Abstract
Background
People with cancer are 1.4 times more likely to be unemployed than people without a cancer diagnosis. Therefore, it is important to investigate whether programmes to enhance the return‐to‐work (RTW) process for people who have been diagnosed with cancer are effective. This is an update of a Cochrane review first published in 2011 and updated in 2015.
Objectives
To evaluate the effectiveness of non‐medical interventions aimed at enhancing return to work (RTW) in people with cancer compared to alternative programmes including usual care or no intervention.
Search methods
We searched CENTRAL (the Cochrane Library), MEDLINE, Embase, CINAHL, PsycINFO and three trial registers up to 18 August 2021. We also examined the reference lists of included studies and selected reviews, and contacted authors of relevant studies.
Selection criteria
We included randomised controlled trials (RCTs) and cluster‐RCTs on the effectiveness of psycho‐educational, vocational, physical or multidisciplinary interventions enhancing RTW in people with cancer. The primary outcome was RTW measured as either RTW rate or sick leave duration measured at 12 months' follow‐up. The secondary outcome was quality of life (QoL).
Data collection and analysis
Two review authors independently assessed RCTs for inclusion, extracted data and rated certainty of the evidence using GRADE. We pooled study results judged to be clinically homogeneous in different comparisons reporting risk ratios (RRs) with 95% confidence intervals (CIs) for RTW and mean differences (MD) or standardised mean differences (SMD) with 95% CIs for QoL.
Main results
We included 15 RCTs involving 1477 people with cancer with 19 evaluations because of multiple treatment groups. In this update, we added eight new RCTs and excluded seven RCTs from the previous versions of this review that were aimed at medical interventions. All included RCTs were conducted in high‐income countries, and most were aimed at people with breast cancer (nine RCTs) or prostate cancer (two RCTs).
Risk of bias
We judged nine RCTs at low risk of bias and six at high risk of bias. The most common type of bias was a lack of blinding (9/15 RCTs).
Psycho‐educational interventions
We found four RCTs comparing psycho‐educational interventions including patient education and patient counselling versus care as usual. Psycho‐educational interventions probably result in little to no difference in RTW compared to care as usual (RR 1.09, 95% CI 0.96 to 1.24; 4 RCTs, 512 participants; moderate‐certainty evidence). This means that in the intervention and control groups, approximately 625 per 1000 participants may have returned to work. The psycho‐educational interventions may result in little to no difference in QoL compared to care as usual (MD 1.47, 95% CI −2.38 to 5.32; 1 RCT, 124 participants; low‐certainty evidence).
Vocational interventions
We found one RCT comparing vocational intervention versus care as usual. The evidence was very uncertain about the effect of a vocational intervention on RTW compared to care as usual (RR 0.94, 95% CI 0.78 to 1.13; 1 RCT, 34 participants; very low‐certainty evidence). The study did not report QoL.
Physical interventions
Four RCTs compared a physical intervention programme versus care as usual. These physical intervention programmes included walking, yoga or physical exercise. Physical interventions likely increase RTW compared to care as usual (RR 1.23, 95% CI 1.08 to 1.39; 4 RCTs, 434 participants; moderate‐certainty evidence). This means that in the intervention group probably 677 to 871 per 1000 participants RTW compared to 627 per 1000 in the control group (thus, 50 to 244 participants more RTW). Physical interventions may result in little to no difference in QoL compared to care as usual (SMD −0.01, 95% CI −0.33 to 0.32; 1 RCT, 173 participants; low‐certainty evidence). The SMD translates back to a 1.8‐point difference (95% CI −7.54 to 3.97) on the European Organisation for Research and Treatment of Cancer Quality of life Questionnaire Core 30 (EORTC QLQ‐C30).
Multidisciplinary interventions
Six RCTs compared multidisciplinary interventions (vocational counselling, patient education, patient counselling, physical exercises) to care as usual. Multidisciplinary interventions likely increase RTW compared to care as usual (RR 1.23, 95% CI 1.09 to 1.33; 6 RCTs, 497 participants; moderate‐certainty evidence). This means that in the intervention group probably 694 to 844 per 1000 participants RTW compared to 625 per 1000 in the control group (thus, 69 to 217 participants more RTW). Multidisciplinary interventions may result in little to no difference in QoL compared to care as usual (SMD 0.07, 95% CI −0.14 to 0.28; 3 RCTs, 378 participants; low‐certainty evidence). The SMD translates back to a 1.4‐point difference (95% CI −2.58 to 5.36) on the EORTC QLQ‐C30.
Authors' conclusions
Physical interventions (four RCTs) and multidisciplinary interventions (six RCTs) likely increase RTW of people with cancer. Psycho‐educational interventions (four RCTs) probably result in little to no difference in RTW, while the evidence from vocational interventions (one RCT) is very uncertain.
Psycho‐educational, physical or multidisciplinary interventions may result in little to no difference in QoL.
Future research on enhancing RTW in people with cancer involving multidisciplinary interventions encompassing a physical, psycho‐educational and vocational component is needed, and be preferably tailored to the needs of the patient.
Keywords: Humans, Male, Breast Neoplasms, Breast Neoplasms/therapy, Exercise Therapy, Prostatic Neoplasms, Prostatic Neoplasms/therapy, Return to Work
Plain language summary
What type of intervention works best to help people with cancer get back to work?
Key messages
– Multidisciplinary and physical interventions are likely to be helpful for people with cancer to get back to work.
– Psycho‐educational interventions likely result in little to no difference in getting back to work, and we are uncertain about the effect of vocational interventions.
What is this review about?
Each year, more people survive after diagnosis and treatment for cancer. Many cancer survivors live well, although they can continue to experience long‐lasting problems such as fatigue, pain and depression. These long‐term effects can cause problems with their participation in working life. Therefore, cancer is a significant cause of absence from work, unemployment and early retirement. People with cancer, their families and society at large all carry part of this burden. In this Cochrane review, we evaluated how well people with cancer can be helped to get back to work.
What did we want to find out?
We wanted to find out if non‐medical interventions are better than usual care to improve getting back to work. We also wanted to find out if those interventions led to better quality of life. We considered four types of intervention:
– psycho‐educational interventions (people with cancer learnt about physical side effects, stress and coping, and they took part in group discussions);
– vocational interventions (aimed at work‐related issues);
– physical interventions (people with cancer took part in physical exercises such as walking); and
– multidisciplinary interventions (vocational counselling, patient education, patient counselling, physical exercises or combinations of these).
What did we do?
We searched for studies that looked at interventions aimed at improving getting people with cancer back to paid employment (employee or self‐employed). We compared and summarised the results of the studies, and rated our confidence in the evidence, based on factors such as study methods and study size.
What did we find?
We found 15 studies that involved 1477 people with cancer that measured getting back to work. All studies were conducted in high‐income countries. Nine studies were aimed at people with breast cancer and two studies at men with prostate cancer.
– Psycho‐educational interventions probably result in little to no difference in getting back to work or quality of life.
– Physical interventions and multidisciplinary interventions likely lead to more people with cancer getting back to work than when they received care as usual. A physical intervention will probably help 50 to 244 per 1000 people returning to work on top of the average of 627 per 1000 people who return to work without intervention. A multidisciplinary intervention will probably help 69 to 219 people per 1000 more return to work. They may result in little to no difference in quality of life.
– We are uncertain about the effects of vocational interventions on getting people back to work.
What are the limitations of the evidence?
We are moderately confident that physical and multidisciplinary interventions likely increase the number of people with cancer getting back to work. Our confidence was reduced because some studies did not clearly report how they were conducted. We have little confidence in the evidence about psycho‐educational interventions, and we have no confidence in the evidence about vocational interventions. The main reasons for reducing our confidence were that studies used methods that were likely to introduce errors in their results, and we found only one very small study on vocational interventions. Further research could change the results of this review.
How up to date is the evidence?
The evidence is up to date to August 2021.
Summary of findings
Summary of findings 1. Psycho‐educational interventions compared to care as usual for people with cancer.
| Psycho‐educational interventions compared to care as usual for people with cancer | ||||||
| Patient or population: people with cancer Setting: hospital, home Intervention: psycho‐educational interventions Comparison: care as usual | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | What happens | |
| Risk with care as usual | Risk with psycho‐educational interventions | |||||
|
RTW Follow‐up: 12 months |
625 per 1000 | 681 per 1000 (593 to 774) | RR 1.09 (0.96 to 1.24) | 512 (4 RCTs) | ⊕⊕⊕⊝ Moderatea | Psycho‐educational interventions probably result in little to no difference in RTW. |
|
QoL Follow‐up: 12 months |
— | MD 1.47 higher (2.38 lower to 5.32 higher) | — | 124 (1 RCT) | ⊕⊕⊝⊝ Lowa,b | Psycho‐educational interventions may result in little to no difference in QoL. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; QoL: quality of life; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio; RTW: return to work. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
a Downgraded one level due to risk of bias (methodological limitations such as possible selection bias, no intention‐to‐treat analysis, lack of baseline similarity between groups). b Downgraded one level due to imprecision (sample size fewer than 400 participants).
Summary of findings 2. Vocational interventions compared to care as usual for people with cancer.
| Vocational interventions compared to care as usual for people with cancer | ||||||
| Patient or population: people with cancer Setting: online intervention Intervention: vocational interventions Comparison: care as usual | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | What happens | |
| Risk with care as usual | Risk with vocational interventions | |||||
|
RTW Follow‐up: 12 months |
1000 per 1000 | 940 per 1000 (780 to 1000) | RR 0.94 (0.78 to 1.13) | 34 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | The evidence is very uncertain about the effect of vocational interventions on RTW. |
| QoL | — | — | — | — | — | No RCTs report QoL. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; QoL: quality of life; RR: risk ratio; RTW: return to work. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
a Downgraded one level due to risk of bias (methodological limitations such as possible selection bias, effect of co‐interventions, compliance unclear). b Downgraded two levels due to very serious imprecision (the optimal information size criterion of at least 196 participants for each sample separately was not met (α = 0.05; desired power = 0.80; relative improvement of 20%; control group risk of 63%; data from one very small trial)).
Summary of findings 3. Physical interventions compared to care as usual for people with cancer.
| Physical interventions compared to care as usual for people with cancer | ||||||
| Patient or population: people with cancer Setting: hospital, home, community Intervention: physical interventions Comparison: care as usual | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | What happens | |
| Risk with care as usual | Risk with physical interventions | |||||
| RTW Follow‐up: 12 months | 627 per 1000 | 771 per 1000 (677 to 871) | RR 1.23 (1.08 to 1.39) | 434 (4 RCTs) | ⊕⊕⊕⊝ Moderatea | Physical interventions likely increase RTW. |
| QoL Follow‐up: 12 months | — | SMD 0.01 lower (0.33 lower to 0.32 higher) | — | 173 (1 RCT) | ⊕⊕⊝⊝ Lowb,c | Physical interventions may result in little to no difference in QoL. For SMDs, 0.2 represents a small effect, 0.5 a moderate effect and 0.8 a large effect (Cohen 1988). The SMD translates back to 1.8‐point difference on the EORTC QLQ‐C30d. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; EORTC QLQ‐C30: European Organisation for Research and Treatment of Cancer Quality of life Questionnaire core 30; QoL: quality of life; RR: risk ratio; RTW: return to work; SMD: standardised mean difference. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
a Downgraded one level due to imprecision (the optimal information size criterion of at least 196 participants for each sample separately was not met (α = 0.05; desired power = 0.80; relative improvement of 20%; control group risk of 63%)). b Downgraded one level due to risk of bias (methodological limitations such as possible performance bias and detection bias and possible co‐interventions). c Downgraded one level due to imprecision (sample size fewer than 400 participants). d The EORTC QLQ‐C30 is a scale from 0 to 100. A group‐level change score on the EORTC QLQ‐C30 ranging from 4 to 10 points is reported to be clinically relevant in people with cancer (Musoro 2020).
Summary of findings 4. Multidisciplinary interventions compared to care as usual for people with cancer.
| Multidisciplinary interventions compared to care as usual for people with cancer | ||||||
| Patient or population: people with cancer Setting: hospital, home Intervention: multidisciplinary interventions Comparison: care as usual | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | What happens | |
| Risk with care as usual | Risk with multidisciplinary interventions | |||||
|
RTW Follow‐up: 12 months |
625 per 1000 | 776 per 1000 (694 to 844) | RR 1.23 (1.09 to 1.33)a | 497 (6 RCTs) | ⊕⊕⊕⊝ Moderateb | Multidisciplinary interventions likely increase RTW. |
|
QoL Follow‐up: 12 months |
— | SMD 0.07 higher (0.14 lower to 0.28 higher) | — | 378 (3 RCTs) | ⊕⊕⊝⊝ Lowc,d | Multidisciplinary interventions may result in little to no difference in QoL. For SMDs, 0.2 represents a small effect, 0.5 a moderate effect and 0.8 a large effect (Cohen 1988). The SMD translates back to 1.4‐point difference on the EORTC QLQ‐C30e. |
| * The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; EORTC QLQ‐C30: European Organisation for Research and Treatment of Cancer Quality of life Questionnaire core 30; QoL: quality of life; RR: risk ratio; RTW: return to work; SMD: standardised mean difference. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
a RR of 1.23 was recalculated from OR of 2.00 (Analysis 4.1). See Data synthesis for our rationale. b Downgraded one level due to risk of bias (methodological limitations such as possible selection bias, possible co‐interventions). c Downgraded one level due to risk of bias (methodological limitations such as possible selection bias, no intention‐to‐treat analysis in the two largest trials, possible co‐interventions). d Downgraded one level due to imprecision (sample size fewer than 400 participants). e The EORTC QLQ‐C30 is a scale from 0 to 100. A group‐level change score on the EORTC QLQ‐C30 ranging from 4 to 10 points is reported to be clinically relevant in people with cancer (Musoro 2020).
Background
Description of the condition
The number of people who survive cancer is increasing due to the sustained improvements in strategies to detect cancer early and treat it effectively (American Cancer Society 2021; de Boer 2014; Ferlay 2018). Since the population is ageing in most countries and cancer survival is prolonged, the prevalence of cancer survivors is expected to rise further (Miller 2019). In the absence of other competing causes of death, 67% of adults now diagnosed with cancer can expect to be alive five years after diagnosis (American Cancer Society 2021).
Cancer diagnoses in working‐age people are becoming more common, with almost half of adults with cancer aged less than 65 years (Allemani 2018; Howlander 2019). Each year an estimated 17.0 million new cases of cancer are diagnosed worldwide (American Cancer Society 2021), and thus approximately eight million working‐age people are diagnosed with cancer each year.
Many people with cancer do well in general terms after diagnosis and treatment. However, a significant proportion of people with cancer continue to experience physical, emotional and social problems such as fatigue, pain, cognitive deficits, anxiety and depression, which may become chronic or persistent (Cooper 2013; Shapiro 2018). These long‐term physical and psychological effects of cancer or its treatment may cause impairments that diminish social functioning, including obtaining or retaining employment (Cooper 2013; Gragnano 2021; Mehnert 2013; Shapiro 2018; Taskila 2007). Fortunately, many people with cancer are both willing and able to return to work (RTW) following treatment (Butow 2020; Taskila 2007) without residual disabilities (Steiner 2010).
Returning to work is important for both people with cancer and society. From the viewpoint of society, it is economically imperative to encourage people to RTW whenever possible (Verbeek 2007). From the individual point of view, employment is an important component of quality of life (QoL) (de Boer 2014; Duijts 2017; Tamminga 2020). Work is invaluable as it can provide a sense of purpose, dignity and an income, thus enabling people to support themselves and their families. There is strong evidence that good work is beneficial for physical and mental health, whereas unemployment and long‐term sickness absence have a harmful impact (Marmot 2012). This also applies to people with cancer who consider returning to work very important (Blinder 2020; Mehnert 2013; Verbeek 2007), because it is regarded as a marker of complete recovery (Spelten 2002) and regaining normality (Blinder 2020). Moreover, returning to work can improve the QoL of people with cancer (Duijts 2017; Tamminga 2020), and it can have a positive effect on self‐esteem and social or family roles (Tamminga 2020; Tan 2021; Verbeek 2007).
Since 1980, several studies have documented the impact of cancer on employment, and they have reported approximately 60% (ranging from 30% to 93%) of people with cancer returning to work after one to two years (Blinder 2020; Mehnert 2011; Spelten 2002; Taskila 2007). However, people with cancer can experience problems getting back to work (Butow 2020; Feuerstein 2007). Overall, cancer survivors are 1.4 times more likely to be unemployed than non‐cancer controls, although the rate differs depending on the diagnosis (de Boer 2009a). Some studies have stated that people with cancer may experience impairments in mental and physical health as a result of their illness, and that these impairments sometimes lead to a decrease in their ability to work (Duijts 2014). More specifically, work ability of people with cancer who work at the time of their diagnosis is severely impaired in the first months of treatment but does improve in the months afterwards (de Boer 2008).
Therefore, it is important to provide employed people with cancer with programmes to support their RTW process, which is underlined by a report by the European Agency for Safety and Health at Work (EU OSHA 2018). One earlier Macmillan report has also proposed that successful vocational rehabilitation can have a major impact on the capability of people with cancer to RTW and remain in work life (Macmillan 2013).
There has been increasing interest in improving RTW outcomes for people with cancer (de Boer 2020). Thus, it is reasonable to expect that more studies of interventions aimed at enhancing RTW for people with cancer have been conducted since the publication of the first and second versions of this review in 2011 and 2015 (de Boer 2011; de Boer 2015). Of note, as per our previous review versions, medical and pharmacological interventions may enhance RTW (de Boer 2015). However, for this latest review version, medical and pharmacological treatments are no longer included in our review scope as we have opted to focus on interventions that can be implemented to enhance RTW in people with cancer, regardless of medical treatment.
Description of the intervention
RTW of people with cancer can be targeted by interventions (EU OSHA 2018).
Psycho‐educational interventions such as counselling, education in long‐term effects of cancer, training in coping skills and problem‐solving therapy (PST), aim to ameliorate the psychological consequences of the diagnosis and treatment of cancer on the ability to work.
Vocational interventions include any type of intervention focused on employment. Vocational interventions might be person‐directed or work‐directed. Person‐directed vocational interventions are aimed at the patient and incorporate programmes which are designed to target and improve work‐related abilities and function to enhance RTW. Work‐directed vocational interventions are aimed at the workplace and include workplace adjustments such as modified work hours, modified work tasks, or modified workplace and improved communication with or between managers, colleagues and health professionals.
Physical interventions include any type of physical training (such as walking or running), physical exercises (such as arm lifting or yoga) or training of bodily functions (such as vocal training). They aim to ameliorate the physical consequences of the diagnosis and treatment of cancer on the ability to work.
Multidisciplinary interventions encompass any combination of psycho‐educational, vocational and physical interventions. They aim to amend the psychological, vocational and physical consequences of the diagnosis and treatment of cancer on the ability to work.
How the intervention might work
Psycho‐educational interventions and physical interventions aim, respectively, to ameliorate the psychological and physical consequences of the diagnosis and treatment of cancer on the ability to work. Thus, the RTW of people with cancer can be enhanced. Vocational interventions are aimed at RTW or improving the workplace, thereby enhancing RTW. Multidisciplinary interventions aim to amend the psychological, vocational and physical consequences of the diagnosis and treatment of cancer on the ability to work. This is likely to enhance RTW in people with cancer.
Why it is important to do this review
It is vital to know what interventions are effective in enhancing RTW in people with cancer, considering the impact of diagnosis and treatment of cancer on the capability of people with cancer to RTW and remain in work life. Since the first and second versions of this review, published in 2011 and 2015 (de Boer 2011; de Boer 2015), several studies on the effectiveness of interventions that enhance the RTW of people with cancer have been published indicating the need for a further update of the review.
Objectives
To evaluate the effectiveness of non‐medical interventions aimed at enhancing return to work (RTW) in people with cancer compared to alternative programmes including usual care or no intervention.
Methods
Criteria for considering studies for this review
Types of studies
We included all eligible randomised controlled trials (RCTs) and cluster‐RCTs.
Types of participants
We included adults (aged 18 years and older) who had been diagnosed with any type of cancer and were in paid employment (employee or self‐employed) at the time of diagnosis.
Types of interventions
We included interventions that aimed to enhance RTW but were not medical (e.g. not surgery, not pharmaceutical). Interventions may have been carried out either with an individual or in a group, in a clinical setting or in the community. Interventions should primarily have focused on different factors that influence RTW, such as coping (in psycho‐educational interventions), workplace adjustments (in vocational interventions), physical exercises (in physical interventions), or a combination of those factors (in multidisciplinary interventions). Therefore, we divided interventions into the following.
Psycho‐educational: interventions that included any type of psycho‐educational intervention such as counselling, education, training in coping skills, and PST, undertaken by any qualified professional (e.g. psychologist, social worker, or oncology nurse).
Vocational: interventions that included any type of intervention focused on employment. Vocational interventions might have been person‐directed or work‐directed. Person‐directed vocational interventions are aimed at the patient and are designed to target and improve work‐related abilities and function to enhance RTW. They include vocational and occupational rehabilitation programmes. Work‐directed vocational interventions are aimed at the workplace (workplace adjustments such as modified work hours, modified work tasks or modified workplace) and improved communication with or between managers, colleagues and health professionals.
Physical: interventions that included any type of physical training (such as walking), physical exercises (such as arm lifting) or training of bodily functions (such as vocal training).
Multidisciplinary: any combination of psycho‐educational, vocational and physical interventions.
Types of outcome measures
Primary outcomes
-
Return to work (RTW), including return to either full‐ or part‐time employment, to the same or a reduced role, and to either the previous job or any new employment. We extracted two types of RTW data:
dichotomous data, such as the number of people who returned to employment (yes/no);
time‐to‐event data, such as the number of days between reporting sick and any work resumption, or the number of days on sick leave during the follow‐up period.
We extracted outcome data from the follow‐up measurement. When study authors reported multiple follow‐up measurements, we extracted the 12‐month follow‐up data as this is the time point where we expect most patients will have ended their treatment and have had the opportunity to RTW.
Secondary outcomes
Quality of life (QoL), including overall QoL, physical QoL and emotional QoL measured with validated and unvalidated questionnaires.
We extracted outcome data from the follow‐up measurement. When study authors reported multiple follow‐up measurements, we extracted the 12‐month follow‐up data. We extracted the 12‐month rate because this is the time point where we expect most people will have ended their treatment and have had the opportunity to RTW.
Search methods for identification of studies
This is the second update of the Cochrane review: "Interventions to enhance return‐to‐work for cancer patients" (de Boer 2011; de Boer 2015). We considered studies published in any language.
Electronic searches
We identified relevant trials from the following sources.
Cochrane Central Register of Controlled Trials (CENTRAL) (the Cochrane library) (up to 18 August 2021)
MEDLINE (Ovid) (1966 to 18 August 2021)
Embase (Ovid) (1947 to 18 August 2021)
CINAHL (EBSCO) (1983 to 18 August 2021)
PsycINFO (Ovid) (1806 to 8 August 2021)
ClinicalTrials.gov (clinicaltrials.gov/) (accessed 18 August 2021)
Trialregister.nl (www.trialregister.nl/) (accessed 18 August 2021)
isrctn.com (formerly controlled‐trials.com) (accessed 18 August 2021)
We selected cancer‐related and work‐related search terms from an earlier meta‐analysis on cancer and employment (de Boer 2006). We based all systematic searches in electronic databases on the MEDLINE search strategy (Appendix 1) using the revised Cochrane RCT filter (Robinson 2002), and the support of Cochrane Work in designing the search strategy and running the searches. We adapted the search to fit the updated specifications for CENTRAL (Appendix 2), Embase (Appendix 3), CINAHL (Appendix 4), and PsycINFO (Appendix 5).
Searching other resources
We checked the reference lists of all studies that we retrieved as full papers and the reference lists of all retrieved systematic and narrative reviews in order to identify other potentially eligible studies. We wrote to the corresponding authors of all identified studies that fulfilled our inclusion criteria but provided insufficient data to request any additional published or unpublished study that may be relevant to this Cochrane review.
Data collection and analysis
Selection of studies
One review author (AdB) independently screened all titles and abstracts of records identified by the search strategy for inclusion and appropriateness based on the selection criteria. Other review authors (JB, JH) independently screened half each of all titles and abstracts for inclusion and appropriateness based on the selection criteria. Review authors were not blinded to the name(s) of the author(s), institution(s) or publication sources at any level. If the title and abstract provided sufficient information to decide that it did not satisfy the inclusion criteria, we excluded the record. When there was a difference of opinion, a third review author (ST, JB or JH) arbitrated. As per the earlier versions of this review, we excluded studies that did not measure our primary outcome (RTW). One review author (AdB) independently examined all full‐text articles to determine which fulfilled all inclusion criteria, with the exception of the studies on which she was an investigator. The other review authors (ST, JB, JH) independently examined a third each of all full‐text articles on which they were not an author, to determine which fulfilled all inclusion criteria. Where necessary, we contacted study authors for further information. A third review author (ST, JB, or JH) arbitrated in case of a difference of opinion. We documented the reasons for exclusion at the full‐text stage and listed them in the Characteristics of excluded studies table.
Data extraction and management
We constructed a data extraction form that enabled the review authors to independently extract the following data from the RCTs: study type; setting; country; recruitment; randomisation; blinding; funding; inclusion and exclusion criteria; number of participants; participant characteristics including diagnosis, medical treatment, socio‐demographic data and employment situation at baseline; intervention (content, duration, provider, discipline, context); co‐interventions; follow‐up time and follow‐up measurements; number of participants lost to follow‐up; RTW outcome measures used, statistical methods and results for each RTW outcome measure at each follow‐up measurement point for each group. Review authors did not extract data from a study on which they were an investigator. We summarised the diagnoses in diagnostic groups such that if at least 50% of the participants had a specific diagnosis, then we included the RCT in that specific cancer diagnostic group; otherwise, we designated it as mixed diagnoses. We discussed all the results of data extraction, and entered study data relevant to this review into RevMan 2022.
We entered the details of the interventions into Table 5.
