Abstract
Background
Ixodes scapularis and Ixodes pacificus ticks are the principal vectors of the agent of Lyme disease and several other tick-borne diseases in Canada. Tick surveillance data can be used to identify local tick-borne disease risk areas and direct public health interventions. The objective of this article is to describe the seasonal and spatial characteristics of the main Lyme disease vectors in Canada, and the tick-borne pathogens they carry, using passive and active surveillance data from 2020.
Methods
Passive and active surveillance data were compiled from the National Microbiology Laboratory Branch (Public Health Agency of Canada), provincial and local public health authorities, and eTick (an online, image-based platform). Seasonal and spatial analyses of ticks and their associated pathogens are presented, including infection prevalence estimates.
Results
In passive surveillance, I. scapularis (n=7,534) were submitted from all provinces except Manitoba and British Columbia, while I. pacificus (n=718) were submitted only from British Columbia. No ticks were submitted from the Territories. The seasonal distribution of I. scapularis submissions was bimodal, but unimodal for I. pacificus. Four tick-borne pathogens were identified in I. scapularis (Borrelia burgdorferi, Anaplasma phagocytophilum, Babesia microti and Borrelia miyamotoi) and one in I. pacificus (B. miyamotoi). In active surveillance, I. scapularis (n=688) were collected in Ontario, Québec and New Brunswick. Five tick-borne pathogens were identified: B. burgdorferi, A. phagocytophilum, B. microti, B. miyamotoi and Powassan virus.
Conclusion
This article provides a snapshot of the distribution of I. scapularis and I. pacificus and their associated human pathogens in Canada in 2020, which can help assess the risk of exposure to tick-borne pathogens in different provinces.
Keywords: Ixodes scapularis, Ixodes pacificus, surveillance, Borrelia, Anaplasma, Babesia, Powassan virus
Introduction
Ixodes scapularis and Ixodes pacificus ticks can transmit several bacterial, viral and protozoan pathogens to humans ((1)). The geographic range and population of I. scapularis is increasing in southern central and eastern Canada ((2,3)), due to climate and environmental changes that have enhanced habitat suitability for ticks in more areas ((4,5)). These changes can further alter tick behaviour and extend their periods of activity, which can increase exposure to tick-borne diseases (TBD) ((1,6)). To reduce the burden from TBD, the continued range expansion of ticks in Canada must be met with increased capacity for and awareness of TBD prevention and surveillance ((1)). Tick surveillance data inform the environmental risk of Lyme disease (LD), which can guide public health authorities in targeting prevention and control efforts and support LD diagnostics by healthcare professionals ((7)).
The causative agent of LD, Borrelia burgdorferi, is transmitted by I. scapularis in central and eastern Canada and by I. pacificus in British Columbia. Reported incidence of LD in people has increased more than 10-fold (from 144 to 1,615 cases) from 2009 to 2020 ((8)). Additional TBD, transmitted by I. scapularis or I. pacificus, are emerging in Canada; including anaplasmosis ((9)), babesiosis ((10)), hard tick-borne relapsing fever ((11)) and Powassan virus disease ((12)).
Passive tick surveillance has been used since the 1990s to identify I. scapularis and I. pacificus tick populations and the presence of tick-borne pathogens ((13,14)). Active tick surveillance began in the 2000s to detect areas with established tick populations where LD risk may become endemic (LD risk areas) ((15)). Efforts to summarize passive and active tick surveillance annually at the national level began in 2019 ((16)), providing a baseline for TBD risk that over time will facilitate the identification of current trends and enable the projection of future trends.
The objective of this surveillance report is to summarize the geographic and seasonal characteristics of the main LD vectors in Canada, I. scapularis and I. pacificus, collected through passive and active surveillance in 2020. This article will also summarize the prevalence and spatial distribution of their associated human pathogens.
Methods
Data sources
This report uses two types of surveillance data from ten different providers. Passive tick surveillance data was provided by the National Microbiology Laboratory (NML) Branch of the Public Health Agency of Canada (PHAC), British Columbia Centre for Disease Control (BCCDC), Alberta Health, Saskatchewan Ministry of Health, and eTick. Active tick surveillance data were provided by Thunder Bay District Health Unit, Kingston, Frontenac and Lennox & Addington Public Health, Laboratoire de santé publique du Québec, New Brunswick Department of Health and New Brunswick Provincial Veterinary Laboratory.
Passive tick surveillance
Passive tick surveillance is the voluntary submission by the public of ticks (or their images) to medical or veterinary clinics, regional public health authorities or other institutions (e.g. university laboratory) for species identification and laboratory testing ((13)). This analysis was limited to I. scapularis and I. pacificus ticks collected within Canada in 2020, although several other tick species were also identified. Ticks could be submitted at any point during the year. Ticks with a location of acquisition outside of Canada, with a submitter's history of travel to another province, or from within Canada but could not be geocoded were excluded. Ticks were submitted individually (single submission) or in groups of two or more (multiple submission). Provinces with five or fewer ticks submitted for species identification and laboratory testing were excluded from the study to avoid misinterpretation of results. No ticks were submitted from Northwest Territories, Nunavut or Yukon as no passive surveillance programs exist for I. scapularis and I. pacificus.
Since 2009, regional passive tick surveillance programs have been gradually discontinued in several jurisdictions (e.g. Nova Scotia, southwestern Québec and eastern Ontario) dependent on laboratory capacity and as I. scapularis populations have become established. However, ticks (or their images) acquired in these jurisdictions could be submitted by the public directly to NML or to eTick.
eTick is a validated, web-based, community-science passive surveillance system for tick identification ((17)). Individuals submit images of ticks they encounter to the online platform, which are then examined by trained personnel for species identification. The system began in 2017 in Québec, with five additional provinces added by 2020 (Saskatchewan, Ontario, Newfoundland and Labrador, New Brunswick and Nova Scotia). Similar to provincial tick surveillance data sources, eTick collects information on location of acquisition, date of collection, submitter travel history, tick host, tick species and tick instar. All ticks from eTick were classified as single submissions, as users must upload images of each tick individually.
