Abstract
Rationale: The SubPopulations and InteRmediate Outcome Measures in COPD Study (SPIROMICS) is a prospective cohort study that enrolled 2981 participants with the goal of identifying new chronic obstructive pulmonary disease (COPD) subgroups and intermediate markers of disease progression. Individuals with COPD and obstructive sleep apnea (OSA) experience impaired quality of life and more frequent exacerbations. COPD severity also associates with computed tomography scan-based emphysema and alterations in airway dimensions.
Objectives: The objective was to determine whether the combination of lung function and structure influences the risk of OSA among current and former smokers.
Methods: Using 2 OSA risk scores, the Berlin Sleep Questionnaire (BSQ), and the DOISNORE50 (Diseases, Observed apnea, Insomnia, Snoring, Neck circumference > 18 inches, Obesity with body mass index [BMI] > 32, R = are you male, Excessive daytime sleepiness, 50 = age ≥ 50) (DIS), 1767 current and former smokers were evaluated for an association of lung structure and function with OSA risk.
Measurements and Main Results: The study cohort's mean age was 63 years, BMI was 28 kg/m2, and forced expiratory volume in 1 second (FEV1) was 74.8% predicted. The majority were male (55%), White (77%), former smokers (59%), and had COPD (63%). A high-risk OSA score was reported in 36% and 61% using DIS and BSQ respectively. There was a 9% increased odds of a high-risk DIS score (odds ratio [OR]=1.09, 95% confidence interval [CI]:1.03–1.14) and nominally increased odds of a high-risk BSQ score for every 10% decrease in FEV1 %predicted (OR=1.04, 95%CI: 0.998–1.09). Lung function-OSA risk associations persisted after additionally adjusting for lung structure measurements (%emphysema, %air trapping, parametric response mapping for functional small airways disease, , mean segmental wall area, tracheal %wall area, dysanapsis) for DIS (OR=1.12, 95%CI:1.03–1.22) and BSQ (OR=1.09, 95%CI:1.01–1.18).
Conclusions: Lower lung function independently associates with having high risk for OSA in current and former smokers. Lung structural elements, especially dysanapsis, functional small airways disease, and tracheal %wall area strengthened the effects on OSA risk.
Keywords: COPD, lung function, CT-scan measurements, DOISNORE50
Introduction
This article contains supplemental material.
Current and former smokers with preserved lung function may have respiratory symptoms, sleep disorders including obstructive sleep apnea (OSA), and decreased quality of life.1-3 Individuals withchronic obstructive pulmonary disease (COPD) and OSA experience impaired quality of life, more frequent respiratory exacerbations,4,5 and increased risk of mortality if not treated with continuous positive airway pressure (CPAP), compared to those with COPD alone.6 Those with COPD and OSA also have evidence of increased airway wall thickness and increased airway wall thickness has separately been associated with increased risk for respiratory symptoms and exacerbations.7,8 While informative, the existing literature is limited by the lack of sleep-related questionnaires in combination with quantitative computed tomography (CT) scan parameters among people with COPD or current and former smokers.
While the combination of COPD and OSA is associated with adverse effects, worse lung function (lower forced expiratory volume in 1 second [FEV1] percentage predicted [%pred]) is associated with a better measure of OSA severity, specifically a lower apnea-hypopnea index (AHI).9 A potential mechanism for this beneficial association might be that individuals with advanced COPD have increased lung volumes due to air trapping and hyperinflation which provides tension that opens and stabilizes the upper airways. This mechanism is supported by CT-based studies of lung structure showing that in smokers with OSA, increased emphysema and air trapping are associated with a lower AHI.10 Dysanapsis is a CT scan-based structural measure of the mismatch between airway tree caliber to lung size that if low, is a known risk factor for COPD progression that might contribute to OSA risk and severity among those with heavier smoking histories and COPD.11
While OSA is typically diagnosed with a polysomnogram (PSG) in COPD, those who need to have a sleep study are often identified with office questionnaires. The Berlin Sleep Questionnaire (BSQ) is a commonly used predictive tool and has 85%-96% positive predictive value (PPV) in a sleep clinic population12 that has not been validated in a population of current and former smokers. A newer tool, the DOISNORE50 (Diseases, Observed apnea, Insomnia, Snoring, Neck circumference > 18 inches, Obesity with body mass index [BMI] > 32, R = are you male, Excessive daytime sleepiness, 50 = age ≥ 50) (DIS) tested as a patient-administered questionnaire has a PPV of 84% in a sleep clinic population that considers additional factors not included in the BSQ, specifically comorbid cardiovascular disease, age, gender, and neck circumference.13,14 DIS has also been associated with increased odds of inpatient medical emergency team activation among those at risk for OSA admitted to the hospital14 and may give additional insight into why some people with or at risk for OSA have more adverse effects than others.