1. Summary of characteristics of included studies.
| Study | Country | Diagnosis | Design | Number of participants (intervention vs control) | Intervention(s) | Control | Type |
| Berglund 1994 | Sweden | Breast | RCT | 81 vs 73 | Physical training, patient education and training of coping skills regarding RTW | Care as usual | Multidisciplinary |
| Burgio 2006 | USA | Prostate | RCT | 28 vs 29 | Biofeedback behavioural training | Care as usual | Physical |
| Greidanus 2021 | Netherlands | Breast | RCT | 24 vs 10 | Employer education, web‐based intervention | Care as usual | Vocational |
| Grunfeld 2019 | UK | Breast, gynaecological, prostate or colorectal | RCT | 26 vs 21 | Workbook with psychological and RTW guidance and support | Care as usual | Multidisciplinary |
| Hass 2017 | Germany | Breast | RCT | 53 vs 63 | Telephone call, goal‐ and problem‐solving conversation | Care as usual | Psycho‐educational |
| Hubbard 2013 | UK | Breast | RCT | 7 vs 11 | Physical, occupational, psycho‐educational support services | Booklet work and cancer | Multidisciplinary |
| Jong 2018 | Netherlands | Breast | RCT | 29 vs 17 | Yoga | Care as usual | Physical |
| Lepore 2003 | USA | Prostate | RCT | 41 vs 20 | Patient education | Care as usual | Psycho‐educational |
| 43 vs 20 | Patient education plus group discussion | ||||||
| Maguire 1983 | UK | Breast | RCT | 42 vs 46 | Physical training, individual counselling and encouragement of RTW | Care as usual | Multidisciplinary |
| Mijwel 2019 | Sweden | Breast | RCT | 62 vs 26 | High‐load resistance exercises plus high‐intensity interval exercises | Flyer with generic information about exercise | Physical |
| 59 vs 26 | Moderate‐intensity aerobic exercise plus high‐intensity interval exercises | ||||||
| Purcell 2011 | Australia | People receiving radiotherapy | RCT | 43 vs 48 | Postradiotherapy fatigue education | Flyer with generic information about fatigue | Psycho‐educational |
| 21 vs 24 | Preradiotherapy and postradiotherapy fatigue education | ||||||
| Singer 2018 | Germany | Head and neck, breast, urinary, female genital | Cluster‐RCT | 115 vs 78 | Stepped care, screening for distress, counselling | Care as usual | Psycho‐educational |
| Tamminga 2013 | Netherlands | Breast | RCT | 57 vs 63 | Vocational support, counselling, education, multidisciplinary, RTW advice. | Care as usual | Multidisciplinary |
| van Waart 2015 | Netherlands | Breast | RCT | 51 vs 27 | Low‐intensity physical activity programme, home‐based | Care as usual | Physical |
| 53 vs 27 | Moderate‐ to high‐intensity, combined resistance and aerobic exercise programme | ||||||
| Zaman 2021 | Netherlands | Gastrointestinal | RCT | 36 vs 34 | Tailored psychosocial work‐related support, in‐hospital | Care as usual | Multidisciplinary |
RCT: randomised controlled trial; RTW: return to work.
When an article reported more than one intervention and compared each intervention against a control group, we entered each intervention as a separate evaluation. In case of a cluster‐RCT, the size of each trial was reduced to its 'effective sample size'. The effective sample size of a single intervention group in a cluster‐randomised trial is its original sample size divided by a quantity called the 'design effect'. The design effect is approximately 1 + (M − 1) × ICC, where M is the original sample size and ICC the intraclass correlation coefficient (Higgins 2011).
Assessment of risk of bias in included studies
Teams of two review authors per study independently assessed the risk of bias of the included RCTs using Cochrane's RoB 1 tool by adapting the procedures described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Review authors did not assess risk of bias in a study in which they were an investigator. To maintain consistency and transparency with previous review versions (de Boer 2011; de Boer 2015), we used the same risk‐of‐bias assessment methods in all the included studies while acknowledging the fact that our methods are considered as non‐standard in Cochrane reviews. As per the earlier versions of this review (de Boer 2011; de Boer 2015), we assessed each included RCT based on 10 domains of risk of bias: 1. adequacy of sequence generation; 2. allocation concealment; 3. blinding; 4. how incomplete outcome data (dropouts) were addressed; 5. use of intention‐to‐treat (ITT) analysis; 6. evidence of selective outcome reporting; 7. similarity of baseline characteristics; 8. similarity or avoidance of co‐interventions; 9. acceptability of compliance and 10. similarity of the timing of outcome assessments. Although these domains of risk of bias are not standard in Cochrane reviews, we considered these domains as important indicators of lower risk of bias in this research field.
For cluster‐RCTs, we assessed five additional domains of risk of bias (Higgins 2011): 1. recruitment bias (differential participant recruitment in clusters for different interventions); 2. baseline imbalance; 3. loss of clusters; 4. incorrect analysis and 5. comparability with individually randomised trials.
We judged RCTs to have a low overall risk of bias when we judged five or more of the domains to have a low risk of bias.
We followed any disagreement about the criteria with a discussion until we reached consensus. If we could not resolve the difference of opinion, we consulted a third review author. We discussed all the results of the two independent review authors and reported one final assessment of risk of bias for each RCT.
Measures of treatment effect
For dichotomous data, such as RTW measured as the number of people who returned to employment, we used risk ratios (RRs) as the measure of treatment effect. For continuous RTW variables, such as the number of days on sick leave during the follow‐up period, we used mean differences (MDs) when RCTs used a similar scale, and standardised mean differences (SMDs) when RCTs used different scales or different time spans. For continuous QoL outcomes, we used MDs when RCTs used a similar scale, and SMDs when studies used different scales. For SMDs, 0.2 represents a small effect, 0.5 a moderate effect and 0.8 a large effect (Cohen 1988). All estimates included a 95% confidence interval (CI). For future updates, if we identify study data reporting RTW as time‐to‐event data, we will plot them as hazard ratios (HRs); if Kaplan‐Meier curves are presented, we will extract the data from the graphs and calculate HRs.
Unit of analysis issues
When an article reported more than one intervention and compared each intervention against a control group, we analysed each intervention as a separate evaluation. The number of participants in the control group was divided over the separate evaluations.
For Singer 2018, the sample size of this cluster‐RCT was reduced to its 'effective sample size' using the formula 1 + (M − 1) × ICC. The reported ICC was 0.03. Therefore, M was (115 participants + 78 participants)/(6 + 7 wards) = 193/13 = 14.85, making the equation 1 + (14.85 − 1) × 0.03 = 1.42. Based on these adjustments for clustering, the recalculated events and sample size for intervention arm were: 99/1.42 = 70 events and 115/1.42 = 81 participants; the recalculated events and sample size for control were: 68/1.42 = 48 events and 78/1.42 = 55 participants.
Dealing with missing data
We contacted the authors of the following RCTs to obtain key data that were missing from their reports that we needed to determine eligibility, or to input for meta‐analysis, or both: Hass 2017; Jones 2005; Rogers 2009 (this study has been excluded for this update); Singer 2018; Tamminga 2013; van Egmond 2016; Wiggins 2009. All these authors kindly provided the information we requested. If statistics were missing, such as standard deviations (SDs) we calculated them from other available statistics such as P values according to the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). In the case of missing outcome data (i.e. when participants were lost to follow‐up, or failed to provide data at a particular time point), participants were kept in the groups to which they were originally randomised, but only the available data was used and only participants who provided data at a particular time point were included in the denominator.
Assessment of heterogeneity
We first decided whether RCTs were sufficiently homogeneous to be able to synthesise the results into one summary measure. We defined RCTs to be sufficiently homogeneous when they had similar designs, similar interventions and similar outcomes measured at the same follow‐up point.
We considered the following categories of interventions as sufficiently similar to be combined: psycho‐educational, vocational, physical, and multidisciplinary interventions.
We considered both RTW outcomes and sick leave duration outcomes as similar enough to be combined. We considered general or overall QoL outcomes measured with different instruments similar enough to be merged. We combined different diagnoses within one analysis because we hypothesised that the mechanism of RTW interventions was similar over the different cancer diagnoses.
We visually inspected forest plots for similarity of point estimates and for any overlapped 95% CIs. We also quantified statistical heterogeneity with the I² statistic and assessed it using the Chi2 test. We considered studies to be statistically heterogeneous if the I² statistic was greater than 50% (Higgins 2011).
Assessment of reporting biases
We assessed publication bias with a funnel plot when at least 10 RCTs were available in the meta‐analysis, because when there are fewer studies the power of the tests is low.
Data synthesis
We pooled RCTs with sufficient data that we judged to be clinically homogeneous with Review Manager (RevMan 2022). We considered it to be implausible that intervention effects across studies were identical, and therefore we combined RCTs using a random‐effects model.
For RTW outcomes, we aimed to combine rates of RTW, which is a dichotomous measure, and the number of days on sick leave, which is a continuous measure. Therefore, we calculated effect sizes in order to enter them in the same comparison. For studies with continuous measures, we first calculated the SMD using Review Manager (RevMan 2022). We subsequently expressed SMDs as log odds ratios (logORs) by multiplying them by 1.814 (Chinn 2000), as recommended by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) (Appendix 6). For studies with dichotomous RTW rates, we recalculated the odds ratios (ORs) into logORs (Appendix 6).
Next, we calculated for both types of RCTs the standard errors (SE) of the logORs from the 95% CI of the logORs. We used the formula: SE = (upper limit logOR − lower limit logOR)/3.92, as is the recommended method in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We then used these logORs and their SEs as input into the meta‐analysis using the generic inverse variance method as implemented in RevMan 2022.
Since RTW rates in people with cancer are much higher than 10%, the ORs overestimate the treatment effect considerably (Higgins 2011). Therefore, as shown in Analysis 4.1, we recalculated the ORs back into RRs using the formula RR = OR/(1 − p0 + (p0 × OR)), where p0 is the baseline risk (Grant 2014). We set the baseline risk (p0) of the unexposed group of RTW at 0.63, which is the mean risk for RTW from the literature (de Boer 2009a).
4.1. Analysis.

Comparison 4: Multidisciplinary interventions versus care as usual, Outcome 1: Return to work (odds ratios)
For studies in which the OR was not directly available, we recalculated the OR using the number of events and sample sizes per group.
For QoL, we pooled calculated SMDs using Review Manager (RevMan 2022).
Subgroup analysis and investigation of heterogeneity
We intended to perform further subgroup analyses according to diagnosis. However, the number of RCTs in the subgroups was too low to perform such subgroup analyses.
Sensitivity analysis
We intended to analyse how sensitive our results were to the risk of bias in the included RCTs. However, there was an insufficient number of RCTs available per comparison to perform such an analysis.
We performed a sensitivity analysis excluding the studies from which we transformed the data and in which the OR was recalculated.
Summary of findings and assessment of the certainty of the evidence
In the summary of findings tables created by GRADEpro GDT, we included RTW and QoL reported at 12‐month follow‐up. Two review authors (AdB, JB) independently assessed the certainty of the body of evidence using the GRADE approach per comparison and per outcome, as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Starting from an assumed level of high certainty for all studies, we downgraded the certainty by one to three levels depending on the seriousness of the violations in each domain. Review authors did not assess the certainty of the evidence of a study on which they were an author. A third review author (JH) resolved any disagreements.
To assess the risk of bias for a comparison, we considered the risk of bias tables for each study in that comparison. We downgraded the certainty of the evidence if there were one or more limitations in the following domains: risk of bias, consistency, directness of the evidence, precision of the pooled estimate and the possibility of publication bias.
Thus, we rated the level of certainty of evidence as high, moderate, low or very low.
High certainty: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
Results
Description of studies
Results of the search
Figure 1 shows the PRISMA study flow diagram of included and excluded articles. Through a comprehensive literature search of electronic databases, we identified 14,950 potentially relevant records with most (46%) retrieved by MEDLINE. We also checked the reference lists of 23 retrieved systematic and narrative reviews to identify additional potentially eligible studies (Beck 2003; De Backer 2009; Egan 2013; Fors 2011; Gudbergsson 2015; Haaf 2005; Harvey 1982; Hersch 2009; Hoving 2009; Irwin 2004; Kirshbaum 2007; Kirshblum 2001; Koczwara 2016; Lamore 2019; Liu 2009; McNeely 2006; McQueen 2017; Oldervoll 2004; Scott 2013; Silver 2013; Stanton 2006; Steiner 2010; van der Molen 2009), and found four additional records for potentially eligible studies. We screened the reference lists of all RCTs that we retrieved as full‐text papers in order to identify further potentially eligible RCTs but did not identify any additional RCTs. After removing duplicates, we screened 9432 potentially relevant references for eligibility. We excluded 9315 references based on the title and abstract. We retrieved full‐text reports of the remaining 117 references for comprehensive assessment. Eventually, we included 15 RCTs in this review update.
1.
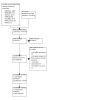
PRISMA flow diagram.
We contacted the corresponding authors of seven studies that fulfilled our inclusion criteria but with insufficient data to request any additional relevant published or unpublished study data. Based on the information kindly provided by the authors, we included two studies (Singer 2018; Tamminga 2013), and excluded the remaining five studies (Emmanouilidis 2009; Gordon 2005; Rogers 2009; van Egmond 2016; Wiggins 2009).
We also identified nine ongoing RCTs (NCT01780064; NCT03666936; NCT04214912; NCT04322695; NCT04469205; NCT04846972; Sheppard 2019; Touillaud 2021; Zegers 2021).
See Characteristics of included studies; Characteristics of excluded studies; and Characteristics of ongoing studies tables.
Included studies
Characteristics and participants
We included 15 RCTs. Of these, four had multiple study arms, and thus we included 19 evaluations of interventions. These RCTs included 1477 participants. Table 5 provides an overview of the main characteristics of the 15 RCTs and 19 evaluations. All RCTs were conducted in high‐income countries, mostly from Europe (UK, three; Sweden, two; the Netherlands, five; Germany, two), while another two RCTs were conducted in the USA and one in Australia. Nine RCTs involved people with breast cancer (Berglund 1994; Greidanus 2021; Hass 2017; Hubbard 2013; Jong 2018; Maguire 1983; Mijwel 2019; Tamminga 2013; van Waart 2015). Two RCTs involved men with prostate cancer (Burgio 2006; Lepore 2003), one involved people with gastrointestinal cancer (Zaman 2021), and three RCTs concerned groups of people with a mix of cancer diagnoses (Grunfeld 2019; Purcell 2011; Singer 2018).
Funding sources were charities (Berglund 1994; Greidanus 2021; Hubbard 2013; Jong 2018; Maguire 1983; Mijwel 2019; van Waart 2015; Zaman 2021), and national institutes (Burgio 2006; Grunfeld 2019; Hass 2017; Lepore 2003; Purcell 2011; Singer 2018; Tamminga 2013). For further details regarding the study populations and settings, see the Characteristics of included studies table and Table 5.
Type of return‐to‐work interventions
We reported the results of four psycho‐educational interventions, one vocational intervention, four physical interventions and six multidisciplinary interventions, which were combinations of psycho‐educational, vocational, and physical interventions.
Psycho‐educational interventions
Lepore 2003 included one study arm on patient education alone and one on a combination of patient education and group discussion. The intervention that only included patient education involved lectures delivered by an expert on subjects including physical adverse effects, stress and coping, which was compared with care as usual. In the second intervention group, group discussions to improve coping were added to the patient education and also compared to care as usual.
Purcell 2011 described education aimed at teaching participants self‐care behaviours to reduce cancer‐related fatigue. The preradiotherapy programme was delivered one week prior to radiotherapy planning and the postradiotherapy programme was delivered one to two weeks after radiotherapy completion.
Hass 2017 involved telephone follow‐up care after oncological rehabilitation. The basic conversation style aimed at goal‐ and problem‐solving, and the physical and psychological well‐being of the participants was assessed. In addition, the follow‐up telephone call focussed on strengthening and maintaining the motivation to carry out health‐promoting behaviour and support of everyday transfer.
Singer 2018 used a stepped care model in the intervention arm. In the first step, each participant was screened for distress (including depression, anxiety, pain, fatigue and financial difficulties). The doctor talked to participants with severe distress about the screening results in step two, and if the participant and doctor decided that further support was necessary, the participant was referred to the hospital's services in step three.
Vocational intervention
Greidanus 2021 involved an online toolbox targeted at the participant's employer. The toolbox was a web‐based intervention with succinct information, interactive communication videos, conversation checklists and links to reliable external sources. The content of the toolbox was tailored per RTW phase and per cancer survivor experience type.
Physical interventions
Burgio 2006 combined physical exercise with biofeedback‐assisted behavioural training.
Jong 2018 involved a 12‐week yoga programme that started before chemotherapy.
The supervised exercise intervention in Mijwel 2019 included one study arm on exercise plus resistance training and one arm on exercise plus overall endurance training session. The intervention lasted 16 weeks and was followed by a personal programme.
In van Waart 2015, one arm received a home‐based, low‐intensity, self‐managed physical activity programme and one arm received a moderate‐ to high‐intensity, combined resistance and aerobic exercise programme supervised by specially trained physiotherapists.
Multidisciplinary interventions
The six included multidisciplinary interventions all involved a vocational component such as vocational counselling, in combination with patient education, patient counselling, physical exercises, or a combination of these.
In Maguire 1983, a nurse advised people with breast cancer on exercise, examined arm movements, checked exercises, and encouraged RTW and becoming socially active.
Berglund 1994 combined training of coping skills regarding RTW with physical activity exercises.
In Hubbard 2013, a case manager working in a multidisciplinary team referred people with cancer to physical, occupational or psychological support services.
In Tamminga 2013, an oncology nurse or medical social worker working in a multidisciplinary team provided participants with vocational support, counselling, education and RTW advice.
Grunfeld 2019 provided a four‐week guided workbook intervention consisting of structured sections and activities to provide guidance and support to participants. Participants incorporated all elements from the workbook into a personal RTW plan.
In Zaman 2021, participants received tailored work‐related support depending on the severity of their work‐related problems. Topics included disease‐related problems, the importance of work, contact with the work environment and possible support interventions.
Setting, design and outcomes
Ten RCTs were conducted in a hospital (Burgio 2006; Hubbard 2013; Jong 2018; Lepore 2003; Maguire 1983; Mijwel 2019; Purcell 2011; Singer 2018; Tamminga 2013; Zaman 2021), two RCTs at home (Grunfeld 2019; Hass 2017), one RCT at home and in the community (van Waart 2015), one RCT online (Greidanus 2021), and one RCT did not report the setting (Berglund 1994).
Fourteen RCTs employed an RCT design (Berglund 1994; Burgio 2006; Greidanus 2021; Grunfeld 2019; Hass 2017; Hubbard 2013; Jong 2018; Lepore 2003; Maguire 1983; Mijwel 2019; Purcell 2011; Tamminga 2013; van Waart 2015; Zaman 2021), and one was a cluster‐RCT (Singer 2018).
Thirteen RCTs measured RTW as event rates (Berglund 1994; Burgio 2006; Greidanus 2021; Grunfeld 2019; Hass 2017; Jong 2018; Lepore 2003; Maguire 1983; Mijwel 2019; Purcell 2011; Singer 2018; Tamminga 2013; van Waart 2015; Zaman 2021). One RCT reported time‐to‐event data, such as number of days between reporting sick and any work resumption or the number of days on sick leave during the follow‐up period (Hubbard 2013). Nine RCTs measured QoL as a secondary outcome (Berglund 1994; Burgio 2006; Grunfeld 2019; Hubbard 2013; Lepore 2003; Mijwel 2019; Purcell 2011; Tamminga 2013; Zaman 2021). Seven of these measured QoL using validated questionnaires: European Organisation for Research and Treatment of Cancer (EORTC) (Grunfeld 2019; Mijwel 2019), 36‐item Short Form (SF‐36) (Burgio 2006; Lepore 2003; Tamminga 2013; Zaman 2021), and Functional Assessment of Cancer Therapy – Breast (FACT‐B) (Hubbard 2013), and one used an unvalidated questionnaire (Berglund 1994).
For further details regarding the content of the interventions and settings, see the Characteristics of included studies table and Table 5.
Excluded studies
We excluded 102 full‐text articles. Reasons for exclusion were: ineligible population (13 records), ineligible study design (nine records), ineligible intervention (nine records) and ineligible outcomes (71 records).
Seven RCTs from the first two versions of this review were excluded in this latest review version as they assessed solely the effects of medical interventions on RTW (Ackerstaff 2009; Emmanouilidis 2009; Friedrichs 2010; Hillman 1998; Johnsson 2007; Kornblith 2009; Lee 1992), because in this update we focussed on interventions that could be implemented to enhance RTW regardless of medical treatment (see Background). We also excluded one RCT that was included in the first two versions of this review because the RTW outcome was reported as a change in number of sick leave days (Rogers 2009), which is an ineligible outcome.
For a detailed description of the reasons for exclusion, see Characteristics of excluded studies table.
Risk of bias in included studies
For results of risk of bias assessment of RCTs, see the risk of bias graph (Figure 2), which is an overview of the review authors' judgements about each risk of bias item presented as percentages across all included RCTs. Figure 3 shows the risk of bias summary of each risk of bias item for each included RCT.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Eleven RCTs reported adequate random sequence generation (low risk of bias; Berglund 1994; Burgio 2006; Greidanus 2021; Grunfeld 2019; Hubbard 2013; Jong 2018; Maguire 1983; Mijwel 2019; Purcell 2011; Tamminga 2013; Zaman 2021). These authors reported having used random numbers generated by a computer or random number tables. Four RCTs were at unclear risk of bias as they did not report their randomisation procedure (Hass 2017; Lepore 2003; Singer 2018; van Waart 2015).
Five RCTs reported adequate allocation concealment (low risk of bias; Burgio 2006; Lepore 2003; Mijwel 2019; Purcell 2011; Tamminga 2013). According to our judgement, allocation was adequately concealed in these RCTs because a research nurse or an independent interviewer performed the randomisation. The other RCTs did not report allocation concealment (unclear risk of bias).
Blinding
Lepore 2003 reported blinding of the interviewer assessing the outcomes and blinding of the participants for the hypothesis. Eight RCTs explicitly reported that participants, intervention providers, outcome assessors, or combinations were not blinded (high risk of bias; Burgio 2006; Greidanus 2021; Grunfeld 2019; Hass 2017; Jong 2018; Mijwel 2019; van Waart 2015; Zaman 2021). The remaining six RCTs did not provide any information on blinding (unclear risk of bias). Risk of bias due to lack of blinding was considered less serious for the outcome RTW (an objective outcome) than for QoL (a subjective outcome).
Incomplete outcome data
Eleven RCTs reported reasons for dropout of participants and thus addressed the issue of incomplete outcome data and possible attrition bias (low risk of attrition bias). Burgio 2006, Purcell 2011, Singer 2018, and Tamminga 2013 did not provide information about participants with missing data (unclear risk of attrition bias).
Six RCTs performed ITT analyses between the two randomised groups even if the participants changed over to the other group (low risk of bias; Greidanus 2021; Grunfeld 2019; Mijwel 2019; Tamminga 2013; van Waart 2015; Zaman 2021). There were no ITT analyses (e.g. per‐protocol analysis) in five RCTs (high risk of bias; Berglund 1994; Burgio 2006; Hubbard 2013; Lepore 2003; Maguire 1983). The remaining RCTs did not report if ITT analyses were performed (unclear risk of bias).
Selective reporting
Thirteen RCTs were free of selective reporting of the outcomes because the authors reported all outcomes described in the methods (low risk of reporting bias). Two RCTs did not clearly describe reporting (unclear risk of reporting bias; Hass 2017; van Waart 2015). We further cross‐checked the available published protocols or trial registers (or both) of the following RCTs and found no selective reporting of outcomes: Greidanus 2021; Grunfeld 2019; Hubbard 2013; Jong 2018; Mijwel 2019; Purcell 2011; Singer 2018; Tamminga 2013; van Waart 2015; Zaman 2021. The other RCTs did not have any published protocols available.
Other potential sources of bias
Baseline characteristics of the participants were similar in five RCTs (low risk of bias; Greidanus 2021; Mijwel 2019; Tamminga 2013; van Waart 2015; Zaman 2021). One RCT stated that baseline characteristics were similar but did not report the actual data (low risk of bias; Maguire 1983). One RCT provided no baseline characteristics, so similarity is unknown (unclear risk of bias; Berglund 1994). Four RCTs included a heterogeneous group of participants but clearly only performed the analyses on employment outcomes in participants employed at baseline; separate data on the similarity of baseline characteristics on these groups of employed participants were not given (unclear risk of bias; Burgio 2006; Hass 2017; Jong 2018; Lepore 2003). Baseline characteristics were not similar in four RCTs (high risk of bias; Grunfeld 2019; Hubbard 2013; Purcell 2011; Singer 2018).
Co‐interventions were avoided or similar in both groups in six RCTs (low risk of bias; Burgio 2006; Grunfeld 2019; Lepore 2003; Maguire 1983; Purcell 2011; Zaman 2021), and not reported in the other RCTs (unclear risk of bias).
Compliance with the intervention was not always reported but was satisfactory in those five RCTs that did report it (low risk of bias; Burgio 2006; Maguire 1983; Mijwel 2019; Tamminga 2013; Zaman 2021). The remaining RCTs did not report compliance (unclear risk of bias).
Follow‐up time was similar in all RCTs except for Hubbard 2013 (high risk of bias) and Maguire 1983 (unclear risk of bias).
For the cluster‐RCT, Singer 2018, we assessed five additional domains of risk of bias: recruitment bias (differential participant recruitment in clusters for different interventions) (low risk of bias); baseline imbalance (low risk of bias); loss of clusters (none) (low risk of bias); incorrect analysis (low risk of bias) and comparability with individually randomised trials (unclear risk of bias).
Overall risk of bias
We rated nine RCTs as having a low overall risk of bias because they had five or more domains at low risk of bias (Burgio 2006; Greidanus 2021; Grunfeld 2019; Lepore 2003; Maguire 1983; Mijwel 2019; Purcell 2011; Tamminga 2013; Zaman 2021). We rated six RCTs at high overall risk of bias because they had six or more domains at unknown or high risk of bias (Berglund 1994; Hass 2017; Hubbard 2013; Jong 2018; Singer 2018; van Waart 2015) (Figure 3).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
The 15 included RCTs evaluated the effects of four types of interventions in participants with cancer: psycho‐educational, vocational, physical, and combinations of psycho‐educational, vocational and physical interventions.
Psycho‐educational interventions
See Table 1.
The four RCTs assessing the effects of psycho‐educational interventions included 512 participants with 282 participants in the intervention groups and 230 in the control groups (Hass 2017; Lepore 2003; Purcell 2011; Singer 2018). Two arms of one RCT reported in the same article compared the effect of a psycho‐educational intervention or a psycho‐educational intervention combined with group discussion to care as usual (Lepore 2003). Similarly, two arms of a second RCT compared the effects of either postradiotherapy or preradiotherapy and postradiotherapy fatigue education to care as usual (Purcell 2011). Hass 2017 involved telephone follow‐up care after oncological rehabilitation. Singer 2018 used a stepped‐care model in the intervention arm which included screening and counselling for distress.