Ticks acquired and submitted in Saskatchewan, Ontario, Québec, Newfoundland and Labrador, New Brunswick, Nova Scotia and Prince Edward Island were tested for A. phagocytophilum, B. burgdorferi, B. miyamotoi and B. microti at NML or University of Saskatchewan using the methods previously described ((16,18)). Ticks from BCCDC were tested only for B. burgdorferi and B. miyamotoi ((14)). Laboratory results for ticks from Alberta Health were not available. Specimens from tick records submitted through eTick were not routinely requested for testing of tick-borne pathogens but could be forwarded onto a laboratory for this purpose at the request of local public health authorities.
Active tick surveillance
In active surveillance, ticks are collected from the environment using drag sampling or by capturing host mammals that are then examined for ticks. This analysis used I. scapularis ticks collected during drag sampling from 7 sites in Ontario, 24 sites in Québec and 14 sites in New Brunswick. Drag sampling takes place in late spring/summer (May through July) and fall (September through November), with some sites visited during both periods.
All ticks were tested at NML for A. phagocytophilum, B. microti, B. burgdorferi, B. miyamotoi and Powassan virus. Ticks were collected and tested using the methods previously described ((16,18,19)).
Analysis
Tick characteristics
For passive surveillance, descriptive statistics were calculated for submission type (sample-based or image-based), tick species, province of acquisition, instar (larva, nymph, adult female or adult male), level of engorgement (unfed or engorged), host (human, dog, cat or other) and month of collection. Where date of collection was not available, the date the sample was received was used to ascertain the month of collection. For active surveillance, descriptive statistics were calculated for province of collection and instar (larva, nymph, adult female or adult male). All data were cleaned and analysed in R (version 4.0.2).
Ticks that were acquired in Canada in passive surveillance were mapped using QGIS (version 3.8.1) based on their location of acquisition, except for ticks from Alberta that were mapped to the centroid of the forward sortation area (the first three characters of the postal code) of acquisition. Ticks from submitters with a history of travel in the previous 14 days within the same province as the locality of acquisition were geocoded to the location of exposure during travel. Ticks from submitters with multiple travel locations listed were not mapped. In active surveillance, the location of tick dragging was geocoded and mapped.
Infection prevalence
To account for pooled testing of ticks from some jurisdictions for passive surveillance, maximum likelihood estimates (MLE) of prevalence were calculated in Excel (version 16.0) with 95% confidence intervals (CI) using the PooledInfRate add-in (version 4.0) ((20,21)). This estimates the probability of infection for an individual tick in the population using the results of testing of the pooled samples (i.e. a group of one or more ticks submitted and tested together). Co-infection prevalence was calculated among single submissions only to ascertain true co-infections; that is, two or more pathogens in a single tick. Where ticks were not tested in pools, prevalence was the number of positive ticks divided by the number of ticks tested.
Results
Passive surveillance tick characteristics
In 2020, a total of 8,252 ticks were submitted from nine provinces (Table 1, Figure 1). Ticks from Manitoba were excluded as five or fewer ticks were submitted. No ticks were submitted from Northwest Territories, Nunavut or Yukon. The majority (71.49%) of ticks were sample-based submissions (n=5,899) and the remainder were image-based submissions (n=2,353). Ticks from Ontario and Québec comprised 77.24% of all ticks submitted. The majority (96.80%) of ticks were from single submissions, but there were 109 multiple submissions (range: 2–6 ticks per submission; median: 2).
Table 1. Number of Ixodes pacificus and Ixodes scapularis ticks collected through passive surveillance by province, Canada, 2020.
| Province | Tick species (number of ticks) |
Type of surveillance (number of ticks)a |
Type of submission (number of submissions)b |
||||
|---|---|---|---|---|---|---|---|
| Ixodes pacificus | Ixodes scapularis | Total | Sample-based | Image-based | Single submissions | Multiple submissions | |
| British Columbia | 718 | 0 | 718 | 718 | N/A | 670 | 22 |
| Alberta | 0 | 81 | 81 | 81 | N/A | 81 | 0 |
| Saskatchewan | 0 | 12 | 12 | 7 | 5 | 12 | 0 |
| Ontario | 0 | 5,139 | 5,139 | 3,713 | 1,426 | 4,964 | 68 |
| Québec | 0 | 1,235 | 1,235 | 809 | 426 | 1,208 | 12 |
| Newfoundland and Labrador | 0 | 14 | 14 | 4 | 10 | 14 | 0 |
| New Brunswick | 0 | 646 | 646 | 516 | 130 | 634 | 6 |
| Nova Scotia | 0 | 392 | 392 | 36 | 356 | 392 | 0 |
| Prince Edward Island | 0 | 15 | 15 | 15 | N/A | 13 | 1 |
| Total | 718 | 7,534 | 8,252 | 5,899 | 2,353 | 7,988 | 109 |
Abbreviation: N/A, not applicable
a Sample-based submissions are physical tick specimens; image-based submissions are images submitted to eTick
b Single submissions consist of one tick; multiple submissions consist of two or more ticks submitted together by the same individual
Figure 1.
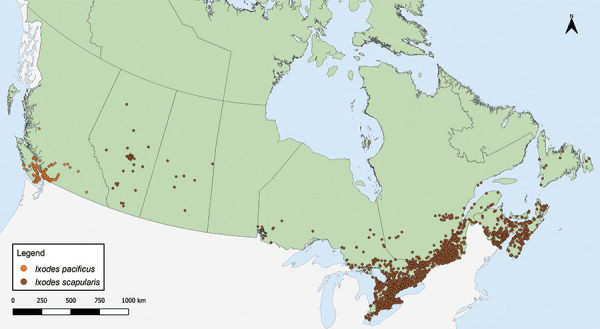
Ixodes pacificus and Ixodes scapularis ticks submitted through passive tick surveillance, Canada, 2020a
a Each dot represents the probable location of acquisition for an I. pacificus (n=718) or I. scapularis (n=7,397) tick submitted through passive surveillance. Ticks from Alberta Health were mapped to the centroid of the forward sortation area (first three characters of the postal code) of acquisition. One hundred and thirty-seven ticks were not mapped because the probable location of acquisition could not be determined
Tick instar, level of engorgement and host were available for 100% of I. pacificus. Tick instar, level of engorgement and host were available for 89.66%, 67.60% and 99.92% of I. scapularis, respectively. The majority of ticks submitted were adult female ticks (I. pacificus: 97.21%; I. scapularis: 92.36%) (Table 2). Adult males, nymphs and larvae were submitted less frequently. Overall, 8.91% of I. pacificus and 41.76% of I. scapularis were engorged. Humans were the most common host among I. pacificus and I. scapularis (90.39% and 82.98%, respectively) followed by dogs (8.91% and 13.34%, respectively).
