Abstract
Insulin is used as a therapeutic agent in patients with diabetes, and cutaneous lipohypertrophy (LH) and localized insulin‐derived amyloidosis (LIDA) are well‐known adverse effects associated with insulin injections. The clinical implications, management, assessment methods, and pathological differentiation of LH and LIDA have been recently updated. This review was to update our knowledge of the pathological differentiation, effects of insulin absorption, hypoglycemic events, and recent assessment methods for LH and LIDA. A scoping review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta Analyses extension for Scoping Reviews guidelines. Original studies and case reports in English were also included. PubMed and Scopus databases were searched for keywords to identify papers published up to January 2022. A total of 113 studies were identified through a database search, and 31 were eligible for inclusion in this scoping review. In the 31 studies included in this review, patients with type 2 diabetes had high frequencies of LH and LIDA. LH outcome parameters were assessed using pathological findings and imaging. LIDA is mainly determined by pathological methods, such as hematoxylin and eosin and Congo red staining. Several in vitro and in vivo LIDA models of LIDA have been developed. These results suggest that pathological analysis is required to identify LH and LIDA. It is important to consider LIDA, as it likely influences insulin adsorption and glycemic control. Although several studies have evaluated the LIDA process, little is known about the mechanisms underlying the development of adverse effects associated with insulin injections.
Keywords: diabetes mellitus, insulin injection, lipohypertrophy, localized insulin‐derived amyloidosis
A Preferred Reporting Items for Systematic Preferred Reporting Items for Systematic Reviews and Meta Analyses extension for Scoping Reviews flowchart of the current review is presented. A total of 31 articles were included in the review.
Key points
Lipohypertrophy (LH) and localized insulin‐derived amyloidosis (LIDA), which are different pathologies, are difficult to distinguish macroscopically, and a definitive diagnosis requires histological evaluation of invasive biopsy samples.
Although several studies have evaluated the LIDA process in vivo and in vitro, little is known about the mechanisms underlying the development of adverse effects associated with insulin injection.
Further studies are required to determine the in vivo pathologic processes of LH and LIDA.
1. INTRODUCTION
Diabetes mellitus (DM) is an epidemic, with the number of global patients predicted to rise to 783 million by 2045. 1 All patients with type I and almost 30% with type Ⅱ DM rely on exogenous insulin injections. Allergic reactions to insulin, insulin‐induced cutaneous lipohypertrophy (LH), and localized insulin‐derived amyloidosis (LIDA) are well‐known adverse effects associated with insulin injection. 2 , 3 , 4
Insulin‐induced LH is one of the most common complications of subcutaneous insulin injections. LH involves the thickened swelling of the tissue, which can be either soft or firm. The prevalence of LH was considerably higher in patients with insulin dependence. It is estimated that 50% of the patients with type I diabetes develop LH over the course of insulin injections. LH is related to the lipogenic and anabolic properties of insulin. 5 , 6 Compared with normal adipose tissue, insulin absorption was reduced by 21%–27% with insulin injection into the LH region, leading to significantly higher insulin doses. 7 , 8 In addition, Gentile et al. reported that high HbA1c values and unexpected hypoglycemic events were significantly associated with LH. 7
LIDA is a nodular type of subcutaneous amyloidosis that was first described in 1983. 9 It is considered that long‐term administration, repeated and same‐site injections of insulin are the major predisposing factors for LIDA. In the insulin‐injected sites, amyloid fibril protein is derived from insulin and forms an amyloid deposition; as a result, almost all LIDA are observed with palpable masses. 2 , 3 LIDA causes poor glycemic control and increased insulin dose requirements because of impaired insulin absorption. 2 , 9
Against this background, an accurate understanding and diagnosis of LH and LIDA are essential because of their different implications and clinical management. Clinically, the mechanisms underlying the development of LH and LIDA are poorly understood. The purpose of this study was to update our knowledge of the pathological differentiation, effects of insulin absorption and hypoglycemic events, and recent methods of assessment between LH and LIDA.
2. METHODS
2.1. Study design and methodology
A scoping review was performed following a previously reported framework 10 and recently revised methods. 11 This scoping review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta Analyses extension for Scoping Reviews (PRISMA‐ScR) checklist. 12
2.2. Scoping review question
The specific questions that guided this scoping review were, “What kinds of pathologies have been reported for LH or LIDA?” and “How do the mechanisms that induce LH or LIDA occur?”
2.3. Scoping review objective
The objectives of this study were (a) to summarize the differences in clinicopathological characteristics between LH and LIDA and (b) to suggest the possibility of noninvasive differential methods for nurses. This scoping review will be useful to a variety of clinicians, including nurses, who are involved in the management of glycemic control.
2.4. Eligibility criteria
We limited our search to human and animal research articles published in English. The current review included studies on the pathology of LH and LIDA. Published original articles and case reports written in English were included in this scoping review. Conference abstracts, galleries, literature reviews, and meta‐analyses were also excluded. Articles not associated with the pathology of LH or LIDA were also excluded.