We hypothesize that the combination of lung function and lung structure influences the risk of sleep apnea among current and former smokers who have COPD or individuals with a prior heavy smoking history. To test this hypothesis, we evaluated how lung function and different CT-based lung structure measures associate with risk of OSA using 2 methods, the BSQ and the DIS, to identify those at risk. The DIS was used as it includes additional comorbidities that largely exist in this population and may differentially identify those at risk for OSA compared to the BSQ. Understanding associations between lung function, structure, and risk of OSA would provide evidence supporting the early recognition of concomitant COPD and OSA to improve outcomes. Further, identifying whether the newer DIS shows different associations than the established BSQ will help determine which questionnaire to use.
Methods
The Subpopulations and Intermediate Outcome Measures in COPD Study (SPIROMICS) is a prospective, multicenter cohort study that enrolled 2981 participants aged 40–80 years old across 4 strata (never users of cigarettes [≤1 pack years], current or ex-users [≥20 pack years] without airway obstruction as defined by FEV1 to forced vital capacity (FVC) ratio ≥0.70, mild-to-moderate COPD, and severe COPD based on Global initiative for chronic Obstructive Lung Disease [GOLD] spirometric grades)15 with goals of identifying new COPD subgroups and intermediate markers of disease progression.16 Spirometry was performed following American Thoracic Society recommendations17 and lung CT scans were performed across sites based on a prespecified protocol.18 Cross-sectional analyses were performed with the 1767 participants who were current or former users of cigarettes with available OSA scoring variables, lung function, CT scan, and covariate data.
OSA risk was evaluated through the BSQ administered during the baseline clinic visit and the calculated DIS.13 The BSQ includes questions in 3 categories: snoring, excessive daytime sleepiness, and hypertension/obesity. Having at least 2 of 3 categories scored positive on the BSQ was considered high-risk for OSA. The DIS was not administered during SPIROMICS, however, analogous collected information on several questionnaires that were administered as part of SPIROMICS was used to calculate the score. The DIS calculation has been previously validated against PSG14 and included: a history of atrial fibrillation, stroke, hypertension, observed apnea, insomnia, snoring, neck circumference (>18 inches in males and >17 inches in females), obesity (BMI >32kg/m2), male gender, excessive daytime sleepiness, and age ≥50 years. The DIS was dichotomized with a score of ≥6 indicating a high risk of having OSA. Parameters used in the DIS were determined based on association with a PSG result in the initial validation work.14 Neither the BSQ nor the DIS are validated in specific COPD populations. Work is ongoing to validate the DIS in the COPD population. We used the BSQ and the DIS to evaluate findings across 2 validated measures of OSA risk.
Continuous variables for the COPD Assessment Test (CAT)19 and the St George’s Respiratory Questionnaire (SGRQ)20 were evaluated using linear regression models. Annualized rates of exacerbations requiring treatment (antibiotics and/or corticosteroids prescribed by a health care provider) and severe exacerbations (exacerbations requiring an emergency department visit or hospitalization) were assessed using zero-inflated negative binomial models.21 The OSA risk scores were the primary predictors and were evaluated individually in models of COPD severity that included standard covariates: age, sex, current smoking status, and smoking pack years.
Linear regression models were fit for each lung structure measure with OSA risk as the primary predictor. Lung structure measures included functional small airways disease (% parametric response mapping for functional small airways disease [PRMfSAD])22; air trapping (% <-856 Hounsfield units [HU] at residual volume); emphysema (% <-950 HU at total lung capacity); and airway dimensions23 including tracheal wall area, mean wall area percentage of the segmental airways (%airway wall area), and dysanapsis (1-SD change).11 Additional covariates of CT scanner model, BMI, height, and study site were included in lung structure models. Models were performed with and without FEV1 %pred.