Psycho‐educational interventions probably result in little to no difference in RTW compared to care as usual (RR 1.09, 95% CI 0.96 to 1.24; 4 RCTs, 512 participants; moderate‐certainty evidence; Analysis 1.1).
1.1. Analysis.

Comparison 1: Psycho‐educational interventions versus care as usual, Outcome 1: Return to work
One study reported QoL (Lepore 2003). Psycho‐educational interventions may result in little to no difference in QoL compared to care as usual (MD 1.47, 95% CI −2.38 to 5.32; 1 RCT, 124 participants; low‐certainty evidence; Analysis 1.2).
1.2. Analysis.

Comparison 1: Psycho‐educational interventions versus care as usual, Outcome 2: Quality of life
Vocational intervention
See Table 2.
Greidanus 2021 involved a web‐based intervention targeted at the employer. The evidence is very uncertain about the effect of a vocational intervention on RTW compared to care as usual (RR 0.94, 95% CI 0.78 to 1.13; 1 RCT, 34 participants; very low‐certainty evidence; Analysis 2.1). Greidanus 2021 did not report QoL.
2.1. Analysis.

Comparison 2: Vocational interventions versus care as usual, Outcome 1: Return to work
Physical interventions
See Table 3.
The four RCTs assessing the effects of physical interventions included 434 participants with 282 participants in the intervention groups and 152 in the control groups. Two arms of one RCT compared the effect of a home‐based, low‐intensity, self‐managed physical activity programme, or a moderate‐ to high‐intensity, combined resistance and aerobic exercise programme, to care as usual (van Waart 2015). Similarly, two arms of Mijwel 2019 included one study arm on exercise plus resistance training and one arm on exercise plus overall endurance training session compared to care as usual. Burgio 2006 combined physical exercise with biofeedback‐assisted behavioural training. Jong 2018 involved a 12‐week yoga programme that started before chemotherapy.
Physical interventions likely increase RTW compared to care as usual (RR 1.23, 95% CI 1.08 to 1.39; 4 RCTs, 434 participants; moderate‐certainty evidence; Analysis 3.1).
3.1. Analysis.

Comparison 3: Physical interventions versus care as usual, Outcome 1: Return to work
One study reported QoL (Mijwel 2019). Physical interventions may result in little to no difference in QoL compared to care as usual (SMD −0.01, 95% CI −0.33 to 0.32; 1 RCT, 173 participants; low‐certainty evidence; Analysis 3.2).
3.2. Analysis.

Comparison 3: Physical interventions versus care as usual, Outcome 2: Quality of life
Multidisciplinary interventions
See Table 4.
The six RCTs assessing the effects of multidisciplinary interventions included 497 participants with 249 participants in the intervention groups and 248 in the control groups. In Maguire 1983, a nurse advised people with breast cancer on exercise and encouraged RTW. Berglund 1994 combined the training of coping skills regarding RTW with physical activity exercises. In Hubbard 2013, a case manager referred participants to physical, occupational or psychological support services. Tamminga 2013 provided participants with vocational support, counselling, education and RTW advice in the clinic. Grunfeld 2019 offered participants a workbook with structured activities to provide guidance and support. In Zaman 2021, participants received tailored psychosocial work‐related support.
Multidisciplinary interventions likely increase RTW compared to care as usual (RR 1.23 (after recalculation from OR 2.00, 95% CI 1.29 to 3.08; see Data synthesis), 95% CI 1.09 to 1.33; 6 RCTs, 497 participants; moderate‐certainty evidence; Analysis 4.1). We performed a sensitivity analysis excluding the study with transformed data (Hubbard 2013), and the study with recalculated data (Tamminga 2013). Exclusion of these two RCTs resulted in a similar outcome (RR 1.25, 95% CI 1.10 to 1.36; 4 RCTs, 359 participants).
Three RCTs reported QoL (Berglund 1994; Tamminga 2013; Zaman 2021). Multidisciplinary interventions may result in little to no difference in QoL compared to care as usual (SMD 0.07, 95% CI −0.14 to 0.28; 3 RCTs, 378 participants; low‐certainty evidence; Analysis 4.2).
4.2. Analysis.

Comparison 4: Multidisciplinary interventions versus care as usual, Outcome 2: Quality of life
Discussion
Summary of main results
Fifteen RCTs met the inclusion criteria of this Cochrane review with 1477 participants with cancer.
We found evidence from six RCTs that multidisciplinary interventions likely increase RTW of people with cancer. Furthermore, results from four RCTs showed that physical interventions likely increase RTW.
We concluded from four RCTs that psycho‐educational interventions probably result in little to no difference in RTW, while the evidence from one vocational RCT on RTW is very uncertain. Psycho‐educational, physical or multidisciplinary interventions may result in little to no difference in QoL.
Overall completeness and applicability of evidence
The included RCTs were reported over a 40‐year time span. While one study was conducted in the 1970s and reported in the 1980s (Maguire 1983), we could not find any RCTs that were performed in the 1980s and only one RCT that was conducted in the 1990s (Berglund 1994). Therefore, two included RCTs were performed between 2000 and 2010 (Burgio 2006; Lepore 2003), and 11 included RCTs after 2010 (Greidanus 2021; Grunfeld 2019; Hass 2017; Hubbard 2013; Jong 2018; Mijwel 2019; Purcell 2011; Singer 2018; Tamminga 2013; van Waart 2015; Zaman 2021). During these 40 years, medical treatment for cancer has changed enormously (Miller 2019). For this reason, older RCTs may describe medical treatments that are no longer used (Berglund 1994; Maguire 1983). In addition, the way in which psycho‐educational and multidisciplinary interventions are performed today differs considerably from what is described in the older included RCTs. Nowadays, psycho‐educational and multidisciplinary interventions are more evidence‐based, more cognitive behavioural therapy‐oriented, more often including activities and sport, briefer, and more targeted than 20 to 30 years ago.
This Cochrane review considered people from the USA, Australia and Europe. Social security systems and labour markets differ widely between countries and thus the employment rates may be impacted differently between people with cancer in different countries. However, in all included RCTs the effects of the interventions were compared in the same country and within an RCT study design and, therefore, the influence of a social security system was equal within RCTs. However, when generalising the results from one country to another, the potential effect of a particular country's social security system should still be considered on the RTW outcomes of the RCT. For the generalisation of the results of this review to countries outside Europe or the USA, cultural differences regarding employment and cancer disclosure should be taken into account (Guo 2021).
People with breast cancer were the most studied diagnosis group with nine RCTs including more than 50% of people with breast cancer (Berglund 1994; Greidanus 2021; Hass 2017; Hubbard 2013; Jong 2018; Maguire 1983; Mijwel 2019; Tamminga 2013; van Waart 2015), two RCTs involved men with prostate cancer (Burgio 2006; Lepore 2003), one involved people with gastrointestinal cancer (Zaman 2021), and three concerned people with mixed cancer diagnoses (Grunfeld 2019; Purcell 2011; Singer 2018).
Breast cancer is the most prevalent cancer diagnosis within the working population followed by blood and lymph cancers, prostate cancer, thyroid cancer and colorectal cancer (European Commission 2023; Short 2005). Although we found RCTs aimed at people with breast, prostate and gastrointestinal cancer, we did not find any RCTs on people diagnosed with blood and lymph cancers; thyroid cancer; or less‐prevalent cancer diagnoses including brain cancer, bone cancer and gynaecological cancers. We consider that the mechanisms of the psycho‐educational, vocational, physical and multidisciplinary RTW interventions may be similar regardless of the type of cancer diagnosis and disease stage, and thus people with other types of cancer may experience the same benefits from any of these interventions aimed at improving RTW.
However, long‐term and late effects of specific treatments for specific cancers, such as solid versus non‐solid tumours, may differ and play a role in the RTW process. Gender differences may also play a role in the RTW process (Wennman‐Larsen 2021), and, therefore, the results of studies on women with breast cancer might only partly apply to people with other diagnoses. However, we did not find gender differences for loss of paid employment after colorectal cancer (de Wind 2021). Ultimately, we do not know the role of gender because of the lack of evidence.
Although most multidisciplinary interventions did have a vocational component, we only found one RCT solely focusing on employment issues. This is remarkable because one would expect interventions aimed at RTW to consist of work‐related components, such as work adjustments or involvement of the supervisor. One scoping review including 14 vocational interventions concluded that vocational rehabilitation interventions, such as making work plans, vocational consultations and training, and interventions aimed at employers, were all associated with increased odds for RTW (Guo 2021). However, this scoping review also showed that few vocational studies are evaluated in RCTs (Guo 2021).
Our findings showed that both multidisciplinary interventions, of which many contained physical training or activation elements, and physical interventions were effective in enhancing RTW in people with cancer. It could be possible that the physical element of the multidisciplinary intervention is the effective element, for instance, because it reduces physical fatigue and thereby enhances RTW (van Waart 2015). It is also possible that the psycho‐educational or vocational element of the multidisciplinary interventions are the effective part, or in combination. Finally, the effective component could be different for each person and interventions should be tailored to the experienced problems of the individual patient (Zaman 2021).
Quality of the evidence
Nine of the 15 RCTs did not implement blinding of providers, participants or outcome assessors, or the blinding was unclear. It can be argued that blinding is not feasible in this type of trial and that lack of blinding should not be considered a weakness, but the absence of blinding can be associated with bias even though blinding is not feasible. However, blinding of the outcome assessors and blinding of the participants to the hypothesis of the study are possible. The possibility for bias should therefore be taken into account. Unfortunately, most reports did not discuss blinding.
In this Cochrane review, we analysed 15 RCTs involving 1477 participants with cancer. However, the number of participants analysed in the individual studies was generally low, with nine RCTs providing fewer than 50 participants in each group, thus limiting the power of the studies. In addition, we assessed four different types of interventions, each of which contained several subtypes of intervention. Most of these subtypes only described one type of intervention. Therefore, it was not possible to perform subgroup analyses according to diagnosis or study risk of bias.
For physical interventions, we included four RCTs with 434 participants and for multidisciplinary interventions, we included six RCTs with 497 participants. We concluded that there is moderate‐certainty evidence that physical and multidisciplinary interventions likely improve RTW more than care as usual. However, for most other comparisons and outcomes, we further downgraded the certainty of the evidence due to too few available RCTs, risk of bias, indirect results and imprecise effect estimates. GRADE assessments are partly based on subjective judgements and are not definite. Nevertheless, GRADE does provide a transparent and consistent classification of the quality of evidence for relevant comparisons and outcomes.
One methodological consideration is the lack of information on baseline characteristics for the participants analysed in this review. In four of 15 included RCTs, the authors analysed data on RTW only for the subgroup of participants who provided these data (i.e. the employed participants with cancer in their study) (Burgio 2006; Jong 2018; Lepore 2003; Maguire 1983). However, the study authors reported baseline characteristics only for the total group of participants with cancer in these studies, including the retired participants and homemakers (i.e. people who spend their time looking after their home and family). Therefore, we could not extract and report the baseline characteristics of the employed participants with cancer that we included in our analyses. As these baseline characteristics have not been reported, we could not check if both groups within these RCTs had equal distributions of age, sex and education, which could have influenced the effects of the intervention on RTW. However, because all RCTs were randomised, we assume that the distributions of age, sex and education in both groups of employed participants in each RCT were similar.
Potential biases in the review process
We sought to conduct a comprehensive and transparent review. We performed the entire process of study search, study selection, data extraction and management, and two review authors independently assessed risk of bias of included RCTs and we discussed all results until we reached consensus.
We scored some domains at unclear risk of bias (see risk of bias graph (Figure 2) and risk of bias summary (Figure 3)). This implies that the primary publications did not supply enough details to assess this point. Ideally, the number of domains assessed as unclear should be reduced by obtaining supplementary information from the authors. For simplicity, we chose to complete our risk of bias assessment based solely on the information printed in the primary papers. The author of the cluster‐RCT provided data (Singer 2018). Therefore, it is unclear if a unit of analysis error could have occurred.
We searched for eligible RCTs in nine electronic databases using 130 keywords or combinations of keywords whilst imposing no restrictions on language or publication date. We supplemented our systematic search process with checking the reference lists of included studies and selected reviews. Despite a highly comprehensive search strategy, we did not search the World Health Organization's International Clinical Trials Registry Platform (ICTRP). Some records from ICTRP were retrieved through other sources included in the search (e.g. CENTRAL, ClinicalTrials.gov), but not all ICTRP records would have been available on the date of search through these sources or via those search interfaces. The omission of ICTRP may have reduced the sensitivity of the search for all eligible trial records, and we will include ICTRP in future updates of this review.
We documented in duplicate and discussed all reasons for exclusion and inclusion of all 7847 potentially relevant studies. Two review authors independently extracted data and performed risk of bias assessment using an eight‐page form with each included study. We combined the contents of these forms in one consensus form, and a third review author gave advice in case of uncertainties. In case of any missing data or doubt on the correctness of data, we contacted the original authors. Seven contacted authors replied and provided the requested data. Therefore, we consider we have made every effort to minimise the risk of bias due to the review process.
Our search date is August 2021. This is potentially an important limitation of this review update as it is highly possible that more RCTs have been published since August 2021, which could have impacted the applicability of our research.
Agreements and disagreements with other studies or reviews
In this Cochrane review update, we conclude that both physical and multidisciplinary interventions are likely to enhance RTW for people with cancer.
In an earlier Cochrane review it was similarly concluded that for workers with chronic back pain, physical conditioning has a small effect on reducing sick leave compared to care as usual after 12 months' follow‐up (Schaafsma 2013). One Cochrane review of people with depression found low‐certainty evidence that supervised strength exercise may reduce sickness absence compared to relaxation (Nieuwenhuijsen 2020). The same review also found that multidisciplinary interventions combining a work‐directed intervention with a clinical intervention reduced the number of days on sick leave compared to a clinical intervention alone (Nieuwenhuijsen 2020). This is similar to the results of our review in which the multidisciplinary interventions were conducted within a hospital setting. Another Cochrane review assessed the effectiveness of vocational rehabilitation programmes compared to care as usual on RTW of people with multiple sclerosis (Amatya 2019; Khan 2009). Results of the included studies showed low‐quality evidence to support vocational rehabilitation for people with multiple sclerosis to increase work participation. One Cochrane review on inflammatory arthritis found evidence for interventions combining vocational counselling, advice or education having an effect on job loss, work absenteeism and work functioning (Hoving 2014). Similarly, one Cochrane review on interventions to support RTW for people with coronary heart disease found that combined interventions may increase RTW for up to six months and probably reduce the time away from work (Hegewald 2019). The results of these earlier Cochrane reviews are in line with this review because the effective multidisciplinary studies in this review contained a vocational rehabilitation component, and were combined with psycho‐educational or physical components. In addition, the effective component could be different for each individual patient and interventions might have to be tailored to the experienced problems of the individual patient to be most effective (Tan 2021).
One systematic review including a meta‐analysis on the efficacy of multidisciplinary interventions on RTW for people on sick leave due to low‐back pain indicated that multidisciplinary interventions, including behaviour‐oriented physiotherapy, cognitive behavioural therapy, behavioural medicine, light mobilisation, rehabilitation problem‐solving therapy and behavioural graded activity or personal information, more effectively improve RTW than the alternatives (Norlund 2009). One systematic review of interventions to retain chronically ill occupationally active employees in work aimed to determine if these findings could be transferred to cancer survivors. The authors concluded that it is advised that working cancer survivors should be offered tailored, multidisciplinary interventions (Stapelfeldt 2019). One systematic review was conducted of controlled studies of rehabilitation interventions with work outcomes for women with breast cancer. Only one study, with a multidisciplinary intervention, showed a significant difference in work outcomes when compared to usual care (Algeo 2021).
These results are also in agreement with our meta‐analysis, which showed that multidisciplinary interventions are more effective than alternative programmes in improving RTW, and should be offered to people with cancer.
The RCTs we found in our literature search mostly involved person‐directed interventions aimed at the participants. We found only one work‐directed vocational intervention that was aimed at the employer. However, we found no interventions that were aimed at the workplace and included workplace adjustments, such as modified work hours, modified work tasks, or modified workplaces or improved communication with or between managers, colleagues and health professionals. An earlier systematic review on workplace‐based RTW interventions found strong evidence that work disability duration is significantly reduced by work accommodation offers and contact between healthcare providers and the workplace (Franche 2005). The same review also found moderate‐quality evidence that work disability duration is reduced by interventions that include early contact with affected workers by their workplaces, ergonomic work site visits and the presence of an RTW co‐ordinator (Franche 2005).
In this Cochrane review, we found that psycho‐educational interventions are likely to result in little to no difference in RTW compared to care as usual in enhancing RTW in people with cancer. An earlier meta‐analysis found that cognitive behaviour training has a positive effect on QoL, depression and anxiety in adult cancer survivors but that patient education does not (Osborn 2006). Results from our review show that interventions with patient education can have a positive effect in RTW of people with cancer, when they are part of a multidisciplinary intervention.
This review is an update of de Boer 2015. Compared to the earlier version of the review, we have included eight new RCTs (Greidanus 2021; Grunfeld 2019; Hass 2017; Jong 2018; Mijwel 2019; Singer 2018; van Waart 2015; Zaman 2021). Furthermore, we have excluded medical and pharmacological RCTs in this update because we decided to focus on interventions that can be launched to enhance RTW in people with cancer, regardless of medical treatment. In addition, we expected that non‐medical or non‐pharmacological interventions can be implemented more easily because the medical or pharmaceutical interventions usually have other primary outcomes, so their implementation would depend on those other outcomes. This resulted in the exclusion of seven medical studies (Ackerstaff 2009; Emmanouilidis 2009; Friedrichs 2010; Hillman 1998; Johnsson 2007; Kornblith 2009; Lee 1992). We also excluded one RCT because the outcome measure was change in number of sick leave days instead of number of sick leave days (Rogers 2009). In this update, we have entered each intervention as a separate evaluation when an RCT reported more than one intervention (multiple study arms) and compared each intervention against a control group. In the first version of this review, we entered these evaluations (study arms) as different studies (de Boer 2011). The inclusion of the eight new studies and exclusion of the seven medical studies did not change the conclusions of this review update compared to the first and second version of this review with regard to psycho‐educational and multidisciplinary interventions, but we now concluded that physical interventions are likely to increase RTW (de Boer 2011; de Boer 2015).
Authors' conclusions
Implications for practice.
We found evidence from six randomised controlled trials (RCTs) that multidisciplinary interventions combining physical training, psycho‐educational, vocational elements, or combinations of these are likely to increase return to work (RTW) of people with cancer. The most apparent setting for these interventions would be the hospital, because all multidisciplinary providers are located there, and it is the main focal point for the patients. However, it might be difficult to introduce vocational intervention elements or employer‐directed elements into hospital‐based interventions because of privacy regulations. Interventions conducted in a hospital setting are feasible for people recently diagnosed with cancer who are engaged in curative treatment and who are expected to have sufficient recovery to RTW (Zaman 2021). Other possible settings for multidisciplinary RTW interventions for people with cancer would be multidisciplinary rehabilitation outpatient services in the community or reintegration teams at large workplaces or multinational corporations. Furthermore, we need to provide effective guidelines for employers needing to facilitate a person with cancer returning to work. Thus, it is possible to find ways to improve RTW for people who survive cancer.
Furthermore, results from four physical intervention RCTs using walking, yoga or physical training, showed that it is likely that physical interventions increase RTW. These interventions can be offered both inside and outside the hospital setting.
Overall, we advise that working cancer survivors should be offered tailored, multidisciplinary interventions or physical interventions to enhance their RTW.
Implications for research.
Multidisciplinary and physical interventions are likely to enhance RTW for people with cancer. Most research so far has been conducted on people with breast cancer or prostate cancer. Research should additionally focus on people with other prevalent diagnoses of cancer in the working population, such as colorectal cancer, and blood or lymph cancers. Other important patient characteristics, such as age, education and ethnicity, should also be measured to assess the effect of those characteristics on the RTW outcomes of the interventions.
Most included RCTs were performed on highly educated people or those with relatively secure jobs. More research is needed in lower‐educated people and those in less‐secure jobs because they might be the most vulnerable and at the highest risk of job loss.
Future research on enhancing RTW in people with cancer involving multidisciplinary interventions should encompass a physical, psycho‐educational and vocational component, and be preferably tailored to the individual needs of the patient. The vocational component should not be just patient‐oriented but should also be directed at the work environment, including work adjustments and supervisors, although this might be difficult in a hospital setting. With regard to psycho‐educational interventions, it is unclear whether patient education or patient counselling is effective. Both interventions should be compared against each other and care as usual.
We found only one vocational intervention aimed at enhancing RTW in people with cancer for this review, although one would expect the largest impact on RTW from this type of intervention. Future research should focus more on vocational interventions focused on employment. Vocational interventions might be person‐directed, that is, aimed at the patient to encourage RTW, meaning vocational rehabilitation or occupational rehabilitation, or they might be work‐directed, that is, aimed at the workplace by means of workplace adjustments such as modified work hours, modified work tasks, or modified workplaces and improved communication with or between managers, colleagues and health professionals.
So far, not all studies comparing the effect of an intervention on RTW with care as usual or an alternative intervention have been conducted using an RCT design (Guo 2021). Consequently, there is uncertainty about effectiveness. Therefore, all studies evaluating the effect of an intervention on RTW should employ an RCT design, although this might sometimes be difficult in daily practice. These RCTs should perform intention‐to‐treat analyses. In some cases, a cluster‐RCT design might have to be chosen in which the providers of the intervention or the settings were randomised and not the participants. In addition, the studies described in this review were all relatively small, and thus we need RCTs with a much greater number of recruited participants.
With regard to outcome measures, many more clinical trials should incorporate RTW measures. Currently, many trials are being conducted evaluating the effect of physical exercise on physical fitness, fatigue or quality of life, but only a few of these studies will evaluate effects on sick leave duration or RTW, although it is reasonable to expect these interventions to be beneficial for employment. In future research, work‐related outcome measures other than the rate of participants returning to work should be used, because this measure is a valid but broad and general indication of RTW. Other work‐related outcome measures that are more precise measures of RTW could include the total number of days of sick leave from first day of sick leave until first day of return. Reporting both these outcomes will make it easier to combine data in the future. Furthermore, a significant proportion of people with cancer successfully RTW but face other difficulties at work that would manifest as more days missed at work and lower performance or productivity. Therefore, measures of work once back at work, including work retention, quality of working life (e.g. de Jong 2018), and work productivity should be considered. Studies also need to define RTW, for example, is it return to full‐time or part‐time work or return to the same job or a different job (Ravinskaya 2021).
RTW is an important outcome measure for people with cancer indicating recovery and return to normality. In this Cochrane review, we found no difference in quality of life in the included RCTs when people had returned to work. This would imply that probably most people with cancer RTW only when it is feasible. After returning to work, it is important that people with cancer are able to remain working. Research beyond RTW, focusing on work retention and factors associated with it, is needed (Dorland 2017; Moskowitz 2014). Based on these factors associated with remaining at work, researchers should develop new interventions for work retention of people with cancer.
We only found studies from high‐income countries. More research is needed to examine what the barriers are to implementing the findings from this review in middle‐ and low‐income countries and to assess if findings would differ if RCTs were conducted in a different setting.
Finally, many treatments for cancer take several months and result in long‐lasting adverse effects. This might influence the effectiveness of interventions aimed at RTW of people with cancer. Furthermore, work disability can be episodic. Given these fluctuations in work absence, we need studies with long‐term follow‐up.
Feedback
Wrong classification of intervention in Burgio 2006 study, 15 December 2015
Summary
Thank you for a very interesting and important review. While I think your work is of the utmost high quality, I was nonetheless rather surprised when reading through it carefully I noticed a possible error regarding the categorisation of one included study. You state in the Abstract: “Five RCTs involved multidisciplinary interventions in which vocational counselling was combined with patient education, patient counselling, and biofeedback‐assisted behavioral training or physical exercises.” This implies that the study by Burgio 2006 would have vocational counselling AND biofeedback‐assisted behavioral training, i.e. a combination of the two. This seems not to be the case. I have read through the Burgio 2006 paper and their intervention clearly does not have a vocational counselling component. Could it be that you have made a mistake in categorising this study and that you should in fact remove this study from the comparison Multidisciplinary physical, psycho‐educational and vocational interventions versus Care as usual? This might affect the results of your meta‐analyses also? Heidi Miettinen
Reply
Thank you very much for pointing this out. We realise that the statement in the abstract is misleading because it was meant to refer to any combination of the interventions and not to the combination of vocational rehabilitation with the other interventions. We have now changed this sentence into: "Five RCTs involved multidisciplinary interventions in which vocational counselling, patient education, patient counselling, biofeedback‐assisted behavioral training and/or physical exercises were combined." We hope that this resolves the confusion.
Contributors
Heidi Mietttinen (provided feedback), Riitta Sauni (feedback editor), Angela de Boer (review author)
What's new
| Date | Event | Description |
|---|---|---|
| 28 March 2024 | Amended | Fixed typographical error in PLS section of review |
History
Protocol first published: Issue 1, 2009 Review first published: Issue 2, 2011
| Date | Event | Description |
|---|---|---|
| 5 March 2024 | New citation required and conclusions have changed | We excluded seven RCTs from the first two versions of this review, because in this update we focussed on interventions which can be launched to enhance return to work (RTW) regardless of medical treatment. However, the excluded RCTs concerned solely medical and pharmaceutical interventions. Burgio 2006 has been reclassified as a physical intervention study. We added eight new studies for this update. We found evidence from six RCTs that multidisciplinary interventions are likely to increase RTW of people with cancer, in line with our earlier publications of this review. New findings in this updated review are that results from four RCTs on physical interventions showed that it is likely that physical interventions increase RTW. |
| 5 March 2024 | New search has been performed | This is the second update of this review. The searches were updated on 18 August 2021. |
| 4 July 2017 | Feedback has been incorporated | Confusion about classification of the intervention in one study. No change in conclusions |
| 14 July 2015 | New citation required but conclusions have not changed | We excluded non‐randomised studies. We reorganised comparisons in such a way that we included studies with multiple arms as different evaluations rather than different studies. Four new studies met the inclusion criteria. |
| 15 June 2015 | New search has been performed | We updated our literature search to 25 March 2014. |
| 18 December 2012 | Amended | We recalculated odd ratios (ORs) into risk ratios (RRs) for medical interventions. |
| 4 July 2012 | Amended | We recalculated ORs into RRs. |
| 15 February 2012 | Amended | We amended errors in references. |
| 8 February 2011 | Amended | A minor edit was made for Issue 3, 2011. |
| 14 April 2008 | Amended | We converted to a new review format. |
Acknowledgements
We are grateful to MC Jong, LQ Rogers, S Singer, S Tamminga and S Duijts for kindly providing further information about their studies.
