Abstract
The indications for cochlear implantation have expanded over time due to evidence demonstrating identification and implantation of appropriate cochlear implant (CI) candidates lead to significant improvements in speech recognition and quality of life (QoL). However, clinical practice is variable, with some providers using outdated criteria and others exceeding current labeled indications. As a results, only a fraction of those persons who could benefit from CI technology receive it. This document summarizes the current evidence for determining appropriate referrals for adults with bilateral hearing loss into CI centers for formal evaluation by stressing the importance of treating each ear individually and a “revised 60/60 rule”. By mirroring contemporary clinical practice and available evidence, these recommendations will also provide a standardized testing protocol for CI candidates using a team-based approach that prioritizes individualized patient care. This manuscript was developed by the Adult Cochlear Implantation Candidacy Task Force of the American Cochlear Implant Alliance using review of the existing literature and clinical consensus.
Keywords: cochlear implant, candidacy, adult, recommendation, evidence, protocol
Background
Over the last several decades, improvements in cochlear implant (CI) technology, increased awareness of the technology, and changes in candidacy criteria have led to a rapid growth of evidence in the literature regarding the benefit of CI in adults. As such, recommendations for CI candidacy and referral continue to evolve. For instance, unlike historical CI recipients with bilateral profound sensorineural hearing loss (SNHL), CI candidates may now have an audiogram that mirrors or overlaps that seen in a hearing aid (HA) candidate and may have hearing in the contralateral ear up to and including normal hearing. Yet, the low utilization of CIs suggests hearing health care providers unfamiliar with current candidacy criteria may be relying on older, stricter criteria for referring potential CI candidates.
To assist in the standardization of practice, CI manufacturers have relied upon device labeling from national healthcare governing bodies (i.e., Food and Drug Administration (FDA). However, labeling has not kept pace with clinical practice. Many commercial payers use FDA labeling to inform their coverage policies, while the Center for Medicare and Medicaid Services (CMS) has a coverage determination policy that dictates payment for CI services. As a result, some patients who may benefit from CI technology yet fall outside of FDA labeling and/or CMS candidacy criteria are prevented from pursuing the technology. The inconsistency among clinical best practice, labeling, and coverage determination policies can create wide variability in practice methods and may lead to the inconsistent provision of CI services on a national, regional, and even a local level. Using established evidence, the recommendations summarized in this manuscript will serve to standardize the approach and testing protocol for determining adult candidacy for a CI and highlight the importance of a multi-disciplinary team-based approach for individualized patient care.
Underutilization of cochlear implantation
An estimated 466 million persons worldwide live with disabling hearing loss (> 6.1% of the world’s population). Over 40 million people in the United States (US) alone suffer from disabling hearing loss (4.6%)1,2. In persons 12-years-and-older, the prevalence of bilateral severe-to-profound hearing loss is estimated to be approximately two million1 making hearing loss the third most prevalent chronic health condition in the US1–3. With the advent of the first multi-channel CI system in 1985, guidelines requiring bilateral severe-to-profound SNHL to qualify for CI became the standard of care. It would be expected that hundreds of thousands if not millions of persons would have been implanted over the last four decades. However, as of 2015, 170,252 US adults have been implanted out of an estimated 1,337,144 traditional audiometric CI candidates for a utilization rate of 12.7%4. Moreover, fewer than one million devices have been implanted worldwide5.
The under-penetration of CIs among adult candidates is multifactorial and may in part be explained by poor awareness among clinicians and consumers alike. Unfamiliarity with current candidacy criteria, lack of awareness of referral processes and clear referral pathways to a CI center, and financial incentives to sell hearing instruments may all contribute6,7. Additionally, hearing healthcare providers currently performing adult CI evaluations demonstrate considerable variability in their testing methodologies and in the definition of a “good candidate”, leading to inconsistent referrals8. Lastly, patients unaware of CI technology, or those unfamiliar with candidacy criteria, may lack the impetus to discuss their options for hearing rehabilitation with their primary care physician, and vice versa. Collectively, these factors likely result in missed opportunities for persons with hearing impairment to be considered for CI.9
As technology has improved and outcomes data have been analyzed, candidacy has gradually expanded to include patients with increasing amounts of residual acoustic hearing and higher aided speech recognition scores (Table 1). Evidence now demonstrates FDA device labeling is not always consistent with current clinical practice. An increasing number of patients with hearing loss who fall outside of labeling guidelines are receiving CIs10 and often demonstrate significant benefit11. For example, increasing numbers of patients with asymmetric SNHL (ASNHL) are undergoing CI in their poorer hearing ear and demonstrate significant benefit in both the implanted ear only condition and when using the CI and the contralateral HA together (bimodal, binaural condition)11–14. In addition, adults with unilateral severe-to-profound SNHL (USNHL) in the poorer ear and thresholds better than or equal to 20 dB HL in the unaffected ear (single-sided deafness; SSD) are now undergoing CI, and the results have been positive15–18. Recipients demonstrate improved speech understanding in noise, improved sound source localization, reduction in tinnitus, improved QoL, and increased quality of hearing compared with other available technologies for SSD such as bone conduction implants and contralateral routing of signal HAs19–24. Furthermore, using a CI in one ear does not alter or decrease performance on the contralateral side, even in cases of normal acoustic hearing25,26.
Table 1.
Evolution of Cochlear Implant Candidacy (compiled from FDA PMA database) https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm
| Criteria | 1985 | 1990 | 1998 | 2000 | 2014 | 2019 | 2020 | 2022 |
|---|---|---|---|---|---|---|---|---|
| Age at implant | 18 yr + | 2 yr + | 18 mo + | 12 mo + | 12 mo + | Adults & Children 5yr + (SSD, AHL) (Med-El) | 9 mo + (Cochlear) | Adults & Children 5yr + (UHL/SSD) (Cochlear) |
| Onset of hearing loss | Post-linguistic | Post-linguistic adults Pre- & post-linguistic children | Pre- & Post- linguistic | Pre- & Post- linguistic | Pre- & Post- linguistic | Pre- & Post- linguistic | Pre- & Post- linguistic | Pre- & Post- linguistic |
| Degree of hearing loss | Profound | Profound | Adults: Severe to profound SNHL (All companies) Peds: Profound | Adults: Moderate to profound SNHL in both ears Peds: Severe to profound: 2 yr + Profound: < 2 yr | Adults: EAS & Hybrid: Normal to moderate SNHL in low to mid frequencies; severe to profound HL in high frequencies (Med-El & Cochlear) | SSD: Profound SNHL, one ear Normal or mild SNHL, contralateral ear Asymmetrical HL: Profound SNHL, one ear Mild to mod severe SNHL, contralateral ear 1 mo HA trial (Med-El) | Adults: Moderate to profound SNHL in both ears (all companies for traditional implants) Peds: Severe to profound: 2 yr + Profound: < 2 yr | SSD: Severe to profound SNHL in one ear, normal or near normal hearing in contralateral ear; At least 2 weeks to 1month wearing CROS device or suitable hearing device. |
| Speech Scores | 0% | 0% | Adults: ≤40% | Adults: Sentences score ≤ 50% in ear to be implanted, ≤ 60% in best aided condition (Cochlear) Peds: ≤30% LNT/MLNT | EAS/Hybrid: CNC word score > 10% but < 60% in ear to be implanted (Med-EL); < 80% CNC words in contralateral ear (Cochlear) | ≤5% correct on CNC word score (Med-El) | ≤5% correct on CNC word score (Cochlear) |
CROS = contralateral routing of signal; mo = months; SNHL = sensorineural hearing loss; SSD = single sided deafness; UHL = unilateral hearing loss; yr = years
There have been two versions of a recommended Minimum Speech Test Battery (MSTB)27 and a third is currently in development. While both versions recommend inclusion of a variety of test conditions (ear-specific and bilaterally aided; sentences in quiet and both fixed and pseudo-adaptive noise, and monosyllabic words), the suggested materials have evolved over time. Specifically, HINT sentences were replaced by AzBio Sentences28 which were shown to be more ecologically valid29. However, the MSTB never specifically suggested how to use the recommended materials to determine CI candidacy. As the criteria for CI continue to broaden, the materials and methods used to test potential CI candidates remain highly variable and open to interpretation, even for traditional, bilaterally deafened candidates8. This variability has led to inconsistencies between testing centers making comparison of objective outcomes between patients more difficult and may impair the successful prognostication of postoperative outcomes.30
In addition to objective measures, such as speech recognition scores and severity of hearing loss, determining whether a patient is a CI candidate relies on a center’s familiarity with outcomes, expanding criteria, and comfort recommending implantation for those outside of FDA indications. Although a few authors have published evidence-based criteria for recommending a CI31, there is limited guidance regarding how other factors such as cognitive ability, willingness to participate in an aural rehabilitation program, duration of hearing loss, history of amplification use among others should be used in the candidacy process.
By developing and implementing standardized recommendations for assessing and confirming CI candidacy, uniformity in testing protocols will be improved, the number of potential CI recipients referred for testing will be increased, and consistency between those considered CI candidates amongst centers will be enhanced. Ultimately this standardization will lead to increased penetration of CI technology to those who could most benefit.
Methods
The purpose of this paper is to provide evidence-based recommendations for CI candidacy identification and referrals based on a comprehensive review of the literature. The recommendations encompassed in this paper were developed following a predetermined methodological approach which included: (1) determining the need for a set of formal recommendations regarding adult cochlear implant candidacy and navigation of the CI referral pathway by the American Cochlear Implant Alliance (ACI Alliance) Board of Directors (BOD); (2) the formation of a team of subject matter experts in the field to serve as co-authors and the designation of a lead author; (3) numerous teleconferences to establish the rationale and methodology for development of the recommendations; (4) performing a comprehensive literature review assisted by medical librarians identifying the most up-to-date and state-of-the-art manuscripts on the topics of CI candidacy and referral guidelines; (5) the formation of the recommendations and the creation of an evidence-based pathway as guided by assimilation of the data based on the literature review; (6) a period of review and public comment by the ACI Alliance BOD followed by a unanimous vote of endorsement.
The comprehensive literature review was conducted with the help of medical librarians using a combination of keywords related to cochlear implants from multiple databases including PubMed, Cochrane Library, Dyna Med, Scopus, EMBASE, and Google Scholar. The articles were reviewed by the authors based on relevance and strength of evidence. The relevant articles were grouped into subtopics (i.e., aided speech recognition testing, candidacy referral recommendations), and the recommendations were formulated based upon discussion, analysis, and data synthesis by all authors.
