Abstract
Hepatitis E virus (HEV) is an important public health problem and is responsible for both acute and chronic viral hepatitis. Public health implications of HEV are derived from its transmission route, either water-borne or food-borne, and its zoonotic potential. Not only in developing countries, but HEV cases are also found in a high number in developed countries. The spread of HEV to the environment might pollute surface waters, which could act as the source of infection for both humans and animals. Identification of the virus in animal products suggests the circulation of HEV within water and food chains. High seroprevalence and circulation of HEV in livestock, in particular pigs, as well as in environmental samples warrants further investigation into pig markets. HEV virulence in different environments and meat supply chains could shed light on the possible sources of infection in humans and the degree of occupational risk. The purpose of this review is to discuss HEV infections with an emphasis on livestock- and environment-related risk factors, and food-borne, water-borne, and zoonotic transmissions.
Keywords: Hepatitis E virus, livestock, zoonotic transmission, water-borne, environment
Introduction
Hepatitis E virus (HEV), one of main contributors to global acute viral hepatitis, poses a significant public health concern with high prevalence in developing countries. It was long- neglected, although has now also emerged as an important virus in the developed countries [1- 4]. In areas with heavy seasonal rainfall, high evaporation rates, and dense population, HEV could cause outbreaks of acute hepatitis [5-7]. Based on a report from the World Health Organization (WHO), approximately 20 million cases of HEV are found annually across countries. Of the total reported hepatitis cases, an estimated 3.3 million infections were asymptomatic. In 2015, according to the WHO estimation, approximately there were 44,000 HEV-associated deaths worldwide [8]. Moreover, HEV mortality rate among women during pregnancy is possible to reach 30% [9-12].
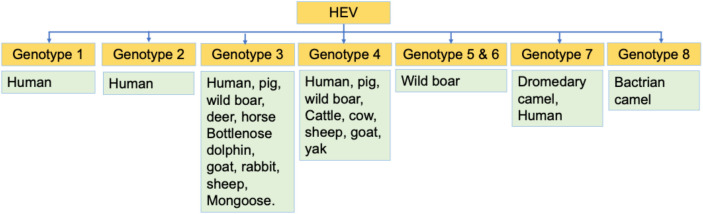
HEV is a non-enveloped single-stranded positive-sense RNA virus with a diameter around 27—34 nm that belongs to the family Hepeviridae, covering two genera: Orthohepevirus (infect birds as well as mammals) and Piscihepevirus (infect fish). Further classification of genus Orthohepevirus results in four known species, they are Orthohepevirus A, B, C, and D. Orthohepevirus A includes at least eight distinct HEV genotypes (HEV-1 to HEV-8) infecting both humans and animals [13-15]. HEV-1 and HEV-2 are responsible for a wide-scale water- borne epidemics in developing countries [1, 6]. Meanwhile, HEV-3 and HEV-4 are more causing impacts of relatively small outbreaks in developed countries [1, 6]. The species causing HEV infection in swine belongs to the Orthohepevirus A [14]. The HEV-1 and HEV-2 are constrained to humans; HEV-3 circulates in humans, deer, rabbits, mongooses, and swine; HEV-4 circulates in humans, sheep, cow, goat, cattle, yak, wild boar, and pig; HEV-5 and HEV-6 are found in wild boars; HEV-7 has been identified in dromedary camel; and genotype 8 has been isolated recently in Bactrian camel [15-17]. Figure 1 presents different HEV genotypes circulate in humans and animals.
Figure 1. Orthohepevirus A genotypes and hosts.
In developing countries, the HEV infection has caused 20-25% of acute hepatitis cases [18], and in the general population, the mortality ranges from 0.2 to 1% [15]. The viral infection is especially prevalent in Asia and Africa [18, 19]. In Africa, high number of HEV infection cases has been documented last year in animals with HEV genotypes closer to those infecting humans, where some animals may act as virus reservoirs suggesting the possibility of zoonotic transmission [20]. In developed countries, the HEV cases have been increased recently mainly owing to the consumption of undercooked meat, and is a silent threat which need much epidemiological investigations and appropriate mitigation strategies to be adopted [18, 21, 22]. In the present scenario, among European population, HEV is being considered as a growing zoonotic infection [23].
Autochthonous HEV causes considerable clinical problems in developed countries, where several animals including wild boar, wild deer and domestic swine act as reservoirs of HEV-3 and HEV-4 genotypes. Humans acquire HEV infections by consuming foods made of meat from infected animals that are not properly cooked, especially the liver [24]. Not much data are available with regards to HEV prevalence and contamination in food, especially in developing countries, and investigations on such issues therefore would elucidate a better understanding of food-borne impact of this important virus and its maintenance [25].
In most cases, the fecal-oral route is involved in the HEV transmission through ingestion of contaminated food and drinking water. HEV contamination of food sources and surface waters could occur through solid and liquid residues of infected animals, which act as zoonotic reservoirs. The food-borne transmission of HEV is primarily due to insufficiently cooked pig products, although living in close contact with animals or irrigation water are the known risk factors of HEV infection in both healthy as well as immunocompromised individuals [26-28]. The HEV may also transmit via other routes such as mother-to-child vertical transmission or blood transfusion [24, 29-34]. The transmission of HEV from person-to-person is infrequent [34-36]. HEV infection is an important public health issue and wide distribution of HEV in humans, animals and environmental as well as associated zoonotic concerns warrants the strengthening of One Health strategies to control and prevent HEV infection [3, 23, 25, 28-30]. This review discusses the importance of HEV infections and associated risk factors, with a particular focus on pig-associated transmission, zoonotic transmission, environmental contamination, and water-borne outbreaks.
HEV infection in livestock in particular pigs
HEV is a zoonotic virus that can be transmitted from animals to humans. Data suggested that the most significant reservoir for HEV genotypes responsible for infection in human is pigs [37, 38]. In 1997, the HEV was identified for the first time in pigs, and pigs are recognized as the main reservoir of HEV-3 along with HEV-4 [39]. Some studies have isolated and characterized HEV from pigs to evidence the infection of HEV and determined the prevalence of anti-HEV antibodies in livestock [40-84]. HEV infection in pigs normally occurs during early life, and the virus shedding peaks at about the age of 3 months old. People occupationally exposed to pigs have increased probability of the past HEV infection by almost threefold [51]. Other than pigs, anti-HEV antibodies have been identified from a wide spectrum of species such as cattle, dogs, goats, deer, donkeys, and sheep [37, 38, 44, 45].