We would like to acknowledge the contribution of Jos Verbeek, Monique Frings‐Dresen, Taina Taskila and Michael Feuerstein as authors of the previous two versions of this review.
Cochrane Work supported the authors in the development of this review update. Joost Daams kindly conducted the search update and we are very grateful for the help of Christina Tikka Mischke. Jan Hoving is a member of Cochrane Work but was not involved in the editorial process or decision‐making for this review update. The following people conducted the editorial process for this update.
Sign‐off Editor (final editorial decision): Lisa Bero, University of Colorado Anschutz Medical Campus
Managing Editor (selected peer reviewers, collated peer‐reviewer comments, provided editorial guidance to authors, edited the article): Joey Kwong, Cochrane Central Editorial Service
Editorial Assistant (conducted editorial policy checks and supported editorial team): Leticia Rodrigues, Cochrane Central Editorial Service
Copy Editor (copy‐editing and production): Anne Lawson, Cochrane Central Production Service
Peer‐reviewers (provided comments and recommended an editorial decision): Bogda Koczwara, Flinders University and Flinders Medical Centre, Adelaide, Australia (clinical/content review); Ingrid G Boelhouwer, Department of Applied Psychology, Amsterdam University of Applied Sciences, Amsterdam, The Netherlands (clinical/content review); Chia Jie Tan, University of Utah College of Pharmacy (clinical/content review), Elanga Andrea Ornela, Communication officer at eBASE Africa (consumer review); Kerry Dwan, Rachel Richardson, Sofia Tsokani, Cochrane Methods Support Unit (methods reviews); Robin Featherstone, Cochrane Evidence Production & Methods Directorate (search review)*. *One additional peer reviewer provided search peer review, but chose not to be publicly acknowledged.
Appendices
Appendix 1. MEDLINE search strategy
| 1 | exp neoplasms/ |
| 2 | cancer*.tw. |
| 3 | neoplasm*.tw. |
| 4 | carcinoma*.tw. |
| 5 | oncolog*.tw. |
| 6 | malignan*.tw. |
| 7 | tumor.tw. |
| 8 | tumour.tw. |
| 9 | tumors.tw. |
| 10 | tumours.tw. |
| 11 | leukemia*.tw. |
| 12 | sarcoma*.tw. |
| 13 | lymphoma*.tw. |
| 14 | melanoma*.tw. |
| 15 | blastoma*.tw. |
| 16 | radiotherapy.tw. |
| 17 | chemotherapy.tw. |
| 18 | or/1‐17 |
| 19 | (rtw or return‐to‐work).tw. |
| 20 | employment/ |
| 21 | employment.tw. |
| 22 | unemployment/ |
| 23 | unemployment.tw. |
| 24 | unemployed.tw. |
| 25 | retirement.tw. |
| 26 | sick leave/ |
| 27 | sick leave.tw. |
| 28 | Sickness absence.tw. |
| 29 | absenteeism/ |
| 30 | absenteeism.tw. |
| 31 | exp work/ |
| 32 | exp occupations/ |
| 33 | occupational medicine/ |
| 34 | occupational health/ |
| 35 | occupational health services/ |
| 36 | disability management.tw. |
| 37 | rehabilitation, vocational/ |
| 38 | occupation*.tw. |
| 39 | rehabilitation/ |
| 40 | neoplasms/rh |
| 41 | vocational*.tw. |
| 42 | work ability.tw. |
| 43 | work capacity.tw. |
| 44 | work activity.tw. |
| 45 | work disability.tw. |
| 46 | work rehabilitation.tw. |
| 47 | work status.tw. |
| 48 | work retention.tw. |
| 49 | workability.tw. |
| 50 | employability.tw. |
| 51 | employable.tw. |
| 52 | employee*.tw. |
| 53 | or/19‐52 |
| 54 | randomized controlled trial.pt. |
| 55 | controlled clinical trial.pt. |
| 56 | randomized.ab. |
| 57 | placebo.ab. |
| 58 | drug therapy.fs. |
| 59 | randomly.ab. |
| 60 | trial.ab. |
| 61 | groups.ab. |
| 62 | or/54‐61 [Adaptation Box 3c Chptr 4 tech suppl Cochrane Handbook] |
| 63 | and/18,53,62 |
| 64 | exp animals/ not humans.sh. |
| 65 | 63 not 64 |
| 66 | limit 65 to yr="2014‐current" |
Appendix 2. CENTRAL search strategy
| Title Abstract Keyword (cancer OR neoplasm* OR carcinoma* OR oncolog* OR malignan* OR tumor* OR tumour* OR leukemi* OR sarcom* OR lymphom* OR melanom* OR blastom* OR radiotherapy OR chemotherapy) | |
| AND | |
| Title Abstract Keyword ("return to work" OR employment OR unemployment OR unemployed OR retirement OR "sick leave" OR "Sickness absence" OR absenteeism OR occupation* OR vocational* OR "work ability" OR "work capacity" OR "work activity" OR "work disability" OR "work rehabilitation" OR "work status" OR "work retention" OR workability OR employability OR employable OR employee*) | |
| NOT | |
| Title Abstract Keyword (primary prevention OR smoking cessation OR palliative OR Metastasis OR terminal) | |
| Limit to publication years 2014 ‐ 2020 |
Appendix 3. Embase search strategy
| 1 | cancer.mp. |
| 2 | *Neoplasm/ or neoplasm*.mp. |
| 3 | carcinoma*.mp. |
| 4 | oncolog*.mp. |
| 5 | malignan*.mp. |
| 6 | tumor*.mp. |
| 7 | tumour*.mp. |
| 8 | leukemi*.mp. |
| 9 | sarcom*.mp. |
| 10 | lymphom*.mp. |
| 11 | melanom*.mp. |
| 12 | blastom*.mp. |
| 13 | radiotherapy.mp. |
| 14 | chemotherapy.mp. |
| 15 | or/1‐14 |
| 16 | exp Work Resumption/ or return to work.mp. |
| 17 | exp Employment/ or exp Employment Status/ or employment.mp. |
| 18 | exp Unemployment/ or unemployment.mp. |
| 19 | unemployed.mp. |
| 20 | retirement.mp. |
| 21 | (sick leave or Sickness absence or absenteeism).mp. |
| 22 | (vocational* or work ability or work capacity or work activity or work disability or work rehabilitation or work status or work retention or workability or employability or employable or employee*).mp. |
| 23 | exp Vocational Rehabilitation/ or exp Work Disability/ |
| 24 | disability management.mp. |
| 25 | exp Vocational Rehabilitation/ or work rehabilitation.mp. |
| 26 | occupation.mp. |
| 27 | or/16‐26 |
| 28 | Randomized controlled trial/ |
| 29 | Controlled clinical study/ |
| 30 | random$.ti,ab. |
| 31 | randomization/ |
| 32 | intermethod comparison/ |
| 33 | placebo.ti,ab. |
| 34 | (compare or compared or comparison).ti. |
| 35 | ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab. |
| 36 | (open adj label).ti,ab. |
| 37 | ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab. |
| 38 | double blind procedure/ |
| 39 | parallel group$1.ti,ab. |
| 40 | (crossover or cross over).ti,ab. |
| 41 | ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab. |
| 42 | (assigned or allocated).ti,ab. |
| 43 | (controlled adj7 (study or design or trial)).ti,ab. |
| 44 | (volunteer or volunteers).ti,ab. |
| 45 | human experiment/ |
| 46 | trial.ti. |
| 47 | or/28‐46 |
| 48 | (random$ adj sampl$ adj7 ("cross section$" or questionnaire$1 or survey$ or database$1)).ti,ab. not (comparative study/ or controlled study/ or randomi?ed controlled.ti,ab. or randomly assigned.ti,ab.) |
| 49 | Cross‐sectional study/ not (randomized controlled trial/ or controlled clinical study/ or controlled study/ or randomi?ed controlled.ti,ab. or control group$1.ti,ab.) |
| 50 | (((case adj control$) and random$) not randomi?ed controlled).ti,ab. |
| 51 | (Systematic review not (trial or study)).ti. |
| 52 | (nonrandom$ not random$).ti,ab. |
| 53 | "Random field$".ti,ab. |
| 54 | (random cluster adj3 sampl$).ti,ab. |
| 55 | (review.ab. and review.pt.) not trial.ti. |
| 56 | 'we searched'.ab. and (review.ti. or review.pt.) |
| 57 | 'update review'.ab. |
| 58 | (databases adj4 searched).ab. |
| 59 | or/48‐58 [Adaptation Box 3e Chptr 4 tech suppl Cochrane Handbook] |
| 60 | 47 not 59 |
| 61 | and/15,27,60 |
| 62 | (rat or rats or mouse or mice or swine or porcine or murine or sheep or lambs or pigs or piglets or rabbit or rabbits or cat or cats or dog or dogs or cattle or bovine or monkey or monkeys or trout or marmoset$1).ti. and animal experiment/ |
| 63 | Animal experiment/ not (human experiment/ or human/) |
| 64 | 62 or 63 |
| 65 | 61 not 64 |
| 66 | limit 65 to yr="2014‐current" |
Appendix 4. CINAHL search strategy
| 1 | MW (cancer or neoplasm* or oncolog* or malignan* or tumor* or tumour* or leukemi* or sarcom* or lymphom* or melanom* or blastom* or radiotherapy or chemotherapy) |
| 2 | TI (cancer or neoplasm* or oncolog* or malignan* or tumor* or tumour* or leukemi* or sarcom* or lymphom* or melanom* or blastom* or radiotherapy or chemotherapy) |
| 3 | AB (cancer or neoplasm* or oncolog* or malignan* or tumor* or tumour* or leukemi* or sarcom* or lymphom* or melanom* or blastom* or radiotherapy or chemotherapy) |
| 4 | S1 or S2 or S3 |
| 5 | MW (reemployment or "return to work" or employment or unemployment or unemployed or retirement or sick leave or sickness absence or absenteeism or vocational* or work ability or work capacity or work activity or work disability or work rehabilitation or work status or work retention or workability or employability or employable or employee* or disability management or occupation) |
| 6 | TI (reemployment or "return to work" or employment or unemployment or unemployed or retirement or sick leave or sickness absence or absenteeism or vocational* or work ability or work capacity or work activity or work disability or work rehabilitation or work status or work retention or workability or employability or employable or employee* or disability management or occupation) |
| 7 | AB (reemployment or "return to work" or employment or unemployment or unemployed or retirement or sick leave or sickness absence or absenteeism or vocational* or work ability or work capacity or work activity or work disability or work rehabilitation or work status or work retention or workability or employability or employable or employee* or disability management or occupation) |
| 8 | S5 or S6 or S7 |
| 9 | MH randomized controlled trials |
| 10 | MH double blind studies |
| 11 | MH single blind studies |
| 12 | MH random assignment |
| 13 | MH pretest posttest design |
| 14 | MH cluster sample |
| 15 | TI (randomised OR randomized) |
| 16 | AB (random*) |
| 17 | TI (trial) |
| 18 | MH (sample size) AND AB (assigned OR allocated OR control) |
| 19 | MH (placebos) |
| 20 | PT (randomized controlled trial) |
| 21 | AB (control W5 group) |
| 22 | MH (crossover design) OR MH (comparative studies) |
| 23 | AB (cluster W3 RCT) |
| 24 | S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 |
| 25 | S4 and S8 and S24 |
| 26 | MH animals+ |
| 27 | MH (animal studies) |
| 28 | TI (animal model*) |
| 29 | S26 or S27 or S28 |
| 30 | MH (human) |
| 31 | S29 NOT S30 |
| 32 | S25 NOT S31 |
| 33 | 2014 ‐ 2020 |
Appendix 5. PsycINFO search strategy
| 1 | cancer.mp. |
| 2 | *Neoplasm/ or neoplasm*.mp. |
| 3 | carcinoma*.mp. |
| 4 | oncolog*.mp. |
| 5 | malignan*.mp. |
| 6 | tumor*.mp. |
| 7 | tumour*.mp. |
| 8 | leukemi*.mp. |
| 9 | sarcom*.mp. |
| 10 | lymphom*.mp. |
| 11 | melanom*.mp. |
| 12 | blastom*.mp. |
| 13 | radiotherapy.mp. |
| 14 | chemotherapy.mp. |
| 15 | or/1‐14 |
| 16 | reemployment/ or return to work.mp. |
| 17 | exp Employment/ or exp Employment Status/ or employment.mp. |
| 18 | exp Unemployment/ or unemployment.mp. |
| 19 | unemployed.mp. |
| 20 | retirement.mp. |
| 21 | (sick leave or Sickness absence or absenteeism).mp. |
| 22 | (vocational* or work ability or work capacity or work activity or work disability or work rehabilitation or work status or work retention or workability or employability or employable or employee*).mp. |
| 23 | exp Vocational Rehabilitation/ or work related illnesses/ |
| 24 | disability management.mp. |
| 25 | exp Vocational Rehabilitation/ or work rehabilitation.mp. |
| 26 | occupation.mp. |
| 27 | or/16‐26 |
| 28 | 15 and 27 |
| 29 | limit 28 to human |
| 30 | limit 29 to yr="2014‐current" |
Appendix 6. LogOR transformations and odds ratio recalculations
| Study ID | OR | OR low | OR high | log OR | SE | ||
| Tamminga 2013a | 1.2957 | 0.481 | 3.4898 | 0.2591 | 0.5055 | ||
| Study ID | SMD | SMD low | SMD high | log OR (lnOR) | logOR low | logOR high | SE |
| Hubbard 2013b | 0.6 | −0.37 | 1.58 | 1.0884 | −0.6712 | 2.86612 | −0.902372449 |
|
logOR low: lower limit log OR; logOR high: upper limit log OR; OR: odds ratio; SE: standard error; SMD: standardised mean difference. a The sample size of Tamminga 2013 has been adjusted because the authors report: "(RTW) rates were 86% and 83%, respectively (p = 0.61), when patients who died within the follow‐up period or those with a life expectancy of only a few months were excluded… Of the patients who did not return to work (intervention versus control group), 2 versus 2 died, 3 versus 1 had a life expectancy of few months." Therefore, eight participants have been excluded. Hence, the intervention group included 57 participants (instead of 62) and the control group 63 (instead of 66). The recalculation of the RR is: 49 of 57 RTW in the intervention group (86%) and 52 of 63 RTW in the control group (83%). This results in RR of 1.0415 (95% confidence interval (CI) 0.8923 to 1.2156; P = 0.6062). This P value was reported in the article but not the RR. With this adjusted sample size, an OR has been recalculated: OR 1.2957 (95% CI 0.4810 to 3.4898). This results in a P value of 0.6084. This results in a log OR 0.2591 and SE 0.5055. These have been entered in Analysis 4.1. b The direction of the continuous outcome was reversed in the calculation of the SMD when the higher score indicated a negative outcome (sick leave days). | |||||||
Data and analyses
Comparison 1. Psycho‐educational interventions versus care as usual.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Return to work | 4 | 512 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.96, 1.24] |
| 1.2 Quality of life | 1 | 124 | Mean Difference (IV, Random, 95% CI) | 1.47 [‐2.38, 5.32] |
Comparison 2. Vocational interventions versus care as usual.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Return to work | 1 | 34 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.78, 1.13] |
Comparison 3. Physical interventions versus care as usual.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Return to work | 4 | 434 | Risk Ratio (IV, Random, 95% CI) | 1.23 [1.08, 1.39] |
| 3.2 Quality of life | 1 | 173 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.33, 0.32] |
Comparison 4. Multidisciplinary interventions versus care as usual.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Return to work (odds ratios) | 6 | 497 | Odds Ratio (IV, Random, 95% CI) | 2.00 [1.29, 3.08] |
| 4.2 Quality of life | 3 | 378 | Std. Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.14, 0.28] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Berglund 1994.
| Study characteristics | ||
| Methods | RCT Setting: not reported. Efron's method for randomisation of small groups: group sizes were forced towards equality by proportionately increasing the probability of assignment to the smaller group. |
|
| Participants | Inclusion criteria: people with cancer (80% breast cancer, 8% ovarian cancer); aged < 75 years; curative treatment for a primary tumour; inclusion within 2 months after postoperative treatment with radio‐ or chemotherapy Exclusion criteria: not reported |
|
| Interventions |
Intervention group (n = 87): during first 4 weeks, participants met twice a week, once for information and once for physical training. The last 3 weeks were devoted to 1 session of coping skills training each week. An oncology nurse specialised in psychosocial issues conducted the sessions. She was accompanied by a specialist of the theme dealt with at each session. Physical training: exercises to increase mobility, muscle strength, fitness, relaxation. Instruction for relaxation at home. Information: effects of treatment, diet, development trough crises, alternative treatment. Coping: role plays, how to handle attitudes towards cancer, meeting people asking too much, problem situations at hospital, anxiety and how to handle it. Intervention lasted 7 weeks Sessions: 11 sessions of 2 hours each Provider: oncology nurse and specialist Setting: not reported Control group (n = 89) 36 participants received single information session (oncologist and dietician information included in the intervention session); 53 participant received care as usual. |
|
| Outcomes |
Primary outcome measure (RTW outcomes) Work status: number not working Registered by: participant at baseline; 3, 6, 12 months Secondary outcome measure (QoL outcomes) Problems with QoL – 2 items Registered by: participant at baseline; 8–12 weeks; 3, 6, 12 months |
|
| Funding | Swedish Cancer Foundation | |
| Objectives of the study | To investigate the short‐term gains of the starting again programme over a follow‐up of 1 year | |
| Country | Sweden | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Efron's method for randomisation of small samples. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There are no systematic differences between groups in the number and the way participants were lost from the study. |
| Selective reporting (reporting bias) | Low risk | All outcomes from methods were reported. |
| ITT analysis? | High risk | Not performed. |
| Baseline similarity? | Unclear risk | No baseline characteristics reported. |
| Co‐interventions avoided or similar? | Unclear risk | Not reported. |
| Compliance? | Unclear risk | Not reported. |
| Similar follow‐up time? | Low risk | All outcomes were measured 8–12 weeks postintervention. |
Burgio 2006.
| Study characteristics | ||
| Methods | RCT, hospital setting | |
| Participants | Inclusion criteria: men with prostate cancer; ambulatory, continent, identified for the study ≥ 1 week prior to surgery, elected for radical prostectomy, prostate cancer Exclusion criteria: > 2 episodes of urinary incontinence in previous 6 months, incontinence, prior prostectomy, impaired mental status, < 1 week prior to surgery |
|
| Interventions |
Intervention group (n = 28): single session of biofeedback‐assisted behavioural training, including pelvic floor muscle control and exercise. Use of rectal probe to provide information on rectal pressure. Feedback and verbal instructions and reinforcement. Daily home practice Intervention lasted 6 months Sessions: 1 session + daily at home Provider: not reported Setting: hospital and at home Control group (n = 29) Brief verbal instructions to interrupt the urinary stream during voiding |
|
| Outcomes |
Primary outcome measure (RTW outcomes) RTW rate at 6 months (results for participants with paid employment at baseline): number returned to work Registered by: participant at baseline and 6 months Secondary outcome measure (QoL outcomes) MOS‐SF Registered by: participant at baseline and 6 months |
|
| Funding | National Institute for Diabetes and Digestive and Kidney Diseases, National Institutes of Health. | |
| Objectives of the study | To test the effectiveness of preoperative biofeedback‐assisted behavioural training for hastening the recovery of urinary control, decreasing the severity of postoperative incontinence and improving QoL in the 6 months following radical prostatectomy. | |
| Country | USA | |
| Notes | Results for participants with paid employment at baseline for 102 participants | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated numbers. |
| Allocation concealment (selection bias) | Low risk | Randomised schedule was implemented by research nurse, so the interventionists would be blinded to next group assignment. |
| Blinding (performance bias and detection bias) All outcomes | High risk | No blinding to participants or interventionists. Blinding of data handling people or researchers or outcome assessors (participants) unknown. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Work‐related outcomes: no information was provided for participants with missing data and no non‐response analysis. For the work‐related data for people working at baseline, no attrition/exclusion statistics were given. |
| Selective reporting (reporting bias) | Low risk | All outcomes from methods are reported. |
| ITT analysis? | High risk | Work‐related outcomes: no information was provided for participants with incomplete data and no ITT analysis. For the work‐related data for people working at baseline, no attrition/exclusion statistics were given. |
| Baseline similarity? | Unclear risk | Not reported for group of participants employed at baseline. |
| Co‐interventions avoided or similar? | Low risk | No co‐interventions. |
| Compliance? | Low risk | 70% were still doing exercises at home after 6 months. |
| Similar follow‐up time? | Low risk | The same time points (6 months). |
Greidanus 2021.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Inclusion criteria: aged 18–63 years; diagnosed with cancer < 2 years earlier; in paid employment under a temporary (> 6 months remaining) or permanent contract on a full‐time, part‐time, or flexible basis; currently fully or partially sick‐listed for < 1 year; able to complete 3 questionnaires in the following 6 months (as assessed by their medical specialist on the basis of their current health and expectations about their health in the future); and able to understand, speak and read Dutch sufficiently to fill out the questionnaires. Cancer survivors who had not informed their employer about their diagnosis of cancer were excluded, in order not to put them under unintended pressure to make that disclosure through the procedures of this study |
|
| Interventions |
Intervention group: an online toolbox targeted at the participant's employer. The Toolbox is a web‐based intervention with succinct information, interactive communication videos, conversation checklists and links to reliable external sources. The content of the toolbox is tailored per RTW phase and per cancer survivor experience type. 3 predefined cancer survivor experience types are distinguished: 1. an emotional cancer survivor, in which intense emotions such as sadness and anger can alternate quickly; 2. a cancer survivor who wants little attention for their health situation, and wants to be involved in work for as long as possible and RTW as quickly as possible; and 3. a cancer survivor who starts looking differently at work and life, and gives other priorities due to their illness. The toolbox is accessible via a URL that was not traceable using any online search engine throughout the study period. The toolbox targets the most important employer actions for successful RTW selected by employers and cancer survivors and was based on the Resource Dependence Institutional Cooperation (RDIC) model. Participants randomised to the intervention (n = 24) were asked to inform their employer about the online toolbox, either by e‐mail with attached secured PDF or by printed letter (both supplied by the research team). The PDF and letter complied fully with privacy and security regulations, and contained information about the participant's involvement in the study, the URL of the toolbox and a short promotional video to persuade the employer to visit the toolbox. The employer was asked to watch the video and to use the online toolbox during the participant's sick leave and RTW. Setting: online Duration: – Provider: online Control group: care as usual Participants randomised to the control group (n = 11) were not able to inform their employer about the online toolbox for a period of 6 months. Consequently, they received "care as usual" from their employer. After this follow‐up period of 6 months, they were able to inform their employer about the online toolbox, using the same materials as the intervention group. |
|
| Outcomes | RTW at 6 months | |
| Funding | Dutch Cancer Society, grant number UVA 2014–7153 | |
| Objectives of the study | To assess feasibility of a future definitive RCT on the effectiveness of the MiLES intervention in terms of recruitment, reach, and acceptability of the study protocol. Secondary aims were: 1. to obtain preliminary results on the effectiveness of the MiLES intervention on successful RTW of cancer survivors, and 2. to determine the sample size needed in a future definitive RCT on the effectiveness of the MiLES intervention | |
| Country | Netherlands | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The electronic data‐capture system Castor was used to randomise participants into the intervention or control group, with the allocation ratio set at 2:1. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Neither the participants nor the research team were blinded for the randomisation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The response rate for the T1 and T2 questionnaires was 97% (n = 34) in both cases. |
| Selective reporting (reporting bias) | Low risk | All outcomes from methods were reported. |
| ITT analysis? | Low risk | Data were analysed according to the ITT principle. |
| Baseline similarity? | Low risk | At baseline, the intervention and control groups differed significantly on 1 prognostic factor for RTW: participants randomised to the control group had undergone chemotherapy more often than those randomised to the intervention group. |
| Co‐interventions avoided or similar? | Unclear risk | Insufficient information. |
| Compliance? | Unclear risk | 88% sent MiLES invitation to employer, but unclear what happened then. |
| Similar follow‐up time? | Low risk | Same measurement points. |
Grunfeld 2019.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Inclusion criteria: received a diagnosis breast, gynaecological, prostate or colorectal cancer; had not been classified as having metastatic disease or recurrence; ≥ 2 weeks post‐treatment initiation; aged 18–70 years; working at the time of diagnosis; not working at time of recruitment but intended to RTW. | |
| Interventions |
Intervention group (n = 26):the WorkPlan package was a 4‐week guided workbook intervention consisting of structured sections and activities to provide guidance and support to patients. The workbook was broken down into chapters. Chapter 1 focussed on thinking about illness and treatment (based around the illness perceptions component of the Self‐Regulation Model) and includes causes of cancer, symptoms, beliefs about efficacy of treatment and consequences of living as a survivor of cancer. It then explores the participant's beliefs about the impact of cancer and treatment on their ability to function in the workplace, including suggestions for management. The chapter concludes by examining participant's emotional reactions to treatment and support/strategies to manage these. Chapter 2 focused on setting and achieving goals (based on Goal Theory) including the goal‐setting process, identifying and overcoming barriers and using support. Chapter 3 worked on building confidence, including ways to boost confidence. This chapter concluded by examining fatigue and ways to identify and manage fatigue triggers. Chapter 4 focussed on developing an action plan for returning to work and outlined how to initiate discussions and deal with difficult questions within the workplace. Participants incorporated all elements from the workbook into a personal RTW plan, which they were encouraged to develop in the final week. A resources section signposted participants towards relevant avenues of further support. Multiple copies of the RTW planning page were available to encourage changes to be made when necessary, and these plans were used as a tool when meeting with employers to aid discussion around returning to work. Service users in the original pilot work (of the materials and study design) were concerned about raising work‐related issues too early with their employer and stated that they would prefer to engage with their workplace after completing the intervention, when they felt better able to represent their view and had formed a RTW plan. Therefore, the intervention did not have a specific employer component, but rather the workbook promoted skills to enable communication with employers. Setting: at home Duration: 4 weeks/ 120 minutes per week Provider: researchers Control group (n = 21): care as usual |
|
| Outcomes |
Primary outcomemeasure Time to RTW and RTW rates and 12 months' follow‐up Secondary outcome measure QoL VAS 0–100 at 12 months' follow‐up |
|
| Funding | National Institute for Health Research (NIHR) under its Research for Patient Benefit (RfPB) Programme (Grant Reference Number PB‐PG‐0613‐31088). | |
| Objectives of the study | The primary objective of the study was to trial the WorkPlan intervention and data collection materials to determine if the materials were acceptable to participants and whether participants were able to provide full answers. | |
| Country | UK | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Following the assessment interview the researchers used an online and text‐based randomisation system (Sealed Envelope Ltd) to randomise participants at a ratio of 1:1. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) All outcomes | High risk | No blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There are no systematic differences between the study groups in the number and the way participants were lost from the study. |
| Selective reporting (reporting bias) | Low risk | All outcomes from methods were reported. |
| ITT analysis? | Low risk | ITT performed. |
| Baseline similarity? | High risk | Small number of participants and differences at baseline seemed to exist in diagnosis, sex, education. |
| Co‐interventions avoided or similar? | Low risk | Participants in both groups were able to access other information and support relating to work and were, therefore, asked to record any resources or information they used during the trial. |
| Compliance? | Unclear risk | Study authors' comment: although we examined engagement and acceptability of the intervention (through the interviews), we did not test the fidelity of the intervention as part of this feasibility trial. |
| Similar follow‐up time? | Low risk | Same measurement points (6 months). |
Hass 2017.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Inclusion criteria: people with breast cancer who went through inpatient care in an oncological rehabilitation clinic; good understanding of the German language; sufficient cognitive and physical resilience Exclusion criteria: metastatic tumour stage or relevant internal concomitant diseases |
|
| Interventions |
Intervention group (n = 53): telephone follow‐up care after oncological rehabilitation First, the establishment of contact with recording the physical and psychological well‐being of the participant as well as possible problems in these areas and the employment situation. In the second section of the follow‐up, telephone call was about strengthening and maintaining the motivation to do health‐promoting behaviour and support of everyday transfer. The basic conversation style can be used as goal‐ and problem‐solving oriented, accepting and motivating. Setting: telephone call Duration: 6 months, 5 sessions, duration 15 minutes Provider: psychologist Control group (n = 63): care as usual |
|
| Outcomes | RTW rate at 6 months | |
| Funding | Die Arbeitsgemeinschaft für Krebsbekämpfung NRW (ARGE). | |
| Objectives of the study | To determine the feasibility and the evaluation of the therapeutic effects (especially in terms of coping with illness, exercise, diet and RTW) of standardised telephone follow‐up care after oncological rehabilitation. | |
| Country | Germany | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Participant and intervention staff were not blinded because the intervention encompassed counselling by phone. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts addressed. |
| Selective reporting (reporting bias) | Unclear risk | Not reported. |
| ITT analysis? | Unclear risk | Not reported. |
| Baseline similarity? | Unclear risk | Not reported. |
| Co‐interventions avoided or similar? | Unclear risk | Not reported. |
| Compliance? | Unclear risk | Not reported. |
| Similar follow‐up time? | Low risk | Same measurement points (6 months). |
Hubbard 2013.