The authors chosen to write these recommendations all have substantial experience in the CI field and as a result have conflicts of interest with the various cochlear implant manufacturers and other relevant enterprises. These relationships have been explicitly reported herein per journal guidelines. Furthermore, none of the authors received financial compensation for the writing of this manuscript, nor will any of the authors receive remuneration incentives for any outcomes following implementation of these recommendations.
Cochlear Implant Evaluation Referral Considerations
Currently, there are no established criteria for routine office-based audiometry to determine who is an appropriate CI candidate. Several authors have published parameters to help determine the appropriateness of an adult CI referral and predict the likelihood the candidate will qualify. Gubbels et al. used audiometric findings to identify patients who are likely to meet CI candidacy following formal testing32. Specifically, patients with low frequency (250, 500, 1000 Hz) thresholds greater than 75 dB HL and/or a monosyllabic word recognition test score of less than 40% have a greater than 80% probability of meeting CMS criteria at the time of publication (i.e., < 40% sentence recognition score bilaterally in the best-aided condition). When using only a monosyllabic word score < 32%, 86% of the patients met CMS criteria. In patients with private insurance, the accuracy of the model remained strong (> 80%) if the monosyllabic word recognition test score was <45%.
Zwolan et al. performed a similar analysis in which audiometric data were used to predict patients who would qualify for CI under the same CMS criteria.33 When only those patients who met traditional CI indications (i.e., consistent with Medicare coverage guidelines of <40% bilateral sentence recognition score at the time of study publication) were considered, 95% had a preoperative pure-tone-average (PTA) in their better hearing ear of 60 dB HL or greater and 92% had an unaided monosyllabic word score of 60% or lower. When applied retrospectively to a large sample of adult CI candidates, this “60/60 guideline” yielded a 96% sensitivity rate (i.e., candidates met both criteria) and a 65% specificity rate (i.e., non-candidates did not meet the 60/60 criteria).
While the Zwolan paper uses a 60/60 guideline in the better hearing ear as a guideline for referral, due to FDA approval for cochlear implantation in cases of asymmetrical hearing loss and single-sided deafness, a “revised 60/60 guideline” is recommended where post-lingually deafened adults are referred for CI evaluations when one or both ears demonstrate a monosyllabic word score that is less than or equal to 60% correct and the unaided PTA (500, 1000, and 2000 Hz) is greater than or equal to 60 dB HL. It is important clinicians recognize use of this guideline will only capture the most clear-cut CI candidates and may overlook candidates who still meet FDA criteria with steeply sloping hearing loss.
Using objective audiological data will help hearing health professionals make high-yield and appropriate CI evaluation referrals. However, it is important to recognize that other studies demonstrate a poor correlation between unaided speech recognition scores and eventual CI candidacy34. Therefore, if a patient demonstrates limited benefit from appropriately fit hearing aids, it is important to consider referral for a formal CI evaluation regardless of the performance on any single test or group of testing measures.
In summary, hearing health professionals should use the revised 60/60 guideline and refer any patient with an unaided PTA of 60 dB HL or greater and an unaided word score of 60% or less in one or both ears for formal CI evaluation. Importantly, those patients falling outside of the revised 60/60 guideline or those falling outside of traditional CI labeling who do not receive adequate benefit from their current technology (i.e., unilateral SNHL, asymmetric SNHL, low frequency thresholds in the normal to mild range) should also be referred for a formal CI evaluation. While other information will be reviewed by the CI team prior to candidacy determination, using audiometric parameters such as these ensures a greater number of potential CI candidates will undergo consideration. If the patient is deemed a non-candidate following formal CI candidacy evaluation, the referring hearing health professional should monitor the patient’s performance and re-refer with worsening performance or ongoing hearing difficulties.
Cochlear Implant Candidacy Considerations
The adult CI candidacy evaluation should involve a multi-disciplinary team with experience treating CI patients. Typically, a CI team includes a CI surgeon(s) and audiologist(s) specializing in CI. Based on the complexity of the candidate’s case, the pre-, peri-, and/or postoperative needs of the candidate, and/or the customs of a particular CI center, additional team members may include a rehabilitation specialist (auditory-based therapist), a neuro-radiologist, psychologists/neuropsychologists, social worker(s), previously implanted peers, and family members/caregivers.
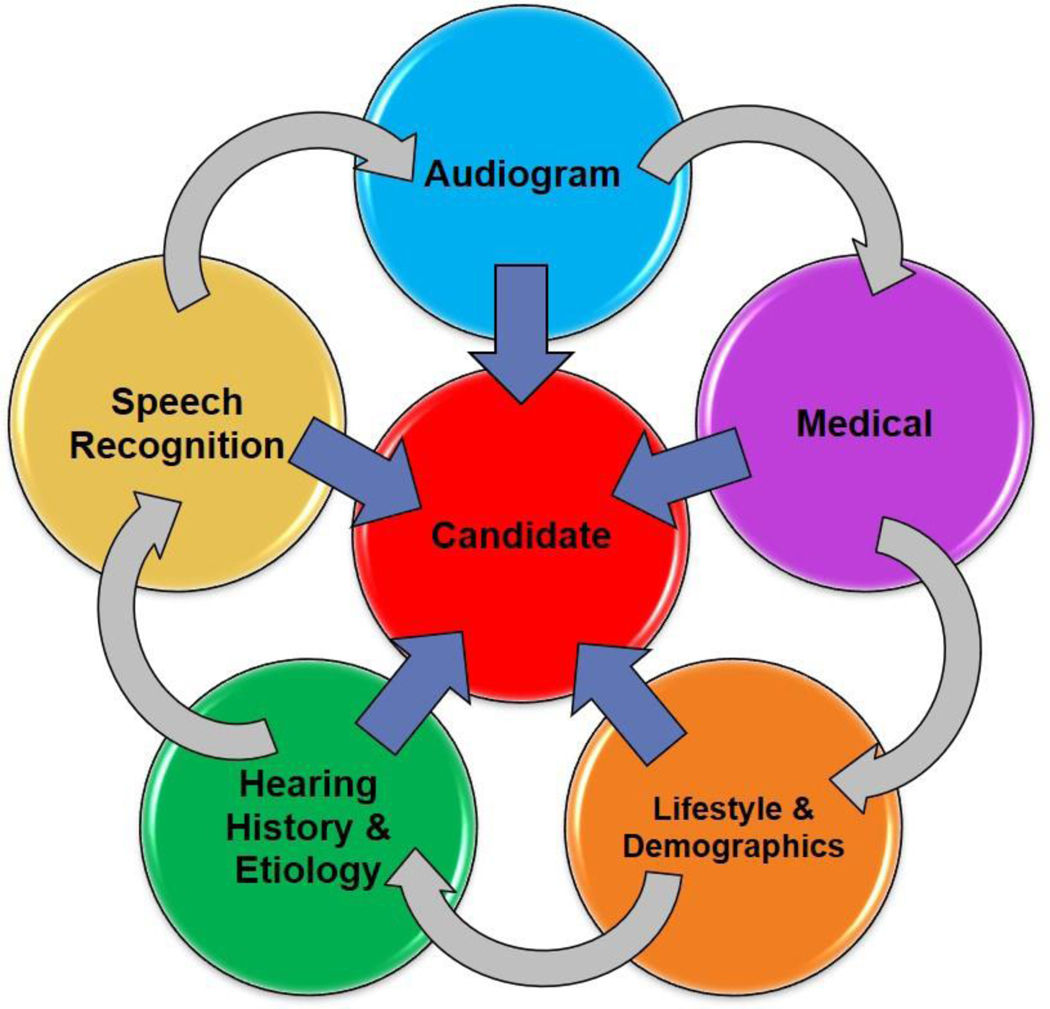
In addition to audiometric thresholds and aided sentence recognition in quiet8,34, other information must be obtained during the hearing health history including ear-specific aided speech recognition using monosyllabic words and sentences in noise, etiology and duration of hearing loss, history of amplification, patient’s occupation and/or social hearing needs, demographics (i.e., social support, age, hearing needs such as occupation), decrement in hearing specific and/or overall quality of life (QoL), motivation, and underlying medical factors. While specific recommendations based on these variables may differ between individual clinics, this holistic approach towards the CI candidate has been shown to be beneficial as part of healthy aging in CI recipients35 (Figure 1).
Figure 1.
Factors to consider in an evaluation for a CI candidate.
Multiple hearing health variables have been shown to correlate with CI outcomes including duration of deafness36–41, HA usage42, age at implantation43, and etiology of hearing loss36,39,42. There is evidence that additional demographic variables may also be determinants of future performance following CI. Tang and colleagues44 showed factors including cohabitation with a spouse or family member(s), familiarity with technology, emotional intelligence, and adherence to postoperative aural rehabilitation programs can influence speech outcomes following CI. Therefore, during the CI evaluation process it is critical to discuss factors such as resources available to the CI candidate and family, a family’s willingness to provide a strong support system, a commitment to post-implantation (re)habilitation, the proximity of the patient to the CI audiologist and/or qualified (re)habilitation provider, and the willingness and ability of the patient to wear the device.
Demographics and Hearing Needs
While a diagnostic audiogram provides objective information, it does not accurately represent the patient’s functional status as a result of their hearing loss. Specifically, it does not reflect the impact the hearing loss has on the patient in their everyday life (i.e., auditory fatigue, cognitive load, etc.) resulting from routine communication disabilities. It is well known that severe-to-profound hearing loss can have significant consequences on one’s mental health, social inclusivity, and overall QoL45. Research demonstrates the advantages CIs have over HAs on the recipient’s QoL in multiple domains including psychosocial health, functional health, and social inclusion46,47. Despite the superiority of CI over HAs in these domains and others, CI penetration in the US has remained stagnant9. Some evidence even suggests that the duration of severe-to-profound hearing loss prior to CI surgery is increasing, further exacerbating the deleterious effects on the patient’s QoL.48 While a CI may not be the optimal approach for every patient who qualifies audiometrically, understanding each person’s hearing needs, communication goals, and QoL detriment are essential in providing additional insight for counseling on realistic expectations. Furthermore, each candidate must demonstrate a motivation for and commitment to the entire CI process.