Table 2. Instar, level of engorgement and host of Ixodes pacificus and Ixodes scapularis ticks submitted through passive surveillance, Canada, 2020a.
| Characteristics | Tick species | |||
|---|---|---|---|---|
| Ixodes pacificus | Ixodes scapularis | |||
| n | % | n | % | |
| Instar | ||||
| Larva | 0 | 0 | 9 | 0.13 |
| Nymph | 1 | 0.14 | 284 | 4.20 |
| Adult female | 698 | 97.21 | 6,239 | 92.36 |
| Adult male | 19 | 2.65 | 223 | 3.30 |
| Total | 718 | 100 | 6,755 | 100 |
| Level of engorgement | ||||
| Engorged | 64 | 8.91 | 2,127 | 41.76 |
| Unfed | 654 | 91.09 | 2,966 | 58.24 |
| Total | 718 | 100 | 5,093 | 100 |
| Host | ||||
| Human | 649 | 90.39 | 6,247 | 82.98 |
| Dog | 64 | 8.91 | 1,004 | 13.34 |
| Cat | 3 | 0.42 | 132 | 1.75 |
| Otherb | 2 | 0.28 | 145 | 1.93 |
| Total | 718 | 100 | 7,528 | 100 |
a Data are presented for all ticks where available, regardless of whether the tick was part of a single or a multiple submission
b Includes environment, horse, rabbit and other unspecified animal
Month of acquisition and tick instar was available for 100% of I. pacificus and 89.66% of I. scapularis. (Figure 2). Adult I. scapularis ticks submitted peaked in May and October through November, while adult I. pacificus submitted peaked only in May. Only 0.14% of I. pacificus submitted were nymphs, while 4.20% of I. scapularis submitted were nymphs, peaking in June. Larvae of I. scapularis (0.13%) were submitted June through September; no I. pacificus larvae were submitted.
Figure 2.
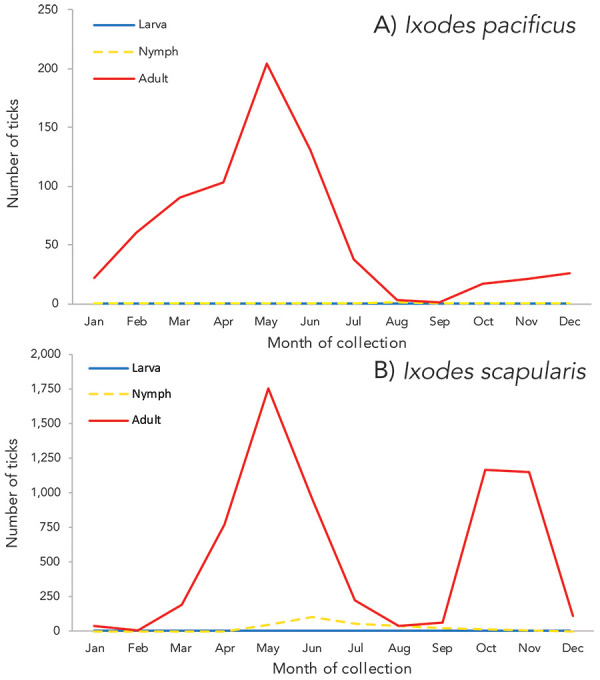
Number of Ixodes pacificus and Ixodes scapularis ticks submitted through passive surveillance, by month and tick instar, Canada, 2020a
a Data are presented for I. pacificus (n=718) and I. scapularis (n=6,755) ticks submitted through passive surveillance. Month of submission or tick instar was not available for I. scapularis (n=779)
Passive surveillance infection prevalence
Data on laboratory testing were available for 98.27% of I. pacificus and 98.20%–98.40% of I. scapularis from sample-based submissions, depending on pathogen. The most prevalent pathogen was B. burgdorferi, detected in 17.19% of I. scapularis (95% CI: 16.17–18.26) (Table 3). Other tick-borne pathogens (A. phagocytophilum, B. microti and B. miyamotoi) and co-infections were estimated to have a prevalence rate of less than 1%. Among I. pacificus, only B. miyamotoi was identified (0.14%, 95% CI: 0.01–0.68).
Table 3. Prevalence of Anaplasma phagocytophilum, Babesia microti, Borrelia burgdorferi and Borrelia miyamotoi infection in Ixodes pacificus and Ixodes scapularis ticks submitted through passive surveillance, Canada, 2020a,b.
| Pathogen | Infection prevalence | |||
|---|---|---|---|---|
| Ixodes pacificus | Ixodes scapularis | |||
| Single agent | Maximum likelihood estimate | |||
| % | 95% CI | % | 95% CI | |
| Anaplasma phagocytophilum | N/A | N/A | 0.87 | 0.64–1.15 |
| Babesia microti | N/A | N/A | 0.02 | 0–0.09 |
| Borrelia burgdorferi | 0 | 0–0.54 | 17.19 | 16.17–18.26 |
| Borrelia miyamotoi | 0.14 | 0.01–0.68 | 0.49 | 0.33–0.71 |
| Total single agent | 0.14 | 0.01–0.68 | 18.21 | 17.16–19.29 |
| Co-infection | Co-infection rate | |||
| % | Number co-infected ticks/number ticks tested | % | Number co-infected ticks/number ticks tested | |
|
Anaplasma phagocytophilum +
Babesia microti |
N/A | N/A | 0 | 0/4,874 |
|
Anaplasma phagocytophilum +
Borrelia burgdorferi |
N/A | N/A | 0.12 | 6/4,874 |
|
Anaplasma phagocytophilum +
Borrelia miyamotoi |
N/A | N/A | 0.02 | 1/4,874 |
|
Babesia microti +
Borrelia burgdorferi |
N/A | N/A | 0 | 0/4,882 |
|
Babesia microti +
Borrelia miyamotoi |
N/A | N/A | 0 | 0/4,883 |
|
Borrelia burgdorferi +
Borrelia miyamotoi |
0 | 0/705 | 0.14 | 7/4,882 |
| Total co-infected | 0 | 0/705 | 0.29 | 14/4,883 |
Abbreviations: CI, confidence interval; N/A, not tested
a All I. pacificus (n=718) were not tested for A. phagocytophilum and B. microti. All I. scapularis from Alberta or submitted through eTick were not tested for any pathogen
b Number of I. scapularis ticks tested: A. phagocytophilum (n=5,090), B. microti (n=5,100), B. burgdorferi (n=5,098), B. miyamotoi (n=5,094). Number of I. pacificus ticks tested: B. burgdorferi (n=705), B. miyamotoi (n=705)
Prevalence of B. burgdorferi was higher in multiple submissions of I. scapularis (32.31%, 95% CI: 25.27–40.34) than from single submissions (16.71%, 95% CI: 15.69–17.78). Infection prevalence did not differ significantly by submission type for any other pathogen. Ixodes scapularis submitted from human hosts did not have significantly different infection prevalence compared to I. scapularis submitted from non-human hosts.