| Study characteristics | ||
| Methods | RCT Setting: hospital and community |
|
| Participants | Inclusion criteria: women with breast cancer; aged 18–65 years; in paid employment or self‐employed; living or working in Lothian or Tayside, Scotland; diagnosed with an invasive breast cancer tumour or ductal carcinoma in situ; treated first with surgery Exclusion criteria: first, women who worked in large companies were excluded but later included when the recruitment criteria were changed |
|
| Interventions |
Intervention group (n = 7): Working Health Services established by the Scottish centre with a multidisciplinary approach whereby case‐management was used to assess individuals' needs to enable work retention or return through signposting or direct referral for a range supportive services according to need, such as physiotherapy, occupational therapy, occupational health nurse, occupation health doctor, counsellor or psychological therapy and complementary therapy. Participants were allocated a case manager who conducted a telephone assessment. Setting: hospital and telephone interview Provider: case manager and referral to physiotherapy, occupational therapy, occupational health nurse, occupation health doctor, counsellor or psychological therapy and complementary therapy. Control group (n = 11): no formal employment support. Participants received a copy of the booklet work and cancer published by Macmillan. |
|
| Outcomes |
Primary outcome measure (RTW) Number of days off work due to ill health within the first 6 months after surgery. Duration of sick leave in the 4 weeks before the date of 6‐ and 12‐month follow‐up. Left or remained in employment, job role and hours worked. Secondary outcome measure (QoL outcomes) FACT‐B Registered by: participants Follow‐up time: 6 and 12 months |
|
| Funding | MacMillan Cancer Support and Scottish Centre for Healthy Working Lives | |
| Objectives of the study | To assess the feasibility and acceptability of an existing case management vocational rehabilitation service for women with breast cancer. It was anticipated that participants referred to the vocational rehabilitation service would experience fewer days off work due to sickness in the first 6 months postsurgery, lower levels of fatigue and increased QoL. | |
| Country | Scotland, UK | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Statistician‐computer. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Assessors were blinded but the blinding status of the participants was unknown. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Incomplete data addressed. |
| Selective reporting (reporting bias) | Low risk | All outcomes from methods were reported. |
| ITT analysis? | High risk | No, per‐protocol analysis, although all randomised participants followed their intervention. |
| Baseline similarity? | High risk | No tests performed. They seemed very different. |
| Co‐interventions avoided or similar? | Unclear risk | Not described. |
| Compliance? | Unclear risk | 2/7 actually received interventions and referrals. |
| Similar follow‐up time? | High risk | Quote: "Due to variation in local clinical processes, service size and workforce capacity, the recruitment process and timeframe differed in each of the three hospitals." |
Jong 2018.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Inclusion criteria: women aged 18–70 years with stage I–III breast cancer who were scheduled for (neo) adjuvant chemotherapy; understood and spoke Dutch language and had access to telephone and internet. Exclusion criteria: women who had received previous treatment with cytostatics; presence of metastasis or other malignancies; unresectable tumours; deafness; serious psychiatric or cognitive problems; participating in other yoga or stress‐reduction programmes at the time. |
|
| Interventions |
Intervention group (n = 29): entered the yoga programme 1–2 weeks before start of chemotherapy Setting: hospital Provider: yoga instructors Duration: 12 weeks, weekly sessions of 75 minutes Control group (n = 17): care as usual |
|
| Outcomes | RTW at 6 months | |
| Funding | Pink Ribbon (Grant: 2011,W016.C97). | |
| Objectives of the study | To investigate the effects of a yoga programme during chemotherapy on fatigue and QoL in women with breast cancer when added to care as usual compared with care as usual only. | |
| Country | Netherlands | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised using separate randomisation lists for each study centre, as generated by the computerised Random Allocation Software Program with a random block size of 10 to guarantee balanced allocation. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Participants, yoga instructors and assessors were not blinded because the intervention encompassed yoga interventions. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts addressed. |
| Selective reporting (reporting bias) | Low risk | All outcomes from methods were reported. |
| ITT analysis? | Unclear risk | Stated as per‐protocol analysis, but all analyses were on the numbers that were randomised. |
| Baseline similarity? | Unclear risk | Unknown for subgroup who were employed at baseline. |
| Co‐interventions avoided or similar? | Unclear risk | Not reported. |
| Compliance? | Unclear risk | Not all women followed all sessions. |
| Similar follow‐up time? | Low risk | Same measurement points. |
Lepore 2003.
| Study characteristics | ||
| Methods | RCT Setting: hospital |
|
| Participants | Inclusion criteria: men with localised prostate cancer; no history of other cancer; primary residence within 1 hour's drive from hospital; non‐metastatic disease Exclusion criteria: not reported |
|
| Interventions |
Intervention group 1 (n = 41): education only. 6 weekly 1‐hour lectures delivered by an expert on prostate cancer biology (oncologist), control physical adverse effects (urologist), nutrition (dietician), stress and coping (oncology nurse), relationships and sexuality (clinical psychologist), follow‐up care and future health concerns (urologist). Printed material. Intervention lasted 6 weeks Sessions: 6 sessions of 1 hour Providers: oncologist, urologist, dietician, oncology nurse, clinical psychologist. Setting: not reported Intervention group 2 (n = 43): education plus discussion. Lecture series and 45 additional minutes of group discussion (male clinical psychologist), discussion on how lecture topic was relevant to the group. Female family member's own discussion with female oncology nurse. Intervention lasted 6 weeks Sessions: 6 sessions of 1 hour 45 minutes Providers: oncologist, urologist, dietician, oncology nurse, clinical psychologist. Setting: not reported Control group (n = 40): nothing beyond standard medical care. |
|
| Outcomes |
Primary outcome measure (RTW outcomes) Employment status only for those working at baseline: number returned to work and in steady employment Registered by: participant at baseline (2 months post‐treatment), 2 weeks, 6 months, 12 months Secondary outcome measure (QoL outcomes) SF‐36 Registered by: participant at baseline (2 months post‐treatment), 2 weeks, 6 months, 12 months |
|
| Funding | National Institute of Health | |
| Objectives of the study | To compare QoL outcomes in participants receiving standard medical care (control) or 1 of 2 types of group education interventions | |
| Country | USA | |
| Notes | Results for working participants only in trial of 250 participants | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sealed envelopes. |
| Allocation concealment (selection bias) | Low risk | Randomisation was carried out by an interviewer who was blinded to experimental condition and did not participate in the interventions. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Interviewer blinded at baseline; participants were not informed about the hypothesis. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition was unrelated to experimental condition. Reasons for dropout were reported. |
| Selective reporting (reporting bias) | Low risk | All outcomes from methods were reported. |
| ITT analysis? | High risk | No ITT analyses conducted in work‐related outcomes. |
| Baseline similarity? | Unclear risk | Not reported for group of participants employed at baseline. |
| Co‐interventions avoided or similar? | Low risk | Co‐interventions avoided. |
| Compliance? | Unclear risk | No report of participants' compliance about the intervention. |
| Similar follow‐up time? | Low risk | Timing of outcomes same in each group. |
Maguire 1983.
| Study characteristics | ||
| Methods | RCT Setting: hospital |
|
| Participants | Inclusion criteria: women with breast cancer, mastectomy Exclusion criteria: not reported |
|
| Interventions |
Intervention group (n = 42): within a few days of surgery the nurse advised on exercise, looked at participant's scar, discussed how participant felt about losing a breast, demonstrated breast prosthesis. After discharge at home, the nurse examined arm movements, checked exercises, clarified how participant felt about scar, encouraged being open with her partner. Nurse encouraged RTW and becoming socially active. She followed the participant up every 2 months to monitor the progress until participant adapted well. Intervention lasted several months Sessions: ≥ 2 sessions Provider: oncology nurse Setting: hospital, home Control group (n = 46): care normally given by the surgical unit |
|
| Outcomes |
Primary outcome measure (RTW outcomes) Employment status rate: number returned to work Registered by: participant at 12–18 months postsurgery |
|
| Funding | Cancer Research Campaign and the North West Regional Health Authority | |
| Objectives of the study | To assess if a specialist nurse improved the physical and social recovery of participants after mastectomy | |
| Country | UK | |
| Notes | Results for working participants only in trial of 152 participants. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Half of the weeks were designed as counselling weeks and the other half as control weeks using a random number table. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasons for dropouts reported. |
| Selective reporting (reporting bias) | Low risk | All outcomes from methods were reported. |
| ITT analysis? | High risk | No ITT analysis. |
| Baseline similarity? | Low risk | Stated in the article that baseline characteristics were similar. |
| Co‐interventions avoided or similar? | Low risk | No co‐interventions or similar visits to social worker. |
| Compliance? | Low risk | Stated in the article that each participant in the counsel group was advised and counselled by the nurse. |
| Similar follow‐up time? | Unclear risk | Broad follow‐up measurement point: 12–18 months. |
Mijwel 2019.
| Study characteristics | ||
| Methods | 3‐armed RCT | |
| Participants | Women aged 18–70 years, diagnosed with I–IIIa stage breast cancer, planned to receive adjuvant chemotherapy | |
| Interventions |
Intervention groups (both): the exercise groups undertook supervised exercise sessions in an exercise clinic twice‐weekly for 16 weeks and the session duration was approximately 60 minutes. HIIT was combined either with resistance training or incorporated as part of an overall endurance training session (AT‐HIIT) in order to obtain similar exercise durations in both groups. Intervention group 1 (RT‐HIIT) (n = 62): high‐load resistance exercises targeting the major muscle groups consisting of 2 or 3 sets of 8–12 repetitions at an initial intensity of 70% of their estimated 1‐RM, progressing to 80% of 1‐RM. The RT‐HIIT sessions concluded with 3 × 3‐minute bouts of high‐intensity interval exercise on a cycle ergometer interspersed with 1 minute of recovery. Intervention group 2 (AT‐HIIT) (n = 59) initiated each session with 20 minutes of moderate intensity continuous aerobic exercise followed by the same high‐intensity interval exercise as in RT‐HIIT. Directly after the completion of the 16‐week exercise programme, participants in the exercise groups were offered a written, basic, physical activity on prescription by nurses involved in the exercise trial. During the follow‐up, in collaboration with a national gym organisation, Friskis & Svettis, these participants were offered a 1‐to‐1 exercise counselling session with a professional health educator which they were able to use at any time during the follow‐up period and were offered the opportunity to purchase gym cards at a reduced rate. Participants were also invited to an additional 2 or 3 motivational exercise sessions per year during the follow‐up period (7 in total during the follow‐up period between 2014 and 2017). Duration of entire intervention: twice‐weekly for 16 weeks, plus gym cards and motivational exercise sessions Number of sessions: 32 Duration of sessions: approximately 60 minutes Providers: exercise supervisors, professional health educator, nurses Locations: exercise clinic, gym Control group (n = 52): written information about physical activity at the initiation of the intervention period about exercise recommendations for people with cancer according to the American College of Sports Medicine guidelines. |
|
| Outcomes |
Primary outcome measure RTW, operationalised as the proportion of participants who had partially or completely returned to work at 12 months (i.e. from 100% sick leave to 50%, 25%, or 0% sick leave) Secondary outcome measure Overall QoL measured by the EORTC‐QLQ‐C30 at 12 months |
|
| Funding | Swedish foundations; Cancerfonden, CancerRehabFonden, The Swedish Society for Medical Research, as well as Radiumhemmets Research Funds. | |
| Objectives of the study | To investigate and compare the effects of the OptiTrain exercise interventions on the primary outcome: cancer‐related fatigue and secondary outcomes: health‐related QoL, symptoms, physiological outcomes, and RTW 12 months following the commencement of chemotherapy in women with breast cancer, i.e. 9 months after completion of the intervention. | |
| Country | Sweden | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly allocated by the Clinical Studies Unit at Radiumhemmet, Karolinska University Hospital (Stockholm, Sweden) to RT‐HIIT, AT‐HIIT or care as usual at a 1:1:1 ratio using a computer‐generated assignment program blinded to the research team. |
| Allocation concealment (selection bias) | Low risk | Participants were randomly allocated by the Clinical Studies Unit at Radiumhemmet, Karolinska University Hospital (Stockholm, Sweden) to RT‐HIIT, AT‐HIIT or care as usual at a 1:1:1 ratio using a computer‐generated assignment program blinded to the research team. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Participants, exercise supervisors and outcome assessors were not blinded to group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All dropouts addressed during the entire study. |
| Selective reporting (reporting bias) | Low risk | All outcomes from methods were reported. |
| ITT analysis? | Low risk | ITT was possible without imputing data. |
| Baseline similarity? | Low risk | There were no differences between intervention groups regarding participant characteristics. |
| Co‐interventions avoided or similar? | Unclear risk | Doubt about how "opportunity to purchase gym cards at a reduced rate" also financially stimulated to exercise after 16‐week training programme. |
| Compliance? | Low risk | Attendance to the 16‐week exercise intervention for participants in the RT‐HIIT group was 68% and AT‐HIIT group was 63%. |
| Similar follow‐up time? | Low risk | Same measurement points. |
Purcell 2011.
| Study characteristics | ||
| Methods | Factorial RCT Setting: hospital |
|
| Participants | Inclusion criteria: people undergoing outpatient radiotherapy treatment; aged ≥ 18 years; booked for ≥ 20 days of radiotherapy for cancer treatment Exclusion criteria: low performance status (Karnofsky level < 60/100 requiring at least considerable assistance and frequent medical care); undergoing treatment with palliative intent; undergoing other concurrent cancer treatments (e.g. chemotherapy); involvement in other programmes or research specifically targeting fatigue; inability to complete questionnaires due to cognitive or literacy levels |
|
| Interventions | RFES programme, CAN‐FIT, aimed to reduce participant's level of fatigue by employing self‐care behaviours. Programme components included a PowerPoint presentation, and a participant handbook, a goal‐setting sheet and progress diary. Session content addressed radiotherapy and its processes, potential treatment adverse effects including fatigue, and behavioural strategies to reduce fatigue including activity modification, participation in exercise/activity, maintaining weight/nutrition, sleep hygiene tips and relaxation techniques. 2 follow‐up telephone calls using a structured script were provided 2 and 4 weeks after each education session to reinforce information. Session duration: 60 minutes Provider: multidisciplinary team Setting: via hospital Participants recruited to the study were randomly allocated into: 1. pre‐ and post‐RFES sessions; 2. pre‐RFES session; 3. post‐RFES session; 4. no RFES session (i.e. standard care only). Post‐RFES vs no post‐RFES Post‐RFES (n = 43): RFES delivered 1–2 weeks after the completion of radiotherapy Control (n = 48): a 1‐page flyer provided with generic information about fatigue Pre‐RFES or post‐RFES intervention vs no RFES Pre‐RFES or post‐RFES (n = 21): RFES delivered 1 week prior to radiotherapy planning or 1–2 weeks after the completion of radiotherapy Control/standard care (n = 24): a 1‐page flyer provided with generic information about fatigue *RTW outcomes for 'Pre‐REFS vs no pre‐REFS' were reported as the number of hours of paid work and not the number of people who participated in paid work. |
|
| Outcomes | Employment status: full time, part‐time, casual, not working (baseline) Outcome measure: Health and Labour questionnaire: participation in paid work (yes/no) Data collected at 6‐week follow‐up |
|
| Funding | Queensland Health Cancer Control Team, Queensland Health, Health Practitioner Research Scheme, Princess Alexandria Hospital Cancer Collaborative Group: indirect funding and no direct involvement | |
| Objectives of the study | To assess if providing pre‐REFS, post‐REFS, or pre‐ and post‐REFS reduces the severity of fatigue of people with cancer experienced 6 weeks after radiotherapy | |
| Country | Australia | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated. |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelope. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Assessors were blinded but the blinding status of the participants was unknown. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported. |
| Selective reporting (reporting bias) | Low risk | All outcomes from methods were reported. |
| ITT analysis? | Unclear risk | Not reported. |
| Baseline similarity? | High risk | Not on education. |
| Co‐interventions avoided or similar? | Low risk | Similar. |
| Compliance? | Unclear risk | Mainly self‐management intervention, unclear whether participants completed or not. |
| Similar follow‐up time? | Low risk | 6 weeks' follow‐up. |
Singer 2018.
| Study characteristics | ||
| Methods | Cluster‐RCT, randomisation of wards | |
| Participants | Inclusion criteria: people with head and neck, breast, urinary, female genital and other cancers; aged ≥ 18 years and written informed consent Inclusion criteria wards: people with cancer in the wards were treated if standard psycho‐social care had been established, i.e. a psycho‐oncologist or social worker was called if a doctor or nurse considered this was needed for a patient Exclusion criteria: insufficient command of German language and when the cancer diagnosis was not confirmed after t1 Exclusion criteria wards: wards with a liaison service, i.e. where a psycho‐oncologist visited every participant |
|
| Interventions |
Intervention group (n = 115): stepped care model was not a single, isolated intervention but a complex care model. It had 3 steps. Step 1: each participant was screened for distress (including depression, anxiety, pain, fatigue and financial difficulties). The results were electronically computed, graphically visualised and fed back to the physician in charge. Step 2: the doctor talked to participants with severe distress about the screening results, and together, the doctor and participant decided on further support. We assumed that, for some participants, this step might have been enough to reduce their distress. All doctors had been trained in person how to read and interpret screening results and how to incorporate them into their consultations. They also learnt to acknowledge participants' preferences regarding psychosocial support, and they had been informed about the hospital's services. Step 3: if the participant and doctor decided that further support was necessary, the participant was referred to the hospital's services. If financial difficulties were also present, a social worker was involved. The support provided by the social workers was always tailored to the needs of each individual participant. Frequently, they provide information about the possibility of medical and vocational rehabilitation and support in completing the relevant application forms. If the participant was experiencing urgent financial hardship, they could help the participant obtain extra money from the German Cancer Aid or local charities. If the participant was at risk of becoming disabled, they informed the participant how a disability certificate could be obtained, and what financial and practical advantages this may imply. They prepared for the participant's discharge from the hospital by ensuring he or she could be cared for at home, if needed. They also provided information about outpatient social services for further support, e.g. regarding early retirement and childcare. Setting: hospital Providers: doctors, social workers Control group (n = 78): standard care |
|
| Outcomes |
Primary outcome RTW at 6 months |
|
| Funding | German Federal Ministry of Health within the framework Research within the German National Cancer Plan (no. NKP‐332‐026). | |
| Objectives of the study | To examine whether multidisciplinary stepped psycho‐social care decreases financial problems and improves RTW in people with cancer | |
| Country | Germany | |
| Notes | Specific domains of risk of bias assessment for cluster‐RCT (Higgins 2011)
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Assessors were blinded but the blinding status of the participants is unknown. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described for the subgroup analysis of participants employed at baseline. |
| Selective reporting (reporting bias) | Low risk | All outcomes from methods were reported. |
| ITT analysis? | Unclear risk | Not stated. |
| Baseline similarity? | High risk | In the subgroup analyses, groups differed in age and sex (50% vs 28% female). |
| Co‐interventions avoided or similar? | Unclear risk | Not reported. |
| Compliance? | Unclear risk | Not described what interventions were actually carried out and what participants received. |
| Similar follow‐up time? | Low risk | Same measurement points. |
Tamminga 2013.
| Study characteristics | ||
| Methods | RCT Setting: hospital |
|
| Participants | Inclusion criteria: women with cancer (64% breast cancer, 23% cervix); aged 18–60 years; treated with curative intent and expected 1‐year survival rate of approximately 80%; had paid work; were on sick leave Exclusion criteria: not sufficiently able to speak, read or write Dutch; severe mental illness; other severe comorbidity; primary diagnosis of cancer > 2 months previously |
|
| Interventions |
Intervention group (n = 57): delivering patient education and support at the hospital, as part of usual psycho‐oncological care; improving communication between the treating physician and the occupational physician, and drawing up a concrete and gradual RTW plan in collaboration with the participant, occupational physician and employer. Session duration: 4 meetings lasting 15 minutes; spread across a maximum of 14 months Provider: oncology nurse or medical social worker Setting: hospital Control group (n = 63): care as usual |
|
| Outcomes |
Primary outcomemeasure RTW rate at 12‐months follow‐up of participants alive and with a life expectancy of more than a few months at 12 months. Same for those who died within the follow‐up period or those with a life expectancy of only a few months. Number of calendar days between the first day of sickness and the first day at work (either full‐time or part‐time) that was sustained for ≥ 4 weeks. Registered by: participant at baseline, and 6 and 12 months of follow‐up Secondary outcome measure (QoL outcomes) SF‐36 |
|
| Funding | Stichting Instituut GAK and part of the research programme Pathways to work. | |
| Objectives of the study | To determine the effect of a hospital‐based work support intervention for people with cancer on RTW and QoL | |
| Country | Netherlands | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified, computer generated. |
| Allocation concealment (selection bias) | Low risk | Computer generated for each participant after inclusion. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Assessors were not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unable to determine numbers of dropout, incomplete data not reported. |
| Selective reporting (reporting bias) | Low risk | All outcomes from methods were reported. |
| ITT analysis? | Low risk | Yes. |
| Baseline similarity? | Low risk | Tested, no differences found. |
| Co‐interventions avoided or similar? | Unclear risk | Not described. |
| Compliance? | Low risk | 8/68 participants did not receive the intervention. |
| Similar follow‐up time? | Low risk | 12 months in both groups. |
van Waart 2015.
