Abstract
Background
COVID‐19 limitations have hindered the implementation of new technologies by preventing proctors from coming to the site. We share our first experience of magnetic resonance imaging (MRI)‐guided focused ultrasound (MRgFUS) treatment with an international remote online proctorship, and develop and evaluate the methodology of remote MRgFUS proctorship.
Methods
This single‐center, nonrandomized controlled prospective study included 94 patients: 27 with essential tremor (ET) and 67 with tremor‐dominant Parkinson's disease (PD). The coming of proctors was impossible, so we arranged for the remote participation of proctors from the United Kingdom, Spain, and Israel. A total of 38 patients (40.4%) received telemedicine‐proctored treatment (proctor group) and 56 received their treatment independently (solo group). We used the Clinical Rating Scale for Tremor (CRST) for ET patients and the Unified Parkinson's Disease Rating Scale (UPDRS) Part III for PD patients.
Results
In patients with ET, success rates were 81.8% (proctor group) and 100% (solo group) (p = 0.22). CRST reduction on the treated side was 71.43% [65.83%; 80.56%] (proctor group) versus 60.87% [53.99; 79.58] (solo group) (p = 0.19). None of the patients showed worsening of tremors within 1 year. In patients with PD, the success rates were 92.6% (proctor group) and 100% (solo group) (p = 0.08). The UPDRS Part III improvement was 30.1% (proctor group) versus 39.9% (solo group) (p = 0.003). The 1‐year recurrence rate was 40% (proctor group) and 17.5% (solo group) (p = 0.04). No complications were observed at 6 months.
Conclusions
We developed a feasible and safe methodology for telemedicine remote online‐proctored MRgFUS treatment. No significant difference was observed between the solo and developed remote proctor protocols in terms of complication rate, effect, and long‐term results; however, UPDRS Part III improvement was better in the PD solo group. This study demonstrated that the MRgFUS international proctorship can be performed successfully remotely.
Keywords: essential tremor, MRgFUS, Parkinson's disease, proctor, telemedicine
General infrastructure for the magnetic resonance imaging‐guided focused ultrasound in the telemedicine mode.

Highlights
This study proposes the introduction of advances in information technology to launch and conduct online remote magnetic resonance imaging‐guided focused ultrasound neurosurgery because of the proctor's absence owing to COVID‐19 restrictions.
We developed a working protocol for remote proctorship that can be used in the future and assessed its effectiveness and safety.
1. INTRODUCTION
The COVID‐19 pandemic has not only affected the health and healthcare system in general but has also led to extensive economic, social, and political changes worldwide. Travel restrictions have slowed global economies; teachers and students have mostly moved to online digital and distance learning, which might have led to certain challenges. 1
The last decade has brought multiple breakthroughs in medical technologies for surgical interventions, but clinicians and their teams should be well prepared to use any of these technologies. Manufacturers usually provide special training programs for doctors to develop their theoretical and practical skills. A better solution is to collaborate with a proctor (a person who supervises and provides advice for initial interventions). 2 This partnership can mitigate the learning curve of complex procedures. 3 As manufacturers are mindful of their image and results, they prohibit launching product programs without assistance and supervision. Usually, 20–25 procedures are performed under supervision according to the manufacturer's policy, and then the team achieves “solo” status, which means that the team is authorized to perform procedures independently.
Magnetic resonance imaging‐guided focused ultrasound (MRgFUS) treatment is an alternative to surgical and radiological interventions, such as deep brain stimulation, stereotactic radiofrequency ablation, and gamma‐knife thalamotomy, for the treatment of movement disorders. This method is based on two physical phenomena: the thermal effect of focused ultrasound waves in the tissue and magnetic resonance, which allow visualization with a real‐time thermometry function. 4 The device was created in 2001, and treatment of the world's first nine patients with functional brain disorders was reported in 2009. 5 This method was approved in the United States in 2016, 6 Europe and Israel in 2017, 7 and Russia in 2017. 8
Travel restrictions due to the COVID‐19 pandemic have become a major problem in the introduction of innovations. In this article, we share our first experience of launching an MRI‐guided focused ultrasound thalamotomy program with an international remote online proctorship, as well as its efficacy, safety, and short‐term results.
The first MRgFUS neurosurgery center in Russia was opened in Ufa on May 5, 2020, under the scientific and methodological guidance of, and in close collaboration with, the Research Center of Neurology and the National Society for Parkinson's Disease and Movement Disorders.
The study was aimed at developing a methodology of remote proctorship in MRgFUS and evaluating the feasibility, efficacy, and safety of an online proctored MRgFUS program.
2. METHODS
2.1. Study design
This was a nonrandomized single‐center prospective study. Randomization was impossible as we were not approved to work “solo,” that is, independently at the study start. We enrolled 94 patients with essential tremor (ET) or tremor‐dominant Parkinson's disease (PD), who underwent MRgFUS thalamotomy. After 38 treatment procedures, the team was certified “solo”; therefore, 38 patients were treated under online telemedicine proctor supervision (“proctor” group) and 56 without supervision (“solo” group).
This 21‐month study started on May 5, 2020 with the first online proctored procedure at the first Russian MRgFUS neurosurgery center—Intelligent Neurosurgery Clinic of V. S. Buzaev Memorial International Medical Centre in Ufa, Russia.