Tick-borne pathogens were largely found in southern and eastern Ontario, southern Québec and southern New Brunswick (Figure 3, Figure 4, and Table 4). Borrelia burgdorferi-infected I. scapularis were found in six provinces: Saskatchewan, Ontario, Québec, Newfoundland and Labrador, New Brunswick and Nova Scotia. Three quarters of B. burgdorferi-infected I. scapularis submissions were within previously identified LD risk areas (74.88%; 644/860). Lyme disease risk areas are localities in which there is evidence of reproducing populations of known tick vector species (particularly I. scapularis and I. pacificus) and the likely transmission of B. burgdorferi ((22)). Most multiple submissions came from LD risk areas (76.15%; 83/109), of which 51.81% were infected with B. burgdorferi (43/83).
Figure 3.
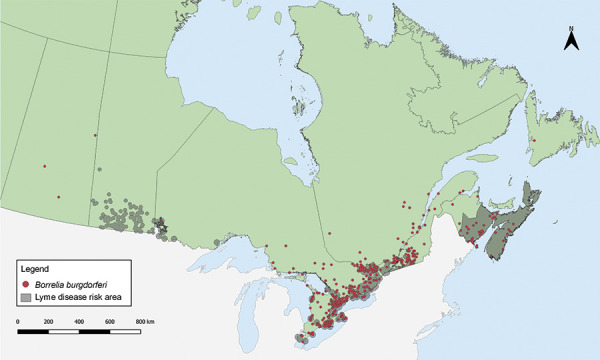
Ixodes scapularis ticks submitted through passive surveillance infected with Borrelia burgdorferi, Canada, 2020a,b
a Each dot represents the probable location of acquisition of at least one I. scapularis (n=860) single or multiple tick submission submitted through passive surveillance that was infected with B. burgdorferi. Eight ticks were not mapped because the probable location of acquisition could not be determined
b Lyme disease risk areas are identified by the provinces as of 2021 using the methods described in the 2016 national Lyme disease case definition ((22)). On the map, risk areas are identified as hatched gray areas
Figure 4.
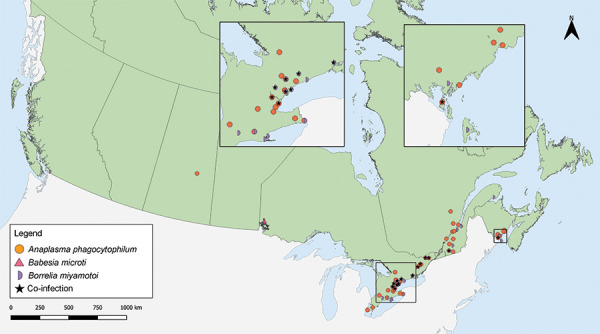
Ixodes pacificus and Ixodes scapularis ticks submitted through passive surveillance infected with Anaplasma phagocytophilum, Babesia microti, Borrelia miyamotoi and co-infections, Canada, 2020a
a Each symbol represents the probable location of acquisition of an I. pacificus (n=1) or I. scapularis (n=67) single or multiple tick submission submitted through passive surveillance that tested positive for A. phagocytophilum (n=42), B. microti (n=1), B. miyamotoi (n=25) or a co-infection (n=14). Co-infections were limited to only single submissions of ticks and include B. burgdorferi + B. miyamotoi (n=7), B. burgdorferi + A. phagocytophilum (n=6) and A. phagocytophilum + B. miyamotoi (n=1) all in I. scapularis. Two ticks with A. phagocytophilum and one tick with B. miyamotoi were not mapped because the probable location of acquisition could not be determined
Table 4. Prevalence of Anaplasma phagocytophilum, Babesia microti, Borrelia burgdorferi and Borrelia miyamotoi infection in Ixodes scapularis and Ixodes pacificus ticks submitted through passive surveillance, by province, Canada, 2020a.
| Province | Infection prevalence Maximum likelihood estimate |
|||||||
|---|---|---|---|---|---|---|---|---|
| Anaplasma phagocytophilum | Babesia microti | Borrelia burgdorferi | Borrelia miyamotoi | |||||
| % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | |
| Ixodes pacificus | ||||||||
| British Columbia | N/A | N/A | N/A | N/A | 0 | 0–0.54 | 0.14 | 0.01–0.68 |
| Ixodes scapularis | ||||||||
| Alberta | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Saskatchewan | 14.29 | 0.85–51.51 | 0 | 0–35.43 | 42.86 | 12.96–77.51 | 0 | 0–35.43 |
| Ontario | 0.73 | 0.49–1.04 | 0.03 | 0–0.13 | 17.78 | 16.56–19.04 | 0.46 | 0.28–0.72 |
| Québec | 1.24 | 0.63–2.19 | 0 | 0–0.47 | 19.50 | 16.87–22.35 | 0.62 | 0.23–1.36 |
| Newfoundland and Labrador | 0 | 0–48.99 | 0 | 0–48.99 | 25.00 | 1.52–73.74 | 0 | 0–48.99 |
| New Brunswick | 1.17 | 0.48–2.40 | 0 | 0–0.74 | 8.97 | 6.72–11.68 | 0.58 | 0.15–1.57 |
| Nova Scotia | 0 | 0–9.64 | 0 | 0–9.64 | 25.00 | 13.03–40.81 | 0 | 0–9.64 |
| Prince Edward Island | 0 | 0–20.15 | 0 | 0–20.15 | 0 | 0–20.15 | 0 | 0–20.15 |
| Total | 0.87 | 0.45–1.15 | 0.02 | 0–0.09 | 17.19 | 16.17–18.26 | 0.49 | 0.33–0.71 |
Abbreviations: CI, confidence interval; N/A, not tested
a Number of ticks tested: British Columbia (n=705), Alberta (n=0), Saskatchewan (n=7), Ontario (n=3,705–3,713), Québec (n=809), Newfoundland and Labrador (n=4), New Brunswick (n=514–516), Nova Scotia (n=36), Prince Edward Island (n=15)
Anaplasma phagocytophilum was found in I. scapularis (0.87%) in four provinces: Saskatchewan, Ontario, Québec, and New Brunswick (Figure 4, Table 4). Borrelia miyamotoi was found in British Columbia, Ontario, Québec and New Brunswick. Babesia microti was found only in Ontario. Co-infections were found in Ontario, Québec and New Brunswick.