2.5. Information sources
PubMed and Scopus bibliographic databases were searched in January 2022.
2.6. Search strategy
Search terms and medical subject heading terms related to amyloidosis, LH, insulin, pathology, and diabetes were used and combined using the Boolean operators “AND” and “AND NOT.” The search strategy was as follows: LH and insulin and (pathology or pathophysiology) and (diabetes or diabetes mellitus) not Alzheimer's disease, not islets (LH) and (amyloidosis or amyloid or amyloid fibrils or amyloid lumps) and insulin and (pathology or pathophysiology) and (diabetes or diabetes mellitus) not Alzheimer's disease not islets (LIDA) for PubMed; ((KEY(lipohypertrophy)) AND (((insulin)) AND (pathology)) AND (diabetes) AND NOT (“Alzheimer's disease”) AND NOT (islets)) (LH) and ((KEY(amyloid)) AND (((insulin)) AND (pathology)) AND (diabetes) AND (skin) AND NOT (“Alzheimer's disease”) AND NOT (islets)) (LIDA) for Scopus.
2.7. Selection of sources of evidence
The search results were extracted into an extraction spreadsheet in Microsoft Excel, and duplicates were removed. Titles and abstracts were screened by three researchers (KM, HT, and EK) independently, and those that did not meet the inclusion criteria were excluded. Potentially eligible full‐text articles were screened for inclusion by three independent reviewers (KM, HT, and EK) according to the inclusion criteria. Articles lacking outcomes for pathology or imaging, including magnetic resonance imaging (MRI), ultrasonographic (US) images, infrared (IR) images, and computed tomography (CT) of LH or LIDA were excluded because, in this scoping review, we focused on the pathology of LH or LIDA in the skin. Disagreements regarding study selection were resolved through discussion.
2.8. Data‐charting process
Three researchers (KM, HT, and EK) developed a data charting form to determine the variables to extract. Data were extracted by three researchers (KM, HT, and EK). Discrepancies in the extracted data were resolved through discussions between the three authors.
2.9. Data items
The following information was extracted: (a) study authors, year of publication, and country; (b) study design/participants; (c) DM type, (d) characteristics; (e) parts of LH or LIDA; (f) outcomes related to pathologies; (g) outcomes related to imaging; (h) outcomes related to others.
3. RESULTS
3.1. Selection of sources of evidence
The initial search yielded a total of 113 studies. After removing the duplicates (n = 32), 81 articles remained. After title and abstract screening, 31 papers were excluded (13 studies did not investigate the skin, six studies were conference abstracts, one study was a gallery, five studies were literature reviews, and six studies were meta‐analyses). Among the 50 remaining papers, 19 were excluded after full‐text screening: two studies did not investigate amyloidosis or LH, one study did not investigate subcutaneous tissue, nine studies did not involve DM, one study did not investigate the outcomes of pathology or imaging, and four studies were not written in English. In total, 31 articles were included in this scoping review. Of these, 26 papers described human studies, one paper reported a human in vivo study, two papers were in vitro studies, and two studies were in vivo studies. Among them, five papers were on LH and 26 papers were on LIDA. There were two case reports and three original papers on LH. There were 22 case reports and four original papers on LIDA. The PRISMA‐ScR flowchart for this review is shown in Figure 1.
Figure 1.
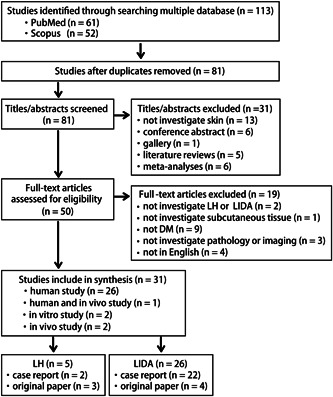
Flowchart of this scoping review. A Preferred Reporting Items for Systematic Reviews and Meta Analyses extension for Scoping Reviews flowchart of the current review is presented. A total of 31 articles were included in the review. DM, diabetes mellitus; LH, lipohypertrophy; LIDA, localized insulin‐derived amyloidosis.
3.2. Characteristics of the source of evidence for LH in this scoping review
The characteristics of the included studies are summarized in Table 1. Among the five studies included in this scoping review, three were original papers and two were case studies. One prospective study was published in 2018 from Canada, 13 and one observational study was published in 2020 from Thailand. 14 An open, multicenter randomized control trial was published in 2020 from Italy. 7 Two case reports were published, one in 2005 15 from Japan and the other in 2018 from Italy. 16
Table 1.