Due to the strong effect of FEV1 %pred on OSA risk and lung structure, logistic regression models for OSA risk were first fit with FEV1 %pred as the main predictor of interest, and then further adjusted for each of the lung structure measures individually. Finally, a multivariable logistic regression model was fit including covariates listed above with FEV1 %pred, air trapping, emphysema, PRMfSAD, dysanapsis, and airway dimensions. Analyses were performed using SAS version 9.4 (SAS Institute Inc, Cary, North Carolina).
Results
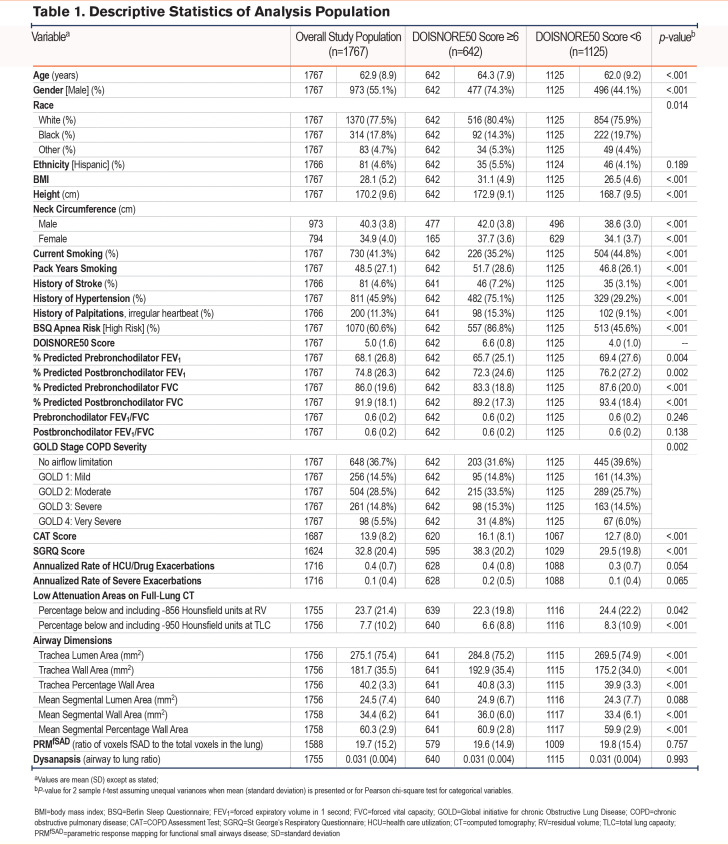
The study cohort had a mean age of 63 years (standard deviation [SD] 8.9). The majority were male (55%), the mean BMI was 28 kg/m2(SD 5.2), and the mean neck circumference was 40.3 cm (SD 3.8) in men and 34.9 cm (SD 4.0) in women. The cohort consisted of current (41%) and former (59%) smokers with a mean smoking history of 48.5 pack years (SD 27.1) and a history of atrial fibrillation, hypertension, and stroke in 11%, 46%, and 5% of the cohort, respectively. The mean postbronchodilator FEV1 was 74.8% predicted (SD 26.3) and 63% had airflow obstruction, 68% of mild-to-moderate severity based on GOLD criteria.15
The mean DIS score in SPIROMICS participants was 5.0 (SD 1.6). A high-risk OSA score was reported in 36% and 61% of participants using the DIS (≥6) and the BSQ (≥2 positive categories), respectively. Participants with a high risk of OSA measured by the DIS were more frequently male, had an older age, higher BMI, and a larger neck circumference when compared to low risk (Table 1). High-risk individuals were more likely to be former cigarette users but had a higher pack-year smoking history. Participants with a high risk of OSA were more likely to have airflow obstruction and had a lower FEV1 %pred, despite having lower percentage emphysema on a CT scan. Those with high-risk OSA scores had thicker airways with greater tracheal wall and airway wall areas when compared to those with lower-risk scores.
