Abstract
The study sought to determine the prevalence of persistent long COVID symptoms such as anxiety, depression, dizziness, chest pain, sleep difficulty, palpitations, weight loss, and hair loss among coronavirus disease 2019 (COVID-19) survivors worldwide and to discuss the potential pathogeneses. Potential studies were searched in three databases (PubMed, Scopus, and Web of Science) as of January 30, 2021. Data on study characteristics, patient characteristics during the follow-up, the number of patients with persistent long COVID symptoms and total COVID-19 survivors were collected according to PRISMA guidelines. To assess the quality of studies, the Newcastle-Ottawa scale was used. The estimated prevalence of each long COVID symptom and the association between COVID-19 severity and the occurrence of prolonged symptoms was assessed, if appropriate. The global prevalence of prolonged anxiety was 15.76% (95%CI: 6.36%, 25.15%). Chest pain persisted in 10.36% (239/3,224) of COVID-19 patients (95%CI: 4.92%, 15.80%). Prolonged depression was found in 24 of 548 COVID-19 survivors with an estimated prevalence of 4.32% (95%CI: 2.62%, 6.03%) and dizziness was presented in 4.83% (118/2,219, 95%CI: 1.50%, 8.16%) after recovery. Hair loss was complained by 527 of 2,251 recovered patients (cumulative prevalence of 24.76%, 95%CI: 19.60%, 29.91%), while weight loss was identified in 37 cases among 452 COVID-19 survivors (8.19%, 95%CI: 5.66%, 10.71%). Prolonged palpitation was experienced by 19.38% (211/1,926) survivors with 95%CI: 2.40%, 41.16%. Sleep difficulty was found in 541 of 2,622 COVID-19 survivors (17.87%, 95%CI: 7.55%, 28.20%). The association between COVID-19 severity and the occurrence of persistent long COVID symptoms was not analyzed due to the lack of data. In conclusion, persistent psychological symptoms are frequently reported among COVID-19 survivors. Follow-up studies with a longer duration and larger population are warranted to assess the extent of prolonged symptoms and the quality of life of COVID-19 survivors. Despite various potential pathogeneses that have been hypothesized, a definitive mechanism is yet to be addressed. PROSPERO registration: CRD42021247172
Keywords: COVID-19, follow-up study, prolonged symptom, long COVID, systematic review
Introduction
The coronavirus disease 2019 (COVID-19) was declared a global pandemic on March 11th, 2020. The disease, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has caused more than 131 million confirmed cases and more than 2.8 million deaths, worldwide [1]. Not only causing socioeconomic disruption [2, 3], COVID-19 has also affected the healthcare system [4-7]. These concerns, along with the risk of discrimination [8] and stigmatization [9, 10] among patients and healthcare workers [11], can lead to psychological problems [12].
A study found that depressed mood (14.9%), anxiety (14.8%), post-traumatic stress disorder (PTSD) (32.2%), insomnia (12.1%), and irritability (12.8%) were frequently reported after severe acute respiratory syndrome (SARS) or Middle East respiratory syndrome (MERS) infection [13]. This could imply that SARS-CoV-2 infection may have a similar course of persistent long COVID symptoms to SARS or MERS infection following recovery of disease [13]. The global pandemic also has triggered a social discriminatory crisis and stigmatization against suspected, confirmed, and recovered COVID-19 patients [12]. Fear, panic, and misinformation about the transmission of COVID-19 have become reasons why verbal abuse and violent acts have occurred against COVID-19 patients and hospital workers [14]. One study reported that among 4,172 COVID-19 survivors in Wuhan, 615 patients had experienced depression, and 528 patients had anxiety with risk factors such as being female, living alone, had low income and had chronic comorbid disease [15]. In South Korea, a study reported that 10% and 50% of COVID-19 patients developed depression and PTSD, respectively one month after discharge from the hospital [16]. Another study reported that being female was significantly related to depressive emotion and PTSD while being retired and having good social support reduced the risk of psychological distress during the early convalescence of COVID-19 (14-days quarantine after hospital discharge) [17].
Several studies found that palpitations either as cardiovascular sequelae or long COVID disturbance have been reported during COVID-19 follow-up [18-20]. Sleep disturbance was also a concern not only during the follow-up but also since hospitalization with acute COVID-19. Longer hospitalization and higher depression rate were found in COVID-19 patients with poor sleep quality [21]. A study found that, after 38 days post-recovery, insomnia was found in 56.3% (89/158) of COVID-19 survivors [22]. Numerous studies have been conducted to identify the persistent symptoms in COVID-19 survivors, however, a detailed pooled analysis of the long COVID symptoms is scarce. Therefore, this systematic review sought to assess (a) the global estimated prevalence of persistent long COVID symptoms among COVID-19 survivors, including anxiety, depression, dizziness, chest pain, sleep difficulty, palpitations, weight loss, and hair loss, and (b) the association of COVID-19 severity during initial infection and persistent long COVID symptoms post-recovery.
Methods
Registration and protocol
We conducted a systematic review to estimate the prevalence of persistent long COVID symptoms such as anxiety, depression, dizziness, chest pain, sleep difficulty, palpitations, weight loss, and hair loss. The study protocol was registered to PROSPERO (CRD42021247172) and no ethical clearance was required. We followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) in searching the databases and reporting the results (Supplementary material) [23].
Eligibility criteria of studies
Observational studies reporting at least one persistent long COVID symptom (anxiety, depression, dizziness, chest pain, sleep difficulty, palpitations, weight loss, and hair loss) in COVID-19 survivors were considered eligible. All editorials, commentaries, reviews, case reports, and case series with less than 10 patients were excluded. RT-PCR of SARS-CoV-2 RNA from nasal or oropharyngeal swab samples must be used to confirm COVID-19 diagnosis. Diagnosis based on clinical symptoms without nucleic acid testing and suspected cases were excluded. A COVID-19 survivor was defined based on discharge criteria from either WHO or China’s National Health Commission [24, 25]. Persistent symptoms were described as symptoms that were presented since hospitalization or during the infection period which prolonged until the patient was discharged.