Summary of collected data with lipohypertrophy in this scoping review.
| Source/country | Study design/participants | DM type | Characteristics (LH participants) | Parts | Outcome parameters | |
|---|---|---|---|---|---|---|
| Pathologies | Imaging | |||||
| Kapeluto et al. 13 /Canada | Prospective study, n = 103 |
Type 1 8% Type 2 92% |
75.0 ± 11.8 years Male 58% BMI 28.3 ± 6.1 kg/m2 HbA1c 8.0% ± 1.1% |
- | Visual inspection and palpation (discrete palpable dermal nodules or swellings of variable consistency) | US (heterogeneous in echotexture, absence of vascularity, absence of capsule) |
| Gentile et al. 16 /Italy | Case report, n = 1 | Type 2 |
71 years Female BMI 33.3 kg/m2 HbA1c 9.3% |
Abdomen | Swellings (umbilicated, hyperchromic and cleft) | US (thickening of the dermis) |
| Fujikura et al. 15 /Japan | Case report, n = 1 | Type 2 |
82 years Female BMI 21.3 kg/m2 HbA1c 8.3% |
Abdomen |
HE (excess mature adipocytes in the dermal reticular layer) SEM (hypertrophic adipocytes, numerous small lipid droplets at the periphery of hypertrophic adipocytes) |
- |
| Gentile et al. 7 /Italy | Open, multicenter, randomized, case–control study, n = 718 (with LH), and 509 (without LH) | Type 2 |
61 ± 16 years Male 41.6% BMI 29.1 ± 4.2 kg/m2 HbA1c 9.6 ± 1.1% |
Abdomen 38.3% Arms 35.8% Thighs 33.3% Buttocks 26.2% |
Inspection and palpation (features of four types of LH: small nodule, large nodule, flat plastron, flat nodule) | US (features of three types of LH: hyper, iso, iso‐hypo) |
| Thewjitcharoen et al. 14 /Thailand | Cross‐sectional study, n = 149 (with LH), and 251 (without LH) | Type 1 14%a Type 2 86%a |
64.8 ± 14.2 years Male 52.3% BMI 25.9 ± 4.8 kg/m2 HbA1c 7.8% ± 1.5% |
Abdomen 96.0% Mixed 4.0% |
Inspection and palpation (Grade 1: LH without visible skin lesion but increased palpable density of subcutaneous tissue, Grade 2: serve hypertrophy with increased density of the injection site) | US (localized areas of decreased subcutaneous fat thickness and increased heterogeneous echoes) |
Note: Data are expressed as the mean ± standard deviation.
Abbreviations: BMI, body mass index; CSⅡ, continuous subcutaneous insulin infusion therapy; DM, diabetes mellitus; HE, hematoxylin and eosin staining; HS, healthy subject; LH, lipohypertrophy; SEM, scanning electron microscopy; US, ultrasonography.
Values are shown as a percentage of the total participants.
Among the five papers in this scoping review, patients with type 2 diabetes had a high frequency of LH, with the abdomen being the most common site. Males accounted for almost half of the total, except for case studies; therefore, sex differences could not be confirmed. In all but one case study, the mean body mass index (BMI) value was greater than 25.0. Moreover, the mean HbA1c value was greater than 6.5% in all but one study that did not include this information.
3.3. Outcome parameters with LH in this scoping review
In this scoping review, outcome parameters were divided into two categories: pathology and imaging. Macroscopic evaluations, such as inspection and/or palpation, and microscopic evaluations, such as hematoxylin and eosin (HE) staining and scanning electron microscopy (SEM), were utilized in the pathological category. In three original studies and one case report, inspection and/or palpation were performed to confirm LH. LH was defined by microscopic evaluations as the following: “discrete palpable dermal nodules or swellings of variable consistency” 13 or “features of four types of LH: small nodule, large nodule, flat plastron, flat nodule” 7 or “swellings (umbilicated, hyperchromic and cleft)”. 16 One original study divided LHs into two grades, “Grade 1: LH without visible skin lesion but the increased palpable density of subcutaneous tissue, Grade 2: severe hypertrophy with increased density of the injection site.” 14 HE staining and SEM were performed in one case report to confirm LH; HE staining showed “excess mature adipocytes in the dermal reticular layer” and SEM showed “hypertrophic adipocytes, numerous small lipid droplets at the periphery of hypertrophic adipocytes.” 15
US images were included in the imaging category. In three original studies and one case report, US images were evaluated to confirm LH. LH was observed by US images as “heterogeneous in echotexture, absence of vascularity, absence of capsule.” 13 One original study divided LH into different types based on three features, “Hyper, Iso, and Iso‐hypo,” 7 and one case report evaluated the thickening of the dermis. 16
3.4. In vitro or in vivo studies on LH
Unfortunately, no in vitro or in vivo studies focusing on LH have been identified in this review. Therefore, the pathophysiological processes that lead to LH remain unknown.
3.4.1. Characteristics of the source of evidence for LIDA in humans in this scoping review
The characteristics of the included studies are provided in the Supporting Information: Table. Among the 22 studies included in this scoping review, 21 were case reports and one was an original paper. Five studies were published from Japan. Six studies were published from the United States. Three studies each were published from Germany and India. Two studies were published from the United Kingdom. One study each was published from the Netherlands, Spain, and Canada.