Anti-HEV antibodies seroprevalence
Exposure to animal feces and intake of improperly cooked pork can cause HEV infection. Due to limited data in resource-deficient countries, a study evaluated whether pigs could potentially act as a source of HEV infections among Vietnamese population [46]. Liver specimens from domestic pigs and plasma specimens from pork-meat-exposed humans and pigs were compared with unexposed controls. The study found that persons occupationally acquainted with pork or pig meat had high seroprevalence compared to unexposed individuals. Approximately 12.3% of liver tissues from pigs were positive for HEV-3 suggesting that exposure to pork meat and pigs is a risk of HEV infection.
A cross-sectional investigation in Lao People’s Democratic Republic revealed the prevalence of anti-HEV IgG antibody in slaughter pigs reached 54.0% (136/252), in professionals exposed to pigs was 41.0% (57/139), and 18.1% (38/210) was in non-risk controls [52]. Furthermore, contact with young piglets was a major risk factor as compared to contact with the older slaughter pigs [52]. Another recent study published from ten US states witnessed the prevalence of HEV-IgG antibody reaching 40% of 5,033 serum samples from market-weight pigs at 25 slaughterhouses, of which the HEV RNA was found in 6.3% with the virus belonging to HEV-3 genotype [53].
European countries studies also report a high prevalence of HEV-infected pigs [54-57]. In Germany, a total of 1,072 domestic pigs’ sera were evaluated against anti-HEV specific antibodies and the overall seroprevalence was 49.8% [54]. Another study from Germany reported the overall seroprevalence of anti-HEV IgG in domestic pigs’ sera of 68.6%. In the Netherlands, the overall anti-HEV seroprevalence in different kind of pigs raised on organic farms (n=417, 89%) was significantly higher than those raised on conventional farms (n=265, 72%) [58]. A study from Scotland found that the seroprevalence of anti-HEV specific antibodies were IgG (29%), IgM (29%), and IgA (36.9%) in slaughter-age pigs [56]. In Switzerland, HEV seroprevalence of 58.1% was documented in domestic pigs [57]. In Bulgaria, HEV seroprevalence in domestic pigs was 60% and the seroprevalence in slaughter-aged pigs 73.6% [21]. A study found that 43.7% of 48 samples from Iberian pigs possessed anti-HEV antibodies [59].
Studies from other countries also reported a high prevalence of HEV in the pig population [21, 51, 58]. In China, the overall seroprevalence of anti-HEV antibodies was 64.7% in swine and the prevalence of HEV was higher in swine farmers than that of the general population [78]. A systematic review and meta-analysis of studies published within the last decade (2010 to 2019) reported a high prevalence of HEV infection among domestic pig population in China [79]. The study recommended to avoid mixed feeding of different stages as an effort to reduce the infection rate of HEV in pigs which could consequently reduce the risks of zoonotic transmission from pigs to humans [79]. In Singapore, serological as well as molecular characterization study of HEV in imported pigs within the years of 2000-2019 demonstrated the presence of HEV in live pig and post-slaughter samples [80]. This study suggested the importance of regular monitoring of the prevalent HEV strains and evaluation of the genetic diversity of HEVs in the imported pigs to further evaluate the association of the role of pigs on transmitting HEV to humans [80].
In one study conducted in Japan, of 160 serum samples collected from pigs, 72.5% (116/160) were IgG positive and IgM negative, while 23.8% (38/160) were positive for both IgG as well as IgM [81]. A recent study, anti-HEV IgG seroprevalence reached 66% in slaughterhouse staff, 51% in pig-farmers, and 38% in pork meat vendors [46] and those who had exposure to pigs or pork meat had high chances of HEV infection when compared with unexposed individuals [46]. A study from Abruzzo, Italy, a reportedly hyperendemic region having the highest HEV seroprevalence in humans, out of 233 blood samples collected from different local pig slaughterhouses between 87.3% and 100% of serum samples were found positive for anti-HEV antibodies (IgG), such high seroprevalence in pigs suggests the intense circulation of HEV in the region [82]. Previously published studies showed that pigs successively receive HEV infection on farms, where a higher prevalence was found among older pigs [26, 83, 84].
HEV RNAs prevalence
Constant contact and consumption of pig and pig products increase risk and hence warrants proper inspection and surveillance [59, 60]. Raw viscera have more feasibility to harbor HEV in comparison with pork, where ground pork and pig liver could act as a potential source of HEV [60, 61]. In one study, HEV RNAs with positivity values of 3.1% (4/129), 6.1% (7/114), and 1.2% (2/170) were found in renal, hepatic, and blood samples, respectively, suggesting higher transmission risk of consuming pig organs [61]. Similarly, investigation in Southern Italy, found that 99 pork and 63 wild boar sausages and salami collected from the market have been detected with HEV belonging to HEV-3 genotype [62]. However, the mere presence of HEV in samples may rarely matter than the infectivity of HEV and there should be a robust system to evaluate the infectivity of HEV in food samples. In this regard, cell culture-based systems are reported to have promising prospects [63].
A study concluded that HEV RNA in pigs and retail pork livers in Canada was comparable to that of Europe and the USA [64]. In Brazil, HEV RNA has been detected in 0.8% (6/713) of serum samples from backyard pigs in southern part of the county and falling in three different genotypes related to human HEV strains indicating backyard pigs as a reservoir of HEV and thus in need for infection and spillover control from backyard farms [65].
In a small study from Switzerland, HEV RNA was detected in 11.1% (10/90) meat products, 18.9% (7/37) liver sausages, and 5.7% (3/53) raw meat sausages with viral loads of up to 5.54 log10 genome copies per gram and the viruses belonged to HEV-3 [66]. The foregoing study indicates the HEV contamination in ready-to-eat meat products on the retail market, hence the necessity for developing efficient diagnostic methods for easy detection [66]. A study that detected and quantified HEV RNA in ready-to-eat raw pork sausages in the Netherlands found that 14.5% (46/316) samples were positive for HEV RNA with the average viral load was 2.76 to 4.5 log10 genome copies per 5 grams [67]. These findings act as the basis for risk assessment and risk management with respect to pork and pork product consumption and raw pork sausages as a risk factor for HEV infection among Dutch population [67]. Various ingredients related to pigs and piggeries are also considered as a possible risk for HEV transmission. Transmission of HEV also has been identified in slurry samples from swine farming activities in Italy where 75% (18/24 samples) were positive containing HEV RNA [68]. Therefore, strategies of improving the safety standard in swine farming and educating individuals involved in pork meat production on the zoonotic risk could minimize risk for HEV infection in human.
In Bangkok, HEV RNA was observed in 0.2% of fresh meat in the markets and 3.9% in the slaughterhouse samples [69]. Fecal and bile samples were found more common than liver, pork, and intestine samples hence posing a risk of zoonosis. A study in Estonia found that domestic pigs, wild boars, pig farmworkers, and hunters were infected with HEV and this indicates the important attention for direct contact between the animals and the persons or handlers [70]. Both direct contact and environmental contaminations could contribute to not only HEV transmission in livestock but also in humans [71].