The MRgFUS program was launched remotely because the proctors failed to come in person owing to COVID‐19 quarantine restrictions. In April 2020, Insightec Exablate 4000 MRgFUS equipment was installed. At that time, 32 patients were on the waiting list. As the COVID‐19 pandemic was unpredictable, we were not sure how long the travel restrictions would last. For ethical reasons, we started all the first cases with proctorship and did not randomize the patients into the “proctor” and “solo” groups.
2.2. Patients
All patients who were treated in our clinic from May 5, 2020, to March 2022 were included in the study. During this time, 94 procedures were performed; 27 patients with ET and 67 patients with PD were enrolled. Patients were not contraindicated to receive this type of treatment, including a significant decrease in cognitive function, administration of anticoagulants or antiplatelet agents, tumors and vascular malformations of the brain, and contraindications to MRI (claustrophobia, installed MRI‐incompatible pacemaker, etc.).
All patients were followed up and evaluated for baseline demographics, brain computed tomography and MRI characteristics, procedural outcomes, and complications. We used the Clinical Rating Scale for Tremor (CRST) 9 , 10 to evaluate the clinical degree of tremor in patients with ET, and the Unified Parkinson's Disease Rating Scale (UPDRS) Part III 11 to measure the severity of motor impairments in patients with PD. We used the UPDRS Part III scale in patients with PD because MRgFUS decreases both tremor and rigidity. Therefore, CRST in isolation could give inaccurate data on the result of the treatment of Parkinson's patients. The CRST was calculated before treatment, after each sonication, immediately after the operation, and at follow‐up; the same was done for the UPDRS Part III in patients with PD medication status was “off” when conducting UPDRS. All adverse reactions and complications were recorded during and after the procedures.
All patients were informed of the treatment technique, prior intervention experience, and remote proctorship. All participants signed a written consent form to participate in the research and agreed to share their personal data and stream videos with our international team members. None of the patients had contraindications.
2.3. Clinical definitions
We used the Consensus Statement of the Movement Disorder Society on Tremor definitions of tremors and diagnostic criteria for diagnosis and classification. 12 “An attempt” was defined as a situation in which the patient was placed on the MRgFUS table and the first sonication was performed.
“Procedural success” was defined as a decrease in tremor as measured by the CRST or UPDRS Part III scale on the treated side after the procedure, a minimal clinically significant improvement on the UPDRS Part III motor examination for PD patients (3.25 points), or CRST for ET patients, as defined in previous studies. 13
We also used a subjective scale of our team members' consensus in assessing the results: “excellent,” when movement disorder symptoms were eliminated and goals were achieved; “good,” when some insignificant symptoms remained; and “compromise,” when some positive result was achieved, but we had to stop the procedure because the risk seemed to outweigh the expected benefit. These three categories were considered “successful.” The result was considered “unsuccessful” if no benefit was observed after the operation. None of the patients showed worsening symptoms.
MRI‐guided focused ultrasound treatment (MRgFUS): Using the Insightec Exablate neurosurgical system with 1024 of 650 kHz piezoelectric elements, a controlled noninvasive thermal effect on tissues is exhibited with the highest accuracy (error 0.50–0.75 mm), without the use of ionizing radiation, incisions, and the need for anesthesia. 14
2.4. Remote proctorship
Insightec's policy and common sense both require the supervision of proctors during the first steps of the learning curve. As the proctors could not arrive in person for the opening of the operating room on May 5, 2020 due to the COVID‐19 pandemic, we implemented the world's first telemedicine MRgFUS technology by creating a virtual presence of Insightec proctors.
In addition to the traditional videoconference routine, we addressed three technical issues regarding the first telemedicine‐focused ultrasound treatment.
First, to ensure the safety and constant visual monitoring of the patient's condition, we had to safely stream real‐time videos of the patient's status in the MRI room during treatment. Moreover, the international team had to assess the neurological tests performed by our neurological team. However, we could not place the cameras directly inside the MRI room because of the constant magnetic field. During the first three procedures, a Microsoft Web camera was placed on the ceiling of the MRI room; however, during the temperature measurement of the sonication target point the MRI signal was extremely noisy, and the only solution was to install the equipment in front of the observation window (camera 2 in Figure 1). We streamed the webcam video to the video conference. Additionally, during the neurological tests, we used a portable MacBook Pro laptop with a built‐in camera (portable laptop 2 in Figure 1).
Figure 1.
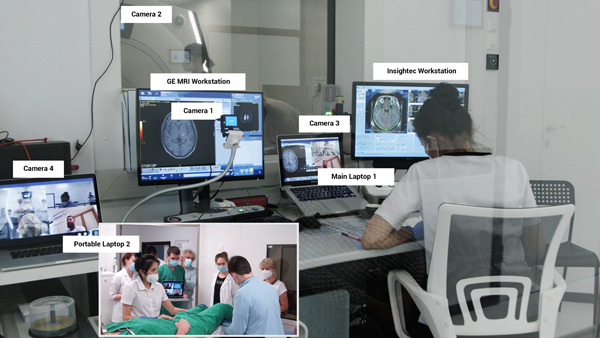
Position of cameras.