Information sources and search strategy
Three databases (PubMed, Scopus, and Web of Science) were searched as of January 30th, 2021. The searches were limited to 2019-2021 and only articles written in English were considered eligible. The search strategies were as follows. PubMed ([Title]("SARS-CoV-2" OR "COVID-19" OR "Wuhan coronavirus" OR "Wuhan virus" OR "novel coronavirus" OR "nCoV" OR "severe acute respiratory syndrome coronavirus 2" OR "coronavirus disease 2019" OR "2019-nCoV" OR "2019 novel coronavirus" OR "SARS 2") AND ([Title]("prolong*" OR "follow-up" OR "persistent" OR "sequelae" OR "consequen*" OR "prospective" OR "cohort" OR "long-term" OR "follow*" OR "longitudinal"). Web of Science ([Title]("SARS-CoV-2" OR "COVID-19" OR "Wuhan coronavirus" OR "Wuhan virus" OR "novel coronavirus" OR "nCoV" OR "severe acute respiratory syndrome coronavirus 2" OR "coronavirus disease 2019" OR "2019-nCoV" OR "2019 novel coronavirus" OR "SARS 2") AND ([Title]("prolong*" OR "follow-up" OR "persistent" OR "sequelae" OR "consequen*" OR "prospective" OR "cohort" OR "long-term" OR "follow*" OR "longitudinal"). Scopus ([Title]("SARS-CoV-2" OR "COVID-19" OR "Wuhan coronavirus" OR "Wuhan virus" OR "novel coronavirus" OR "nCoV" OR "severe acute respiratory syndrome coronavirus 2" OR "coronavirus disease 2019" OR "2019-nCoV" OR "2019 novel coronavirus" OR "SARS 2") AND ([Title]("prolong*" OR "follow-up" OR "persistent" OR "sequelae" OR "consequen*" OR "prospective" OR "cohort" OR "long-term" OR "follow*" OR "longitudinal").
Study selection and data extraction
Information for relevant articles was imported to EndNote X9 (Thompson Reuters, Philadelphia, PA, USA) with duplicated references among the three databases removed. Initial screening of titles and abstracts was done to identify eligible articles. Two authors (MF and MI) downloaded and reviewed the full text of potentially eligible articles based on eligibility criteria and the availability of the data. Data from the eligible articles and supplementary materials were extracted and the list of references was retrieved for further relevant studies.
Study characteristics of the eligible articles including author(s), year of study, study site and country, study design, time of follow-ups conducted after discharge, number of COVID-19 patients who were followed, number of COVID-19 patients with prolonged specific long COVID symptom, and severity of the COVID-19 during admission in the hospitals were collected.
Outcomes
There were two main outcomes in this study: (a) global prevalence of persistent long COVID symptoms such as anxiety, depression, dizziness, chest pain, sleep difficulty, palpitations, weight loss and hair loss in COVID-19 survivors; and (b) associations of COVID-19 severity with the presence of persistent long COVID symptoms. We also discussed the possible mechanisms of persistent long COVID symptoms in COVID-19 survivors.
Risk of bias assessment
The quality of each study was assessed by using the Newcastle-Ottawa scale (NOS) [26]. Nine characteristics of a study were evaluated in NOS, including four items for sample selection, one item for group comparison and three items for the outcome. The scores ranged between 0 to 9 and the quality of the study was classified as low (≤ 4), moderate (5–6), and high (≥7).
Data synthesis and statistical analysis
The estimated prevalence of each persistent long COVID symptoms was calculated as the number of patients who had prolonged symptoms divided by the total number of patients with or without the specific long COVID symptom during the follow-up and expressed as frequency (%) and 95% confidence interval (95%CI). The associations of the COVID-19 severity during infection and the risk of persistent long COVID symptoms were calculated and expressed as odds ratios (ODs) and 95% CI. Forest plots were used to visualize the data.
The heterogeneity of the pooled data was evaluated by the Q-test. Data was analyzed using a random-effects model. The publication bias was assessed by using Egger's test with p<0.05 considered as publication bias. Review Manager version 5.3 (the Cochrane Collaboration) was used to analyze the data [27].
Results
Study eligibility results
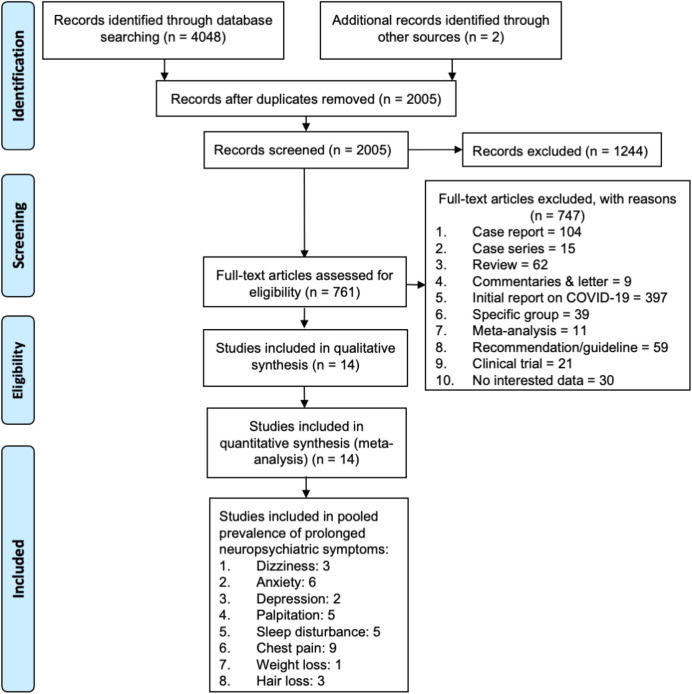
The search resulted in 4,050 eligible articles, with 2,045 duplicates. The titles and abstracts of the remaining 2,005 references were screened, and 1,244 articles were excluded. Screening of the full-text of 761 studies excluded an additional 747 references for reasons such as being reviews, case series, case reports, initial reports on COVID-19, letter/commentaries, studies on specific groups, recommendations, and studies with insufficient data. The final screening process yielded 14 articles which were included in the meta-analysis (Figure 1).