The number of viral particles existing in the environment has been considered significant during the transmission process [71]. Transmission via porcine blood ingredient during meat production is found feasible [72]. Positive results for HEV RNA were observed in 91.6% (33/36) batches of liquid products from a non-heating process and 29.1% (7/24) batches of powder products from a spray-drying process [72].
A study from Finland reported that 11.9% of domestic pigs were HEV RNA positive. HEV contamination was found in fattening pigs and weaning pigs, where the occurrence was more common in the first than the latter (14.8% vs. 12.5%) [73]. A study in Italy found that 64.6% pigs were positive for HEV RNA in at least one sample, and the HEV genome was detected in 51.1%, 33.3%, and 20.8% of bile samples, feces and livers, respectively [74]. Another study from four abattoirs in Italy revealed a high seroprevalence (76.8%) of anti-HEV antibodies in pigs using ELISA and 3.6% (21/585) were found HEV RNA positive in either fecal or hepatic samples by real-time RT-PCR [75]. This study also found circulation of HEV-3 and a novel unclassified HEV subtype was noticed from phylogenetic analyses [75]. A study from Denmark reported fecal samples of 49.5% Danish pig population to be positive for HEV RNA [76]. In France, the farm-level HEV seroprevalence was 65%, and 31% in the slaughter-aged pigs [77]. Furthermore, 4% of pig livers were HEV RNA positive [77]. The prevalence of HEV RNA in different samples from previous studies is presented in Table 1.
Table 1. The prevalence of IgG, IgM and HEV RNA in different pig samples around the globe.
| Antibody | Country | Animal | Sample | Positive | Total tested | Prevalence (%) | Reference |
|---|---|---|---|---|---|---|---|
| IgG | |||||||
| Lao PDR | Slaughter pigs | Serum | 136 | 252 | 54.0 | [52] | |
| US | Market-weight pigs at slaughterhouses | Serum | 2014 | 5,033 | 40.0 | [53] | |
| Germany | Pigs | Serum | 354 | 516 | 68.6 | [55] | |
| Germany | Pigs | Meat-juice | 134 | 198 | 67.7 | [55] | |
| Scotland | Slaughter-age pigs | Serum | 51 | 176 | 29.0 | [56] | |
| Bulgaria | Domestic pigs | Blood | 260 | 433 | 60.0 | [21] | |
| Bulgaria | Slaughter-aged pigs | Blood | 245 | 333 | 73.6 | [21] | |
| Spain | Iberian pigs | Serum | 21 | 48 | 43.8 | [59] | |
| Germany | Domestic pigs | Serum | 534 | 1072 | 49.8 | [54] | |
| Netherlands | Pigs raised on organic farms | Serum | 37 | 417 | 8.9 | [58] | |
| Netherlands | Pigs raised on conventional farms | Serum | 191 | 265 | 72.1 | [58] | |
| Netherlands | Pigs raised on free-range | Serum | 124 | 164 | 75.6 | [58] | |
| Switzerland | Domestic pigs | Serum | 1163 | 2,001 | 58.1 | [57] | |
| IgM | |||||||
| Germany | Pigs | Serum | 36 | 516 | 7.0 | [55] | |
| Scotland | Slaughter-age pigs | Serum | 51 | 176 | 29.0 | [56] | |
| RNA | |||||||
| Vietnam | Domestic pigs | Pig liver tissues | 26 | 210 | 12.4 | [46] | |
| US | Market-weight pigs at slaughterhouses | serum | 318 | 5,033 | 6.3 | [53] | |
| Scotland | Slaughter-age pigs | Serum | 72 | 162 | 44.4 | [56] | |
| Spain | Iberian pigs | Serum | 0 | 48 | 0.0 | [59] |
The cross-species transmission is of importance since it dominates the cases of HEV infection in developed countries [49, 50]. The involvement of other animal species in HEV transmission complicates control of the infection. In Spain, more than 10% samples from wild boar and 16% samples from red deer were positive for HEV RNA and approximately 57% samples of wild boar and 12.8% samples from red deer were positive for anti-HEV antibodies [59].
Altogether, there are strong evidence that indicate the prevalence of HEV in the livestock in particular pigs is high in both developed and developing countries. This poses high risk of zoonotic transmission from pigs to humans. Therefore, the regulations of farm, slaughterhouses, pork meat industries need to be strengthened. In addition, animal models for HEV infection might be important for further studies [85-87].
HEV in environmental samples
The persistence and transmission of HEV in the environment are currently underreported [88]. HEV-1 and HEV-2 are mostly found in places with limited resources as well as low sanitation leading to contamination of water supplies and food [1, 16, 17]. In India, for example, the HEV outbreaks were caused by highly polluted water between 1978 and 2013 [24]. Previously, the role of water in the HEV transmission has only been suspected without confirmation. However, detection of HEV-3 in shellfish and multiple shellfish-related outbreaks have recently triggered the discourse on water-borne HEV outbreak among scientists [89, 90].
Studies from developed countries also have showed a high HEV seroprevalence in individuals exposed to water or those who consume shellfish [91, 92]. Besides the evidence of HEV infection in humans via the intake of seafood (mussels and shellfish), captive dolphins, which generally feed on fish, have been reported to be positive for anti-HEV antibody and HEV RNA (genotype 3) [93], in which this finding raises concerns of environmental contamination of food or wastewater as a source of HEV.
To date, the waterborne HEV-3 transmission remains to be elucidated. A current study in Germany recognized that work-related contact with wastewater can be associated with autochthonous hepatitis E, indicating the possible role of water in transmitting HEV-3 [94]. Animal and human hosts of HEV-3 might pollute wastewater matrices by the secretion of feces. The release of HEV particles to the environment can pollute surface waters, which could possibly be an HEV infection source for animals and humans [88].