Audiologic Evaluation
Hearing History
Obtaining an accurate and comprehensive hearing health history is critical to identifying appropriate CI candidates and understanding factors that may influence outcomes post-operatively. Information regarding the etiology of hearing loss (if known), rate of hearing loss progression (supplemented by prior audiometric testing where available), history of amplification, otologic history (i.e., prior operations and/or pathology), and post-implant expectations must be obtained during the clinic evaluation. Additionally, age at onset of hearing loss and its relationship to language acquisition must be queried as these factors may help predict postoperative outcomes and/or device use compliance49,50. Specifically, adult candidates with pre-lingual onset of deafness demonstrate large inter-individual performance variability and levels of satisfaction and may be at increased risk for device non-use51,52.
Careful attention to duration of deafness is essential when discussing realistic expectations with the candidate. Duration of deafness can be difficult to accurately assess in some patients. An understanding of the estimated amount of time that the inner ear and auditory cortex has been without meaningful stimulation is critical as it is an important predictor of post-operative objective success. Lack of stimulation may be due to severe-to-profound hearing loss, lack of adequate amplification, and/or failure to engage in auditory-rich environments. As discussed above, longer durations of post-lingual deafness are associated with poorer outcomes after CI36–41. However, successful outcomes following CI in cases of prolonged durations of deafness (> 30 years) have been reported53. Therefore, duration of deafness in bilaterally deafened adults with a post-lingual onset should not be an absolute contraindication for CI, but rather an important consideration in setting appropriate post-implantation expectations.
In addition to age of onset and duration of deafness, the duration and consistency of amplification use in impaired ears must be determined. Even with consistent amplification, long-term severe-to-profound hearing loss can lead to auditory deprivation and may impact outcomes following CI40,41,54. Critically, the fit and settings of current amplification must be verified by the CI audiologist as many potential CI candidates arrive with inappropriately fit HAs at the time of the evaluation8.
The etiology of the candidate’s hearing loss should be obtained and documented. CI outcomes can vary widely among recipients based on the etiology of hearing loss and must be reviewed with the patient55–59. While very few etiologies of deafness are contraindications to CI surgery, they may impact expected outcomes and must be considered when discussing realistic expectations with the patient.
During the hearing health history, the CI candidate should be asked to share the impact the hearing loss has on their daily life. It is important to understand how the hearing loss impairs or affects the candidate’s ability to work, communicate with friends and family, or interact socially. It is helpful to ask patient-specific, closed-ended questions that clarify the impact of their hearing loss (i.e., “Can you use a telephone with one or both ears?”, “Can you watch television without subtitles?”, “Can you go out to dinner with friends and keep up with the conversation?”, “Do you feel your HAs provide sufficient benefit?”). Many clinics are using validated screening questionnaires such as the Hearing Handicap Inventory for the Elderly Screening Test (HHIE-S)60 to better understand the impact of the candidate’s hearing loss. This should be repeated post-operatively and shared with the patient by discussing areas of benefit and areas that the patient could benefit from additional rehabilitation.
Diagnostic Unaided Audiologic Evaluation
A diagnostic audiogram is required as part of CI candidacy assessment. Objective assessment of the ear under consideration for CI as well as the contralateral ear is important for managing post-operative expectations for residual hearing61, determining electrode array selection62–64, determining the optimal surgical approach65, consideration for the use of intraoperative tools such as electrocochleography (ECochG)66, and future amplification of the implanted or non-implanted ear. Results of the diagnostic audiogram must be used together with the other components of the CI evaluation discussed below as part of a holistic candidacy approach (see above) (Figure 1).
Consistent with the Minimum Reporting Standards for Adult Cochlear Implantation, a standard set of measures must be performed and documented for each patient67. Pre-operative air conduction (AC) pure tone thresholds including 125, 250, 500, 750, 1000, 1500, 2000, 3000, 4000, 6000, and 8000 Hz are performed via insert earphones for each ear. Bone conduction (BC) pure tone thresholds including 250, 500, 1000, 1500, 2000, 3000 and 4000 Hz are performed for each ear. High frequency AC thresholds (2000, 3000, 4000, 6000, and 8000 Hz) are often in the severe-to-profound range and may not be measurable using standard audiometry. These are assigned the value of 120 dB for documentation purposes.
Significant residual low-frequency hearing in either ear (but especially in the ear-to-be-implanted) should not serve as an exclusion for CI candidacy. When low frequency thresholds (125, 250, and 500 Hz) are in the normal-to-moderately-severe range, hearing preservation is possible following CI. Electric and acoustic stimulation (EAS) using an acoustic component with a CI ear-level sound processor should be considered and discussed if functional hearing preservation is achieved. Functional hearing is defined as hearing in the low frequency thresholds that can be adequately amplified (i.e., meeting targets on real-ear measurements). Patients using acoustic plus electric hearing demonstrate improved speech perception outcomes68, speech understanding in noise69–72, binaural cues such as summation and squelch73, sound source localization70,74, melody recognition and music appreciation69,75,76, and perceived quality of speech77.
Other objective measures such as tympanometry and acoustic reflex thresholds are performed when clinically indicated to screen for the presence of middle ear dysfunction (i.e., middle ear atelectasis, chronic serous otitis, acute otitis media, etc.)78. While not a contraindication to CI surgery, middle ear dysfunction may delay the implantation process and/or affect the decision regarding which ear to implant.
Unaided Speech Recognition Testing
As previously discussed, unaided speech recognition testing has historically served as a guide for CI referral. Significant variability exists in the testing methodologies (i.e., presentation level, recorded vs. monitored live voice, number of stimuli). Without standardization, the reliability of using unaided testing to identify CI candidates is diminished and the ability to compare results between practices is mitigated. The recommendation for best practice is to use recorded speech measures. When a patient is non-English-speaking, recommendation for CI evaluation may be made based on pure tone audiometry and reported amplification benefit.
Hearing Aid Fitting and Evaluation
CI candidacy evaluations must be performed using appropriately fitted and verified HAs. The term ‘appropriately fitted and verified’ refers to the use of real-ear or simulated real-ear measures conducted in a test box or on-ear to confirm the devices meet prescriptive targets for sufficient audibility (i.e., DSL adult, NAL-NL1)79,80. Verification can be performed on the patient’s personal hearing instruments or clinic-owned instruments programmed to the patient’s most recent hearing test81. For CI candidates with underfit or poorly fit HAs (e.g., +/− >5 dB from the prescriptive targets), the audiologist will make recommendations regarding appropriate adjustments. Functional aided thresholds to determine audibility with the HA(s) can be included but are not essential and should not be the only verification method used.
Aided Speech Recognition Testing
Historically, sentence recognition testing has been the standard for aided speech measures in CI candidacy testing (Table 1). However, Sladen and colleagues82 suggest sentence recognition scores less reflect how well a person can detect and process spectral and temporal components of speech, and more about how well one can use “top-down processing” to “fill in missing pieces” . Top-down processing can also be altered by cognitive resources83,84. Previous studies show that post-lingually deafened adults score much higher on sentence testing than on word testing during CI evaluation85,86.
Previous work demonstrates when monosyllabic word recognition scores are used for CI candidacy qualification, performance outcomes show significant improvement from pre- to post-CI. Furthermore, Sladen et al.82 showed a trend for improved performance on CNC word testing when less restrictive criteria (< 40% CNC) are used for candidacy qualification rather than more restrictive criteria (< 30% CNC)87. Data from Dunn et al.88 demonstrate in patients with bilateral deafness who undergo implantation in their worse ear, using a CNC score of ≤ 50% in the ear to be implanted had a 99.7% sensitivity for identifying candidates who ultimately qualified based on previous CMS criteria (≤ 40% sentence recognition testing). Pre to Postoperative CNC word score comparisons in the implanted ear demonstrated a significant improvement for those who scored up to 50% preoperatively. Previous CI clinical trials investigating EAS have used a best-aided CNC score of ≤ 60% in the ear to be implanted for candidacy inclusion, and a CNC word score of ≤ 80% in the contralateral ear72,89–91.
These data and those from Sladen et al.82 suggest monosyllabic word recognition testing is more ecological than sentence testing and is as sensitive in predicting CI candidates. Moreover, sentence recognitions tasks are not useful for tracking CI performance outcomes over time due to the large numbers of CI users who achieve ceiling performance early during their post-operative course (60% of users scored > 80% by 3 months post-CI). Conversely, CNC scores improved significantly at each of the time periods between surgery and 12-months post-CI with no patients scoring over 80% by the 12-month interval.
Aided Speech Recognition Testing Recommendations
Aided speech recognition testing should be conducted following recommended clinical guidelines outlined in the Minimum Speech Test Battery27 and Minimum Reporting Standards for Adult Cochlear Implantation67 to ensure standardization across centers. To test, a speaker is situated approximately 39 inches (1 meter) from the floor and the center of the listener’s head. Best aided speech recognition testing is defined as the speech perception score in individual ear(s) using optimized hearing aid(s) on a monosyllabic word test (Consonant-nucleus-consonant, CNC)92 . The target presentation level for stimuli is 60 dB A93. When unaided hearing thresholds are 60 dB HL or better in the non-test ear, either plugging and muffing or masking using speech-shaped noise is performed. Any patient who scores ≤ 50% on CNC in the poorer hearing ear should be considered for CI88 unless contraindicated by hearing history, etiology, or other pertinent factors gathered during the CI evaluation. The CNC word score in the contralateral ear should not be considered when determining candidacy using this test. Importantly, the clinical trials investigating CI for USNHL did not stipulate the CNC score in the contralateral ear.
It is important to note our recommendation is to use CNC word testing to determine CI candidacy. Because CNC word scores are not used for device labeling or insurance criteria, best aided connected speech testing (best aided defined as speech recognition testing in the individual ear[s] using optimized hearing aid[s]) should be performed using AzBio sentences86 to determine if a patient qualifies for coverage for their CI. To date, no consensus exists on the SNR recommended for testing, and varies between clinics8. Our recommendation is to test the ear to be implanted in the best aided condition using AzBio sentences in noise starting with a +10 dB SNR using a 10-talker babble (AzBio Sentences presented at 65 dB A and noise presented at 55 dB A). To further evaluate hearing status and to meet insurance qualification requirements, the clinician should consider decreasing the adversity (sentences presented in quiet at 60 dB A) or increasing the adversity (sentences in +5 dB SNR with sentences presented at 65 dB A and noise presented at 60 dB A) of the listening condition as needed (Table 2).
Table 2.