Among the 22 papers in this scoping review, as was in the case of patients with LH, those with LIDA also had type 2 diabetes more frequently than those with type 1 diabetes, and amyloidosis was most commonly observed in the abdomen. Although sex differences were not clear, there were more males than female patients.
3.5. Outcome parameters with LIDA in this scoping review
Eighteen (81.8%) of 22 studies performed HE staining and reported “foreign body giant cells,” “eosinophilic,” “inflammation,” “amorphous,” and “keratosis” as observations. 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 Twenty (90.9%) of 22 studies performed Congo red staining and reported green birefringence when viewed with polarized light, consistent with amyloid deposits. 3 , 9 , 17 , 18 , 19 , 20 , 21 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 Fifteen (68.1%) of 22 studies performed immunohistochemical (IHC) staining for insulin and reported that LIDA was positive for insulin. 3 , 9 , 17 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 28 , 30 , 32 , 33 , 35 Six (27.2%) of 22 studies performed IHC staining for amyloid P; five studies reported positive results 20 , 26 , 28 , 30 , 33 and one study reported negative results. 25 One study performed IHC staining for direct fast scarlet 4BS and reported positive results. 22 Four (18.1%) of 22 studies performed electron microscope analysis. SEM showed “only the Bremsstrahlung, but a few particles gave tile spectra of sulfur or calcium,” 28 transmission electron microscopy (TEM) showed “closely packed microfibrils, and cytoplasmic fibrillary inclusions were found within some of the giant cells,” 28 electron microscopic observation (not stated if SEM or TEM) revealed “protein fibrils were concentrated adjacent to the dark globular proteins,” 21 “a typical spear‐like fibrillar structure,” 9 and “fibrils consistent with amyloid” 33 as observations. Two (9.0%) of 22 studies evaluated LIDA using the US, and reported “a solid complex lobulated lesion within the subcutaneous tissue of the left lower quadrant measuring,” 17 and “irregular and ill‐defined nodules, hypoechoic, heterogeneous and hypovascular” as observations. 22 Three (13.6%) of the 22 studies evaluated LIDA using MRI and reported that both T1‐ and T2‐weighted images showed low signal intensity, while fat‐suppressed T2‐weighted images showed slightly higher signal intensity than subcutaneous fat. There was minimal gadolinium enhancement on fat‐suppressed T1WI,” 22 “unlike fat, they had low signal intensity on T1‐weighted and T2‐weighted images,” 3 “T1 and T2‐weighted sequences revealed hypointense enhancement, while the postcontrast T1‐sequence showed inhomogeneous enhancement with peripheral hyperintensity and central hypointensity,” 29 as observations. Three (13.6%) of 22 studies evaluated LIDA using CT, and reported “irregular with ill‐defined nodules, heterogeneous,” 22 “homogeneous mass,” 34 and “a round mass in the subcutaneous region and inhomogeneous shadow” 25 as observations.
3.6. In vitro or in vivo studies on LIDA
As an in vitro model, Manno et al. reported that human insulin incubated with an acidic solution (pH 1.6) at high temperatures (between 50°C and 70°C) led to the formation of amyloid fibrils. 37 Another group reported that vigorous stirring of insulin at 37°C for 24 h under pH 7.0 or pH 1.8 conditions resulted in the formation of amyloid fibrils. 38 Störkel et al. attempted to create a model of amyloid fibrils in rats and administered porcine insulin daily into subperitoneal adipose tissue using a catheter. After 6 weeks, they observed amyloid fibril formation by HE staining, Congo red staining, and electron microscopy. 9 Chinisaz et al. presented an animal model in which insulin was incubated at 57°C for 24 h and amyloid fibrils were confirmed by Congo red staining and TEM, and these amyloid fibrils were continuously subcutaneously administered to mice for 21 days. 39 These two animal models observed amyloid mass formation but did not examine insulin resistance at the amyloid mass site. Nakamura et al. evaluated insulin resistance in an animal model in which mice were given amyloid fibrils (prepared by incubating insulin at pH 2.5°C and 55°C for 48 h) for 7 days, and reported a decrease in insulin effect similar to LIDA. 40
4. DISCUSSION
4.1. Summary of evidence
This scoping review focuses on the pathological characteristics of LH and LIDA, and the pathological processes leading to LH and LIDA. To date, this evidence has not been summarized in detail. The final article count was 31, and only five papers demonstrated the pathophysiological processes leading to LIDA in in vitro and in vivo studies, highlighting the paucity of relevant literature in this field. The remaining articles reported the pathophysiology of LH or LIDA and suggested that LH and LIDA, which are different pathologies, are difficult to distinguish by macroscopic evaluation and that definitive diagnosis requires histological evaluation of invasive biopsy samples.