HEV has been identified in urban sewage samples in various countries such as in Spain, Italy, and the Netherlands [95-97]. Recently, several studies also have been conducted to determine HEV in urban sewages in European countries [98-106]. In Germany, wastewater samples were found positive by RT-qPCR for genotypes HEV-3c and 3f, where HEV-3c was identified as the most dominant genotype [107]. In addition, approximately 75% of samples from the urban river showed the detection of HEV RNA [107]. In another study, HEV prevalence was monitored in effluent and influent water in drinking and wastewater treatment plants (WWTPs) [108]. The data suggested that the prevalence of HEV in inflowing water samples varied based among WWTPs but no HEV was identified in effluent water [108]. A large-scale of nine years-study (2011-2019) was conducted that covered 48 different WWTPs in 20 distinct regions of Italy [106]. Out of 1,374 sewage samples, among 74 samples detected with HEV RNA, 56 and 18 of which belonged to HEV-3 and HEV-1, respectively. HEV-3 strains were detected throughout the investigation period while HEV-1 strains was detected only in 2011- 2012 suggesting that HEV-3 was the prevalent genotype [106]. In a 5-year integrated surveillance that included environmental and human, 169 HEV cases were confirmed with an annual occurrence of 0.72 cases/1,000,000 [105]. Among 65 HEV RNA-positive samples, 66%, 32%, and 1% were detected to be HEV-3, HEV-1, and HEV-4, respectively [105]. A study of water samples from the Arias–Arenales River in Salta city, Argentina found that HEV RNA was detected in 1.6% of the tested samples where the viruses belonged to HEV-3 while the prevalence of anti-HEV IgG was 9% [109]. A study analyzing 250 water and 68 sediment samples from the Sinos River, Brazil along with 50 pork products sold around the river found that HEV-3 was identified in 36% of food samples and HEV was not identified in water or sediment [110].
Altogether, there are robust evidence to demonstrate that HEV is widespread in both developed and developing countries indicating a significant threat to public health worldwide. Zoonotic-transmission has caused HEV infections in humans in the developed nations and this requires the development of vaccines to prevent the spread to humans [111]. A study since the 90s in European countries clearly indicated that HEV frequently infects the European communities, and some animal species such as deer, wild boars, and pigs serves as reservoirs for the virus [22]. Identification of virus in polluted pork products and mussels suggesting the transmission of HEV strains from water to food chains.
HEV in water and water-borne outbreaks
Genotypes 1 and 2 of HEV are mainly reported in Asia including Afghanistan, India, Bangladesh, China, Nepal, and Pakistan, some African countries such as Chad, the Central African Republic, Nigeria, and Sudan, as well as Mexico [112]. Analysis of drinking and sewage water samples from Faisalabad, Pakistan showed the presence of HEV-1 strain [113]. In Italy, HEV-1 and HEV-2 were identified in sewage water samples from WWTPs [96]. In Spain, HEV genome was detected from slaughterhouse sewage mainly from pigs and the viruses had 92-94% nucleotide similarity with those strain detected from the humans [114]. A high positivity rate of 20/46 (43.5%) of HEV RNA was also reported in sewage samples from Barcelona, Spain [115].
Another study from Spain showed the presence of both HEV-1 and HEV-3 genotypes in water samples from WWTPs [116], and sewage [117]. In Colombia, of the total sixty environmental samples, the HEV genome was detected in 23.3% (7/30) of drinking water plants/creek and in 16.7% (5/30) of sewage samples [118]. HEV-1 and possibly HEV-2 have caused several water-borne outbreaks in many parts of the world [3, 24, 119].
HEV prevention strategies
Considering the importance and wide circulation of HEV in humans and animals, global public health priority needs to be given for enhancing monitoring and surveillance as well as adopting adequate mitigation strategies for evading and managing HEV and its associated zoonotic significance [120]. HEV 239, Hecolin® vaccine (Xiamen Innovax Biotech, China) is purchasable in private market in China, but it has not received WHO approval for its use in endemic settings and disease outbreaks worldwide. In HEV outbreaks, the two important preventive approaches comprise the provision of drinking clean water and improving the sanitary disposal of human waste. Implementing these approaches in a timely manner in regions where the HEV epidemic occurs is a challenging issue [121].
Globally, the anti-HEV antibodies (IgM and IgG) seroprevalence has been recorded both in humans and animals, and HEV RNA also has been identified from environmental samples. Some contributing factors on the increasing HEV infection rate include low socioeconomic status, poor hygiene, low access to clean water, lack of proper sanitation, and the lack of commercial access to a hepatitis E vaccine especially in the high endemic regions. Global availability of the effective vaccine to tackle future HEV outbreaks, larger analysis of magnitude of the worldwide burden, improving diagnostics and epidemiological methodologies, improving standards of water quality, hygiene, and sanitation in endemic regions along with implementation of One Health approach are needed for effective prevention and control of HEV. Awareness with regards to the prevalence and spatial distribution of HEV in livestock animals, especially in pigs and strengthening of HEV testing in boars, along with controlling environmental contamination of the virus could play vital role in implementing appropriate prevention and control strategies to avoid the animals-to-humans transmission of HEV.
Conclusion and future prospects
In the past, the HEV was considered an infection of developing countries only. But after the discovery of new HEV genotypes, different animal reservoirs, the cross-species transmission have changed the understanding of HEV worldwide and it became a significant global public health problem. Since a few years ago, the cases of HEV have been increased in developed countries, which are mainly transmitted due to the consumption of uncooked food. However, other modes of transmissions also contribute significantly.
Genotype 1 and 2 of HEV are responsible for large outbreaks in low-income countries, while HEV-3 and HEV-4 are mainly responsible for sporadic cases in the developed countries. In developed countries, exposure to animal feces and consumption of undercooked pork meat could have cause significant number of hepatitis E. Therefore, the cross-species transmission is considered the leading cause of HEV infection in the developed countries. Identification of virus in polluted pork products and mussels indicate the circulation of HEV strains from water to food chain, and thus necessitate a careful assessment of swine herds and food safety. Although the waterborne HEV-3 transmission remains uncertain, the occupational contact with wastewater might be associated with autochthonous hepatitis E, suggesting the possible role of water in transmitting HEV-3. HEV has been identified in urban sewage samples in countries around the globe.
A better understanding of the viral dynamics and disease progress by developing models that are amicable to natural host or disease pathology to support future research and minimize risks. Special attention should be paid to vulnerable and high-risk groups including pregnant women. In the absence of specific knowledge and lack of interventions, general preventive measures can be helpful. Safety measures and disease awareness are of worth importance while traveling to HEV endemic areas. There is a dire need for effective treatment and commercially available vaccines to prevent and control HEV infection with particular emphasis on low- income countries as well as adopting the concept of One Health approach.
Acknowledgments
The authors acknowledge their respective universities/institutes/organizations.
Ethics approval
Not required.
Conflict of interest
All the authors declare that there are no conflicts of interest.
Funding
This study received no external funding.
Underlying data
All data underlying the results are available as part of the article and no additional source data are required.
How to cite
Ahmad T, Jin H, Dhama K, et al. Hepatitis E virus in pigs and the environment: An updated review of public health concerns. Narra J 2022; 2 (2): e78 - http://doi.org/10.52225/narra.v2i2.78.