Minimum Speech Test Battery (MSTB)
| Stimuli | Competing signal | Aided listening condition | Appropriate speech recognition measures | ||
|---|---|---|---|---|---|
| Left | Right | Bilateral | |||
| Monosyllabic words in quiet (60 dBA) | X | X | Consonant-Nucleus-Consonant (CNC) |
||
| Sentences in quiet (60 dBA) | X | X | AzBio Sentences | ||
| Sentences in noise (65 dBA) | Multi-talker speech babble +5 to +10 dB signal-to-noise ratio | X | X | X | AzBio Sentences in Noise; BKB-Sentences in Noise |
While clinically significant improvements in speech perception in noise can be achieved after implantation for patients qualifying for CI in noise conditions listed above, improvements tend to be smaller as SNR becomes more adverse94,95. Specifically, persons qualifying for CI in only the +5 dB SNR condition can derive significant benefit from their device, but objective outcomes are more variable96–98. These data provide useful counseling tools for patients considering CI, but consideration of listening needs and goals should be discussed with each candidate on an individualized basis. Varying the SNR represents real-life listening situations96,99,100 and can be beneficial for determining the SNR at which substantial difficulty is noted. Finally, testing AzBio Sentences in the person’s everyday listening condition is recommended for postoperative comparison to the person’s then everyday listening condition. The everyday listening condition is defined as testing with the optimized hearing configuration typical of a patient’s everyday listening (e.g., unoccluded, unilateral or bilateral hearing aid(s), bimodal, unilateral or bilateral CI(s), EAS with contralateral HA).
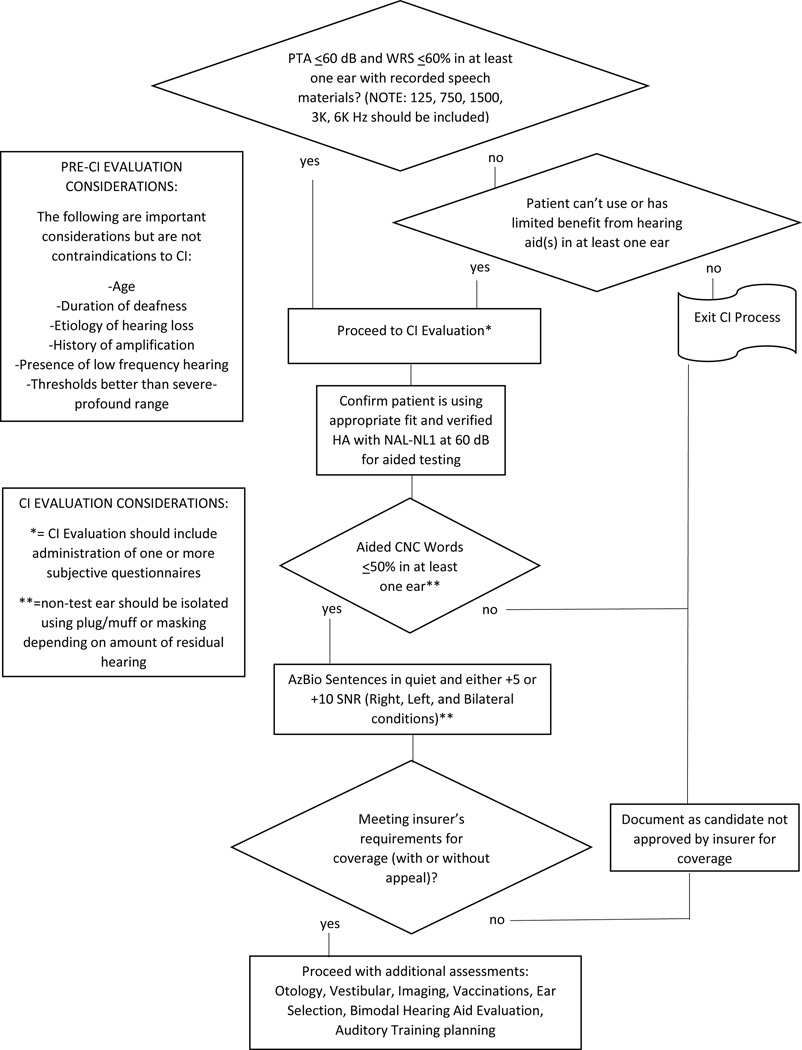
Figure 2 summarizes the recommendations for determining CI candidacy using routine audiometry to screen potential candidates for referral, and aided speech recognition to verify candidacy and potentially coverage.
Figure 2.
Flowchart summarizing the protocol for cochlear implant candidacy testing in an adult with bilateral hearing loss.
Preoperative Medical Evaluation
CI has proven successful in patients well into their eighth and ninth decades, and age alone should not preclude evaluation for CI or CI surgery if the patient is otherwise medically fit101–103. In patients with terminal illness, chronic health conditions compromising the administration of safe anesthesia, or at the extremes of age (>95 years), best clinical judgement and shared decision making should be used when considering the provision of CI technology. Additionally, numerous authors have published on the relationship between cochlear implantation and cognitive status in patients over the age of 65104–106. Cognitive evaluations such as the mini mental status exam (MMSE) and Montreal Cognitive Assessment (MoCA), tests of verbally based stimuli and responses (i.e., digit span, Stroop), and comparable visually based tests such as the Brief Visuospatial Memory Test have been evaluated. While these tests may be helpful in assessing CI candidates and in tracking changes in cognition from pre- to post-CI, cognitive testing is not required prior to CI, and there are no recommendations for its inclusion during CI evaluation in this manuscript. Certain considerations must be given to circumstances unique to the aging person. These include possible degeneration of the central auditory system, declining cognitive status, diminished central receptive or expressive language function, and coexisting medical, fine motor, and psychosocial disorders. While none of these issues are absolute contraindications to CI, they should be discussed with the candidate and their support system (friends, family, caregivers) during the evaluation process since they may impact both expectations and outcomes104,107,108.
The medical history should include a thorough medical assessment of the candidate’s comorbidities and cardiovascular health. Patients with chronic diseases such as diabetes, chronic obstructive pulmonary disease, kidney failure, atrial fibrillation, and coronary artery disease can safely undergo CI surgery. However, communication with the primary care physician or specialist is necessary to obtain surgical clearance and perioperative recommendations, which may include conscious sedation rather than general anesthesia109–111. The evaluation must also include an otologic and neurotologic history and physical examination including microscopic otoscopy including an assessment of current and previous vestibular function. Up to 35% of adults over age 40 have vestibular dysfunction112,113. Formal vestibular testing prior to CI has been advocated by some to help mitigate the risk of bilateral vestibular hypofunction following CI114. This practice is not uniformly applied and is not mandatory115. Furthermore, no consensus exists regarding who to test or which test(s) to perform. Pre-operative vestibular testing should therefore be performed at the discretion of the implant team and/or when warranted by the patient’s history.
Like vestibular disturbance, the otologic history should also query for the presence of tinnitus. While some data support the suppression of tinnitus following CI116,117, the perception of tinnitus can persist following CI and reasonable expectations should be included as part of counseling.
Imaging
There is debate regarding the optimal imaging modality to assess cochlear, middle ear, and mastoid anatomy prior to CI surgery. High resolution computed tomography (HRCT) and/or magnetic resonance imaging (MRI) have both been used. While the data suggest preoperative imaging rarely affects surgical decision making,118 imaging ordered in preparation for CI surgery remains at the discretion of the surgeon. Like any diagnostic tool, cost/benefit analysis and risk assessment must be considered in each case.
Ear Selection
The decision of which ear to implant is nuanced and creating a formulaic approach leading to specific recommendations is not possible. Often the decision is based on objective CI testing, but other factors can contribute such as patient preference, medical evaluation, preoperative imaging, duration of severe-to-profound hearing loss, amplification history, and audiometric findings42,119,120. Although the poorer hearing ear was routinely selected in the past, dogmatic methodology should not take precedence in all cases over these other factors119,121,122. This less rigid approach may lead to a recommendation for implantation of the better hearing ear in some cases when it also meets candidacy. For instance, when the examination indicates significant pathology in one ear, implantation of the uninvolved ear (which may be the better hearing ear) is often indicated. However, in cases with an unrevealing history, normal examination and imaging, and symmetric hearing thresholds, the poorer performing ear on CI candidacy testing is routinely selected for CI.
Vaccinations
It is important that the vaccination history of CI candidates is reviewed by the CI team. The Centers for Disease Control (CDC) recommends all adult CI candidates (≥ 19 years old) be immunized against pneumococcal meningitis. The Task Force recommendation is to follow CDC guidelines prior to (and subsequent to when relevant) cochlear implantation and these guidelines are summarized in Table 3. Vaccination recommendations may differ between patients based on age, medical history, and/or previous vaccinations. Additional details regarding vaccination recommendations in CI patients can be found on the CDC website123,124.
Table 3.
Pneumococcal meningitis vaccination recommendations for adult CI candidates and adults who have previously received a CI
| Pneumococcal meningitis vaccine |
|---|
| 19–64 years with no previous vaccinations/unknown vaccination history − Single dose PCV20 OR − Single dose PCV15 followed by single dose of PPSV23 at least 8 weeks later ≥65 years − Single dose PCV20 OR − Single dose PCV15 followed by single dose of PPSV23 at least 8 weeks later *The incremental public health benefits of providing PCV15 or PCV20 to adults who have received PCV13 only or both PCV13 and PPSV23 have not been evaluated. |
| ≥ 1 dose of PPSV23 but no PCV13, PCV15, or PCV20 − Single dose of PCV15 or PCV20 at least 1 year after last PPSV23 dose *When PCV15 is used in those with history of PPSV23 receipt, it need not be followed by another dose of PPSV23. |
| Previous dose of PCV13 or PCV15 but not PPSV23 − Single dose of PPSV23 at least 8 weeks after dose of PCV13 or PCV15 *The incremental public health benefits of providing PCV15 or PCV20 to adults who have received PCV13 only or both PCV13 and PPSV23 have not been evaluated. These adults should complete the previously recommended PPSV23 series. |
Note: Vaccination schedule should be completed two weeks or more before surgery.
PCV 13 = pneumococcal conjugate vaccine 13 valent (Prevnar 13™); PCV15 = pneumococcal conjugate vaccine 15 valent (Vaxneuvance™); PCV20 = pneumococcal conjugate vaccine 20 valent (Prevnar 20™); PPSV23 = pneumococcal polysaccharide vaccine (Pneumovax 23™)
Counseling and Therapy
Prior to surgery, the CI team must attempt to understand the patient’s goals and expectations as well as those of the caregiver(s) and others involved in the candidate’s support system. Data suggest a patient’s expectations before CI may influence their postoperative QoL after surgery, with those who report lower performance expectations showing higher postoperative QoL.125 Importantly, preoperative expectations do not appear to impact post-CI speech recognition scores126. Although there is no validated measure to assess expectations prior to CI surgery, many CI centers have developed their own pre-operative CI questionnaire that is administered to and discussed with the candidate and their support system during the evaluation process.