Second, telemetry from the Insightec MRgFUS equipment had to be transmitted to an international team of engineers to monitor how consistent the chosen treatment tactics were with the equipment settings and, if necessary, to adjust the settings to achieve the best possible result. As this was the first focused ultrasound treatment via telemedicine, we had no ready‐to‐use or approved technical solutions. We analyzed the available technologies and chose Virtual Network Computing (VNC) and Port Address Translation to secure remote connection sessions for proctors from the United Kingdom, Spain, Israel, and St. Petersburg during treatment (Figure 2).
Figure 2.
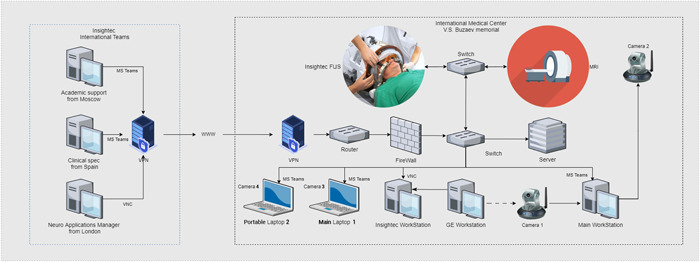
General infrastructure for the magnetic resonance imaging‐guided focused ultrasound in the telemedicine mode.
Third, the patient's MRI images had to be transferred to the international team to assess the effectiveness of the treatment and suggest corrections to our strategy and tactics. As the General Electric MRI device is a closed system with no available data interfaces, we were unable to connect any image capture technology to obtain images and transmit them in a manner similar to HDMI to USB adapters. Instead, we used a camera aimed at the MRI monitor to stream the video to all participants (camera 1 in Figure 1). This stream was not used for clinical decisions; rather, it was used to guide our MRI technicians in console settings. The real‐time maximum quality MRI image was transferred to an Insightec Exablate 4000 (Insightec Workstation, Figure 1) according to the Insightec device protocol. The images were then streamed from the Insightec console to the proctors over the VNC (Figure 2). Microsoft Teams was used as the platform for video conferencing among all participants (Figure 2). The choice of this technology was determined by the corporate standards of Insightec, and its functionality met all our requirements. Four cameras were used: camera 1 for the MRI console, camera 2 for the MRI room, camera 3 for the neurosurgeon, and camera 4 for neurological tests and temporary close‐up examinations inside the MRI room. “Portable” laptop 2 with camera 4 was placed in the MRI room only when the MRI machine was stopped (Figure 1).
The neurosurgeon, radiologist, and MRI technician communicated with proctors using “main” laptop 1 (Figure 2). Using a mouse, the neurosurgeon and Insightec proctors controlled the console of the Insightec Workstation over the VNC. The radiologist and MRI technician controlled the console of the MRI workstation.
By resolving these three issues, we ensured an adequate safety level for patients during the world's first telemedicine‐focused ultrasound treatment.
2.5. Statistical analysis
R language (version 4.1.1; 2021‐08‐10) was used for data analysis. Continuous variables are presented as medians and quartiles [Q1; Q3]. Categorical variables are presented as frequencies (%). A Shapiro–Wilk normality test was performed. Due to the small sample size, a nonparametric Wilcoxon's test was used to compare the groups. To assess the surgical effect using the CRST and UPDRS scales, we used the Wilcoxon test for paired data. To analyze linear dependencies, a correlation test was used to determine the correlation coefficient, R. Statistical significance was set at p < 0.05.
3. RESULTS
Of the 94 patients treated using MRgFUS in our center, 38 patients (40.4%) underwent MRgFUS thalamotomy under an online international proctorship. Of these 94 patients, 27 had ET and 67 had tremor‐dominant PD (Figure 3).
Figure 3.
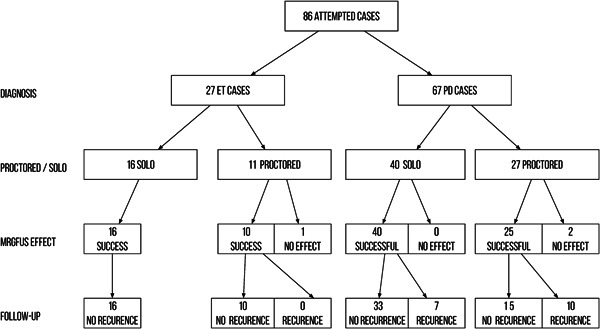
Flow diagram of the treatments.
The median age of the treated patients was 61.5 [50.0; 68.3] years (range, 21–82 years), and no age difference was observed between the men and women (p = 0.88, Wilcoxon's test).
After the procedure, all patients were monitored on a regular basis with a median follow‐up time of 109.0 [53.0; 231.0] days (maximum 625 days).
3.1. ET
Twenty‐seven patients with severe refractory ET (17 males and 10 females) underwent MRgFUS thalamotomies. The baseline clinical characteristics of the patients with ET are shown in Table 1. Tremor duration ranged from 3 to 58 years (median, 26.0 [18.5; 34.8] years). Severity was assessed using the CRST. Median skull density ratio (SDR, Skull Score) was 0.5 [0.4; 0.6], ranging from 0.34 to 0.69, with less dense bones of the skull (higher Skull Score) tended to increase the operation time.
Table 1.