References
- 1.Hakim MS, Wang W, Bramer WM, et al. The global burden of hepatitis E outbreaks: a systematic review. Liver Int 2017; 37(1):19–31. [DOI] [PubMed] [Google Scholar]
- 2.Aslan AT, Balaban HY. Hepatitis E virus: Epidemiology, diagnosis, clinical manifestations, and treatment. World J Gastroenterol 2020; 26(37):5543–5560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pallerla SR, Harms D, Johne R, et al. Hepatitis E virus infection: circulation, molecular epidemiology, and impact on global health. Pathogens 2020; 9(10):856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jing W, Liu J, Liu M.. The global trends and regional differences in incidence of HEV infection from 1990 to 2017 and implications for HEV prevention. Liver Int 2021; 41(1):58–69. [DOI] [PubMed] [Google Scholar]
- 5.Kamar N, Bendall R, Legrand-Abravanel F, et al. Hepatitis E. Lancet 2012; 379(9835):2477–2488. [DOI] [PubMed] [Google Scholar]
- 6.Carratala A, Joost S.. Population density and water balance influence the global occurrence of hepatitis E epidemics. Sci Rep 2019; 9(1):10042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Azman AS, Ciglenecki I, Wamala JF, et al. Hepatitis E should be considered a neglected tropical disease. PLoS Negl Trop Dis 2019; 13(7):e0007453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hepatitis E. Available from: https://www.who.int/news-room/fact-sheets/detail/hepatitis-e. Accessed: 1 December 2021.
- 9.Boccia D, Guthmann JP, Klovstad H, et al. High mortality associated with an outbreak of hepatitis E among displaced persons in Darfur, Sudan. Clin Infect Dis 2006; 42(12):1679–1684. [DOI] [PubMed] [Google Scholar]
- 10.Bhatnagar G, Sharma S, Kumar A, et al. Reduced glutathione in hepatitis E infection and pregnancy outcome. J Obstet Gynaecol Res 2016; 42(7):789–795. [DOI] [PubMed] [Google Scholar]
- 11.Perez-Gracia MT, Suay-Garcia B, Mateos-Lindemann ML. Hepatitis E and pregnancy: current state. Rev Med Virol 2017; 27(3):e1929. [DOI] [PubMed] [Google Scholar]
- 12.Ahmad T, Hui J, Musa TH, et al. Seroprevalence of hepatitis E virus infection in pregnant women: a systematic review and meta-analysis. Ann Saudi Med 2020; 40(2):136–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Purdy MA, Harrison TJ, Jameel S, et al. ICTV Virus Taxonomy Profile: Hepeviridae. J Gen Virol 2017; 98(11):2645–2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sooryanarain H, Meng XJ. Swine hepatitis E virus: Cross-species infection, pork safety and chronic infection. Virus Res 2020;284:197985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thakur V, Ratho RK, Kumar S, et al. Viral hepatitis E and chronicity: a growing public health concern. Front Microbiol 2020;11:577339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sridhar S, Teng JLL, Chiu TH, et al. Hepatitis E virus genotypes and evolution: emergence of camel hepatitis E variants. Int J Mol Sci 2017;18(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Primadharsini PP, Nagashima S, Okamoto H.. Genetic variability and evolution of hepatitis E virus. Viruses 2019;11(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marrone G, Biolato M, Mercurio G, et al. Acute HEV hepatitis: clinical and laboratory diagnosis. Eur Rev Med Pharmacol Sci 2019; 23(2):764–770. [DOI] [PubMed] [Google Scholar]
- 19.Raji YE, Toung OP, Mohd Taib N, et al. A systematic review of the epidemiology of Hepatitis E virus infection in South - Eastern Asia. Virulence 2021; 12(1):114–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Modiyinji AF, Bigna JJ, Kenmoe S, et al. Epidemiology of hepatitis E virus infection in animals in Africa: a systematic review and meta-analysis. BMC Vet Res 2021; 17(1):50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takova K, Koynarski T, Minkov I, et al. Increasing hepatitis E virus seroprevalence in domestic pigs and wild boar in Bulgaria. Animals (Basel) 2020; 10(9):1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clemente-Casares P, Ramos-Romero C, Ramirez-Gonzalez E, et al. Hepatitis E Virus in Industrialized countries: the silent threat. Biomed Res Int 2016;2016:9838041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mrzljak A, Dinjar-Kujundzic P, Jemersic L, et al. Epidemiology of hepatitis E in South-East Europe in the “One Health” concept. World J Gastroenterol 2019; 25(25):3168–3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khuroo MS, Khuroo MS, Khuroo NS. Transmission of hepatitis E virus in developing countries. Viruses 2016; 8(9):253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Di Cola G, Fantilli AC, Pisano MB, et al. Foodborne transmission of hepatitis A and hepatitis E viruses: A literature review. Int J Food Microbiol 2021;338:108986. [DOI] [PubMed] [Google Scholar]
- 26.Bouwknegt M, Frankena K, Rutjes SA, et al. Estimation of hepatitis E virus transmission among pigs due to contact-exposure. Vet Res 2008; 39(5):40. [DOI] [PubMed] [Google Scholar]
- 27.Van der Poel WH. Food and environmental routes of Hepatitis E virus transmission. Curr Opin Virol 2014;4:91–96. [DOI] [PubMed] [Google Scholar]
- 28.EFSA Panel on Biological Hazards (BIOHAZ), Ricci A, Allende A, et al. Public health risks associated with hepatitis E virus (HEV) as a food-borne pathogen. Efsa j 2017; 15(7):e04886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meng XJ. Zoonotic and foodborne transmission of hepatitis E virus. Semin Liver Dis 2013; 33(1):41–49. [DOI] [PubMed] [Google Scholar]
- 30.Park WJ, Park BJ, Ahn HS, et al. Hepatitis E virus as an emerging zoonotic pathogen. J Vet Sci 2016; 17(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khuroo MS, Kamili S, Jameel S.. Vertical transmission of hepatitis E virus. Lancet 1995; 345(8956):1025–1026. [DOI] [PubMed] [Google Scholar]
- 32.Patra S, Kumar A, Trivedi SS, et al. Maternal and fetal outcomes in pregnant women with acute hepatitis E virus infection. Ann Intern Med 2007; 147(1):28–33. [DOI] [PubMed] [Google Scholar]
- 33.Khuroo MS, Kamili S, Khuroo MS. Clinical course and duration of viremia in vertically transmitted hepatitis E virus (HEV) infection in babies born to HEV-infected mothers. J Viral Hepat 2009; 16(7):519–523. [DOI] [PubMed] [Google Scholar]