Patient-reported outcome measures (PROMs) assessing the real-world benefits and improvements in QOL following cochlear implantation have been popularized to complement the objective information gained from speech-centered outcome measures. Examples of these PROMs include Speech, Spatial and Qualities Questionnaire (SSQ)127, and a version of the Cochlear Implant Quality of Life Profile (CIQOL-35 or CIQOL-10)128. The CIQOL-35 has recently been validated and is more psychometrically sound and comprehensive than these other tests for assessing QOL in adult CI users129. While preoperative CIQOL-35 scores are not correlated with objective outcomes following CI and are not used for candidacy determination, collecting these data using a standardized instrument will allow large-scale QOL studies in the future. Therefore, the authors suggest the CIQOL-35 be administered pre-operatively and at 3- and 12-months post-CI. CI satisfaction has been shown to correlate with self-assessed improvements in hearing disability, auditory perception, speech perception, and ease of communication. However, satisfaction following CI is not exclusively related to objective determinants and may also be related to positive self-esteem, less severe symptoms of depression, and the use of humor 130. Furthermore, measuring subjective benefit can guide counseling and aural rehabilitation and initiate changes in CI programming to be used in various real-life listening situations.
Aural Rehabilitation
During the CI evaluation process, it is important for the hearing health professional to discuss the importance of post-operative rehabilitation following CI. The candidate’s motivation and support system to participate in rehabilitation services following implantation should be assessed. As more is learned about the patient’s goals, an individualized listening therapy plan can be developed and could include referrals to additional specialists (e.g., speech language pathologist). Despite evidence suggesting aural rehabilitation training exercises improve outcomes in CI recipients131,132, formal aural rehabilitation training programs not often incorporated as part of the follow-up care. Aural rehabilitation can occur through the CI clinic, guided by a speech pathologist or audiologist. For centers without these services, patients can be given the appropriate materials to use at home. For those patients with computer and internet access, self-directed computer-based training should be encouraged as research demonstrates that doing so results in improved speech recognition and CI-related QoL133.
Other Considerations
Insurance Coverage
It is important to recognize that ‘candidacy’ and ‘coverage’ are not synonymous. As previously discussed, clinical best practice dictates many candidates fall outside of FDA-labeled indications and CMS coverage policies. While National Coverage Determination (NCD) policies published by CMS specify rigid guidelines for unaided audiometric thresholds and aided speech recognition testing, it is important to recognize these definitions may be more stringent than clinical best practice. Additionally, since each state administers its own Medicaid program, differences exist in CI coverage for adults 21 years and older. As outcomes have improved and candidacy criteria continue to broaden beyond payer guidelines, policies and associated determination of candidacy serve to confuse the issue of candidacy versus coverage, which may restrict appropriate education and access.
Off-Label Considerations
Off-label use of a medical device is the application of the device for a purpose not included as an indication in the FDA-approved device labeling. Clinicians often recommend CI for patients falling outside of FDA-approved criteria when the advantages outweigh the disadvantages. Over 75% of CI surgeons in the US self-report that they perform “off-label” CI surgery10. Off-label implantation requires the clinician be well informed about the product, its use on “firm scientific rationale and sound medical evidence”, keep the patient’s best interests at the forefront of the decision-making process, and use best knowledge and judgment.
Summary/Guidelines for Identification of CI Candidates
Any patient with hearing loss who gains limited benefit from their current HA(s) and desires improvement in hearing should be referred for a CI evaluation. Referrals for borderline candidates, even if the candidate does not quality, provides an opportunity for counseling and documents a baseline to monitor for future hearing loss progression.
CI should not be considered a “last resort”. Candidates should not wait for “something better”. CI is currently the most effective option for the management of sensorineural hearing loss not optimally managed by HAs.
The presence of residual acoustic hearing should not deter referral for CI candidacy evaluation. Residual hearing is not a contraindication for CI surgery, and if maintained following CI can lead to better speech understanding in noise, appreciation of music, and improved sound quality among other benefits. Additionally, EAS listening strategies can be implemented post-operatively.
Ear-specific CI candidacy must be considered as many studies have demonstrated the benefit of CI in cases of UHL/SSD and AHL.
A “revised 60/60” criteria can be used as a clinical benchmark for referral for a CI evaluation whereby each ear is considered individually for CI rather than using the better ear as the reference point. If a patient has a PTA ≥ 60 dB HL and an unaided monosyllabic word recognition score ≤ 60% in the worse hearing ear the patient should be referred for CI evaluation. It should be remembered patients falling outside the “traditional” 60/60 criteria or the “revised” 60/60 criteria may still qualify for CI and should not be excluded from CI evaluation.
Patient-specific factors must be considered in identifying appropriate CI candidates and can be helpful in counseling prior to surgery. These factors include demographic information, etiology of deafness, duration of hearing loss, HA history, and the candidate’s physical/mental/emotional support system. While these factors deserve consideration during the evaluation process, they are rarely absolute contraindications for surgery.
CI candidacy testing begins with CNC monosyllabic word testing in each ear using optimized hearing aids. Candidacy is recommended by a score of ≤ 50% in the ear-to-be implanted, regardless of performance in the contralateral ear. AzBio sentence recognition testing is then performed in the ear to be implanted and used to determine qualification for insurance coverage. “Best aided” should be interpreted as the score for the ear considered for CI fitted optimized hearing aids.
To determine if a candidate qualifies for insurer’s coverage for the CI surgery and device, best aided connected speech testing should be performed in the ear to be implanted using AzBio sentences played with a 10-talker babble in +10 dB SNR. To further evaluate hearing status and qualification of insurer’s requirements, the clinician should consider decreasing the adversity (sentences obtained in quiet at 60 dB A) or increasing the adversity (AzBio in +5 dB SNR with sentences presented at 65 dB A and noise presented at 60 dB A) of the listening condition as appropriate.
Appropriate recommendations for fitting hearing technology in the contralateral, non-implanted ear, should be discussed to allow for optimization of binaural hearing, use of compatible accessories, etc.
Hearing related disability can be assessed using a variety of QOL instruments. While optional and unrelated to behavioral outcomes post-CI, measuring subjective benefit can guide counseling and aural rehabilitation and initiate changes in CI programming based on various real-life listening situations. The CIQOL-35 is a validated questionnaire specific to CI patients, and administration is suggested pre-implantation and at 3-months and 12-months post-CI. The CIQOL-35 can be administrated annually thereafter where appropriate to ensure the patient is progressing and meeting stated goals.
Patients not meeting current FDA labeling and CMS requirements should still be considered for a CI. Evidence-based medicine should guide clinical decision making by the multidisciplinary CI team. Consideration of insurance coverage should be included during counseling as a supplement to clinical recommendations but should not be used as deterrent for referral for a CI evaluation or for CI candidacy.
Implementation of an aural rehabilitation program must be encouraged as it is essential to maximize outcomes following CI.
There is no “bad” CI referral. Even if a patient does not initially qualify, the evaluation process can be educational for the patient, can provide a baseline for comparison in the future, and may result in optimization of HA technology and/or the provision of assistive listening devices.
Conclusion
Untreated and undertreated hearing loss can have negative effects on quality of life. Any patient unable to benefit from, or who perceives dissatisfaction with amplification should be referred for a formal CI evaluation. Currently, adult CI candidacy determination involves consideration of both medical and audiological criteria. Appropriate identification of CI candidates has been shown to lead to positive outcomes in multiple objective and subjective domains. Given the success of CI technology as a treatment for disabling hearing loss, it is imperative to expand access for all appropriate candidates. These evidence-based recommendations outline a standardized method for the identification of potential CI candidates, and for the pre- and post-operative evaluation of objective and subjective outcomes.
Acknowledgments
DMZ serves on the Surgical Advisory Boards for MED-EL Corporation and Advanced Bionics Corporation, is course director and faculty for Institute for Cochlear Implant Training and is a consultant for Cochlear Corporation. SMP is a consultant for Pipeline Therapeutics and faculty for the International Institute for Cochlear Implant Training. SAS is a consultant for and receives study funding from Cochlear Corporation, is on the advisory board for Envoy Medical, is course director and faculty for Institute for Cochlear Implant Training and is on the Editorial Board of the Hearing Journal. CCD serves on the Advisory Board for Cochlear Americas, MED-EL Corporation, Envoy Medical, and iotaMotion Inc., is a course director and faculty for Institute for Cochlear Implant Training, is a consultant for Advanced Bionics, and received grant funding from NIH/NIDCD and MED-EL Corporation.
Footnotes
Level of Evidence: N/A
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
References:
- 1.Goman AM, Lin FR. Prevalence of hearing loss by severity in the United States. American Journal of Public Health. 2016;106(10):1820–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deafness and hearing loss. https://www.who.int/news-room/fact-sheets/detail/deafness-and-hearing-loss. Accessed June 23, 2021.
- 3.Li-Korotky HS. Age-related hearing loss: Quality of care for quality of life. Gerontologist. 2012;52(2):265–271. [DOI] [PubMed] [Google Scholar]
- 4.Nassiri AM, Sorkin DL, Carlson ML. Current Estimates of Cochlear Implant Utilization in the United States. Otology & neurotology. 2022;43(5):e558–e562. [DOI] [PubMed] [Google Scholar]
- 5.What Are Cochlear Implants for Hearing? | NIDCD. https://www.nidcd.nih.gov/health/cochlear-implants. Accessed June 23, 2021.