Figure 1. Flowchart of the results of literature search according to PRISMA.
Among the 14 studies, three studies were included in meta-analysis to calculate the prevalence of prolonged dizziness [28-30], six studies for persistent anxiety [28-33], two studies for persistent depression [16, 30], five studies for palpitation [28, 29, 34-36], five studies for sleep disturbance [28, 29, 31, 33, 37], nine articles for prolonged chest pain [28-32, 34, 35, 38, 39], one study for weight loss [37] and three studies for hair loss [28, 30, 40]. The summary of studies included is presented in Table 1.
Table 1. The prevalence of persistent long covid symptoms among covid-19 survivors.
| Symptom | Year | Study design | Country | Days from discharge to follow-up | Followed up COVID-19 | Association between COVID-19 severity and occurrence of symptoms | NOS | Ref | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prolonged symptom | Total patient | % | Mild- moderate | Total | % | Severe | Total | % | |||||||
| Dizziness | 2021 | Cohort | China | 153 (146–160) | 101 | 1655 | 6.10 | 92 | 1538 | 5.98 | 9 | 117 | 7.69 | 8 | [28] |
| 2020 | Cohort | USA | 38 (21–49) | 3 | 26 | 11.54 | 8 | [29] | |||||||
| 2021 | Prospective | China | 97 (95–102) | 14 | 538 | 2.60 | 8 | [30] | |||||||
| Total | 118 | 2219 | 5.32 | ||||||||||||
| Anxiety | 2021 | Cohort | China | 153 (146–160) | 367 | 1617 | 22.70 | 331 | 1506 | 21.98 | 36 | 111 | 32.43 | 8 | [28] |
| 2020 | Cohort | USA | 38 (21–49) | 8 | 26 | 30.77 | 8 | [29] | |||||||
| 2021 | Retrospective | China | 30 | 20 | 304 | 6.58 | 16 | 243 | 6.58 | 4 | 61 | 6.56 | 7 | [31] | |
| 2021 | Prospective | China | 97 (95–102) | 35 | 538 | 6.51 | 8 | [30] | |||||||
| 2020 | Prospective | China | 14 | 17 | 337 | 5.04 | 7 | [32] | |||||||
| 2020 | Cohort | Italy | 23 (20-29) | 55 | 185 | 29.73 | 8 | [33] | |||||||
| Total | 502 | 3007 | 16.69 | ||||||||||||
| Depression | 2021 | Prospective | China | 97 (95–102) | 23 | 538 | 4.28 | 8 | [30] | ||||||
| 2020 Total | Prospective | South Korea | 25 (13–50) | 1 24 | 10 548 | 10.00 4.38 | 8 | [16] | |||||||
| Palpitation | 2021 | Cohort | China | 153 (146–160) | 154 | 1655 | 9.31 | 141 | 1538 | 9.17 | 13 | 117 | 11.11 | 8 | [28] |
| 2020 | Cohort | USA | 38 (21–49) | 6 | 26 | 23.08 | 8 | [29] | |||||||
| 2020 | Prospective | China | 14 | 3 | 131 | 2.29 | 2 | 62 | 3.23 | 1 | 69 | 1.45 | 7 | [34] | |
| 2020 | Prospective | China | 28 | 1 | 38 | 2.63 | 7 | [35] | |||||||
| 2020 | Prospective | China | 90 | 47 | 76 | 61.84 | 8 | [36] | |||||||
| Total | 211 | 1926 | 10.96 | ||||||||||||
| Sleep | 2021 | Cohort | China | 153 (146–160) | 437 | 1655 | 26.40 | 406 | 1538 | 26.40 | 31 | 117 | 26.50 | 8 | [28] |
| difficulties | 2020 | Cohort | USA | 38 (21–49) | 7 | 26 | 26.92 | 8 | [29] | ||||||