| Study characteristics | ||
| Methods | 3‐arm RCT | |
| Participants | People with breast cancer scheduled to undergo adjuvant chemotherapy Exclusion criteria: serious orthopaedic, cardiovascular, or cardiopulmonary conditions; had malnutrition; had serious psychiatric or cognitive problems; did not have basic fluency in Dutch |
|
| Interventions |
Intervention group 1 (n = 51): Onco‐Move was a home‐based, low‐intensity, individualised, self‐managed physical activity programme, as proposed by Mock, to which behavioural reinforcement techniques were added. These comprised written information that was tailored to the individual's preparedness to exercise according to the Transtheoretical model, and an activity diary that was discussed at each chemotherapy cycle. Specially trained nurses encouraged participants to engage in ≥ 30 minutes of physical activity per day, 5 days per week, with an intensity level of 12–14 on the Borg Scale of perceived exertion. Intervention group 2 (n = 53): OnTrack was a moderate‐ to high‐intensity, combined resistance and aerobic exercise programme and was supervised by specially trained physiotherapists. The participants attended 2 sessions per week. 6 large muscle groups were trained for 20 minutes per session, with 2 series of 8 repetitions at 80% of the 1‐RM. 1‐RM testing was repeated every 3 weeks. Each session incorporated 30 minutes of aerobic exercises, with an intensity of 50–80% of the maximal workload as estimated by the Steep Ramp Test. The intensity was adjusted using the Borg Scale, with a threshold < 12 for increase and > 16 for decrease of intensity. Participants in this group were also encouraged to be physically active 5 days each week for 30 minutes per session and to keep an activity diary. Duration of entire intervention: both interventions started with the first cycle of chemotherapy and continued until 3 weeks after the last cycle. Number of sessions: group 1: 5 days a week; group 2: 2 days a week Duration of sessions: group 1: 30 minutes; group 2: 50 minutes Provider: group 2: physiotherapist Location: group 1: home; group 2: not reported Control group (n = 54): care as usual varied according to hospital guidelines and preferences, but did not involve routine exercise. |
|
| Outcomes | RTW at 6 months | |
| Funding | Alpe d'Huzes/Dutch Cancer Society Grant No. ALPE‐2009‐4299, the CZ Fund, Zilveren Kruis Achmea, and the Comprehensive Cancer Centre of the Netherlands. | |
| Objectives of the study | To evaluate the effectiveness of a home‐based, low‐intensity physical activity programme (Onco‐Move) and a supervised, moderate‐ to high‐intensity, combined resistance and aerobic exercise programme (OnTrack) in maintaining or enhancing physical fitness and minimising fatigue in people undergoing adjuvant chemotherapy. | |
| Country | Netherlands | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Most likely participants, physiotherapists and assessors not blinded, given the nature of the study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All dropouts described. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes from methods were reported. However, RTW was not a planned outcome in trial protocol. |
| ITT analysis? | Low risk | All ITT analysis. |
| Baseline similarity? | Low risk | Quote: "Baseline characteristics were balanced across groups (Table 2)." |
| Co‐interventions avoided or similar? | Unclear risk | Not described. Dose adjustments but what other interventions happened in the groups was not reported (e.g. other forms of exercise). |
| Compliance? | Unclear risk | No clear rules for compliance set. Considerable percentage of participants did not have the entire intervention. |
| Similar follow‐up time? | Low risk | 6 months was the same for all groups. |
Zaman 2021.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Inclusion criteria: diagnosed with a primary gastrointestinal cancer (i.e. a malignancies in the digestive tract system, ranging from the oesophagus to the colorectum) that was treatable with curative intent (all treatments were included); aged 18–63 years; in paid work (including temporary and flexible contracts and self‐employed) at time of diagnosis Exclusion criteria: had a severe mental disorder or other severe comorbidities (observed by the nurses who included eligible participants, not from the self‐reported patient questionnaire) |
|
| Interventions |
Intervention group (n = 36): tailored work‐related support intervention encompassed 3 individual meetings of psychosocial work‐related support. The first was to inform participants about work during and after treatment, to identify any work‐related problems they might already have and to make a plan for their RTW or to stay in work. The first meeting was scheduled before the treatment began, the second a maximum of 6 months after the first and the third (if necessary) a maximum of 9 months after the first. Each meeting lasted approximately 30 minutes. The first and second meetings needed to be face‐to‐face as per protocol; the third could be conducted by telephone. Topics discussed were the importance of work, contact with the work environment, transparency about their diagnosis with employer or colleagues (or both), and the process of reporting sick under Dutch law. The intervention itself was split into 3 types of work‐related support (A, B and C); this was in order to respond to the participant's individual needs, since work‐related problems can differ in severity. Based on contributing factors to such problems as described in a decision diagram. For example, in support A, fatigue, pain and lack of support from family and friends; in support B, lack of support in work environment, neuropsychological problems; and in support C, a combination of factors. These factors were assessed in the participant's baseline questionnaire (T0). Based on the answers and the decision diagram, the researcher referred the participant to the tailored type of support, of A, B or C. Within each of these, the type of healthcare professional assigned to provide supportive work‐related care was tailored to the severity of the participant's work‐related problems and the healthcare professional considered the type of work of the participant. In support type A, this was an oncology nurse; in type B, an Oncological Occupational Physician; and in type C, a multidisciplinary team (including at least an oncology nurse, the treating physician, and an Oncological Occupational Physician). Setting: hospital Duration: 3 sessions of 30 minutes, maximum 9 months Provider: the type of healthcare professional assigned to provide supportive work‐related care was tailored to the severity of the participant's work‐related problems and the healthcare professional considered the type of work of the participant. Control group (n = 34): care as usual |
|
| Outcomes |
Primary outcome RTW at 12 months Secondary outcome QoL |
|
| Funding | Dutch Cancer Society | |
| Objectives of the study | To study the effectiveness on RTW of an early tailored work‐related support intervention in participants diagnosed with curative gastrointestinal cancer | |
| Country | Netherlands | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Using a computerised web‐based randomisation program, ALEA, this randomisation was conducted centrally by the research team for all participating hospitals. Used the biased‐coin principle, with a threshold of 2. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Participants, healthcare professionals and researchers were not blinded to the group assignment. 1 researcher contacted the healthcare professional and the participant after randomisation, so that the first meeting could be scheduled (when participant was randomised to the intervention group). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcomes addressed. |
| Selective reporting (reporting bias) | Low risk | All outcomes from methods were reported. |
| ITT analysis? | Low risk | ITT performed. |
| Baseline similarity? | Low risk | Similar. |
| Co‐interventions avoided or similar? | Low risk | Similar. |
| Compliance? | Low risk | Participants complied. |
| Similar follow‐up time? | Low risk | Same follow‐up time. |
1‐RM: one repetition maximum; AT‐HIIT: aerobic training – high‐intensity interval training; EORTC‐QLQ‐C30: European Organisation for Research and Treatment of Cancer Quality of Life Core 30; FACT‐B: Functional Assessment of Cancer Therapy – Breast; HIIT: high‐intensity interval training; ITT: intention to treat; MOS‐SF: Medical Outcomes Studies‐Short Form; n: number of participants; QoL: quality of life; RCT: randomised controlled trial; RFES: radiotherapy fatigue education and support; RT‐HIIT: resistance training – high‐intensity interval training; RTW: return‐to‐work; SF‐36: 36‐item Short Form; VAS: visual analogue scale.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ackerstaff 2009 | Medical intervention |
| Adamsen 2009 | No RTW outcomes measured. |
| Berglund 2003 | No RTW outcomes measured. |
| Bertheussen 2012 | No control group. |
| Bird 2010 | No RTW outcomes measured. |
| Bloom 2008 | No RTW outcomes measured. |
| Borget 2007 | Not an RCT. |
| Brocki 2014 | No RTW outcomes measured. |
| Broemer 2019 | No RTW outcomes measured. |
| Budin 2008 | Outcome was not sick leave or RTW but vocational environment scale. |
| Burak 2002 | Outcome was not sick leave or RTW but return to normal activity. |
| Böttcher 2013 | Not an RCT. |
| Bürger 2011 | No RTW outcomes measured. |
| Cadmus‐Bertram 2013 | No RTW outcomes measured. |
| Cain 1986 | Outcome was not sick leave or RTW but vocational environment scale. |
| Capone 1980 | Not an RCT. |
| Chan 2005 | No RTW outcomes measured. |
| Cherrier 2013 | No RTW outcomes measured. |
| Cho 2006 | No RTW outcomes measured. |
| Egan 2013 | Review. |
| Emmanouilidis 2009 | Medical intervention |
| Eyigor 2010 | No RTW outcomes measured. |
| Fassoulaki 2000 | No RTW outcomes measured. |
| Fauser 2019 | Not all participants employed at baseline:89.7% of intervention group and 77.0% of control group was on sick leave or unemployed at baseline. |
| Fors 2011 | Review. |
| Friedrichs 2010 | Medical intervention |
| Gordon 1980 | Not a randomised study. |
| Gordon 2005 | No RTW outcomes measured. |
| Greco 2021 | Ineligible population. |
| Greer 1992 | Outcome was not sick leave or RTW but vocational environment scale. |
| Griffith 2009 | No RTW outcomes measured. |
| Gyllensten 2019 | Medical intervention. |
| Hansen 2020 | No RTW outcomes measured. |
| Harrison‐Paul 2006 | No RTW outcomes measured. |
| Hartmann 2007 | No RTW outcomes measured. |
| Hegel 2011 | No RTW outcomes measured. |
| Heim 2007 | No RTW outcomes measured. |
| Hillman 1998 | Medical intervention. |
| Huri 2015 | No RTW outcomes measured. |
| Høybye 2010 | No RTW outcomes measured. |
| Ibrahim 2017 | Not all participants employed at baseline. |
| Janson 2005 | No RTW outcomes measured for intervention. |
| Ji 2019 | No RTW outcomes measured. |
| Jiang 2009 | Outcome was not sick leave or RTW but includes normal routine activity. |
| Johansson 2020 | Not all participants employed at baseline. |
| Johnsson 2007 | Medical intervention. |
| Jones 2005 | No RTW outcomes measured. |
| Jørgensen 2009 | No RTW outcomes measured. |
| Kornblith 2009 | Medical intervention. |
| Korstjens 2008 | No RTW outcomes measured. |
| Kwiatkowski 2014 | No RTW outcomes measured. |
| Lauchlan 2011 | No RTW outcomes measured. |
| Lee 1992 | Medical intervention. |
| Lee 2009 | No cancer. |
| Luo 2018 | Not all participants employed at baseline |
| Madore 2014 | No RTW outcomes measured. |
| May 2009 | No RTW outcomes measured. |
| McNeely 2008 | No RTW outcomes measured. |
| Meneses 2007 | No RTW outcomes measured. |
| Meraviglia 2013 | No RTW outcomes measured. |
| Mock 1994 | No RTW outcomes measured. |
| Mourgues 2014 | No RTW outcomes measured. |
| Norager 2006 | No RTW outcomes measured. |
| Norouzi 2017 | No RTW outcomes measured. |
| Nowrouzi 2009 | No intervention. |
| O'Brien 2014 | No RTW outcomes measured. |
| Paolucci 2019 | Not all participants employed at baseline |
| Persson 2010 | No cancer. |
| Poppelreuter 2009 | No RTW outcomes measured. |
| Rogers 2009 | RTW outcome was mean change in sick leave days. |
| Rotstein 1989 | Only half of the participants were employed at baseline. |
| Salonen 2011 | No RTW outcomes measured. |
| Scherz 2017 | Not all participants employed at baseline. |
| Scott 2013 | Ineligible design. |
| Seibaek 2009 | No RTW outcomes measured. |
| Seiler 2005 | Outcome was not sick leave or RTW but return to normal daily activity. |
| Semple 2009 | No RTW outcomes measured. |
| Shelton 2009 | No RTW outcomes measured. |
| Sherer 1997 | Ineligible design. |
| Sherman 2010 | No RTW outcomes measured. |
| Sherman 2012 | No RTW outcomes measured. |
| Shimada 2007 | No RTW outcomes measured. |
| Silver 2013 | Review. |
| van Egmond 2016 | Not all participants employed at baseline. |
| van Waart 2018 | No RTW outcomes measured. |
| Vos 2006 | No RTW outcomes measured. |
| Wenzel 1995 | Outcome was not sick leave or RTW but vocational environment scale. |
| Wienert 2019 | No RTW outcomes measured. |
| Wiggins 2009 | No RTW outcomes measured. |
| Yang 2020 | No RTW outcomes measured. |
| Yun 2017 | No RTW outcomes measured. |
RCT: randomised controlled trial; RTW: return to work.
Characteristics of ongoing studies [ordered by study ID]
NCT01780064.
| Study name | Psychosocial support to facilitate the return to employment of women with breast cancer (APAPI) |
| Methods | RCT |
| Participants | 128 participants Women aged ≥ 18 years with work at the time of diagnosis; presenting a unilateral breast cancer exclusively local extension; having received surgery; reporting of adjuvant chemotherapy (with or without trastuzumab); reporting for radiotherapy or not (if radiotherapy, it will be done on site investigator); in work at time of diagnosis (employees, traders and professionals), participant affiliated to a social security scheme; consent signed by the participant before the implementation of any specific procedure to study |
| Interventions | Intervention group: interviews with a psychologist (at cure 1 of chemotherapy treatment, at cure 6 of chemotherapy treatment, at last radiotherapy session and 3 months after the end of radiotherapy) and questionnaires Control group: routine monitoring with questionnaires |
| Outcomes | Comparison of the effect of early individualised psychosocial assets versus a standard support on social inequalities in the rate of return to work (follow‐up 24 months) |
| Starting date | February 2013 |
| Contact information | |
| Notes |
NCT03666936.
| Study name | Facilitate the return to work of cancer survivors |
| Methods | RCT |
| Participants | 30 adults with cancer diagnosis and employed at the time of diagnosis |
| Interventions | Cancer survivors enrolled in the study could receive support from the occupational therapist or from the social care professional (or both) to overcome the difficulties encounter at work or to facilitate work reintegration. |
| Outcomes | Ratio of participants who have returned to work/not returned to work |
| Starting date | 1 May 2018 |
| Contact information | stefania.costi@unimore.it |
| Notes |
NCT04214912.
| Study name | Personalized survivor care plan for oral cancer patients – effects on physical‐psychological functions and return‐to‐work |
| Methods | RCT |
| Participants | 300 people with newly diagnosed oral cancer with surgery, who are at work in time of diagnosis |
| Interventions | Personalized Survivor Care Plan (PSCP) will deliver based on participants' distress, concerns and care needs in each interview or assessment (or both). For the factors or concerns about RTW assessment, study authors will develop and test a modified instrument for the study purpose. For each assessment, the computer‐assisted assessment will help the oral cancer educator to immediately determine the participants' care distress and care needs. It will provide the direction for caring of their personalised distress, concerns and needs. |
| Outcomes | RTW assessed using valid assessment tools |
| Starting date | 1 December 2017 |
| Contact information | laiyhwk@ntu.edu.tw |
| Notes |
NCT04322695.
| Study name | A rehabilitation education care program on return to work among head and neck cancer survivors |
| Methods | RCT |
| Participants | 80 people with head and neck cancer |
| Interventions | Behavioural: Rehabilitation Education Care Program containing the following domains: assessment and detection of disability; home exercise; activities to improve mobility; dietary management; patient education; and vocational counselling. |
| Outcomes | Return to Work Barrier Scale (RTWBS) |
| Starting date | 30 March 2020 |
| Contact information | |
| Notes |
NCT04469205.
| Study name | Study to assess the impact of personalized coaching on the time period and quality of return to work after breast cancer (OPTICOACH) |
| Methods | RCT |
| Participants | 200 people with breast cancer |
| Interventions | Intervention group will receive RTW coaching sessions. The intervention consists of 3 individual coaching sessions with a certified professional coach. This personalised accompaniment will complete the standard accompaniment offered to all participants. |
| Outcomes | RTW rate |
| Starting date | September 2020 |
| Contact information | delphine.hequet@curie.fr |
| Notes |
NCT04846972.
| Study name | Facilitate and sustain return to work after breast cancer (FASTRACS‐RCT) |
| Methods | Realist RCT |
| Participants | Women aged 18–55 years with first invasive non‐metastatic breast cancer (treated by surgery and chemotherapy in an adjuvant or neoadjuvant situation) and in paid employment at the time of diagnosis. |
| Interventions | Behavioural: FASTRACS intervention Intervention (4 steps):
|
| Outcomes | Sustained RTW |
| Starting date | |
| Contact information | jean‐baptiste.fassier@univ‐lyon1.fr |
| Notes |
Sheppard 2019.
| Study name | Beyond Cancer |
| Methods | Prospective feasibility design allows determination of change in primary (work status) as well as secondary outcome measures work capacity and perceived support at work |
| Participants | Breast cancer survivors of working age, unable to work in their prediagnosis capacity for > 3 months, their employers and a historical usual care group |
| Interventions | Key intervention elements: an evidence‐based biopsychosocial assessment and health coaching programme, employer education and support, and RTW planning and monitoring |
| Outcomes | Return to social function, including work |
| Starting date | Not reported |
| Contact information | Not reported |
| Notes |
Touillaud 2021.
| Study name | DISCO trial |
| Methods | 2 × 2 factorial, multicentre, phase III RCT |
| Participants | 432 women diagnosed with primary localised invasive breast carcinoma and eligible for adjuvant chemotherapy, hormonotherapy, radiotherapy, or a combination of these. Gave written informed consent |
| Interventions | 4 arms Web‐based connected device (evolving target number of daily steps and an individualised, semisupervised, adaptive programme of 2 walking and 1 muscle strengthening sessions per week in autonomy) Therapeutic patient education (1 educational diagnosis, 2 collective educational sessions, 1 evaluation) Combination of both interventions Control All participants will receive the international physical activity recommendations |
| Outcomes | Assessments (baseline, 6 and 12 months) will include physical fitness tests, anthropometrics measures, body composition (computer tomography scan, bioelectrical impedance), self‐administered questionnaires (physical activity profile (Recent Physical Activity Questionnaire), quality of life (European Organization for Research and Treatment of Cancer Quality‐Of‐Life Questionnaire‐30, EQ‐5D‐5L), fatigue (Piper Fatigue Scale‐12), social deprivation (Evaluation of Deprivation and Inequalities in Health Examination Centres), lifestyle, physical activity barriers, occupational status) and biological parameters (blood draw) |
| Starting date | Not reported |
| Contact information | Not reported |
| Notes |
Zegers 2021.
| Study name | STEPS |
| Methods | Multicentre RCT |
| Participants | 236 working‐age cancer survivors with an employment contract |
| Interventions | Usual care group or an intervention group receiving a multidisciplinary rehabilitation programme, combining occupational therapy facilitating work retention (e.g. energy management and self‐efficacy training) and reintegration consultation addressing work‐related issues (e.g. RTW planning and discussing workplace or task modifications with the supervisor). |
| Outcomes | Questionnaires at baseline, and after 6 and 12 months, assessing work‐ and health‐related outcomes |
| Starting date | Not reported |
| Contact information | Not reported |
| Notes |
RCT: randomised controlled trial; RTW: return to work.
Differences between protocol and review
2024 update
For this updated review version, we made several amendments from the protocol (de Boer 2009b).
We excluded seven RCTs from the first two versions of this review (de Boer 2011; de Boer 2015), because in this update we focussed on interventions that can be launched to enhance RTW in people with cancer, regardless of medical treatment. These seven excluded RCTs concerned solely medical and pharmaceutical interventions.
Accordingly, the objectives of this review version have been changed to: To evaluate the effectiveness of non‐medical interventions aimed at enhancing return to work (RTW) in people with cancer compared to alternative programmes including usual care or no intervention. See Objectives.
We did not use the OSH‐ROM database because it is not supported by our organisation.
We did not used the DARE database because no new records have been published to DARE since 2015.
We visually inspected forest plots for similarity of point estimates to indicate statistical heterogeneity. We assessed publication bias by visually inspecting funnel plot asymmetry when at least 10 RCTs were available in the meta‐analysis, because when there are fewer studies the power of the tests is low. This is in line with the Cochrane Handbook for Systematic Reviews of Interventions. However, this is a deviation from the protocol when we stated a minimum of five studies was needed for a funnel plot test (de Boer 2009b).
We did not pool the QoL outcomes of Purcell 2011, because the outcomes were reported as medians and IQRs.
We excluded Rogers 2009, which was included in previous review versions (de Boer 2011; de Boer 2015), because the RTW outcomes were reported as a mean change in the number of sick leave days instead of the number of sick leave days.
For cluster‐RCTs, we assessed five additional domains of risk of bias (Richardson 2016): recruitment bias (differential participant recruitment in clusters for different interventions); baseline imbalance; loss of clusters; incorrect analysis; and comparability with individually randomised trials.
Note: in the next update of this review, we will assess risk of bias in included studies using the Cochrane RoB 2 tool as per guidance in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2023).
2015 update
For the 2015 updated review version (de Boer 2015), we made several amendments.
We excluded non‐randomised studies because it was clear that randomised studies are feasible and have been conducted. This proved that our earlier understanding was mistaken in that it would be difficult to randomise in this context. This had been the main reason for including non‐randomised studies.
Work retention was not considered a primary outcome because the aim of this review was to evaluate the effectiveness of interventions aimed at enhancing RTW not on work retention. The primary outcome was instead: RTW measured as event data such as the number of people who RTW.
We added physical interventions to the type of interventions included in this review due to clinical relevance. They were not listed in the protocol.
Subgroup analyses according to setting and quality of study were no longer planned.
Contributions of authors
Update 2024
AdB is the main review author. She designed and conducted the search strategy, ran the analyses, wrote the draft of the updated review and revised it against editorial and peer‐review comments.
ST checked studies for eligibility, conducted data extraction and assessed included studies for risk of bias. She provided feedback during the review development process.
JB conducted the searches with the clinical librarian, checked studies for eligibility, conducted data extraction, assessed included studies for risk of bias, performed GRADE data synthesis and provided feedback during the review development process.
JH checked studies for eligibility, conducted data extraction, assessed included studies for risk of bias and provided feedback during the review development process.
Original review and 2015 update
AdB was the main review author and was involved with all aspects of the protocol. She wrote the protocol and the review. She designed and conducted the search strategy.
TT, ST, MF‐D, MF and JV contributed to the draft version of the protocol and review.
AdB and TT screened eligible studies, conducted the quality assessment of eligible studies and extracted data from the included studies.
JV and AdB conducted the data synthesis.
JV performed GRADE data synthesis.
Sources of support
Internal sources
-
Coronel Institute of Occupational Health, Department of Public and Occupational Health, Netherlands
Personnel
-
Cochrane Occupational Safety and Health Review Group, Finland
Personnel
-
University of Birmingham, UK
Personnel
-
Uniformed Services University of the Health Sciences, USA
Personnel
-
Centre for Workforce Effectiveness, The Work Foundation, London, UK
Personnel
External sources
-
SIG Pathways to Work. University Research Programme, Netherlands
Personnel
-
Finnish Work Environment Fund, Finland
Personnel
-
COST Action CANWON IS1211, Other
Meetings
Declarations of interest
AdB: no relevant interests; involved in conducting Tamminga 2013 (GAK (social insurance) Foundation), Greidanus 2021 (Dutch Cancer Society), Zaman 2021 (Dutch Cancer Society); Tamminga 2013: co‐author, academic medical hospital Amsterdam; Greidanus 2021: co‐author, academic medical hospital Amsterdam; Zaman 2021: co‐author, academic medical hospital Amsterdam.
ST: no relevant interests; involved in conducting Tamminga 2013 (GAK (social insurance) Foundation), Greidanus 2021 (Dutch Cancer Society); Tamminga 2013: co‐author; academic medical hospital Amsterdam; Greidanus 2021: co‐author, academic medical hospital Amsterdam.
JB: Knowledge Institute of the Dutch Association of Medical Specialists (Senior Advisor); former Managing Editor of Cochrane Work, Amsterdam UMC, Amsterdam, Netherlands.
JH: no relevant interests; currently Coordinating Editor of Cochrane Work, Amsterdam UMC, Amsterdam, Netherlands.
JB and JH excluded themselves entirely from the editorial process to ensure independence. Review authors were not involved in the study selection, data extraction, risk of bias or GRADE assessment for studies where they were also involved as study co‐authors.