- 6.Nassiri AM, Marinelli JP, Sorkin DL, Carlson ML. Barriers to Adult Cochlear Implant Care in the United States: An Analysis of Health Care Delivery. Seminars in hearing. 2021;42(4):311–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marinelli JP, Carlson ML. Barriers to Access and Health Care Disparities Associated With Cochlear Implantation Among Adults in the United States. Mayo Clinic proceedings. 2021;96(3):547–549. [DOI] [PubMed] [Google Scholar]
- 8.Prentiss S, Snapp H, Zwolan T. Audiology Practices in the Preoperative Evaluation and Management of Adult Cochlear Implant Candidates. JAMA Otolaryngology - Head and Neck Surgery. 2020;146(2):136–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sorkin DL. Cochlear implantation in the world’s largest medical device market: utilization and awareness of cochlear implants in the United States. Cochlear Implants International. 2013;14 Suppl 1(Suppl 1):S4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carlson ML, Sladen DP, Gurgel RK, Tombers NM, Lohse CM, Driscoll CL. Survey of the American Neurotology Society on Cochlear Implantation: Part 1, Candidacy Assessment and Expanding Indications. Otology and Neurotology. 2018;39(1):e12–e19. [DOI] [PubMed] [Google Scholar]
- 11.Van Loon MC, Smits C, Smit CF, Hensen EF, Merkus P. Cochlear Implantation in Adults with Asymmetric Hearing Loss: Benefits of Bimodal Stimulation. Otology and Neurotology. 2017;38(6):e100–e106. [DOI] [PubMed] [Google Scholar]
- 12.Dillon MT, Buss E, Rooth MA, et al. Cochlear Implantation in Cases of Asymmetric Hearing Loss: Subjective Benefit, Word Recognition, and Spatial Hearing. Trends in Hearing. 2020;24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Firszt JB, Reeder RM, Holden LK, et al. Results in adult cochlear implant recipients with varied asymmetric hearing: A prospective longitudinal study of speech recognition, localization, and participant report. Ear and Hearing. 2018;39(5):845–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sladen DP, Carlson ML, Dowling BP, et al. Cochlear implantation in adults with asymmetric hearing loss: Speech recognition in quiet and in noise, and health related quality of life. Otology and Neurotology. 2018;39(5):576–581. [DOI] [PubMed] [Google Scholar]
- 15.Hansen MR, Gantz BJ, Dunn C. Outcomes After Cochlear Implantation for Patients With Single-Sided Deafness, Including Those With Recalcitrant Ménière’s Disease. Otology & Neurotology. 2013;34(9):1681–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zeitler DM, Dorman MF. Cochlear Implantation for Single-Sided Deafness: A New Treatment Paradigm. Journal of Neurological Surgery, Part B: Skull Base. 2019;80(2):178–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dorman MF, Natale SC, Butts AM, Zeitler DM, Carlson ML. The Sound Quality of Cochlear Implants: Studies with Single-sided Deaf Patients. Otology & Neurotology. 2017;38(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Selleck AM, Brown KD, Park LR. Cochlear Implantation for Unilateral Hearing Loss. Otolaryngologic clinics of North America. 2021;54(6):1193–1203. [DOI] [PubMed] [Google Scholar]
- 19.Arndt S, Aschendorff A, Laszig R, et al. Comparison of pseudobinaural hearing to real binaural hearing rehabilitation after cochlear implantation in patients with unilateral deafness and tinnitus. Otology & Neurotology. 2011;32(1):39–47. [DOI] [PubMed] [Google Scholar]
- 20.Buss E, Dillon MT, Rooth MA, et al. Effects of Cochlear Implantation on Binaural Hearing in Adults With Unilateral Hearing Loss. Trends in Hearing. 2018;22:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dillon MT, Buss E, Anderson ML, et al. Cochlear Implantation in Cases of Unilateral Hearing Loss: Initial Localization Abilities. Ear and hearing. 2017;38(5):611–619. [DOI] [PubMed] [Google Scholar]
- 22.Galvin JJ, Fu QJ, Wilkinson EP, et al. Benefits of cochlear implantation for single-sided deafness: Data from the House Clinic-University of Southern California-University of California, Los Angeles Clinical Trial. Ear and Hearing. 2019;40(4):766–781. [DOI] [PubMed] [Google Scholar]
- 23.Távora-Vieira D, Rajan GP, Van De Heyning P, Mertens G. Evaluating the Long-Term Hearing Outcomes of Cochlear Implant Users with Single-Sided Deafness. Otology and Neurotology. 2019;40(6):E575–E580. [DOI] [PubMed] [Google Scholar]
- 24.Van De Heyning P, Vermeire K, Diebl M, Nopp P, Anderson I, De Ridder D. Incapacitating unilateral tinnitus in single-sided deafness treated by cochlear implantation. Annals of Otology, Rhinology and Laryngology. 2008;117(9):645–652. [DOI] [PubMed] [Google Scholar]
- 25.Sullivan CB, Al-Qurayshi Z, Zhu V, et al. Long-term audiologic outcomes after cochlear implantation for single-sided deafness. Laryngoscope. 2020;130(7):1805–1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deep NL, Spitzer ER, Shapiro WH, Waltzman SB, Roland JT, Friedmann DR. Cochlear Implantation in Adults With Single-sided Deafness: Outcomes and Device Use. Otology and Neurotology. 2021;42(3):414–423. [DOI] [PubMed] [Google Scholar]
- 27.Minimum Speech Test Battery (MSTB) For Adult Cochlear Implant Users 2011 New MSTB User Manual.; 2011.
- 28.Spahr AJ, Dorman MF. Performance of Subjects Fit With the Advanced Bionics CII and Nucleus 3G Cochlear Implant Devices. Archives of Otolaryngology–Head & Neck Surgery. 2004;130(5):624–628. [DOI] [PubMed] [Google Scholar]
- 29.Gifford RH, Shallop JK, Peterson AM. Speech recognition materials and ceiling effects: considerations for cochlear implant programs. Audiology & neuro-otology. 2008;13(3):193–205. [DOI] [PubMed] [Google Scholar]
- 30.Zhao EE, Dornhoffer JR, Loftus C, et al. Association of Patient-Related Factors with Adult Cochlear Implant Speech Recognition Outcomes: A Meta-analysis. JAMA Otolaryngology - Head and Neck Surgery. 2020;146(7):613–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leigh JR, Moran M, Hollow R, Dowell RC. Evidence-based guidelines for recommending cochlear implantation for postlingually deafened adults. https://doi-org.offcampus.lib.washington.edu/103109/1499202720161146415. 2016;55:S3-S8. [DOI] [PubMed]
- 32.Gubbels SP, Gartrell BC, Ploch JL, Hanson KD. Can routine office-based audiometry predict cochlear implant evaluation results? Laryngoscope. 2017;127(1):216–222. [DOI] [PubMed] [Google Scholar]
- 33.Zwolan TA, Schvartz-Leyzac KC, Pleasant T. Development of a 60/60 Guideline for Referring Adults for a Traditional Cochlear Implant Candidacy Evaluation. Otology and Neurotology. 2020;41(7):895–900. [DOI] [PubMed] [Google Scholar]
- 34.McRackan TR, Fabie JE, Burton JA, Munawar S, Holcomb MA, Dubno JR. Earphone and Aided Word Recognition Differences in Cochlear Implant Candidates. Otology and Neurotology. 2018;39(7):e543–e549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Illg A, Bojanowicz M, Lesinski-Schiedat A, Lenarz T, Büchner A. Evaluation of the bimodal benefit in a large cohort of cochlear implant subjects using a contralateral hearing aid. Otology and Neurotology. 2014;35(9):e240–e244. [DOI] [PubMed] [Google Scholar]
- 36.Beyea JA, McMullen KP, Harris MS, et al. Cochlear implants in adults: Effects of age and duration of deafness on speech recognition. Otology and Neurotology. 2016;37(9):1238–1245. [DOI] [PubMed] [Google Scholar]
- 37.Derinsu U, Yüksel M, Geçici CR, Çiprut A, Akdeniz E. Effects of residual speech and auditory deprivation on speech perception of adult cochlear implant recipients. Auris Nasus Larynx. 2019;46(1):58–63. [DOI] [PubMed] [Google Scholar]
- 38.Dierickx C, Jacquemin L, Boon E, et al. Predictive factors of speech understanding in adults with cochlear implants. B-ENT. 2016;12(3):219–226. [PubMed] [Google Scholar]
- 39.Holden LK, Finley CC, Firszt JB, et al. Factors affecting open-set word recognition in adults with cochlear implants. Ear and Hearing. 2013;34(3):342–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim H, Kang WS, Park HJ, et al. Cochlear Implantation in Postlingually Deaf Adults is Time-sensitive Towards Positive Outcome: Prediction using Advanced Machine Learning Techniques. Scientific Reports. 2018;8(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tyler RS, Summerfield AQ. Cochlear implantation: Relationships with research on auditory deprivation and acclimatization. Ear and Hearing. 1996;17(3 SUPPL.). [DOI] [PubMed] [Google Scholar]
- 42.Lazard DS, Vincent C, Venail F, et al. Pre-, Per- and Postoperative Factors Affecting Performance of Postlinguistically Deaf Adults Using Cochlear Implants: A New Conceptual Model over Time. PLoS ONE. 2012;7(11):e48739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leung J, Wang NY, Yeagle JD, et al. Predictive models for cochlear implantation in elderly candidates. Archives of Otolaryngology - Head and Neck Surgery. 2005;131(12):1049–1054. [DOI] [PubMed] [Google Scholar]
- 44.Tang L, Thompson CB, Clark JH, Ceh KM, Yeagle JD, Francis HW. Rehabilitation and psychosocial determinants of cochlear implant outcomes in older adults. Ear and Hearing. 2017;38(6):663–671. [DOI] [PubMed] [Google Scholar]
- 45.Carlsson PI, Hjaldahl J, Magnuson A, et al. Severe to profound hearing impairment: Quality of life, psychosocial consequences and audiological rehabilitation. Disability and Rehabilitation. 2015;37(20):1849–1856. [DOI] [PubMed] [Google Scholar]
- 46.Cohen SM, Labadie RF, Dietrich MS, Haynes DS. Quality of life in hearing-impaired adults: The role of cochlear implants and hearing aids. Otolaryngology - Head and Neck Surgery. 2004;131(4):413–422. [DOI] [PubMed] [Google Scholar]
- 47.Francis HW, Chee N, Yeagle J, Cheng A, Niparko JK. Impact of cochlear implants on the functional health status of older adults. Laryngoscope. 2002;112(8 I):1482–1488. [DOI] [PubMed] [Google Scholar]
- 48.Appelbaum EN, Yoo SS, Perera RA, Coelho DH. Duration of Eligibility Prior to Cochlear Implantation: Have We Made Any Progress? Otology and Neurotology. 2017;38(9):1273–1277. [DOI] [PubMed] [Google Scholar]