Active surveillance tick characteristics
In 2020, I. scapularis (n=688) were collected in three provinces in active surveillance: New Brunswick (n=445), Ontario (n=128) and Québec (n=115). Adult males (n=264/688; 38.37%) and females (n=214/688; 31.10%) were collected most often, followed by nymphs (n=209/688; 30.38%) and larva (1/688; 0.15%).
Active surveillance infection prevalence
Laboratory testing results were available for 99.27% of I. scapularis. The most prevalent pathogen was B. burgdorferi (29.28%), present in Ontario, Québec and New Brunswick (Table 5). Anaplasma phagocytophilum (4.54%) was found in ticks in Ontario and New Brunswick. The remaining pathogens were found in less than 0.5% of I. scapularis: three B. miyamotoi-positive and one B. microti-positive ticks were found in New Brunswick, and one tick with Powassan virus (deer tick lineage) was found in Québec. The site locations where I. scapularis was collected in active surveillance are shown in Figure 5.
Table 5. Infection prevalence of Ixodes scapularis ticks collected in active surveillance, by province, Canada, 2020.
| Province | Infection prevalence | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Anaplasma phagocytophilum | Babesia microti | Borrelia burgdorferi | Borrelia miyamotoi | Powassan virus | ||||||
| Proportion positive ticka | % | Proportion positive tick | % | Proportion positive tick | % | Proportion positive tick | % | Proportion positive tick | % | |
| Ontario | 2/128 | 1.56 | 0/128 | 0 | 53/128 | 41.41 | 0/128 | 0 | 0/128 | 0 |
| Québec | 0/110 | 0 | 0/110 | 0 | 40/110 | 36.36 | 0/110 | 0 | 1/110 | 0.91 |
| New Brunswick | 29/445 | 6.52 | 1/445 | 0.22 | 107/445 | 24.04 | 3/445 | 0.67 | 0/445 | 0 |
| Total | 31/683 | 4.54 | 1/683 | 0.15 | 200/683 | 29.28 | 3/683 | 0.44 | 1/683 | 0.15 |
a Proportion positive tick equals the number of positive tick divided by the number of ticks tested
Figure 5.
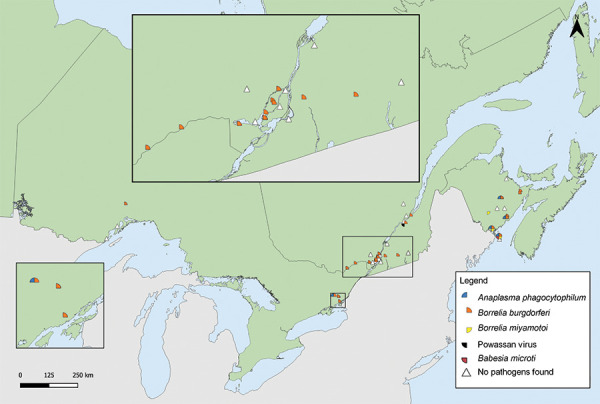
Ixodes scapularis ticks with associated pathogens collected through active surveillance, Canada, 2020a,b
a Each symbol represents an active surveillance site where A. phagocytophilum (n=31), B. microti (n=1), B. burgdorferi (n=200), B. miyamotoi (n=3), or Powassan virus (n=1) were found in I. scapularis ticks. There were 17 sites where no tick-borne pathogens were identified in I. scapularis ticks
b Number of ticks tested: Ontario (n=128), Québec (n=110) and New Brunswick (n=445)
Discussion
In 2020, I. scapularis and I. pacificus were submitted in passive surveillance from nine provinces. Only I. pacificus were submitted in British Columbia. The majority of ticks were female adults and obtained from human hosts. Among ticks that were tested, 18.21% of I. scapularis and 0.14% of I. pacificus were infected with at least one tick-borne pathogen, mainly B. burgdorferi. In active surveillance, five tick-borne pathogens (A. phagocytophilum, B. burgdorferi, B. miyamotoi, B. microti and Powassan virus) were identified among the I. scapularis collected in Ontario, Québec and New Brunswick.
From passive surveillance, 5,899 ticks were sample-based submissions, a decrease of 44% from the 10,549 ticks submitted in 2019 ((16)), which could be due, in part, to impacts from the coronavirus disease 2019 (COVID-19) pandemic. Beginning in spring 2020, COVID-19 pandemic restrictions affected traditional passive surveillance, as health units, medical clinics and veterinary clinics were limited in their ability to accept physical tick specimens at some locations (e.g. Simcoe Muskoka District Health Unit) ((23)). The decrease in submissions could also be due to changes to sample-based submission programs and greater emphasis on image-based submission programs in most jurisdictions. Active surveillance was also affected by pandemic restrictions, as in-person activities like field surveillance were limited (e.g. Institut national de santé publique du Québec) ((24)). Data from the Canadian Lyme Sentinel Network, which was included in the 2019 report ((16)), was unavailable in 2020 as Canadian Lyme Sentinel Network activities were suspended (personal communication, C. Guillot, 2022).
In passive surveillance, ticks were submitted every month, but submissions followed distinct species-specific patterns influenced by location and weather. Despite fewer ticks submitted to passive surveillance than in 2019 ((16)), the same bimodal peaks for I. scapularis adults that have been shown historically in central and eastern Canada ((13,25–27)) were observed in 2020. For I. pacificus, a single springtime peak was observed as shown previously in British Columbia ((14,16)) and the western United States ((28)). While risk of exposure to ticks was present year-round, exposure to tick-borne pathogens is dependent on infection prevalence and attachment time.