- 34.Hewitt PE, Ijaz S, Brailsford SR, et al. Hepatitis E virus in blood components: a prevalence and transmission study in southeast England. Lancet 2014; 384(9956):1766–1773. [DOI] [PubMed] [Google Scholar]
- 35.Schlosser B, Stein A, Neuhaus R, et al. Liver transplant from a donor with occult HEV infection induced chronic hepatitis and cirrhosis in the recipient. J Hepatol 2012; 56(2):500–502. [DOI] [PubMed] [Google Scholar]
- 36.Matsui T, Kang JH, Matsubayashi K, et al. Rare case of transfusion-transmitted hepatitis E from the blood of a donor infected with the hepatitis E virus genotype 3 indigenous to Japan: Viral dynamics from onset to recovery. Hepatol Res 2015; 45(6):698–704. [DOI] [PubMed] [Google Scholar]
- 37.Perez-Gracia MT, Garcia M, Suay B, et al. Current knowledge on hepatitis E. J Clin Transl Hepatol 2015; 3(2):117–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sridhar S, Lau SK, Woo PC. Hepatitis E: A disease of reemerging importance. J Formos Med Assoc 2015; 114(8):681–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meng XJ, Purcell RH, Halbur PG, et al. A novel virus in swine is closely related to the human hepatitis E virus. Proc Natl Acad Sci U S A 1997; 94(18):9860–9865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu X, Saito M, Sayama Y, et al. Seroprevalence and molecular characteristics of hepatitis E virus in household-raised pig population in the Philippines. BMC Vet Res 2015;11:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Adelabu OA, Chuks Iweriebor B, Nwodo UU, et al. Incidence and molecular characterization of hepatitis E virus from swine in Eastern Cape, South Africa. Adv Virol 2017;2017:1073253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim YH, Park BJ, Ahn HS, et al. Detection of hepatitis E virus genotypes 3 and 4 in pig farms in Korea. J Vet Sci 2018; 19(2):309–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sasaki Y, Haruna M, Uema M, et al. Prevalence and phylogenetic analysis of hepatitis e virus among pigs in Japan. Jpn J Infect Dis 2018; 71(1):75–78. [DOI] [PubMed] [Google Scholar]
- 44.Tei S, Kitajima N, Takahashi K, et al. Zoonotic transmission of hepatitis E virus from deer to human beings. Lancet 2003; 362(9381):371–373. [DOI] [PubMed] [Google Scholar]
- 45.Geng J, Wang L, Wang X, et al. Potential risk of zoonotic transmission from young swine to human: seroepidemiological and genetic characterization of hepatitis E virus in human and various animals in Beijing, China. J Viral Hepat 2011; 18(10):e583–590. [DOI] [PubMed] [Google Scholar]
- 46.Hoan NX, Huy PX, Sy BT, et al. High Hepatitis E virus (HEV) positivity among domestic pigs and risk of HEV Infection of individuals occupationally exposed to pigs and pork meat in Hanoi, Vietnam. Open Forum Infect Dis 2019; 6(9):ofz306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yazaki Y, Mizuo H, Takahashi M, et al. Sporadic acute or fulminant hepatitis E in Hokkaido, Japan, may be food-borne, as suggested by the presence of hepatitis E virus in pig liver as food. J Gen Virol 2003;84(Pt 9):2351–2357. [DOI] [PubMed] [Google Scholar]
- 48.Mykytczuk O, Harlow J, Bidawid S, et al. Prevalence and molecular characterization of the hepatitis E virus in retail pork products marketed in Canada. Food Environ Virol 2017; 9(2):208–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meng XJ. Hepatitis E virus: animal reservoirs and zoonotic risk. Vet Microbiol 2010;140(3-4):256–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mirazo S, Gardinali NR, Cecilia D, et al. Serological and virological survey of hepatitis E virus (HEV) in animal reservoirs from Uruguay reveals elevated prevalences and a very close phylogenetic relationship between swine and human strains. Vet Microbiol 2018;213:21–27. [DOI] [PubMed] [Google Scholar]
- 51.Salines M, Andraud M, Rose N.. From the epidemiology of hepatitis E virus (HEV) within the swine reservoir to public health risk mitigation strategies: a comprehensive review. Vet Res 2017; 48(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Khounvisith V, Tritz S, Khenkha L, et al. High circulation of Hepatitis E virus in pigs and professionals exposed to pigs in Laos. Zoonoses Public Health 2018; 65(8):1020–1026. [DOI] [PubMed] [Google Scholar]
- 53.Sooryanarain H, Heffron CL, Hill DE, et al. Hepatitis E Virus in Pigs from Slaughterhouses, United States, 2017-2019. Emerg Infect Dis 2020; 26(2):354–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baechlein C, Schielke A, Johne R, et al. Prevalence of Hepatitis E virus-specific antibodies in sera of German domestic pigs estimated by using different assays. Vet Microbiol 2010;144(1-2):187–191. [DOI] [PubMed] [Google Scholar]
- 55.Wacheck S, Werres C, Mohn U, et al. Detection of IgM and IgG against hepatitis E virus in serum and meat juice samples from pigs at slaughter in Bavaria, Germany. Foodborne Pathog Dis 2012; 9(7):655–660. [DOI] [PubMed] [Google Scholar]
- 56.Crossan C, Grierson S, Thomson J, et al. Prevalence of hepatitis E virus in slaughter-age pigs in Scotland. Epidemiol Infect 2015; 143(10):2237–2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Burri C, Vial F, Ryser-Degiorgis MP, et al. Seroprevalence of hepatitis E virus in domestic pigs and wild boars in Switzerland. Zoonoses Public Health 2014; 61(8):537–544. [DOI] [PubMed] [Google Scholar]
- 58.Rutjes SA, Bouwknegt M, van der Giessen JW, et al. Seroprevalence of hepatitis E virus in pigs from different farming systems in The Netherlands. J Food Prot 2014; 77(4):640–642. [DOI] [PubMed] [Google Scholar]
- 59.Kukielka D, Rodriguez-Prieto V, Vicente J, et al. Constant hepatitis E Virus (HEV) circulation in wild boar and red deer in Spain: an increasing concern source of HEV zoonotic transmission. Transbound Emerg Dis 2016; 63(5):e360–368. [DOI] [PubMed] [Google Scholar]
- 60.Harrison L, Ramos TM, Wu X, et al. Presence of hepatitis E virus in commercially Available pork products. Int J Food Microbiol 2021;339:109033. [DOI] [PubMed] [Google Scholar]
- 61.Geng Y, Zhao C, Guo T, et al. Detection of hepatitis E virus in raw pork and pig viscera as food in hebei province of China. Foodborne Pathog Dis 2019; 16(5):325–330. [DOI] [PubMed] [Google Scholar]
- 62.Montone AMI, De Sabato L, Suffredini E, et al. Occurrence of HEV-RNA in Italian regional pork and wild boar food products. Food Environ Virol 2019; 11(4):420–426. [DOI] [PubMed] [Google Scholar]