4.2. Characteristics of LH and LIDA in pathophysiology
Allergic reactions to insulin, insulin‐induced cutaneous LH, and LIDA are adverse effects associated with insulin injection. 2 , 3 , 4 Unfortunately, insulin‐induced cutaneous LH and LIDA remain overlooked complications in diabetes care. Several previous studies have shown altered insulin absorption at sites of LH. 5 , 41 , 42 Moreover, previous research has reported that LIDA causes poor glycemic control and increased insulin dose requirements because of impaired insulin absorption. 3 , 43 In this scoping review, there were six duplicate articles on LH and LIDA in the screening process; therefore, it is possible that the researchers themselves were not able to clearly distinguish between them. Therefore, understanding the pathophysiological characteristics of these complications is important in the nursing care of patients with diabetes. LH and LIDA are defined as “discrete palpable dermal nodules or swellings of variable consistency” 13 and “nodular on palpation, with a soft to firm,” 3 , 31 respectively, by findings at macroscopic evaluations such as inspection and/or palpation. Inspection and palpation are easy to use in clinical settings; however, it is difficult to distinguish between LH and LIDA. The results of this scoping review also showed that histological evaluation is the gold standard for a definitive diagnosis. Only anti‐insulin antibody‐positive result 3 , 9 , 17 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 28 , 30 , 32 , 33 , 35 or Congo red staining 3 , 9 , 17 , 18 , 19 , 20 , 21 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 can confirm LIDA. Interestingly, noninvasive imaging devices, such as MRI and US, have recently been reported as assessment tools for LH and LIDA. LH was observed by US images as “heterogeneous in echotexture, absence of vascularity, absence of capsule” and “localized areas of decreased subcutaneous fat thickness and increased heterogeneous echoes.” 14 Meanwhile, LIDA was observed by MRI imaging as “T1‐ and T2‐weighted images show hypointensity” 29 and US imaging as “hypovascular.” 22 Among these noninvasive imaging devices, US is an effective tool that is available to nurses in clinical settings in various fields, such as detecting aspiration, 44 US‐guided peripheral intravenous insertion, 45 and confirmation of endotracheal tube placement. 46 Therefore, the daily assessment of the insulin injection site by a US device may be useful for detecting the signs of LH or LIDA.
4.3. Cause mechanisms of LIDA
Several studies have attempted to establish LIDA models in vitro and in vivo. In vitro, it was shown that amyloid fibrils are formed by incubating insulin under acidic conditions (pH 1.6) at 50–70°C. 37 It was also reported that amyloid fibrils are formed by incubating insulin with shaking at pH 7.0°C and 37°C, under conditions more closely resembling the physiological conditions. 38 As shown in in vitro studies, pH, temperature, and shaking (vibration) are considered to be important factors in amyloid fibril formation. Ohno et al. reported that amyloid fibril formation was observed by rapid mixing stimulation (850 rpm) and suggested that vibration or shaking may occur during the transportation of insulin preparation. 47 In this scoping review, we found three animal models (in vivo), although two studies using animal models in which animals were injected in vitro generated amyloid fibrils. 9 , 39 , 40 In vitro and in vivo studies indicated that amyloid fibrils may be formed in insulin preparation rather than inside the body. However, we could not confirm whether LIDA formed outside or inside the body. To clarify this point, an analysis using a model of repeated insulin administration in animals is needed.
4.4. Implications for practice and future perspectives
This scoping review clarified that the pathophysiological evidence in LH or LIDA is called an “insulin ball.” 3 In comparison with normal adipose tissue, insulin absorption is reduced in the LH region 7 , 8 and impaired in the LIDA region. 2 , 9 Therefore, understanding the pathophysiological characteristics of these complications is important in the nursing care of patients with diabetes. Unfortunately, the results of the scoping review showed that LH and LIDA were difficult to distinguish by macroscopic evaluation, and that definitive diagnosis required histological evaluation of invasive biopsy samples. Our results for the scoping review also showed that daily assessment of the insulin injection site using a US device may be useful for detecting signs of LH or LIDA. The establishment of a noninvasive assessment tool for cases that are difficult to distinguish by macroscopic evaluation means that assessment should be performed early, enabling the establishment of appropriate care for patients with diabetes. Therefore, this scoping review has important implications for future studies on providing care to patients with diabetes.
4.5. Limitations
This scoping review has some limitations. We did not extract information on the quality of evidence provided by the authors and the quality of the studies was not assessed systematically in this scoping review. Additionally, we cannot rule out the possibility that articles on mixed‐etiology hypertrophy were excluded if they did not employ the terms used in the search strategy. These processes may have affected the selection of evidence. Furthermore, this scoping review did not extract information on the involvement of disease duration or insulin injections in the development of LH or LIDA. In the future, the involvement of disease duration and insulin injections in the development of LH and LIDA should also be considered.
5. CONCLUSION
This scoping review summarizes the evidence regarding the pathologies of LH and LIDA and the pathological processes leading to the adverse effects associated with insulin injection. The articles included were 31, and only five papers demonstrated the pathological processes leading to LIDA through in vitro and in vivo studies, highlighting the paucity of relevant literature in this field. The remaining articles reported the pathophysiology of LH or LIDA and suggested that LH and LIDA, which are different pathologies, are difficult to distinguish macroscopically, and a definitive diagnosis requires histological evaluation with invasive biopsy samples. Further studies for determining the pathological process in vivo, especially in the human body, are needed, as relevant literature remains sparse.