The proportion of ticks submitted from dogs or cats increased from 8.9% in 2019 to 15.1% in 2020 ((16)). This increase is likely from including data from eTick: whereas sample-based passive surveillance programs in some localities (e.g. health units, municipalities) are restricted to ticks from human hosts only, image-based passive surveillance has no such restriction, leading to a greater proportion of ticks from animal hosts when eTick data was included in this report.
Compared to 2019 ((16)), province and pathogen-specific infection prevalence estimates were similar, but geographic distribution was more limited in some cases (e.g. I. scapularis with A. phagocytophilum were limited to only the southernmost parts of New Brunswick compared to 2019). Several factors influence infection prevalence estimates from year-to-year or between provinces, including annual variation in weather, surveillance effort, habitat suitability, presence of established vector and reservoir populations and interactions between humans, ticks and the environment. Because of small sample sizes tested (n=<10), infection prevalence estimates from Saskatchewan and Newfoundland and Labrador should be interpreted with caution.
Ixodes pacificus (found in British Columbia) historically have low rates of B. burgdorferi infection ((14,16)), while B. burgdorferi infection prevalence in I. scapularis found in central and eastern Canada is typically higher ((18,25,29)); both trends continued to be observed in 2020. Jacob et al. ((30)) report higher infection prevalence among companion animals of several tick-borne pathogens compared to our estimates; however, participating veterinary clinics in that study were skewed towards areas with higher or emerging risk of TBD, likely leading to overestimation of the province-level infection prevalence. The one-year study also concluded in spring 2020, thus not accounting for the effects of pandemic restrictions on tick exposure for the remainder of 2020.
The majority of B. burgdorferi-infected I. scapularis had probable location of acquisition within LD risk areas ((8,22)). The remaining B. burgdorferi-infected I. scapularis may be adventitious ticks carried by migrating birds or mammals ((15)) or collected from areas with emerging LD risk. Provinces routinely review LD risk areas based on new surveillance data according to the 2016 case definition ((22)).
Despite limited opportunities for active field surveillance due to ongoing COVID-19 pandemic restrictions, over 600 I. scapularis were collected in drag sampling from 45 sites across Ontario, Québec and New Brunswick. Five tick-borne pathogens were identified, ranging in prevalence from 0.15% to 29.28%. This was the first detection of Powassan virus (deer tick lineage) in active surveillance in Québec ((24)), which has previously been identified in small numbers of Ixodes spp. in Manitoba, Ontario and New Brunswick ((12,31)).
In addition to single-agent infection with B. burgdorferi and the four other tick-borne pathogens, three distinct types of co-infections were identified. Surveillance beyond LD for other TBD is warranted to monitor the emergence and spread of these pathogens, especially as suitable habitat for Ixodes spp. is predicted to increase due to changes in climate and environment ((1,32,33)).
Co-infections have been reported to varying extents in ticks found in Canada ((16,18)) and the United States ((34)). Humans who are co-infected may experience a greater number and duration of symptoms compared to single-agent infections ((35,36)). Many factors influence the risk of co-infection, including attachment time, but preventing tick bites can help prevent transmission of all TBDs.
Strengths and limitations
This article presents a snapshot of infection prevalence and range estimates for the main LD vectors in Canada. While traditional passive surveillance programs have been discontinued or limited to specific hosts in some regions, incorporating data from eTick allows broader geographic and host representation from these regions in this summary. Combining passive and active surveillance also allows the strengths and weaknesses of the systems to complement each other. For example, while active surveillance is limited in geographic and temporal scope, passive surveillance programs gather data from large areas throughout the year.
There are several limitations to this study. Due to competing public health priorities, passive surveillance programs and the effort of active surveillance vary across Canada. As previously noted, COVID-19 pandemic restrictions affected public health services and surveillance in 2020, resulting in fewer sample-based submissions to passive surveillance and active surveillance that was less geographically representative compared to the previous year ((16)). Shifts in passive tick surveillance programs (e.g. limits on tick host or location of acquisition of tick; discontinuation of regional or provincial programs) have also limited the number of submissions. While digital platforms like eTick offer timely tick identification, tick specimens are not routinely requested for tick-borne pathogen testing from imaging identification platforms ((17)). Recall bias in reporting locality of acquisition and travel history in passive surveillance might create uncertainty as to the exact location where ticks were found. Finally, there are likely other active surveillance programs conducted in 2020 not included here in this summary if ticks were not sent for pathogen testing at NML. Furthermore, the number of larvae included in active surveillance is an underestimate, since our dataset only includes ticks sent for testing, for which larvae are rarely sent. These underestimates of the number of ticks may affect the accuracy of infection prevalence of various pathogens.
Conclusion
Ixodes scapularis and I. pacificus were identified across Canada in passive and active surveillance, some of which were infected with B. burgdorferi, the LD pathogen, but also with emerging tick-borne pathogen(s). Healthcare professionals and the public should be aware that there is a risk of exposure to infected ticks outside of known LD risk areas, even if the risk is low in those areas. The identification of new tick-borne pathogens in several jurisdictions in active surveillance may help public health authorities update their prevention strategies, as some of those emerging tick-borne illnesses, like Powassan virus disease, may have infection transmission patterns that differ from LD. As climate change alters the habitat and seasonality of tick vectors, continued surveillance can help in timely identification of new risk areas for LD and other emerging TBD, and directing public health interventions towards these at-risk areas.
Acknowledgements
We thank all those involved with tick collection and testing at regional, provincial, and national levels, including Thunder Bay District Health Unit; Kingston, Frontenac and Lennox & Addington Public Health; and members of the public who submitted ticks. We thank M Stefopulos (PHAC) for assistance in creating Figure 3. In addition to co-author J Savage, the composition of the team involved in collection and processing of eTick data included several students as well as the following people: C Jardine (Department of Pathobiology, University of Guelph, Guelph, Ontario [ON]); K Clow (Department of Population Medicine, University of Guelph, Guelph, ON); M Kulkarni (School of Epidemiology and Public Health, University of Ottawa, Ottawa, ON); J Nocera (Faculty of Forestry and Environmental Management, University of New Brunswick, Fredericton, New Brunswick [NB]); S Heard (Department of Biology, University of New Brunswick, Fredericton, NB); E Jenkins, M Voordouw (Department of Veterinary Microbiology, University of Saskatchewan, Saskatoon, Saskatchewan); D Shutler, K Hillier (Department of Biology, Acadia University, Wolfville, Nova Scotia); J Bowden (Natural Resources Canada, Canadian Forest Service, Atlantic Forestry Centre, Corner Brook, Newfoundland and Labrador); P Chuard, J Bouffard (Department of Biology and Biochemistry, Bishop’s University, Sherbrooke, Québec).