- 63.Cook N, D’Agostino M, Johne R.. Potential approaches to assess the infectivity of hepatitis E Virus in pork products: a review. Food Environ Virol 2017; 9(3):243–255. [DOI] [PubMed] [Google Scholar]
- 64.Wilhelm B, Fazil A, Rajic A, et al. Risk Profile of hepatitis E Virus from pigs or pork in Canada. Transbound Emerg Dis 2017; 64(6):1694–1708. [DOI] [PubMed] [Google Scholar]
- 65.da Silva MS, Silveira S, Caron VS, et al. Backyard pigs are a reservoir of zoonotic hepatitis E virus in southern Brazil. Trans R Soc Trop Med Hyg 2018; 112(1):14–21. [DOI] [PubMed] [Google Scholar]
- 66.Moor D, Liniger M, Baumgartner A, et al. Screening of ready-to-eat meat products for hepatitis E virus in Switzerland. Food Environ Virol 2018; 10(3):263–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Boxman ILA, Jansen CCC, Zwartkruis-Nahuis AJT, et al. Detection and quantification of hepatitis E virus RNA in ready to eat raw pork sausages in the Netherlands. Int J Food Microbiol 2020;333:108791. [DOI] [PubMed] [Google Scholar]
- 68.La Rosa G, Della Libera S, Brambilla M, et al. Hepatitis E virus (Genotype 3) in slurry samples from swine farming activities in Italy. Food Environ Virol 2017; 9(2):219–229. [DOI] [PubMed] [Google Scholar]
- 69.Intharasongkroh D, Sa-Nguanmoo P, Tuanthap S, et al. Hepatitis E Virus in pork and variety meats sold in fresh markets. Food Environ Virol 2017; 9(1):45–53. [DOI] [PubMed] [Google Scholar]
- 70.Ivanova A, Tefanova V, Reshetnjak I, et al. Hepatitis E virus in domestic pigs, wild boars, pig farm workers, and hunters in Estonia. Food Environ Virol 2015; 7(4):403–412. [DOI] [PubMed] [Google Scholar]
- 71.Andraud M, Dumarest M, Cariolet R, et al. Direct contact and environmental contaminations are responsible for HEV transmission in pigs. Vet Res 2013;44:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Boxman ILA, Jansen CCC, Hägele G, et al. Porcine blood used as ingredient in meat productions may serve as a vehicle for hepatitis E virus transmission. Int J Food Microbiol 2017;257:225–231. [DOI] [PubMed] [Google Scholar]
- 73.Kantala T, Heinonen M, Oristo S, et al. Hepatitis E virus in young pigs in Finland and characterization of the isolated partial genomic sequences of genotype 3 HEV. Foodborne Pathog Dis 2015; 12(3):253–260. [DOI] [PubMed] [Google Scholar]
- 74.Di Bartolo I, Ponterio E, Castellini L, et al. Viral and antibody HEV prevalence in swine at slaughterhouse in Italy. Vet Microbiol 2011;149(3-4):330–338. [DOI] [PubMed] [Google Scholar]
- 75.Chelli E, Suffredini E, De Santis P, et al. Hepatitis E virus occurrence in pigs slaughtered in Italy. Animals (Basel) 2021; 11(2):277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Breum S, Hjulsager CK, de Deus N, et al. Hepatitis E virus is highly prevalent in the Danish pig population. Vet Microbiol 2010;146(1-2):144–149. [DOI] [PubMed] [Google Scholar]
- 77.Rose N, Lunazzi A, Dorenlor V, et al. High prevalence of Hepatitis E virus in French domestic pigs. Comp Immunol Microbiol Infect Dis 2011; 34(5):419–427. [DOI] [PubMed] [Google Scholar]
- 78.Liang H, Su S, Deng S, et al. The prevalence of hepatitis E virus infections among swine, swine farmers and the general population in Guangdong Province, China. PLoS One 2014; 9(2):e88106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen Y, Gong QL, Wang Q, et al. Prevalence of hepatitis E virus among swine in China from 2010 to 2019: A systematic review and meta-analysis. Microb Pathog 2021;150:104687. [DOI] [PubMed] [Google Scholar]
- 80.Wang Y, Toh X, Ong J, et al. Serological prevalence and molecular characterization of hepatitis E virus in imported pigs in Singapore (2000-2019). Transbound Emerg Dis 2021. [DOI] [PubMed] [Google Scholar]
- 81.Motoya T, Umezawa M, Goto K, et al. High prevalence of hepatitis E virus infection among domestic pigs in Ibaraki Prefecture, Japan. BMC Vet Res 2019; 15(1):87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Martino C, Rampacci E, Pierini I, et al. Detection of anti-HEV antibodies and RNA of HEV in pigs from a hyperendemic Italian region with high human seroprevalence. Eur J Public Health 2021; 31(1):68–72. [DOI] [PubMed] [Google Scholar]
- 83.Sakano C, Morita Y, Shiono M, et al. Prevalence of hepatitis E virus (HEV) infection in wild boars (Sus scrofa leucomystax) and pigs in Gunma Prefecture, Japan. J Vet Med Sci 2009; 71(1):21–25. [DOI] [PubMed] [Google Scholar]
- 84.Haider N, Khan MSU, Hossain MB, et al. Serological evidence of hepatitis E virus infection in pigs and jaundice among pig handlers in Bangladesh. Zoonoses Public Health 2017; 64(7):572–577. [DOI] [PubMed] [Google Scholar]
- 85.Sayed IM, Meuleman P.. Updates in Hepatitis E virus (HEV) field; lessons learned from human liver chimeric mice. Rev Med Virol 2020; 30(2):e2086. [DOI] [PubMed] [Google Scholar]
- 86.Sayed IM, Elkhawaga AA, El-Mokhtar MA. In vivo models for studying Hepatitis E virus infection; Updates and applications. Virus Res 2019;274:197765. [DOI] [PubMed] [Google Scholar]
- 87.Yang C, Hao X, Li Y, et al. Successful establishment of hepatitis E virus infection in pregnant BALB/c Mice. Viruses 2019; 11(5):451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fenaux H, Chassaing M, Berger S, et al. Transmission of hepatitis E virus by water: An issue still pending in industrialized countries. Water Res 2019;151:144–157. [DOI] [PubMed] [Google Scholar]
- 89.Said B, Ijaz S, Kafatos G, et al. Hepatitis E outbreak on cruise ship. Emerg Infect Dis 2009; 15(11):1738–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Crossan C, Baker PJ, Craft J, et al. Hepatitis E virus genotype 3 in shellfish, United Kingdom. Emerg Infect Dis 2012; 18(12):2085–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mansuy JM, Saune K, Rech H, et al. Seroprevalence in blood donors reveals widespread, multi-source exposure to hepatitis E virus, southern France, October 2011. Euro Surveill 2015; 20(19):27–34. [PubMed] [Google Scholar]
- 92.Mansuy JM, Gallian P, Dimeglio C, et al. A nationwide survey of hepatitis E viral infection in French blood donors. Hepatology 2016; 63(4):1145–1154. [DOI] [PubMed] [Google Scholar]
- 93.Montalvo Villalba MC, Cruz Martinez D, Ahmad I, et al. Hepatitis E virus in bottlenose dolphins Tursiops truncatus. Dis Aquat Organ 2017; 123(1):13–18. [DOI] [PubMed] [Google Scholar]