| 2021 | Retrospective | China | 30 | 21 | 304 | 6.91 | 12 | 243 | 4.94 | 9 | 61 | 14.75 | 7 | [31] | |
| 2020 | Prospective | Iran | 28 | 25 | 452 | 5.53 | 21 | 400 | 5.25 | 4 | 52 | 7.69 | 9 | [37] | |
| 2020 | Cohort | Italy | 23 (20-29) | 51 | 185 | 27.57 | 8 | [33] | |||||||
| Total | 541 | 2622 | 20.63 | ||||||||||||
| Chest pain | 2021 | Cohort | China | 153 (146–160) | 75 | 1655 | 4.53 | 65 | 1538 | 4.23 | 10 | 117 | 8.55 | 8 | [28] |
| 2020 | Cohort | USA | 38 (21–49) | 10 | 26 | 38.46 | 8 | [29] | |||||||
| 2020 | Retrospective | Iran | 91 (±15.5) | 9 | 52 | 17.31 | 8 | [38] | |||||||
| 2021 | Retrospective | China | 30 | 15 | 304 | 4.93 | 7 | 243 | 2.88 | 8 | 61 | 13.11 | 7 | [31] | |
| 2020 | Prospective | China | 14 | 4 | 131 | 3.05 | 3 | 62 | 4.84 | 1 | 69 | 1.45 | 7 | [34] | |
| 2021 | Prospective | China | 97 (95–102) | 66 | 538 | 12.27 | 8 | [30] | |||||||
| 2020 | Prospective | China | 14 | 28 | 337 | 8.31 | 7 | [32] | |||||||
| 2020 | Prospective | Italy | 60.3 (±13.6) | 31 | 143 | 21.68 | 7 | [39] | |||||||
| 2020 | Prospective | China | 28 | 1 | 38 | 2.63 | 7 | [35] | |||||||
| Total | 239 | 3224 | 7.41 | ||||||||||||
| Weight | 2020 | prospective | Iran | 28 | 37 | 452 | 8.19 | 31 | 400 | 7.75 | 6 | 52 | 11.54 | 9 | [37] |
| loss | Total | 37 | 452 | 8.19 | |||||||||||
| Hair loss | 2021 | Cohort | China | 153 (146–160) | 359 | 1655 | 21.69 | 331 | 1538 | 21.52 | 28 | 117 | 23.93 | 8 | [28] |
| 2021 | Prospective | China | 97 (95–102) | 154 | 538 | 28.62 | 8 | [30] | |||||||
| 2020 | Prospective | Japan | 108 (±23) | 14 | 58 | 24.14 | 7 | [40] | |||||||
| Total | 527 | 2251 | 23.41 | ||||||||||||
Prevalence of persistent long COVID symptoms in COVID-19 survivors
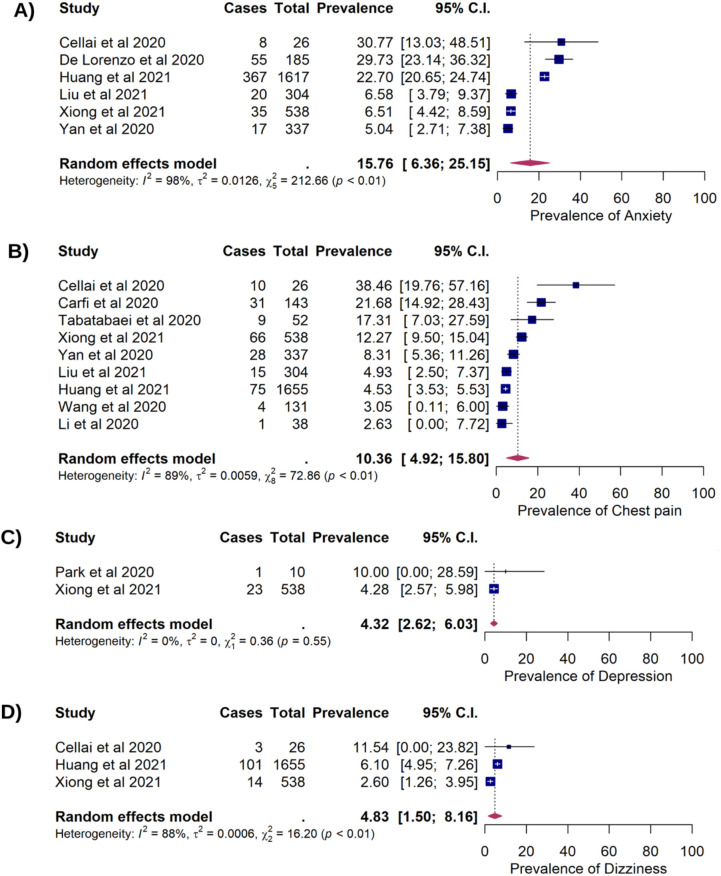
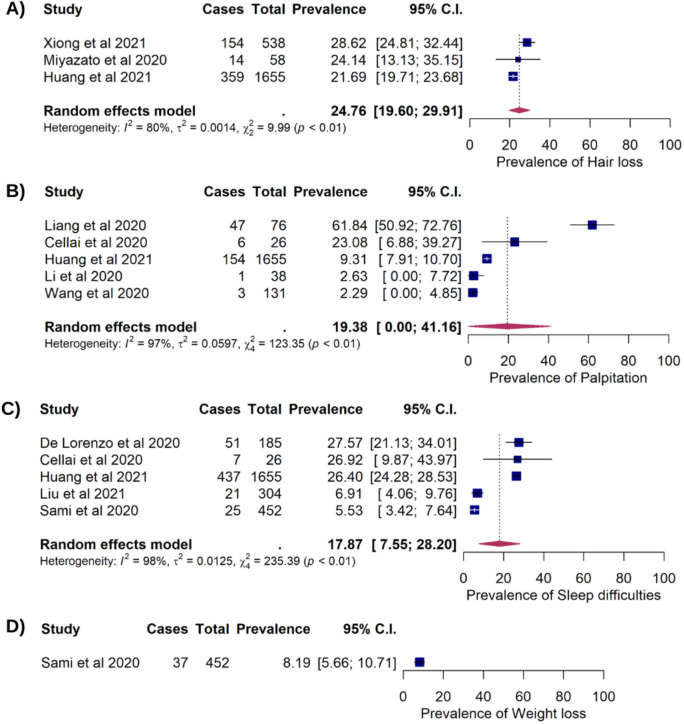
Six studies which included 3,007 COVID-19 patients reported prolonged anxiety among 502 patients after recovery, with an estimated prevalence of 15.76% (95%CI: 6.36%, 25.15%). Nine studies reported prolonged chest pain in 239 of 3,224 COVID-19 patients (10.36%, 95%CI:4.92%, 15.80%). Prolonged depression presented in 24 of 548 COVID-19 patients which corresponded to a pooled prevalence of 4.32% (95%CI: 2.62%, 6.03%). The estimated prevalence of persistent dizziness among COVID-19 survivors was 4.83% (118/2,219 95%CI: 1.50%, 8.16%) (Figure 2). Hair loss was found in three studies consisting of 2,251 COVID-19 survivors, of which 527 patients were reported having the prolonged symptom (cumulative prevalence of 24.76%, 95%CI: 19.60%, 29.91%). Persistent palpitation was reported in 19.38% (211/1,926) survivors with 95%CI: 2.40%, 41.16%. Sleep difficulty was identified in 541 out of 2,622 COVID-19 survivors (17.87%, 95%CI: 7.55%, 28.20%) and weight loss was reported in 8.19% survivors (37/452, 95%CI: 5.66%, 10.71%) (Figure 3).
