Abstract
In conjunction with other health promotion strategies, vaccination of coronavirus disease 2019 (COVID-19) is a strategy to alleviate the burden of infection. The aim of this study was to determine the differences in antibody response strength between individuals who received COVID-19 vaccination and those who had a natural infection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). A cross-sectional study was conducted among post-natural confirmed COVID-19 infection and immunized people in Bali, Indonesia. The vaccination was using Sinovac-CoronaVac with two-weeks interval between the two vaccine doses. To measure the level of anti-Spike receptor binding domain (SRBD) of SARS-CoV-2 antibody, we used Roche electro- chemiluminescence immunoassay (ECLIA) platform. Blood samples were obtained before and 28 days after first immunization in the vaccinated group, as well as two weeks after hospital discharge in the confirmed COVID-19 patients based on real-time reverse transcription polymerase chain reaction (RT-PCR). A total of 58 confirmed COVID-19 patients and 60 vaccinated individuals were included. On the 28th day after the initial vaccination, the seroconversion rate among vaccinated individuals was 91.67%. The mean titer of anti-SRBD SARS-CoV-2 antibody among vaccinated participants was 63.62±82.57 IU/mL (ranged between 0 IU/mL and 250 IU/mL). The mean titer among naturally infected group was 188.47±94.57 IU/mL (ranged between 4.25 IU/mL to 250 IU/mL) regardless the severity of COVID-19. Our data suggested that the titer of anti- SRBD SARS-CoV-2 antibody was significantly higher in naturally infected individuals compared to those who received COVID-19 vaccination (p<0.001). These data suggest that not all individuals vaccinated with Sinovac COVID-19 had protective level of anti- SRBD SARS-CoV-2 antibody and booster dose of heterologous vaccine maybe required.
Keywords: COVID-19, anti-SRBD quantitative, natural infection, vaccine, CoronaVac
Introduction
Coronavirus disease 2019 (COVID-19) is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which was first identified on December 2019 and proclaimed a global pandemic by the World Health Organization (WHO). By December 15th, 2021, it has infected over 270 million people worldwide and caused deaths in over 5 million people [1]. As of December 15th, 2021, Indonesia was also at risk from the COVID-19 pandemic, with more than four million confirmed cases and over 140.000 deaths [2]. The infection manifests in a variety of ways, ranging from asymptomatic to life-threatening diseases with a high fatality rate particularly in people with comorbidities [3]. High mortality rate (37.5%) was reported in Sudan with the most death reported during the first 24 hours of infection [4]. The high mortality was not only reported in the low-middle income countries but also in high-income countries [5, 6].
Since the morbidity and mortality is profoundly remarkable, many efforts have been made to limit the number of COVID-19 cases, including the enforcement of health protocols and the nationwide distribution of the COVID-19 vaccination [7]. However, no particular anti-SARS- CoV-2 medications have yet been discovered, and SARS-CoV-2 variants have spread. Under the Emergency Use Authorization from The United States Food and Drug Administration (FDA), several COVID-19 vaccines have been approved and available globally in 2021 including the vaccines from Pfizer-BioNTech, Moderna, Janssen and Janssen vaccine, AztraZeneca, Sinopharm, Bharat Biotech and Sinovac [8]. In Indonesia, the COVID-19 vaccine program started on January 2021 with Sinovac at first, followed by AztraZeneca vaccine. This study was conducted to compare the seroconversion strength (the titer level) of neutralizing antibody (anti-Spike receptor binding domain (SRBD)) between post-COVID-19 vaccination and natural SARS-CoV-2 infection. In this study, we assessed the titer of antibody among individuals vaccinated with CoronaVac (Sinovac Life Sciences, Beijing, China). This is because CoronaVac was the first vaccine approved in Indonesia, where its effectiveness and protective ability are being questioned as compared to other vaccines.
Methods
A cross-sectional study was conducted between March and June 2021 in two immunization centers (Warmadewa Clinic and Wantilan DPRD Vaccine Center) and three hospitals (Puri Raharja Hospital, Kasih Ibu Hospital, and Bhayangkara Hospital) that provided health services for confirmed COVID-19 patients in Bali Province, Indonesia. All COVID-19 patients were diagnosed based on real-time reverse transcription polymerase chain reaction (RT-PCR) from nasopharyngeal swab samples. The inclusion criteria were adults COVID-19 confirmed with real-time reverse transcription polymerase chain reaction (RT-PCR) patients who came for review, approximately 14 days after discharge from the hospital. Three mL of venous blood was collected from the post-COVID-19 patients during their follow-up appointment. We did not evaluate the COVID-19 severity.