Competing interests
None.
Funding
This study was supported by the Public Health Agency of Canada. Passive surveillance from British Columbia was supported by the BC Centre for Disease Control Foundation. Passive surveillance in Saskatchewan is partially funded by the Government of Saskatchewan. Passive and active surveillance from Québec was supported by the Ministère de la Santé et des Services sociaux (MSSS).
References
- 1.Bouchard C, Dibernardo A, Koffi J, Wood H, Leighton PA, Lindsay LR. N Increased risk of tick-borne diseases with climate and environmental changes. Can Commun Dis Rep 2019;45(4):83–9. 10.14745/ccdr.v45i04a02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robinson EL, Jardine CM, Koffi JK, Russell C, Lindsay LR, Dibernardo A, Clow KM. Range expansion of Ixodes scapularis and Borrelia burgdorferi in Ontario, Canada, from 2017 to 2019. Vector Borne Zoonotic Dis 2022;22(7):361–9. 10.1089/vbz.2022.0015 [DOI] [PubMed] [Google Scholar]
- 3.Ogden NH, Koffi JK, Pelcat Y, Lindsay LR. Environmental risk from Lyme disease in central and eastern Canada: a summary of recent surveillance information. Can Commun Dis Rep 2014;40(5):74–82. 10.14745/ccdr.v40i05a01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ogden NH, Mechai S, Margos G. Changing geographic ranges of ticks and tick-borne pathogens: drivers, mechanisms and consequences for pathogen diversity. Front Cell Infect Microbiol 2013;3:46. 10.3389/fcimb.2013.00046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ogden NH, Radojević M, Wu X, Duvvuri VR, Leighton PA, Wu J. Estimated effects of projected climate change on the basic reproductive number of the Lyme disease vector Ixodes scapularis. Environ Health Perspect 2014;122(6):631–8. 10.1289/ehp.1307799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ogden NH, Ben Beard C, Ginsberg HS, Tsao JI. Possible effects of climate change on Ixodid ticks and the pathogens they transmit: predictions and observations. J Med Entomol 2021;58(4):1536–45. 10.1093/jme/tjaa220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gasmi S, Ogden NH, Leighton PA, Adam-Poupart A, Milord F, Lindsay LR, Barkati S, Thivierge K. Practices of Lyme disease diagnosis and treatment by general practitioners in Quebec, 2008-2015. BMC Fam Pract 2017;18(1):65. 10.1186/s12875-017-0636-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Public Health Agency of Canada. Lyme disease: Surveillance. Ottawa, ON: PHAC; 2023. [Accessed 2022 June 16]. https://www.canada.ca/en/public-health/services/diseases/lyme-disease/surveillance-lyme-disease.html
- 9.Campeau L, Roy V, Petit G, Baron G, Blouin J, Carignan A. Identification of an unusual cluster of human granulocytic anaplasmosis in the Estrie region, Québec, Canada in 2021. Can Commun Dis Rep 2022;48(5):188–95. 10.14745/ccdr.v48i05a02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allehebi ZO, Khan FM, Robbins M, Simms E, Xiang R, Shawwa A, Lindsay LR, Dibernardo A, d’Entremont C, Crowell A, LeBlanc JJ, Haldane DJ. Lyme Disease, Anaplasmosis, and Babesiosis, Atlantic Canada. Emerg Infect Dis 2022;28(6):1292–4. 10.3201/eid2806.220443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kadkhoda K, Dumouchel C, Brancato J, Gretchen A, Krause PJ. Human seroprevalence of Borrelia miyamotoi in Manitoba, Canada, in 2011-2014: a cross-sectional study. CMAJ Open 2017;5(3):E690–3. 10.9778/cmajo.20170070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanderson M, Lindsay LR, Campbell TM, Morshed M. A case of Powassan encephalitis acquired in southern Quebec. CMAJ 2018;190(50):E1478–80. 10.1503/cmaj.180905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ogden NH, Trudel L, Artsob H, Barker IK, Beauchamp G, Charron DF, Drebot MA, Galloway TD, O’Handley R, Thompson RA, Lindsay LR. Ixodes scapularis ticks collected by passive surveillance in Canada: analysis of geographic distribution and infection with Lyme borreliosis agent Borrelia burgdorferi. J Med Entomol 2006;43(3):600–9. 10.1093/jmedent/43.3.600 [DOI] [PubMed] [Google Scholar]
- 14.Morshed MG, Lee MK, Boyd E, Mak S, Fraser E, Nguyen J, Dykstra EA, Otterstatter M, Henry B, Galanis E. Passive tick surveillance and detection of Borrelia species in ticks from British Columbia, Canada: 2002-2018. Vector Borne Zoonotic Dis 2021;21(7):490–7. 10.1089/vbz.2020.2743 [DOI] [PubMed] [Google Scholar]
- 15.Ogden NH, Bouchard C, Kurtenbach K, Margos G, Lindsay LR, Trudel L, Nguon S, Milord F. Active and passive surveillance and phylogenetic analysis of Borrelia burgdorferi elucidate the process of Lyme disease risk emergence in Canada. Environ Health Perspect 2010;118(7):909–14. 10.1289/ehp.0901766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilson CH, Gasmi S, Bourgeois AC, Badcock J, Chahil N, Kulkarni MA, Lee MK, Lindsay LR, Leighton PA, Morshed MG, Smolarchuk C, Koffi JK. Surveillance for Ixodes scapularis and Ixodes pacificus ticks and their associated pathogens in Canada, 2019. Can Commun Dis Rep 2022;48(5):208–18. 10.14745/ccdr.v48i05a04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koffi JK, Savage J, Thivierge K, Lindsay LR, Bouchard C, Pelcat Y, Ogden NH. Evaluating the submission of digital images as a method of surveillance for Ixodes scapularis ticks. Parasitology 2017;144(7):877–83. 