Baseline clinical characteristics of essential tremor patients.
| Variable | Overall,a n = 27 | Solo,a n = 16 | Proctor,a n = 11 | p Valueb |
|---|---|---|---|---|
| Age (years) | 49.0 (35.0, 64.0) | 56.0 (38.2, 63.5) | 39.0 (33.0, 62.5) | 0.5 |
| Skull area | 317.0 (301.0, 356.0) | 316.0 (282.5, 344.5) | 329.5 (315.5, 376.2) | 0.3 |
| Skull score | 0.5 (0.4, 0.6) | 0.5 (0.4, 0.6) | 0.5 (0.4, 0.6) | >0.9 |
| Disease duration (years) | 26.0 (18.5, 34.8) | 30.0 (22.5, 45.0) | 20.0 (17.5, 24.0) | 0.045 |
| Sex | 0.7 | |||
| Female | 10.0 (37.0) | 5.0 (31.2) | 5.0 (45.5) | |
| Male | 17.0 (63.0) | 11.0 (68.8) | 6.0 (54.5) | |
| Successful result | 0.4 | |||
| No | 1.0 (3.7) | 0 | 1.0 (9.1) | |
| Yes | 26.0 (96.3) | 16.0 (100.0) | 10.0 (90.9) | |
| Number of sonications | 5.0 (4.0, 7.0) | 5.0 (4.5, 7.0) | 5.5 (4.2, 7.8) | 0.9 |
Data are presented as median (interquartile range) or n (%).
Wilcoxon's rank sum test; Fisher's exact test; Wilcoxon's rank sum exact test.
In the majority of cases (23 of 27 patients), a unilateral intervention was performed: the left thalamus (right‐side symptoms) was targeted in 17 patients and the right thalamus (left‐side symptoms) in six patients. The literature describes cases of successive bilateral ET treatments with a 6–9‐month interval between procedures. 15 In our study, two patients were treated successively and two patients were treated on both sides within a single procedure. The baseline clinical characteristics of the patients are summarized in Table 1.
One patient with ET (3.8%) under proctorship did not respond to 15 trial sonications; no effective target was found in the typical location of the ventralis intermediate nucleus (VIM) nucleus, and the patient was discharged without any results and without complications. In the remaining 26 patients, the effect was considered satisfactory (any reduction in contralateral tremor). In the proctor group, the overall success rate was 10/11 (81.8%), and in the solo group, 16/16 (100%), χ 2 p = 0.2191. The overall CRST score reduction was 37.51% (V = 351, p < 0.001), and on the side of the body corresponding to the focus of destruction (contralateral to the intervention, “treatment” side) CRST score reduction reached 64.73% (V = 325, p < 0.001). A boxplot of the CRST reduction (%) on the treatment side is shown in Figure 4. On the control side, the difference in CRST before and after treatment was not significant (V = 15, p > 0.05). In the proctor group, the CRST score reduction on the treated side was 71.43% [65.83%; 80.56%] versus 60.87% [53.99%; 79.58%] in the solo group (p = 0.19).
Figure 4.
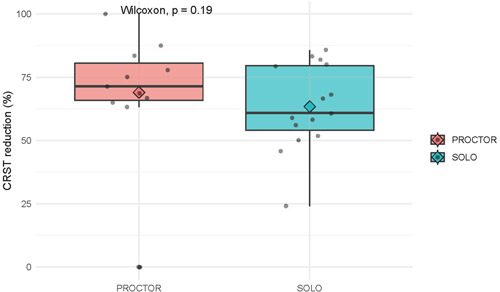
Boxplot of Clinical Rating Scale for Tremor (CRST) reduction (%) on the treatment side. Wilcoxon, p = 0.19.
The median duration of the operation, from the first to the last sonication, was 115.07 [81.02; 132.28] min, with the fastest operation taking 30.7 min and the longest one, 189.1 min.
Regarding intraoperative adverse reactions, one patient had an abnormal reaction to cold water inside the helmet that manifested as chills and short‐term respiratory arrest; headache was observed in 11 patients, dizziness in four, nausea in three, vomiting in two, numbness of the tongue and oral region in one, and ataxia in one. These symptoms were short‐term and disappeared immediately after the patients were transferred to the ward. Gait instability that disappeared completely after 2 weeks was observed in five patients after the intervention, which is consistent with the data of Cacho‐Asenjo et al. 16 Regarding anamnesis, 27 (100%) patients received drug therapy, but due to side effects and insufficient effectiveness, 26 patients stopped taking these drugs even before applying MRgFUS treatment. Among the reasons for discontinuation of drug therapy, propranolol was canceled due to low efficacy at low doses, with an increase in dosage due to general weakness and a decrease in blood pressure and pulse rate; topiramate due to low efficiency with prolonged use, lethargy, and insomnia; primidone was not available in the country; and alprazolam caused drowsiness, lethargy, and addiction in one patient. In this addiction case, we eliminated the tremor and discontinued alprazolam with the help of a narcologist. None of the patients required antitremor therapy after MRgFUS treatment or 1 year later. During the observation period, none of the 26 successfully treated patients showed recurrence (after the complete disappearance of hyperkinesis) or an increase in tremor (after its postoperative decrease).