Adult over the age of 18 years who intended to receive the CoronaVac were recruited. All individuals who were seropositive before the vaccination were excluded. Before the first dose of vaccination, 3 mL venous blood sample was aseptically collected from all individuals. The whole blood then underwent centrifugation for 10 minutes with 3000 rpm. Subjects who tested positive for COVID-19 from this blood sample were excluded. The vaccination group received CoronaVac for two doses at a two-weeks interval. On day 28th after the first dose, additional 3 mL of venous blood was re-collected from each individual. Individuals who confirmed positive for COVID-19 between the first dose and the second blood collection were excluded.
Roche electrochemiluminescence immunoassay (ECLIA) method using Elecsys® Anti-SARS-CoV-2 S immunoassay was used to measure specific anti-SRBD antibody titer following the manufacturer's instruction (Roche Diagnostics International Ltd, Rotkreuz, Switzerland) [9]. The platform was run using an automatic Roche COBAS® E411 immunoassay analyzer (Roche Diagnostics International Ltd, Rotkreuz, Switzerland). To have better understanding of titer distribution, the antibody titer of anti-SRBD were classified into 0–50, 51–100, 101–200 and >200 IU/mL. In addition, the titer was also classified as protective and non-protective using a cut-off of 15 IU/mL [10].
The titer of anti-SRBD antibody were compared between the two groups using Student t-test. The analysis was conducted using Stata (StataCorp LLC, Texas, USA).
Results
A total of 58 hospitalized COVID-19 confirmed patients and 60 participants who had Sinovac immunization were recruited. The baseline data of the subject are presented in Table 1. The naturally infection group's median age was 53.52 years (ranged between 18.01–78.09 years), while the vaccine group's median age was 44.06 years (ranged between 19.00–62.09 years). For both groups, females were predominant (65.5% and 58.3% in natural infection group and COVID-19 vaccination group, respectively).
Table 1. Characteristics and the titer of anti-SRBD SARS-CoV-2 antibody between vaccinated and naturally SARS-CoV-2 infected individuals (n=118).
| Variable | Natural infection n (%), (n=58) | COVID-19 vaccination n (%), (n=60) |
|---|---|---|
| Age, median (IQR), year | 53.52 (18.01–78.09) | 44.06 (19.00–62.09) |
| Age group, year | ||
| 18-40 | 11 (18.9) | 26 (43.3) |
| >40–60 | 31 (52.4) | 31 (51.7) |
| >60 | 16 (27.6) | 3 (5.0) |
| Gender | ||
| Male | 20 (34.4) | 25 (41.7) |
| Female | 38 (65.5) | 35 (58.3) |
| Anti-SRBD antibody, mean (±SD), IU/mL | 188.47±94.57 | 63.62±82.57 |
| Antibody titer group, IU/mL | ||
| 0–50 | 12 (20.6) | 38 (63.3) |
| 51–100 | 2 (3.4) | 9 (15.0) |
| 101–200 | 5 (8.6) | 6 (10.0) |
| >200 | 39 (67.2) | 7 (11.7) |
| Antibody titer group | ||
| Protective | 52 (89.7) | 36 (60.0) |
| Non-protective | 6 (10.3) | 24 (40.0) |
The maximum detectable antibody titer was 250 IU/mL.
Among the vaccinated group, 91.6% (55/60) individuals were seroconverted while all individual who naturally infected were seroconverted. The individual titers of anti-SRBD SARS-CoV-2 antibody for both vaccinated and naturally infected are present in Figure 1. Our analysis found that the mean titer of anti-SRBD SARS-CoV-2 antibody was significantly higher in naturally infected individuals as compared to those who received COVID-19 vaccination; mean 188.47±94.57 IU/mL (ranges: 4.25–250) vs 63.62±82.57 IU/mL (ranges: 0–250), respectively with p<0.001.
Figure 1.