- 94.Faber M, Askar M, Stark K.. Case-control study on risk factors for acute hepatitis E in Germany, 2012 to 2014. Euro Surveill 2018;23(19). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pina S, Jofre J, Emerson SU, et al. Characterization of a strain of infectious hepatitis E virus isolated from sewage in an area where hepatitis E is not endemic. Appl Environ Microbiol 1998; 64(11):4485–4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.La Rosa G, Pourshaban M, Iaconelli M, et al. Molecular detection of hepatitis E virus in sewage samples. Appl Environ Microbiol 2010; 76(17):5870–5873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rutjes SA, Lodder WJ, Lodder-Verschoor F, et al. Sources of hepatitis E virus genotype 3 in The Netherlands. Emerg Infect Dis 2009; 15(3):381–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rodriguez-Manzano J, Miagostovich M, Hundesa A, et al. Analysis of the evolution in the circulation of HAV and HEV in eastern Spain by testing urban sewage samples. J Water Health 2010; 8(2):346–354. [DOI] [PubMed] [Google Scholar]
- 99.Kokkinos PA, Ziros PG, Mpalasopoulou A, et al. Molecular detection of multiple viral targets in untreated urban sewage from Greece. Virol J 2011;8:195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Masclaux FG, Hotz P, Friedli D, et al. High occurrence of hepatitis E virus in samples from wastewater treatment plants in Switzerland and comparison with other enteric viruses. Water Res 2013; 47(14):5101–5109. [DOI] [PubMed] [Google Scholar]
- 101.Myrmel M, Lange H, Rimstad E.. A 1-Year Quantitative survey of noro-, adeno-, human boca-, and hepatitis E viruses in raw and secondarily treated sewage from two plants in Norway. Food Environ Virol 2015; 7(3):213–223. [DOI] [PubMed] [Google Scholar]
- 102.Smith DB, Paddy JO, Simmonds P.. The use of human sewage screening for community surveillance of hepatitis E virus in the UK. J Med Virol 2016; 88(5):915–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang H, Sikora P, Rutgersson C, et al. Differential removal of human pathogenic viruses from sewage by conventional and ozone treatments. Int J Hyg Environ Health 2018; 221(3):479–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Matos A, Mesquita JR, Goncalves D, et al. First detection and molecular characterization of hepatitis E virus in water from wastewater treatment plants in Portugal. Ann Agric Environ Med 2018; 25(2):364–367. [DOI] [PubMed] [Google Scholar]
- 105.Alfonsi V, Romano L, Ciccaglione AR, et al. Hepatitis E in Italy: 5 years of national epidemiological, virological and environmental surveillance, 2012 to 2016. Euro Surveill 2018; 23(41): 1700517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Iaconelli M, Bonanno Ferraro G, Mancini P, et al. Nine-year nationwide environmental surveillance of hepatitis e virus in urban wastewaters in Italy (2011-2019). Int J Environ Res Public Health 2020; 17(6):2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Beyer S, Szewzyk R, Gnirss R, et al. Detection and characterization of hepatitis E Virus genotype 3 in wastewater and urban surface waters in Germany. Food Environ Virol 2020; 12(2):137–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cuevas-Ferrando E, Randazzo W, Perez-Cataluna A, et al. HEV Occurrence in waste and drinking water treatment plants. Front Microbiol 2019;10:2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pisano MB, Lugo BC, Poma R, et al. Environmental hepatitis E virus detection supported by serological evidence in the northwest of Argentina. Trans R Soc Trop Med Hyg 2018; 112(4):181–187. [DOI] [PubMed] [Google Scholar]
- 110.Heldt FH, Staggmeier R, Gularte JS, et al. Hepatitis E virus in surface water, sediments, and pork products marketed in southern Brazil. Food Environ Virol 2016; 8(3):200–205. [DOI] [PubMed] [Google Scholar]
- 111.Song YJ, Park WJ, Park BJ, et al. Hepatitis E virus infections in humans and animals. Clin Exp Vaccine Res 2014; 3(1):29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nelson KE, Labrique AB, Kmush BL. Epidemiology of genotype 1 and 2 hepatitis e virus Infections. Cold Spring Harb Perspect Med 2019;9(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ahmad T, Anjum S, Sadaf Zaidi NU, et al. Frequency of hepatitis E and hepatitis A virus in water sample collected from Faisalabad, Pakistan. Ann Agric Environ Med 2015; 22(4):661–664. [DOI] [PubMed] [Google Scholar]
- 114.Pina S, Buti M, Cotrina M, et al. HEV identified in serum from humans with acute hepatitis and in sewage of animal origin in Spain. J Hepatol 2000; 33(5):826–833. [DOI] [PubMed] [Google Scholar]
- 115.Clemente-Casares P, Pina S, Buti M, et al. Hepatitis E virus epidemiology in industrialized countries. Emerg Infect Dis 2003; 9(4):448–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bofill-Mas S, Albinana-Gimenez N, Clemente-Casares P, et al. Quantification and stability of human adenoviruses and polyomavirus JCPyV in wastewater matrices. Appl Environ Microbiol 2006; 72(12):7894–7896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Clemente-Casares P, Rodriguez-Manzano J, Girones R.. Hepatitis E virus genotype 3 and sporadically also genotype 1 circulate in the population of Catalonia, Spain. J Water Health 2009; 7(4):664–673. [DOI] [PubMed] [Google Scholar]
- 118.Baez PA, Lopez MC, Duque-Jaramillo A, et al. First evidence of the Hepatitis E virus in environmental waters in Colombia. PLoS One 2017; 12(5):e0177525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Maila HT, Bowyer SM, Swanepoel R.. Identification of a new strain of hepatitis E virus from an outbreak in Namibia in 1995. J Gen Virol 2004;85(Pt 1):89–95. [DOI] [PubMed] [Google Scholar]
- 120.Kirkwood CD, Dobscha KR, Steele AD. Hepatitis E should be a global public health priority: recommendations for improving surveillance and prevention. Expert Rev Vaccines 2020; 19(12):1129–1140. [DOI] [PubMed] [Google Scholar]
- 121.Teshale EH, Hu DJ. Hepatitis E: Epidemiology and prevention. World J Hepatol 2011; 3(12):285–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data underlying the results are available as part of the article and no additional source data are required.