3.2. PD
Sixty‐seven patients with tremor‐dominant PD (48 males and 19 females) underwent MRgFUS thalamotomies. The criteria for selecting patients for surgery included several factors: tremor resistance to levodopa therapy, the short effect of levodopa‐containing drugs (less than 2 h), the presence of complications of levodopa therapy (asymmetric dyskinesia, more pronounced on the operated side), refusal to take levodopa‐containing drugs (levodopa phobia), and use of anti‐parkinsonian drugs from other groups. The baseline clinical characteristics of the patients with PD are shown in Table 2. The disease duration ranged from 3 to 37 years (median, 5.0 [4.0, 9.0] years). The severity was assessed by the UPDRS Part III scale. The median skull density ratio (SDR, Skull Score) was 0.5 [0.4; 0.6], ranging from 0.32 to 0.70.
Table 2.
Baseline clinical characteristics of Parkinson's disease patients.
| Variables | Overalla | Solo, n = 40a | Proctor, n = 27a | p Valueb |
|---|---|---|---|---|
| Age (years) | 63.0 (55.0, 70.0) | 63.0 (58.5, 69.5) | 64.0 (51.2, 72.0) | 0.7 |
| Skull area | 348.0 (333.0, 365.8) | 346.0 (333.5, 365.0) | 354.0 (322.0, 365.5) | 0.8 |
| Skull score | 0.5 (0.4, 0.6) | 0.5 (0.4, 0.6) | 0.5 (0.4, 0.6) | >0.9 |
| Disease duration (years) | 5.0 (4.0, 9.0) | 6.0 (5.0, 11.2) | 4.5 (3.8, 5.2) | 0.058 |
| Sex | 0.8 | |||
| Female | 19.0 (28.4) | 11.0 (27.5) | 8.0 (29.6) | |
| Male | 48.0 (71.6) | 29.0 (72.5) | 19.0 (70.4) | |
| Thalamus side | 0.3 | |||
| Left | 38.0 (56.7) | 25.0 (62.5) | 13.0 (48.1) | |
| Right | 29.0 (43.3) | 15.0 (37.5) | 14.0 (51.9) | |
| Success | 0.2 | |||
| No | 2.0 (3.0) | 0 | 2.0 (7.4) | |
| Yes | 65.0 (97.0) | 40.0 (100.0) | 25.0 (92.6) | |
| Number of sonications | 6.0 (5.0, 8.0) | 6.0 (5.0, 9.0) | 6.0 (5.0, 8.0) | 0.6 |
| UPDRS Part III before | 54.0 (43.0, 65.0) | 56.0 (46.5, 71.5) | 47.5 (38.2, 59.5) | 0.035 |
| UPDRS Part III after | 33.0 (24.0, 40.8) | 35.5 (26.0, 39.8) | 29.5 (24.0, 47.2) | >0.9 |
Abbreviation: UPDRS, Unified Parkinson's Disease Rating Scale.
Data are presented as median (interquartile range) or n (%).
Wilcoxon's rank sum exact test; Wilcoxon's rank sum test; Pearson's χ 2 test; Fisher's exact test.
In all 67 cases, a unilateral intervention was performed: the left thalamus (right‐sided symptoms) was targeted in 38 patients and the right thalamus (left‐sided symptoms) in 29 patients.
In the PD proctor group, procedural success was achieved in 25/27 (92.6%) and in the PD solo group, in 40/40 (100%; p = 0.08, χ 2 test). The 1‐year recurrence rate was 10/25 (40%) in the proctor group and 7/40 (17.5%) in the solo group (p = 0.04, χ 2 test). In the whole cohort of PD patients, the UPDRS Part III score after the operation significantly decreased from 54.0 [43.0; 65.0] to 33.0 [24.0; 40.8] (Wilcoxon's signed rank test, p < 0.001). A boxplot of the UPDRS Part III before and immediately (1 h) after the operation is shown in Figure 5. The proctors did not approve of pallidothalamic tractotomy (PTT) as a target.
Figure 5.
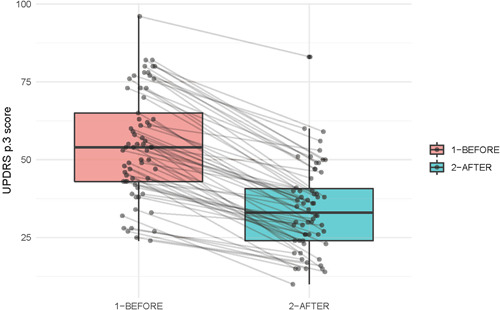
Boxplot of Unified Parkinson's Disease Rating Scale (UPDRS) Part III before and immediately (1 h) after the operation. Wilcoxon, p < 0.001.
In the solo group, we used the VIM nucleus target in 30 cases and the PTT target in 25 cases (including 15 patients with both targets). After publications 17 , 18 , 19 on successful treatments with PTT targets, we added PTT to our therapies. In 7/30 (23%) VIM nucleus target patients and 3/25 (12%) PTT target patients, we observed some recurrence of tremor 1 year after the operation. One patient underwent successful reoperation. One patient had dysarthria within 1 year, and two patients had significant nontremor disease progression with dystonia.
In the proctor group, the VIM target was used in all 27 cases; in two cases, the operation was assessed as ineffective, and 10 patients (37%) showed a recurrence of tremor 1 year after successful treatment. Five patients with tremor recurrence underwent successful MRgFUS reoperation and one patient was treated with deep brain stimulation.