Edited (no change to conclusions)
References
References to studies included in this review
Berglund 1994 {published data only}
- Berglund G, Bolund C, Gustafsson UL, Sjödén P-O. One-year follow-up of the 'Starting Again' group rehabilitation programme for cancer patients. European Journal of Cancer 1994;30A(12):1744-51. [DOI] [PubMed] [Google Scholar]
Burgio 2006 {published data only}
- Burgio KL, Goode PS, Urban DA, Umlauf MG, Locher JL, Bueschen A, et al. Preoperative biofeedback assisted behavioral training to decrease post-prostatectomy incontinence: a randomized, controlled study. Journal of Urology 2006;175(1):196-201. [DOI] [PubMed] [Google Scholar]
Greidanus 2021 {published data only}
- Greidanus MA, Rijk AE, Boer AG, Bos ME, Plaisier PW, Smeenk RM, et al. A randomised feasibility trial of an employer-based intervention for enhancing successful return to work of cancer survivors (MiLES intervention). BMC Public Health 2021;21(1):1433. [PMID: 10.1186/s12889-021-11357-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
Grunfeld 2019 {published data only}
- Grunfeld EA, Schumacher L, Armaou M, Woods PL, Rolf P, Sutton AJ, et al. Feasibility randomised controlled trial of a guided workbook intervention to support work-related goals among cancer survivors in the UK. BMJ Open 2019;9(1):e022746. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hass 2017 {published data only}
- Hass HG, Muthny F, Stepien J, Lerch J, Marwitz C, Schröck R, et al. Effects of a phone-based follow-up care after inpatient rehabilitation for breast cancer patients – a randomized controlled trial [Effekte der telefonischen Nachsorge in der onkologischen Rehabilitation nach Brustkrebs – Ergebnisse einer randomisierten Studie]. Rehabilitation 2017;56(3):189-97. [DOI] [PubMed] [Google Scholar]
Hubbard 2013 {published data only}
- Hubbard G, Gray NM, Ayansina D, Evans JM, Kyle RG. Case management vocational rehabilitation for women with breast cancer after surgery: a feasibility study incorporating a pilot randomised controlled trial. Trials 2013;14:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jong 2018 {published and unpublished data}
- Jong MC, Boers I, Schouten van der Velden AP, Meij SV, Göker E, Timmer-Bonte AN, et al. A randomized study of yoga for fatigue and quality of life in women with breast cancer undergoing (neo) adjuvant chemotherapy. Journal of Alternative and Complementary Medicine 2018;24(9-10):942-53. [DOI] [PubMed] [Google Scholar]
Lepore 2003 {published data only}
- Lepore SJ, Helgeson VS, Eton DT, Schulz R. Improving quality of life in men with prostate cancer: a randomized controlled trial of group education interventions. Health Psychology 2003;22(5):443-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Maguire 1983 {published data only}
- Maguire P, Brooke M, Tait A, Thomas C, Sellwood R. The effect of counselling on physical disability and social recovery after mastectomy. Clinical Oncology 1983;9(4):319-24. [PubMed] [Google Scholar]
Mijwel 2019 {published data only}
- Mijwel S, Jervaeus A, Bolam KA, Norrbom J, Bergh J, Rundqvist H, et al. High-intensity exercise during chemotherapy induces beneficial effects 12 months into breast cancer survivorship. Journal of Cancer Survivorship 2019;13(2):244-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Purcell 2011 {published data only}
- Purcell A, Fleming J, Burmeister B, Bennett S, Haines T. Is education an effective management strategy for reducing cancer-related fatigue? Supportive Care in Cancer 2011;19(9):1429-39. [DOI] [PubMed] [Google Scholar]
Singer 2018 {published and unpublished data}
- Singer S, Roick J, Meixensberger J, Schiefke F, Briest S, Dietz A, et al. The effects of multi-disciplinary psycho-social care on socio-economic problems in cancer patients: a cluster-randomized trial. Support Care Cancer 2018;26(6):1851-9. [DOI] [PubMed] [Google Scholar]
Tamminga 2013 {published and unpublished data}
- Tamminga SJ, Verbeek JH, Bos MM, Fons G, Kitzen JJ, Plaisier PW, et al. Effectiveness of a hospital-based work support intervention for female cancer patients – a multi-centre randomised controlled trial. PLOS One 2013;8(5):e63271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamminga SJ, Verbeek JH, Bos MM, Fons G, Kitzen JJ, Plaisier PW, et al. Two-year follow-up of a multi-centre randomized controlled trial to study effectiveness of a hospital-based work support intervention for cancer patients. Journal of Occupational Rehabilitation 2019;29(4):701-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
van Waart 2015 {published data only}
- Waart H, Stuiver MM, Harten WH, Geleijn E, Kieffer JM, Buffart LM, et al. Effect of low-intensity physical activity and moderate- to high-intensity physical exercise during adjuvant chemotherapy on physical fitness, fatigue, and chemotherapy completion rates: results of the PACES randomized clinical trial. Journal of Clinical Oncology 2015;33(17):1918-27. [DOI] [PubMed] [Google Scholar]
Zaman 2021 {published data only}
- Zaman AC, Tytgat KM, Klinkenbijl JH, Boer FC, Brink MA, Brinkhuis JC, et al. Effectiveness of a tailored work-related support intervention for patients diagnosed with gastrointestinal cancer: a multicenter randomized controlled trial. Journal of Occupational Rehabilitation 2021;31(2):323-38. [DOI: 10.1007/s10926-020-09920-z] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Ackerstaff 2009 {published and unpublished data}
- Ackerstaff AH, Balm AJ, Rasch CR, Boer JP, Wiggenraad R, Rietveld DH, et al. First-year quality of life assessment of an intra-arterial (RADPLAT) versus intravenous chemoradiation phase III trial. Head & Neck 2009;31(1):77-84. [DOI] [PubMed] [Google Scholar]
Adamsen 2009 {published data only}
- Adamsen L, Quist M, Andersen C, Møller T, Herrstedt J, Kronborg D, et al. Effect of a multimodal high intensity exercise intervention in cancer patients undergoing chemotherapy: randomised controlled trial. BMJ 2009;339:b3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
Berglund 2003 {published data only}
- Berglund G, Petersson LM, Eriksson KR, Häggman M. "Between men": patient perceptions and priorities in a rehabilitation program for men with prostate cancer. Patient Education and Counselling 2003;49(3):285-92. [DOI] [PubMed] [Google Scholar]
Bertheussen 2012 {published data only}
- Bertheussen GF, Kaasa S, Hokstad A, Sandmæl JA, Helbostad JL, Salvesen Ø, et al. Feasibility and changes in symptoms and functioning following inpatient cancer rehabilitation. Acta Oncologica 2012;51(8):1070-80. [DOI] [PubMed] [Google Scholar]
Bird 2010 {published data only}
- Bird L, Arthur A, Niblock T, Stone R, Watson L, Cox K. Rehabilitation programme after stem cell transplantation: randomized controlled trial. Journal of Advanced Nursing 2010;66(3):607-15. [DOI] [PubMed] [Google Scholar]
Bloom 2008 {published data only}
- Bloom JR, Stewart SL, D'Onofrio CN, Luce J, Banks PJ. Addressing the needs of young breast cancer survivors at the 5 year milestone: can a short-term, low intensity intervention produce change? Journal of Cancer Survivorship 2008;2(3):190-204. [DOI] [PubMed] [Google Scholar]
Borget 2007 {published data only}
- Borget I, Corone C, Nocaudie M, Allyn M, Iacobelli S, Schlumberger M, et al. Sick leave for follow-up control in thyroid cancer patients: comparison between stimulation with thyrogen and thyroid hormone withdrawal. European Journal of Endocrinology 2007;156(5):531-8. [DOI] [PubMed] [Google Scholar]
Böttcher 2013 {published data only}
- Böttcher HM, Steimann M, Ullrich A, Rotsch M, Zurborn KH, Koch U, et al. Evaluation of a vocationally oriented concept within inpatient oncological rehabilitation [Evaluation eines berufsbezogenen Konzepts im Rahmen der stationären onkologischen Rehabilitation]. Die Rehabilitation 2013;52(5):329-36. [DOI] [PubMed] [Google Scholar]
Brocki 2014 {published data only}
- Brocki BC, Andreasen J, Nielsen LR, Nekrasas V, Gorst-Rasmussen A, Westerdahl E. Short and long-term effects of supervised versus unsupervised exercise training on health-related quality of life and functional outcomes following lung cancer surgery – a randomized controlled trial. Cancer 2014;83(1):102-8. [DOI] [PubMed] [Google Scholar]
Broemer 2019 {published data only}
- Broemer L, Esser P, Koranyi S, Friedrich M, Leuteritz K, Wiegand S, et al. A group intervention to promote work ability in patients with head and neck cancer. Laryngo-Rhino-Otologie 2019;98(3):175-82. [DOI] [PubMed] [Google Scholar]
Budin 2008 {published data only}
- Budin WC, Hoskins CN, Haber J, Sherman DW, Maislin G, Cater JR, et al. Breast cancer: education, counseling, and adjustment among patients and partners: a randomized clinical trial. Nursing Research 2008;57(3):199-213. [DOI] [PubMed] [Google Scholar]
Burak 2002 {published data only}
- Burak J, Hollenbeck ST, Zervos EE, Hock KL, Kemp LC, Young DC. Sentinel lymph node biopsy results in less postoperative morbidity compared with axillary lymph node dissection for breast cancer. American Journal of Surgery 2002;183(1):23-7. [DOI] [PubMed] [Google Scholar]
Bürger 2011 {published data only}
- Bürger W, Streibelt M. Who benefits from stepwise occupational reintegration provided under the statutory pension insurance scheme? [Wer profitiert von Stufenweiser Wiedereingliederung in Trägerschaft der gesetzlichen Rentenversicherung?]. Die Rehabilitation 2011;50(3):178-85. [DOI] [PubMed] [Google Scholar]
Cadmus‐Bertram 2013 {published data only}
- Cadmus-Bertram L, Littman AJ, Ulrich CM, Stovall R, Ceballos RM, McGregor BA, et al. Predictors of adherence to a 26-week viniyoga intervention among post-treatment breast cancer survivors. Journal of Alternative and Complementary Medicine 2013;19(9):751-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cain 1986 {published data only}
- Cain EN, Kohorn EI, Quinlan DM, Latimer K, Schwartz PE. Psychological benefits of a cancer support group. Cancer 1986;57(1):183-9. [DOI] [PubMed] [Google Scholar]
Capone 1980 {published data only}
- Capone MA, Good RS, Westie KS, Jacobson AF. Psychosocial rehabilitation of gynecologic oncology patients. Archives of Physical Medical and Rehabilitation 1980;61(3):128-32. [PubMed] [Google Scholar]
Chan 2005 {published data only}
- Chan YM, Lee PW, Fong DY, Fung AS, Wu LY, Choi AY, et al. Effect of individual psychological intervention in Chinese women with gynecologic malignancy: a randomized controlled trial. Journal of Clinical Oncology 2005;23(22):4913-24. [DOI] [PubMed] [Google Scholar]
Cherrier 2013 {published data only}
- Cherrier MM, Anderson K, David D, Higano CS, Gray H, Church A, et al. A randomized trial of cognitive rehabilitation in cancer survivors. Life Sciences 2013;93(17):617-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cho 2006 {published data only}
- Cho OH, Yoo YS, Kim NC. Efficacy of comprehensive group rehabilitation for women with early breast cancer in South Korea. Nursing Health Science 2006;8(3):140-6. [DOI] [PubMed] [Google Scholar]
Egan 2013 {published data only}
- Egan MY, McEwen S, Sikora L, Chasen M, Fitch M, Eldred S. Rehabilitation following cancer treatment. Disability and Rehabilitation 2013;35(26):2245-58. [DOI] [PubMed] [Google Scholar]
Emmanouilidis 2009 {published data only}
- Emmanouilidis N, Müller JA, Jäger MD, Kaaden S, Helfritz FA, Güner Z, et al. Surgery and radioablation therapy combined: introducing a 1-week-condensed procedure bonding total thyroidectomy and radioablation therapy with recombinant human TSH. European Journal of Endocrinology 2009;161(5):763-9. [DOI] [PubMed] [Google Scholar]
- Emmanouilidis N, Schrem H, Winkler M, Klempnauer J, Scheumann GF. Long-term results after treatment of very low-, low-, and high-risk thyroid cancers in a combined setting of thyroidectomy and radio ablation therapy in euthyroidism. International Journal of Endocrinology 2013;2013:769473. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eyigor 2010 {published data only}
- Eyigor S, Karapolat H, Yesil H, Uslu R, Durmaz B. Effects of Pilates exercises on functional capacity, flexibility, fatigue, depression and quality of life in female breast cancer patients: a randomized controlled study. European Journal of Physical and Rehabilitation Medicine 2010;46(4):481-7. [PubMed] [Google Scholar]
Fassoulaki 2000 {published data only}
- Fassoulaki A, Sarantopoulos C, Melemeni A, Hogan Q. EMLA reduces acute and chronic pain after breast surgery for cancer. Regional Anesthesia and Pain Medicine 2000;25(4):350-5. [DOI] [PubMed] [Google Scholar]
Fauser 2019 {published data only}
- Fauser D, Wienert J, Beinert T, Schmielau J, Biester I, Kruger HU, et al. Work-related medical rehabilitation in patients with cancer – postrehabilitation results from a cluster-randomized multicenter trial. Cancer 2019;125(15):2666-74. [DOI] [PubMed] [Google Scholar]
Fors 2011 {published data only}
- Fors EA, Bertheussen GF, Thune I, Juvet LK, Elvsaas IK, Oldervoll L, et al. Psychosocial interventions as part of breast cancer rehabilitation programs? Results from a systematic review. Psychooncology 2011;20(9):909-18. [DOI] [PubMed] [Google Scholar]
Friedrichs 2010 {published data only}
- Friedrichs B, Tichelli A, Bacigalupo A, Russell NH, Ruutu T, Shapira MY, et al. Long-term outcome and late effects in patients transplanted with mobilised blood or bone marrow: a randomised trial. Lancet Oncology 2010;11(4):331-8. [DOI] [PubMed] [Google Scholar]
Gordon 1980 {published data only}
- Gordon WA, Freidenbergs I, Diller L, Hibbard M, Wolf C, Levine L, et al. Efficacy of psychosocial intervention with cancer patients. Journal of Consulting and Clinical Psychology 1980;48(6):743-59. [DOI] [PubMed] [Google Scholar]
Gordon 2005 {published and unpublished data}
- Gordon LG, Scuffham P, Battistutta D, Graves N, Tweeddale M, Newman B. A cost-effectiveness analysis of two rehabilitation support services for women with breast cancer. Breast Cancer Research and Treatment 2005;94(2):123-33. [DOI] [PubMed] [Google Scholar]
Greco 2021 {published data only}
- Greco CE, Strauser D, Rahkyung K, Fine E, Liptak C. An investigation of self-determined work motivation among young adult survivors of central nervous system cancer. Oncology Issues 2021;36(6):52-61. [Google Scholar]
Greer 1992 {published data only}
- Greer S, Moorey S, Baruch JD, Watson M, Robertson BM, Mason A, et al. Adjuvant psychological therapy for patients with cancer: a prospective randomised trial. BMJ 1992;304(6828):675-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Griffith 2009 {published data only}
- Griffith K, Wenzel J, Shang J, Thompson C, Stewart K, Mock V. Impact of a walking intervention on cardiorespiratory fitness, self-reported physical function, and pain in patients undergoing treatment for solid tumors. Cancer 2009;115(20):4874-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gyllensten 2019 {published data only}
- Gyllensten H, Koinberg I, Carlstrom E, Olsson LE, Hansson Olofsson E. Economic evaluation of a person-centred care intervention in head and neck oncology: results from a randomized controlled trial. Supportive Care in Cancer 2019;27(5):1825-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hansen 2020 {published data only}
- Hansen A, Pedersen CB, Jarden JO, Beier D, Minet LR, Sogaard K. Effectiveness of physical therapy- and occupational therapy-based rehabilitation in people who have glioma and are undergoing active anticancer treatment: single-blind, randomized controlled trial. Physical Therapy 2020;100(3):564-74. [DOI] [PubMed] [Google Scholar]
Harrison‐Paul 2006 {published data only}
- Harrison-Paul J, Drummond AE. A randomised controlled trial of occupational therapy in oncology: challenges in conducting a pilot study. British Journal of Occupational Therapy 2006;69(3):130-3. [Google Scholar]
Hartmann 2007 {published data only}
- Hartmann U, Muche R, Reuss-Borst M. Effects of a step-by-step inpatient rehabilitation programme on quality of life in breast cancer patients. A prospective randomised study. Onkologie 2007;30(4):177-82. [DOI] [PubMed] [Google Scholar]
Hegel 2011 {published data only}
- Hegel MT, Lyons KD, Hull JG, Kaufman P, Urquhart L, Li Z, et al. Feasibility study of a randomized controlled trial of a telephone-delivered problem-solving-occupational therapy intervention to reduce participation restrictions in rural breast cancer survivors undergoing chemotherapy. Psychooncology 2011;20(10):1092-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Heim 2007 {published data only}
- Heim ME, Malsburg ML, Niklas A. Randomized controlled trial of a structured training program in breast cancer patients with tumor-related chronic fatigue. Onkologie 2007;30(8-9):429-34. [DOI] [PubMed] [Google Scholar]
Hillman 1998 {published data only}
- Hillman RE, Walsh MJ, Wolf GT, Fisher SG, Hong WK. Functional outcomes following treatment for advanced laryngeal cancer. Part I – voice preservation in advanced laryngeal cancer. Part II – laryngectomy rehabilitation: the state of the art in the VA System. Research Speech-Language Pathologists. Department of Veterans Affairs Laryngeal Cancer Study Group. Annals of Otology, Rhinology and Laryngology 1998;172:1-27. [PubMed] [Google Scholar]
Huri 2015 {published data only}
- Huri M, Huri E, Kayihan H, Altuntas O. Effects of occupational therapy on quality of life of patients with metastatic prostate cancer. A randomized controlled study. Saudi Medical Journal 2015;36(8):954-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Høybye 2010 {published data only}
- Høybye MT, Dalton SO, Deltour I, Bidstrup PE, Frederiksen K, Johansen C. Effect of Internet peer-support groups on psychosocial adjustment to cancer: a randomised study. British Journal of Cancer 2010;102(9):1348-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ibrahim 2017 {published data only}
- Ibrahim M, Muanza T, Smirnow N, Sateren W, Fournier B, Kavan P, et al. Time course of upper limb function and return-to-work post-radiotherapy in young adults with breast cancer: a pilot randomized control trial on effects of targeted exercise program. Journal of Cancer Survivorship 2017;11(6):791-9. [DOI] [PubMed] [Google Scholar]
Janson 2005 {published data only}
- Janson M, Carlsson P, Haglind E, Anderberg B. Data validation in an economic evaluation of surgery for colon cancer. International Journal of Technology Assessment in Health Care 2005;21(2):246-52. [PubMed] [Google Scholar]
Ji 2019 {published data only}
- Ji W, Kwon H, Lee S, Kim S, Hong JS, Park YR, et al. Mobile health management platform-based pulmonary rehabilitation for patients with non-small cell lung cancer: prospective clinical trial. JMIR Mhealth Uhealth 2019;7(6):e12645. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jiang 2009 {published data only}
- Jiang J, Zheng X, Qin J, Zheng M, Mao Q, Zhang Z, et al. Health-related quality of life after hand-assisted laparoscopic and open radical nephrectomies of renal cell carcinoma. International Urology and Nephrology 2009;41(1):23-7. [DOI] [PubMed] [Google Scholar]
Johansson 2020 {published data only}
- Johansson M, Finizia C, Persson J, Tuomi L. Cost-effectiveness analysis of voice rehabilitation for patients with laryngeal cancer: a randomized controlled study. Support Care Cancer 2020;20:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Johnsson 2007 {published data only}
- Johnsson A, Fornander T, Olsson M, Nystedt M, Johansson H, Rutqvist LE. Factors associated with return to work after breast cancer treatment. Acta Oncologica 2007;46(1):90-6. [DOI] [PubMed] [Google Scholar]
Jones 2005 {published and unpublished data}
- Jones WG, Fossa SD, Mead GM, Roberts JT, Sokal M, Horwich A, et al. Randomized trial of 30 versus 20 Gy in the adjuvant treatment of stage I Testicular Seminoma: a report on Medical Research Council Trial TE18, European Organisation for the Research and Treatment of Cancer Trial 30942 (ISRCTN18525328). Journal of Clinical Oncology 2005;23(6):1200-8. [DOI] [PubMed] [Google Scholar]
Jørgensen 2009 {published data only}
- Jørgensen IL, Frederiksen K, Boesen E, Elsass P, Johansen C. An exploratory study of associations between illness perceptions and adjustment and changes after psychosocial rehabilitation in survivors of breast cancer. Acta Oncologica 2009;48(8):1119-27. [DOI] [PubMed] [Google Scholar]
Kornblith 2009 {published data only}
- Kornblith AB, Huang HQ, Walker JL, Spirtos NM, Rotmensch J, Cella D. Quality of life patients with endometrial cancer undergoing laparoscopic international federation of gynecology and obstetrics staging compared with laparotomy: a Gynecologic Oncology Group study. Journal of Clinical Oncology 2009;27(32):5337-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Korstjens 2008 {published data only}
- Korstjens I, May AM, Weert E, Mesters I, Tan F, Ros WJ, et al. Quality of life after self-management cancer rehabilitation: a randomized controlled trial comparing physical and cognitive-behavioral training versus physical training. Psychosomatic Medicine 2008;70(4):422-9. [DOI] [PubMed] [Google Scholar]
Kwiatkowski 2014 {published data only}
- Kwiatkowski F, Bignon Y, Leger-Enreille A. Long-term improved quality of life by a 2-week group physical and educational intervention shortly after breast cancer chemotherapy completion. Results of the "Programme of Accompanying women after breast Cancer treatment completion in Thermal resorts" (PACTHE). Annals of Physical and Rehabilitation Medicine 2014;57:e161. [DOI] [PubMed] [Google Scholar]
Lauchlan 2011 {published data only}
- Lauchlan DT, McCaul JA, McCarron T, Patil S, McManners J, McGarva J. An exploratory trial of preventative rehabilitation on shoulder disability and quality of life in patients following neck dissection surgery. European Journal of Cancer Care 2011;20(1):113-22. [DOI] [PubMed] [Google Scholar]
Lee 1992 {published data only}
- Lee MS, Love SB, Mitchell JB, Parker EM, Rubens RD, Watson JP, et al. Mastectomy or conservation for early breast cancer: psychological morbidity. European Journal of Cancer 1992;28A(8-9):1340-4. [DOI] [PubMed] [Google Scholar]
Lee 2009 {published data only}
- Lee WL, Liu WM, Cheng MH, Chao HT, Fuh JL, Wang PH. Uterine vascular occlusion in management of leiomyomas: laparoscopy vs laparotomy. Journal of Minimal Invasive Gynecology 2009;16(5):562-8. [DOI] [PubMed] [Google Scholar]
Luo 2018 {published data only}
- Luo Z, Wang L, Sikorskii A, Wyatt G. Healthcare service utilization and work-related productivity in reflexology intervention for advanced breast cancer women. Supportive Care in Cancer 2018;27(8):2837-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Madore 2014 {published data only}
- Madore S, Kilbourn K, Valverde P, Borrayo E, Raich P. Feasibility of a psychosocial and patient navigation intervention to improve access to treatment among underserved breast cancer patients. Supportive Care in Cancer 2014;22(8):2085-93. [DOI] [PubMed] [Google Scholar]
May 2009 {published data only}
- May A, Korstjens I, Weert E, den Borne B, Hoekstra-Weebers J, Schans CP, et al. Long-term effects on cancer survivors' quality of life of physical training versus physical training combined with cognitive-behavioral therapy: results from a randomized trial. Supportive Care in Cancer 2009;17(6):653-63. [DOI] [PubMed] [Google Scholar]
McNeely 2008 {published data only}
- McNeely ML, Parliament MB, Seikaly H, Jha N, Magee DJ, Haykowsky MJ, et al. Effect of exercise on upper extremity pain and dysfunction in head and neck cancer survivors: a randomized controlled trial. Cancer 2008;113(1):214-22. [DOI] [PubMed] [Google Scholar]
Meneses 2007 {published data only}
- Meneses KD, McNees P, Loerzel VW, Su X, Zhang Y, Hassey LA. Transition from treatment to survivorship: effects of a psychoeducational intervention on quality of life in breast cancer survivors. Oncology Nursing Forum 2007;34(5):1007-16. [DOI] [PubMed] [Google Scholar]
Meraviglia 2013 {published data only}
- Meraviglia M, Stuifbergen A, Parsons D, Morgan S. Health promotion for cancer survivors: adaptation and implementation of an intervention. Holistic Nursing Practice 2013;27(3):140-7. [DOI] [PubMed] [Google Scholar]
Mock 1994 {published data only}
- Mock V, Burke MB, Sheehan P, Creaton EM, Winningham ML, McKenney-Tedder S, et al. A nursing rehabilitation program for women with breast cancer receiving adjuvant chemotherapy. Oncology Nursing Forum 1994;21(5):899-907. [PubMed] [Google Scholar]
Mourgues 2014 {published data only}
- Mourgues C, Gerbaud L, Leger S, Auclair C, Peyrol F, Blanquet M, et al. Positive and cost-effectiveness effect of spa therapy on the resumption of occupational and non-occupational activities in women in breast cancer remission: a French multicentre randomised controlled trial. European Journal of Oncology Nursing 2014;18(5):505-11. [DOI] [PubMed] [Google Scholar]
Norager 2006 {published data only}
- Norager CB, Jensen MB, Madsen MR, Qvist N, Laurberg S. Effect of darbepoetin alfa on physical function in patients undergoing surgery for colorectal cancer. A randomized, double-blind, placebo-controlled study. Oncology 2006;71(3-4):212-20. [DOI] [PubMed] [Google Scholar]
Norouzi 2017 {published data only}
- Norouzi H, Rahimian-Boogar I, Talepasand S. Effectiveness of mindfulness-based cognitive therapy on posttraumatic growth, self-management and functional disability among patients with breast cancer. Nursing Practice Today 2017;4(4):190-202. [Google Scholar]
Nowrouzi 2009 {published data only}
- Nowrouzi B, Lightfoot N, Cote K, Watson R. Workplace support for employees with cancer. Current Oncology 2009;16(5):15-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Brien 2014 {published data only}
- O'Brien L, Loughnan A, Purcell A, Haines T. Education for cancer-related fatigue: could talking about it make people more likely to report it? Supportive Care in Cancer 2014;22(1):209-15. [DOI] [PubMed] [Google Scholar]
Paolucci 2019 {published data only}
- Paolucci T, Bernetti A, Paoloni M, Capobianco SV, Bai AV, Lai C, et al. Therapeutic alliance in a single versus group rehabilitative setting after breast cancer surgery: psychological profile and performance. BioResearch Open Access 2019;8(1):101-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Persson 2010 {published data only}
- Persson P, Brynhildsen J, Kjølhede P, Hysterectomy Multicentre Study Group in South-East Sweden. Short-term recovery after subtotal and total abdominal hysterectomy – a randomised clinical trial. BJOG 2010;117(4):469-78. [DOI] [PubMed] [Google Scholar]
Poppelreuter 2009 {published data only}
- Poppelreuter M, Weis J, Bartsch HH. Effects of specific neuropsychological training programs for breast cancer patients after adjuvant chemotherapy. Journal of Psychosocial Oncology 2009;27(2):274-96. [DOI] [PubMed] [Google Scholar]
Rogers 2009 {published and unpublished data}
- Rogers LQ, Hopkins-Pryce P, Vicari S, Pamenter R, Courneya KS, Markwell S, et al. A randomized trial to increase physical activity in breast cancer survivors. Medical Science Sports Exercises 2009;41(4):935-46. [DOI] [PubMed] [Google Scholar]
Rotstein 1989 {published data only}
- Rotstein S, Nilsson B, Gustavson-Kadaka E, Andersson S. Long-term follow-up of sickness periods in breast cancer patients primarily treated with surgery and radiotherapy or surgery only. Acta Oncologica 1989;28(6):817-22. [DOI] [PubMed] [Google Scholar]
Salonen 2011 {published data only}
- Salonen P, Kellokumpu-Lehtinen PL, Tarkka MT, Koivisto AM, Kaunonen M. Changes in quality of life in patients with breast cancer. Journal of Clinical Nursing 2011;20(1-2):255-66. [DOI] [PubMed] [Google Scholar]
Scherz 2017 {published data only}
- Scherz N, Bachmann-Mettler I, Chmiel C, Senn O, Boss N, Bardheci K, et al. Case management to increase quality of life after cancer treatment: a randomized controlled trial. BMC Cancer 2017;17(1):223. [DOI] [PMC free article] [PubMed] [Google Scholar]
Scott 2013 {published data only}
- Scott DA, Mills M, Black A, Cantwell M, Campbell A, Cardwell CR, et al. Multidimensional rehabilitation programmes for adult cancer survivors. Cochrane Database of Systematic Reviews 2013, Issue 3. Art. No: CD007730. [DOI: 10.1002/14651858.CD007730.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Seibaek 2009 {published data only}
- Seibaek L, Petersen L. Nurse-led rehabilitation after gynaecological cancer surgery: preliminary results from a clinically controlled, prospective questionnaire study. Supportive Care in Cancer 2009;17(5):601-5. [DOI] [PubMed] [Google Scholar]
Seiler 2005 {published data only}
- Seiler CA, Wagner M, Bachmann T, Redaelli CA, Schmied B, Uhl W, et al. Randomized clinical trial of pylorus-preserving duodenopancreatectomy versus classical Whipple resection – long term results. British Journal of Surgery 2005;92(5):547-56. [DOI] [PubMed] [Google Scholar]
Semple 2009 {published data only}
- Semple C, Dunwoody L, Kernohan W, McCaughan E. Development and evaluation of a problem-focused psychosocial intervention for patients with head and neck cancer. Supportive Care in Cancer 2009;17(4):379-88. [DOI] [PubMed] [Google Scholar]
Shelton 2009 {published data only}
- Shelton ML, Lee JQ, Morris GS, Massey PR, Kendall DG, Munsell MF, et al. A randomized control trial of a supervised versus a self-directed exercise program for allogeneic stem cell transplant patients. Psycho-Oncology 2009;18(4):353-9. [DOI] [PubMed] [Google Scholar]
Sherer 1997 {published data only}
- Sherer M, Meyers CA, Bergloff P. Efficacy of postacute brain injury rehabilitation for patients with primary malignant brain tumors. Cancer 1997;80(2):250-7. [PubMed] [Google Scholar]
Sherman 2010 {published data only}
- Sherman KA, Heard G, Cavanagh KL. Psychological effects and mediators of a group multi-component program for breast cancer survivors. Journal of Behavioral Medicine 2010;33(5):378-91. [DOI] [PubMed] [Google Scholar]
Sherman 2012 {published data only}
- Sherman DW, Haber J, Hoskins CN, Budin WC, Maislin G, Shukla S, et al. The effects of psychoeducation and telephone counseling on the adjustment of women with early-stage breast cancer. Applied Nursing Research 2012;25(1):3-16. [DOI] [PubMed] [Google Scholar]
Shimada 2007 {published data only}
- Shimada Y, Chida S, Matsunaga T, Sato M, Hatakeyama K, Itoi E. Clinical results of rehabilitation for accessory nerve palsy after radical neck dissection. Acta Otolaryngology 2007;127(5):491-7. [DOI] [PubMed] [Google Scholar]
Silver 2013 {published data only}
- Silver JK, Baima J, Newman R, Galantino ML, Shockney LD. Cancer rehabilitation may improve function in survivors and decrease the economic burden of cancer to individuals and society. Work 2013;46(4):455-72. [DOI] [PubMed] [Google Scholar]
van Egmond 2016 {published and unpublished data}
- Egmond MP, Duijts SF, Jonker MA, Beek AJ, Anema JR. Effectiveness of a tailored return to work program for cancer survivors with job loss: results of a randomized controlled trial. Acta Oncologica 2016;55(9-10):1210-9. [DOI] [PubMed] [Google Scholar]
van Waart 2018 {published data only}
- Waart H, Dongen JM, Harten WH, Stuiver MM, Huijsmans R, Hellendoorn-van Vreeswijk J, et al. Cost-utility and cost-effectiveness of physical exercise during adjuvant chemotherapy. European Journal of Health Economics 2018;19(6):893-904. [DOI] [PubMed] [Google Scholar]
Vos 2006 {published data only}
- Vos PJ, Visser AP, Garssen B, Duivenvoorden HJ, Haes HC. Effects of delayed psychosocial interventions versus early psychosocial interventions for women with early stage breast cancer. Patient Education and Counselling 2006;60(2):212-9. [DOI] [PubMed] [Google Scholar]
Wenzel 1995 {published data only}
- Wenzel LB, Robinson SE, Blake DD. The effects of problem-focused group counselling for early-stage gynecologic cancer patients. Journal of Mental Health Counselling 1995;17(1):81-93. [Google Scholar]
Wienert 2019 {published data only}
- Wienert J, Bethge M. Work-related medical rehabilitation in cancer rehabilitation – short-term results from a cluster-randomized multicenter-trial. Rehabilitation (Stuttg) 2019;58(3):181-90. [DOI] [PubMed] [Google Scholar]
Wiggins 2009 {published and unpublished data}
- Wiggins MS, Simonavice EM. Quality of life benefits in cancer survivorship with supervised exercise. Psychological Reports 2009;104(2):421-4. [DOI] [PubMed] [Google Scholar]
Yang 2020 {published data only}
- Yang S, Wang JD, Chang JH. Occupational therapy to improve quality of life for colorectal cancer survivors: a randomized clinical trial. Supportive Care in Cancer 2020;28(3):1503-11. [DOI] [PubMed] [Google Scholar]
Yun 2017 {published data only}
- Yun YH, Kim YA, Lee MK, Sim JA, Nam BH, Kim S, et al. A randomized controlled trial of physical activity, dietary habit, and distress management with the Leadership and Coaching for Health (LEACH) program for disease-free cancer survivors. BMC Cancer 2017;17(1):298. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
NCT01780064 {published data only}
- NCT01780064. Psychosocial support to facilitate the return to employment of women with breast cancer (APAPI). clinicaltrials.gov/study/NCT01780064 (first received 29 January 2013).