Of those with high-risk DIS scores, 87% were high-risk on the BSQ. Conversely, only 52% of those with high-risk BSQ scores had high-risk DIS scores. This discordance between the DIS compared to the BSQ (Kappa 0.36 [95% CI: 0.32, 0.40]) was primarily due to the inclusion of male sex and history of stroke or hypertension (see Table A1 in the online supplement). Since male sex accounted for high-risk DIS scores, we report participant characteristics by sex in the overall cohort in Table A2 in the online supplement. Men were older than women, less likely to be currently smoking, and had a higher pack-year smoking history. Men also reported fewer symptoms based on the CAT score, fewer exacerbations, better SGRQ scores, greater air trapping, emphysema, and functional small airways disease compared to women while women had thicker tracheal walls (Table A2 in the online supplement).
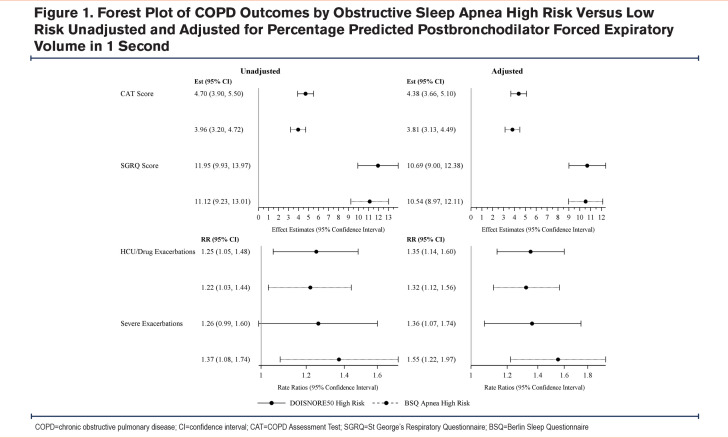
As expected, based on prior literature, having a high-risk OSA score was adversely associated with FEV1 %pred, mean CAT, SGRQ scores, and exacerbation rates in unadjusted and adjusted models (Figure 1). Those with high risk of OSA scores based on the DIS showed increased air trapping (estimate 0.25, 95% CI: 0.03, 0.47), tracheal wall area (estimate 0.51, 95% confidence interval [CI]: 0.16, 0.86), airway wall area (estimate 0.40, 95% CI: 0.08, 0.71), PRMfSAD (estimate 0.23, 95% CI: 0.04, 0.41), and decreased dysanapsis (estimate -0.1195, 95% CI: -0.2222, -0.0168) compared to those with a low risk. There was no association between a DIS high risk of OSA and emphysema. Those with a high-risk OSA BSQ score had an increased average %tracheal wall area (estimate 0.46, 95% CI: 0.14, 0.79), however, they had no significant association with air trapping, emphysema, airway wall area, or PRMfSAD. There were no associations with a high risk of OSA using either score when stratified by GOLD grades (data not shown).15 Because of the known strong association between lung structure measures on CT and lung function,8 we added FEV1 %pred to the models. We found that the association between a high-risk OSA score, based on both the DIS (estimate 0.43, 95% CI: 0.08, 0.78) and the BSQ (estimate 0.41, 95% CI: 0.09, 0.73), and %tracheal wall area was the only lung structural association to remain significant after adjustment for lung function.
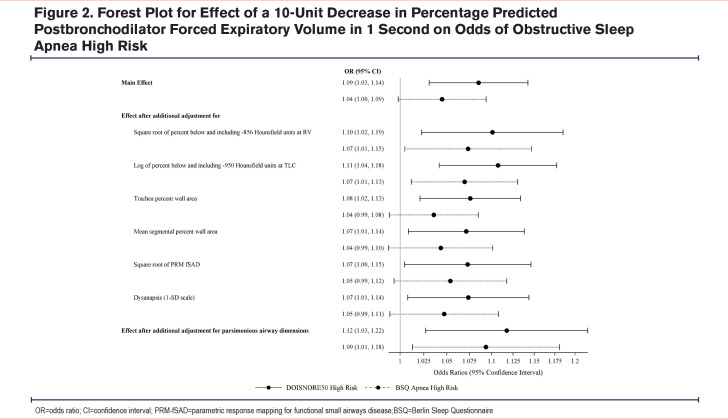
Because we found that FEV1 strongly attenuated the association between OSA and CT measures, further analysis targeted the association between FEV1%pred and having a high risk for OSA. There was a 9% increased odds of a high-risk DIS score (OR 1.09, 95% CI: 1.03, 1.14) and a nominally increased odds of a high-risk BSQ score for every 10% decrease in FEV1 %pred (OR 1.04, 95% CI: 0.998, 1.09).