AUTHOR CONTRIBUTIONS
Study design: Kanae Mukai, Hiromasa Tanno, and Emi Kanno. Data collection: Hiromasa Tanno, Kanae Mukai, and Emi Kanno. Data analysis: Kanae Mukai, Hiromasa Tanno, and Emi Kanno. Study supervision: Junko Sugama and Toshihiko Yanagita. Manuscript writing: Hiromasa Tanno, Kanae Mukai, and Emi Kanno.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
None.
Supporting information
Supporting information.
ACKNOWLEDGMENTS
The authors thank the following board members of collaborative projects between Japan Academy of Nursing Science and The Japanese Pharmacological Society who were involved in the development of the projects: Dr. Natsuko Seto, Dr. Kimie Takehara, Dr. Tomoko Akase, Dr. Makoto Oe, Dr. Keisuke Nakanishi, Dr. Fumiya Hisano, Dr. Ai Ibuki, Dr. Mayu Fukuda, Dr. Sayuri Nakamura, Dr. Yuko Matsui, Dr. Tomomi Horiguchi, Dr. Terumi Ueda, and Ms. Naoko Kageura.
Mukai K, Tanno H, Sugama J, Yanagita T, Kanno E. Differences in clinicopathological characteristics between lipohypertrophy and localized insulin‐derived amyloidosis: a scoping review. Chronic Dis Transl Med. 2024;10:22‐30. 10.1002/cdt3.98
Kanae Mukai and Hiromasa Tanno contributed equally to this study.
DATA AVAILABILITY STATEMENT
All of the data are available from the corresponding author upon reasonable request.
REFERENCES
- 1. International Diabetes Federation . IDF Diabetes Atlas. 10th ed. International Diabetes Federation; 2021. https://www.diabetesatlas.org [Google Scholar]
- 2. Blanco M, Hernández MT, Strauss KW, Amaya M. Prevalence and risk factors of lipohypertrophy in insulin‐injecting patients with diabetes. Diabetes Metab. 2013;39(5):445‐453. 10.1016/j.diabet.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 3. Nagase T, Katsura Y, Iwaki Y, et al. The insulin ball. Lancet. 2009;373(9658):184. 10.1016/S0140-6736(09)60041-6 [DOI] [PubMed] [Google Scholar]
- 4. Nilsson MR. Insulin amyloid at injection sites of patients with diabetes. Amyloid. 2016;23(3):139‐147. 10.1080/13506129.2016.1179183. [DOI] [PubMed] [Google Scholar]
- 5. Johansson UB, Amsberg S, Hannerz L, et al. Impaired absorption of insulin aspart from lipohypertrophic injection sites. Diabetes Care. 2005;28(8):2025‐2027. 10.2337/diacare.28.8.2025. [DOI] [PubMed] [Google Scholar]
- 6. Mokta J, Mokta K, Panda P. Insulin lipodystrophy and lipohypertrophy. Indian J Endocrinol Metab. 2013;17(4):773‐774. 10.4103/2230-8210.113788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gentile S, Guarino G, Corte TD, et al. Insulin‐induced skin lipohypertrophy in Type 2 diabetes: a multicenter regional survey in Southern Italy. Diabetes Ther. 2020;11(9):2001‐2017. 10.1007/s13300-020-00876-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Famulla S, Hövelmann U, Fischer A, et al. Insulin injection into lipohypertrophic tissue: blunted and more variable insulin absorption and action and impaired postprandial glucose control. Diabetes Care. 2016;39(9):1486‐1492. 10.2337/dc16-0610. [DOI] [PubMed] [Google Scholar]
- 9. Störkel S, Schneider HM, Müntefering H, Kashiwagi S. Iatrogenic, insulin‐dependent, local amyloidosis. Lab Invest. 1983;48(1):108‐111. [PubMed] [Google Scholar]
- 10. Arksey H, O'Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8(1):19‐32. 10.1080/1364557032000119616. [DOI] [Google Scholar]
- 11. Daudt HM, van Mossel C, Scott SJ. Enhancing the scoping study methodology: a large, inter‐professional team's experience with Arksey and O'Malley's framework. BMC Med Res Methodol. 2013;13(1):48. 10.1186/1471-2288-13-48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA‐ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467‐473. 10.7326/M18-0850. [DOI] [PubMed] [Google Scholar]
- 13. Kapeluto JE, Paty BW, Chang SD, Meneilly GS. Ultrasound detection of insulin‐induced lipohypertrophy in Type 1 and Type 2 diabetes. Diabetic Med. 2018;35(10):1383‐1390. 10.1111/dme.13764. [DOI] [PubMed] [Google Scholar]