- 49.van Dijk JE, van Olphen AF, Langereis MC, Mens LHM, Brokx JPL, Smoorenburg GF. Predictors of cochlear implant performance. International Journal of Audiology. 1999;38(2):109–116. [DOI] [PubMed] [Google Scholar]
- 50.Friedland DR, Venick HS, Niparko JK. Choice of ear for cochlear implantation: the effect of history and residual hearing on predicted postoperative performance. Otology and Neurotology. 2003;24(4):582–589. [DOI] [PubMed] [Google Scholar]
- 51.Lammers MJW, Versnel H, Topsakal V, Van Zanten GA, Grolman W. Predicting Performance and Non-Use in Prelingually Deaf and Late-Implanted Cochlear Implant Users. Otology and Neurotology. 2018;39(6):e436–e442. [DOI] [PubMed] [Google Scholar]
- 52.Duchesne L, Millette I, Bhérer M, Gobeil S. Auditory performance and subjective benefits in adults with congenital or prelinguistic deafness who receive cochlear implants during adulthood. Cochlear Implants International. 2017;18(3):143–152. [DOI] [PubMed] [Google Scholar]
- 53.Moon IS, Park S, Kim HN, et al. Is there a deafness duration limit for cochlear implants in post-lingual deaf adults? Acta Oto-Laryngologica. 2014;134(2):173–180. [DOI] [PubMed] [Google Scholar]
- 54.Sampaio ALL, Araújo MFS, Oliveira CACP. New Criteria of Indication and Selection of Patients to Cochlear Implant. International Journal of Otolaryngology. 2011;2011:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shearer AE, Eppsteiner RW, Frees K, et al. Genetic Variants in the Peripheral Auditory System Significantly Affect Adult Cochlear Implant Performance. Hearing research. 2017;348:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lalwani AK, Budenz CL, Weisstuch AS, Babb J, Roland T, Waltzman SB. Predictability of cochlear implant outcome in families. The Laryngoscope. 2009;119(1):131–136. [DOI] [PubMed] [Google Scholar]
- 57.Lee SY, Shim YJ, Han JH, et al. The molecular etiology of deafness and auditory performance in the postlingually deafened cochlear implantees. Scientific reports. 2020;10(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Altuntaş OM, Özkan B, Bajin D, Sennaroğlu G, Sennaroğlu L. Long-Term Outcome of Cochlear Implantation in Post-meningitic Deafnes. The journal of international advanced otology. 2021;17(6):500–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Heinze-Köhler K, Lehmann EK, Hoppe U. Depressive symptoms affect short- and long-term speech recognition outcome in cochlear implant users. European archives of oto-rhino-laryngology : official journal of the European Federation of Oto-Rhino-Laryngological Societies (EUFOS) : affiliated with the German Society for Oto-Rhino-Laryngology - Head and Neck Surgery. 2021;278(2):345–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Feltner C, Wallace IF, Kistler CE, Coker-Schwimmer M, Jonas DE. Screening for Hearing Loss in Older Adults: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA. 2021;325(12):1202–1215. [DOI] [PubMed] [Google Scholar]
- 61.Zanetti D, Nassif N, Redaelli De Zinis LO. Factors affecting residual hearing preservation in cochlear implantation. Acta otorhinolaryngologica Italica : organo ufficiale della Societa italiana di otorinolaringologia e chirurgia cervico-facciale. 2015;35(6):433–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hassepass F, Aschendorff A, Bulla S, et al. Radiologic Results and Hearing Preservation With a Straight Narrow Electrode via Round Window Versus Cochleostomy Approach at Initial Activation. Otology and Neurotology. 2015;36(6):993–1000. [DOI] [PubMed] [Google Scholar]
- 63.Wanna GB, O’Connell BP, Francis DO, et al. Predictive factors for short- and long-term hearing preservation in cochlear implantation with conventional-length electrodes. The Laryngoscope. 2018;128(2):482–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brant JA, Ruckenstein MJ. Electrode selection for hearing preservation in cochlear implantation: A review of the evidence. World journal of otorhinolaryngology - head and neck surgery. 2016;2(3):157–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Havenith S, Lammers MJW, Tange RA, et al. Hearing preservation surgery: cochleostomy or round window approach? A systematic review. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2013;34(4):667–674. [DOI] [PubMed] [Google Scholar]
- 66.Yin LX, Barnes JH, Saoji AA, Carlson ML. Clinical Utility of Intraoperative Electrocochleography (ECochG) During Cochlear Implantation: A Systematic Review and Quantitative Analysis. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2021;42(3):363–371. [DOI] [PubMed] [Google Scholar]
- 67.Adunka OF, Gantz BJ, Dunn C, Gurgel RK, Buchman CA. Minimum Reporting Standards for Adult Cochlear Implantation. Otolaryngology - Head and Neck Surgery. 2018;159(2):215–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Adunka OF, Dillon MT, Adunka MC, King ER, Pillsbury HC, Buchman CA. Hearing preservation and speech perception outcomes with electric-acoustic stimulation after 12 months of listening experience. Laryngoscope. 2013;123(10):2509–2515. [DOI] [PubMed] [Google Scholar]
- 69.Dorman MF, Gifford RH, Spahr AJ, McKarns SA. The benefits of combining acoustic and electric stimulation for the recognition of speech, voice and melodies. Audiology and Neurotology. 2008;13(2):105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dunn CC, Perreau A, Gantz B, Tyler RS. Benefits of localization and speech perception with multiple noise sources in listeners with a short-electrode cochlear implant. Journal of the American Academy of Audiology. 2010;21(1):44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gantz BJ, Dunn CC, Oleson J, Hansen MR. Acoustic plus electric speech processing: Long-term results. Laryngoscope. 2018;128(2):473–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pillsbury HC, DIllon MT, Buchman CA, et al. Multicenter US Clinical Trial with an Electric-Acoustic Stimulation (EAS) System in Adults: Final Outcomes. Otology and Neurotology. 2018;39(3):299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gifford RH, Dorman MF, Sheffield SW, Teece K, Olund AP. Availability of binaural cues for bilateral implant recipients and bimodal listeners with and without preserved hearing in the implanted ear. Audiology and Neurotology. 2014;19(1):57–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gifford RH, Grantham DW, Sheffield SW, Davis TJ, Dwyer R, Dorman MF. Localization and interaural time difference (ITD) thresholds for cochlear implant recipients with preserved acoustic hearing in the implanted ear. Hearing Research. 2014;312:28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gantz BJ, Turner C, Gfeller KE. Acoustic plus electric speech processing: Preliminary results of a multicenter clinical trial of the Iowa/Nucleus Hybrid implant. Audiology and Neurotology. 2006;11(SUPPL. 1):63–68. [DOI] [PubMed] [Google Scholar]
- 76.Gfeller KE, Olszewski C, Turner C, Gantz B, Oleson J. Music perception with cochlear implants and residual hearing. Audiology and Neurotology. 2006;11(SUPPL. 1):12–15. [DOI] [PubMed] [Google Scholar]
- 77.Gfeller K, Turner C, Oleson J, et al. Accuracy of cochlear implant recipients on pitch perception, melody recognition, and speech reception in noise. Ear and Hearing. 2007;28(3):412–423. [DOI] [PubMed] [Google Scholar]
- 78.Schairer KS, Patrick Feeney M, Sanford CA. Acoustic reflex measurement. Ear and hearing. 2013;34 Suppl 1(SUPPL. 1). [DOI] [PubMed] [Google Scholar]
- 79.Johnson EE, Dillon H. A comparison of gain for adults from generic hearing aid prescriptive methods: impacts on predicted loudness, frequency bandwidth, and speech intelligibility. Journal of the American Academy of Audiology. 2011;22(7):441–459. [DOI] [PubMed] [Google Scholar]
- 80.Keidser G, Dillon H, Flax M, Ching T, Brewer S. The NAL-NL2 Prescription Procedure. Audiology research. 2011;1(1):e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Turton L, Souza P, Thibodeau L, et al. Guidelines for Best Practice in the Audiological Management of Adults with Severe and Profound Hearing Loss. Seminars in hearing. 2020;41(3):141–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sladen DP, Gifford RH, Haynes D, et al. Evaluation of a revised indication for determining adult cochlear implant candidacy. Laryngoscope. 2017;127(10):2368–2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Heinrich A, Henshaw H, Ferguson MA. The relationship of speech intelligibility with hearing sensitivity, cognition, and perceived hearing difficulties varies for different speech perception tests. Frontiers in psychology. 2015;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Moberly AC, Castellanos I, Mattingly JK. Neurocognitive factors contributing to cochlear implant candidacy. Otology and Neurotology. 2018;39(10):e1010–e1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sladen DP, Zappler A. Older and younger adult cochlear implant users: speech recognition in quiet and noise, quality of life, and music perception. American journal of audiology. 2015;24(1):31–39. [DOI] [PubMed] [Google Scholar]
- 86.Spahr AJ, Dorman MF, Litvak LM, et al. Development and validation of the AzBio sentence lists. Ear and hearing. 2012;33(1):112–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Balkany T, Hodges A, Menapace C, et al. Nucleus Freedom North American clinical trial. Otolaryngology - Head and Neck Surgery. 2007;136(5):757–762. [DOI] [PubMed] [Google Scholar]
- 88.Dunn CC. Cochlear implant candidacy: CNC word score. [Google Scholar]
- 89.Dunn CC, Oleson J, Parkinson A, Hansen MR, Gantz BJ. Nucleus Hybrid S12: Multicenter Clinical Trial Results. Laryngoscope. 2020;130(10):E548–E558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gantz BJ, Dunn C, Oleson J, Hansen M, Parkinson A, Turner C. Multicenter clinical trial of the Nucleus Hybrid S8 cochlear implant: Final outcomes. Laryngoscope. 2016;126(4):962–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Roland JT, Gantz BJ, Waltzman SB, Parkinson AJ. United States multicenter clinical trial of the cochlear nucleus hybrid implant system. Laryngoscope. 2016;126(1):175–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Peterson G, Lehiste I. Revised CNC lists for auditory tests. The Journal of speech and hearing disorders. 1962;27:62–70. [DOI] [PubMed] [Google Scholar]