During the procedure, six patients had headaches, two had dizziness, three had nausea, one had vomiting, one had numbness of the tongue and oral region, and one had transitory dysarthria. Symptoms resolved immediately after the procedure. One patient had ataxia at 1‐year follow‐up. One patient died 2 years after the operation, and the cause of death was unrelated to PD or MRgFUS (an accident). Before MRgFUS, all 67 patients underwent PD therapy with levodopa‐containing medications, dopamine receptor agonists, amantadine derivatives, and MAO inhibitors. Unfortunately, PD is a steadily progressive disease, MRgFUS surgery is a symptomatic treatment, and we cannot confirm the withdrawal of drug therapy after treatment. However, in many patients, the daily dose of anti‐parkinsonian drugs was reduced 1 month after treatment (17 patients). Only two patients discontinued anti‐parkinsonian therapy because of the temporary absence of clinical manifestations of the disease. One of these patients returned to taking dopamine receptor agonists because of the development of Parkinsonism on the other side. During the annual follow‐up after surgery, after 12 months, all patients returned to the previous level of taking anti‐parkinsonian drugs but maintained the described clinical improvement in decreasing tremor intensity and UPDRS.
4. DISCUSSION
This study demonstrated that online proctorship for the MRgFUS procedure was effective and safe. No publications with keywords “MRgFUS,” “proctor”, or “telemedicine” were found in Google Scholar, Pubmed, or ScienceDirect. The MRgFUS device manufacturer, Insightec, reported our case with telemedical proctorship as the first in their world practice.
The overall success rate of ET shortly after the program launch was 26/27 (96.3%). The ET group had no cases of the recurrence of tremor in the proctor or solo groups. This result corresponds to the published nonproctored operations experience, where tremor suppression after MRgFUS thalamotomy for ET was stably maintained for 2 years, and late complications were usually not observed after treatment. 20 , 21
In the PD proctor group, procedural success was achieved in 92.6% of patients, and in the solo group, 100%, with a significant decrease in the UPDRS Part III score after the operation; this corresponds to the results of Eisenberg et al. 22 The 1‐year recurrence rate in the proctor (40%) and solo groups (17.5%) was comparable with a published report, where two of 26 patients had full recurrence of tremor and eight of 26 patients had partial recurrence. 23
In ET, the reduction of the CRST score was not statistically different between the proctor and solo groups, but in PD, the results in the solo group were significantly better. A boxplot of the UPDRS Part III reduction (%) in the solo and proctor groups is shown in Figure 6. This can be explained by the fact that proctors were limited to a strict protocol using the VIM nucleus target, whereas other targets for the treatment of Parkinson's are described in the literature, such as PTT or subthalamotomy (STN). 24 In 2014, Magara et al. demonstrated that the feasibility, safety, and accuracy of MRgFUS with PTT targets are comparable with radiofrequency ablation, but white matter requires more thermal exposure. 25 Tremor‐dominant PD is a good indication for MRgFUS thalamotomy and has been validated in several clinical trials. 26 Bond et al. reported differences in the clinical outcomes between patients with PD who underwent unilateral MRgFUS thalamotomy and those who underwent a sham procedure. They found that the median tremor scores in 20 patients improved by 62% from baseline, and the median UPDRS motor scores while on medication also improved by 8 points from baseline after MRgFUS thalamotomy, which was much better than the outcome of the sham procedure. 25 Martínez‐Fernández et al. used a different target, 27 they demonstrated a reduction in the MDS‐UPDRS Part III off‐medication score on the treated side by 53%, rigidity by 71%, akinesia by 37%, and tremors by 77% 6 months after STN. This emphasizes the need for more flexible proctorship to achieve a high success rate.
Figure 6.
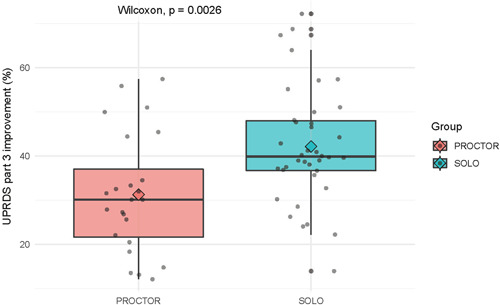
Boxplot of Unified Parkinson's Disease Rating Scale (UPDRS) Part III reduction (%) in solo and proctor groups. Wilcoxon, p = 0.0026.
This was a single‐center, nonrandomized, controlled prospective study. We compared a sample of patients who were treated during proctorship with those subsequently treated independently by us alone. They are two different samples, and the most important bias is the learning effect, which can increase effectiveness in later cases (solo group). However, we showed that the results in earlier cases did not significantly differ from those treated by more experienced surgeons. Patients could not be randomized because proctorship is essential during the launch and for a certain number of initial cases (after the learning curve, it is no longer required). Another concern regarding the credibility of the results was related to the controls. For comparison, we used patients operated on with online proctors and those operated on by an experienced team without proctors. A study could be done comparing online with on‐site proctoring. However, such a design was impossible owing to pandemic travel bans. A single‐center, nonrandomized study with a relatively small number of patients may be regarded as a drawback of this report, but also as the potency of a center to introduce this complex and highly sophisticated online program. Under these conditions, we found a solution implemented by the subsequent centers that launched the technology. We hope that our experience in this critical situation will help others. This study shows that online proctorship is effective and safe in terms of immediate procedural success. A large randomized multicenter study is needed to analyze the cost‐effectiveness and long‐term clinical outcomes of online and offline proctorships.