Figure 2. Forest plots showing of the long COVID symptoms in COVID-19 survivors. (A) The prevalence of anxiety (15.76%; 95%CI: 6.36%, 25.15%); p=0.001; pHet <0.001; pEgger=0.085); (B) The prevalence of chest pain (10.36%; 95%CI: 4.92%, 15.80%]; p<0.001; pHet<0.001; pEgger<0.001); (C) The prevalence of depression (4.32%; 95%CI: 2.62%. 6.03%; p<0.001; pHet=0.547; pEgger<0.001); (D) The prevalence of dizziness (4.83%; 95%CI: 1.50%, 8.16%; p=0.004; pHet<0.001; pEgger=0.320).
Figure 3. Forest plots showing the long COVID symptoms in COVID-19 survivors (A) The prevalence of hair loss (24.76%; 95%CI: 19.60%, 29.91%; p<0.001; pHet=0.006; pEgger=0.875; (B) The prevalence of palpitation (19.38; 95%CI: 2.40, 41.16; p=0.081; pHet<0.001; pEgger=0.185; (C) The prevalence of sleep difficulties (17.87%; 95%CI: 7.55%, 28.20%); p<0.001; pHet<0.001; pEgger=0.328. (D) The prevalence of weight loss (8.19%; 95%CI: 5.66%, 10.71%).
Association of COVID-19 severity and persistent long COVID symptoms
Due to the scarcity of data relating to persistent long COVID symptoms among mild-moderate and severe COVID-19 patients, the association between COVID-19 severity and the risk of persistent long COVID symptoms was unable to be determined.
Discussion
The COVID-19 outbreak is an ongoing health crisis with concerns about patients' health status that arise not only during the initial infection but also post-recovery. Studies have reported persistent symptoms and prolonged lung function, physical and psychological problems in discharged patients [30, 39, 41-43].
Psychological issues during the COVID-19 pandemic were common due to social isolation, concern about transmitting the disease, and the fear of stigma and discrimination society [44]. Long-term quarantine and loneliness among COVID-19 patients might increase psychological distress, including severe depression and insomnia, leading to suicidal thoughts and behaviour [45]. While psychological distress increased universally among the general population [46, 47], this is apparent only in the initial period of the pandemic before swift recovery to baseline [48], indicating prolonged distress as a specific attribute of long COVID.
The host immune response also plays an important role in cognitive-behavioural changes in COVID-19 infection. High levels of interleukin (IL)-1β, IL-6, IL-4, IL-10, tumor necrosis factor-alpha (TNF-α), chemokine (C-C-motif) ligand 2 (CCL2), granulocyte-macrophage colony-stimulating factor (GM-CSF) and interferon (IFN)-γ suggests a cytokine dysregulation in SARS-CoV-2 infection that might lead to altered neurotransmitter signalling [49]. Cytokine dysregulation, particularly of IL-1β, IL-6, IL-10, IFN-γ and TNF-α are known to be associated with the development and progression of psychological disorders, including depressive mood and anxiety [50-52]. There is evidence of SARS-CoV-2 causing changes in the brain parenchyma and blood vessels [53, 54]. These changes may induce brain inflammation, blood-brain-barrier dysfunction, neurotransmitter impairment and hypothalamic-pituitary adrenal (HPA) axis disruption which might induce neurological manifestation such as brain fog, tinnitus, and poor sleep quality [22].
Weight loss of more than 5% of initial body weight was found among 31% (48/156) hospitalized and 21% (12/57) non-hospitalized COVID-19 patients after recovery in Milan, Italy [55]. Several mechanisms may be involved in weight loss among COVID-19 patients. Vascular leakage combined with endothelial barrier disruption in COVID-19 patients may result in an inflammatory cascade, disrupting tissue homeostasis [56]. The presence of abundant acute phase proteins and cytokines in COVID-19 patients may cause dysregulation of metabolism [57-59], proteolysis [59-61], and the hypothalamus pathway, thereby increasing resting energy expenditure and muscle catabolism [62], that could further cause weight loss [63, 64]. Psychological problems also contribute to decreased food intake, consequently aggravating the COVID-19 patients' malnutrition [65-67]. As a result, these conditions contribute to tissue degeneration [68]. Additionally, appetite loss, fatigue, changes in smell and taste, anosmia, ageusia, and fever all contribute to malnutrition and cachexia [69, 70], which can result in anabolic failure and subsequent weight loss [71].
Chest pain is commonly complained by COVID-19 survivors [72]. Another study reported that chest pain was experienced by 60% of 430 patients on initial infection and 32.6% of 370 patients after COVID-19 recovery [73]. It is crucial to understand whether persistent chest pain in post-COVID-19 is due to musculoskeletal, cardiovascular, or lung lesions. As symptoms on chest are self-defined by the patient, sometimes it is also termed as chest tightness which is related to musculoskeletal symptoms or chest burn as cardiovascular symptoms[74]. Coronary microvascular ischemia has been reported to cause persistent chest pain in patients that have recovered from COVID-19 [75]. Chest discomfort is also attributed to pulmonary lesions, such as lung fibrosis[76], mild pleural effusion and bronchiectasis[77].
Data on hair loss in COVID-19 survivors is still limited and may be associated with androgenetic alopecia in men [78] or telogen effluvium in women [79]. Hair loss in COVID-19 patients could also be explained by vascular leakage caused by stress, as well as endothelial and epithelial scalp cell death, which could trigger an inflammatory cascade by disrupting homeostasis, such as IL-1 [80, 81]. Inflammatory mediators are cytotoxic to hair cells [82, 83] and high IL-1 level has been linked to hair loss [84, 85].