To understand how CT-based measures of lung structure may change the association between high risk for OSA and FEV1 %pred these covariates were added to the regression model. Lung function-OSA risk associations persisted and were strengthened after additionally adjusting for lung structure measurements when determined using both the DIS (OR 1.12, 95% CI: 1.03, 1.22) and the BSQ (OR 1.09, 95% CI: 1.01, 1.18) (Figure 2).
Discussion
Our integrative study of OSA risk scores, comprehensive COPD outcomes, and CT lung structure in the SPIROMICS cohort provides confirmatory evidence of the deleterious associations between OSA risk and multiple clinical and physiologic measures of COPD severity while providing novel insights into the interplay between lung structure measures and OSA risk that related to measures of COPD. We found that individuals with high-risk OSA scores had lower lung function, and thicker upper and lower airways. However, we found that lung function strongly attenuated the effects of most lung structure measures on OSA risk with the exception of %tracheal wall area.
The incidence of OSA in those with COPD has been reported to be between 14%-66% based on severity of obstructive ventilatory defect.6,24,25 Individuals with both COPD and OSA have more respiratory symptoms and exacerbations as well as poorer quality of life.4,5 While having lung disease measured by a decrease in lung function (FEV1) is a known risk factor for having OSA,6 what had remained largely unknown was the contributions of lung structure on risk of OSA and its deleterious impact on COPD severity, independent of lung function impairment.
In line with previous reports,4,5,26 we demonstrate that individuals at high risk for OSA who previously used or are currently smoking tobacco with more than a 20 pack-year history experienced increased symptoms, impaired respiratory quality of life, and an increased frequency of exacerbations. Hence, while we were unable to confirm a diagnosis of OSA using PSG in these participants, we were indeed able to recapitulate the adverse effects of being at risk for OSA on COPD outcomes using 2 different OSA risk measures. It has been reported that those with severe emphysema have a less severe AHI9,>10 which may be why OSA is largely unrecognized in this population. Consistent with prior reports, we found that those at high risk for OSA had lower measures of quantitative emphysema on CT scan, however, severity of OSA based on the AHI was lacking. Further, it is hypothesized that individuals with increased air trapping and hyperinflation may experience a protective effect on the AHI due to pull on the upper airway allowing maintained patency. Contrary to this hypothesis, we showed that those with increased air trapping were at high risk for OSA. This may, in part, be due to the low overall median %air trapping in this cohort which may not translate to hyperinflation and the theoretic protective effect.
The novelty of this study is that we were able to identify those at high risk for OSA using 2 scores which were then applied to integrative approaches focused on determining the contribution of lung function and lung structure on determining OSA risk. For the DIS, every 10% decrease in FEV1 was associated with a 9% increased odds of having a high-risk OSA score, and that association was even stronger when lung structural measures were applied to the model with a 12% increased odds of high-risk DIS. Hence, having incremental impairments in lung function is independently associated with being at high risk for OSA in these current and former heavy users of cigarettes. This effect persisted by degree of dysanapsis, emphysema, air trapping, functional small airways disease, and tracheal wall area despite their individual structural effects on OSA risk. Of these, only tracheal wall area remained associated with being at high risk for OSA when lung function was considered in our models. Increased trachea wall area may be due to smooth muscle hypertrophy or tissue fibrosis and remodeling in the setting of chronic inflammation due to smoking, COPD, and potentially intermittent hypoxia. The tracheal changes seen here may be a correlate to a similar phenomenon of remodeling and thickening of the upper airway which is known to be associated with obstructive sleep apnea.27
The associations differed between the OSA risk score estimates, as evident by the stronger lung function and structural associations found for the DIS compared to the BSQ. It is important to note that although designed to identify OSA, sleep questionnaires also identify other breathing-related sleep disorders, some unique to those with COPD. Hence, the notable difference here could be related to the presence of multiple nocturnal arousals and comorbidities. The addition of sex to the score was a likely driver in the differences of OSA risk estimated between the 2 scores as men have a higher risk for OSA28 and often greater smoking histories compared to women.29 In this study, those at high risk for OSA based on the DIS had a higher pack-year smoking history which may have contributed to the extent of lung structural abnormalities. The inclusion of age, sex, neck circumference, and comorbidities in the DIS score also led to fewer participants being considered at high risk for OSA compared to the BSQ. We included sex and pack-year smoking histories into our models to address their individual impacts on OSA and COPD risk and severity. Atrial fibrillation and stroke are comorbidities that were considered on the DIS but not the BSQ; however, both questionnaires included hypertension, but compared to the self-reported data in the BSQ, the DIS used data extracted from the SPIROMICS database.