In conclusion, we developed a feasible and safe methodology for telemedicine remote online‐proctored MRgFUS treatment. No procedure‐related complications were observed after telemedicine‐proctored treatment in the entire cohort of patients. In patients with ET, no significant differences were observed between the treatment effects in the solo and proctor groups in terms of complication rates, effects, and long‐term results.
In our case, the learning curve of treatment under proctorship included 38 patients, after which our medical center received a solo license. Obtaining a solo license took more time than usual (20–25 procedures) as online is more challenging than offline; moreover, this first‐in‐practice experience reasonably required more support. At the clinical level, this study demonstrated that with today's impressive development of the Internet, we can do multiple things online and that the MRgFUS international proctorship can be performed successfully remotely.
AUTHOR CONTRIBUTIONS
Igor V. Buzaev: Research project (conception, organization, execution); statistical analysis (design, execution); manuscript preparation (writing of the first draft). Rezida M. Galimova: Research project (conception, organization, execution); statistical analysis (design, execution, review, and critique); manuscript preparation (review and critique). Dinara I. Nabiullina: Research project (execution); statistical analysis (review and critique); manuscript preparation (review and critique). Sergey N. Illarioshkin: Research project (organization); statistical analysis (review and critique); manuscript preparation (review and critique). Naufal S. Zagidullin: Statistical analysis (review and critique); manuscript preparation (review and critique). Shamil M. Safin: Manuscript preparation (review and critique).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest. Professor Igor V. Buzaev is a member of Chronic Diseases and Translational Medicine editorial board and is not involved in the peer review process of this article.
ETHICS STATEMENT
This study was approved by the Ethical Committee of the Research Center of Neurology (Moscow). All patients were informed about the treatment technique, number of cases operated before them, and remote proctorship. They all signed a written consent to participate in the research and agreed to share personal data and broadcast video to our international team members and were fully aware of the treatment, results, and risks.
ACKNOWLEDGMENTS
The authors thank Research Center of Neurology, Moscow, Russia; National Society of Movement disorders; and Parkinson Disease Research and Bashkir State Medical University for academic support.
Buzaev IV, Galimova RM, Nabiullina DI, Illarioshkin SN, Zagidullin NS, Safin SM. Magnetic resonance imaging‐guided focused ultrasound thalamotomy launch with remote telemedicine international proctorship. Chronic Dis Transl Med. 2024;10:40‐50. 10.1002/cdt3.92
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Schnitzler L, Janssen LMM, Evers SMAA, et al. The broader societal impacts of COVID‐19 and the growing importance of capturing these in health economic analyses. Int J Technol Assess Health Care. 2021;37:e43. 10.1017/S0266462321000155 [DOI] [PubMed] [Google Scholar]
- 2. Broering DC, Berardi G, El Sheikh Y, Spagnoli A, Troisi RI. Learning curve under proctorship of pure laparoscopic living donor left lateral sectionectomy for pediatric transplantation. Ann Surg. 2020;271(3):542‐548. 10.1097/SLA.0000000000002948 [DOI] [PubMed] [Google Scholar]
- 3. Gurevich S, John R, Kelly RF, et al. Avoiding the learning curve for transcatheter aortic valve replacement. Cardiol Res Pract. 2017;2017:7524925. 10.1155/2017/7524925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Galimova RM, Illarioshkin SN, Buzaev IV, Kachemaeva OV. Terapiya dvigatel'ny'x narushenij metodom fokusirovannogo ul'trazvuka pod kontrolem magnitno‐rezonansnoj tomografii. Rekomendacii dlya vrachej‐nevrologov po otboru pacientov [Therapy of motor disorders by the method of focused ultrasound under the control of magnetic resonance imaging. recommendations for neurologists on selection of patients] [in Russian]. Byul Nac ob‐va po izuch bolezni Parkinsona i rasstrojstv dvizheniya. 2020;1:9‐15. [Google Scholar]
- 5. Martin E, Jeanmonod D, Morel A, Zadicario E, Werner B. High‐intensity focused ultrasound for noninvasive functional neurosurgery. Ann Neurol. 2009;66(6):858‐861. [DOI] [PubMed] [Google Scholar]
- 6. FDA . FDA approves first MRI‐guided focused ultrasound device to treat essential tremor. 2016. Accessed February 13, 2022. https://www.fda.gov/news-events/press-announcements/fda-approves-first-mri-guided-focused-ultrasound-device-treat-essential-tremor
- 7. Zaaroor M, Sinai A, Goldsher D, Eran A, Nassar M, Schlesinger I. Magnetic resonance‐guided focused ultrasound thalamotomy for tremor: a report of 30 Parkinson's disease and essential tremor cases. J Neurosurg. 2018;128(1):202‐210. 10.3171/2016.10.JNS16758 [DOI] [PubMed] [Google Scholar]
- 8.Registracionnoe udostoverenie na medicinskoe izdelie ot 13.02.2017 No RZN 2017/5378, F.S.P.N.V.S.Z. (ROSZDRAVNADZOR), Editor. 2017.