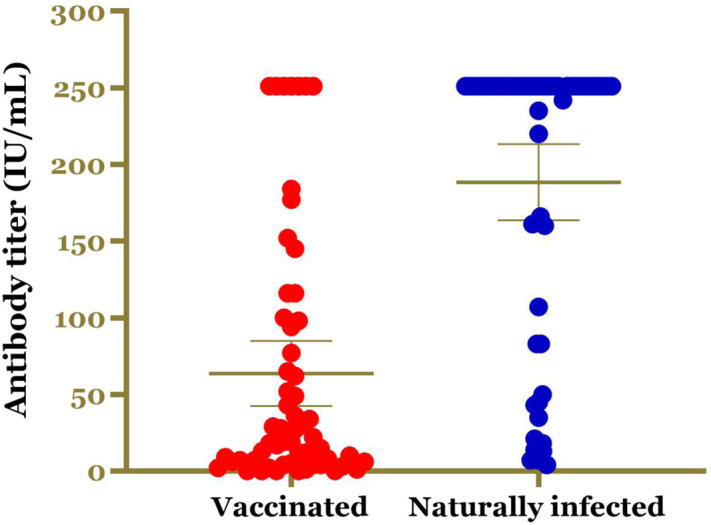
The individual titers of anti-SRBD SARS-CoV-2 antibody in CoronaVac vaccinated and naturally SARS-CoV-2 infected group.
Based on the distribution of the antibody titer, 67.2% of naturally infected individuals had antibody titer of >200 IU/mL while only 11.7% of vaccinated individuals had the same titer (Table 1). In addition, our data found that 89.7% of naturally infected individuals had protective level of anti-SRBD SARS-CoV-2 antibody while only 60.0% (36/60) vaccinated individuals had protective antibody (Table 1).
Discussion
Despite the widespread use of preventive tools, the ongoing COVID-19 pandemic has resulted in substantial morbidity and mortality worldwide. As a result, the vaccine is the last and most effective approach for reducing COVID-19 incidence. The development of COVID-19 vaccines began right after the genetic material for SARS-CoV-2 became accessible in early 2020. Inactivated vaccines (Sinovac and Sinopharm), mRNA vaccines (Moderna and BioNTech Pfizer), protein recombinant vaccines (Novavax), and viral vector vaccines (AstraZeneca and Johnson and Johnson) [11] have all been approved by WHO as Emergency Use Authorization [11]. Regardless vaccination platform used, it must be able to stimulate both the cellular and humoral immune systems. In Indonesia, vaccination with the CoronaVac has been started since January 2021.
In a published trial investigating the efficacy of inactivated COVID-19 vaccine (3 g/0.5 mL aluminum hydroxide diluent), seroconversion rate or immunogenicity was observed in 46% of the total subjects analyzed on the following 28 days after the first injection [12]. The second administration of the vaccine increased the seroconversion rate up to 92% [12]. The geometric mean titers of neutralizing antibodies observed after 14 days post-3rd dose injection in groups receiving 2.5, 5, and 10µg/dose inactivated vaccines were 316 (95% CI: 218–457), 206 (95% CI: 123–343), and 297 (95% CI: 208–424), respectively. Meanwhile, in this present study, the seroconversion rate of 91.67% among vaccinated individuals were found. We argue that the discrepancy in seroconversion could be due to different study populations, different vaccine doses, and vaccine schedules.
SARS-CoV-2 infection is neutralized by SRBD antibody. Neutralizing antibodies to SARS-CoV-2 can be detected using a variety of approaches, including neutralizing antibodies to live SARS-CoV-2, neutralizing antibodies to pseudo virus, surrogate viral neutralizing antibody (sVNT), and the ECLIA. Our present study found that anti-SRBD SARS-CoV-2 antibody titers in the vaccinated group were lower than those in the infected group. This could be ascribed to the common fact that a live virus induces a stronger immune response than an inactivated virus. In a previously published report, the majority of patients (99.1%; n=195) remained seropositive 61 days following the beginning of the illness [12], where the antibody titer of severe, mild, and asymptomatic individuals was 1:10. Unfortunately, as the limitation of this study, the antibody was only measured once in the infected group, and without considering COVID-19 severity.
The virus's virulence is likely to be reduced owing to the presence of numerical process during the production of an inactivated vaccine [13]. This could explain why inactivated vaccines, as well as other vaccinations, are less effective at eliciting an immune response than spontaneous infection. This present study quantitatively measured the anti-SARS-CoV-2 14 days after disease resolution. A published work reported that in mild COVID-19, the neutralizing antibodies were found in 94% of 175 patients two weeks of symptom onset [15]. Indeed, there is another study reported the neutralizing antibody occurred on the sixth day from the disease onset [14].