10.1017/S0031182017000117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dibernardo A, Cote T, Ogden NH, Lindsay LR. The prevalence of Borrelia miyamotoi infection, and co-infections with other Borrelia spp. in Ixodes scapularis ticks collected in Canada. Parasit Vectors 2014;7:183. 10.1186/1756-3305-7-183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schillberg E, Lunny D, Lindsay LR, Nelder MP, Russell C, Mackie M, Coats D, Berry A, Young Hoon KN. Distribution of Ixodes scapularis in northwestern Ontario: results from active and passive surveillance activities in the Northwestern Health Unit catchment area. Int J Environ Res Public Health 2018;15(10):2225. 10.3390/ijerph15102225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Biggerstaff BJ. PooledInfRate, Version 4.0: a Microsoft® Office Excel© Add-In to compute prevalence estimates from pooled samples. Fort Collins, CO: CDC; 2009. https://www.cdc.gov/westnile/resourcepages/mosqSurvSoft.html [Google Scholar]
- 21.Walter SD, Hildreth SW, Beaty BJ. Estimation of infection rates in population of organisms using pools of variable size. Am J Epidemiol 1980;112(1):124–8. 10.1093/oxfordjournals.aje.a112961 [DOI] [PubMed] [Google Scholar]
- 22.Public Health Agency of Canada. Lyme disease 2016 case definition. Ottawa, ON: PHAC; 2022. [Accessed 2022 Aug 31]. https://www.canada.ca/en/public-health/services/diseases/lyme-disease/surveillance-lyme-disease/case-definition.html
- 23.Simcoe Muskoka District Health Unit. Passive Tick Surveillance. Barrie, ON: SMDHU; 2021. [Accessed 2022 Oct 20]. https://www.simcoemuskokahealthstats.org/topics/environment/vectorborne-disease/passive-tick-surveillance
- 24.Institut national de santé publique du Québec. Résultats de surveillance de la maladie de Lyme : année 2020. Montréal, QC: INSPQ; 2022. [Accessed 2022 Aug 15]. https://www.inspq.qc.ca/zoonoses/maladie-de-lyme/resultats-de-surveillance-2020
- 25.Nelder MP, Russell CB, Dibernardo A, Clow KM, Johnson S, Cronin K, Patel SN, Lindsay LR. Monitoring the patterns of submission and presence of tick-borne pathogens in Ixodes scapularis collected from humans and companion animals in Ontario, Canada (2011-2017). Parasit Vectors 2021;14(1):260. 10.1186/s13071-021-04750-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gasmi S, Ogden NH, Leighton PA, Lindsay LR, Thivierge K. Analysis of the human population bitten by Ixodes scapularis ticks in Quebec, Canada: increasing risk of Lyme disease. Ticks Tick Borne Dis 2016;7(6):1075–81. 10.1016/j.ttbdis.2016.09.006 [DOI] [PubMed] [Google Scholar]
- 27.Lewis J, Kirby AM, Harris KD, Filiaggi CL, Foley-Eby A, Mann M, Lieske D, Lloyd VK. Monitoring risk: tick and Borrelia burgdorferi public participatory surveillance in the Canadian Maritimes, 2012–2020. Pathogens 2021;10(10):1284. 10.3390/pathogens10101284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu G, Pearson P, Dykstra E, Andrews ES, Rich SM. Human-biting Ixodes ticks and pathogen prevalence from California, Oregon, and Washington. Vector Borne Zoonotic Dis 2019;19(2):106–14. 10.1089/vbz.2018.2323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Institut national de santé publique du Québec. Portrait de la maladie de Lyme au Québec: 2006-2019. Montréal, QC: INSPQ; 2022. [Accessed 2022 Oct 11]. https://www.inspq.qc.ca/publications/2844
- 30.Jacob AE, Weese JS, Rosseau J, Clow KM. Spatial patterns of Borrelia burgdorferi, Borrelia miyamotoi and Anaplasma phagocytophilum detected in Ixodes spp. ticks from Canadian companion animals, 2019-2020. Zoonoses Public Health 2022;69(8):944–55. 10.1111/zph.12992 [DOI] [PubMed] [Google Scholar]
- 31.Smith K, Oesterle PT, Jardine CM, Dibernardo A, Huynh C, Lindsay R, Pearl DL, Bosco-Lauth AM, Nemeth NM. Powassan virus and other arthropod-borne viruses in wildlife and ticks in Ontario, Canada. Am J Trop Med Hyg 2018;99(2):458–65. 10.4269/ajtmh.18-0098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kotchi SO, Bouchard C, Brazeau S, Ogden NH. Earth observation-informed risk maps of the Lyme disease vector Ixodes scapularis in Central and Eastern Canada. Remote Sens 2021;13(3):524. 10.3390/rs13030524 [DOI] [Google Scholar]
- 33.Leighton PA, Koffi JK, Pelcat Y, Lindsay LR, Ogden NH. Predicting the speed of tick invasion: an empirical model of range expansion for the Lyme disease vector Ixodes scapularis in Canada. J Appl Ecol 2012;49(2):457–64. 10.1111/j.1365-2664.2012.02112.x [DOI] [Google Scholar]
- 34.Aliota MT, Dupuis AP 2nd, Wilczek MP, Peters RJ, Ostfeld RS, Kramer LD. The prevalence of zoonotic tick-borne pathogens in Ixodes scapularis collected in the Hudson Valley, New York State. Vector Borne Zoonotic Dis 2014;14(4):245–50. 10.1089/vbz.2013.1475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krause PJ, McKay K, Thompson CA, Sikand VK, Lentz R, Lepore T, Closter L, Christianson D, Telford SR, Persing D, Radolf JD, Spielman A; Deer-Associated Infection Study Group . Disease-specific diagnosis of coinfecting tickborne zoonoses: babesiosis, human granulocytic ehrlichiosis, and Lyme disease. Clin Infect Dis 2002;34(9):1184–91. 10.1086/339813 [DOI] [PubMed] [Google Scholar]
- 36.Diuk-Wasser MA, Vannier E, Krause PJ. Coinfection by Ixodes tick-borne pathogens: ecological, epidemiological, and clinical consequences. Trends Parasitol 2016;32(1):30–42. 10.1016/j.pt.2015.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]