- 93.Skinner MW, Holden LK, Holden TA, Demorest ME, Fourakis MS. Speech recognition at simulated soft, conversational, and raised-to-loud vocal efforts by adults with cochlear implants. The Journal of the Acoustical Society of America. 1997;101(6):3766–3782. [DOI] [PubMed] [Google Scholar]
- 94.Kelsall D, Lupo E, Biever A. Longitudinal outcomes of cochlear implantation and bimodal hearing in a large group of adults: A multicenter clinical study. American Journal of Otolaryngology - Head and Neck Medicine and Surgery. 2021;42(1). [DOI] [PubMed] [Google Scholar]
- 95.Zhang E, Coelho DH. Beyond sentence recognition in quiet for older adults: Implications for cochlear implant candidacy. Otology and Neurotology. 2018;39(8):979–986. [DOI] [PubMed] [Google Scholar]
- 96.Mudery JA, Francis R, McCrary H, Jacob A. Older individuals meeting medicare cochlear implant candidacy criteria in noise but not in quiet: Are these patients improved by surgery? In: Otology and Neurotology. Vol 38. Lippincott Williams and Wilkins; 2017:187–191. [DOI] [PubMed] [Google Scholar]
- 97.Friedland DR, Kozlowski K, Runge CL. Cochlear Implant Performance in Candidates With Moderate Hearing Loss Qualifying in Noise. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2021;42(10):1484–1491. [DOI] [PubMed] [Google Scholar]
- 98.Lundberg EMH, Strong D, Anderson M, Kaizer AM, Gubbels S. Do Patients Benefit From a Cochlear Implant When They Qualify Only in the Presence of Background Noise? Otology and Neurotology. 2021;42(2):251–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gifford RH, Shallop JK, Peterson AM. Speech recognition materials and ceiling effects: Considerations for cochlear implant programs. Audiology and Neurotology. 2008;13(3):193–205. [DOI] [PubMed] [Google Scholar]
- 100.Hughes ML, Neff DL, Simmons JL, Moeller MP. Performance outcomes for borderline cochlear implant recipients with substantial preoperative residual hearing. Otology and Neurotology. 2014;35(8):1373–1384. [DOI] [PubMed] [Google Scholar]
- 101.Eshraghi AA, Rodriguez M, Balkany TJ, et al. Cochlear implant surgery in patients more than seventy-nine years old. Laryngoscope. 2009;119(6):1180–1183. [DOI] [PubMed] [Google Scholar]
- 102.Wong DJY, Moran M, O’Leary SJ. Outcomes After Cochlear Implantation in the Very Elderly. Otology and Neurotology. 2016;37(1):46–51. [DOI] [PubMed] [Google Scholar]
- 103.Carlson ML, Breen JT, Gifford RH, et al. Cochlear implantation in the octogenarian and nonagenarian. Otology & Neurotology. 2010;31(8):1343–1349. [DOI] [PubMed] [Google Scholar]
- 104.Gurgel RK, Duff K, Foster NL, Urano KA, deTorres A. Evaluating the Impact of Cochlear Implantation on Cognitive Function in Older Adults. The Laryngoscope. 2022;132 Suppl 7(Suppl 7):S1–S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Castiglione A, Benatti A, Velardita C, et al. Aging, Cognitive Decline and Hearing Loss: Effects of Auditory Rehabilitation and Training with Hearing Aids and Cochlear Implants on Cognitive Function and Depression among Older Adults. Audiology & neuro-otology. 2016;21 Suppl 1(1):21–28. [DOI] [PubMed] [Google Scholar]
- 106.Herzog JA, Buchman CA, Kallogjeri D, et al. Cognitive Assessment in Elderly Cochlear Implant Recipients: Long-Term Analysis. The Laryngoscope. 2022. [DOI] [PubMed] [Google Scholar]
- 107.Mosnier I, Bebear JP, Marx M, et al. Improvement of cognitive function after cochlear implantation in elderly patients. JAMA Otolaryngology - Head and Neck Surgery. 2015;141(5):442–450. [DOI] [PubMed] [Google Scholar]
- 108.Cosetti MK, Pinkston JB, Flores JM, et al. Neurocognitive testing and cochlear implantation: insights into performance in older adults. Clinical interventions in aging. 2016;11:603–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kecskeméti N, Szőnyi M, Küstel M, Gáborján A, Tamás L, Répássy G. Cochlear implantation under local anesthesia: a possible alternative for elderly patients. European Archives of Oto-Rhino-Laryngology. 2019;276(6):1643–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Vincenti V, Plantone F, Ciavarro G, et al. Cochlear implantation under local anesthesia and conscious sedation: an Italian experience. European archives of oto-rhino-laryngology : official journal of the European Federation of Oto-Rhino-Laryngological Societies (EUFOS) : affiliated with the German Society for Oto-Rhino-Laryngology - Head and Neck Surgery. 2021;278(10):3667–3672. [DOI] [PubMed] [Google Scholar]
- 111.Connors JR, Deep NL, Huncke TK, Roland JT. Cochlear Implantation Under Local Anesthesia With Conscious Sedation in the Elderly: First 100 Cases. The Laryngoscope. 2021;131(3):E946–E951. [DOI] [PubMed] [Google Scholar]
- 112.Agrawal Y, Ward BK, Minor LB. Vestibular dysfunction: Prevalence, impact and need for targeted treatment. Journal of Vestibular Research: Equilibrium and Orientation. 2013;23(3):113–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Barbara M, Talamonti R, Benincasa AT, et al. Early assessment of vestibular function after unilateral cochlear implant surgery. Audiology and Neurotology. 2020;25(1–2):50–59. [DOI] [PubMed] [Google Scholar]
- 114.West N, Klokker M, Cayé-Thomasen P. Vestibular Screening Before Cochlear Implantation: Clinical Implications and Challenges in 409 Cochlear Implant Recipients. Otology and Neurotology. 2021;42(2):e137–e144. [DOI] [PubMed] [Google Scholar]
- 115.Parmar A, Savage J, Wilkinson A, Hajioff D, Nunez DA, Robinson P. The role of vestibular caloric tests in cochlear implantation. Otolaryngology Head and Neck Surgery. 2012;147(1):127–131. [DOI] [PubMed] [Google Scholar]
- 116.Hsieh WH, Huang WT, Lin HC. Investigation of the effect of cochlear implantation on tinnitus, and its associated factors. Acta oto-laryngologica. 2020;140(6):497–500. [DOI] [PubMed] [Google Scholar]
- 117.Wang Q, Li JN, Lei GX, et al. Interaction of tinnitus suppression and hearing ability after cochlear implantation. Acta oto-laryngologica. 2017;137(10):1077–1082. [DOI] [PubMed] [Google Scholar]
- 118.Tamplen M, Schwalje A, Lustig L, Alemi AS, Miller ME. Utility of preoperative computed tomography and magnetic resonance imaging in adult and pediatric cochlear implant candidates. Laryngoscope. 2016;126(6):1440–1445. [DOI] [PubMed] [Google Scholar]
- 119.Lassaletta L, Calvino M, Sánchez-Cuadrado I, Pérez-Mora RM, Gavilán J. Which ear should we choose for cochlear implantation in the elderly: The poorer or the better? Audiometric outcomes, quality of sound, and quality-of-life results. Acta Oto-Laryngologica. 2015;135(12):1268–1276. [DOI] [PubMed] [Google Scholar]
- 120.Patki A, Tucci DL. Choice of ear for cochlear implantation: Implant the better- or worse-hearing ear? Laryngoscope. 2015;125(1):5–6. [DOI] [PubMed] [Google Scholar]
- 121.Fielden CA, Mehta RL, Kitterick PT. Choosing which ear to implant in adult candidates with functional residual hearing. Cochlear Implants International. 2016;17:47–50. [DOI] [PubMed] [Google Scholar]
- 122.Pelosi S, Wanna GB, Gifford RH, et al. Unilateral auditory performance before and after bilateral sequential cochlear implantation. Otology and Neurotology. 2013;34(9):1642–1647. [DOI] [PubMed] [Google Scholar]
- 123.Cochlear Implants and Vaccination Recommendations. https://www.cdc.gov/vaccines/vpd/mening/public/dis-cochlear-faq-gen.html. Published 2019. Accessed May 5, 2022.
- 124.Kobayashi M, Farrar JL, Gierke R, et al. Use of 15-Valent Pneumococcal Conjugate Vaccine and 20-Valent Pneumococcal Conjugate Vaccine Among U.S. Adults: Updated Recommendations of the Advisory Committee on Immunization Practices — United States, 2022. MMWR Morbidity and Mortality Weekly Report. 2022;71(4):109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.McRackan TR, Reddy P, Costello MS, Dubno JR. Role of Preoperative Patient Expectations in Adult Cochlear Implant Outcomes. Otology and Neurotology. 2021;42(2):e130–e136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.McRackan TR, Hand BN, Velozo CA, Dubno JR. Association of Demographic and Hearing-Related Factors With Cochlear Implant-Related Quality of Life. JAMA otolaryngology-- head & neck surgery. 2019;145(5):422–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gatehouse S, Noble I. The Speech, Spatial and Qualities of Hearing Scale (SSQ). International Journal of Audiology. 2004;43(2):85–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.McRackan TR, Hand BN, Velozo CA, et al. Cochlear implant quality of life (CIQOL): Development of a profile instrument (CIQOL-35 profile) and a global measure (CIQOL-10 Global). Journal of Speech, Language, and Hearing Research. 2019;62(9):3554–3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.McRackan TR, Hand BN, Velozo CA, Dubno JR. Validity and reliability of the Cochlear Implant Quality of Life (CIQOL)-35 Profile and CIQOL-10 Global instruments in comparison to legacy instruments. Ear and hearing. 2021;42(4):896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kobosko J, Jedrzejczak WW, Pilka E, Pankowska A, Skarzynski H. Satisfaction with Cochlear Implants in Postlingually Deaf Adults and Its Nonaudiological Predictors: Psychological Distress, Coping Strategies, and Self-Esteem. Ear and Hearing. 2015;36(5):605–618. [DOI] [PubMed] [Google Scholar]
- 131.Harris MS, Capretta NR, Henning SC, Feeney L, Pitt MA, Moberly AC. Postoperative Rehabilitation Strategies Used by Adults With Cochlear Implants: A Pilot Study. Laryngoscope Investigative Otolaryngology. 2016;1(3):42–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Schumann A, Hast A, Hoppe U. Speech performance and training effects in the cochlear implant elderly. Audiology and Neurotology. 2014;19:45–48. [DOI] [PubMed] [Google Scholar]
- 133.Dornhoffer JR, Reddy P, Ma C, Schvartz-Leyzac KC, Dubno JR, McRackan TR. Use of Auditory Training and Its Influence on Early Cochlear Implant Outcomes in Adults. Otology Neurotology. 2022;43(2):E165–E173. [DOI] [PMC free article] [PubMed] [Google Scholar]