- 9. Fahn S, Tolosa E, Marin C. Clinical Rating Scale for Tremor. Urban & Schwarzenberg; 1988:225‐234. [Google Scholar]
- 10. Fahn S, Tolosa E, Conceppcion M. Clinical rating scale for tremor. In: Jankovic J, Tolosa E, eds. Parkinson's Disease and Movement Disorders. Williams and Wilkins; 1993:271‐280. [Google Scholar]
- 11. Movement Disorder Society Task Force on Rating Scales for Parkinson's Disease . The Unified Parkinson's Disease Rating Scale (UPDRS): status and recommendations. Mov Disord. 2003;18(7):738‐750. 10.1002/mds.10473 [DOI] [PubMed] [Google Scholar]
- 12. Deuschl G, Bain P, Brin M. Consensus statement of the Movement Disorder Society on Tremor. Mov Disorders. 1998;13(suppl 3):2‐23. 10.1002/mds.870131303 [DOI] [PubMed] [Google Scholar]
- 13. Eisenberg HM, Krishna V, Elias WJ, et al. MR‐guided focused ultrasound pallidotomy for Parkinson's disease: safety and feasibility. J Neurosurg. 2020;135(3):792‐798. [DOI] [PubMed] [Google Scholar]
- 14. INSIGHTEC . Insightec for neurosurgery. Insightec. 2017. Accessed January 25, 2020. http://www.insightec.com/clinical/neurosurgery
- 15. Martínez‐Fernández R, Mahendran S, Pineda‐Pardo JA, et al. Bilateral staged magnetic resonance‐guided focused ultrasound thalamotomy for the treatment of essential tremor: a case series study. J Neurol Neurosurg Psychiatry. 2021;92(9):927‐931. 10.1136/jnnp-2020-325278 [DOI] [PubMed] [Google Scholar]
- 16. Cacho‐Asenjo E, Honorato‐Cia C, Nuñez‐Cordoba JM, et al. Factors associated with headache and nausea during magnetic resonance‐guided focused ultrasound for tremor. Mov Disord Clin Pract. 2021;8(5):701‐708. 10.1002/mdc3.13210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gallay MN, Moser D, Magara AE, Haufler F, Jeanmonod D. Bilateral MR‐guided focused ultrasound pallidothalamic tractotomy for Parkinson's disease with 1‐year follow‐up. Front Neurol. 2021;12:601153. 10.3389/fneur.2021.601153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yamamoto K, Ito H, Fukutake S, et al. Focused ultrasound thalamotomy for tremor‐dominant Parkinson's disease: a prospective 1‐year follow‐up study. Neurol Med Chir. 2021;61:414‐421. 10.2176/nmc.oa.2020-0370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lu H, Wang X, Lou X. Current applications for magnetic resonance‐guided focused ultrasound in the treatment of Parkinson's disease. Chin Med J. 2023;136:780‐787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chang JW, Park CK, Lipsman N, et al. A prospective trial of magnetic resonance‐guided focused ultrasound thalamotomy for essential tremor: results at the 2‐year follow‐up. Ann Neurol. 2018;83(1):107‐114. 10.1002/ana.25126 [DOI] [PubMed] [Google Scholar]
- 21. Gallay MN, Moser D, Jeanmonod D. MR‐guided focused ultrasound cerebellothalamic tractotomy for chronic therapy‐resistant essential tremor: anatomical target reappraisal and clinical results. J Neurosurg. 2021;134:376‐385. 10.3171/2019.12.JNS192219 [DOI] [PubMed] [Google Scholar]
- 22. Eisenberg HM, Krishna V, Elias WJ, et al. MR‐guided focused ultrasound pallidotomy for Parkinson's disease: safety and feasibility. J Neurosurg. 2021;135(3):792‐798. 10.3171/2020.6.JNS192773 [DOI] [PubMed] [Google Scholar]
- 23. Sinai A, Nassar M, Sprecher E, Constantinescu M, Zaaroor M, Schlesinger I. Focused ultrasound thalamotomy in tremor dominant Parkinson's disease: long‐term results. J Parkinsons Dis. 2022;12(1):199‐206. 10.3233/JPD-212810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fasano A, Lozano AM, Cubo E. New neurosurgical approaches for tremor and Parkinson's disease. Curr Opin Neurol. 2017;30(4):435‐446. 10.1097/WCO.0000000000000465 [DOI] [PubMed] [Google Scholar]
- 25. Magara A, Bühler R, Moser D, Kowalski M, Pourtehrani P, Jeanmonod D. First experience with MR‐guided focused ultrasound in the treatment of Parkinson's disease. J Ther Ultrasound. 2014;2:11. 10.1186/2050-5736-2-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bond AE, Shah BB, Huss DS, et al. Safety and efficacy of focused ultrasound thalamotomy for patients with medication‐refractory. JAMA Neurol. 2017;74(12):1412‐1418. 10.1001/jamaneurol.2017.3098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Martínez‐Fernández R, Rodríguez‐Rojas R, Del Álamo M, et al. Focused ultrasound subthalamotomy in patients with asymmetric Parkinson's disease: a pilot study. Lancet Neurol. 2018;17(1):54‐63. 10.1016/S1474-4422(17)30403-9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


