor necrosis factor-mediated cytotoxicity. Oncogene. 1993;8:1075–1081. [PubMed] [Google Scholar]
- 65.Warrell R P, Jr, de Thé H, Wang Z Y, Degos L. Acute promyelocytic leukemia. N Engl J Med. 1993;329:177–189. doi: 10.1056/NEJM199307153290307. [DOI] [PubMed] [Google Scholar]
- 66.Weis K, Rambaud S, Lavau C, Jansen J, Carvalho T, Carmo-Fonseca M, Lamond A, Dejean A. Retinoic acid regulates aberrant nuclear localization of PML-RAR alpha in acute promyelocytic leukemia cells. Cell. 1994;76:345–356. doi: 10.1016/0092-8674(94)90341-7. [DOI] [PubMed] [Google Scholar]
- 67.Wells R A, Catzavelos C, Kamel-Reid S. Fusion of the retinoic acid receptor α to NuMA, the nuclear mitotic apparatus protein, by a variant translocation in acute promyelocytic leukemia. Nat Genet. 1997;17:109–113. doi: 10.1038/ng0997-109. [DOI] [PubMed] [Google Scholar]
- 68.Wells R A, Hummel J L, De Koven A, Zipursky A, Kirby M, Dube I, Kamel-Reid S. A new variant translocation in acute promyelocytic leukaemia: molecular characterization and clinical correlation. Leukemia. 1996;10:735–740. [PubMed] [Google Scholar]
- 69.Yang E, Korsmeyer S J. Molecular thanatopsis: a discourse on the BCL2 family and cell death. Blood. 1996;88:386–401. [PubMed] [Google Scholar]
- 70.Ye B H, Lista F, Lo Coco F, Knowles D M, Offit K, Chaganti R S, Dalla-Favera R. Alterations of a zinc finger-encoding gene, BCL-6, in diffuse large-cell lymphoma. Science. 1993;262:747–750. doi: 10.1126/science.8235596. [DOI] [PubMed] [Google Scholar]
- 71.Yen A, Brown D, Fishbaugh J. Precommitment states induced during HL-60 myeloid differentiation: possible similarities of retinoic acid- and DMSO-induced early events. Exp Cell Res. 1987;173:80–84. doi: 10.1016/0014-4827(87)90333-8. [DOI] [PubMed] [Google Scholar]
- 72.Yen A, Forbes M, DeGala G, Fishbaugh J. Control of HL-60 cell differentiation lineage specificity, a late event occurring after precommitment. Cancer Res. 1987;47:129–134. [PubMed] [Google Scholar]
- 73.Yeyati, P. L., R. Shaknovich, and J. D. Licht. Unpublished data.