The more stringent criteria for being at high risk for OSA using the DIS might have allowed the identification of stronger lung function and structural associations with OSA risk compared to the broader and more inclusive BSQ. This highlights the need to develop OSA predictive tools specific to individuals with heavy smoking histories, those with COPD, or at risk for developing COPD. This is of particular importance as those with respiratory symptoms and frequent exacerbations related to COPD may receive focused care based on cough and dyspnea rather than sleep habits, leading to the underrecognition of OSA in this important at-risk group.
Limitations of this study include the lack of PSG to confirm the diagnosis and provide the type and severity of sleep apnea, lack of adjustment for the use of sedating medications, and the post hoc calculation of the DIS score. This cohort has known cardiac comorbidities that may have a component of central sleep apnea. Lack of PSG does not allow for an understanding of how central versus obstructive sleep apnea, as well as severity of disease, may alter the associations identified in this study. However, regardless of PSG-confirmed OSA, it is important to properly identify those at risk for OSA so that providers are not under or over-ordering a test that is cumbersome, time-consuming, and expensive to perform. Strengths of this study are the comprehensively characterized cohort of individuals with a prior or current history of heavy smoking with available spirometry and CT scans assessed for 2 different OSA risk scores.
We were able to demonstrate the strong association between lung function impairment and being at high risk for OSA, independent of lung structure while also demonstrating for the first time the contribution of lung structure measures, of which tracheal wall area impacts risk, independent of lung function. Our findings suggest that both lung function and structure contribute to the risk of OSA in those with heavy smoking histories and COPD and that the early recognition and treatment of OSA could have a beneficial impact on COPD-related symptoms, exacerbations, quality of life, and mortality.
Abbreviations
Abbreviations: % airway wall area=mean wall area percentage of the segmental airways; %pred=percentage predicted; AHI=apnea-hypopnea index; BMI=body mass index; BSQ=Berlin Sleep Questionnaire; CAT=COPD Assessment Test; CI=confidence interval; COPD=chronic obstructive pulmonary disease; CPAP=continuous positive airway pressure; DIS=DOISNORE tool; FEV1=forced expiratory volume in 1 second; GOLD=Global initiative for chronic Obstructive Lung Disease; HCU=health care utilization; HU=Hounsfield unit; TLC=total lung capacity; OR=odds ratio; OSA=obstructive sleep apnea; PRMfSAD=parametric response mapping for functional small airways disease; PPV=positive predictive value; PSG=polysomnogram; RV=residual volume; SD=standard deviation; SPIROMICS=The SubPopulations and InteRmediate Outcome Measures in COPD Study
Funding Statement
SPIROMICS was supported by contracts from the National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute (NHLBI) (HHSN268200900013C, HHSN268200900014C, HHSN268200900015C, HHSN268200900016C, HHSN268200900017C, HHSN268200900018C, HHSN268200900019C, HHSN268200900020C), grants from the NIH/NHLBI (U01 HL137880 and U24 HL141762), and supplemented by contributions made through the Foundation for the NIH and the COPD Foundation from AstraZeneca/MedImmune, Bayer, Bellerophon Therapeutics, Boehringer-Ingelheim Pharmaceuticals, Inc., Chiesi Farmaceutici S.p.A., Forest Research Institute, Inc., GlaxoSmithKline, Grifols Therapeutics, Inc., Ikaria, Inc., Novartis Pharmaceuticals Corporation, Nycomed GmbH; ProterixBio, Regeneron Pharmaceuticals, Inc., Sanofi, Sunovion, Takeda Pharmaceutical Company, and Theravance Biopharma and Mylan.