This study compiled preliminary data on prolonged symptoms of COVID-19, such as anxiety, depression, dizziness, chest pain, sleep difficulty, palpitations, weight loss, and hair loss. Due to the small number of current studies on persistent symptoms of COVID-19, there is no standard data on the onset and duration of persistent symptoms of COVID-19. However, our study presents basic data on the prevalence and the duration of prolonged symptoms of SARS-CoV-2 infection. The low number of articles included in this meta-analysis significantly influenced the findings of our study and due to the lack of data on severity of COVID-19 in these studies, no association between persistent symptoms and the severity of COVID-19 could be determined. Therefore, further studies with larger populations and subgroup analysis are warranted to ascertain these findings, including determining the association between COVID-19 severity and the presence of prolonged symptoms in COVID-19 survivors.
Conclusions
Persistent long COVID symptoms are prevalent in COVID-19 survivors. The result of our study suggested that longer follow-up studies within a larger population are needed. Supportive care is also necessary to ensure a smooth transition back to the community and increasing the quality of life of COVID-19 patients after discharge.
Acknowledgments
The authors would like to thank Narra Studio Journal Indonesia for assisting the writing processes.
Declarations
Ethics approval
Not required.
Conflict of interest
The authors declare that they have no competing interests.
Funding
This study received no external funding.
Supplementary material
PRISMA checklist is available from: https://doi.org/10.6084/m9.figshare.14371784.
How to cite
Fahriani M, Ilmawan M, Fajar JK, et al Persistence of long COVID symptoms in COVID-19 survivors worldwide and its potential pathogenesis - A systematic review and meta-analysis. Narra J 2021; 1(2): e36. https://doi.org/10.6084/m9.figshare.14371784.
References
- 1.COVID-19 coronavirus pandemic. Available from: https://www.worldometers.info/coronavirus/ (Accessed: 11 March, 2021).
- 2.Nicola M, Alsafi Z, Sohrabi C, et al. The socio-economic implications of the coronavirus pandemic (COVID-19): A review. Int J Surg 2020; 78:185–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martin A, Markhvida M, Hallegatte S, et al. Socio-Economic impacts of COVID-19 on household consumption and poverty. Econ Disaster Clim Chang 2020; 4(3):1–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greene CJ, Burleson SL, Crosby JC, et al. Coronavirus disease 2019: International public health considerations. J Am Coll Emerg Physicians Open 2020; 1(2):70–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lew HL, Oh-Park M, Cifu DX. The war on covid-19 pandemic: role of rehabilitation professionals and hospitals. Am J Phys Med Rehabil 2020; 99(7):571–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giannakeas V, Bhatia D, arkentin MT, et al. Estimating the maximum capacity of COVID-19 cases manageable per day given a health care system’s constrained resources. Ann Intern Med 2020; 173(5):407–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fahriani M, Anwar S, Yufika A, et al. Disruption of childhood vaccination during the COVID-19 pandemic in Indonesia. Narra J 2021; 1(1):e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhanot D, Singh T, Verma SK, et al. Stigma and Discrimination During COVID-19 Pandemic. Front Public Health 2020; 8(829):577018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Badrfam R, Zandifar A.. Stigma Over COVID-19; New conception beyond individual sense. Arch Med Res 2020; 51(6):593–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Badrfam R, Zandifar A.. COVID-19 and melancholia: different perception of the concept of stigma and loss. Iran J Psychiatry 2020; 15(3):264–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh R, Subedi M.. COVID-19 and stigma: Social discrimination towards frontline healthcare providers and COVID-19 recovered patients in Nepal. Asian J Psychiatr 2020; 53:102222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dye TD, Alcantara L, Siddiqi S, et al. Risk of COVID-19-related bullying, harassment and stigma among healthcare workers: an analytical cross-sectional global study. BMJ Open 2020; 10(12):e046620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rogers JP, Chesney E, Oliver D, et al. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry 2020; 7(7):611–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McKay D, Heisler M, Mishori R, et al. Attacks against health-care personnel must stop, especially as the world fights COVID-19. The Lancet 2020; 395(10239):1743–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mei Q, Wang F, Bryant A, et al. Mental health problems among COVID-19 survivors in uhan, China. orld Psychiatry 2021; 20(1):139–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park HY, Jung J, Park HY, et al. Psychological consequences of survivors of COVID-19 pneumonia 1 month after discharge. J Korean Med Sci 2020; 35(47):e409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cai X, Hu X, Ekumi IO, et al. Psychological distress and its correlates among COVID-19 survivors during early convalescence across age groups. Am J Geriatr Psychiatry 2020; 28(10):1030–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun N, ei L, Wang H, et al. Cualitative study of the psychological experience of COVID-19 patients during hospitalization. J Affect Disord 2021; 278:15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nalbandian A, Sehgal K, Gupta A, et al. Post-acute COVID-19 syndrome. Nat Med 2021; 27(4):601–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carvalho-Schneider C, Laurent E, Lemaignen A, et al. Follow-up of adults with noncritical COVID-19 two months after symptom onset. Clin Microbiol Infect 2021; 27(2):258–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akinci T, Basar H Melek. Relationship between sleep quality and the psychological status of patients hospitalised with COVID-19. Sleep Med 2021; 80:167–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iqbal A, Iqbal K, Arshad Ali S, et al. The COVID-19 sequelae: a cross-sectional evaluation of post-recovery symptoms and the need for rehabilitation of covid-19 survivors. Cureus 2021; 13(2):e13080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Plos Med 2009; 6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.World Health Organization. Clinical management of COVID-19: interim guidance, 27 May 2020. In.: World Health Organization; 2020. [Google Scholar]
- 25.China National Health Commission. Chinese clinical guidance for COVID-19 pneumonia diagnosis and treatment. 2020.