Our study had some limitations including the fact that we did not test the titer serially or the severity of the disease of the naturally infected group. We also did not estimate other factors in calculating vaccine response, such as cellular immune responses. Our study tested only confirmed COVID-19 patients with moderate-critical severity on day 28th of symptom onset to compare on the same days after COVID-19 vaccination.
Conclusion
Our study found that naturally SARS-CoV-2 infection produced higher titer of neutralizing antibodies than Sinovac vaccination. Since SARS-CoV-2 could cause unfavorable outcomes, the vaccine is still required. However, a booster dose of COVID-19 with another type of COVID-19 vaccine (i.e., heterologous vaccination) maybe required.
Acknowledgments
Thank to Faculty of Medicine and Health Sciences who fund the study. We also thank to all participants, nurse and the research team.
Ethics approval
The study was approved by Research Committee of Faculty of Medicine and Health Sciences, Warmadewa University (Document number 172/Unwar/FKIK/EC KEPK/IV/2021).
Conflict of interest
The authors declare no conflict of interest.
Funding
This study received no additional funding.
Underlying data
All data underlying the results are available as part of the article.
How to cite
Surawan DP, Sumohadi D, Budhitresna AAGD, et al. Anti-Spike receptor binding domain of SARS-CoV-2 antibody titer disparity between vaccinated and naturally infected individuals. Narra J 2022; 2(1): e71 - https://doi.org/10.52225/narra.v2i1.71.
References
- 1.WHO . Coronavirus (COVID-19) dashboard. Available from: https://covid19.who.int. Accessed: 15 December 2021.
- 2.WHO . Update on coronavirus disease. Available from: https://www.who.int/indonesia/news/novel-coronavirus. Accessed: 15 December 2021.
- 3.Surendra H, Elyazar IR, Djaafara BA, et al. Clinical characteristics and mortality associated with COVID-19 in Jakarta, Indonesia: A hospital-based retrospective cohort study. Lancet Reg Health West Pac 2021; 9:100108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Omar SM, Musa IR, Salah SE, et al. High mortality rate in adult COVID-19 inpatients in Eastern Sudan: A retrospective study. J Multidiscip Healthc 2020; 13:1887-1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Islam N, Shkolnikov VM, Acosta RJ, et al. Excess deaths associated with covid-19 pandemic in 2020: age and sex disaggregated time series analysis in 29 high income countries. BMJ 2021; 373:n1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soria A, Lapadula G, Bonfanti P. COVID-19 mortality and stress to the hospital system from high patient load. JAMA Internal Medicine 2021; 181(8):1134-1134. [DOI] [PubMed] [Google Scholar]
- 7.Le TT, Cramer JP, Chen R, et al. Evolution of the COVID-19 vaccine development landscape. Nat Rev Drug Discov 2020; 19(10):667-668. [DOI] [PubMed] [Google Scholar]
- 8.WHO . Coronavirus disease 2019: vaccines. Available from: https://www.who.int/news-room/questions-and-answers/item/coronavirus-disease-(covid-19)-vaccines. Accessed: 15 December 2021.
- 9.Roche Diagnostics GmbH . Elecsys® Anti-SARS-CoV-2 assay method sheet; 09203095501 V9.0. Available from: https://wwwfdagov/media/137605/download. Accessed: 1 October 2021.
- 10.Tekol SD, Altıntaş MM, Yılmaz E, et al. Detection and evaluation of antibodies to SARS CoV-2 spike protein in healthcare workers after inactivated COVID-19 (CoronaVac) vaccination. Southern Clinics of Istanbul Eurasia 2021; 32(3): 217-222. [Google Scholar]
- 11.Zhang Y, Zeng G, Pan H, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18-59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis 2021; 21(2):181-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xia S, Duan K, Zhang Y, et al. Effect of an inactivated vaccine against SARS-CoV-2 on safety and immunogenicity outcomes: interim analysis of 2 randomized clinical trials. JAMA 2020; 324(10):951-960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zimmermann P, Curtis N. Factors that influence the immune response to vaccination. Clin Microbiol Rev 2019; 32(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suthar MS, Zimmerman MG, Kauffman RC, et al. Rapid generation of neutralizing antibody responses in COVID-19 patients. Cell Rep Med 2020; 1(3):100040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu F, Wang A, Liu M, et al. Neutralizing antibody responses to SARS-CoV-2 in a COVID-19 recovered patient cohort and their implications. medRxiv 2020:2020.2003.2030.20047365. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data underlying the results are available as part of the article.