References
- 1.Woodruff PG,Barr RG,Bleecker E,et al. Clinical significance of symptoms in smokers with preserved pulmonary function. N Engl J Med. 2016;374(19):1811-1821. doi: https://doi.org/10.1056/NEJMoa1505971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Purani H,Friedrichsen S,Allen AM. Sleep quality in cigarette smokers: associations with smoking-related outcomes and exercise. Addict Behav. 2019;90:71-76. doi: https://doi.org/10.1016/j.addbeh.2018.10.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Esen AD,Akpinar M. Relevance of obstructive sleep apnea and smoking: obstructive sleep apnea and smoking. Fam Pract. 2021;38(2):181-186. doi: https://doi.org/10.1093/fampra/cmaa112 [DOI] [PubMed] [Google Scholar]
- 4.Omachi TA,Blanc PD,Claman DM,et al. Disturbed sleep among COPD patients is longitudinally associated with mortality and adverse COPD outcomes. Sleep Med. 2012;13(5):476-483. doi: https://doi.org/10.1016/j.sleep.2011.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zeidler MR,Martin JL,Kleerup EC,et al. Sleep disruption as a predictor of quality of life among patients in the subpopulations and intermediate outcome measures in COPD study (SPIROMICS). Sleep. 2018;41(5):zsy044. doi: https://doi.org/10.1093/sleep/zsy044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marin JM,Soriano JB,Carrizo SJ,Boldova A,Celli BR. Outcomes in patients with chronic obstructive pulmonary disease and obstructive sleep apnea: the overlap syndrome. Am J Respir Crit Care Med. 2010;182(3):325-331. doi: https://doi.org/10.1164/rccm.200912-1869OC [DOI] [PubMed] [Google Scholar]
- 7.Koch AL,Brown RH,Woo H,et al. Obstructive sleep apnea and airway dimensions in chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2020;17(1):116-118. doi: https://doi.org/10.1513/AnnalsATS.201903-220RL [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han MK,Kazerooni EA,Lynch DA,et al. Chronic obstructive pulmonary disease exacerbations in the COPDGene study: associated radiologic phenotypes. Radiology. 2011; 261(1):274-282. doi: https://doi.org/10.1148/radiol.11110173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu J,Zhao Z,Nie Q,et al. Effect of lung function on the apnea-hypopnea index in patients with overlap syndrome: a multicenter cross-sectional study. Sleep Breath. 2020;24(3):1059-1066. doi: https://doi.org/10.1007/s11325-019-01961-w [DOI] [PubMed] [Google Scholar]
- 10.Krachman SL,Tiwari R,Vega ML,et al. Effect of emphysema severity on the apnea-hypopnea index in smokers with obstructive sleep apnea. Ann Am Thorac Soc. 2016;13(7):1129-1135. doi: https://doi.org/10.1513/AnnalsATS.201511-765OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith BM,Kirby M,Hoffman EA,et al. Association of dysanapsis with chronic obstructive pulmonary disease among older adults. JAMA. 2020;323(22):2268-2280. doi: https://doi.org/10.1001/jama.2020.6918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Netzer NC,Stoohs RA,Netzer CM,Clark K,Strohl KP. Using the Berlin questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131(7):485-491. doi: https://doi.org/10.7326/0003-4819-131-7-199910050-00002 [DOI] [PubMed] [Google Scholar]
- 13.Namen AM,Forest DJ,Ahmad ZN,et al. Preoperative sleep questionnaires identify medical emergency team activation in older adults. J Am Med Dir Assoc. 2019;20(10):1340-1343. e2. doi: https://doi.org/10.1016/j.jamda.2019.04.024 [DOI] [PubMed] [Google Scholar]
- 14.Namen AM,Forest D,Saha AK,et al. DOISNORE50: a perioperative sleep questionnaire predictive of obstructive sleep apnea and postoperative medical emergency team activation. A learning health system approach to sleep questionnaire development and screening. J Clin Sleep Med. 2022;18(8):1909-1919. doi: https://doi.org/10.5664/jcsm.10006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management and prevention of COPD. 2021 report. GOLD website. Published 2021. Accessed April 2023. https://goldcopd.org/archived-reports/ [Google Scholar]