- 26.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010; 25(9):603–605. [DOI] [PubMed] [Google Scholar]
- 27.Cochrane T. Review Manager (RevMan) 5.3. Copenhagen: The Nordic Cochrane Centre 2008; 373. [Google Scholar]
- 28.Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet 2021; 397(10270):220–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cellai M, O’Keefe JB. Characterization of prolonged COVID-19 symptoms in an outpatient telemedicine clinic. Open Forum Infect Dis 2020; 7(10):ofaa420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xiong Q, Xu M, Li J, et al. Clinical sequelae of COVID-19 survivors in uhan, China: a single-centre longitudinal study. Clin Microbiol Infect 2021; 27(1):89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu HQ, Yuan B, An YW, et al. Clinical characteristics and follow-up analysis of 324 discharged COVID-19 patients in Shenzhen during the recovery period. Int J Med Sci 2021; 18(2):347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yan N, Wang W, Gao Y, et al. Medium term follow-up of 337 patients with coronavirus disease 2019 (COVID-19) in a Fangcang Shelter Hospital in uhan, China. Front Med (Lausanne) 2020; 7:373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Lorenzo R, Conte C, Lanzani C, et al. Residual clinical damage after COVID-19: A retrospective and prospective observational cohort study. PLoS One 2020; 15(10):e0239570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou L-p, Wang J, ie R-h, et al. The effects of traditional Chinese Medicine as an auxiliary treatment for COVID-19: A systematic review and meta-analysis. The Journal of Alternative and Complementary Medicine 2020. [DOI] [PubMed] [Google Scholar]
- 35.Levy C, Lassailly G, Parmentier E, et al. Caution with the use of lopinavir/ritonavir in severely ill patients for the treatment of SARS-CoV-2: A report of severe jaundice. The American Journal of Gastroenterology 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liang L, Yang B, Jiang N, et al. Three-month Follow-up study of survivors of coronavirus disease 2019 after discharge. J Korean Med Sci 2020; 35(47):e418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sami R, Soltaninejad F, Amra B, et al. A one-year hospital-based prospective COVID-19 open-cohort in the Eastern Mediterranean region: The Khorshid COVID Cohort (KCC) study. PLoS One 2020; 15(11):e0241537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tabatabaei SMH, Rajebi H, Moghaddas F, et al. Chest CT in COVID-19 pneumonia: what are the findings in mid-term follow-up? Emerg Radiol 2020; 27(6):711–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carfi A, Bernabei R, Landi F, et al. Persistent symptoms in patients after acute COVID-19. JAMA 2020; 324(6):603–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miyazato Y, Morioka S, Tsuzuki S, et al. Prolonged and late-onset symptoms of coronavirus disease 2019. Open Forum Infect Dis 2020; 7(11):ofaa507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang Y, Tan C, Xu J, et al. Impact of coronavirus disease 2019 on pulmonary function in early convalescence phase. Respir Res 2020; 21(1):163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu C, Ye L, ia R, et al. Chest computed tomography and clinical follow-up of discharged patients with COVID-19 in enzhou City, Zhejiang, China. Ann Am Thorac Soc 2020; 17(10):1231–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao YM, Shang YM, Song B, et al. Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery. EClinicalMedicine 2020; 25:100463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mazza MG, De Lorenzo R, Conte C, et al. Anxiety and depression in COVID-19 survivors: Role of inflammatory and clinical predictors. Brain Behav Immun 2020; 89:594–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gillett G, Jordan I.. Severe psychiatric disturbance and attempted suicide in a patient with COVID-19 and no psychiatric history. BMJ Case Rep 2020; 13(10):e239191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang J, Lu H, Zeng H, et al. The differential psychological distress of populations affected by the COVID-19 pandemic. Brain Behav Immun 2020; 87:49–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.iu J, Shen B, Zhao M, et al. A nationwide survey of psychological distress among Chinese people in the COVID-19 epidemic: implications and policy recommendations. Gen Psychiatr 2020; 33(2):e100213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Daly M, Robinson E.. Psychological distress and adaptation to the COVID-19 crisis in the United States. J Psychiatr Res 2021; 136:603–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ye Q, Wang B, Mao J.. The pathogenesis and treatment of the cytokine storm’in COVID-19. J Infection 2020; 80(6):607–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hepgul N, Cattaneo A, Agarwal K, et al. Transcriptomics in interferon-a-treated patients identifies inflammation-, neuroplasticity-and oxidative stress-related signatures as predictors and correlates of depression. Neuropsychopharmacology 2016; 41(10):2502–2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hou R, Garner M, Holmes C, et al. Peripheral inflammatory cytokines and immune balance in Generalised Anxiety Disorder: Case-controlled study. Brain Behav Immun 2017; 62:212–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kubistova A, Horacek J, Novak T.. Increased interleukin-6 and tumor necrosis factor alpha in first episode schizophrenia patients versus healthy controls. Psychiatr Danub 2012; 24 Suppl 1:S153–156. [PubMed] [Google Scholar]
- 53.Romero-Sanchez CM, Diaz-Maroto I, Fernandez-Diaz E, et al. Neurologic manifestations in hospitalized patients with COVID-19: The ALBACOVID registry. Neurology 2020; 95(8):e1060–e1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reichard RR, Kashani KB, Boire NA, et al. Neuropathology of COVID-19: a spectrum of vascular and acute disseminated encephalomyelitis (ADEM)-like pathology. Acta Neuropathol 2020; 140(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Di Filippo L, De Lorenzo R, D’Amico M, et al. COVID-19 is associated with clinically significant weight loss and risk of malnutrition, independent of hospitalisation: A post-hoc analysis of a prospective cohort study. Clin Nutr 2021; 40(4):2420–2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Anker MS, Landmesser U, von Haehling S, et al. eight loss, malnutrition, and cachexia in COVID-19: facts and numbers. In.: Wiley Online Library; 2021. [DOI] [PMC free article] [PubMed]