NCT03666936 {published data only}
- NCT03666936. Facilitate the return to work of cancer survivors. clinicaltrials.gov/study/NCT03666936 (first received 20 August 2018).
NCT04214912 {published data only}
- NCT04214912. Personalized survivor care plan for oral cancer patients – effects on physical-psychological functions and return-to-work. clinicaltrials.gov/study/NCT04214912 (first received 27 November 2018).
NCT04322695 {published data only}
- NCT04322695. A rehabilitation education care program on return to work among head and neck cancer survivors. clinicaltrials.gov/study/NCT04322695 (first received 23 March 2020).
NCT04469205 {published data only}
- NCT04469205. Study to assess the impact of personalized coaching on the time period and quality of return to work after breast cancer (OPTICOACH). clinicaltrials.gov/study/NCT04469205 (first received 8 June 2020).
NCT04846972 {published data only}
- NCT04846972. Facilitate and sustain return to work after breast cancer (FASTRACS-RCT). clinicaltrials.gov/study/NCT04846972 (first received 14 April 2021).
Sheppard 2019 {published data only}
- Sheppard DM, Frost D, Jefford M, O'Connor M, Halkett G. 'Beyond Cancer': a study protocol of a multimodal occupational rehabilitation programme to support breast cancer survivors to return work. BMJ Open 2019;6(12):e032505. [DOI] [PMC free article] [PubMed] [Google Scholar]
Touillaud 2021 {published data only}
- Touillaud M, Fournier B, Perol O, Delrieu L, Maire A, Belladame E, et al. Connected device and therapeutic patient education to promote physical activity among women with localised breast cancer (DISCO trial): protocol for a multicentre 2x2 factorial randomised controlled trial. BMJ Open 2021;11(9):e045448. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zegers 2021 {published data only}
- Zegers AD, Coenen P, Bultmann U, Retel V, Kieffer JM, Beek AJ, et al. Supporting participation in paid work of cancer survivors and their partners in the Netherlands: protocol of the SusTained Employability in cancer Patients and their partnerS (STEPS) multi-centre randomized controlled trial and cohort study. BMC Public Health 2021;21(1):1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Algeo 2021
- Algeo N, Bennett K, Connolly D. Rehabilitation interventions to support return to work for women with breast cancer: a systematic review and meta-analysis. BMC Cancer 2021;21(1):895. [DOI] [PMC free article] [PubMed] [Google Scholar]
Allemani 2018
- Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Nikšić M, et al, CONCORD Working Group. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018;391:1023-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Amatya 2019
- Amatya B, Khan F, Galea M. Rehabilitation for people with multiple sclerosis: an overview of Cochrane reviews. Cochrane Database of Systematic Reviews 2019, Issue 1. Art. No: CD012732. [DOI: 10.1002/14651858.CD012732] [DOI] [PMC free article] [PubMed] [Google Scholar]
American Cancer Society 2021
- American Cancer Society. Cancer facts & figures 2021. www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2021.html (accessed 3 May 2021).
Beck 2003
- Beck LA. Cancer rehabilitation: does it make a difference? Rehabilitation Nursing 2003;28(2):42-7. [DOI] [PubMed] [Google Scholar]
Blinder 2020
- Blinder VS, Gany FM. Impact of Cancer on Employment. Journal of Clinical Oncology 2020;38(4):302-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Butow 2020
- Butow P, Laidsaar-Powell R, Konings S, Lim CY, Koczwara B. Return to work after a cancer diagnosis: a meta-review of reviews and a meta-synthesis of recent qualitative studies. Journal of Cancer Survivorship 2020;14(2):114-34. [DOI] [PubMed] [Google Scholar]
Chinn 2000
- Chinn S. A simple method for converting an odds ratio to effect size for use in meta-analysis. Statistics in Medicine 2000;19(22):3127-31. [DOI] [PubMed] [Google Scholar]
Cohen 1988
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd edition. Hillsdale (NJ): Lawrence Erlbaum Associates, Publishers, 1988. [Google Scholar]
Cooper 2013
- Cooper AF, Hankins M, Rixon L, Eaton E, Grunfeld EA. Distinct work-related, clinical and psychological factors predict return to work following treatment in four different cancer types. Psychooncology 2013;22(3):659-67. [DOI] [PubMed] [Google Scholar]
De Backer 2009
- De Backer IC, Schep G, Backx FJ, Vreugdenhil G, Kuipers H. Resistance training in cancer survivors: a systematic review. International Journal of Sports Medicine 2009;30(10):703-12. [DOI] [PubMed] [Google Scholar]
de Boer 2006
- Boer AG, Verbeek JH, Dijk FJ. Adult survivors of childhood cancer and unemployment: a metaanalysis. Cancer 2006;107(1):1-11. [DOI] [PubMed] [Google Scholar]
de Boer 2008
- Boer AG, Verbeek JH, Spelten ER, Uitterhoeve AL, Ansink AC, Reijke TM, et al. Work ability and return-to-work in cancer patients. British Journal of Cancer 2008;98(8):1342-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
de Boer 2009a
- Boer AG, Taskila T, Ojajärvi A, Dijk FJ, Verbeek JH. Cancer survivors and unemployment: a meta-analysis and meta-regression. JAMA 2009;301(7):753-62. [DOI] [PubMed] [Google Scholar]
de Boer 2014
- Boer AG. The European Cancer and Work Network: CANWON. Journal of Occupational Rehabilitation 2014;24(3):393-8. [DOI] [PubMed] [Google Scholar]
de Boer 2020
- Boer AG, Greidanus MA, Dewa CS, Duijts SF, Tamminga SJ. Introduction to special section on: current topics in cancer survivorship and work. Cancer Survivorship 2020;14(2):101-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
de Jong 2018
- Jong M, Tamminga SJ, Es RJ, Frings-Dresen MH, Boer AG. The quality of working life questionnaire for cancer survivors (QWLQ-CS): factorial structure, internal consistency, construct validity and reproducibility. BMC Cancer 2018;18(1):66. [DOI] [PMC free article] [PubMed] [Google Scholar]
de Wind 2021
- Wind A, Tamminga SJ, Bony CA, Diether M, Ludwig M, Velthuis MJ, et al. Loss of paid employment up to 4 years after colorectal cancer diagnosis – a nationwide register-based study with a population-based reference group. Cancers (Basel) 2021;13(12):2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dorland 2017
- Dorland HF, Abma FI, Roelen CA, Stewart RE, Amick BC, Ranchor AV, et al. Work functioning trajectories in cancer patients: results from the longitudinal Work Life after Cancer (WOLICA) study. International Journal of Cancer 2017;141(9):1751-62. [DOI] [PubMed] [Google Scholar]
Duijts 2014
- Duijts SF, Egmond MP, Spelten E, Muijen P, Anema JR, Beek AJ. Physical and psychosocial problems in cancer survivors beyond return to work: a systematic review. Psychooncology 2014;23(5):481-92. [DOI] [PubMed] [Google Scholar]
Duijts 2017
- Duijts SF, Kieffer JM, Muijen P, Beek AJ. Sustained employability and health-related quality of life in cancer survivors up to four years after diagnosis. Acta Oncologia 2017;56(2):174-82. [DOI: 10.1080/0284186X.2016.1266083] [DOI] [PubMed] [Google Scholar]
EU OSHA 2018
- European Agency for Safety and Health at Work. Rehabilitation and Return to Work After Cancer – Instruments and Practices. Luxembourg (Luxembourg): Publications Office of the European Union, 2018. [Google Scholar]
European Commission 2023
- ECIS - European Cancer Information System. https://ecis.jrc.ec.europa.eu/explorer.php?$0-1$1-IT$2-127$4-1,2$3-All$6-0,85$5-1976,2012$7-2$CRatesByCancer$X0_10-ASR_EU_NEW (accessed 8 February 2023).
Ferlay 2018
- Ferlay J, Colombet M, Soerjomataram I, Dyba T, Randi G, Bettio M, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries and 25 major cancers in 2018. European Journal of Cancer 2018;103:356-87. [DOI] [PubMed] [Google Scholar]
Feuerstein 2007
- Feuerstein M, Hansen JA, Calvio LC, Johnson L, Ronquillo JG. Work productivity in brain tumor survivors. Journal of Occupational and Environmental Medicine 2007;49(7):803-11. [DOI] [PubMed] [Google Scholar]
Franche 2005
- Franche RL, Cullen K, Clarke J, Irvin E, Sinclair S, Frank J. Workplace-based return-to-work interventions: a systematic review of the quantitative literature. Journal of Occupational Rehabilitation 2005;15(4):607-31. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Gragnano 2021
- Gragnano A, Miglioretti M, Magon G, Pravettoni G. Work with cancer or stop working after diagnosis? Variables affecting the decision. Work 2021;70(1):177-85. [DOI] [PubMed] [Google Scholar]
Grant 2014
- Grant RL. Converting an odds ratio to a range of plausible relative risks for better communication of research findings. BMJ 2014;348:f7450. [DOI] [PubMed] [Google Scholar]
Gudbergsson 2015
- Gudbergsson SB, Dahl AA, Loge JH, Thorsen L, Oldervoll LM, Grov EK. What is covered by "cancer rehabilitation" in PubMed? A review of randomized controlled trials 1990–2011. Journal of Rehabilitation Medicine 2015;47(2):97-106. [DOI] [PubMed] [Google Scholar]
Guo 2021
- Guo YJ, Tang J, Li JM, Zhu LL, Xu JS. Exploration of interventions to enhance return-to-work for cancer patients: a scoping review. Clinical Rehabilitation 2021;35(12):1674-93. [DOI] [PubMed] [Google Scholar]
Haaf 2005
- Haaf HG. Findings on the effectiveness of rehabilitation [Ergebnisse zur Wirksamkeit der Rehabilitation]. Die Rehabilitation 2005;44(5):259-76. [DOI] [PubMed] [Google Scholar]
Harvey 1982
- Harvey RF, Jellinek HM, Habeck RV. Cancer rehabilitation. An analysis of 36 program approaches. JAMA 1982;247(15):2127-31. [DOI] [PubMed] [Google Scholar]
Hegewald 2019
- Hegewald J, Wegewitz UE, Euler U, Dijk JL, Adams J, Fishta A, et al. Interventions to support return to work for people with coronary heart disease. Cochrane Database of Systematic Reviews 2019, Issue 3. Art. No: CD010748. [DOI: 10.1002/14651858.CD010748] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hersch 2009
- Hersch J, Juraskova I, Price M, Mullan B. Psychosocial interventions and quality of life in gynaecological cancer patients: a systematic review. Psycho-oncology 2009;18(8):795-810. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2023
- Higgins JP, Savović J, Page MJ, Elbers RG, Sterne JA. Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.4 (updated August 2023). Cochrane, 2023. Available from www.training.cochrane.org/handbook.
Hoving 2009
- Hoving JL, Broekhuizen ML, Frings-Dresen MH. Return to work of breast cancer survivors: a systematic review of intervention studies. BMC Cancer 2009;9:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hoving 2014
- Hoving JL, Lacaille D, Urquhart DM, Hannu TJ, Sluiter JK, Frings-Dresen MH. Non-pharmacological interventions for preventing job loss in workers with inflammatory arthritis. Cochrane Database of Systematic Reviews 2014, Issue 11. Art. No: CD010208. [DOI: 10.1002/14651858.CD010208] [DOI] [PMC free article] [PubMed] [Google Scholar]
Howlander 2019
- Howlader N, Noone AM, Krapcho M, Miller D, Brest A, Yu M, et al. SEER Cancer Statistics Review, 1975–2016. seer.cancer.gov/archive/csr/1975_2016/index.html (accessed prior to 12 January 2024).
Irwin 2004
- Irwin ML, Ainsworth BE. Physical activity interventions following cancer diagnosis: methodologic challenges to delivery and assessment. Cancer Investigation 2004;22(1):30-50. [DOI] [PubMed] [Google Scholar]
Khan 2009
- Khan F, Ng L, Turner-Stokes L. Effectiveness of vocational rehabilitation intervention on the return to work and employment of persons with multiple sclerosis. Cochrane Database of Systematic Reviews 2009, Issue 1. Art. No: CD007256. [DOI: 10.1002/14651858.CD007256.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kirshbaum 2007
- Kirshbaum MN. A review of the benefits of whole body exercise during and after treatment for breast cancer. Journal of Clinical Nursing 2007;16(1):104-21. [DOI] [PubMed] [Google Scholar]
Kirshblum 2001
- Kirshblum S, O'Dell MW, Ho C, Barr K. Rehabilitation of persons with central nervous system tumors. Cancer 2001;92(4 Suppl):1029-38. [DOI] [PubMed] [Google Scholar]
Koczwara 2016
- Koczwara B, Freegard S, Siddiquee S, Knott V, Ward P, Olver I, et al. Return to work after cancer – a systematic literature review comparing cancer and non-cancer settings. Supportive Care in Cancer 2016;24(1):S227-8. [Google Scholar]
Lamore 2019
- Lamore K, Dubois T, Rothe U, Leonardi M, Girard I, Manuwald U, et al. Return to work interventions for cancer survivors: a systematic review and a methodological critique. International Journal of Environmental Research and Public Health 2019;16(8):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Liu 2009
- Liu RD, Chinapaw MJ, Huijgens PC, Mechelen W. Physical exercise interventions in haematological cancer patients, feasible to conduct but effectiveness to be established: a systematic literature review. Cancer Treatment Reviews 2009;35(2):185-92. [DOI] [PubMed] [Google Scholar]
Macmillan 2013
- Macmillan Cancer Support. Making the shift – providing specialist work support to people with cancer. www.macmillan.org.uk/Documents/GetInvolved/Campaigns/WorkingThroughCancer/Making-the-shift-specialist-work-support-for-people-with-cancer.pdf (accessed 27 August 2015).
Marmot 2012
- Marmot M, Allen J, Bell R, Bloomer E, Goldblatt P, Consortium for the European Review of Social Determinants of Health and the Health Divide. WHO European review of social determinants of health and the health divide. Lancet 2012;380(9846):1011-29. [DOI] [PubMed] [Google Scholar]
McNeely 2006
- McNeely ML, Campbell KL, Rowe BH, Klassen TP, Mackey JR, Courneya KS. Effects of exercise on breast cancer patients and survivors: a systematic review and meta-analysis. Canadian Medical Association Journal 2006;175(1):34-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
McQueen 2017
- McQueen J, McFeely G. Case management for return to work for individuals living with cancer: a systematic review. International Journal of Therapy & Rehabilitation 2017;24(5):203-10. [Google Scholar]
Mehnert 2011
- Mehnert A. Employment and work-related issues in cancer survivors. Critical Reviews in Oncology/Hematology 2011;77(2):109-30. [DOI] [PubMed] [Google Scholar]
Mehnert 2013
- Mehnert A, Boer A, Feuerstein M. Employment challenges for cancer survivors. Cancer 2013;119(Suppl 11):2151-9. [DOI] [PubMed] [Google Scholar]
Miller 2019
- Miller KD, Nogueira L, Mariotto AB, Rowland JH, Yabroff KR, Alfano CM, et al. Cancer treatment and survivorship statistics, 2019. CA Cancer Journal for Clinicians 2019;69(5):363-85. [DOI] [PubMed] [Google Scholar]
Moskowitz 2014
- Moskowitz MC, Todd BL, Chen R, Feuerstein M. Function and friction at work: a multidimensional analysis of work outcomes in cancer survivors. Journal of Cancer Survivorship: Research and Practice 2014;8(2):173-82. [DOI] [PubMed] [Google Scholar]
Musoro 2020
- Musoro JZ, Coens C, Greimel E, King MT, Sprangers MA, Nordin A, et al, EORTC Gynecological and Quality of Life Groups. Minimally important differences for interpreting European Organisation for Research and Treatment of Cancer (EORTC) Quality of life Questionnaire core 30 scores in patients with ovarian cancer. Gynecologic Oncology 2020;159(2):515-21. [DOI: 10.1016/j.ygyno.2020.09.007] [DOI] [PubMed] [Google Scholar]
Nieuwenhuijsen 2020
- Nieuwenhuijsen K, Verbeek JH, Neumeyer-Gromen A, Verhoeven AC, Bültmann U, Faber B. Interventions to improve return to work in depressed people. Cochrane Database of Systematic Reviews 2020, Issue 10. Art. No: CD006237. [DOI: 10.1002/14651858.CD006237] [DOI] [PMC free article] [PubMed] [Google Scholar]
Norlund 2009
- Norlund A, Ropponen A, Alexanderson K. Multidisciplinary interventions: review of studies of return to work after rehabilitation for low back pain. Journal of Rehabilitation Medicine 2009;41(3):115-21. [DOI] [PubMed] [Google Scholar]
Oldervoll 2004
- Oldervoll LM, Kaasa S, Hjermstad MJ, Lund JA, Loge JH. Physical exercise results in the improved subjective well-being of a few or is effective rehabilitation for all cancer patients? European Journal of Cancer 2004;40(7):951-62. [DOI] [PubMed] [Google Scholar]
Osborn 2006
- Osborn RL, Demoncada AC, Feuerstein M. Psychosocial interventions for depression, anxiety, and quality of life in cancer survivors: meta-analyses. International Journal of Psychiatry in Medicine 2006;36(1):13-34. [DOI] [PubMed] [Google Scholar]
Ravinskaya 2021
- Ravinskaya M, Verbeek JH, Langendam M, Daams JG, Hulshof CT, Madan I, et al. Extensive variability of work participation outcomes measured in randomized controlled trials: a systematic review. Journal of Clinical Epidemiology 2021;142:60-99. [DOI] [PubMed] [Google Scholar]
RevMan 2022 [Computer program]
- Review Manager (RevMan). Version 4.12.0. The Cochrane Collaboration, 2022. Available at revman.cochrane.org.
Richardson 2016
- Richardson M, Garner P, Donegan S. Cluster randomised trials in Cochrane reviews: evaluation of methodological and reporting practice. PLOS One 2016;11(3):e0151818. [DOI] [PMC free article] [PubMed] [Google Scholar]
Robinson 2002
- Robinson KA, Dickersin K. Development of a highly sensitive search strategy for the retrieval of reports of controlled trials using PubMed. International Journal of Epidemiology 2002;31(1):150-3. [DOI] [PubMed] [Google Scholar]
Schaafsma 2013
- Schaafsma FG, Whelan K, Beek AJ, Es-Lambeek LC, Ojajärvi A, Verbeek JH. Physical conditioning as part of a return to work strategy to reduce sickness absence for workers with back pain. Cochrane Database of Systematic Reviews 2013, Issue 8. Art. No: CD001822. [DOI: 10.1002/14651858.CD001822.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shapiro 2018
- Shapiro CL. Cancer survivorship. New England Journal of Medicine 2018;379(25):2438-50. [DOI] [PubMed] [Google Scholar]
Short 2005
- Short P, Vasey JJ, Tuncelli K. Employment pathways in a large cohort of adult cancer survivors. Cancer 2005;103(6):1292-301. [DOI] [PubMed] [Google Scholar]
Spelten 2002
- Spelten ER, Sprangers MA, Verbeek JH. Factors reported to influence the return to work of cancer survivors: a literature review. Psycho-oncology 2002;11(2):124-31. [DOI] [PubMed] [Google Scholar]
Stanton 2006
- Stanton AL. Psychosocial concerns and interventions for cancer survivors. Journal of Clinical Oncology 2006;24(32):5132-7. [DOI] [PubMed] [Google Scholar]
Stapelfeldt 2019
- Stapelfeldt CM, Klaver KM, Rosbjerg RS, Dalton SO, Bültmann U, Labriola M, et al. A systematic review of interventions to retain chronically ill occupationally active employees in work: can findings be transferred to cancer survivors? Acta Oncologica 2019;58(5):548-65. [DOI] [PubMed] [Google Scholar]
Steiner 2010
- Steiner JF, Nowels CT, Main DS. Returning to work after cancer: quantitative studies and prototypical narratives. Psycho-oncology 2010;19(2):115-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tamminga 2020
- Tamminga SJ, Jansen LP, Frings-Dresen MH, Boer AG. Long-term employment status and quality of life after cancer: a longitudinal prospective cohort study from diagnosis up to and including 5 years post diagnosis. Work 2020;66(4):901-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tan 2021
- Tan FS, Shorey S. Experiences of women with breast cancer while working or returning to work: a qualitative systematic review and meta-synthesis. Support Care Cancer 2022;30(4):2971-82. [DOI] [PubMed] [Google Scholar]
Taskila 2007
- Taskila T, Lindbohm ML. Factors affecting cancer survivors' employment and work ability. Acta Oncologica 2007;46(4):446-51. [DOI] [PubMed] [Google Scholar]
van der Molen 2009
- Molen LA, Rossum MA, Burkhead LM, Smeele LE, Hilgers FJ. Functional outcomes and rehabilitation strategies in patients treated with chemoradiotherapy for advanced head and neck cancer: a systematic review. European Archives of Oto-rhino-laryngology 2009;266(6):889-900. [DOI] [PubMed] [Google Scholar]
Verbeek 2007
- Verbeek J, Spelten E. Work. In: Feuerstein M, editors(s). Handbook of Cancer Survivorship. Berkeley (CA): Springer, 2007. [Google Scholar]
Wennman‐Larsen 2021
- Wennman-Larsen A, Svärd V, Alexanderson K, Friberg E. Factors of decisive importance for being in work or not during two years after breast cancer surgery: content analysis of 462 women's open answers. BMC Womens Health 2021;21(1):332. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
de Boer 2009b
- Boer AG, Taskila T, Tamminga SJ, Frings‐Dresen MH, Feuerstein M, Verbeek JH. Interventions to enhance return-to-work for cancer patients. Cochrane Database of Systematic Reviews 2009, Issue 1. Art. No: CD007569. [DOI: 10.1002/14651858.CD007569] [DOI] [Google Scholar]
de Boer 2011
- Boer AG, Taskila T, Tamminga SJ, Frings-Dresen MH, Feuerstein M, Verbeek JH. Interventions to enhance return-to-work for cancer patients. Cochrane Database of Systematic Reviews 2011, Issue 2. Art. No: CD007569. [DOI: 10.1002/14651858.CD007569.pub2] [DOI] [PubMed] [Google Scholar]
de Boer 2015
- Boer AG, Taskila TK, Tamminga SJ, Feuerstein M, Frings-Dresen MH, Verbeek JH. Interventions to enhance return-to-work for cancer patients. Cochrane Database of Systematic Reviews 2015, Issue 9. Art. No: CD007569. [DOI: 10.1002/14651858.CD007569.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]