- 14. Thewjitcharoen Y, Prasartkaew H, Tongsumrit P, et al. Prevalence, risk factors, and clinical characteristics of lipodystrophy in insulin‐treated patients with diabetes: an old problem in a New Era of modern insulin. Diabetes Metab Syndr Obes. 2020;13:4609‐4620. 10.2147/DMSO.S282926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fujikura J, Fujimoto M, Yasue S, et al. Insulin‐induced lipohypertrophy: report of a case with histopathology. Endocr J. 2005;52(5):623‐628. 10.1507/endocrj.52.623. [DOI] [PubMed] [Google Scholar]
- 16. Gentile S, Strollo F, Corte TD, Marino G, Guarino G, Italian Study Group on Injection Techniques . Skin complications of insulin injections: a case presentation and a possible explanation of hypoglycaemia. Diabetes Res Clin Pract. 2018;138:284‐287. 10.1016/j.diabres.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 17. Aghighi M, Linos K. Insulin‐induced amyloidosis in a diabetic patient. J Cutan Pathol. 2022;49(10):845‐849. 10.1111/cup.14087. [DOI] [PubMed] [Google Scholar]
- 18. Tummidi S, Balakrishna P, Gupta Y. Insulin‐induced amyloidoma—A cytological catch. Diagn Cytopathol. 2021;49(3):424‐428. 10.1002/dc.24616. [DOI] [PubMed] [Google Scholar]
- 19. Carll T, Antic T. An abdominal wall mass of exogenous insulin amyloidosis in setting of metastatic sarcoma. J Cutan Pathol. 2020;47(4):406‐408. 10.1111/cup.13613. [DOI] [PubMed] [Google Scholar]
- 20. Iwaya K, Zako T, Fukunaga J, et al. Toxicity of insulin‐derived amyloidosis: a case report. BMC Endocr Disord. 2019;19(1):61. 10.1186/s12902-019-0385-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Katzman BD, Traum P, Medline PB. New histologic finding of amyloid insulin bodies at an insulin injection site in a patient with diabetes. Am J Dermatopathol. 2018;40(7):527‐530. 10.1097/DAD.0000000000001052 [DOI] [PubMed] [Google Scholar]
- 22. Tanio N, Nozaki T, Matsusako M, Starkey J, Suzuki K. Imaging characteristics of subcutaneous amyloid deposits in diabetic patients: the “insulin ball. Skeletal Radiol. 2018;47(1):85‐92. 10.1007/s00256-017-2749-8 [DOI] [PubMed] [Google Scholar]
- 23. Kudo‐Watanuki S, Kurihara E, Yamamoto K, Mukai K, Chen KR. Coexistence of insulin‐derived amyloidosis and an overlying acanthosis nigricans‐like lesion at the site of insulin injection. Clin Exp Dermatol. 2013;38(1):25‐29. 10.1111/j.1365-2230.2012.04373.x. [DOI] [PubMed] [Google Scholar]
- 24. Endo JO, Röcken C, Lamb S, Harris RM, Bowen AR. Nodular amyloidosis in a diabetic patient with frequent hypoglycemia: sequelae of repeatedly injecting insulin without site rotation. J Am Acad Dermatol. 2010;63(6):e113‐e114. 10.1016/j.jaad.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 25. Shikama Y, Kitazawa J, Yagihashi N, et al. Localized amyloidosis at the site of repeated insulin injection in a diabetic patient. Intern Med. 2010;49(5):397‐401. 10.2169/internalmedicine.49.2633. [DOI] [PubMed] [Google Scholar]
- 26. Yumlu S, Barany R, Eriksson M, Röcken C. Localized insulin‐derived amyloidosis in patients with diabetes mellitus: a case report. Hum Pathol. 2009;40(11):1655‐1660. 10.1016/j.humpath.2009.02.019. [DOI] [PubMed] [Google Scholar]
- 27. Albert SG, Obadiah J, Parseghian SA, Yadira Hurley M, Mooradian AD. Severe insulin resistance associated with subcutaneous amyloid deposition. Diabetes Res Clin Pract. 2007;75(3):374‐376. 10.1016/j.diabres.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 28. Dische FE, Wernstedt C, Westermark GT, et al. Insulin as an amyloid‐fibril protein at sites of repeated insulin injections in a diabetic patient. Diabetologia. 1988;31(3):158‐161. 10.1007/BF00276849. [DOI] [PubMed] [Google Scholar]
- 29. Desai SS, Rizzo MG, Rush AJ, Rosenberg AE, Al Maaieh M. Amyloidoma: a review and case report. Skeletal Radiol. 2021;50(2):437‐444. 10.1007/s00256-020-03560-3 [DOI] [PubMed] [Google Scholar]
- 30. Bernárdez C, Schärer L, Molina‐Ruiz AM, Requena L. Nodular amyloidosis at the sites of insulin injections. J Cutan Pathol. 2015;42(7):496‐502. 10.1111/cup.12501. [DOI] [PubMed] [Google Scholar]
- 31. Nandeesh B, Rajalakshmi T, Shubha B. Cutaneous amyloidosis and insulin with coexistence of acanthosis nigricans. Indian J Pathol Microbiol. 2014;57(1):127‐129. 10.4103/0377-4929.130920. [DOI] [PubMed] [Google Scholar]