- 57.Laaksonen DE, Niskanen L, Nyyssonen K, et al. C-reactive protein and the development of the metabolic syndrome and diabetes in middle-aged men. Diabetologia 2004; 47(8):1403–1410. [DOI] [PubMed] [Google Scholar]
- 58.Ventre J, Doebber T, Xu M, et al. Targeted disruption of the tumor necrosis factor-alpha gene: metabolic consequences in obese and nonobese mice. Diabetes 1997; 46(9):1526–1531. [DOI] [PubMed] [Google Scholar]
- 59.Virgens IP, Santana NM, Lima SC, et al. Can COVID-19 be a risk for cachexia for patients during intensive care? Narrative review and nutritional recommendations. 2020:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zamir O, Hasselgren P-O, Kunkel SL, et al. Evidence that tumor necrosis factor participates in the regulation of muscle proteolysis during sepsis. 1992; 127(2):170–174. [DOI] [PubMed] [Google Scholar]
- 61.Clowes Jr G, Hirsch E, George BC, et al. Survival from sepsis. The significance of altered protein metabolism regulated by proteolysis inducing factor, the circulating cleavage product of interleukin-1. 1985; 202(4):446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.ahlin-Larsson B, ilkinson DJ, Strandberg E, et al. Mechanistic Links Underlying the Impact of C-Reactive Protein on Muscle Mass in Elderly. Cell Physiol Biochem 2017; 44(1):267–278. [DOI] [PubMed] [Google Scholar]
- 63.Fruebis J, Tsao TS, Javorschi S, et al. Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc Natl Acad Sci U S A 2001; 98(4):2005–2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Selvin E, Paynter NP, Erlinger TP. The effect of weight loss on C-reactive protein: a systematic review. Arch Intern Med 2007; 167(1):31–39. [DOI] [PubMed] [Google Scholar]
- 65.Zhang C, Xu Z, Li JW, et al. Cytokine release syndrome in severe COVID-19: interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. Int J Antimicrob Agents 2020; 55(5):105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Di--Filippo L, De--Lorenzo R, D’Amico M, et al. COVID-19 is associated with clinically significant weight loss and risk of malnutrition, independent of hospitalisation: A post-hoc analysis of a prospective cohort study. 2021; 40(4):2420–2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bedock D, Bel Lassen P, Mathian A, et al. Prevalence and severity of malnutrition in hospitalized COVID-19 patients. Clin Nutr ESPEN 2020; 40:214–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Deshmukh V, Motwani R, Kumar A, et al. Histopathological observations in COVID-19: a systematic review. J Clin Pathol 2021; 74(2):76–83. [DOI] [PubMed] [Google Scholar]
- 69.Morley JE, Kalantar-Zadeh K, Anker SD. COVID-19: a major cause of cachexia and sarcopenia? J Cachexia Sarcopenia Muscle 2020; 11(4):863–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Trachootham D, Thongyen S, Lam-Ubol A, et al. Simultaneously Complete but Not Partial Taste and Smell Losses were Associated with SAR-CoV-2 Infection. 2021. [DOI] [PMC free article] [PubMed]
- 71.Anker SD, Negassa A, Coats AJ, et al. Prognostic importance of weight loss in chronic heart failure and the effect of treatment with angiotensin-converting-enzyme inhibitors: an observational study. Lancet 2003; 361(9363):1077–1083. [DOI] [PubMed] [Google Scholar]
- 72.Greenhalgh T, Knight M, A’Court C, et al. Management of post-acute covid-19 in primary care. BMJ 2020; 370:m3026. [DOI] [PubMed] [Google Scholar]
- 73.Galal I, Hussein AARM, Amin MT, et al. Determinants of persistent post-COVID-19 symptoms: value of a novel COVID-19 symptom score. The Egyptian Journal of Bronchology 2021; 15(1):10. [Google Scholar]
- 74.Davis HE, Assaf GS, McCorkell L, et al. Characterizing long COVID in an International Cohort: 7 months of symptoms and their impact. medRxiv 2021:2020.2012.2024.20248802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vallejo N, Teis A, Mateu L, et al. Persistent chest pain after recovery of COVID-19: microvascular disease-related angina? European Heart Journal-Case Reports 2021; 5(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shaw B, Daskareh M, Gholamrezanezhad A.. The lingering manifestations of COVID-19 during and after convalescence: update on long-term pulmonary consequences of coronavirus disease 2019 (COVID-19). Radiol Med 2021; 126(1):40–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Spinner CD, Gottlieb RL, Criner GJ, et al. Effect of remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID-19: a randomized clinical trial. Jama 2020; 324(11):1048–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Goren A, Vano-Galvan S, ambier CG, et al. A preliminary observation: Male pattern hair loss among hospitalized COVID-19 patients in Spain - A potential clue to the role of androgens in COVID-19 severity. J Cosmet Dermatol 2020; 19(7):1545–1547. [DOI] [PubMed] [Google Scholar]
- 79.Mieczkowska K, Deutsch A, Borok J, et al. Telogen effluvium: a sequela of COVID-19. Int J Dermatol 2021; 60(1):122–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cavalli G, De Luca G, Campochiaro C, et al. Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study. Lancet Rheumatol 2020; 2(6):e325–e331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Theobald SJ, Simonis A, Kreer C, et al. The SARS-CoV-2 spike protein primes inflammasome-mediated interleukin-1-beta secretion in COVID-19 patient-derived macrophages. 2020.
- 82.Huang M, Dulon D, Schacht JJAoO, Rhinology, et al. Outer hair cells as potential targets of inflammatory mediators. 1990; 99(6_suppl):35–38. [DOI] [PubMed] [Google Scholar]
- 83.Mahe YF, Buan B, Billoni N, et al. Pro-inflammatory cytokine cascade in human plucked hair. Skin Pharmacol 1996; 9(6):366–375. [DOI] [PubMed] [Google Scholar]
- 84.Hoffmann R, Eicheler W, Wenzel E, et al. Interleukin-1-induced inhibition of hair growth in vitro is mediated by cyclic AMP. 1997; 108(1):40–42. [DOI] [PubMed] [Google Scholar]
- 85.Hoffmann R, Happle RJD. Does lnterleukin-1 Induce Hair Loss? 1995; 191(4):273–275. [DOI] [PubMed] [Google Scholar]