- 16.Couper D,LaVange LM,Han M,et al. Design of the subpopulations and intermediate outcomes in COPD study (SPIROMICS). Thorax. 2014;69(5):491-494. doi: https://doi.org/10.1136/thoraxjnl-2013-203897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller MR,Hankinson J,Brusasco V,et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319-338. doi: https://doi.org/10.1183/09031936.05.00034805 [DOI] [PubMed] [Google Scholar]
- 18.Sieren JP,Newell JD,Barr RG,et al. SPIROMICS protocol for multicenter quantitative computed tomography to phenotype the lungs. Am J Respir Crit Care Med. 2016;194(7):794-806. doi: https://doi.org/10.1164/rccm.201506-1208PP [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones PW,Harding G,Berry P,Wiklund I,Chen W-H,Kline Leidy N. Development and first validation of the COPD Assessment Test. Eur Respir J. 2009;34(3):648-654. doi: https://doi.org/10.1183/09031936.00102509 [DOI] [PubMed] [Google Scholar]
- 20.Jones PW,Quirk FH,Baveystock CM. The St George's Respiratory Questionnaire. Respir Med. 1991;85 Suppl B:25-31. doi: https://doi.org/10.1016/S0954-6111(06)80166-6 [DOI] [PubMed] [Google Scholar]
- 21.Han MK,Quibrera PM,Carretta EA,et al. Frequency of exacerbations in patients with chronic obstructive pulmonary disease: an analysis of the SPIROMICS cohort. Lancet Respir Med. 2017;5(8):619-626. doi: https://doi.org/10.1016/S2213-2600(17)30207-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galban CJ,Han MK,Boes JL,et al. Computed tomography-based biomarker provides unique signature for diagnosis of COPD phenotypes and disease progression. Nat Med. 2012; 18(11):1711-1715. doi: https://doi.org/10.1038/nm.2971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oelsner EC,Ortega VE,Smith BM,et al. A genetic risk score associated with chronic obstructive pulmonary disease susceptibility and lung structure on computed Tomography. Am J Respir Crit Care Med. 2019;200(6):721-731. doi: https://doi.org/10.1164/rccm.201812-2355OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soler X,Gaio E,Powell FL,et al. High prevalence of obstructive sleep apnea in patients with moderate to severe chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2015; 12(8):1219-1225. doi: https://doi.org/10.1513/annalsats.201407-336oc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanders MH,Newman AB,Haggerty CL,et al. Sleep and sleep-disordered breathing in adults with predominantly mild obstructive airway disease. Am J Respir Crit Care Med. 2003; 167(1):7-14. doi: https://doi.org/10.1164/rccm.2203046 [DOI] [PubMed] [Google Scholar]
- 26.Naranjo M,Willes L,Prillaman BA,Quan SF,Sharma S,et al. Undiagnosed OSA may significantly affect outcomes in adults admitted for COPD in an inner-city hospital. Chest. 2020;158(3):1198-1207. doi: https://doi.org/10.1016/j.chest.2020.03.036 [DOI] [PubMed] [Google Scholar]
- 27.Schellenberg JB,Maislin G,Schwab RJ. Physical findings and the risk for obstructive sleep apnea. The importance of oropharyngeal structures. Am J Respir Crit Care Med. 2000;162(2 Pt 1):740-748. doi: https://doi.org/10.1164/ajrccm.162.2.9908123 [DOI] [PubMed] [Google Scholar]
- 28.Senaratna CV,Perret JL,Lodge CJ,et al. Prevalence of obstructive sleep apnea in the general population: a systematic review. Sleep Med Rev. 2017;34:70-81. doi: https://doi.org/10.1016/j.smrv.2016.07.002 [DOI] [PubMed] [Google Scholar]
- 29.Thun MJ,Carter BD,Feskanich D,et al. 50-year trends in smoking-related mortality in the United States. N Engl J Med. 2013;368(4):351-364. doi: https://doi.org/10.1056/NEJMsa1211127 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This article contains supplemental material.