- 32. Sie MP, van der Wiel HE, Smedts FM, de Boer AC. Human recombinant insulin and amyloidosis: an unexpected association. Neth J Med. 2010;68(3):138‐140. [PubMed] [Google Scholar]
- 33. Lonsdale‐Eccles AA, Gonda P, Gilbertson JA, Haworth AE. Localized cutaneous amyloid at an insulin injection site. Clin Exp Dermatol. 2009;34(8):e1027‐e1028. 10.1111/j.1365-2230.2009.03711.x. [DOI] [PubMed] [Google Scholar]
- 34. Grunes D, Rapkiewicz A, Simsir A. Amyloidoma secondary to insulin injection: cytologic diagnosis and pitfalls. Cytojournal. 2015;12:15. 10.4103/1742-6413.161602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Arora S, Agrawal NK, Shanthaiah DM, et al. Early detection of cutaneous complications of insulin therapy in type 1 and type 2 diabetes mellitus. Prim Care Diabetes. 2021;15(5):859‐864. 10.1016/j.pcd.2021.06.004. [DOI] [PubMed] [Google Scholar]
- 36. Kranc CL, Wagner R, Joy NM, Feldman F, Reid DC. Cutaneous insulin‐derived amyloidosis presenting as hyperkeratotic nodules. Cutis. 2021;107(1):E6‐E9. 10.12788/cutis.0161. [DOI] [PubMed] [Google Scholar]
- 37. Manno M, Craparo EF, Podestà A, et al. Kinetics of different processes in human insulin amyloid formation. J Mol Biol. 2007;366(1):258‐274. 10.1016/j.jmb.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 38. Iannuzzi C, Borriello M, Portaccio M, Irace G, Sirangelo I. Insights into insulin fibril assembly at physiological and acidic pH and related amyloid intrinsic fluorescence. Int J Mol Sci. 2017;18(12):2551. 10.3390/ijms18122551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chinisaz M, Ebrahim‐Habibi A, Yaghmaei P, Parivar K, Dehpour AR. Generating local amyloidosis in mice by the subcutaneous injection of human insulin amyloid fibrils. Exp Ther Med. 2014;8(2):405‐408. 10.3892/etm.2014.1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nakamura M, Misumi Y, Nomura T, et al. Extreme adhesion activity of amyloid fibrils induces subcutaneous insulin resistance. Diabetes. 2019;68(3):609‐616. 10.2337/db18-0846. [DOI] [PubMed] [Google Scholar]
- 41. Young RJ, Hannan WJ, Frier BM, Steel JM, Duncan LJP. Diabetic lipohypertrophy delays insulin absorption. Diabetes Care. 1984;7(5):479‐480. 10.2337/diacare.7.5.479. [DOI] [PubMed] [Google Scholar]
- 42. Gentile S, Agrusta M, Guarino G, et al. Metabolic consequences of incorrect insulin administration techniques in aging subjects with diabetes. Acta Diabetol. 2011;48(2):121‐125. 10.1007/s00592-009-0172-x [DOI] [PubMed] [Google Scholar]
- 43. Nagase T, Iwaya K, Iwaki Y, et al. Insulin‐derived amyloidosis and poor glycemic control: a case series. Am J Med. 2014;127(5):450‐454. 10.1016/j.amjmed.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 44. Miura Y, Tamai N, Kitamura A, et al. Diagnostic accuracy of ultrasound examination in detecting aspiration and pharyngeal residue in patients with dysphagia: a systematic review and meta‐analysis. Jpn J Nurs Sci. 2021;18(2):e12396. 10.1111/jjns.12396. [DOI] [PubMed] [Google Scholar]
- 45. Tran QK, Fairchild M, Yardi I, Mirda D, Markin K, Pourmand A. Efficacy of ultrasound‐guided peripheral intravenous cannulation versus standard of care: a systematic review and meta‐analysis. Ultrasound Med Biol. 2021;47(11):3068‐3078. 10.1016/j.ultrasmedbio.2021.07.002 [DOI] [PubMed] [Google Scholar]
- 46. Chen WT, Wang MY, Jiang TT, et al. Transtracheal ultrasound for confirmation of endotracheal tube placement in the intensive care unit: a systematic review and meta‐analysis. Eur Rev Med Pharmacol Sci. 2022;26(22):8224‐8233. 10.26355/eurrev_202211_30354 [DOI] [PubMed] [Google Scholar]
- 47. Ohno Y, Seki T, Kojima Y, et al. Investigation of factors that cause insulin precipitation and/or amyloid formation in insulin formulations. J Pharm Health Care Sci. 2019;5(1):22. 10.1186/s40780-019-0151-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Data Availability Statement
All of the data are available from the corresponding author upon reasonable request.


